Abstract
Background:
The Adult Autism Subthreshold Spectrum (AdAS Spectrum) is a recently developed instrument tailored to assess the broad range of full-threshold as well as sub-threshold manifestations related to the autism spectrum. Although it has proved to be a valuable instrument for quantitative assessment of autistic symptoms, the AdAS Spectrum still lacks validated diagnostic thresholds.
Objective:
The aim of this study was to define the best cut-off scores of the AdAS Spectrum for determining the presence of subthreshold autistic traits as well as a clinically significant autism spectrum disorder (ASD).
Methods:
Our sample was composed of 39 patients with full-blown ASD, 73 subjects with autistic traits, and 150 healthy controls. Subjects were evaluated by trained psychiatrists, who performed a clinical diagnosis according to DSM-5 and then assessed with the AdAS Spectrum and the Autism Spectrum Quotient.
Results:
Our results showed that the most discriminant cut-off scores were 70 for identifying subjects with full-blown ASD, and 43 for determining the presence of significant autistic traits.
Conclusion:
The threshold values proposed here showed satisfying levels of specificity and sensibility, as well as a good agreement with the diagnosis according to DSM-5 criteria, confirming the validity of the AdAS Spectrum as a psychometric tool for measuring ASD-related conditions in the clinical and general population.
Keywords: Autistic traits, Autism Spectrum Disorder, Psychometric instrument, AdAS Spectrum, Diagnostic threshold, RAADS
1. INTRODUCTION
Autism spectrum disorder (ASD) is a condition characterized by an impairment in social communication and interactions, as well as in restricted interests and repetitive behaviours with a significant impact on global functioning. The diagnosis of ASD encompasses patients with very different levels of impairment, with or without the presence of intellectual disabilities and language development alterations [1]. In the last few decades, a growing number of data pointed out the presence of a quantitative more than a qualitative difference between the old categories of Autistic and Asperger's Disorder described in DSM-IV TR [2-4]. In DSM-5, these diagnoses have been merged into the broader label of ASD [4, 5]. This choice went in the direction of a dimensional approach to psychopathology, according to the purpose of classifying psychiatric disorders on the basis of both common clinical presentations and shared pathophysiology, in the framework of a link between symptomatic manifestations and brain functioning [1, 4-7]. However, DSM-5 classi-fication of ASD does not encompass the broader spectrum of ASD milder (or atypical) manifestations and subthreshold autistic traits, whose relevance is increasingly gaining attention in the recent literature [5, 8-12]. Autistic traits are a set of features (such as impaired social skills, atypical and restricted interests, aloof personality) similar to, although less severe of, ASD typical presentation. The relevance of autistic traits was highlighted for the first time amongst first degree relatives of ASD probands, leading to the conceptualization of a “broad autism phenotype” and corroborating the hypothesis of a genetic liability linked to these features [5, 8-14]. However, further studies stressed how autistic traits seem to be continuously distributed also amongst the general population, and in particular amongst some high-risk groups (such as university students of scientific courses) [15-18], as well as in clinical samples of patients with other kinds of psychiatric disorders [19-24]. The recent interest in investigating autistic traits is justified by the body of evidence that highlighted how such features, also when subthreshold, may be associated with significant clinical correlates, including a higher vulnerability towards the development of psychiatric disorders and suicidal ideation and behaviours [20, 25, 26].
At the same time, while ASD, as a neurodevelopmental disorder, is a condition generally detected and clinically addressed in childhood, a wide number of studies highlighted the need of providing more attention to detect ASD (as well as autistic traits) also among adults [5, 19, 27]. In particular, some authors stressed that milder forms of ASD may be under-recognized during childhood, and thus patients will eventually come to clinical attention only when developing, in their adulthood, other disorders in comorbidity [5, 19, 20]. In these cases, frequently the underlying ASD is not investigated by clinicians, remaining undiagnosed and untreated, while leading to higher treatment resistance and worse outcomes, including chronicization and suicidality risk [5, 19, 20]. In this framework, Dell'Osso et al. [28] recently developed and validated the Adult Autism Subthreshold Spectrum (AdAS Spectrum), an instrument with the purpose of evaluating the presence of full-threshold and/or subthreshold autism spectrum symptoms and traits in adults without intellectual disabilities and language development alterations. Although other questionnaires have been previously developed for measuring autistic traits amongst adults, such as the Autism Spectrum Quotient (AQ) and the Ritvo Autism and Asperger´s Diagnostic Scale (RAADS) [15, 29], the AdAS Spectrum is, to date, the only questionnaire for assessing the presence of the entire range of autism spectrum manifestations amongst adults developed after the publication of the DSM-5. Therefore, it is specifically tailored to the new diagnostic criteria, including the greater importance given to the altered reactivity to sensory inputs in ASD patients [28]. It is noteworthy that studies in this field are increasingly stressing how females with ASD may show significantly different clinical presentations than those typically reported amongst males, on whose basis the diagnostic criteria of ASD have been established [28, 30]. In particular, amongst females with ASD, different kinds of restricted interests and repetitive behaviours were reported, such as reading fiction, spending time with animals and a specific focus on food and diet [23, 30, 31]. Moreover, females are more frequently aware of their own social difficulties, while showing higher levels of social anxiety (which is, noticeably, a disorder with a greater prevalence amongst females and associated with an impairment of the social brain) [32-35] and adopting a specific kind of coping strategies that typically features the imitation of peers' behaviours [5, 30, 36-38]. In light of this data, during the development of the AdAS Spectrum, specific attention has been provided to include in the questionnaire items investigating female-specific manifestations of ASD/autistic traits. The AdAS Spectrum proved to be a valuable instrument for quantitative assessment of autistic traits, reporting excellent reliability (Kuder-Richardson's coefficient of 0.964 for the total score, and above 0.75 for all single domain scores) and a good convergent validity with previous scales, such as the AQ (Pearson's r correlation=0.77) and the RAADS (Pearson's r correlation=0.83). In the last few years, it has been used in many studies with satisfying results [5, 17, 22-26], and a Spanish version has been validated [39]. However, to date, the AdAS Spectrum lacks a validated threshold for also establishing qualitatively the presence of ASD and autistic traits. The presence of a validated threshold would allow a better employment of the instrument as a screening tool in clinical and non-clinical settings. The aim of this study was to evaluate the best thresholds of the AdAS Spectrum for determining the presence of subthreshold autistic traits as well as clinically significant ASD in a clinical setting and in healthy controls (HC).
