Abstract
Background:
It has been proposed that autistic individuals are at an increased risk of type 1 and type 2 diabetes. Improved understanding of diabetes prevalence in autistic persons will help inform resource allocation for diabetes-related public health measures for this patient group.
Objective:
To conduct a systematic review of published literature pertaining to type 1 and type 2 diabetes prevalence in autistic individuals, including comparison with their non-autistic peers.
Methods:
Eligibility criteria included studies investigating the prevalence of diabetes in autistic individuals, as well as having been published in the English language. A systematic search of online databases (MEDLINE, PsycINFO, CINAHL, EMBASE and PubMed) was conducted on 4th April 2020. Additional approaches included the ancestry method, grey literature searches and expert consultation. Studies were qualitatively analysed with reporting quality appraised.
Results:
19 eligible studies were identified, 7 of which provided type-specific diabetes prevalence data. Of 15 studies that included a non-autistic control group, 9 reported a higher diabetes prevalence among autistic persons, with a statistically significant difference in 4 studies. Studies demonstrating a higher diabetes prevalence in autistic groups had higher average study population sizes and reporting quality ratings.
Conclusion:
It is uncertain whether diabetes is significantly more prevalent in autistic persons relative to their non-autistic peers, though larger studies suggest a trend in this direction. Nevertheless, diabetes is a significant public health issue for the autistic community, which may require a tailored approach for identification and management. Prospero database registration number: CRD42019122176.
Keywords: Autism, Asperger, Psychiatric, Diabetes, Epidemiology, Systematic review
1. INTRODUCTION
Autism Spectrum Disorders (hereafter referred to as autism) manifest as persistent atypicalities in social communication and interaction, and a repertoire of restricted and repetitive patterns of behaviour, present since early development [1]. Recent epidemiological studies estimate rates of approximately 0.95% (95% CI 0.82-1.08) in child [2] and 0.76% (95% CI 0.51-1.12) in adult populations [3]. People with autism frequently have co-occurring Intellectual Disability (ID) [4] and/or mental health conditions, sometimes requiring hospital admission [5]. However, less is known about the rates of common physical health conditions within this population [6].
Diabetes Mellitus (DM) is a metabolic disorder characterised by chronic hyperglycaemia and disturbances of carbohydrate, fat and protein metabolism resulting from defects in insulin secretion, insulin action or both [7]. There are three main forms of DM – Type 1 (T1DM), Type 2 (T1DM) and gestational diabetes mellitus (GDM). T1DM occurs secondary to the destruction of insulin-producing beta cells (usually via autoimmune-mediated pathology), whereas T2DM occurs secondary to insulin resistance and relative insulin deficiency [7]. In 2019, the International Diabetes Federation estimated that since 2000, the prevalence of DM (both T1DM and T2DM, diagnosed and undiagnosed) in adults aged between 20 and 79 years has increased from 151 million (4.6% of the global population) to 463 million (9.3%) [8]. The respective prevalence’s of T1DM and T2DM also vary considerably both within and across countries. For T1DM, genetic susceptibility appears to be the primary aetiological factor, though environmental contributions such as viral infection or diet are thought also to have a role. In contrast, while genetics also contribute to T2DM, the relative contributions of other factors are well established, including age, socioeconomic status, lifestyle factors (such as weight, physical activity, smoking, alcohol), ethnicity and genetic risk [9].
Though the association between maternal DM and offspring autism has been subjected to a systematic review, revealing a significant association between the conditions [10], similar evaluations of the association between DM and autism in the same individual have not yet been conducted. Evidence suggests that autism is related to immune system dysfunction, including neuroinflammation, increased autoantibodies, or aberrations in immune cells, cytokines and immunoglobulins [11, 12]. Therefore, individuals with autism may be at greater risk of disorders with autoimmune pathophysiology, such as T1DM, as well as asthma, inflammatory bowel disease and atopic dermatitis [13]. Concerning T2DM, individuals with autism are known to be at greater risk of metabolic complications associated with its development, including obesity and dyslipidaemia [6, 14], and higher rates of antipsychotic prescribing [15], which could conceivably contribute to a greater prevalence of T2DM within this group.
This systematic review evaluates the current evidence examining the prevalence of diabetes among individuals with autism. Understanding the prevalence of both T1DM and T2DM among autistic persons will help inform resource allocation for diabetes-related public health measures and management approaches specific to this population.
2. MATERIALS AND METHODS
2.1. Study Selection
Studies were included provided they satisfied all the following criteria: (a) investigation of the prevalence of DM (either or both of T1DM and T2DM) in individuals with autism (b) published in English language. Exclusion criteria included conference proceedings. No specific limitations were imposed pertaining to the study duration or the study population size (though case reports and case series were excluded as they cannot reasonably be considered investigations of prevalence).
In the conduction of the review, several studies were identified that measured the association of DM with autism via the prevalence of autism in persons with DM, or the development of DM over time in autistic persons (rather than the prevalence). Such studies do not satisfy inclusion criteria, but are still discussed in the findings, within the section entitled ‘Summary of Relevant Excluded Studies.’
2.2. Systematic Search Strategy
The PubMed, MedLine, CINAHL, PsycINFO and EMBASE databases were searched from their respective inceptions from 4th April 2020. Titles and abstracts were searched for the following terms: (autis* OR PDD* OR Kanner* OR Asperger* OR ASD* OR pervasive development* or ASC* or neurodevelopmental dis*) AND (diabet* OR hyperglyc* OR glucose* OR DM). All titles and abstracts of articles that remained, following the removal of duplicates, were screened against the inclusion criteria by two investigators (ST + RC). If papers potentially satisfied inclusion criteria, full texts were accessed. In instances of uncertainty between ST and RC regarding the eligibility of an article, the final decision was made by TB. The ancestry method was utilised to identify additional studies within the references of eligible papers. Grey literature searches included Google Scholar and manual searches. Experts in the field were consulted to identify any additional published or unpublished data; for all articles identified via expert consultation, the full texts of the articles were assessed. A separate search was conducted by the University Hospitals of Leicester library services on 28th April 2020 to find studies not identified by the above methods. The systematic review was registered in 2019 on PROSPERO: CRD42019122176, with a subsequent revision submitted and authorised in 2020.
