Key Points
Question
Is a patient’s mental health status associated with discordance between patient and physician assessments of psoriasis severity?
Findings
In this cohort study of 502 patients with psoriasis, analysis of repeated cross-sectional data from a tertiary psoriasis service in London found discordance in patient and physician psoriasis severity ratings at 39% of the appointments. Twenty-six percent of the patients underestimated their psoriasis severity, and 13% of patients overestimated their psoriasis severity, compared with physician ratings.
Meaning
Although patient assessments of disease severity remain important for informing appropriate treatment decisions, the data from this study suggest that recognition of anxiety and depression among individuals with psoriasis is important for interpretation of the assessments.
Abstract
Importance
The emerging paradigm of treat-to-target in psoriasis requires accurate monitoring of treatment response. The commonly used physician global assessment tool does not capture the patient’s perception of their disease. Patient assessments facilitate shared decision-making and foster patient-centered care; however, recent research reports a discordance between patient- and physician-reported psoriasis severity. Understanding the factors underlying this discordance may improve treatment satisfaction and disease outcomes.
Objectives
To evaluate the discordance between patient- and physician-reported measures of psoriasis severity and assess the association with patient mental health status.
Design, Setting, and Participants
A cohort study using repeated cross-sectional analysis of real-world longitudinal data was conducted at a large specialist psoriasis service serving London and Southeast England. A total of 502 patients attending the psoriasis service between May 12, 2016, and November 1, 2018, were included. Data analysis was conducted July 22 to October 22, 2019.
Main Outcomes and Measures
Psoriasis severity was assessed on each visit with identical 5-point physician and patient global assessment scales (clear/nearly clear, mild, moderate, severe, and very severe). Each patient completed validated self-report screens for depression and anxiety on each visit.
Results
Longitudinal data from 502 individuals with psoriasis (1985 total observations) were available. A total of 339 patients (68%) were men, 396 (79%) were White, mean (SD) age was 47 (13) years, and 197 patients (39%) had concurrent psoriatic arthritis, 43 (9%) screened positive for depression, and 49 (10%) screened positive for anxiety. There was discordance between physician and patient measures of disease severity in 768 of 1985 office appointments (39%); on 511 visits (26%) patients rated their psoriasis as less severe and on 257 visits (13%) patients rated their psoriasis as more severe compared with their physician. Individuals who screened positive for depression or anxiety were more likely to overestimate their psoriasis severity compared with their physician (relative risk ratio: depression, 2.7; 95% CI, 1.6-4.5; anxiety, 2.1; 95% CI, 1.3-3.4). These findings remained statistically significant after adjustment for age, ethnicity, sex, body mass index, smoking, number of comorbidities, treatment modality, and presence of psoriatic arthritis.
Conclusions and Relevance
The findings of this cohort study suggest that discordance between patient and physician assessments of psoriasis severity is associated with patients’ mental health status. Recognition of anxiety and depression in individuals with psoriasis appears to be important when interpreting patient-reported outcome measures and informing appropriate treatment decisions.
This cohort study examines the ratings of physicians compared with the ratings of patients on the severity of psoriasis in patients with depression and anxiety.
Introduction
Psoriasis is a common inflammatory skin condition affecting 2% to 4% of the global population.1 Psoriasis is associated with reduced quality of life and substantial morbidity, including psoriatic arthritis, cardiovascular disease, and obesity.2 The management of psoriasis has been improved by the availability of biologic drugs, such that skin clearance is now a realistic treatment goal.3 This advance has opened avenues for treat-to-target approaches, whereby target disease activity end points are used to support the introduction and modification of treatments, with the ultimate aim of improving clinical and cost-effectiveness outcomes.4 Because the introduction and continuation of biologic treatments is informed by clinical assessments of disease severity,5,6,7 ensuring current measures are accurate and relevant is vital.
Physician Global Assessment (GA) and Psoriasis Area Severity Index (PASI) are physician-rated disease activity measures commonly used in real-world practice and clinical trials and recommended in treatment guidelines.5,6,7 Although data are limited in psoriasis,8,9,10 studies have identified discordance between physician and patient measures of disease severity in chronic inflammatory diseases, for example, among one-third of patients with rheumatoid arthritis.11,12,13 Because this discordance is associated with patient dissatisfaction, lower treatment adherence, and poorer disease outcomes,11,12,13 an improved understanding of the underlying factors is important.
Evidence suggests that pain and fatigue lead patients with arthritis to overestimate their disease activity relative to their physicians.14,15,16,17,18 Patient-physician discordance in ratings of psoriatic and rheumatoid arthritis has also been associated with poor mental health.14,16,17 This latter finding warrants further investigation in psoriasis owing to the high prevalence of depression and anxiety, particularly among individuals with worse psoriasis.17,19 A previous pilot study also showed that psychological interventions, such as mindfulness-based cognitive therapy used as an adjunct to usual psoriasis therapy, may improve self-assessed psoriasis severity.20
This real-world repeated cross-sectional study therefore aimed to (1) evaluate the extent of discordance between physician- and patient-reported measures of psoriasis severity, (2) examine whether discordance is associated with adverse mental health status, and (3) explore the interaction between adverse mental health status and psoriasis disease severity.
Methods
Study Participants
All participants were recruited during dermatology outpatient visits between May 12, 2016, and November 1, 2018, in a large specialist psoriasis center serving London and South East England. Longitudinal clinical data were combined with information collected from the same patients as part of the Integrating Mental and Physical Healthcare: Research Training and Services (IMPARTS) screening program.21 Participants first completed self-report questionnaires via a tablet device including a patient GA and questions on depression and anxiety. They subsequently underwent clinical assessments by their physician as part of their routine care. Participants attended multiple appointments (between 1 and 8) during the study.