2. MATERIALS AND METHODS
2.1. Participants and Procedures
For the aims of this study, we evaluated a convenient sample consecutively recruited between May 2015 and April 2016 at 4 Italian University Departments of Psychiatry (Catania, Napoli, Pavia, Pisa), coordinated by the University of Pisa. The sample was composed of three groups: patients with full-blown ASD, subjects with autistic traits and HC. The first group was recruited amongst adult outpatients with a full-blown ASD diagnosis according to DSM-5 criteria, without intellectual or language impairment, who were following a treatment program for ASD. Subjects with autistic traits were recruited among university students. Subjects in this group satisfied at least one of the DSM-5 symptomatologic criteria for ASD, without showing a clinically significant functional impairment related to the presence of autistic traits. All subjects were clinically assessed according to DSM-5 criteria, and were requested to fulfill the AdAS Spectrum and the AQ questionnaire. All subjects enrolled in this research received clear information about the study and had the opportunity to ask questions before they provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki, and the local ethics Committees approved all recruitment and assessment procedures (Date of approval: 26/03/2015; ID number: 2015551).
2.2. Measures
The AdAS Spectrum is a self-report instrument developed and validated by Dell’Osso et al. [28] with the aim to assess the broad spectrum of autism symptoms and traits in adults with average intelligence and without language impairment. It was tailored to evaluate not only full-threshold manifestations but also atypical, non-clinical subthreshold traits, as well as gender-specific features. The questionnaire is declined in 7 domains, for a total of 160 dichotomous items (yes/no). The AdAS Spectrum domains allow an independent investigation of autism spectrum core features, and in particular: the presence of symptoms since childhood/adolescence (“Childhood/adolescence” domain); the impairment in verbal and non-verbal communication (“Verbal communication” and “Non-verbal communication” domains); empathy alterations (“Empathy” domain); manifestations of rigidity and difficulties in adjusting to the change (“Inflexibility and adherence to routine” domain); ruminative thinking and narrow interests (“Restrictive interests and rumination”); altered reactivity to sensory inputs (“Hyper-hypo reactivity to sensory input” domain).
The Autism Spectrum Quotient (AQ) is one of the most employed questionnaires for evaluating autistic traits amongst adults. It is composed of 50 items with responses organized in a 4-point Likert scale. It features 5 subscales: “Social skills, “Communication, “Attention to detail, “Attention switching and “Imagination. This instrument demonstrated excellent alpha coefficients in the validation study; the most used cut-off for significant autistic symptoms was 32 [15].
2.3. Statistical Analyses
Chi-square tests and ANOVA analyses were employed for comparing socio-demographic variables, AdAS Spectrum and AQ scores amongst groups, followed by Bonferroni post-hoc tests. Two Receiver-Operating Characteristics (ROC) curves were utilized to find the threshold values of AdAS Spectrum total score that best identify the subjects meeting the DSM-5 criteria for full-blown ASD as well as the subjects with subthreshold autistic traits. According to the definition of accuracy, the ROC analysis was built on the basis of sensitivity and specificity for different cut-offs. The Cohen K was used to calculate the degree of agreement with the DSM-5 of AdAS Spectrum and AQ. According to Wheelwright et al. [40], we adopted an AQ score of 23 for evaluating the presence of milder autistic traits, while for the presence of significant autistic traits, we employed the previously validated threshold of 32 [15]. In the scientific literature, a Cohen K > 0.60 is considered adequate [41].
