Key Points
Question
What is the long-term outcome of the Ross procedure in young and middle-aged adults?
Findings
In this cohort study including 1431 adult patients undergoing the Ross procedure, survival at 10 and 15 years was 95.1% and 88.5%, respectively. Reintervention on either the homograft or autograft 15 years after surgery was uncommon, valve-related events were rare, and valve function was excellent as determined by echocardiography.
Meaning
The Ross procedure should be considered in young adults with active lifestyles and good cardiac health who are in need of aortic valve replacement, and patients should be referred to a specialized center with a dedicated Ross program.
Abstract
Importance
There is no ideal valve substitute for young adults requiring aortic valve replacement. Multicenter data supporting use of the Ross procedure with respect to long-term postoperative valve–related mortality and reintervention, as well as function of the autograft and pulmonary homograft, are needed.
Objective
To determine the long-term clinical and echocardiographic outcomes in young and middle-aged patients undergoing the Ross procedure.
Design, Setting, and Participants
A retrospective multicenter international cohort study with a median follow-up period of 9.2 years was conducted in 5 experienced centers regularly performing the Ross procedure. Consecutive patients aged 18 to 65 years were included by each center between 1991 and 2018.
Main Outcomes and Measures
Survival and autograft-related and homograft-related reintervention. Serial echocardiographic measurements of valve function were analyzed using mixed-effects modeling.
Results
During the study period, 1431 patients (74.3% men; n = 1063) were operated on at a median age of 48.5 years (mean [SD], 47.7 [9.5]; range, 18.1-65; interquartile range, 42.7-54.0). Implantation techniques were root inclusion in 355 (24.9%), root replacement in 485 (34.0%), and subcoronary implantation in 587 (41.1%). Right ventricular outflow tract reconstruction was performed with homografts in 98.6% (n = 1189) and bioprostheses in 1.4% (n = 17). Ten patients (0.7%) died before discharge. Median follow-up was 9.2 years (13 015 total patient-years). Survival after 10 and 15 years was 95.1% (95% CI, 93.8%-96.5%) and 88.5% (95% CI, 85.9%-91.1%), respectively. Freedom from autograft and homograft reintervention after 15 years was 92.0% and 97.2%, respectively. Late events were autograft endocarditis in 14 patients (0.11% per patient-year), homograft endocarditis in 11 patients (0.08% per patient-year), and stroke in 37 patients (0.3% per patient-year).
Conclusions and Relevance
Given its excellent short-term and long-term outcome in young and middle-aged adults in this study, the Ross procedure should be considered in young and middle-aged adults who require aortic valve replacement. Patients should be referred to an experienced center with a program dedicated to the Ross procedure.
This study evaluates the long-term clinical and echocardiographic outcomes in young and middle-aged patients undergoing pulmonary autograft procedure.
Introduction
Surgical alternatives for aortic valve replacement (AVR) in young and middle-aged adults are limited. Mechanical and bioprosthetic valves have benefits and drawbacks, but both are suboptimal in terms of survival and comorbidity. The pulmonary autograft procedure (Ross procedure hereafter) has been the only alternative, restoring life expectancy to that of a sex- and age-matched general population for at least 15 years after the operation.1,2,3,4 Several single-center studies with extensive follow-up indicated that patients younger than 50 years who have active lifestyles, a long life expectancy, and a potential childbearing desire are better served with the Ross procedure.1,2,5,6,7,8,9,10 Despite excellent results, the Ross procedure remains an underused treatment, limited to experienced centers and surgeons. Its limited use is often attributed to the complexity of the procedure and concerns about increased risks of early mortality and late reintervention.11,12,13 The 2017 guidelines14 on the management of valvular heart disease do not recommend the Ross procedure in middle-aged patients and advise consideration only in selected young patients with contraindications or unwillingness to anticoagulation (class IIb, evidence level C). The Society of Thoracic Surgeons 2013 aortic valve and ascending aorta management guidelines15 do not recommend the Ross procedure (class III, evidence level C) for middle-aged or older adults. However, evidence indicating clear benefits of the Ross procedure in selected young and middle-aged patients is accumulating.16,17 We present an international, multicenter study focusing on the characteristics and clinical and echocardiographic outcomes of the Ross procedure in adult patients aged 18 to 65 years.
Methods
Study Data
Patients operated on in 1 of 5 high-volume centers in Australia, Belgium, Brazil, Canada, and Germany were pooled (eAppendix 1 in the Supplement). All consecutive young and middle-aged patients who were aged 18 through 65 years at time of surgery and electively underwent the Ross procedure between January 1991 and December 2018 were included. Indications for the Ross procedure have been described by multiple reports by each center, including exact surgical techniques.10,18,19,20,21,22 Bicuspid aortic valves, isolated severe aortic regurgitation, concomitant coronary artery bypass surgery, or replacement of the ascending aorta were not considered exclusion criteria. Patients who underwent emergency (<24-hour) operation or concomitant mitral valve replacement or had an aortic dissection were excluded. Each center prospectively and independently registered procedural and clinical outcomes as well as echocardiographic measurements according to center-specific protocol.10,18,19,20,21,22 Anonymized individual patient data were merged into a single data set and analyzed by an independent biostatistician (G.P.) from the Erasmus University Medical Center. The institutional review boards of all participating centers reviewed and approved this study. All participants provided written informed consent.
End Points
Valve-related events and outcomes were reported according to recommended guidelines.23 Late all-cause mortality was thus defined as any death occurring beyond 30 days after surgery. Homografts were used in 98.5% of all patients to reconstruct the right ventricular outflow tract. Reintervention was defined as reintervention on either the autograft or homograft after the initial procedure, regardless of concomitant procedures involving other (cardiac) structures. The peak transvalvular gradient and regurgitation grades were determined from a multiwindow perspective using m-mode Doppler echocardiography on a yearly basis. Aortic regurgitation (AR) was scored on a scale from 0 (none) to 4 (severe) according to the guidelines for the assessment of valvular stenosis and regurgitation.24
Statistical Analysis
Continuous data are presented as means with standard deviation or medians with range, after testing for normality using the Kolmogorov-Smirnov test. Categorical data are presented as absolute count with percentages. Descriptive analyses were performed with SPSS, version 23.0 (IBM SPSS Inc). Actuarial estimates of freedom from death and reintervention were performed using Kaplan-Meier techniques. Mixed-effects models were used to analyze repeated measurements as gradient and dimensions. Multivariable proportional hazards models were used to investigate factors associated with the clinical end points. Owing to the low event rate and the high number of candidate predictors, a penalized likelihood approach was used for the multivariable Cox regression models. To account for missing covariate data, we used a multiple imputation approach. Associations between baseline characteristics and outcomes of interest were tested using the Wald test. The reported P values are not adjusted for multiple testing. The regression analysis and mixed-effect models were performed using R software, version 3.6.1 (the R Foundation). A more detailed description of the statistical analysis is provided in eAppendix 2 in the Supplement. All authors had direct access to any aspect of the data and take responsibility for its integrity.