Because IMPARTS is a clinical initiative used in routine care, participants did not require formal consent to take part in the study. Everyone was informed that their anonymized data might be used for research purposes and could opt out at any time. Ethical approval for the present study was given by the IMPARTS Research Ethics Committee (REC reference 11/H0802-7), and ethical approval for IMPARTS was given by NHS Research Ethics Committee. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Measures
In measurement of discordance, psoriasis severity was quantified using identical physician and patient GA scales. This 5-point scale (1, clear/nearly clear; 2, mild; 3, moderate; 4, severe; and 5, very severe) had no specific descriptors relating to redness, thickness, or scaling.22,23 We elected to use the physician’s assessment as the standard against which to evaluate discordance because it is the standard measure used in clinical guidelines.5,7 Psoriasis severity discordance was measured by subtracting the patient assessment from the physician assessment and categorizing the ratings as overestimate (the patient reported a greater level of psoriasis severity compared with the physician by at least 1 point on the scale); neutral (the patient and physician reported the same category of psoriasis severity); or underestimate (the patient reported a lower level of psoriasis severity compared with the physician by at least 1 point on the scale). We chose a difference of 1 point following evidence, albeit using varied GA measures showing that differences of 1 point correspond to meaningful differences in physician-assessed outcome measures, such as the PASI.4,23 Although a difference of 2 or more points resulted in smaller groups of participants in each discordant category, we also assessed this more stringent threshold in a sensitivity analysis. In addition, psoriasis severity was evaluated with the PASI, a clinician-rated measure of lesion area coverage and appearance.22
Screening for probable major depressive disorder (herein, depression) was conducted with the Patient Health Questionnaire-2 (PHQ-2)24 and PHQ-9.25 Screening for probable generalized anxiety disorder (GAD) (herein, anxiety) was screened with the Generalized Anxiety Disorder scale (GAD-7).26 Details are provided in the eMethods in the Supplement.
Covariates were recorded at each appointment. Demographic information included age, sex, and race/ethnicity. Race/ethnicity was coded into 6 categories (White; Asian or Asian British; Black or Black British; Chinese, Japanese, Korean, or Indochinese; mixed; and other). We further adjusted for clinical factors previously shown to influence treatment outcomes. These included smoking status (current smoker or nonsmoker),27 body mass index (BMI),28 psoriatic arthritis diagnosis (yes or no),29 number of comorbid conditions (0, 1-2, 3-4, and ≥5),30 current treatment (none, topical only, nonbiologic systemic, and biologic systemic).31
Statistical Analysis
Psoriasis disease severity (patient GA and physician GA) was summarized as counts and percentages across all appointments over follow-up. Demographic and clinical covariates were summarized for each participant’s first appointment. Discordance was summarized (counts and percentages) for the overall sample, as well as for subsamples defined by the presence of depression and anxiety. The weighted κ value was calculated.32
Multivariable multinomial logistic regression models were used to assess the association between mental health status and patient-physician psoriasis severity discordance. The outcome was the 3-category measure of the defined discordance (overestimate, neutral, or underestimate), with neutral treated as the base category. Depression and anxiety, as defined by PHQ-9 and GAD-7 screening tools, were entered as binary variables in separate models, given the substantial overlap between these conditions. To investigate whether mental health was more strongly associated with discordance among patients with more severe clinician-assessed psoriasis, we also included a term representing the interaction between probable depression and the continuous PASI score. The statistical significance of the interaction effect (main effects and interaction term) was tested with a 2-sided Wald test.
All models were adjusted for age, sex, race/ethnicity, smoking status, BMI, psoriatic arthritis, current treatment, and number of comorbidities. A sandwich estimator was used to adjust SEs to take into account clustering owing to repeated appointments per participant.33 Estimates are reported as relative risk ratios with associated 95% CIs and predicted probabilities estimated with other covariates set to their sample mean values. Findings were considered significant at P ≤ .05. Models were estimated in Stata, version 15 (StataCorp).34
Results
Cohort Characteristics
After excluding those with missing information on patient GA or physician GA (n = 65) or covariates (n = 5), the analytical sample comprised 502 participants. Compared with those in the analytical sample, excluded participants tended to be younger (mean age, 42.6 vs 46.9 years; P = .02) and had a higher prevalence of probable depression (16% vs 9%; P = .09) and probable anxiety (18% vs 10%; P = .09) but were similar in terms of sex (32% [included] vs 42% [excluded] female; P = .20).
Table 1 summarizes the analytical sample. Participants included 339 men (68%) and 163 women (32%); most were of White race (396 [79%]), nonsmokers (393 [78%]), and overweight (mean [SD] BMI, 29.4 [6.0], calculated as weight in kilograms divided by height in meters squared). The mean (SD) age was 46.8 (13.1) years. Over a third of participants (197 [39%]) had concurrent psoriatic arthritis, and most were receiving systemic treatment (nonbiologic, 257 [51%]; biologic, 170 [34%]). A total of 221 participants (44%) reported having at least 1 comorbid condition (the most prevalent comorbidities were hypertension, autoimmune disorders, and liver disease).