3. RESULTS
We recruited a total of 262 subjects (Females 51.15%, N = 134; Males 48.85%, N = 128; mean age: 25.66±8.85). The sample was distributed in three groups: 150 HC (HC group; Females: 60%, N = 90; Males: 40%, N = 60; mean age: 26.64±8.82), 39 patients with ASD (ASD group; Females: 48.7%, N = 19; Males: 51.3%; N = 20; mean age: 28.46±11.77) and 73 subjects with autistic traits (AT group; Females: 34.2%, N = 25; Males: 65.8%, N = 48; mean age: 22.14±5.60). The mean age was significantly different amongst groups (F(2.259)= 9.20; p < .001): in particular, the group with autistic traits showed a mean age significantly lower when compared with both the ASD group (p = .007) and the HC (p < .001). No significant gender differences were found between HC and subjects with autistic traits as well as between subjects with autistic traits and patients with ASD, while HC showed a significantly higher proportion of females when compared with the ASD subjects (Chi-square = 13.22, p < .001) ( Table 1). Subjects with ASD reported significantly higher scores on AdAS Spectrum and AQ than subjects with autistic traits. These latters scored significantly higher on both questionnaires than HC, with the exception of AQ “Immagination„ subscale, for which AT group and HC did not report significantly different scores (Tables 2 and 3).
Table 1.
Comparison of sociodemographic variables amongst groups.
| ASD (N= 39) | AT (N=73) | HC (N=150) | ||||
|---|---|---|---|---|---|---|
| mean± DS | mean± DS | mean± DS | F | p | ||
| Age | 28.46±11.77 | 22.14±5.60 | 26.64±8.82 | 9.20 | <.001* | |
| N(%) | N(%) | N(%) | Chi-Square | p | ||
| Sex | F | 19(48.7) | 25(34.2) | 90(60) | 13.22 | <.001# |
| M | 20(51.3) | 48(65.8) | 60(40) | |||
*AT<ASD; AT<HC; p<.05
#♀:HC>ASD; p<.05
Table 2.
Comparison of AdAS Spectrum scores amongst groups.
| AdAS Spectrum |
ASD (N= 39)
mean± DS |
AT (N=73)
mean± DS |
HC (N=150)
mean± DS |
F | p |
Post-Hoc comparisons
(p<.05) |
|---|---|---|---|---|---|---|
| Total | 93.43±18.74 | 60.23±15.66 | 27.79±14.74 | 310.68 | <.001 | ASD>AT>HC |
| Childhood/adolescence | 12.87±3.75 | 8.82±3.25 | 3.74±2.58 | 176.11 | <.001 | ASD>AT>HC |
| Verbal communication | 11.79±3.07 | 5.97±2.43 | 2.82±2.23 | 217.93 | <.001 | ASD>AT>HC |
| Non-verbal communication | 14.74±4.87 | 11.89±4.23 | 5.80±3.60 | 107.57 | <.001 | ASD>AT>HC |