Results
Survival and Morbidity
The total study population consisted of 1431 patients (Australia [n = 201], Belgium [n = 174], Brazil [n = 316], Canada [n = 112], and Germany [n = 628]). Median age at operation was 48.5 years (interquartile range [IQR], 42.7-54.0). Valve morphology was known in 1022 patients, of whom 778 (76.1%) had a bicuspid aortic valve. All baseline characteristics and intraoperative and early outcomes are presented in the Table. Characteristics of the patients in each of the 5 centers are presented in eTable 1 in the Supplement.
Table. Baseline and Surgical Characteristics.
| Characteristic | No. (%) |
|---|---|
| Patients, No. | 1431 |
| Age, mean (SD), y | 47.7 (9.5) |
| <20 | 20 |
| 20-40 | 192 |
| 40-60 | 1114 |
| >60 | 105 |
| Male | 1064 (74.3) |
| Hypertension | 374 (32.0) |
| Total No. | 1167 |
| Diabetes | 43 (4.1) |
| Total No. | 1055 |
| Coronary artery disease | 52 (4.9) |
| Total No. | 1056 |
| Chronical obstructive lung disease | 21 (2.0) |
| Total No. | 1056 |
| Congestive heart failure | 15 (1.2) |
| Total No. | 1230 |
| Dyslipidemia | 223 |
| Total No. | 1056 |
| Peripheral arterial disease | 5 (0.5) |
| Total No. | 1056 |
| Aortic valve morphology | |
| Total No. | 1022 |
| Bicuspid | 778 (76.1) |
| Tricuspid | 191 (18.7) |
| Other/prosthetic | 53 (5.2) |
| Concomitant procedures | |
| CABG | 45 (3.7) |
| Total No. | 1230 |
| Previous cardiac surgery | 85 (5.9) |
| Echocardiographic | |
| LVEDD, mean (SD), mm | 53 (9.5) |
| Total No. | 1198 |
| LVESD, mean (SD), mm | 34 (8.5) |
| Total No. | 1127 |
| LVEF, mean (SD), % | 66 (11.5) |
| Total No. | 967 |
| Aorta valve gradient, mean (SD), mm Hg | |
| Peak | 70 (33) |
| Mean | 44 (21) |
| Aortic valve regurgitation | |
| Total No. | 1117 |
| Aortic regurgitation | |
| None | 141 (12.6) |
| Trace | 363 (32.5) |
| Mild | 237 (21.2) |
| Moderate | 278 (24.9) |
| Severe | 98 (8.8) |
| CPB time, mean (SD), min | 198 (41) |
| Total No. | 1313 |
| Cross-clamp time, mean (SD), min | 173 (36) |
| No. | 1313 |
| RVOT conduit | |
| No. | 1206 |
| Homograft | 1189 (98.6) |
| Pulmonary | 1185 (99.7) |
| Aortic | 4 (0.3) |
| Bioprosthesis | 17 (1.4) |
| In-hospital mortality | 10 (0.7) |
Abbreviations: CABG, coronary artery bypass grafting; CPB, cardio-pulmonary bypass; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; NYHA, New York Heart Association; RVOT, right ventricular outflow tract.
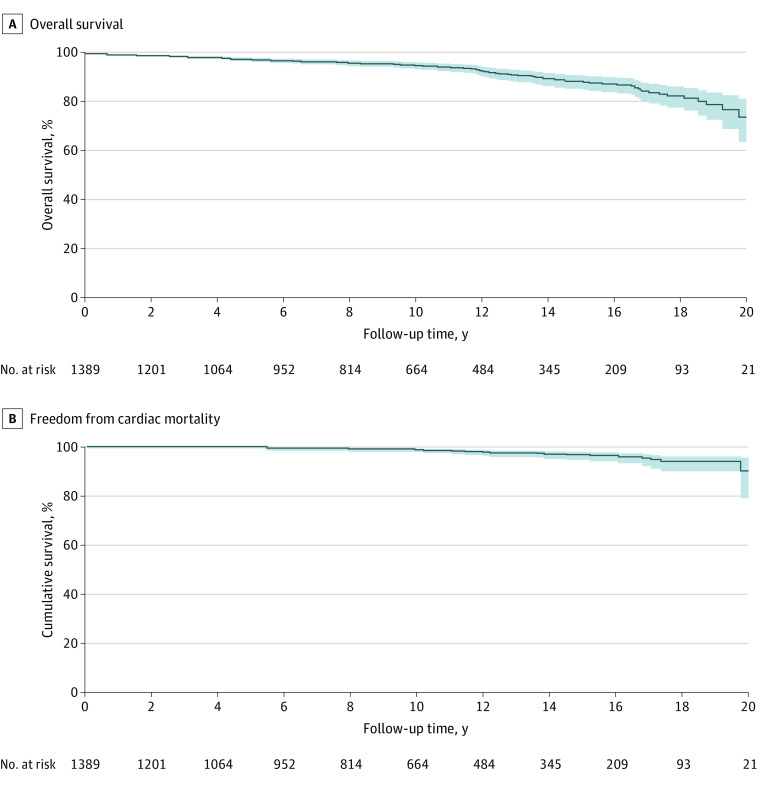
Ten patients (o.7%) had in-hospital mortality. Median follow-up was 9.2 years (13.02 total patient-years; IQR, 4.2-14.0), during which autograft endocarditis occurred in 14 patients (linearized occurrence rate [LOR], 0.11% per patient-year), homograft endocarditis in 11 patients (LOR, 0.08% per patient-year), and stroke in 37 patients (LOR, 0.3% per patient-year). Overall survival after 10 and 15 years was 95.1% (95% CI, 93.8%-96.5%) and 88.5% (95% CI, 85.9%-91.1%), respectively (Figure 1). Figure 2 presents similar survival curves for specific centers. Freedom from cardiac mortality (n = 30) at 10 and 15 years was 98.6% (95% CI, 97.9%-99.4%) and 96.5% (95% CI, 95.0%-98.0%), respectively (Figure 1). Higher age at operation (hazard ratio [HR], 1.07; 95% CI, 1.05-1.08), female sex (HR, 1.42; 95% CI, 1.01-2.01), preoperative comorbidities peripheral vascular disease (HR, 9.42; 95% CI, 3.92-22.63), chronic obstructive pulmonary disease (HR, 5.06; 95% CI, 2.60-9.88), congenital heart disease (HR, 1.92, 95% 1.16-3.20), and congestive heart failure (HR, 1.07; 95% CI, 1.05-1.08) were associated with an increased hazard of late death (eTables 2 and 3 in the Supplement).