Table 1. Sample Characteristics of 502 Patients at First Recorded Appointment .
| Characteristic | No. (%) |
|---|---|
| Continuous variables | |
| Age, mean (SD), y | 46.8 (13.1) |
| BMI, mean (SD) | 29.4 (6.0) |
| PASI score, mean (SD) | 4.3 (5.1) |
| Categorical variables | |
| Sex | |
| Female | 163 (32) |
| Male | 339 (68) |
| Race/ethnicity | |
| White | 396 (79) |
| Asian or Asian British | 65 (13) |
| Black or Black British | 10 (2) |
| Chinese, Japanese, Korean, Indochinese | 15 (3) |
| Mixed | 7 (1) |
| Other | 9 (2) |
| Current smoker | |
| No | 393 (78) |
| Yes | 109 (22) |
| Psoriatic arthritis | |
| No | 305 (61) |
| Yes | 197 (39) |
| Current treatment | |
| None | 65 (13) |
| Topical only | 10 (2) |
| Nonbiologic systemic | 257 (51) |
| Biologic systemic | 170 (34) |
| No. of comorbidities | |
| 0 | 281 (56) |
| 1-2 | 160 (32) |
| 3-4 | 53 (11) |
| ≥5 | 8 (2) |
| Caseness | |
| PHQ-9 | 43 (9) |
| GAD-7 | 49 (10) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); GAD-7, Generalized Anxiety Disorder-7; PASI, Psoriasis Area Severity Index; PHQ-9, Patient Health Questionnaire-9.
Discordance in Psoriasis Severity Ratings
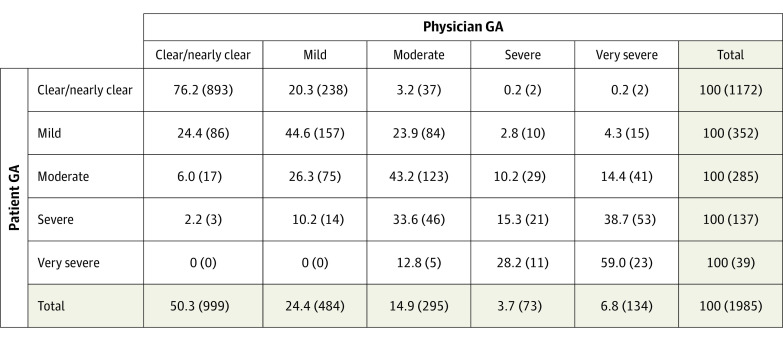
Overall, patient and physician severity assessments tended to be in agreement (Figure 1). At 893 appointments in which the patient rated their psoriasis severity as clear/nearly clear, 76.2% were also assessed by their physician as clear/nearly clear. The weighted κ statistic was 0.58 (95% CI, 0.56-0.60) indicating moderate agreement. Regarding the 3-category measure of discordance, 1217 appointments (61%) resulted in agreement between patient and physician about symptom severity (neutral) (Table 2) and 768 appointments (39%) resulted in disagreements. Where disagreements occurred, patients were more likely to underestimate their psoriasis severity, compared with the physician rating (511 [26%] appointments) rather than overestimate the severity (257 [13%] appointments).
Figure 1. Patient Global Assessment by Physician Global Assessment (1985 Appointments), Row Percentage (No.).
GA indicates global assessment.
Table 2. Discordance in Patient vs Physician Ratings, Overall and by Mental Health Status.
| Sample | Type of discordance, No. (%)a | ||
|---|---|---|---|
| Overestimate | Neutral | Underestimate | |
| All patients (1985 appointments) | 257 (13) | 1217 (61) | 511 (26) |
| Patients by caseness | |||
| Depression (PHQ-9) | |||
| Cases (129 appointments) | 33 (26) | 57 (44) | 39 (30) |
| Noncases (1856 appointments) | 224 (12) | 1160 (62) | 472 (25) |
| Anxiety (GAD-7) | |||
| Cases (155 appointments) | 36 (23) | 80 (52) | 39 (25) |
| Noncases (1830) | 221 (12) | 1137 (62) | 472 (26) |
Abbreviations: GAD-7, Generalized Anxiety Disorder-7 scale; PHQ-9, Patient Health Questionnaire-9.
Discordance indicated by differences of 1 or more categories for patient vs physician ratings.
Compared with physician ratings, patients screening positive for depression or anxiety were more likely to overestimate their psoriasis severity (depression, 33 of 129 appointments [26%]; anxiety, 36 of 155 appointments [23%]) than were patients without depression or anxiety (depression, 224 of 1856 appointments [12%]; anxiety, 221 of 1830 appointments [13%]). To investigate the risk of discordance attributable to mental health, we used multivariable multinomial logistic regression analysis. After adjustment for covariates, depression and anxiety were separately associated with an increased risk of patients overestimating their disease severity, compared with their physician, vs those without depression or anxiety (Figure 2) (relative risk ratio for depression, 2.7; 95% CI, 1.6-4.5; for anxiety, 2.1; 95% CI, 1.3-3.4).
Figure 2. Predicted Probabilities of Discordance Between Patient and Physician Global Assessments by Mental Health Status.