| Empathy | 7.02±2.67 | 3.54±2.56 | 1.51±1.64 | 111.01 | <.001 | ASD>AT>HC |
| Inflexibility and adherence to routine | 24.17±6.34 | 15.95±5.60 | 8.02±4.73 | 167.13 | <.001 | ASD>AT>HC |
| Restrictive interests and rumination | 14.89±2.90 | 9.89±3.26 | 4.08±3.20 | 211.82 | <.001 | ASD>AT>HC |
| Hyper-Hyporeactivity to sensory input | 7.92±3.96 | 4.15±2.49 | 1.80±1.85 | 101.14 | <.001 | ASD>AT>HC |
Table 3.
Comparison of AQ scores amongst groups.
| AQ |
ASD (N= 39)
mean± DS |
AT (N=73)
mean± DS |
HC (N=150)
mean± DS |
F | p |
Post-Hoc comparisons
(p<.05) |
|---|---|---|---|---|---|---|
| Total | 33.61±7.30 | 19.82±6.14 | 12.58±5.26 | 204.99 | <.001 | ASD>AT>HC |
| Social skills | 6.97±2.40 | 3.86±2.55 | 1.44±1.78 | 114.72 | <.001 | ASD>AT>HC |
| Attention switching | 7.95±1.67 | 5.04±1.84 | 3.16±1.75 | 120.95 | <.001 | ASD>AT>HC |
| Attention to detail | 6.67±1.95 | 5.00±2.41 | 4.05±2.14 | 22.84 | <.001 | ASD>AT>HC |
| Communication | 7.08±2.14 | 3.07±1.78 | 1.36±1.47 | 183.87 | <.001 | ASD>AT>HC |
| Immagination | 4.95±2.20 | 2.92±1.77 | 2.47±1.66 | 29.93 | <.001 | ASD>AT |
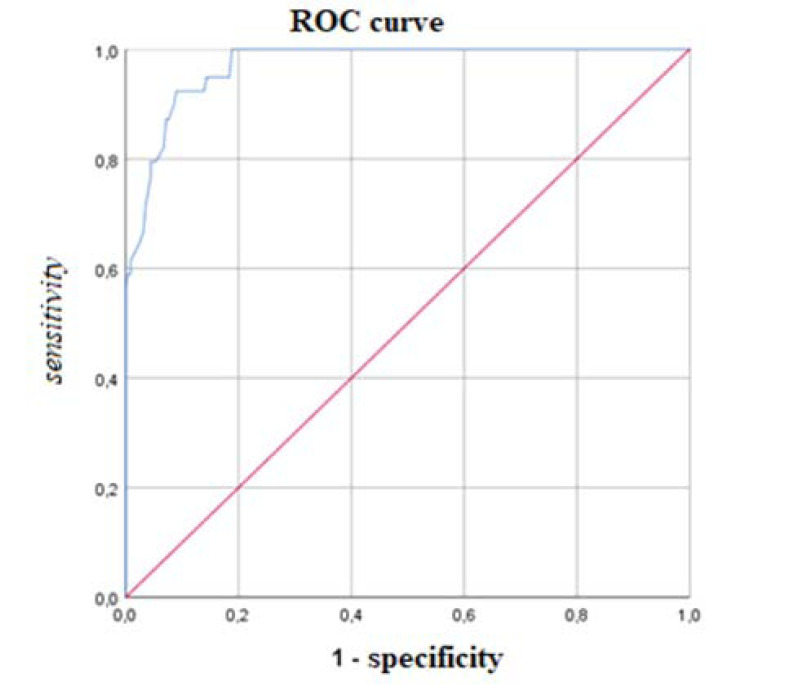
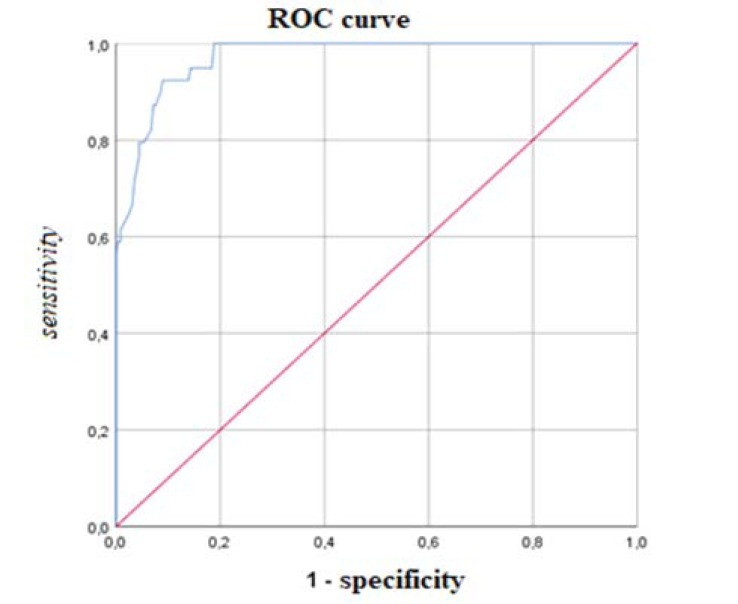
According to the ROC analysis, the most discriminant cut-off of the AdAS Spectrum total score for identifying ASD subject was 70, with an AUC value of 0.971 (p < .001), a sensitivity of 0.923 and a specificity of 0.910 (Fig. 1). The degree of agreement with the DSM-5 diagnosis, according to the Cohen K, was satisfactory (k = 0.706). The most discriminant total score cut-off for identifying subjects with autistic traits was 43, with an AUC value of 0.927 (p<0.001), a sensitivity of 0.849 and a specificity of 0.833 (Fig. 2). Also, in this case, the Cohen K used for calculating the degree of agreement with the DSM-5 criteria was satisfactory (k = 0.651). Cohen K values for the degree of agreement with the DSM-5 of AdAS Spectrum and AQ are reported in Table 4.
Fig. (1).
ROC curve of the AdAS Spectrum threshold score for determining ASD. AUC = 0.971; threshold score = 70.
Fig. (2).
ROC curve of the AdAS Spectrum threshold score for determining autistic traits. AUC = 0.927; threshold score = 43.
Table 4.
Cohen K values for the degree of agreement of AdAS Spectrum and AQ with DSM-5 in identifying subjects with ASD and AT.
