Abstract
Respirable crystalline silica in mineral dust, wood dust, diesel engine exhaust emissions and welding fumes are among the most common process‐generated substances to which millions of workers are exposed daily. The composition of process‐generated substances can vary substantially, depending on the parameters of the underlying processes; for example, the composition and intensity of diesel motor emissions differs among the various generations of diesel engines and working environments (e.g. surface or underground mining). We illustrate how common these occupational exposures are and discuss challenges in estimating their global prevalence and their contribution to the burden of occupational cancer. Estimates of the number and proportion of workers exposed in most countries and on a global scale are generally scarce. A remarkable exception is based on the proactive bottom‐up estimates generated within the European Network for Silica. Actions to reduce exposures and research to fill gaps in knowledge adapted to local settings are warranted to mitigate the occupational cancer burden, especially in under‐researched settings including low‐ and middle‐income countries.
Keywords: diesel engine exhaust, occupational cancer burden, occupational exposures, process‐generated substances, respirable crystalline silica, welding fumes, wood dust
Occupational exposure to respirable crystalline silica, diesel engine exhaust emissions and welding fumes are widespread risk factors for lung cancer and account for approximately half of the occupational lung cancer burden. If employers succeed in controlling workplace exposures to these process‐generated substances, the fraction of lung cancers attributable to occupational exposures could be reduced dramatically.

Abbreviations
- AWES
Australian Work Exposures Study
- CAREX
CARcinogen EXposure, international information system on occupational exposure to carcinogens
- CEE
Central and Eastern Europe
- ECHA
European Chemicals Agency
- ECRHS
European Community Respiratory Health Survey
- EU
European Union
- IARC
International Agency for Research in Cancer
- IMA‐DMP
European Industrial Minerals Association Dust Monitoring Program
- NEPSI
European Network on Silica
- NIOSH
National Institute for Occupational Safety and Health
- NMSC
nonmelanoma skin cancer
- OccIDEAS
Occupational Integrated Database Exposure Assessment System
- OSHA
Occupational Safety and Health Administration
- PAF
population attributable fraction
- RCS
respirable crystalline silica
- REACH
Registration, Evaluation, Authorisation and Restriction of Chemicals
- REL
recommended exposure limit
- REP
risk exposure periods
- RERI
relative excess risk due to interaction
- SCOEL
Scientific Committee on Occupational Exposure Limits
1. The burden of cancer related to occupational exposures
Cancers caused wholly or partly by exposure to carcinogenic agents at work or by circumstances at work can be referred to as occupational cancers. The most frequent occupational cancers are lung cancer, mesothelioma and bladder cancer. Figure 1 shows the numbers of cancers attributable to occupational exposures in the total population and stratified by sex in three nationwide studies in Great Britain, Canada and France [1, 2, 3]. Occupational cancers are preventable by eliminating hazardous substances or enhancing the protection of workers and reducing exposures.
Fig. 1.
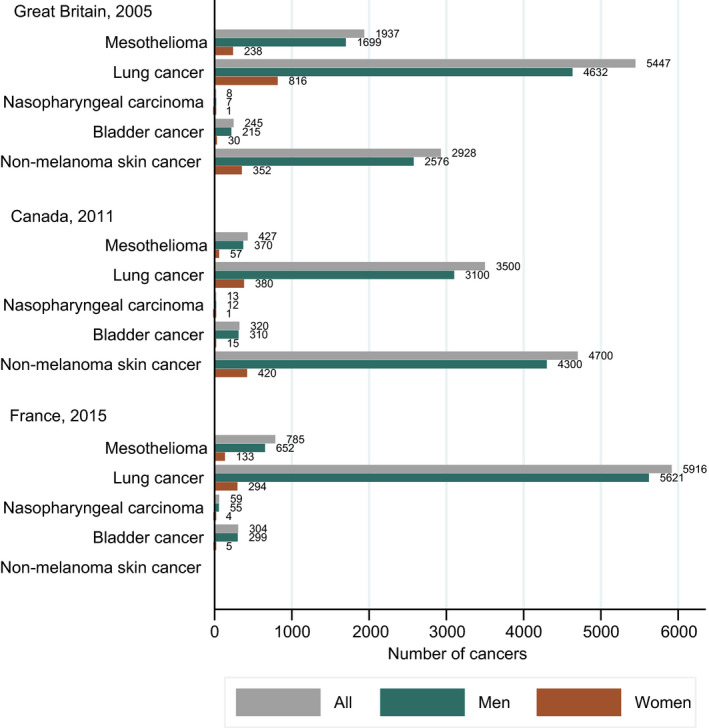
Number of cancer cases attributable to occupational exposures at selected cancer sites in Great Britain [1], Canada [2] and France [3] in the total population and by gender.
In epidemiology, the ‘population attributable fraction’ (PAF) is estimated to assess the public health impact of specific risk factors and to rank them. The PAF, usually presented as a percentage, represents the estimated proportion of cases that would not have occurred if the exposure had not been present. The PAF is directly determined by the magnitude of risk associated with the exposure and the prevalence and level of the exposure in the working population [4].
The overall cancer burden attributable to occupational exposures has most often been estimated between 2% and 5% since the 1980s [1, 2, 3, 5]. Figure 2 shows the PAFs for occupational exposures (total and stratified by sex) for selected cancers in Great Britain, Canada and France [1, 2, 3]. Mesothelioma is mainly associated with exposure to asbestos [6]. Lung cancer is associated with many occupational exposures including asbestos, crystalline silica, diesel engine exhaust and welding fume [6, 7, 8]. Nasopharyngeal cancer is associated with wood dust and formaldehyde [6, 9]. Urinary bladder cancer is associated with exposure to aromatic amines, diesel engine exhaust and exposures in painting and the rubber industry [9]. Nonmelanoma skin cancer (NMSC) is associated with exposure to solar radiation, coal tars and pitches, mineral oils and arsenic [6, 10]. The figure shows a marked difference in PAFs by cancer site with mesothelioma having the highest occupational PAF, followed by lung cancer, nasopharyngeal, bladder and NMSC. There are also large differences in occupational PAFs by sex, with generally larger PAFs among men. Men are indeed 4–5 times more likely to work in primary production jobs with higher occupational exposures compared with women [11, 12]. Finally, it is noticeable that not all cancer types are included in all studies; for example, NMSC is not included in the French study.