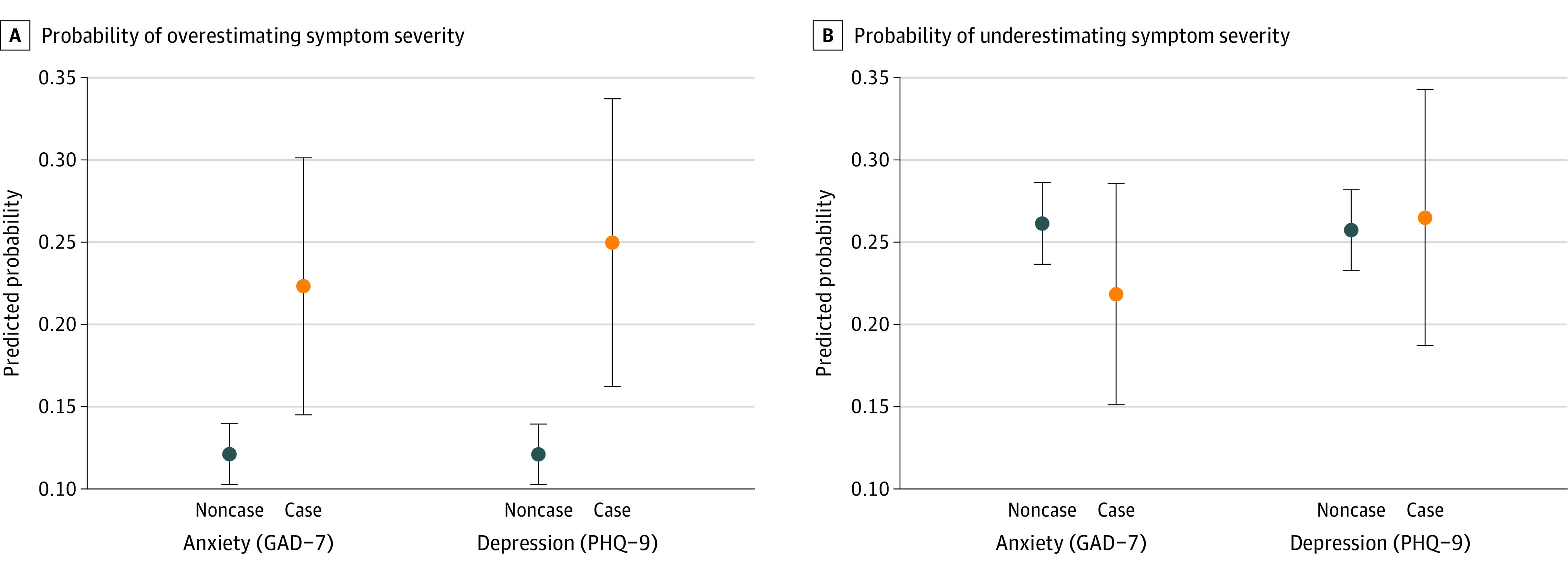
Probability of patient overestimation (A) and underestimation (B) of psoriasis severity compared with physicians. Relative risk ratios with 95% CIs adjusted for clustering of repeated appointments per patient shown. Analysis was adjusted for age, sex, race/ethnicity, body mass index, smoking, psoriatic arthritis, current treatment, and number of comorbidities. GAD-7 indicates Generalized Anxiety Disorder-7; PHQ-9, Patient Health Questionnaire-9.
Interaction Between Mental Health Status and Disease Severity
We investigated the interaction between mental health status and disease severity on patients’ probability of overestimating or underestimating severity, compared with the physician’s GA (Figure 3; eTable 1 in the Supplement). We found that patients with milder psoriasis (PASI score ≤5) and concurrent depression or anxiety were more likely to overestimate their psoriasis severity. Conversely, patients with moderate to severe psoriasis (PASI score >10) and concurrent depression or anxiety were more likely to underestimate their psoriasis severity. This interaction was statistically significant based on a Wald test (χ22 for depression, 8.8; P < .001; χ22 for anxiety, 37.0; P < .001). Thus, psoriasis severity may moderate the association of mental health status with patient-physician discordance.
Figure 3. Predicted Probability of Patient-Clinician Discordance by Continuous Psoriasis Area Severity Index (PASI) Score.
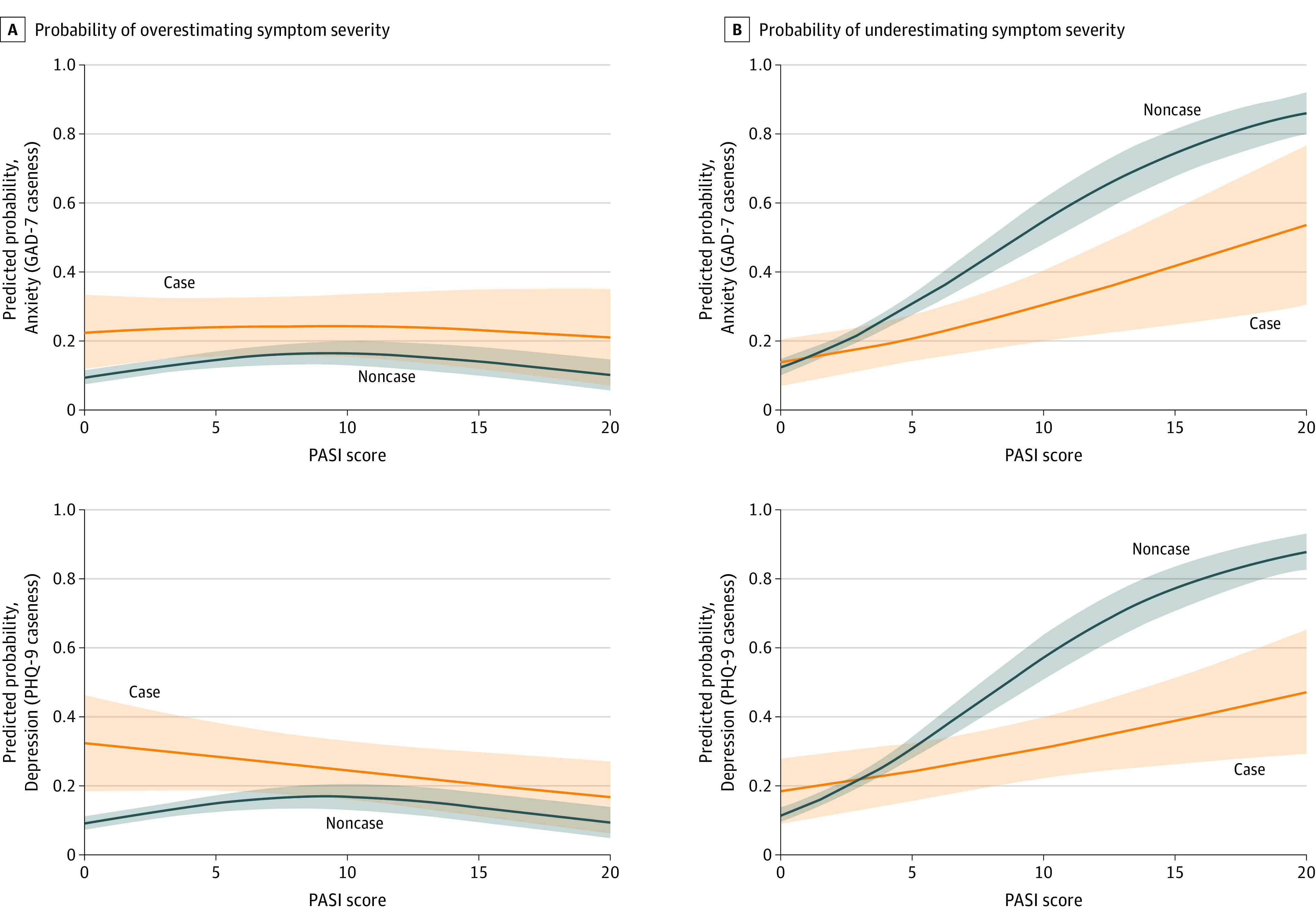
Solid lines show predicted probability that the patient underestimates or overestimates their psoriasis severity compared with the clinician; shaded areas indicate the 95% CIs for these probabilities. Analysis was adjusted was for age, sex, race/ethnicity, body mass index, smoking, psoriatic arthritis, current treatment, number of comorbidities, and cardiovascular comorbidity. GAD-7 indicates Generalized Anxiety Disorder-7; PHQ-9, Patient Health Questionnaire-9.
Sensitivity Analysis
Our findings were consistent when using a more stringent threshold for discordance (a difference of 2 categories between patient and physician GAs). The proportion of patients overestimating or underestimating severity was lower, but the patterns of association with mental health were preserved (eTable 2 and eFigure in the Supplement).
Discussion