| Clinical evaluation according to DSM-5 | AdAS Spectrum (cut-off = 70) | AQ (cut-off = 32) | ||
|---|---|---|---|---|
| K | 95%IC | K | 95%IC | |
| ASD | 0.706 | 0.594-0.817 | 0.650 | 0.506-0.793 |
| AdAS Spectrum (cut-off = 43) | AQ (cut-off = 23) | |||
| K | 95%IC | K | 95%IC | |
| AT | 0.651 | 0.547-0.754 | 0.349 | 0.221-0.476 |
4. DISCUSSION
The aim of this study was to define which score of the AdAS Spectrum should be considered as a threshold value when investigating the presence of autistic traits and ASD amongst adults. Since recently, the AdAS Spectrum was used in both clinical and non-clinical populations only as a quantitative instrument in order to investigate the presence of lifetime autistic traits in the framework of a dimensional approach [5, 22-26]. The AdAS Spectrum has been demonstrated to be a useful instrument for the assessment of the autism spectrum, with the capability of also evaluating specific symptomatological clusters [5, 22-26]. However, a critical flaw of the instrument was the lack of threshold values, which would allow employing the AdAS Spectrum for a qualitative evaluation of the presence/absence of ASD or autistic traits. According to our results, the AdAS Spectrum showed an excellent ability to discriminate the presence of both clinical ASD and subthreshold autistic traits. The best threshold value for identifying full-blown ASD was a score of 70 (which is obtained with a positive endorsement of 70 items of the questionnaire). This value showed a good agreement with the diagnosis performed according to DSM-5 criteria, and it is also associated with good levels of specificity (0.910) and sensibility (0.923). The threshold score for determining autistic traits (43) showed lower values of specificity (0.833) and sensibility (0.849), which are however, still satisfying, especially considering the non-clinical nature of the condition. The reported levels of sensibility and specificity for the AdAS Spectrum thresholds are comparable to those reported by other questionnaires commonly employed for the screening of ASD or autistic traits amongst adults, such as the AQ and the RAADS [15, 29]. However, the AdAS Spectrum is, to date, the only instrument tailored on the DSM-5 criteria for ASD, thus taking into account also the manifestations associated with the hypo- and hyper-reactivity to sensory inputs, which are not properly investigated by other questionnaires [29]. On the other hand, the inclusion of female-specific ASD features makes the AdAS Spectrum an ideal instrument for the assessment of ASD and autistic traits among female adults [28, 30]. The degree of agreement between AdAS Spectrum cut-offs and the clinical evaluation according to DSM-5 was satisfying, with a Cohen K of 0.706 for clinically relevant ASD symptoms and 0.651 for the presence of autistic traits. Lower levels of agreement were reported between clinical evaluations and the AQ, in particular in determining the presence of autistic traits (Cohen K = 0.349). Globally, these results further confirm the validity of the AdAS Spectrum for measuring autism-related conditions, as yet shown by the validation study and by the evaluation of the psychometric properties of the Spanish version [28, 39]. Moreover, our results also strengthen the findings from previous researches, which found AdAS Spectrum scores above 70 in clinical populations of subjects with ASD and AdAS Spectrum scores around 40 in clinical or non-clinical samples of subjects considered at risk for autistic traits [5, 22-26]. The study further corroborates the validity of AdAS Spectrum domains, showing that ASD patients scored significantly higher than the AT group (which scored, in turn, significantly higher than HC) on each dimension measured by the AdAS Spectrum (“Childhood/adolescence”; “Verbal communication”; “Non-verbal communication”; “Empathy”; “Inflexibility and adherence to routine”; “Restrictive interests and rumination”; “Hyper-hypo reactivity to sensory input”). Similar results were found for the AQ “Social skills, “Communication, “Attention to detail, and “Attention switching, subscales, while we did not find significant differences on AQ “Imagination, subscale between AT group and HC. This result is in line with previous studies, which highlighted that Imagination was the AQ subscale with the lowest internal consistency [42]. Moreover, other authors reported similar scores for the imagination dimension, as measured by the AQ, between subjects with a broad autism phenotype and non-autistic groups [42-44]. Noticeably, imagination skills have been found less impaired, in particular among women [42-44]. On the basis of this data, it is possible to hypothesize that imagination difficulties would be associated with more severe presentations of the autism spectrum and/or with specific, eventually, gender-related, autistic phenotypes. Globally, this work confirms the validity of employing the AdAS Spectrum as a psychometric tool for measuring both autistic traits and ASD in clinical settings as well as in the general population. The threshold values of the instrument proposed here showed satisfying levels of specificity and sensibility, as well as a good agreement with the assessment performed by trained psychiatrists, allowing clinicians and researchers to use the questionnaire in different kinds of settings. In clinical settings, the AdAS Spectrum may improve prevention and diagnostic procedures. In this framework, it may allow investigating the presence of ASD or subthreshold autistic traits - whose role in worsening the psychopathological course and the clinical outcome has been variously stressed in the scientific literature – [5, 19, 20] also in clinical populations of patients with other kinds of mental disorders. In research settings, the AdAS Spectrum may be considered a powerful tool for evaluating the presence of both subthreshold and full-threshold autism spectrum from a qualitative as well as a quantitative point of view, in clinical and non-clinical populations, also allowing specific psychopathological profiling of the subjects.