Fig. 2.
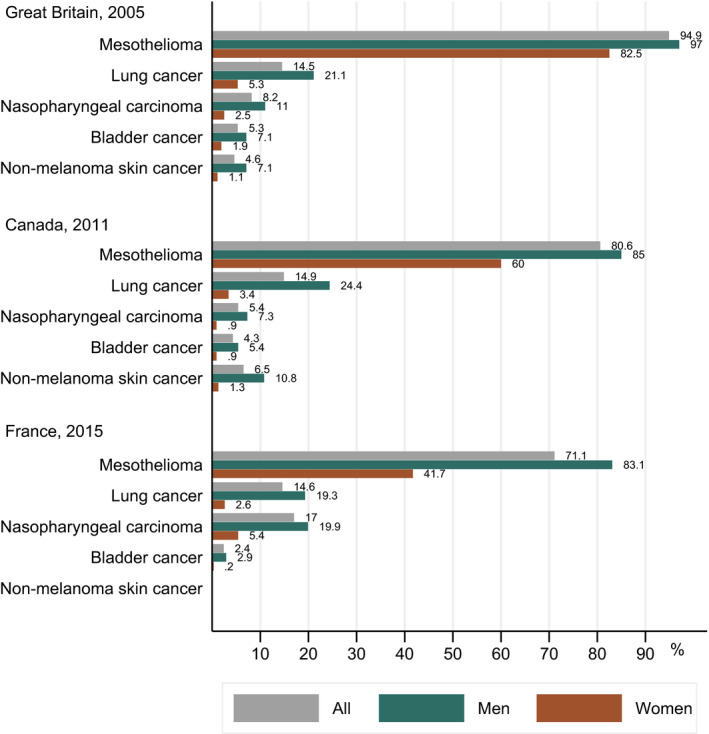
Cancer burden (%) attributable to occupational exposures at selected cancer sites, in Great Britain [1], Canada [2] and France [3] in the total population and by gender.
PAFs are not easily comparable across studies due to differences in the selection of exposure‐cancer combinations, the use of varying criteria to define prevalence and level of exposures encountered, differences in the selection of risk estimates associated with the exposures, etc. For example, the French study included only IARC Group 1 substances (n = 25), the study from Great Britain included in addition Group 2A substances (n = 41) and the Canadian study included 44 exposures whereof two Group 2A (creosotes and night shift work) [1, 2, 3]. The French project was studying the burden of cancer attributable to all modifiable risk factors and therefore assigned ‘second‐hand smoke’ to ‘cancers attributable to tobacco smoking’ rather than occupational exposures. Moreover, solar radiation was considered as a risk factor for cutaneous melanoma attributable to solar ultraviolet radiation, together with the use of sunbeds. NMSCs were not included in the French study or the Global Burden of Disease project, possibly because NMSCs often are not systematically registered and therefore may be severely under‐reported [13, 14, 15]. Other reasons to exclude exposures or exposure‐cancer combinations from the estimation are the lack of exposure prevalence data, or that exposures are no longer relevant or thought to be present (e.g. after asbestos use bans) [16].
The methodology for estimating PAFs has evolved to account for relevant risk exposure periods (REP) defined by the cancer latency for solid tumours (0–50 years) and for haematopoietic cancers (0–20 years), workers turnover (which might differ considerable between industries and especially over time) and proportion of workers exposed to high‐ versus low levels of exposure (however without a clear definition of high and low levels of exposure). Nonetheless, study bias and uncertainty are present. The choice of risk estimates and the employment turnover was identified as the largest contributors to the occupational PAF estimates in the British study [17].
Smoking patterns among workers should be incorporated in the methodology for estimating of the occupational cancer burden in the future. The reason is that many occupational exposures confer stronger effects among smokers. In the SYNERGY project – a large pooled analysis of case–control studies on interactions of smoking and occupational exposure risks relating to lung cancer incidence – an additive scale was used to calculate the relative excess risk due to interaction (RERI) by fitting linear odds ratio (OR) models [18]. The RERI measures the extent to which the effect of both exposures (e.g. smoking and having worked as a painter) combined exceeds the sum of the effects of each considered separately, and a RERI > 0 indicates a positive additive interaction, where the effect of both exposures together exceeds the sum of the two exposures considered separately. Given that smoking is a strong risk factor for lung cancer, this marked difference in smoking habits between men and women especially in the past may, at least partly, explain why we often see less effect of occupational exposures in exposed women than in exposed men [18, 19, 20, 21]. A recent paper by Kulhánová et al. [22] shows that the tobacco‐related cancer burden in Europe differs across countries and genders; the largest and the lowest PAF due to smoking in males occurred in Eastern Europe (35% of all cancer cases) and Northern Europe (21%), while among women this pattern was reversed (16% in Northern Europe and 6% in Eastern. Consequently, it would be beneficial to account for smoking patterns at the relevant period by sex in each country, when estimating the occupational cancer burden.
In this review, we discuss the contribution of process‐generated substances to the occupational cancer burden, with a focus on occupational exposure to crystalline silica, wood dust, diesel engine exhaust and welding fumes. Our aim is to illustrate how common these exposures have been and still remain, to a large extent, in the working environment, and to discuss their contribution to the global occupational cancer burden. We also address the challenges in estimating the global prevalence of these occupational exposures and the subsequent burden of occupational cancer.
2. Process‐generated substances
Process‐generated substances are generated as emissions from combustion or heating processes, abrasion and other processes that physically or chemically modify or degrade the starting material(s) and are, thus, present in various workplaces. Respirable crystalline silica in mineral dust, wood dust, diesel engine exhaust emissions, welding fumes, flour dust, bitumen fumes and rubber curing fumes are some examples of common process‐generated substances affecting millions of workers daily. While a clear definition is not readily available, these process‐generated substances often exist as mixtures and can be of chemical or biological nature. They are by their nature more complex than single chemical substances or single biological species, and they are not seen as manufactured products that can be traded and tracked along a supply chain. Therefore in the EU, these substances are not regulated by the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) of the European Union (EU) REACH regulation in the EU [23], but they are part of the Carcinogens and Mutagens Directive 2004/37/EC [24]. Since 2019, the Risk Assessment Committee of the European Chemicals Agency (ECHA) provides scientific opinions on occupational exposure limits after it took over this responsibility from the Commission's Scientific Committee on Occupational Exposure Limits (SCOEL).