2.3. Data Extraction
For each article, data was extracted pertaining to the year and location of the study, as well as the number of participants, both with an autism diagnosis, and non-autistic controls, where applicable. The methods employed for such assessments were also obtained, as well as information regarding the resultant prevalence estimate for both autism groups and non-autistic controls (where applicable).
2.4. Data Synthesis
The prevalence results obtained were considered both regarding overall findings for DM prevalence across available studies, as well as specific T1DM and T2DM data where reported. Quantitative synthesis (meta-analysis) was considered inappropriate due to the high level of heterogeneity in study design, population, setting and diagnostic criteria used.
2.5. Quality Assessment
All articles that qualified for inclusion were assessed by two investigators (ST + RC) according to the 22-item (34 with subsections) STROBE checklist for cohort, case-control and cross-sectional studies [16], as all studies were of this type. Each of the STROBE items was determined as being complete (1 point), partially complete (0.5), incomplete (0 points) or not applicable [17]. The resultant STROBE score has been expressed as a fraction, as the total applicable items (the denominator) varied between studies, and a corresponding percentage value. No studies qualifying for inclusion were excluded based upon their STROBE score.
3. RESULTS
3.1. Study Characteristics
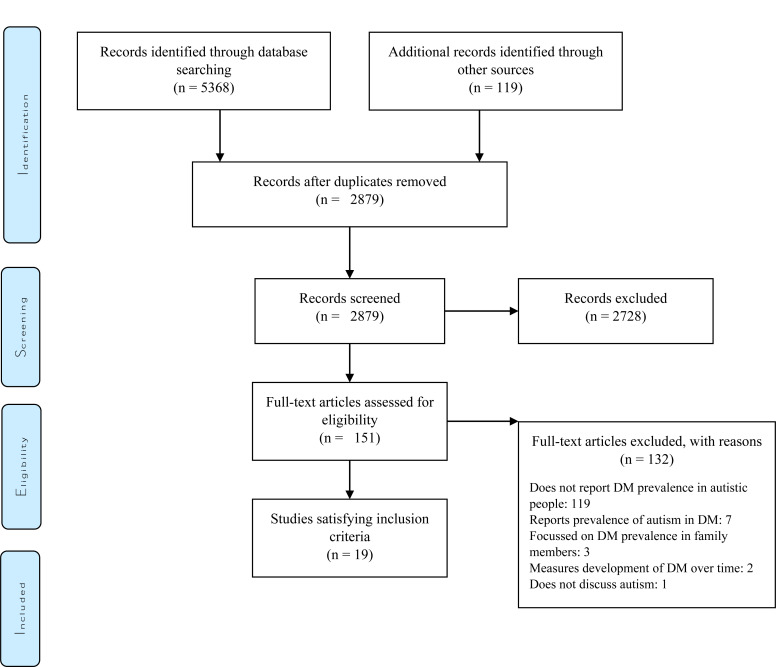
Database searches were conducted on 4th April 2020. The database search yielded a total of n=5368 articles, including n=1535 articles in PubMed, n=1236 articles in MedLine, n=370 articles in CINAHL, n=305 articles in PsycINFO and n=1922 articles in EMBASE. Additional records (n=119) were identified through the ancestry method, grey literature searches and expert consultation techniques. Following duplicate removal, n=2879 articles remained for screening. (Fig. 1) illustrates a PRISMA flow chart summary of the systematic search.
Fig. (1).
PRISMA flow diagram of systematic search [18].
Upon abstract screening, 2728 articles were excluded. Reasons included focusing on unrelated research topics, failing to satisfy inclusion criteria, or being not being published in English. Thus, full texts of 151 articles were assessed, of which 19 qualified for inclusion [6, 13, 19-35](Please refer to the supplementary material for a table of all full-text articles that were assessed). Of the included papers, 10 were identified on the database search [6, 19, 20, 23-28, 34], 6 via the ancestry method [13, 21, 29-31, 35], and 3 via expert consultation [22, 32, 33]. Grey literature searches and the library search failed to identify any additional studies satisfying inclusion criteria.
3.2. General Findings of Included Studies
Table 1 summarises all the eligible studies. Publication dates for the studies ranged from 2006 to 2020. Most studies were conducted in North America (n=12) [6, 13, 20, 21, 23, 25, 27, 30, 31, 34], followed by Sweden (n=2) [26, 28]. The mean participant age across studies that reported such data ranged from 12-48 years. Some studies focussed exclusively on child/adolescent or adult populations, whereas others included both. Most autistic participants in eligible studies were male, ranging from 66-82%. The proportions of autistic participants with co-occurring ID were reported in 9 eligible studies [6, 22, 24, 26-29, 31, 34], with rates ranging from 0-97%. The methodology varied across studies, including clinical/electronic record review [6, 13, 19-23, 25, 27-29, 32, 34, 35], ques-tionnaires [24, 31], surveys [30, 33], and structured interviews [26].
Table 1.