To our knowledge, this represents the first study in psoriasis to characterize the real-world association of both depression and anxiety assessed using validated screening tools with discordance between patient and physician psoriasis severity ratings. These data suggest a substantial (39%) discordance between patients and physicians in their psoriasis severity assessments and highlight patient mental health status as a significant contributor to rating discordance. Patients with depression (major depressive disorder) and anxiety (GAD) were twice as likely to have overestimated their disease severity assessments vs physician rating, compared with patients without depression or anxiety after adjustment for potential demographic and clinical confounders.
These data have important clinical relevance. Shared decision-making between patients and their physicians is necessary for safe and effective care of psoriasis, which often requires lifelong treatment. Decisions about when to start or switch psoriasis therapies in routine practice are currently informed by physician-rated disease severity assessments, such as the Physician Global Assessment and PASI measures.4,5,6 However, data from evaluations of other chronic inflammatory diseases have underscored the importance of considering the patient’s perspective through the use of patient-reported measures; agreement between patient and physician assessments has been reported to improve both treatment adherence and outcomes.8 Our data highlight an unmet need to align physician and patient perceptions of disease severity, which must be addressed to reach a consensus about optimal treatment strategy.35
Because the prevalence of psoriasis continues to increase in the face of limited health service resources,36 virtual clinical review is increasingly important for the efficient and sustainable delivery of health care. In particular, the implementation of virtual consultations has been rapidly accelerated in recent months in response to the coronavirus disease 2019 pandemic. Virtual consults rely on the accurate interpretation of patient-reported disease severity measures. Our data suggest that coexistent depression and anxiety may lead to the overestimation of psoriasis severity by patients compared with their physician, particularly at the less severe end of the disease severity spectrum. If not considered in the correct context, the patient GA thus has the potential to influence clinical decision-making inappropriately. This possibility underscores the importance of concurrent screening for anxiety and depression to ensure that treatment plans accurately address disease activity in addition to mental health comorbidity. A multidisciplinary approach to psoriasis care is thus necessary.