Figure 1. Kaplan-Meier Plots of Freedom From All-Cause Mortality and Cardiac Mortality.
Cumulative freedom from all-cause mortality (A) and cardiac mortality (B) in years after the Ross operation, with the shaded areas representing 95% CIs.
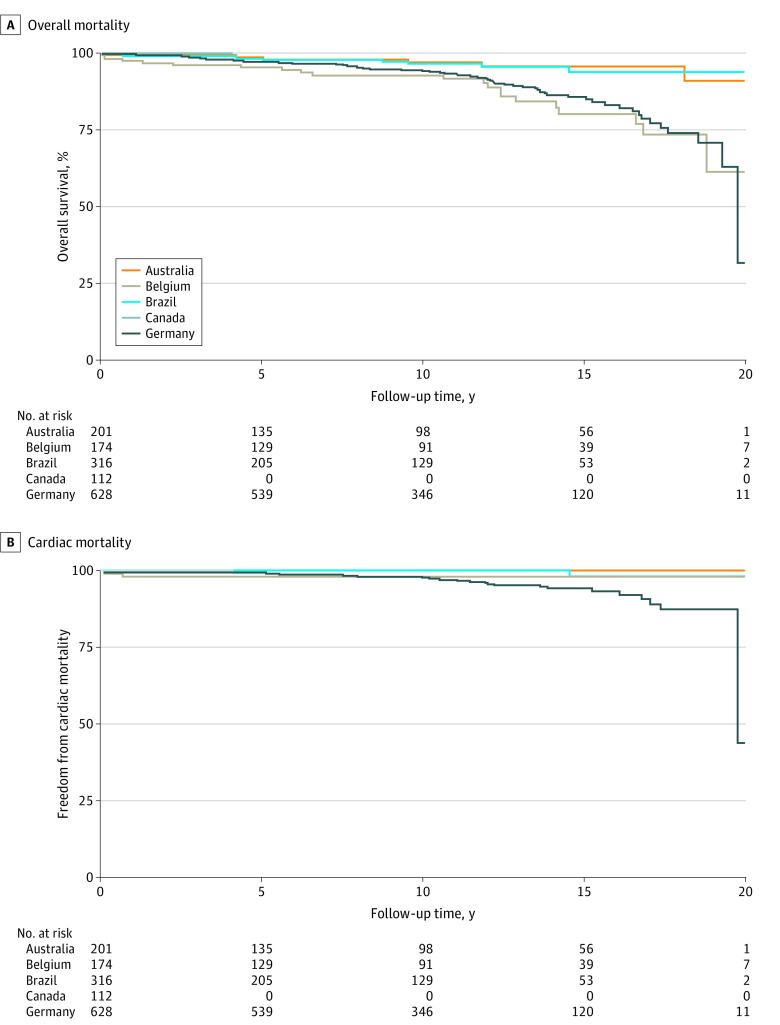
Figure 2. Kaplan-Meier Plots of Freedom From All-Cause and Cardiac Mortality per Center.
Freedom from overall mortality (A) and cardiac mortality (B) are shown for each country.
Reintervention
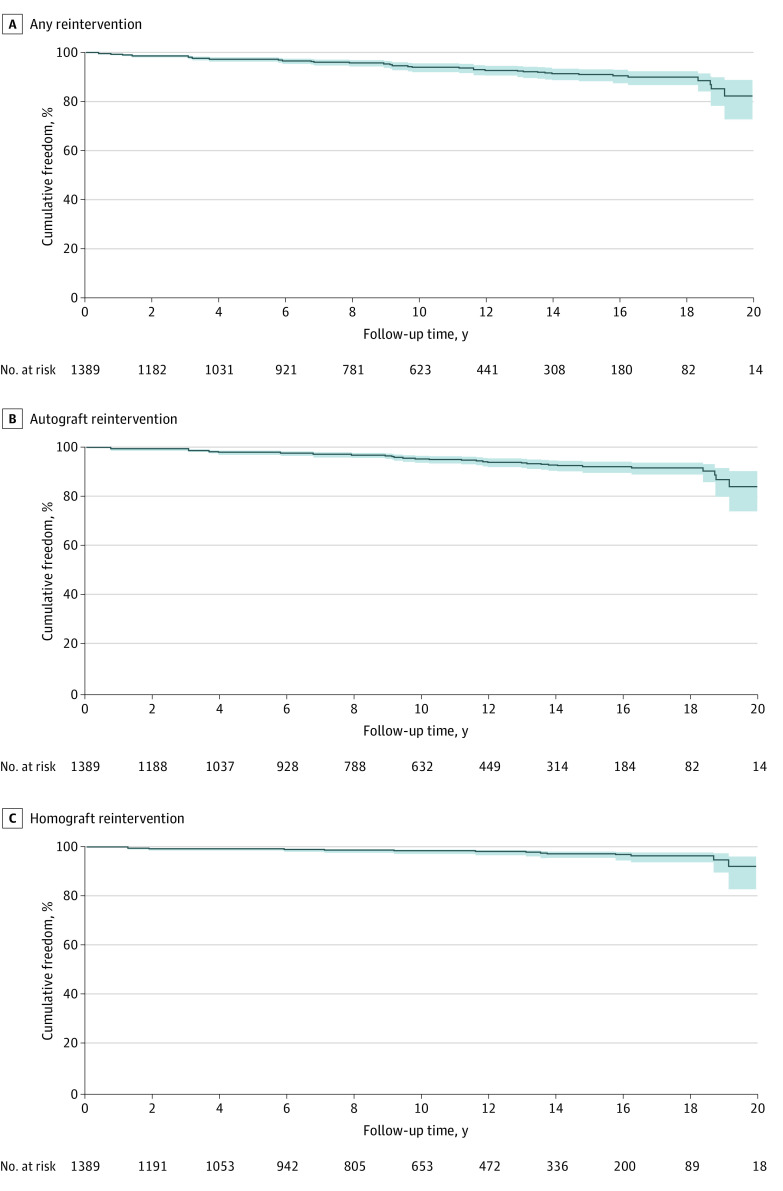
Freedom from any reintervention on the autograft or homograft after 10 and 15 years was 93.9% (95% CI, 92.4%-95.5%) and 90.8% (95% CI, 88.6%-93.1%), respectively (Figure 3; eTable 4 in the Supplement). Freedom from autograft reintervention at 10 and 15 years was 95.0% (95% CI, 93.6%-96.4%) and 92.0% (95% CI, 89.8%-94.2%) (Figure 3). Severe preoperative regurgitation was associated with an increased hazard of autograft reintervention (HR, 3.07; 95% CI, 1.02-9.22) (eTable 4 in the Supplement). Likewise, a lower New York Heart Association functional classification (HR, 0.67; 95% CI, 0.51-0.87), female sex (HR, 0.66; 95% CI, 0.45-0.98), the subcoronary implantation technique (vs the root inclusion technique) (HR, 0.44; 95% CI, 0.25-0.77), and severe comorbidities were associated with a lower hazard of autograft reintervention (eTable 5 in the Supplement). Freedom from homograft reintervention at 10 and 15 years was 98.4% (95% CI, 97.6-99.2) and 97.2% (95% CI, 95.9%-98.5%), respectively (Figure 3; eFigure 1 in the Supplement). Higher age of the homograft donor (HR, 0.97; 95% CI, 0.94-0.99) and larger homograft diameter (HR, 0.77; 95% CI, 0.66-0.90) were associated with a lower hazard of reintervention on the homograft (eTable 6 in the Supplement). Country-specific freedom from reintervention is presented eFigures 1 to 3 in the Supplement.