The composition of a process‐generated substance can vary substantially, depending on the parameters of the underlying process. For instance: differences in recipes for rubber compounding may produce vulcanization or curing fumes that differ dramatically not only in composition, but also in levels of the individual chemicals present in these fumes [25]. Similarly, the composition of organic dusts can differ dramatically when working with organic material with different moisture content [26]. For process‐generated substances such as diesel motor emissions, the composition and intensity will differ among the various generations of diesel engines and environments where human exposure occurs (e.g. surface or underground mining) [27].
For many process‐generated substances, exposed populations are considerably larger when compared to single (chemical) substances. Kauppinen et al. (2000) generated in a Europe Against Cancer project called CAREX (CARcinogen EXposure) the number of workers exposed in the then 15 EU member states in the early 1990s. The CAREX project covered all agents, groups of agents and process‐generated substances which were considered by the International Agency for Research on Cancer (IARC) as known or suspected carcinogenic agents as of February 1995 [28]. The prevalence estimates from the CAREX paper indicated that the number of workers exposed to certain process‐generated substances make up for the majority of exposures to known or suspected carcinogenic chemical agents [29]. The four most prevalent exposures to substances described within the CAREX project included the following process‐generated substances: environmental tobacco smoke > 75% of working time, crystalline silica, diesel engine exhaust and wood dust. These four process‐generated substances resulted in an estimated total of slightly more than 16 million exposed workers in 15 member states, which represented 55% of the total number of workers exposed to 80 known or suspected carcinogenic substances considered.
The CAREX approach has consequently been applied and adapted to national circumstances by experts in industrial hygiene, other experts and data sources in individual countries including Costa Rica, Estonia, Czech Republic, Latvia and Lithuania [30, 31, 32]. In Costa Rica, CAREX was renamed TICAREX and included self‐employed persons and working family members older than 12 years to reflect the national working situation more accurately. The top‐10 substances in TICAREX were largely overlapping with the original CAREX, but it also provided separate numbers for men and women [32]. In the Baltic countries, there were some differences noted such as that exposure to wood dust being more prevalent in Estonia due to large wood and furniture industries, while for Lithuania, the prevalence of benzene exposure was more prevalent due to the presence of oil refineries [30].
A more recent and refined carcinogen surveillance project modelled on the original European CAREX, called CAREX Canada, showed similar prevalence of carcinogen exposures for the year 2006 [33]. The process‐generated substances diesel engine exhaust, crystalline silica, polycyclic aromatic hydrocarbons (PAHs) and wood dust were again located at the top of the list of 40 known or suspected carcinogenic chemical agents considered, and as in Europe, these agents represented half of the workers' exposures.
A study in Quebec made use of CAREX Canada but refined the estimates by including additional workplace monitoring data, research projects, a population survey and radiation protection data [34]. The European and the Costa Rican CAREX methodology was applied with modifications in Nicaragua and Panama and included in addition relevant pesticides. Population censuses provided industry‐ and sex‐specific workforce numbers and experts from governmental agencies, workers' organizations and employers' representatives estimated activity‐ and sex‐specific proportions of exposed workers [35].
The SHEcan project, sponsored by EC DG Employment in Luxembourg and carried out between 2009 and 2011, investigated the socioeconomic, health and environmental impact associated with a range of policy options for amendments to Directive 2004/37/EC (Carcinogens or Mutagens at work) [36]. The purpose of the assessment was to enable the European Commission to initiate informed discussions with stakeholders about potential impacts of changes in legislation. The SHEcan reports included estimates of the number of workers exposed [36].
The relatively recent Australian Work Exposures Study (AWES) conducted a cross‐sectional survey including about 5000 respondents (53% response fraction) from a random sample of the population. Data were collected by trained interviewers using a computer‐assisted telephone interview. An automated expert assessment method (OccIDEAS) assigned exposure to carcinogens based on the general job information and when justified job‐specific modules [37]. The prevalence figures were thereafter extrapolated to the Australian working population by sex. OccIDEAS allows modifying definitions of exposure, for example to define ‘substantial exposure’ of a substance resulting in an alternative prevalence of exposure [38].
Table 1 provides an overview of estimates of number of workers (men and women) and percentages of the workforce exposed to crystalline silica, wood dust, diesel engine exhaust and welding fumes in different regions/countries in different periods.
Table 1.
Overview of studies and other resources estimating number of workers (men and women) and percentages exposed to respirable crystalline silica, wood dust, diesel engine exhaust and welding fumes.