CONCLUSION
However, this work should be considered in light of several limitations. First, our sample was relatively small, and a limited number of demographic features has been taken into account. Moreover, there were significant gender and age differences amongst the groups. These elements prevent us from evaluating possible confounding factors related to demographic features. Further studies in wider samples, which would allow a random sampling and/or a stratification for demographic characteristics, may confirm or eventually improve the definition of the best threshold values for the AdAS Spectrum. Moreover, the AdAS Spectrum is an instrument that includes 160 items, requiring more time than other questionnaires to be fulfilled; it may imply a higher risk of inaccurate responses. However, this feature of the AdAS Spectrum provides an extremely detailed profile of ASD symptomatology, eventually identifying which areas were more impaired than others in the single subject. Finally, although showing a good agreement with the clinical assessment, the AdAS Spectrum remains a self-report instrument, and therefore it is subject to bias. For these reasons, the AdAS Spectrum should not be used, especially in clinical settings, as the only diagnostic tool. On the other hand, this questionnaire can be considered, according to this data, a good screening instrument to confirm and deepen the clinical diagnosis amongst subjects with a suspected autism spectrum. Further studies with the AdAS Spectrum should focus on deepening the knowledge about the prevalence and relevance of ASD and autistic traits among the general population, in high-risk groups and in psychiatric patients, with specific attention on detecting ASD female phenotypes. The AdAS Spectrum domains are useful tools for evaluating the impact and the distribution of specific symptomatological clusters and their eventual association with neurobiological and biochemical features.
ACKNOWLEDGEMENTS
Declared none.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the ethics committee of University of Pisa, Italy (Date of approval: 26/03/2015; id number: 2015551).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
All patients participated on a voluntary basis and gave their informed consent.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.American Psychiatric Association . Diagnostic and statistical manual of mental disorders: DSM-5. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 2.Macintosh K.E., Dissanayake C. Annotation: The similarities and differences between autistic disorder and Asperger’s disorder: a review of the empirical evidence. J. Child Psychol. Psychiatry. 2004;45(3):421–434. doi: 10.1111/j.1469-7610.2004.00234.x. [DOI] [PubMed] [Google Scholar]
- 3.Sanders J.L. Qualitative or quantitative differences between Asperger’s disorder and autism? Historical considerations. J. Autism Dev. Disord. 2009;39(11):1560–1567. doi: 10.1007/s10803-009-0798-0. [DOI] [PubMed] [Google Scholar]
- 4.Dell’Osso L., Luche R.D., Gesi C., Moroni I., Carmassi C., Maj M. From Asperger’s autistischen psychopathen to DSM-5 autism spectrum disorder and beyond: a subthreshold autism spectrum model. Clin. Pract. Epidemiol. Ment. Health. 2016;12:120–131. doi: 10.2174/1745017901612010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dell’Osso L., Muti D., Carpita B., Cremone I.M., Bui E., Gesi C., Carmassi C. The adult autism subthreshold spectrum (AdAS) model: a neurodevelopmental approach to mental disorders. J. Psychopathol. 2018;24(3):118–124. [Google Scholar]
- 6.Carpita B., Marazziti D., Palego L., Giannaccini G., Betti L., Dell’Osso L. Microbiota, immune system and autism spectrum disorders. An integrative model towards novel treatment options. Curr. Med. Chem. 2019 doi: 10.2174/0929867326666190328151539. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7.Carpita B., Muti D., Dell’Osso L. Oxidative stress, maternal diabetes, and autism spectrum disorders. Oxid. Med. Cel. Longev; 2018. p. 3717215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sucksmith E., Allison C., Baron-Cohen S., Chakrabarti B., Hoekstra R.A. Empathy and emotion recognition in people with autism, first-degree relatives, and controls. Neuropsychologia. 2013;51(1):98–105. doi: 10.1016/j.neuropsychologia.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De la Marche W., Noens I., Luts J., Scholte E., Van Huffel S., Steyaert J. Quantitative autism traits in first degree relatives: evidence for the broader autism phenotype in fathers, but not in mothers and siblings. Autism. 2012;16(3):247–260. doi: 10.1177/1362361311421776. [DOI] [PubMed] [Google Scholar]
- 10.Berthoz S., Lalanne C., Crane L., Hill E.L. Investigating emotional impairments in adults with autism spectrum disorders and the broader autism phenotype. Psychiatry Res. 2013;208(3):257–264. doi: 10.1016/j.psychres.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Sucksmith E., Roth I., Hoekstra R.A. Autistic traits below the clinical threshold: re-examining the broader autism phenotype in the 21st century. Neuropsychol. Rev. 2011;21(4):360–389. doi: 10.1007/s11065-011-9183-9. [DOI] [PubMed] [Google Scholar]