Summary of included studies.
| Author and Country | Study Type | Study Population | Autistic Cohort Characteristics | Data Source | Diagnostic Methods | Statistical Methods | Salient Findings | STROBE Score |
|---|---|---|---|---|---|---|---|---|
| T1DM-specific Findings | ||||||||
| Chen et al (2013) [19], Taiwan | Retrospective case-control study. | 1,598 autistic patients and 6,392 age and gender matched controls. | Mean age = 18 years; Age range = Not reported (NR); Male % = 80; ID % = NR; Ethnicity distribution (ED) = NR. | Electronic health records from 1996-2010. | Diagnoses were recorded in electronic records according to ICD-9-CM criteria [36]. | For between-group comparisons, the Pearson’s Chi square test or Fisher’s exact test were used for nominal variables, such as presence of T1DM. A logistic regression model was used to investigate the OR and 95% CI of T2DM in autistic patients. | Autistic patients were borderline significantly (p=0.056) more likely to have T1DM than non-autistic controls (Autistic group: 0.3%, 4/1,598; control group: 0.1%, 4/6,392). Modified OR (after adjusting for age and gender) = 4.00 (95% CI 1.00-16.00). | 17.5/27; 65% |
| Kohane et al (2012) [20], United States | Retrospective case-control study. | 14,381 autistic patients and 2,379,397 non-matched non-autistic controls. | Mean age = NR; Age range = 0-34 years; Male % = 79; ID % = NR; ED = NR. | Electronic healthcare records. | ICD-9 [37]. | Prevalence of DM was compared between autistic cases and controls using chi-square testing. | Autistic patients were significantly (p<0.00001) more likely to have T1DM (Autistic group: n=114, 0.79%; Control group: n=8058, 0.34%; 95% CI 0.3-0.6%). | 16/26; 62% |
| Supekar et al (2017) [21], United States | Cross-sectional study. | 4,790 autistic individuals and 1,842,575 non-matched non-autistic controls. | Mean age = NR; Age range = NR; Male % = NR; ID % = NR; ED = NR. | Electronic hospital database. | ICD-9 [37]. | Descriptive analysis, including percentage values (though not raw data). | The authors reported a T1DM prevalence of 0.42% among the autistic group, and 0.59% among the non-autistic controls. | 18.5/27; 69% |
| Zerbo et al (2015) [13], United States | Retrospective case-control. | 5,565 autistic individuals, and 27,825 controls, matched on age, sex and enrolment time. | Mean age = 12 years; Age range = NR; Male % = 82; ID % = NR; ED = NR. | Electronic health records. | ICD-9-CM [36]. | Chi-square testing was used to evaluate differences between the autistic and non-autistic groups. | Of the autistic group, 0.22% (12/5,565) had T1DM, compared with 0.19% (52/27,825) among the control group. This difference was not statistically significant (OR 1.15; 95% CI 0.62-2.17). | 21/27; 78% |
| T2DM-specific Findings | ||||||||
| Brondino et al (2019) [22], Italy | Cross sectional observational study | 191 autistic patients. No non-autistic controls were included. | Mean age = 24 years; Age range = NR; Male % = 75; ID % = 421; ED = NR. | Clinical records. | Autism diagnosis was according to DSM-5 [1],whereas T2DM was according to ICD-10 [38]. | Descriptive analysis, including frequencies. | Autistic patients had a T2DM prevalence of 0.5% (1/191). | 11/24; 46% |
| Shedlock et al (2016) [23], United States | Retrospective-case control study. | 48,762 autistic children and 243,810 controls matched by age, sex and enrolment time. | Mean age = NR; Age range = NR; Male % = 80; ID % = NR; ED = NR. | Electronic database. | ICD-9-CM [36]. | Conditional logistic regression was used to calculate OR and 95% CI for T2DM in comparing autistic patients and non-autistic controls. | Autistic children were significantly (p≤0.05) more likely to have T2DM (n=515; 1.06%) than non-autistic controls (n=970; 0.40%). Both results represent an underestimate of T2DM prevalence, as insulin treatment was a study exclusion criterion. | 25.5/32; 80% |
| T1DM-and T2DM-specific Findings | ||||||||
| Taggart et al (2013) [24], Northern Ireland | Quantitative questionnaire. | 186 individuals with ID, including autistic persons (though the number with autism is not reported). | No autistic cohort characteristics reported other than entire population having ID (overall study population characteristics reported only). | Postal questionnaire. | No details reported; questionnaire design suggests carers were just asked whether their patient had autism and/or DM. | The precise statistical technique used to examine the association between autism and DM is not reported2. | The authors report that significantly (p<0.05) more people with autism had T1DM (13%) compared with T2DM (5%). Raw data regarding the overall number of autistic patients, as well as those with T1DM and T2DM was not reported. | 17.5/27; 65% |
| Non-specific DM Findings | ||||||||
| Akobirshoev et al (2020) [25], United States. | Retrospective case-control study. | 34,237 adults with autism and 102,711 age and sex-matched controls. | Mean age = 33 years; Age range = NR; Male % = 75; ID % = NR; ED = 65% White non-Hispanic, 11% Black non-Hispanic, 5% Hispanic, 4% Other non-Hispanic, 15% Unknown ethnicity. | Electronic hospital database records from 2004-2014. | Autism diagnosis was according to ICD-9-CM criteria [36]. | Differences across categorical variables between the two groups were assessed using the Chi square test; Differences across continuous variables were assessed using the t-test. Logistic regression was used to assess mortality risk for different medical comorbidities in autistic adults relative to matched controls. | Among adults whom experienced in-hospital mortality, 11.5-13.6% (53-63/462) of the autistic group had a diagnosis of DM, compared to 15.0% (145/967) of the control group. Of these, 53 of the autistic group and 114 of the control group had DM without chronic complications (a statistically significantly [p≤0.05] increased risk in the autistic group). 0-103 of the autistic group and 31 of the control group had DM with chronic complications (a non-statistically significant difference). Overall data for all autistic adults (i.e. including those whom did not experience in-hospital mortality) was not reported. | 13.5/30; 45% |