| Study [Reference] | Location | Year(s) | Number of workers and percentages (%) of exposed to selected process‐generated substances | Total working population | |||
|---|---|---|---|---|---|---|---|
| Respirable crystalline silica | Wood dust | Diesel engine exhaust | Welding fumes | ||||
| CAREX [29] | EU‐15 | 1990–1993 | 3 200 000 (2.3) | 2 600 000 (1.9) | 3 000 000 (2.2) | ‐ | 139 000 000 |
|
Occupational cancer in Britain [56] |
UK | 1990–1993, 1979 | 564 787 (2.0) | 433 834 (1.5) | 473 062 (1.6) | 172 418 (welders) | 28 768 000 a |
| CAREX [30] | Estonia | 1997 | 19 000 (3.1) | 34 000 (5.5) | 21 000 (3.4) | 620 689 | |
| Latvia | 1997 | 19 000 (2.0) | 35 000 (3.8) | 20 000 (2.2) | 928 571 | ||
| Lithuania | 1997 | 40 000 (2.4) | 47 000 (2.8) | 37 000 (2.2) | 1 678 571 | ||
| Czech Republic | 1997 | 170 000 (3.4) | 180 000 (3.6) | 130 000 (2.6) | 5 000 000 | ||
| TICAREX [32] | Costa Rica | 2000 | 27 100 (2.1) | 32 200 (2.5) | 278 000 (21.4) | 1 300 000 | |
| CAREX/SALTRA program [35] | Panama | 2006 | 66 274 (6.9) | 22 091 (2.3) | 258 374 (26.9) | 960 500 | |
| Nicaragua | 2007 | 31 213 (1.5) | 47 860 (2.3) | 407 856 (19.6) | 2 080 899 | ||
| CAREX Canada [33] | Canada | 2006 | 382 000 (2.3) | 338 000 (2.0) | 781 000 (4.6) | 16 800 000 | |
| CAREX Canada [34] | Quebec | 2006 | 57 600 (1.6) | 101 600 (2.8) | 152 900 (4.2) | 3 600 000 | |
| Australian Work Exposures Study (AWES) [37] | Australia | 2011–2012 | 586 900 (6.5) | 478 320 (5.3) | 1 599 700 (17.9) | 8 933 000 | |
| SHECAN (http://www.occupationalcancer.eu/projresults.html) | EU‐25 | 2006 | 5 300 000 (2.6) | Hardwood 3 000 000 (1.4) | 3 600 000 (1.7) | 206 700 000 b | |
| IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 118. Welding, Molybdenum Trioxide and Indium Tin Oxide [51] | Worldwide | 2017 | 110 000 000 (3) | 3 500 000 000 | |||
UK Commission for employment and skills. Working Futures 2010–2020: Main Report. August 2012, page 83.
EUROSTAT Euro‐indicators 37/2007 14 March 2007.
2.1. Respirable crystalline silica
From Table 1, it is clear that the prevalence of occupational exposure to respirable crystalline silica (RCS) is estimated to be between 2% and 3% of the working population, but estimates for Panama, Nicaragua and Australia are much higher at 6–7%. This might be the consequence of different methodological approaches. In Europe, fifteen industry sector organizations and their counterpart trade union federations negotiated a multisectoral social dialogue agreement on (exposure to) crystalline silica that was signed by all parties in October 2006 [39]. This unique agreement resulted among others in a bottom‐up exercise of reporting numbers of workers exposed to crystalline silica at 6200 sites (85% of the total number of sites) of 19 industrial sectors. In the 2018 report of the European Network on Silica (NEPSI), it was estimated that 180 000 workers were exposed to RCS [40]. This is considerably lower than the 320 000 estimated by Kauppinen et al. [29] for the early 1990s, but is most likely due to the large construction and agricultural sectors not being part of NEPSI where exposure to respirable crystalline silica does occur [41, 42].
Evidence of long‐term trends in levels of RCS exposure is abundant. Peters et al. [43] reported an overall downward time trend in RCS exposure levels in Europe of −6% per year across all industries over a time period covering 1976–2009. Analyses of the European Industrial Minerals Association Dust Monitoring Program (IMA‐DMP) that started in 2000 showed overall downward temporal trends of −9.0% and −3.9% per year for, respectively, respirable dust and respirable quartz. No downward trends and even a slight increase were seen within the IMA‐DMP during the most recent global economic crisis between 2008 and 2012. After this period, exposure concentrations started to decline again [44].
The National Institute for Occupational Safety and Health in the United States (NIOSH) estimated the numbers and percentages of workers exposed to respirable crystalline silica at levels of at least 1, 2, 5 and 10 times the NIOSH recommended exposure limit (REL 50 µg·m−3), based on the Occupational Safety and Health Administration (OSHA)'s compliance inspection sampling data from 1979 to 2015. Approximately 100 000 workers were exposed to crystalline silica above the REL, and most (~ 80%) worked in the construction industry [45].
2.2. Wood dust
From Table 1, the estimates of prevalence of exposure to wood dust vary between 1.4% and 5.5% of the workforce. Local differences in construction and availability of wood as construction material as well as difference in definition (only hardwood exposure considered in some estimates) will be underlying these considerable differences in prevalence estimates.
Quite a considerable number of studies on long‐term trends in wood dust exposure concentrations exist. Galea et al. [46] reported an annual decrease of −8% during over a 20‐year period (1985–2005) in wood‐treating industries. An almost similar annual trend of −7% was derived from two cross‐sectional studies in the furniture industry in Denmark over the turn of the century (1997–2004) [47]. Long‐term annual trends of −11% in wood dust exposure concentrations were reported for different industries based on data in OSHA's Integrated Management Information System (1979–1997). A recent study from Sweden in the wood pellet production showed an even steeper decline of −20% per annum [48].
2.3. Diesel engine exhaust emissions
Estimates of the number of workers exposure to diesel engine exhaust emissions vary wildly, with earlier estimates varying between 1% and 3% of the workforce, later estimates around 4–5% and most recent estimates providing unlikely extremely high prevalence estimates of 18–27%. Most likely, methods applied and definitions of what occupational exposure to diesel engine exhaust entails will be accountable for this. The authors explained that very few exposure measurements have been conducted in Nicaragua and Panama, and therefore, they relied heavily on expert judgment (n = 25 in each country) from relevant authorities [35].
A few long‐term trends in levels of exposure to diesel engine exhaust emissions exist. For the U.S. trucking industry, sharp declines in exposure to elemental carbon (as a marker for diesel engine exhaust emissions) were estimated which were job group specific [49]. Exposure levels differed between and within job groups as well as between types of trucking terminal, and regions of the United States. Two large studies in the mining industry also showed diverse trends and large difference between job groups and between surface and underground work [27, 50].
2.4. Welding fumes
Welding‐related exposures were recently classified a human carcinogen by IARC, and it was estimated by the working group during the review process that 11 million workers are exposed as (full‐time) welders, while the total number of workers (part‐time) exposed to welding fumes was estimated to be around 110 million (3% of the worldwide economically active population) [51].
Studies on long‐term trends in exposure to welding fumes are hard to find. For the European Community Respiratory Health Survey (ECRHS) II study, a welding fume algorithm was developed based on a database of 1233 welding fume personal measurements from the Netherlands collected over a 20‐year period [52, 53]. A relatively minor 2–3% annual decrease in welding fume concentrations (halving of concentrations after 20–35 years) was observed. Within the ECRHS study, cumulative exposures in Northern Europe were lower than in Southern Europe most likely as result of differences in welding techniques, available control measures and/or hours welding per day/week, since no difference was seen in average number of years welding in Northern and Southern Europe.