There is a paucity of real-world data on physician-patient discordance of disease severity ratings in psoriasis and the underlying factors contributing to this discordance,14 so our study provides evidence to inform clinical practice. Our rate of discordance (39%) is similar to that reported by Griffiths et al8 from their multinational surveys of 524 dermatologists and 3821 patients with psoriasis. Their retrospective study found that 45% of physician-patient pairs were in disagreement about psoriasis severity (assessed using a 5-point scale: clear, almost clear, mild, moderate, and severe) but did not examine reasons for the discordance. Two studies in trial settings similarly highlighted discordance between patient and physician assessments of psoriasis, but again did not explore the factors contributing to the discrepancy.10,11 There are also reports of discordance between physicians and patients regarding their satisfaction with the level of psoriasis control achieved. Two surveys in the US showed patient-physician discordance in satisfaction with psoriasis control among 18% of patients, and this discordance was associated with higher disease severity and reduced quality of life.2
Although limited data exist, the influence of mental health on patient-physician discordance has mostly been explored in individuals with rheumatic disease.11,15,16,17,18 Barton et al16 reported an association between PHQ-9 depression score and patient-physician disease severity assessment discordance among 223 patients with rheumatoid arthritis. Discordance was found in 36% (compared with 39% in our psoriasis study) and patients overestimated their disease severity compared with physicians’ assessments in 85% of these cases. Consistent with our findings, Barton et al found depressive symptoms (a 5-point increase in PHQ-9 score) to be the strongest predictor of a patient overestimating their psoriasis severity compared with their physician (odds ratio, 1.61; 95% CI, 1.02-2.55).
Eder et al14 analyzed data from 331 individuals attending a psoriatic arthritis clinic and found lower patient-physician (rheumatologist) discordance (15%) for skin assessments compared with our study. However, the scores of patient and physician GAs ranged from 0 to 10 on a numeric rating scale, and a difference of more than 2 points was considered a clinically relevant discordance. Discordance was attributed to increased pain and levels on the Dermatology Life Quality Index, which accounted for 17% (pain) and 14% (quality of life) of the variation in patient-physician discordance. Unlike our findings, those of Eder et al did not identify an important role for mental health in patient-physician discordance of disease severity ratings. However, they used a simple mental component score derived from the Short Form-36 Mental Component Summary health survey as a surrogate for general psychological distress, which accounted for just 1% of variation in patient-physician discordance. A need to improve physician detection and management of psychological distress in patients with psoriasis has also been highlighted.37
Strengths and Limitations
To our knowledge, this represents the largest study to date of patient-physician discordance in psoriasis severity ratings, drawing on real-world clinical data. We were able to adjust for important demographic and clinical confounders and used validated screening tools for depression and anxiety (GAD-7 and PHQ-9), improving on previous studies in which mental health was assessed using the Short Form-36 Mental Component Summary.
In terms of limitations, these data were collected from a single specialist psoriasis clinic comprising patients with moderate to severe psoriasis and so may not be generalizable. The repeated cross-sectional analysis was unable to address questions of causation. There was no information on the stage or duration of treatment, which may help to contextualize the severity of psoriasis (eg, patients experiencing failure of a long-term treatment may perceive their disease severity differently than those starting treatment). Owing to the small sample size, discordance was defined as a difference of 1 category on the patient and physician GAs; however, a difference of 2 categories identified similar findings in sensitivity analyses. Although evidence showed differences of 1 category to be clinically meaningful,4 further work is required to understand what constitutes a clinically significant level of discordance. There are also no current standard definitions for physician and patient GAs; there is a paucity of research on patient-reported measures, and 5-, 6-, or 7-point Likert scales have variously been used for physician GAs in earlier studies.22
Depression and anxiety were assessed using the PHQ-9 and GAD-7 screening tools rather than via clinical interview. However, these screening tools have been shown to be valid and reliable,25,26,38,39 with sensitivity of 89% and specificity of 76% for the PHQ-2 in primary care samples.40 The percentage screening positive for depression (9%) was lower than the 22% reporting a history of depression (ie, current or prior diagnosis) in BADBIR (the UK pharmacovigilance psoriasis registry41) but consistent with the UK General Practice Research Database (13% of those with severe psoriasis reported a history of depression42), the PsoBest registry in Germany (7%43), and the global PSOLAR study (15%44). We were unable to measure pain or fatigue in our sample, which have previously been shown to be predictors of discordance.14 We were also unable to consider characteristics of the assessing dermatologist, such as age, sex, or level of experience, which may have influenced the extent of discordance.
Conclusions
Taken together, our results suggest that discordance between patient and physician measures of psoriasis severity is associated with patient mental health status. Patient-reported disease severity scores should thus be interpreted in the context of comorbid anxiety and depression, which can be facilitated by the routine use of mental health screening tools. The recognition, monitoring, and management of depression and anxiety in psoriasis by multidisciplinary health care teams (preferably encompassing clinical psychology expertise) has the potential to alleviate the substantial mental health burden in psoriasis while aligning clinician and patient perceptions of disease and treatment goals.