| Alabaf et al (2019) [26], Sweden | Case-control study. | 23,049 children (301 with autism), from the Child and Adolescent Twin Study [39]. | Mean age = NR; Age range = 9-12; Male % = NR; ID % = 34; ED = NR. | Autism, Tics and other Comorbidities (A-TAC) [40] interview with parents. | Autism diagnosis was via the A-TAC, based on DSM-IV [41] and ICD-10 [38] criteria. DM diagnosis was based on parental self-report. | The Pearson Chi square or the Fisher’s exact tests were used to test for statistical significance between the prevalence of DM across subgroups. | 0.66% (2/301) of the autistic subgroup had a diagnosis of DM, compared to 0.4% (91/22028) of the control group. This difference was not statistically significant (p≥0.05). | 21.5/28; 77% |
| Croen et al (2015) [6],United States | Retrospective case-control study. | 1,507 autistic adults and 15,070 age and sex matched controls. | Mean age = 29 years; Age range = ≥18 years; Male % = 73; ID % = 19; ED = 66% White non-Hispanic, 11% Asian, 8% Black, 4% White Hispanic, 12% Other. | Electronic health records from January 2008 – December 2012. | Diagnoses were recorded in electronic records according to ICD-9-CM criteria [36] for autism, and ICD-9 criteria [37] for DM. | Prevalence of DM was compared between autistic cases and controls using chi-square tests. Also, a multivariate logistic regression model was run to compare the odds for DM between cases and controls. | Autistic adults were significantly (p<0.001) more likely to have DM than their non-autistic peers (Autistic group: n=114, 7.6%; control group: n=653, 4.3%). Adjusted OR (after adjusting for sex, age and race/ethnicity) = 2.18 (99% CI 1.62-2.93). | 20.5/26; 79% |
| Davignon et al (2018) [27], United States | Retrospective case-control study. | 47,509 children and young adults, including 4,123 with autism, 20,615 with ADHD, 2,156 with DM and 20,615 controls with none of these conditions, matched on age. | Mean age = 18 years; Age range = 14-25 years; Male % = 81; ID % = 13; ED = 54% White non-Hispanic, 16% Asian, 8% African American, 7% White Hispanic, 16% Other. | Electronic health records from 2013-2015. | Diagnoses were recorded in electronic records according to ICD-9 criteria [37]. | Prevalence of DM was compared between autistic cases and controls using chi-square testing. Additionally, a multivariate logistic regression model was run to compare the adjusted odds for DM between cases and controls. | 0.6% (25/4123) of the autistic group had DM; compared to 0.5% (107/20,615) of the ADHD group (Autism vs. ADHD adjusted OR 1.18; 95% CI 0.76-1.83). Due to the control group not having a diagnosis of DM by definition, a comparison between the autistic and control groups is not meaningful. | 24.5/29; 84% |
| Flygare Wallen et al (2018) [28], Sweden | Retrospective case-control study. | 13,921 autistic patients with no ID and 1,996,140 non-autistic non-matched controls4 | Mean age = NR; Age range = NR; Male % = 66; ID % = 05; ED = NR. | Electronic database. | Diagnoses were recorded according to ICD-10 criteria [38]. | Age-adjusted odds ratios with 95% CI’s were calculated using logistic regression analyses, to compare the prevalence of DM different groups. | Autistic patients had a DM prevalence of 2.87% (399/13,921), compared with a control group prevalence of 5.87% (117,148/1,996,140). However, the age-adjusted OR for DM in autistic patients was significantly (p<0.05) greater than for the control group (Autistic males – OR 1.705, 95% CI 1.50-1.93; Autistic females – OR 1.596, CI 1.33-1.92). Both DM prevalence estimates likely represent overestimations, as a diagnosis of DM, hypertension or obesity was an inclusion criterion for the study. | 16/24; 67% |
| Guinchat et al (2015) [29], France | Mixed retrospective and prospective cohort study. | 58 autistic patients (44 male and 14 female), 56 of which had co-occurring ID. No non-autistic controls were included. | Mean age = 16 years; Age range = 11-37 years; Male % = 76; ID % = 97; ED = NR. | Medical records. | ICD-10 criteria [38] confirmed by the Childhood Autism Rating Scale [42]. | Descriptive analysis, including frequencies. | Autistic patients had a DM prevalence of 1.7% (1/58). | 25/28; 89% |
| Gurney et al (2006) [30],United States | Cross-sectional analysis of survey. | 324,000 autistic children and 61,100,0006 non-autistic non-matched controls. | Mean age = NR; Age range = 3-17 years; Male % = 79; ID % = NR; ED = 74% White, 15% Black, 2% Multiracial, 3% Other, 7% Missing. | The 2003-2004 National Survey of Children’s Health (NSCH) [43],a population-based, cross-sectional telephone survey. | No specific diagnostic criteria were used. Autistic cases were identified from asking parents if a health professional has ever told them that their child has autism. | Prevalence values were calculated using the stratified weighted sampling fractions detailed in the NSCH public use data set. A multivariate logistic regression model was used to estimate OR. | Of the autistic group, 0.4% had DM, compared with 0.3% of the control group (raw values are not reported in the article). The difference between the groups was not statistically significant (OR 1.1; 95% CI 0.4-3.1). | 21.5/25; 86% |
| Jones et al (2016) [31], United States | Cross-sectional analysis of cohort. | 92 autistic adults. | Mean age = NR; Age range = 24-51 years; Male % = 75; ID % = 62; ED = NR. | Patient or caregiver questionnaire. | DSM-III [44] or DSM-IV-TR [45] for autism. No specific diagnostic criteria for DM were reported as being used. | Goodness-of-fit testing was used to test for any differences in the frequency of categorical variables, such as DM. | At follow-up, 9.8% (9/92) of patients had DM. Though the researchers did not have a control group for their study, they compared this finding with that of Ford et al.(2013), whom found a DM prevalence of 12% in the general US adult population. | 24.5/30; 82% |
| McDermott et al (2007) [32], United States | Retrospective cohort study. | 1,303 patients with disabilities (45 with autism) and 1,828 non-disabled patients. | Mean age = 28 years; Age range = NR; Male % = 76; ID % = NR; ED = NR for autistic cohort. | Medical records. | ICD-9-CM [36]. | Data was analysed using chi-square testing and logistic regression modelling. | Of the 45 autistic patients within the study population, 0% (0/45) had DM. This compares to a prevalence of 15.5% (202/1303) for the overall disability group and 14.5% (265/1828) for the non-disabled group. | 15/28; 54% |
| McManus et al. (2009) [33], England | Cross-sectional survey. | 19 autistic adults, and 599 non-autistic adults7. | Mean age = 48 years; Age range = 17-78 years; Male % = 79; ID % = NR; ED = 100% White British. | National survey data. | An ADOS [46]score of ≥10 was used as a proxy measure for autism. | No specific statistical methods were applied in comparing the rates of DM between autistic and non-autistic patients; the findings were reported as raw data. | Of the 19 autistic patients, 0% (0/19) had DM. This compares to a prevalence of 6.3% (38/599) for the non-autistic group. | Not applicable8 |