3. Conclusions
The PAF for lung cancer due to occupational exposure has been estimated to be between 18 and 25% in men and 2–6% in women, resulting in lung cancer being the most prevalent occupational cancer [1, 2, 3]. Generally, occupational exposure to asbestos is considered to be contributing the most to the occupational PAF for lung cancer, followed by occupational exposures to respirable crystalline silica, diesel engine exhaust emissions and welding fumes. Table 2 shows examples of occupational PAFs for lung cancer for the selected process‐generated substances and confirms the ranking although the PAFs vary slightly by study and study type. Together exposure to respirable crystalline silica, diesel engine exhaust emissions and welding fumes account for half of the occupational PAF for lung cancer. If employers succeed in controlling workplace exposures to process‐generated substances, the fraction of lung cancers attributable to occupational exposures would be reduced dramatically.
Table 2.
Overview of studies estimating the occupational lung cancer burden (PAFs) related to selected process‐generated substances and the source of the estimated exposure prevalence in different types of studies.
| Reference | Where, when | Population attributable fractions (%) | Source of proportion exposed | |||||
|---|---|---|---|---|---|---|---|---|
| Respirable crystalline silica | Welding fumes | Diesel engine exhaust | ||||||
| Men | Women | Men | Women | Men | Women | |||
| Boffetta et al. [16] | France, 2000 | 0.5 | 0.07 | SUMER 1994 survey | ||||
| Olsson et al. [66] | CEE, 1998–2002 | 4.9 | 2.2 | IARC CEE case–control study | ||||
| De Matteis et al. [67] | Italy, 2002–2005 | 5.7 | – | EAGLE case–control study | ||||
| Rushton et al. 2010 [68] | GB, 2005 | 4.2 | 0.4 | 0.7 | 0.1 | 3.0 | 0.5 | CAREX 1990–1993, Labour Force Survey |
| Labrèche et al. [3] | Canada, 2011 | 4.4 | 0.2 | 2.4 | 0.08 | 4.3 | 0.2 | CAREX Canada |
| Marant Micallef et al. [4] | France, 2015 | 1.5 | 0.1 | 1.4 | 0.1 | SUMER 2003 survey | ||
Kauppinen and coworkers should be applauded for inclusion of suspected carcinogenic agents in the original CAREX project carried out 35 years ago, because the number of known occupational carcinogens has increased over time to 47 agents identified as known occupational carcinogens in 2017, compared with 28 in 2004 [54]. Three of the four selected process‐generated substances discussed in this paper, namely crystalline silica, diesel motor exhaust and welding fumes, were classified by IARC as lung carcinogens (Group 1) only after the creation of CAREX. Welding fume is most often excluded from studies calculating PAFs related to occupational exposures, because it was classified as carcinogenic to the lung only in 2017 and the prevalence of the occurrence of this exposure is not easy to assess. However, exposure to welding fumes will have partly overlapped with other exposures such as hexavalent chromium in several studies.
The original CAREX has inspired many consequent projects to estimate numbers and proportions of workers exposed to carcinogenic agents, and to adapt these estimates for various countries [16, 32, 33, 34, 35, 55, 56]. Nevertheless, CAREX is still being used more than 35 years after its creation, notably in the Global Burden of Disease Project [13, 57]. This may be sufficient if the objective is only to conclude that occupational carcinogens continue contributing to the global cancer burden and to justify the need for ongoing prevention and control initiatives [58]. However, if the objective is to set the departure for controlling hazardous exposures in the workplaces, it is important to conduct workplace exposure studies in more countries than what is currently done. Also, employers, worker's associations and management are more likely to ‘act on what they see’ in local, regional or national studies, and dust monitoring in itself might result already in lower exposure concentrations [59].
Despite evidence of declining exposure in European and North American workplaces [60], comprehensive studies of the effectiveness of workplace interventions for reducing hazardous exposure remain scarce [61]. Recently, Ohlander et al. [61] observed an improvement in the frequency and quality of intervention studies targeting exposure to chemicals and biological agents in the workplace over the last six decades and concluded that it is important to expand the evidence on (cost‐) effectiveness and transferability of interventions to reduce exposure and health effects, in order to reduce occupational ill‐health caused by these exposures.
The prevalence of process‐generated substances and others in the majority of countries including low‐ and middle‐income countries is largely unknown because few studies have been conducted locally [13, 62, 63]. A review discussing the increasing cancer burden in Africa revealed suboptimal implementation of occupational health standards notably in the informal sector, use of outdated technologies in industry and lack of awareness of potential hazards in specific employment structures may give rise to high levels of occupational exposures. Exposures in mining and exposure to pesticides in agriculture and agents arising from the mismanagement of hazardous waste from local, industrial and transboundary sources are of particular concern [64, 65].
Process‐generated substances are by far the most prominent and prevalent occupational exposures to substances even today in Europe, Canada, Australia and a few other countries, where systematic research has been done to estimate the prevalence of occupational exposure. Unfortunately, due to this limited insight, precise estimates of the number of workers exposed (on a global scale) and turnover rates in global workforces are generally not available, and therefore, the estimates of the global burden of cancer due to these exposures will remain rather imprecise and will either overestimate or (more likely) underestimate the importance of carcinogenic exposure in the workplace. Actions to reduce exposures and research to fill gaps in knowledge adapted to local settings are warranted to mitigate the occupational cancer burden, especially in low‐ and middle‐income countries.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
AO and HK conceived the project, AO and HK acquired the data for the tables and figures, AO and HK drafted separate parts of the paper and thereafter agreed on the interpretation and final version.
Disclaimer
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer /World Health Organization.