- 12.Ozonoff S., Young G.S., Belding A., Hill M., Hill A., Hutman T., Johnson S., Miller M., Rogers S.J., Schwichtenberg A.J., Steinfeld M., Iosif A.M. The broader autism phenotype in infancy: when does it emerge? J. Am. Acad. Child Adolesc. Psychiatry. 2014;53(4):398–407.e2. doi: 10.1016/j.jaac.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Losh M., Adolphs R., Poe M.D., Couture S., Penn D., Baranek G.T., Piven J. Neuropsychological profile of autism and the broad autism phenotype. Arch. Gen. Psychiatry. 2009;66(5):518–526. doi: 10.1001/archgenpsychiatry.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carpita B., Carmassi C., Calderoni S., Muscarella A., Massimetti G., Cremone I.M., Gesi C., Conti E., Muratori F., Dell'Osso L. The broad autism phenotype in real-life: clinical and functional correlates of autism spectrum symptoms and rumination among parents of patients with autism spectrum disorder. 2019. [DOI] [PubMed]
- 15.Baron-Cohen S., Wheelwright S., Skinner R., Martin J., Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J. Autism Dev. Disord. 2001;31(1):5–17. doi: 10.1023/A:1005653411471. [DOI] [PubMed] [Google Scholar]
- 16.Skylark W.J., Baron-Cohen S. Initial evidence that non-clinical autistic traits are associated with lower income. Mol. Autism. 2017;8:61. doi: 10.1186/s13229-017-0179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dell’Osso L., Carpita B., Cremone I.M., Muti D., Diadema E., Barberi F.M., Massimetti G., Brondino N., Petrosino B., Politi P., Aguglia E., Lorenzi P., Carmassi C., Gesi C. The mediating effect of trauma and stressor related symptoms and ruminations on the relationship between autistic traits and mood spectrum. Psychiatry Res. 2019;279:123–129. doi: 10.1016/j.psychres.2018.10.040. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki T., Miyaki K., Eguchi H., Tsutsumi A. Distribution of autistic traits and their association with sociodemographic characteristics in Japanese workers. Autism. 2018;22(8):907–914. doi: 10.1177/1362361317716605. [DOI] [PubMed] [Google Scholar]
- 19.Kato K., Mikami K., Akama F., Yamada K., Maehara M., Kimoto K., Kimoto K., Sato R., Takahashi Y., Fukushima R., Ichimura A., Matsumoto H. Clinical features of suicide attempts in adults with autism spectrum disorders. Gen. Hosp. Psychiatry. 2013;35(1):50–53. doi: 10.1016/j.genhosppsych.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Takara K., Kondo T. Comorbid atypical autistic traits as a potential risk factor for suicide attempts among adult depressed patients: a case-control study. Ann. Gen. Psychiatry. 2014;13(1):33. doi: 10.1186/s12991-014-0033-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dell’Osso L., Conversano C., Corsi M., Bertelloni C.A., Cremone I.M., Carpita B., Carbone M.G., Gesi C., Carmassi C. Polysubstance and behavioral addictions in a patient with bipolar disorder: Role of lifetime subthreshold autism spectrum. Case Rep. Psychiatry. 2018;2018:1547975. doi: 10.1155/2018/1547975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dell’Osso L., Cremone I.M., Carpita B., Fagiolini A., Massimetti G., Bossini L., Vita A., Barlati S., Carmassi C., Gesi C. Correlates of autistic traits among patients with borderline personality disorder. Compr. Psychiatry. 2018;83:7–11. doi: 10.1016/j.comppsych.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Dell’Osso L., Carpita B., Gesi C., Cremone I.M., Corsi M., Massimetti E., Muti D., Calderani E., Castellini G., Luciano M., Ricca V., Carmassi C., Maj M. Subthreshold autism spectrum disorder in patients with eating disorders. Compr. Psychiatry. 2018;81:66–72. doi: 10.1016/j.comppsych.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Dell’Osso L., Carpita B., Bertelloni C.A., Diadema E., Barberi F.M., Gesi C., Carmassi C. Subthreshold autism spectrum in bipolar disorder: Prevalence and clinical correlates. Psychiatry Res. 2019;281:112605. doi: 10.1016/j.psychres.2019.112605. [DOI] [PubMed] [Google Scholar]
- 25.Dell’Osso L., Bertelloni C.A., Di Paolo M., Avella M.T., Carpita B., Gori F., Pompili M., Carmassi C. Problematic internet use in university students attending three superior graduate schools in italy: Is autism spectrum related to suicide risk? Int. J. Environ. Res. Public Health. 2019;16(7):E1098. doi: 10.3390/ijerph16071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dell’Osso L., Carpita B., Muti D., Morelli V., Salarpi G., Salerni A., Scotto J., Massimetti G., Gesi C., Ballerio M., Signorelli M.S., Luciano M., Politi P., Aguglia E., Carmassi C., Maj M. Mood symptoms and suicidality across the autism spectrum. Compr. Psychiatry. 2019;91:34–38. doi: 10.1016/j.comppsych.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Dell’Osso L., Lorenzi P., Carpita B. Autistic traits and illness trajectories. Clin. Pract. Epidemiol. Ment. Health. 