| Tyler et al (2011) [34], United States | Retrospective case-control study. | 108 autistic adults and 206 paired non-autistic controls matched for age, sex and insurance status. | Mean age = 29 years; Age range = NR; Male % = 71; ID % = 25; ED = 77% Caucasian, 14% African American, 2% Hispanic, 7% Other. | Electronic healthcare records. | ICD-9 [37]. | Precise details of statistical technique implemented are not reported9. | No significant difference in DM prevalence (p=0.68) between autistic group (n=7; 6.5%) and control group (n=16; 7.8%). | 23/31; 74% |
| Vohra et al (2017) [35], United States | Retrospective matched cohort study. | 1,772 adults with autism and 5,320 non-autistic adult controls matched by age, gender and race. | Mean age = NR; Age range = 22-64 years; Male % = 71; ID % = NR10; ED = 37% White, 21% African American, 42% Other. | Electronic healthcare records from 2000-2008. | ICD-9-CM [36]. | Chi-square tests of association were used for categorical variables, and t-tests for continuous variables, were used to assess differences between the autistic and non-autistic groups. | Of the 1,772 autistic adults, 3.6% (63/1772) had DM, compared with 4.7% (250/5320) for non-autistic controls. This difference was statistically significant (p<0.05). | 20.5/27; 76% |
1 The percentage of the study population with recorded ‘cognitive impairment’, which is a broader term than ID, also encompassing dementia-related illness and brain injury acquired following the developmental period. 2 The authors write ‘A series of chi-squared tests, independent t-tests and one-way ANOVAs were used to examine for significant differences between T1DM and T2DM, gender, age, level of ID, accommodation, BMI and hypertension across a number of quality diabetes care indicators.’ 3 To maintain confidentiality, the authors could not report precise numbers for cells with <11 cases. 4 There were also separate patient groups with ID and no Down syndrome (n= 11,785) and Down syndrome (n= 1282), though these have not been considered for the purposes of this review. 5 The authors report data for a separate group with ID, rather than as part of the autistic group or the non-autistic controls. 6 The sample sizes reported by the authors are US national estimates, based on sampling fractions and weighted extrapolation of parental reports of 483 autistic children withand 84,789 children without autism. 7 Autistic adults were defined as those scoring ≥10 on the Autism Diagnostic Observation Schedule, rather than having a clinical diagnosis of autism. Controls were defined as persons with ADOS scores of ≤9. 8 The autism and DM findings from the Adult Psychiatric Morbidity Survey were not reported in a specific journal article but rather gleaned from the overall dataset, available online, and as such a STROBE score could not be calculated. 9 Authors write ‘Using standard descriptive statistics, the two cohorts were compared in their documentation of selected biophysical data, chronic disease diagnoses, and pharmacotherapeutic data.’ 10 Though 1,231 (70%) of the autistic cohort were classified as having ‘developmental disorders,’
Overall, of 15 studies that compared findings from autistic and non-autistic groups [6, 13, 19-21, 23, 25-28, 30, 32-35], 9 found a heightened prevalence in the autistic group [6, 13, 19, 20, 23, 26-28, 30]. However, the difference was reported as statistically significant in only 4 of these [6, 20, 23, 28]. Furthermore, the studies that found a heightened DM prevalence in autistic persons [6, 13, 19, 20, 23, 26 - 28, 30] had a far greater mean average study population size (Average number of autistic participants = 12,599; Average number of controls = 541,861) than those finding a greater DM prevalence in the control group [21, 25, 32 - 35]’ (Average number of autistic participants = 6,829; Average number of controls = 325,539). Additionally, the studies showing heightened DM prevalence in autistic persons [6, 13, 19, 20, 23, 26-28, 30] had a higher mean average percentage STROBE score rating than those showing a higher DM prevalence in the control group [21, 25, 32-35] (75% vs. 64%), suggesting that the reporting of these studies was of a generally higher quality.
Three studies did not provide raw data pertaining to DM prevalence [21, 24, 30], reporting only percentage values. This data was requested from the corresponding authors, but either reply were not received, or such data was unavailable. However, these studies were included, as they provided prevalence data in some form.
Of the sixteen studies that included both autistic and non-autistic groups within their respective study populations [6, 13, 19-21, 23-28, 30, 32-35], five showed a statistically significant difference between the autistic and non-autistic groups [6, 20, 23, 28, 35], four of which demonstrated a significantly increased rate of DM in the autistic group [6, 20, 23, 28], and one a significantly reduced risk of DM in this group [35].
3.3. Non-Specific DM Findings
Twelve studies did not report type-specific DM data, rather reporting a prevalence rate that likely encompasses both subtypes, though in many instances, this is not explicitly stated. Eight of these studies were based in the United States, including Croen et al. (2015) [6], who reviewed the electronic records of 1,507 autistic adults and 15,070 age- and sex-matched controls, finding a significantly (p<0.001) increased prevalence of DM in autistic people (n=114; 7.6%) relative to their non-autistic peers (n=653; 4.3%). In contrast, Vohra et al. (2017) [35] reported a significantly (p<0.05) reduced DM prevalence in autistic individuals (3.6%; 63/1772) compared to non-autistic controls (4.7%; 250/5320). They suggested that possible reasons for the stark differences in findings compared to Croen et al. (2015) [6] could be the different demographics of different populations studied (Northern California for Croen et al. (2015) [6] vs. Illinois, New York and Texas for Vohra et al. (2017) [35]), the Croen et al. (2015) [6] data being more recent and thus their heightened autism awareness with improved access to associated services, and Vohra et al. (2017) [35] requiring at least two outpatient (or one inpatient) insurance claims in order to code comorbidity.