Figure 3. Kaplan-Meier Plots of Freedom From Reintervention.
Cumulative freedom from any reintervention (A), autograft reintervention (B), and homograft reintervention (C) in years after the Ross operation, with the shaded areas representing 95% CIs.
Echocardiographic Outcomes
In total, 8655 postoperative echocardiographic measurements were available for 1150 (82.8%) (mean echocardiograms available: 7.5; range, 1-20) for a median period of 9.2 years (range, 0.01-25.4 years). eFigures 4 and 5 in the Supplement depict the predicted evolution of gradient and regurgitation grade for the autograft and homograft for a patient with median characteristics. Female sex, a tricuspid native aortic valve, and higher preoperative gradient were significantly associated with a higher autograft gradient (eTable 7 in the Supplement). Moderate (odds ratio [OR], 2.72; 95% CI, 0.97-7.64) or severe (OR, 2.12; 95% CI, 0.56-8.03) preoperative aortic valve regurgitation were not significantly associated with an increased likelihood to develop significant postoperative autograft regurgitation (eTable 8 in the Supplement). Furthermore, the likelihood of developing significant regurgitation was independent from surgical technique (ie, root inclusion vs root replacement vs subcoronary). The predicted prevalence of moderate or severe regurgitation after 20 years was less than 1% in a patient with median characteristics.
eFigures 4 and 5 in the Supplement depict the predicted evolution of the gradient and regurgitation grade for the homograft, respectively. The gradient initially increases during the first postoperative decade, after which it slopes down and plateaus during the second postoperative decade. Female sex was significantly associated with a lower gradient (eTable 9 in the Supplement) but also with an increased likelihood of developing significant regurgitation (OR, 2.63; 95% CI, 1.60-4.32). In contrast, use of a homograft instead of a bioprosthesis (OR, 0.05, 95% 0.01-0.29) and older donor age (OR, 0.97; 95% CI, 0.95-0.99) were associated with a lower likelihood to develop significant regurgitation (eTable 10 in the Supplement).
Discussion
This international multicenter cohort study of consecutive young and middle-aged adults undergoing the Ross procedure shows excellent survival and outstanding clinical and echocardiographic outcomes. Both the autograft and homograft show a stable and predictable evolution of transvalvular gradient during the first 20 years postoperatively, with low rates of reintervention on both the autograft and homograft.
Current Challenges
Approximately 25% of all patients undergoing AVR are currently younger than 60 years, with most receiving mechanical valves.25 With life expectancy expected to further increase, the implantation of an aortic valve substitute that minimizes complications and optimizes quality of life is important. All available valve substitutes have their advantages and disadvantages that need to be considered in counseling patients in a shared decision-making prosthetic valve selection process. This study shows that the Ross procedure is an operation with potentially significant value in terms of survival and quality of life in young and middle-aged adults. The Ross procedure continues to be the only living-valve alternative in young and middle-aged patients with a reported survival that compares with the general population well into the second postoperative decade.4 Despite several studies unanimously indicating superior long-term results of the Ross procedure compared with conventional AVR,1,2,3,5,6,7,8,9,10,22,26 it represents only a modest share of all AVR performed in young and middle-aged patients who could have been eligible.27 Current challenges for a broader use of the Ross procedure are the need for more proctoring, specialized training and education, and better access to the small number of specialized centers.
Early Mortality
One of the main reasons for lagging interest in the procedure has been the complexity of both the initial procedure and reoperation suggesting limited generalizability of the results published by specialized centers.11,12,13 An analysis of the Society of Thoracic Surgeons Adult Cardiac Surgery database suggested a 3-fold increased operative mortality (2.7% vs 0.9%) compared with conventional mechanical AVR.27 However, operative risks have a well-known inverse association with center volume, and a significant proportion of mortality was accountable to centers performing the procedure only sporadically.28 Bouhout et al29 showed by propensity-matched comparison that the Ross procedure yields comparable perioperative outcomes and risks as mechanical AVR. Early mortality in the study was only 0.7% and ranges between 0.4% and 2.3% in experienced centers, which is comparable with many routine surgical procedures.1,2,4,5,10,26,29,30,31,32 Technical complexities can thus be overcome without increased early mortality given sufficient operative volumes.
Late Survival
The Ross procedure is the only aortic valve substitute that is associated with a life expectancy and quality of life comparable with an age- and sex-matched population up to at least 15 years of follow-up.1,2,3,33,34,35 In our study, survival after 15 years was 88.5%, with older age, female sex, and significant preoperative comorbidity as risk factors for reduced survival. Survival at 15 years in large contemporary series ranges between 88% and 97%,1,2,9,10,18,21,36 with survival being reported up to 97% at 20 years in experienced centers.2,18 In contrast, young and middle-aged patients who undergo either mechanical AVR or bioprosthetic AVR both show excess mortality compared with an age-matched general population.37,38,39 This difference can on the one hand be explained by the implanted prosthesis; on the other hand, selection bias may also play a role.