eMethods. Screening for Depression and Anxiety
eReferences
eFigure. Predicted Probabilities of Discordance by Mental Health Status for a Difference of 2+ Categories Between Patient and Physician Global Assessments
eTable 1. Odds Ratios for the Interaction Between Mental Health Status and PASI Score
eTable 2. Discordance in Patient vs Physician Ratings, Overall and by Mental Health Status, Based on a Difference of 2+ Categories
References
- 1.Michalek IM, Loring B, John SM. Global report on psoriasis. World Health Organization; 2016. [Google Scholar]
- 2.Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76(3):377-390. doi: 10.1016/j.jaad.2016.07.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahil SK, Capon F, Barker JN. Update on psoriasis immunopathogenesis and targeted immunotherapy. Semin Immunopathol. 2016;38(1):11-27. doi: 10.1007/s00281-015-0539-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahil SK, Wilson N, Dand N, et al. ; BADBIR study group and the PSORT consortium . Psoriasis treat to target: defining outcomes in psoriasis using data from a real-world, population-based cohort study (the British Association of Dermatologists Biologics and Immunomodulators Register, BADBIR). Br J Dermatol. 2020;182(5):1158-1166. doi: 10.1111/bjd.18333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029-1072. doi: 10.1016/j.jaad.2018.11.057 [DOI] [PubMed] [Google Scholar]
- 6.NICE . Psoriasis: assessment and management. Updated September 1, 2017. Accessed July 2, 2020. https://www.nice.org.uk/guidance/cg153/chapter/Introduction#systemic-therapy
- 7.Smith CH, Jabbar-Lopez ZK, Yiu ZZ, et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol. 2017;177(3):628-636. doi: 10.1111/bjd.15665 [DOI] [PubMed] [Google Scholar]
- 8.Griffiths CEM, Augustin M, Naldi L, et al. Patient-dermatologist agreement in psoriasis severity, symptoms and satisfaction: results from a real-world multinational survey. J Eur Acad Dermatol Venereol. 2018;32(9):1523-1529. doi: 10.1111/jdv.14937 [DOI] [PubMed] [Google Scholar]
- 9.Bushmakin AG, Mamolo C, Cappelleri JC, Stewart M. The relationship between pruritus and the clinical signs of psoriasis in patients receiving tofacitinib. J Dermatolog Treat. 2015;26(1):19-22. doi: 10.3109/09546634.2013.861891 [DOI] [PubMed] [Google Scholar]
- 10.Gordon, K. B., et al. Correlation of Physician-Assessed Psoriasis Area and Severity Index scores with patient-reported Psoriasis Symptoms and Signs Diary scores among patients with moderate-to-severe psoriasis: results from VOYAGE 1 and VOYAGE 2 Studies. J Psoriasis Psoriatic Arthritis. 2019;4(3):147-152. doi: 10.1177/2475530319854781 [DOI] [Google Scholar]
- 11.Neville C, Clarke AE, Joseph L, Belisle P, Ferland D, Fortin PR. Learning from discordance in patient and physician global assessments of systemic lupus erythematosus disease activity. J Rheumatol. 2000;27(3):675-679. [PubMed] [Google Scholar]
- 12.Jackson CA, Clatworthy J, Robinson A, Horne R. Factors associated with non-adherence to oral medication for inflammatory bowel disease: a systematic review. Am J Gastroenterol. 2010;105(3):525-539. doi: 10.1038/ajg.2009.685 [DOI] [PubMed] [Google Scholar]
- 13.Starfield B, Wray C, Hess K, Gross R, Birk PS, D’Lugoff BC. The influence of patient-practitioner agreement on outcome of care. Am J Public Health. 1981;71(2):127-131. doi: 10.2105/AJPH.71.2.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eder L, Thavaneswaran A, Chandran V, Cook R, Gladman DD. Factors explaining the discrepancy between physician and patient global assessment of joint and skin disease activity in psoriatic arthritis patients. Arthritis Care Res (Hoboken). 2015;67(2):264-272. doi: 10.1002/acr.22401 [DOI] [PubMed] [Google Scholar]
- 15.Nicolau G, Yogui MM, Vallochi TL, Gianini RJ, Laurindo IM, Novaes GS. Sources of discrepancy in patient and physician global assessments of rheumatoid arthritis disease activity. J Rheumatol. 2004;31(7):1293-1296. [PubMed] [Google Scholar]
- 16.Barton JL, Imboden J, Graf J, Glidden D, Yelin EH, Schillinger D. Patient-physician discordance in assessments of global disease severity in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2010;62(6):857-864. doi: 10.1002/acr.20132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan NA, Spencer HJ, Abda E, et al. Determinants of discordance in patients’ and physicians’ rating of rheumatoid arthritis disease activity. Arthritis Care Res (Hoboken). 2012;64(2):206-214. doi: 10.1002/acr.20685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Studenic P, Radner H, Smolen JS, Aletaha D. Discrepancies between patients and physicians in their perceptions of rheumatoid arthritis disease activity. Arthritis Rheum. 2012;64(9):2814-2823. doi: 10.1002/art.34543 [DOI] [PubMed] [Google Scholar]
- 19.Tribó MJ, Turroja M, Castaño-Vinyals G, et al. Patients with moderate to severe psoriasis associate with higher risk of depression and anxiety symptoms: results of a multivariate study of 300 Spanish individuals with psoriasis. Acta Derm Venereol. 2019;99(4):417-422. doi: 10.2340/00015555-3114 [DOI] [PubMed] [Google Scholar]
- 20.Fordham B, Griffiths CEM, Bundy C. A pilot study examining mindfulness-based cognitive therapy in psoriasis. Psychol Health Med. 2015;20(1):121-127. doi: 10.1080/13548506.2014.902483 [DOI] [PubMed] [Google Scholar]
- 21.Rayner L, Matcham F, Hutton J, et al. Embedding integrated mental health assessment and management in general hospital settings: feasibility, acceptability and the prevalence of common mental disorder. Gen Hosp Psychiatry. 2014;36(3):318-324. doi: 10.1016/j.genhosppsych.2013.12.004 [DOI] [PubMed] [Google Scholar]