Other United States-based studies adopting electronic records approach include a study by Tyler et al. (2011) [34], who found no significant difference in DM prevalence between 108 autistic adults (n=7; 6.5%) and 206 non-autistic controls matched for age, sex and insurance status (n=16; 7.8%). However, as with many such studies, autistic persons were identified via receiving services through their local healthcare system (in this case, the Cleveland Clinic). Thus, autistic adults not utilising such services are not identified by such approach. Davignon and colleagues (2018) [27] similarly used electronic records to investigate co-occurring physical and psychiatric conditions in autistic children and young adults, comparing them with peer groups with Attention Deficit Hyperactivity Disorder (ADHD), DM, and controls with none of three conditions. DM prevalence rates of 0.6% (25/4123) and 0.5% (107/20,615) were reported for the autistic and ADHD groups, respectively (the control group did not have DM by definition, so could not be meaningfully compared).
McDermott and colleagues (2007) [32] used primary care records to compare individuals with disabilities (including autism, ID, cerebral palsy, spinal cord injury, chronic mental illnesses, visual impairment and hearing impairment) with non-disabled persons. Out of the autistic subgroup (n=45), 0 had co-occurring DM, compared to 15.5% (202/1303) of the overall disability group and 14.5% (265/1828) of the non-disabled cohort. This low prevalence finding may be somewhat attributable to the small size of the autistic cohort, much like the United Kingdom-based survey by McManus et al. (2014) [33], where no autistic individuals with co-occurring DM were identified. Still, the autistic cohort size was similarly small (n=19). However, in contrast, Jones et al. (2015) [31] identified a DM prevalence of 9.8% (n=9) from a cohort of 92 autistic adults, who were interviewed as part of a 25-year outcome study of adult autism.
In Sweden, a large electronic database study by Flygare Wallen et al. (2018) [28], demonstrated a significantly heightened risk of DM in both autistic people as well as those with ID, after adjusting for age. However, the quoted prevalence rate of 2.87% (399/13,921) is likely an overestimate relative to the general Swedish autistic population, as the study inclusion criteria stipulated that participants had to have at least one recorded diagnosis of either DM, hypertension or obesity. Focussing exclusively on Swedish children, Alabaf et al. (2018) [26] interviewed the parents of twins born between 31st June 2002 and 31st December 2006, including 301 autistic children and 22,028 controls with no autism, ID or ADHD. They found a slightly heightened prevalence of DM among autistic children relative to controls (0.66%; 91/22028 vs. 0.4%; 2/301), though this difference was not statistically significant, and both diabetic autistic individuals also had co-occurring ID. Additionally, the different approach to data collection (database vs. parental interview) introduces a further source of bias when making a comparison with Flygare Wallen et al. (2018) [28]. In the United States, Gurney et al. (2006) [30] similarly interviewed parents of autistic vs. non-autistic children, similarly finding a lack of a statistically significant DM prevalence difference between the two cohorts (autistic group 0.4%; non-autistic group 0.3%).
Akobirshoev et al. (2019) [25] examined the electronic discharge records of North American-based autistic adults who had been admitted to hospital, relative to non-autistic age- and sex-matched controls. Overall, 11.5-13.6% (53-63/462) of the autistic group had DM, compared to 15.0% (145/967) of the control group; not a statistically significant result, though autistic persons were significantly (p<0.05) more likely to have DM without chronic complications. A France-based study by Guinchat et al. (2015) [29] also focussed on an inpatient setting, reporting on the medical records of 58 autistic adolescents who had been admitted to a specialist neurobehavioural unit. Interestingly, out of the entire cohort, only 1 patient had a DM diagnosis, which may be surprising given the high rates of antipsychotic prescribing in such a group (in this cohort, 56 patients had been prescribed an antipsychotic).
3.4. Subgroup Analyses
Five studies reported T1DM-specific prevalence data. In a US-based study, Kohane et al. (2012) [20] reviewed the electronic healthcare records of 14,381 autistic patients and 2,379,397 non-autistic peers. They found that autistic patients were significantly (p<0.001) more likely to have T1DM (Autistic group: n=114, 0.79%; Non-autistic group: n=8058, 0.34%). This observation is further supported by the findings of Chen and colleagues [19], who found that autistic patients were borderline significantly (p=0.056) more likely to have T1DM compared to their non-autistic counterparts (Autistic group = 0.3%; 4/1,598 vs. Non-autistic controls = 0.1%; 4/6,392). More recent US-based findings by Zerbo et al (2015) [13] found an increased T1DM prevalence in autistic patients relative to their non-autistic peers, though this difference was not statistically significant (Autistic group = 0.22%; 12/5,565 vs. Non-autistic controls = 0.19%; 52/27,825). In contrast, another study found conversely found a modestly increased risk of T1DM among non-autistic individuals (Autistic group = 0.42%; Non-autistic controls = 0.59%) [21]. In a postal questionnaire study of 186 individuals with ID, Taggart et al. (2013) [24] reported a considerably higher T1DM prevalence of 13% among the autistic subgroup. However, raw data pertaining to the size of the autistic subgroup, the number with T1DM and the subgroups age distribution was not reported or available on request, making it difficult to draw any clear conclusions from such a prevalence finding in isolation.