Multiple propensity score–matched comparisons have unanimously indicated freedom from both death and reintervention in favor of the Ross procedure compared with mechanical AVR.3,4,22,26,29,40,41 In 2018, the group of Skillington18 presented superior survival after 20 years (94% vs 84%) among 275 patients who underwent the Ross procedure who were matched with patients who underwent mechanical AVR.2 Again, mechanical AVR was an independent risk factor for mortality in that study. Sharabiani et al3 showed that in a matched population of young adults who underwent either a Ross procedure, mechanical AVR, or bioprosthetic AVR, the former 2 had comparable hazards for both death and reintervention that were superior to the bioprosthetic group. Andreas et al40 showed that patients who underwent the Ross procedure enjoyed survival comparable with an age- and sex-matched Austrian population. In contrast, observed survival of patients who underwent mechanical AVR was significantly less than predicted, with mechanical AVR again being an independent risk factor for late mortality. Mazine et al26 showed that cardiac- and valve-related mortality was less frequent in patients who underwent the Ross procedure than in a matched group of patients undergoing mechanical AVR. Survival during the first postoperative decade of patients undergoing mechanical AVR has been equated to patients undergoing the Ross procedure in a setting of optimal anticoagulation therapy in terms of intensive monitoring and self-management.4
Reintervention
Potential failure of both valves in a patient who originally only had single-valve disease has been regarded as another major drawback of the Ross procedure.31 Freedom from reintervention in our study after 15 years was 97.2% for the homograft and 92.0% for the autograft. Freedom from reintervention in large contemporary series on the either the pulmonary autograft or homograft after 20 years ranges between 81.8 and 85.0% and 82.6 and 95.0%, respectively.2,9,26,32 Transcatheter pulmonary valve implantation may be able to reduce the frequency of surgical reintervention even further.42 Compared with AVR with bioprostheses or homografts, combined freedom from reintervention on both valves is superior in matched patients who underwent the Ross procedure.1,43,44 Moreover, reintervention after mechanical AVR is not negligible with freedom from reoperation ranging between 82% and 96% after 10 years owing to valve thrombosis, pannus, endocarditis, or paravalvular leakage.45,46,47
Common mechanisms of failure in patients who underwent the Ross procedure are currently better understood and often are associated with dilatation at either the annulus, sinus, or sino-tubular junction.48 Severe preoperative aortic regurgitation was confirmed to be a risk factor for autograft dysfunction. Reoperation can be challenging depending on which valve or valves are involved and the extent of other pathology, but in experienced hands, mortality is low, ranging from 0% to 5.6% as reported in a number of fairly large contemporary series.9,11,12,13,41,49,50 Preoperative echocardiography and chest computed tomographic angiography can identify which patients are especially at risk of late root dilatation, in which case other surgical options may be more appropriate or surgical modifications designed to prevent dilatation can be included in the operative plan. Good initial results have been reported with an autograft support strategy, but solid long-term data are not yet available.18,51 Furthermore, if reoperation on the pulmonary autograft is required, restoration and preservation may sometimes be possible, preserving the benefits of the living valve. Therefore, the risks of reintervention after the Ross procedure are acceptable, certainly manageable, and should not be a reason to avoid the procedure altogether.
Valve-Related Events
One of the major advantages of the Ross procedure is the avoidance of permanent anticoagulation. No other durable AVR substitute that does not require anticoagulation is available. Especially for young and middle-aged patients, there is an unambiguous need for a durable valve alternative without the lifestyle restraints and risks inherent to permanent anticoagulation. In our study, the stroke and overall endocarditis rates were 0.3% and 0.19% per patient-year, respectively, which is comparable with results of other studies and meta-analyses. A 2018 meta-analysis16 in patients who underwent the Ross procedure showed linearized occurrence rates in adults of thromboembolism of 0.17% per patient-year, bleeding of 0.10% per patient-year, right ventricular outflow tract endocarditis of 0.14% per patient-year, and autograft endocarditis of 0.18% per patient-year. The authors concluded that the risks of bleeding and thromboembolism in patients undergoing the Ross procedure are comparable with those in the general population.16 A comparable 2017 meta-analysis52 about mechanical AVR in nonelderly adults (ie, <55 years) indicated higher incidences of thromboembolism (0.90% per patient-year), bleeding (0.85% per patient-year), and endocarditis (0.41% per patient-year).52 Note that the cumulative risks of severe stroke owing to the permanent use of anticoagulation are extensive,38,39,45,46,53 causing young adults who undergo mechanical AVR to experience excess mortality compared with a sex- and age-matched general population.3,22,40,46 Younger age in patients undergoing mechanical AVR is an independent risk factor for mortality.38 Furthermore, self-monitoring and lowering the therapeutic range of international normalized ratio have had a limited effect on reducing thromboembolic or bleeding events and the subsequent hazard of death.54,55 Full discussion of the long-term prospects of the different valve alternatives should be presented to young and middle-aged adults, including the evidence-based superiority of the Ross procedure in terms of valve-related events.
These and other studies1,2,3,5,6,7,8,9,10,22,26 support the need for active proctoring, more surgical training, and education and reference to experienced centers if patients are eligible and willing to undergo the procedure. Careful selection of patients and reference to one of the specialized and experienced centers is essential. Both patients and cardiologists should be aware that for patients with long (>15 years) life expectancy, feasible anatomy, a pregnancy wish, contraindications to anticoagulation, or physically active lifestyles, the Ross procedure can be a more suitable option than mechanical AVR.
Strengths and Limitations
This study is, to our knowledge, the largest pooled analysis of previously described prospectively included young and middle-aged patients undergoing the Ross procedure in experienced centers. Mixed-effects models of serial echocardiographic measurements were used for the first time to identify risk factors for late graft deterioration and mortality. A limitation to these results is that they were achieved by experienced surgeons in highly specialized centers, making it perhaps difficult for less-experienced surgeons to instantly mimic the results. Differences in surgeons and surgical techniques could not completely be accounted for, and follow-up of the Canadian cohort was limited. The positive results after the Ross procedure might be partially explained by the careful selection of patients besides avoidance of anticoagulation and superior valve hemodynamics alone. Future studies should include even longer follow-up, ideally into the third decade, including matched comparisons with patients undergoing mechanical AVR and the general population. Furthermore, new studies addressing the quality of life given permanent oral anticoagulation are needed to more closely determine its effect on long-term quality of life.