Acknowledgements
The authors thank Monika Moissonnier for preparing the figures and Joanne Kim for complementary data from the Canadian study [2]
References
- 1. Rushton L, Bagga S, Bevan R, Brown TP, Cherrie JW, Holmes P, Fortunato L, Slack R, Van Tongeren M, Young C et al. (2010) Occupation and cancer in Britain. Br J Cancer 102, 1428–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Labrèche F, Kim J, Song C, Pahwa M, Ge CB, Arrandale VH, McLeod CB, Peters CE, Lavoué J, Davies HW et al. (2019) The current burden of cancer attributable to occupational exposures in Canada. Prev Med 122, 128–139. [DOI] [PubMed] [Google Scholar]
- 3. Marant Micallef C, Shield KD, Vignat J, Baldi I, Charbotel B, Fervers B, Gilg Soit Ilg A, Guenel P, Olsson A, Rushton L et al. (2019) Cancers in France in 2015 attributable to occupational exposures. Int J Hyg Environ Health 222, 22–29. [DOI] [PubMed] [Google Scholar]
- 4. Mansournia MA & Altman DG (2018) Population attributable fraction. BMJ 360, k757. [DOI] [PubMed] [Google Scholar]
- 5. Doll R & Peto R (1981) The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst 66, 1191–1308. [PubMed] [Google Scholar]
- 6. Straif K, Benbrahim‐Tallaa L, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, Guha N, Freeman C, Galichet L et al. (2009) A review of human carcinogens–Part C: metals, arsenic, dusts, and fibres. Lancet Oncol 10, 453–454. [DOI] [PubMed] [Google Scholar]
- 7. Benbrahim‐Tallaa L, Baan RA, Grosse Y, Lauby‐Secretan B, El Ghissassi F, Bouvard V, Guha N, Loomis D & Straif K (2012) Carcinogenicity of diesel‐engine and gasoline‐engine exhausts and some nitroarenes. Lancet Oncol 13, 663–664. [DOI] [PubMed] [Google Scholar]
- 8. Guha N, Loomis D, Guyton KZ, Grosse Y, El Ghissassi F, Bouvard V, Benbrahim‐Tallaa L, Vilahur N, Muller K & Straif K (2017) Carcinogenicity of welding, molybdenum trioxide, and indium tin oxide. Lancet Oncol 18, 581–582. [DOI] [PubMed] [Google Scholar]
- 9. Baan R, Grosse Y, Straif K, Secretan B, El Ghissassi F, Bouvard V, Benbrahim‐Tallaa L, Guha N, Freeman C, Galichet L et al. (2012) A review of human carcinogens part F: chemical agents and related occupations. Lancet Oncol 10, 1143–1144. [DOI] [PubMed] [Google Scholar]
- 10. El Ghissassi F, Baan R, Straif K, Grosse Y, Secretan B, Bouvard V, Benbrahim‐Tallaa L, Guha N, Freeman C, Galichet L et al. (2009) A review of human carcinogens–part D: radiation. Lancet Oncol 10, 751–752. [DOI] [PubMed] [Google Scholar]
- 11. Eng A, 't Mannetje A, McLean D, Ellison‐Loschmann L, Cheng S & Pearce N (2011) Gender differences in occupational exposure patterns. Occup Environ Med 68, 888–894. [DOI] [PubMed] [Google Scholar]
- 12. Scarselli A, Corfiati M, Di Marzio D, Marinaccio A & Iavicoli S (2018) Gender differences in occupational exposure to carcinogens among Italian workers. BMC Public Health 18, 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. GBD 2016 Occupational Carcinogens Collaborators (2020) Global and regional burden of cancer in 2016 arising from occupational exposure to selected carcinogens: a systematic analysis for the Global Burden of Disease Study 2016. Occup Environ Med 77, 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anselmo Lima C, Sampaio Lima M, Maria Da Silva A, Prado Nunes MA, Macedo Lima MM, Oliveira Santos M, Lyra D Jr & Kleber Alves C (2018) Do cancer registries play a role in determining the incidence of non‐melanoma skin cancers? Eur J Dermatol 28, 169–176. [DOI] [PubMed] [Google Scholar]
- 15. Callens J, Van Eycken L, Henau K & Garmyn M (2016) Epidemiology of basal and squamous cell carcinoma in Belgium: the need for a uniform and compulsory registration. J Eur Acad Dermatol Venereol 30, 1912–1918. [DOI] [PubMed] [Google Scholar]
- 16. Boffetta P, Autier P, Boniol M, Boyle P, Hill C, Aurengo A, Masse R, The G, Valleron AJ, Monier R et al. (2010) An estimate of cancers attributable to occupational exposures in France. J Occup Environ Med 52, 399–406. [DOI] [PubMed] [Google Scholar]
- 17. Hutchings S & Rushton L (2017) Estimating the burden of occupational cancer: assessing bias and uncertainty. Occup Environ Med 74, 604–611. [DOI] [PubMed] [Google Scholar]
- 18. Guha N, Bouaoun L, Kromhout H, Vermeulen R, Brüning T, Behrens T, Peters S, Luzon V, Siemiatycki J, Xu M et al. (2020) Lung cancer risk in painters: results from the SYNERGY pooled case–control study consortium. Occup Environ Med, oemed‐2020‐106770. 10.1136/oemed-2020-106770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Olsson AC, Gustavsson P, Kromhout H, Peters S, Vermeulen R, Bruske I, Pesch B, Siemiatycki J, Pintos J, Bruning T et al. (2011) Exposure to diesel motor exhaust and lung cancer risk in a pooled analysis from case‐control studies in Europe and Canada. Am J Respir Crit Care Med 183, 941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Olsson AC, Vermeulen R, Schuz J, Kromhout H, Pesch B, Peters S, Behrens T, Portengen L, Mirabelli D, Gustavsson P et al. (2017) Exposure‐response analyses of asbestos and lung cancer subtypes in a pooled analysis of case‐control studies. Epidemiology 28, 288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pesch B, Kendzia B, Gustavsson P, Jockel KH, Johnen G, Pohlabeln H, Olsson A, Ahrens W, Gross IM, Bruske I et al. (2012) Cigarette smoking and lung cancer–relative risk estimates for the major histological types from a pooled analysis of case‐control studies. Int J Cancer 131, 1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kulhánová I, Forman D, Vignat J, Espina C, Brenner H, Storm HH, Bauld L & Soerjomataram I (2020) Tobacco‐related cancers in Europe: the scale of the epidemic in 2018. Eur J Cancer 139, 27–36. [DOI] [PubMed] [Google Scholar]
- 23. European Parliament (2006). Regulation (EC) No 1907/2006 of the European Parliament and of the council of 18 December 2006. EUR‐Lex Access to European law.