Only three studies reported T2DM-specific prevalence data. In a US-based retrospective review of the electronic healthcare records of 48,762 autistic children and 243,810 matched non-autistic patients [23], autistic children were significantly (p≤0.05) more likely to have T2DM relative to their non-autistic peers (Autistic group: n=515, 1.06%; Non-autistic group: n=970, 0.40%). However, both results likely represent an underestimate of T2DM prevalence for both groups, as insulin treatment was listed as an exclusion criterion for the study. Brondino et al. (2019) [22] reviewed the clinical records of 191 autistic patients based in Italy, finding a prevalence rate of 0.5% (n=1). It is difficult to draw any firm conclusions from this finding, however, considering the relatively small sample size and lack of a non-autistic comparison group. Much like for T1DM, Taggart et al. (2013) [24] also reported a considerably higher T2DM prevalence than the aforementioned studies, 5%. However, as was the case for their T1DM prevalence rate, key related data to put this percentage value in context was not reported.
3.5. Summary of Relevant Excluded Studies
The most frequent rationale for rejection of articles during full text screening included not reporting prevalence data for DM in autistic people, reporting prevalence data for autism among people with DM, or focusing on maternal or family history of DM with autism in their offspring.
Several studies estimated the prevalence of autism in people with DM, rather than the prevalence of DM in autistic people. Though not satisfying inclusion criteria for this review, these studies similarly measure the association of autism with DM. Freeman et al. (2005) [47] retrospectively reviewed the charts of 984 children attending a Canadian diabetes clinic, estimating an autism prevalence of 0.9% (9/984) in children with T1DM. The authors reported that this was greater than general population prevalence estimates [48]; however, more recent data suggests this finding aligns with the general population [3]. Further studies found equivocal results; Harjutsalo and Tuomilehto (2006) [49] observed no significant difference in autism prevalence between Finnish children with T1DM relative to the general population. Iafusco et al. (2006) [50] suggested that the autism prevalence in patients with T1DM in Italy appeared inversely associated with the incidence trends of T1DM in the geographical areas being observed.
Lemay et al. (2018) [51] investigated this relationship in a large study population (n=61,749) of German and Austrian children and adolescents with T1DM, estimating an autism prevalence of 0.24% (n=150), concluding that this finding did not suggest an elevated autism risk relative to the general population. Two similar studies based in North America, both also involving child populations, also concluded that the prevalence of T1DM in autistic children was similar to the general population [52, 53].
In contrast, Levitt Katz et al. (2005) [54] investigated the prevalence of neuropsychiatric disorders in North American T2DM patients via a retrospective chart review of 237 children. However, the prevalence of autism within this cohort was not reported, as the authors grouped autistic persons together with those with ID, who had a combined prevalence of 4% (10/237) within this cohort.
Chen et al. (2016) [55] measured the development of T2DM over time rather than prevalence per se. Using the Taiwan National Health Insurance Database, they monitored 6,122 autistic adolescents and adults, and 24,488 non-autistic age- and sex- matched peers from 2002-2009 until the end of 2011. They observed that the autistic individuals were significantly (p<0.001) more likely to develop T2DM over the study period than their non-autistic peers (Hazard ratio 3.25 and 95% CI 2.23-4.75, after adjusting for demographic data, antipsychotic use and medical comorbidities). Additionally, prescription of atypical antipsychotics, far more prevalent in the autistic cohort (n=288; 32.2%) than the non-autistic group (n=117; 0.8%), conferred an additional significantly increased risk (p<0.001) of T2DM development.
4. DISCUSSION
This systematic review assessed the current evidence regarding the prevalence of T1DM and T2DM among autistic persons. Though there was not an overwhelmingly clear trend across eligible studies for either T1DM or T2DM, those showing a heightened prevalence of DM in autistic persons (relative to non-autistic controls) had on average larger study populations and had a higher average rated quality of reporting.
The DM prevalence rates reported in both autistic and non-autistic groups were generally substantially lower than the worldwide prevalence of 9.3% reported by the International Diabetes Federation [8]. One factor likely contributing to this finding is that eligible studies tended to focus on younger persons (with the mean participant age ranging from 12-48 year), with fewer representations of older persons for whom the DM prevalence would be higher. Additionally, 16 of the 19 eligible studies were published prior to 2019 [6, 13, 19-21, 23, 24, 27-35]; given that worldwide DM prevalence has more than doubled from 2000 (4.3%) to 2019 (9.3%) [8], one would not expect pre-2019 prevalence estimates to be as high.
There are numerous possible contributory factors to DM risk in autistic persons. There may be a shared genetic linkage, a notion supported by the association between T2DM and GDM in mothers of autistic children [56]. Several conditions associated with autism also confer a greater risk of T2DM, including chromosomal disorders such as Turner’s [57] and Down’s syndromes [34, 58], ID [59], obesity [23] and psychotropic medications, which are more frequently prescribed to autistic individuals [60]. Another potential mechanism conferring DM risk is altered autoimmune cytokine secretion, as elevated secretion of IL-1 and IL-6 relative to healthy controls has been observed in autistic individuals [61], as well as those with T1DM [62, 63] and T2DM [19, 64]. Increased cytokine secretion and immune dysregulation from an early age could contribute to the apoptotic destruction of pancreatic beta-cells, leading to the clinical manifestation of either T1DM or T2DM in later life [55, 64].
Whilst it is likely that factors such as ID, obesity and psychotropic medications contribute to T2DM risk in autistic individuals, behavioural factors may also play a role. Previous research has highlighted that there can be issues in clinical practice with regard to reaching and providing treatment to autistic patients [24], as they can experience barriers to accessing public health information as well as healthcare interventions more generally [65]. Additionally, autistic persons in general, have a higher dependence on others to recognise signs and symptoms, report concerns to health professionals and seek treatment for such conditions [25].
Following diagnosis, DM has a high level of demand on patients, with consistent day-to-day management often required to optimize glycaemic control and minimize complications, which may be difficult for some autistic individuals, especially those with co-occurring ID, who represent a significant subgroup of the autistic population [4]. However, it is also plausible that some features of autism, such as rigidity and a preference for routine, may be beneficial in DM management [51]. Future research should examine the characteristics, needs, experiences, health-related behaviours, and long-term health outcomes of autistic individuals DM, as these may differ from their non-autistic diabetic peers. This will assist the development of public health measures tailored to supporting autistic individuals with diabetes.