- 22.Spuls PI, Lecluse LL, Poulsen ML, Bos JD, Stern RS, Nijsten T. How good are clinical severity and outcome measures for psoriasis? quantitative evaluation in a systematic review. J Invest Dermatol. 2010;130(4):933-943. doi: 10.1038/jid.2009.391 [DOI] [PubMed] [Google Scholar]
- 23.Cappelleri JC, Bushmakin AG, Harness J, Mamolo C. Psychometric validation of the Physician Global Assessment scale for assessing severity of psoriasis disease activity. Qual Life Res. 2013;22(9):2489-2499. doi: 10.1007/s11136-013-0384-y [DOI] [PubMed] [Google Scholar]
- 24.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284-1292. doi: 10.1097/01.MLR.0000093487.78664.3C [DOI] [PubMed] [Google Scholar]
- 25.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097. doi: 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 27.Naldi L. Psoriasis and smoking: links and risks. Psoriasis (Auckl). 2016;6:65-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahil SK, McSweeney SM, Kloczko E, McGowan B, Barker JN, Smith CH. Does weight loss reduce the severity and incidence of psoriasis or psoriatic arthritis? a critically appraised topic. Br J Dermatol. 2019;181(5):946-953. doi: 10.1111/bjd.17741 [DOI] [PubMed] [Google Scholar]
- 29.Smith CH, Yiu ZZN, Bale T, et al. ; British Association of Dermatologists’ Clinical Standards Unit . British Association of Dermatologists guidelines for biologic therapy for psoriasis 2020: a rapid update. Br J Dermatol. 2020;183(4):628-637. doi: 10.1111/bjd.19039 [DOI] [PubMed] [Google Scholar]
- 30.Warren RB, Marsden A, Tomenson B, et al. ; PSORT Consortium and on behalf of the BADBIR Study Group . Identifying demographic, social and clinical predictors of biologic therapy effectiveness in psoriasis: a multicentre longitudinal cohort study. Br J Dermatol. 2019;180(5):1069-1076. doi: 10.1111/bjd.16776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warren RB, Smith CH, Yiu ZZN, et al. Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol. 2015;135(11):2632-2640. doi: 10.1038/jid.2015.208 [DOI] [PubMed] [Google Scholar]
- 32.Cohen J. Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull. 1968;70(4):213-220. doi: 10.1037/h0026256 [DOI] [PubMed] [Google Scholar]
- 33.Rogers WH. Regression standard errors in clustered samples. Stata Technical Bulletin. 1993;13:19-23. [Google Scholar]
- 34.Stata Statistical Software . Release 15. StataCorp LP; 2017. [Google Scholar]
- 35.Selzer RM, Ellen S, McGartland M. Alignment: a conceptual shift from adherence. Intern Med J. 2013;43(8):940-942. doi: 10.1111/imj.12212 [DOI] [PubMed] [Google Scholar]
- 36.Danielsen K, Olsen AO, Wilsgaard T, Furberg A-S. Is the prevalence of psoriasis increasing? a 30-year follow-up of a population-based cohort. Br J Dermatol. 2013;168(6):1303-1310. doi: 10.1111/bjd.12230 [DOI] [PubMed] [Google Scholar]
- 37.Zastrow A, Faude V, Seyboth F, Niehoff D, Herzog W, Löwe B. Risk factors of symptom underestimation by physicians. J Psychosom Res. 2008;64(5):543-551. doi: 10.1016/j.jpsychores.2007.11.010 [DOI] [PubMed] [Google Scholar]
- 38.Levis B, Benedetti A, Thombs BD; DEPRESsion Screening Data (DEPRESSD) Collaboration . Accuracy of Patient Health Questionnaire-9 (PHQ-9) for screening to detect major depression: individual participant data meta-analysis. BMJ. 2019;365:l1476. doi: 10.1136/bmj.l1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He C, Levis B, Riehm KE, et al. The accuracy of the Patient Health Questionnaire-9 algorithm for screening to detect major depression: an individual participant data meta-analysis. Psychother Psychosom. 2020;89(1):25-37. doi: 10.1159/000502294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitchell AJ, Yadegarfar M, Gill J, Stubbs B. Case finding and screening clinical utility of the Patient Health Questionnaire (PHQ-9 and PHQ-2) for depression in primary care: a diagnostic meta-analysis of 40 studies. BJPsych Open. 2016;2(2):127-138. doi: 10.1192/bjpo.bp.115.001685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iskandar IYK, Ashcroft DM, Warren RB, et al. Demographics and disease characteristics of patients with psoriasis enrolled in the British Association of Dermatologists Biologic Interventions Register. Br J Dermatol. 2015;173(2):510-518. doi: 10.1111/bjd.13908 [DOI] [PubMed] [Google Scholar]
- 42.Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146(8):891-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Augustin M, Spehr C, Radtke MA, et al. German psoriasis registry PsoBest: objectives, methodology and baseline data. J Dtsch Dermatol Ges. 2014;12(1):48-57. doi: 10.1111/ddg.12233 [DOI] [PubMed] [Google Scholar]
- 44.Kimball AB, Leonardi C, Stahle M, et al. ; PSOLAR Steering Committee . Demography, baseline disease characteristics and treatment history of patients with psoriasis enrolled in a multicentre, prospective, disease-based registry (PSOLAR). Br J Dermatol. 2014;171(1):137-147. doi: 10.1111/bjd.13013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Screening for Depression and Anxiety
eReferences
eFigure. Predicted Probabilities of Discordance by Mental Health Status for a Difference of 2+ Categories Between Patient and Physician Global Assessments
eTable 1. Odds Ratios for the Interaction Between Mental Health Status and PASI Score
eTable 2. Discordance in Patient vs Physician Ratings, Overall and by Mental Health Status, Based on a Difference of 2+ Categories