- 24. European Parliament (2004) Directive 2004/37/EC of the European Parliament and of the Council of 29 April 2004 on the protection of workers from the risks related to exposure to carcinogens or mutagens at work (Sixth individual Directive within the meaning of Article 16(1) of Council Directive 89/391/EEC), Directive 2004/37/EC.
- 25. Rappaport SM & Fraser DA (1977) Air sampling and analysis in a rubber vulcanization area. Am Ind Hyg Assoc J 38, 205–210. [DOI] [PubMed] [Google Scholar]
- 26. Halstensen AS, Nordby KC, Wouters IM & Eduard W (2007) Determinants of microbial exposure in grain farming. Ann Occup Hyg 51, 581–592. [DOI] [PubMed] [Google Scholar]
- 27. Peters S, de Klerk N, Reid A, Fritschi L, Musk AB & Vermeulen R (2017) Estimation of quantitative levels of diesel exhaust exposure and the health impact in the contemporary Australian mining industry. Occup Environ Med 74, 282–289. [DOI] [PubMed] [Google Scholar]
- 28. CAREX International Information System on Occupational Exposure to Carcinogens. Finnish Institute of Occupational Health. Available from: https://www.ttl.fi/en/carex/
- 29. Kauppinen T, Toikkanen J, Pedersen D, Young R, Ahrens W, Boffetta P, Hansen J, Kromhout H, Maqueda Blasco J, Mirabelli D et al. (2000) Occupational exposure to carcinogens in the European Union. Occup Environ Med 57, 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kauppinen T, Pajarskiene B, Podniece Z, Rjazanov V, Smerhovsky Z, Veidebaum T & Leino T (2001) Occupational exposure to carcinogens in Estonia, Latvia, Lithuania and the Czech Republic in 1997. Scand J Work Environ Health 27, 343–345. [DOI] [PubMed] [Google Scholar]
- 31. Kogevinas MM, De la Orden V, Fernandez F, Kauppinen T & Benavides FG (2000) Exposicion a carcinogenos laborales en Espana: aplicacion de la base de datos CAREX. Arch Prev Riesgos Labor 3, 153–159. [Google Scholar]
- 32. Partanen T, Chaves J, Wesseling C, Chaverri F, Monge P, Ruepert C, Aragón A, Kogevinas M, Hogstedt C & Kauppinen T (2003) Workplace carcinogen and pesticide exposures in Costa Rica. Int J Occup Environ Health 9, 104–111. [DOI] [PubMed] [Google Scholar]
- 33. Peters CE, Ge CB, Hall AL, Davies HW & Demers PA (2015) CAREX Canada: an enhanced model for assessing occupational carcinogen exposure. Occup Environ Med 72, 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Labrèche F, Duguay P, Ostiguy C, Boucher A, Roberge B, Peters CE & Demers PA (2013) Estimating occupational exposure to carcinogens in Quebec. Am J Ind Med 56, 1040–1050. [DOI] [PubMed] [Google Scholar]
- 35. Blanco‐Romero LE, Vega LE, Lozano‐Chavarría LM & Partanen TJ (2011) CAREX Nicaragua and Panama: Worker exposures to carcinogenic substances and pesticides. Int J Occup Environ Health 17, 251–257. [DOI] [PubMed] [Google Scholar]
- 36. Cherrie JW (2011) Website for the SHEcan Research Project. European Union. Available from http://www.occupationalcancer.eu/projresults.html
- 37. Carey RN, Driscoll TR, Peters S, Glass DC, Reid A, Benke G & Fritschi L (2014) Estimated prevalence of exposure to occupational carcinogens in Australia (2011–2012). Occup Environ Med 71, 55–62. [DOI] [PubMed] [Google Scholar]
- 38. Peters S, Carey RN, Driscoll TR, Glass DC, Benke G, Reid A & Fritschi L (2015) The Australian Work Exposures Study: prevalence of occupational exposure to diesel engine exhaust. Ann Occup Hyg 59, 600–608. [DOI] [PubMed] [Google Scholar]
- 39. EU guidelines: Agreement on Workers Health Protection through the Good Handling and Use of Crystalline Silica and Products containing it of 18/12/2017. Available from: https://osha.europa.eu/en/legislation/guidelines/agreement‐on‐workers‐health‐protection‐through‐the‐good‐handling‐and‐use‐of‐crystalline‐silica‐and‐products‐containing‐it
- 40. The European Network on Silica . NEPSI report 2018 Executive summary, European Social Dialogue, Brussels. Available from: https://www.nepsi.eu/news/nepsi‐2018‐report
- 41. Sauvé JF, Beaudry C, Bégin D, Dion C, Gérin M & Lavoué J (2012) Statistical modeling of crystalline silica exposure by trade in the construction industry using a database compiled from the literature. J Environ Monit 14, 2512–2520. [DOI] [PubMed] [Google Scholar]
- 42. Swanepoel AJ, Kromhout H, Jinnah ZA, Portengen L, Renton K, Gardiner K & Rees D (2011) Respirable dust and quartz exposure from three South African farms with sandy, sandy loam, and clay soils. Ann Occup Hyg 55, 634–643. [DOI] [PubMed] [Google Scholar]
- 43. Peters S, Vermeulen R, Portengen L, Olsson A, Kendzia B, Vincent R, Savary B, Lavoue J, Cavallo D, Cattaneo A et al. (2011) Modelling of occupational respirable crystalline silica exposure for quantitative exposure assessment in community‐based case‐control studies. J Environ Monit 13, 3262–3268. [DOI] [PubMed] [Google Scholar]
- 44. Zilaout H, Houba R & Kromhout H (2020) Temporal trends in respirable dust and respirable quartz concentrations within the European industrial minerals sector over a 15‐year period (2002–2016). Occup Environ Med 77, 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Doney BC, Miller WE, Hale JM & Syamlal G (2020) Estimation of the number of workers exposed to respirable crystalline silica by industry: Analysis of OSHA compliance data (1979–2015). Am J Ind Med 63, 465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Galea KS, Van Tongeren M, Sleeuwenhoek AJ, While D, Graham M, Bolton A, Kromhout H & Cherrie JW (2009) Trends in wood dust inhalation exposure in the UK, 1985–2005. Ann Occup Hyg 53, 657–667. [DOI] [PubMed] [Google Scholar]