Conclusions
In this study, excellent short- and long-term outcomes after the Ross procedure are observed in appropriately selected patients treated in experienced centers. The Ross procedure should be considered in young and middle-aged adults who need AVR and can be referred to dedicated centers with expertise in this operation.
eTable 1. Baseline and surgical characteristics per center
eTable 2. Risk factor analysis by penalized Cox regression: All-cause mortality
eTable 3. Risk factor analysis by penalized Cox regression: Cardiac mortality
eTable 4. Risk factor analysis by penalized Cox regression: Freedom from any reintervention
eTable 5. Risk factor analysis by penalized Cox regression: Freedom from autograft reintervention
eTable 6. Risk factor analysis by penalized Cox regression: Freedom from homograft reintervention
eTable 7. Model Coefficients for peak autograft gradient
eTable 8. Odds Ratios, 95% CIs and Univariate Wald tests of covariates with autograft regurgitation
eTable 9. Model Coefficients for peak homograft gradient
eTable 10. Odds Ratios, 95% CIs and Univariate Wald tests of covariates with homograft regurgitation
eFigure 1. Kaplan-Meier plots of overall reintervention per country
eFigure 2. Kaplan-Meier plots of autograft reintervention per country
eFigure 3. Kaplan-Meier plots of homograft reintervention per country
eFigure 4. Effect plots of Autograft gradient and homograft gradient
eFigure 5. Effect plot of Autograft regurgitation and homograft regurgitation
eAppendix 1. List of participating centers
eAppendix 2. In depth description of the statistical analysis
References
- 1.El-Hamamsy I, Eryigit Z, Stevens LM, et al. Long-term outcomes after autograft versus homograft aortic root replacement in adults with aortic valve disease: a randomised controlled trial. Lancet. 2010;376(9740):524-531. doi: 10.1016/S0140-6736(10)60828-8 [DOI] [PubMed] [Google Scholar]
- 2.David TE, David C, Woo A, Manlhiot C. The Ross procedure: outcomes at 20 years. J Thorac Cardiovasc Surg. 2014;147(1):85-93. doi: 10.1016/j.jtcvs.2013.08.007 [DOI] [PubMed] [Google Scholar]
- 3.Sharabiani MT, Dorobantu DM, Mahani AS, et al. Aortic valve replacement and the Ross operation in children and young adults. J Am Coll Cardiol. 2016;67(24):2858-2870. doi: 10.1016/j.jacc.2016.04.021 [DOI] [PubMed] [Google Scholar]
- 4.Mokhles MM, Körtke H, Stierle U, et al. Survival comparison of the Ross procedure and mechanical valve replacement with optimal self-management anticoagulation therapy: propensity-matched cohort study. Circulation. 2011;123(1):31-38. doi: 10.1161/CIRCULATIONAHA.110.947341 [DOI] [PubMed] [Google Scholar]
- 5.Sievers HH, Stierle U, Charitos EI, et al. Fourteen years’ experience with 501 subcoronary Ross procedures: surgical details and results. J Thorac Cardiovasc Surg. 2010;140(4):816-822, 822.e1-822.e5. [DOI] [PubMed] [Google Scholar]
- 6.Klieverik LM, Takkenberg JJ, Bekkers JA, Roos-Hesselink JW, Witsenburg M, Bogers AJ. The Ross operation: a Trojan horse? Eur Heart J. 2007;28(16):1993-2000. doi: 10.1093/eurheartj/ehl550 [DOI] [PubMed] [Google Scholar]
- 7.Charitos EI, Stierle U, Hanke T, Schmidtke C, Sievers HH, Richardt D. Long-term results of 203 young and middle-aged patients with more than 10 years of follow-up after the original subcoronary Ross operation. Ann Thorac Surg. 2012;93(2):495-502. doi: 10.1016/j.athoracsur.2011.10.017 [DOI] [PubMed] [Google Scholar]
- 8.Yacoub MH, Klieverik LM, Melina G, et al. An evaluation of the Ross operation in adults. J Heart Valve Dis. 2006;15(4):531-539. [PubMed] [Google Scholar]
- 9.Martin E, Mohammadi S, Jacques F, et al. Clinical outcomes following the Ross procedure in adults: a 25-year longitudinal study. J Am Coll Cardiol. 2017;70(15):1890-1899. doi: 10.1016/j.jacc.2017.08.030 [DOI] [PubMed] [Google Scholar]
- 10.Mastrobuoni S, de Kerchove L, Solari S, et al. The Ross procedure in young adults: over 20 years of experience in our institution. Eur J Cardiothorac Surg. 2016;49(2):507-512. doi: 10.1093/ejcts/ezv053 [DOI] [PubMed] [Google Scholar]
- 11.Stulak JM, Burkhart HM, Sundt TM III, et al. Spectrum and outcome of reoperations after the Ross procedure. Circulation. 2010;122(12):1153-1158. doi: 10.1161/CIRCULATIONAHA.109.897538 [DOI] [PubMed] [Google Scholar]
- 12.Pettersson GB, Subramanian S, Flynn M, et al. Reoperations after the ross procedure in adults: towards autograft-sparing/Ross reversal. J Heart Valve Dis. 2011;20(4):425-432. [PubMed] [Google Scholar]
- 13.Luciani GB, Favaro A, Casali G, Santini F, Mazzucco A. Reoperations for aortic aneurysm after the Ross procedure. J Heart Valve Dis. 2005;14(6):766-772. [PubMed] [Google Scholar]
- 14.Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(25):e1159-e1195. doi: 10.1161/CIR.0000000000000503 [DOI] [PubMed] [Google Scholar]
- 15.Svensson LG, Adams DH, Bonow RO, et al. Aortic valve and ascending aorta guidelines for management and quality measures. Ann Thorac Surg. 2013;95(6)(suppl):S1-S66. doi: 10.1016/j.athoracsur.2013.01.083 [DOI] [PubMed] [Google Scholar]
- 16.Etnel JRG, Grashuis P, Huygens SA, et al. The Ross procedure: a systematic review, meta-analysis, and microsimulation. Circ Cardiovasc Qual Outcomes. 2018;11(12):e004748. doi: 10.1161/CIRCOUTCOMES.118.004748 [DOI] [PubMed] [Google Scholar]
- 17.Mazine A, El-Hamamsy I, Verma S, et al. Ross procedure in adults for cardiologists and cardiac surgeons: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72(22):2761-2777. doi: 10.1016/j.jacc.2018.08.2200 [DOI] [PubMed] [Google Scholar]