2019;15:94–98. doi: 10.2174/1745017901915010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dell’Osso L., Gesi C., Massimetti E., Cremone I.M., Barbuti M., Maccariello G., Moroni I., Barlati S., Castellini G., Luciano M., Bossini L., Rocchetti M., Signorelli M., Aguglia E., Fagiolini A., Politi P., Ricca V., Vita A., Carmassi C., Maj M. Adult Autism Subthreshold Spectrum (AdAS Spectrum): Validation of a questionnaire investigating subthreshold autism spectrum. Compr. Psychiatry. 2017;73:61–83. doi: 10.1016/j.comppsych.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Ritvo R.A., Ritvo E.R., Guthrie D., Ritvo M.J., Hufnagel D.H., McMahon W., Tonge B., Mataix-Cols D., Jassi A., Attwood T., Eloff J. The Ritvo Autism Asperger Diagnostic Scale-Revised (RAADS-R): a scale to assist the diagnosis of Autism Spectrum Disorder in adults: an international validation study. J. Autism Dev. Disord. 2011;41(8):1076–1089. doi: 10.1007/s10803-010-1133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai M.C., Baron-Cohen S., Buxbaum J.D. Understanding autism in the light of sex/gender. Mol. Autism. 2015;6:24. doi: 10.1186/s13229-015-0021-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dell’Osso L., Abelli M., Carpita B., Pini S., Castellini G., Carmassi C., Ricca V. Historical evolution of the concept of anorexia nervosa and relationships with orthorexia nervosa, autism, and obsessive-compulsive spectrum. Neuropsychiatr. Dis. Treat. 2016;12:1651–1660. doi: 10.2147/NDT.S108912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jobe L.E., White S.W. Loneliness, social relationships, and a broader autism phenotype in college students. Pers. Individ. Dif. 2007;42:1479–1489. doi: 10.1016/j.paid.2006.10.021. [DOI] [Google Scholar]
- 33.Dell’Osso L., Abelli M., Pini S., Carlini M., Carpita B., Macchi E., Gorrasi F., Mengali F., Tognetti R., Massimetti G. Dimensional assessment of DSM-5 social anxiety symptoms among university students and its relationship with functional impairment. Neuropsychiatr. Dis. Treat. 2014;10:1325–1332. doi: 10.2147/NDT.S59348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marazziti D., Abelli M., Baroni S., Carpita B., Piccinni A., Dell’Osso L. Recent findings on the pathophysiology of social anxiety disorder. Clin. Neuropsychiatry. 2014;11:91–100. [Google Scholar]
- 35.Marazziti D., Abelli M., Baroni S., Carpita B., Ramacciotti C.E., Dell’Osso L. Neurobiological correlates of social anxiety disorder: an update. CNS Spectr. 2015;20(2):100–111. doi: 10.1017/S109285291400008X. [DOI] [PubMed] [Google Scholar]
- 36.Kuusikko S., Pollock-Wurman R., Jussila K., Carter A.S., Mattila M.L., Ebeling H., Pauls D.L., Moilanen I. Social anxiety in high-functioning children and adolescents with Autism and Asperger syndrome. J. Autism Dev. Disord. 2008;38(9):1697–1709. doi: 10.1007/s10803-008-0555-9. [DOI] [PubMed] [Google Scholar]
- 37.Hull L., Mandy W., Lai M.C., Baron-Cohen S., Allison C., Smith P., Petrides K.V. Development and Validation of the Camouflaging Autistic Traits Questionnaire (CAT-Q). J. Autism Dev. Disord. 2019;49(3):819–833. doi: 10.1007/s10803-018-3792-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dell’Osso L., Lorenzi P., Carpita B. Camouflaging: Psychopathological meanings and clinical relevance in autism spectrum 4 conditions 5. CNS Spectr. 2020;26:1–8. doi: 10.1017/S1092852920001467. [DOI] [PubMed] [Google Scholar]
- 39.Donati M.A., Berrocal C., Primi C., Petracchi G., Carpita B., Cosci F., Ruiz A., Carmassi C., Dell’Osso L. Measuring subthreshold autistic traits in the general population: Psychometric properties of the Adult Autism Subthreshold Spectrum (AdAS Spectrum) scale. Psychiatry Res. 2019;281:112576. doi: 10.1016/j.psychres.2019.112576. [DOI] [PubMed] [Google Scholar]
- 40.Wheelwright S., Baron-Cohen S., Goldenfeld N., Delaney J., Fine D., Smith R., Weil L., Wakabayashi A. Predicting Autism Spectrum Quotient (AQ) from the Systemizing Quotient-Revised (SQ-R) and Empathy Quotient (EQ). Brain Res. 2006;1079(1):47–56. doi: 10.1016/j.brainres.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 41.Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 42.Pisula E., Kawa R., Szostakiewicz Ł., Łucka I., Kawa M., Rynkiewicz A. Autistic traits in male and female students and individuals with high functioning autism spectrum disorders measured by the Polish version of the Autism-Spectrum Quotient. PLoS One. 2013;8(9):e75236. doi: 10.1371/journal.pone.0075236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bishop D.V., Maybery M., Maley A., Wong D., Hill W., Hallmayer J. Using self-report to identify the broad phenotype in parents of children with autistic spectrum disorders: a study using the Autism-Spectrum Quotient. J. Child Psychol. Psychiatry. 2004;45(8):1431–1436. doi: 10.1111/j.1469-7610.2004.00325.x. [DOI] [PubMed] [Google Scholar]
- 44.Khanjani Z., Azmoodeh S., Mahmoudaliloo M., Noorazar G. A comparison of autistic like traits in the relatives of patients with autism and schizophrenia spectrum disorder. Iran. J. Psychiatry. 2018;13(2):148–153. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.