- 47. Schlünssen V, Jacobsen G, Erlandsen M, Mikkelsen AB, Schaumburg I & Sigsgaard T (2008) Determinants of wood dust exposure in the Danish furniture industry–results from two cross‐sectional studies 6 years apart. Ann Occup Hyg 52, 227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Eriksson K, Bryngelsson IL & Hagström K (2017) Temporal trend in wood dust exposure during the production of wood pellets. Ann Work Expo Health 61, 429–439. [DOI] [PubMed] [Google Scholar]
- 49. Davis ME, Hart JE, Laden F, Garshick E & Smith TJ (2011) A retrospective assessment of occupational exposure to elemental carbon in the U.S. trucking industry. Environ Health Perspect 119, 997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vermeulen R, Coble JB, Lubin JH, Portengen L, Blair A, Attfield MD, Silverman DT & Stewart PA (2010) The Diesel Exhaust in Miners Study: IV. Estimating historical exposures to diesel exhaust in underground non‐metal mining facilities. Ann Occup Hyg 54, 774–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. International Agency for Research on Cancer (2018) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 118. Welding, Molybdenum Trioxide, and Indium Tin Oxide. IARC, Lyon. Available from: https://publications.iarc.fr/569 [PubMed] [Google Scholar]
- 52. Kromhout H, Linnéa L, Jan Paul Z & Manolis K (2004) Quantitative estimation of exposure to welding fumes in an international general population study on asthma. Eur Respir J 24(Suppl 48), 177 [Google Scholar]
- 53. Lillienberg L, Zock JP, Kromhout H, Plana E, Jarvis D, Torén K & Kogevinas M (2008) A population‐based study on welding exposures at work and respiratory symptoms. Ann Occup Hyg 52, 107–115. [DOI] [PubMed] [Google Scholar]
- 54. Loomis D, Guha N, Hall AL & Straif K (2018) Identifying occupational carcinogens: an update from the IARC Monographs. Occup Environ Med 75, 593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Marant Micallef C, Shield KD, Baldi I, Charbotel B, Fervers B, Gilg Soit Ilg A, Guénel P, Olsson A, Rushton L, Hutchings SJ et al. (2018) Occupational exposures and cancer: a review of agents and relative risk estimates. Occup Environ Med 75, 604–614. [DOI] [PubMed] [Google Scholar]
- 56. Van Tongeren M, Jimenez AS, Hutchings SJ, MacCalman L, Rushton L & Cherrie JW (2012) Occupational cancer in Britain. Exposure assessment methodology. Br J Cancer 107 (Suppl 1), S18–S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mosavi‐Jarrahi A, Mohagheghi M, Kalaghchi B, Mousavi‐Jarrahi Y & Noori MK (2009) Estimating the incidence of lung cancer attributable to occupational exposure in Iran. Popul Health Metr 7, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kromhout H, van Tongeren M, Peters CE & Hall AL (2020) Commentary. Occup Environ Med 77, 513–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Basinas I, Sigsgaard T, Bønløkke JH, Andersen NT, Omland Ø, Kromhout H & Schlünssen V (2016) Feedback on measured dust concentrations reduces exposure levels among farmers. Ann Occup Hyg 60, 812–824. [DOI] [PubMed] [Google Scholar]
- 60. Peters S, Vermeulen R, Olsson A, Van Gelder R, Kendzia B, Vincent R, Savary B, Williams N, Woldbaek T, Lavoue J et al. (2012) Development of an exposure measurement database on five lung carcinogens (ExpoSYN) for quantitative retrospective occupational exposure assessment. Ann Occup Hyg 56, 70–79. [DOI] [PubMed] [Google Scholar]
- 61. Ohlander J, Kromhout H & van Tongeren M (2020) Interventions to reduce exposures in the workplace: a systematic review of intervention studies over six decades, 1960–2019. Front Public Health 8, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hashim D & Boffetta P (2014) Occupational and environmental exposures and cancers in developing countries. Ann Glob Health 80, 393–411. [DOI] [PubMed] [Google Scholar]
- 63. Pasetto R, Terracini B, Marsili D & Comba P (2014) Occupational burden of asbestos‐related cancer in Argentina, Brazil, Colombia, and Mexico. Ann Glob Health 80, 263–268. [DOI] [PubMed] [Google Scholar]
- 64. McCormack VA & Schüz J (2012) Africa's growing cancer burden: environmental and occupational contributions. Cancer Epidemiol 36, 1–7. [DOI] [PubMed] [Google Scholar]
- 65. Negatu B, Kromhout H, Mekonnen Y & Vermeulen R (2016) Use of chemical pesticides in Ethiopia: a cross‐sectional comparative study on knowledge, attitude and practice of farmers and farm workers in three farming systems. Ann Occup Hyg 60, 551–566. [DOI] [PubMed] [Google Scholar]
- 66. Olsson AC, Gustavsson P, Zaridze D, Mukeriya A, Szeszenia‐Dabrowska N, Rudnai P, Lissowska J, Fabianova E, Mates D, Bencko V et al. (2011) Lung cancer risk attributable to occupational exposures in a multicenter case‐control study in Central and Eastern Europe. J Occup Environ Med 53, 1262–1267. [DOI] [PubMed] [Google Scholar]
- 67. De Matteis S, Consonni D, Lubin JH, Tucker M, Peters S, Vermeulen R, Kromhout H, Bertazzi PA, Caporaso NE, Pesatori AC et al. (2012) Impact of occupational carcinogens on lung cancer risk in a general population. Int J Epidemiol 41, 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rushton L, Hutchings S & Brown T (2007) The burden of occupational cancer in Great Britain. Technical Annex 6: Lung cancer. Health and Safety Executive. Available from: https://www.hse.gov.uk/research/rrpdf/rr595ann6.pdf