- 18.Skillington PD, Mokhles MM, Takkenberg JJ, et al. The Ross procedure using autologous support of the pulmonary autograft: techniques and late results. J Thorac Cardiovasc Surg. 2015;149(2)(suppl):S46-S52. doi: 10.1016/j.jtcvs.2014.08.068 [DOI] [PubMed] [Google Scholar]
- 19.Sievers HH, Stierle U, Petersen M, et al. Valve performance classification in 630 subcoronary Ross patients over 22 years. J Thorac Cardiovasc Surg. 2018;156(1):79-86.e2. [DOI] [PubMed] [Google Scholar]
- 20.de Kerchove L, Rubay J, Pasquet A, et al. Ross operation in the adult: long-term outcomes after root replacement and inclusion techniques. Ann Thorac Surg. 2009;87(1):95-102. doi: 10.1016/j.athoracsur.2008.09.031 [DOI] [PubMed] [Google Scholar]
- 21.da Costa FD, Takkenberg JJ, Fornazari D, et al. Long-term results of the Ross operation: an 18-year single institutional experience. Eur J Cardiothorac Surg. 2014;46(3):415-422. doi: 10.1093/ejcts/ezu013 [DOI] [PubMed] [Google Scholar]
- 22.Buratto E, Shi WY, Wynne R, et al. Improved survival after the ross procedure compared with mechanical aortic valve replacement. J Am Coll Cardiol. 2018;71(12):1337-1344. doi: 10.1016/j.jacc.2018.01.048 [DOI] [PubMed] [Google Scholar]
- 23.Akins CW, Miller DC, Turina MI, et al. ; STS; AATS; EACTS . Guidelines for reporting mortality and morbidity after cardiac valve interventions. Ann Thorac Surg. 2008;85(4):1490-1495. doi: 10.1016/j.athoracsur.2007.12.082 [DOI] [PubMed] [Google Scholar]
- 24.Lancellotti P, Tribouilloy C, Hagendorff A, et al. ; Scientific Document Committee of the European Association of Cardiovascular Imaging . Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2013;14(7):611-644. doi: 10.1093/ehjci/jet105 [DOI] [PubMed] [Google Scholar]
- 25.Brown JM, O’Brien SM, Wu C, Sikora JA, Griffith BP, Gammie JS. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg. 2009;137(1):82-90. doi: 10.1016/j.jtcvs.2008.08.015 [DOI] [PubMed] [Google Scholar]
- 26.Mazine A, David TE, Rao V, et al. Long-term outcomes of the Ross procedure versus mechanical aortic valve replacement: propensity-matched cohort study. Circulation. 2016;134(8):576-585. doi: 10.1161/CIRCULATIONAHA.116.022800 [DOI] [PubMed] [Google Scholar]
- 27.Reece TB, Welke KF, O’Brien S, Grau-Sepulveda MV, Grover FL, Gammie JS. Rethinking the ross procedure in adults. Ann Thorac Surg. 2014;97(1):175-181. doi: 10.1016/j.athoracsur.2013.07.036 [DOI] [PubMed] [Google Scholar]
- 28.Hughes GC, Zhao Y, Rankin JS, et al. Effects of institutional volumes on operative outcomes for aortic root replacement in North America. J Thorac Cardiovasc Surg. 2013;145(1):166-170. doi: 10.1016/j.jtcvs.2011.10.094 [DOI] [PubMed] [Google Scholar]
- 29.Bouhout I, Noly PE, Ghoneim A, et al. Is the Ross procedure a riskier operation? perioperative outcome comparison with mechanical aortic valve replacement in a propensity-matched cohort. Interact Cardiovasc Thorac Surg. 2017;24(1):41-47. doi: 10.1093/icvts/ivw325 [DOI] [PubMed] [Google Scholar]
- 30.Takkenberg JJ, Klieverik LM, Schoof PH, et al. The Ross procedure: a systematic review and meta-analysis. Circulation. 2009;119(2):222-228. doi: 10.1161/CIRCULATIONAHA.107.726349 [DOI] [PubMed] [Google Scholar]
- 31.Mokhles MM, Rizopoulos D, Andrinopoulou ER, et al. Autograft and pulmonary allograft performance in the second post-operative decade after the Ross procedure: insights from the Rotterdam Prospective Cohort Study. Eur Heart J. 2012;33(17):2213-2224. doi: 10.1093/eurheartj/ehs173 [DOI] [PubMed] [Google Scholar]
- 32.Poh CL, Buratto E, Larobina M, et al. The Ross procedure in adults presenting with bicuspid aortic valve and pure aortic regurgitation: 85% freedom from reoperation at 20 years. Eur J Cardiothorac Surg. 2018;54(3):420-426. doi: 10.1093/ejcts/ezy073 [DOI] [PubMed] [Google Scholar]
- 33.Zacek P, Holubec T, Vobornik M, et al. Quality of life after aortic valve repair is similar to Ross patients and superior to mechanical valve replacement: a cross-sectional study. BMC Cardiovasc Disord. 2016;16:63. doi: 10.1186/s12872-016-0236-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nötzold A, Hüppe M, Schmidtke C, Blömer P, Uhlig T, Sievers H-H. Quality of life in aortic valve replacement: pulmonary autografts versus mechanical prostheses. J Am Coll Cardiol. 2001;37(7):1963-1966. doi: 10.1016/S0735-1097(01)01267-0 [DOI] [PubMed] [Google Scholar]
- 35.Aicher D, Holz A, Feldner S, Köllner V, Schäfers HJ. Quality of life after aortic valve surgery: replacement versus reconstruction. J Thorac Cardiovasc Surg. 2011;142(2):e19-e24. doi: 10.1016/j.jtcvs.2011.02.006 [DOI] [PubMed] [Google Scholar]
- 36.Sievers HH, Stierle U, Charitos EI, et al. A multicentre evaluation of the autograft procedure for young patients undergoing aortic valve replacement: update on the German Ross Registry. Eur J Cardiothorac Surg. 2016;49(1):212-218. doi: 10.1093/ejcts/ezv001 [DOI] [PubMed] [Google Scholar]
- 37.Goldstone AB, Chiu P, Baiocchi M, et al. Mechanical or biologic prostheses for aortic-valve and mitral-valve replacement. N Engl J Med. 2017;377(19):1847-1857. doi: 10.1056/NEJMoa1613792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kvidal P, Bergström R, Hörte LG, Ståhle E. Observed and relative survival after aortic valve replacement. J Am Coll Cardiol. 2000;35(3):747-756. doi: 10.1016/S0735-1097(99)00584-7 [DOI] [PubMed] [Google Scholar]
- 39.Mihaljevic T, Nowicki ER, Rajeswaran J, et al. Survival after valve replacement for aortic stenosis: implications for decision making. J Thorac Cardiovasc Surg. 2008;135(6):1270-1278. doi: 10.1016/j.jtcvs.2007.12.042 [DOI] [PubMed] [Google Scholar]
- 40.Andreas M, Wiedemann D, Seebacher G, et al. The Ross procedure offers excellent survival compared with mechanical aortic valve replacement in a real-world setting. Eur J Cardiothorac Surg. 2014;46(3):409-413. doi: 10.1093/ejcts/ezt663 [DOI] [PubMed] [Google Scholar]
- 41.Karaskov A, Sharifulin R, Zheleznev S, Demin I, Lenko E, Bogachev-Prokophiev A. Results of the Ross procedure in adults: a single-centre experience of 741 operations. Eur J Cardiothorac Surg. 2016;49(5):e97-e104. doi: 10.1093/ejcts/ezw047 [DOI] [PubMed] [Google Scholar]
- 42.Gillespie MJ, McElhinney DB, Kreutzer J, et al. Transcatheter pulmonary valve replacement for right ventricular outflow tract conduit dysfunction after the Ross procedure. Ann Thorac Surg. 2015;100(3):996-1002. doi: 10.1016/j.athoracsur.2015.04.108 [DOI] [PubMed] [Google Scholar]
- 43.David TE, Armstrong S, Maganti M. Hancock II bioprosthesis for aortic valve replacement: the gold standard of bioprosthetic valves durability? Ann Thorac Surg. 2010;90(3):775-781. doi: 10.1016/j.athoracsur.2010.05.034 [DOI] [PubMed] [Google Scholar]
- 44.Smedira NG, Blackstone EH, Roselli EE, Laffey CC, Cosgrove DM. Are allografts the biologic valve of choice for aortic valve replacement in nonelderly patients? Comparison of explantation for structural valve deterioration of allograft and pericardial prostheses. J Thorac Cardiovasc Surg. 2006;131(3):558-564.e4. [DOI] [PubMed] [Google Scholar]
- 45.Ikonomidis JS, Kratz JM, Crumbley AJ III, et al. Twenty-year experience with the St Jude Medical mechanical valve prosthesis. J Thorac Cardiovasc Surg. 2003;126(6):2022-2031. doi: 10.1016/j.jtcvs.2003.07.005 [DOI] [PubMed] [Google Scholar]
- 46.Bouhout I, Stevens LM, Mazine A, et al. Long-term outcomes after elective isolated mechanical aortic valve replacement in young adults. J Thorac Cardiovasc Surg. 2014;148(4):1341-1346.e1. [DOI] [PubMed] [Google Scholar]
- 47.Kulik A, Bédard P, Lam BK, et al. Mechanical versus bioprosthetic valve replacement in middle-aged patients. Eur J Cardiothorac Surg. 2006;30(3):485-491. doi: 10.1016/j.ejcts.2006.06.013 [DOI] [PubMed] [Google Scholar]
- 48.David TE, Ouzounian M, David CM, Lafreniere-Roula M, Manlhiot C. Late results of the Ross procedure. J Thorac Cardiovasc Surg. 2019;157(1):201-208. doi: 10.1016/j.jtcvs.2018.06.037 [DOI] [PubMed] [Google Scholar]
- 49.Kumar SR, Bansal N, Wells WJ, Starnes VA. Outcomes of reintervention on the autograft after Ross procedure. Ann Thorac Surg. 2016;102(5):1517-1521. doi: 10.1016/j.athoracsur.2016.04.059 [DOI] [PubMed] [Google Scholar]
- 50.Charitos EI, Takkenberg JJ, Hanke T, et al. Reoperations on the pulmonary autograft and pulmonary homograft after the Ross procedure: an update on the German Dutch Ross Registry. J Thorac Cardiovasc Surg. 2012;144(4):813-821. doi: 10.1016/j.jtcvs.2012.07.005 [DOI] [PubMed] [Google Scholar]
- 51.David TE, Omran A, Ivanov J, et al. Dilation of the pulmonary autograft after the Ross procedure. J Thorac Cardiovasc Surg. 2000;119(2):210-220. doi: 10.1016/S0022-5223(00)70175-9 [DOI] [PubMed] [Google Scholar]
- 52.Korteland NM, Etnel JRG, Arabkhani B, et al. Mechanical aortic valve replacement in non-elderly adults: meta-analysis and microsimulation. Eur Heart J. 2017;38(45):3370-3377. doi: 10.1093/eurheartj/ehx199 [DOI] [PubMed] [Google Scholar]
- 53.Van Nooten GJ, Caes F, François K, et al. Twenty years’ single-center experience with mechanical heart valves: a critical review of anticoagulation policy. J Heart Valve Dis. 2012;21(1):88-98. [PubMed] [Google Scholar]
- 54.Heneghan C, Ward A, Perera R, et al. ; Self-Monitoring Trialist Collaboration . Self-monitoring of oral anticoagulation: systematic review and meta-analysis of individual patient data. Lancet. 2012;379(9813):322-334. doi: 10.1016/S0140-6736(11)61294-4 [DOI] [PubMed] [Google Scholar]
- 55.Matchar DB, Jacobson A, Dolor R, et al. ; THINRS Executive Committee and Site Investigators . Effect of home testing of international normalized ratio on clinical events. N Engl J Med. 2010;363(17):1608-1620. doi: 10.1056/NEJMoa1002617 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Baseline and surgical characteristics per center
eTable 2. Risk factor analysis by penalized Cox regression: All-cause mortality
eTable 3. Risk factor analysis by penalized Cox regression: Cardiac mortality
eTable 4. Risk factor analysis by penalized Cox regression: Freedom from any reintervention
eTable 5. Risk factor analysis by penalized Cox regression: Freedom from autograft reintervention
eTable 6. Risk factor analysis by penalized Cox regression: Freedom from homograft reintervention
eTable 7. Model Coefficients for peak autograft gradient
eTable 8. Odds Ratios, 95% CIs and Univariate Wald tests of covariates with autograft regurgitation
eTable 9. Model Coefficients for peak homograft gradient
eTable 10. Odds Ratios, 95% CIs and Univariate Wald tests of covariates with homograft regurgitation
eFigure 1. Kaplan-Meier plots of overall reintervention per country
eFigure 2. Kaplan-Meier plots of autograft reintervention per country
eFigure 3. Kaplan-Meier plots of homograft reintervention per country
eFigure 4. Effect plots of Autograft gradient and homograft gradient
eFigure 5. Effect plot of Autograft regurgitation and homograft regurgitation
eAppendix 1. List of participating centers
eAppendix 2. In depth description of the statistical analysis