Healthcare professionals need to bear in mind these challenges when working with autistic people, and be vigilant in terms of screening for DM. They also need to consider DM risk regarding treatment decisions, such as commencement of psychotropic medications with diabetogenic potential, to ensure autistic individuals receive high quality, equitable care. The recently published NHS Long Term Plan [66] outlines plans to pilot a specific health check for autistic individuals, with a view to potentially extending it more widely. The findings of this review suggest a need to incorporate DM screening into such assessments, as it represents a significant public health issue for this patient group.
4.1. Limitations
Though this review focuses on the prevalence of DM in autistic persons, this is just one means of measuring the association between the two conditions. Measuring this association in the form of prevalence is valuable to a healthcare professional-based readership, as it can be readily translated into policy and costings [67]. However, other means of measuring the association between these conditions, such as the development of DM in autistic persons over time, as well as the prevalence of autism in persons with DM, should also be considered, and such articles have been discussed in the Summary of Relevant Excluded Studies section of this review. Another issue meriting further exploration is the prognosis of T1DM and T2DM among autistic individuals relative to their non-autistic peers. Studies could be underestimating the true association if the relative survival following a diagnosis of DM is lower in autistic persons [28]. This theory is supported by the findings of Chen et al. (2016) [55], who observed that the rate of development of T2DM in autistic persons over time was significantly (o <0.001) greater than for their non-autistic peers.
The designs of eligible studies varied widely, including surveys, case-control and cohort studies. Ideally, the association between autism and DM should be explored in large cohort studies where autism can be analysed alongside other conditions in which the prevalence of DM may differ from the general population, such as ID [68], as well as measuring other factors contributory to DM development. A further limitation of most eligible studies is that they focus on autistic individuals who have interacted with primary and/or secondary care services; thus, the DM risk in these persons may not be entirely representative of all autistic individuals. Additionally, there is a considerable heterogeneity across studies in terms of approaches to data collection, including electronic records, questionnaires, surveys and structured interview, representing a further source of bias. Furthermore, the search was limited to English language, which could have overlooked relevant evidence published in foreign language journals. However, whilst most eligible studies were North American in origin, the review nevertheless included studies from many different countries, where access to health services and diagnostic practices may differ considerably.
CONCLUSION
As with any systematic review, the quality is affected by the quality of its constituent studies. Many studies did not report data differentiating the prevalence of DM subtypes, and as such, their findings are somewhat limited in value. We would recommend that future work involving estimating the prevalence of DM, in autistic individuals or otherwise needs to collect T1 and T2 specific data. Moreover, it is essential that such studies also report other data central to DM aetiology, such as the age, ethnicity and gender distributions of their respective study populations.
ACKNOWLEDGEMENTS
The authors would like to thank Tanya McLaven and Stuart Glover at University Hospitals of Leicester for their support with the literature search, and Enzo Cerullo at the University of Leicester for assistance with the manuscript review.
LIST OF ABBREVIATIONS
- 95% CI
95% Confidence Interval
- ADOS
Autism Diagnostic Observation Schedule
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- ICD
International Classification of Diseases
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s website, along with the published article.
CONSENT FOR PUBLICATION
Not applicable.
STANDARDS OF REPORTING
PRISMA guidelines and methodology were followed.
FUNDING
None.
CONFLICT OF INTEREST
T.B. is a member of the Editorial Board of Clinical Practice & Epidemiology in Mental Health.
REFERENCES
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Arlington: American Psychiatric Publishing; 2013. [Google Scholar]
- 2.Lundström S., Reichenberg A., Anckarsäter H., Lichtenstein P., Gillberg C. Autism phenotype versus registered diagnosis in Swedish children: prevalence trends over 10 years in general population samples. BMJ. 2015;350:h1961. doi: 10.1136/bmj.h1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxter A.J., Brugha T.S., Erskine H.E., Scheurer R.W., Vos T., Scott J.G. The epidemiology and global burden of autism spectrum disorders. Psychol. Med. 2015;45(3):601–613. doi: 10.1017/S003329171400172X. [DOI] [PubMed] [Google Scholar]
- 4.Brugha T.S., Spiers N., Bankart J., Cooper S.A., McManus S., Scott F.J., Smith J., Tyrer F. Epidemiology of autism in adults across age groups and ability levels. Br. J. Psychiatry. 2016;209(6):498–503. doi: 10.1192/bjp.bp.115.174649. [DOI] [PubMed] [Google Scholar]
- 5.Tromans S., Chester V., Kiani R., Alexander R., Brugha T. The prevalence of autism spectrum disorders in adult psychiatric inpatients: A systematic review. Clin. Pract. Epidemiol. Ment. Health. 2018;14:177–187. doi: 10.2174/1745017901814010177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Croen L.A., Zerbo O., Qian Y., Massolo M.L., Rich S., Sidney S., Kripke C. The health status of adults on the autism spectrum. Autism. 2015;19(7):814–823. doi: 10.1177/1362361315577517. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. ICD-11 for Mortality and Morbidity Statistics. Geneva: World Health Organization; 2018. [Google Scholar]
- 8.International Diabetes Federation IDF Diabetes Atlas. https://www.diabetesatlas.org
- 9.Forouhi N.G., Wareham N.J. Epidemiology of diabetes. Medicine (Baltimore) 2019;47(1):22–27. doi: 10.1016/j.mpmed.2018.10.004. [DOI] [Google Scholar]
- 10.Xu G., Jing J., Bowers K., Liu B., Bao W. Maternal diabetes and the risk of autism spectrum disorders in the offspring: a systematic review and meta-analysis. J. Autism Dev. Disord. 2014;44(4):766–775. doi: 10.1007/s10803-013-1928-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masi A., Glozier N., Dale R., Guastella A.J. The immune system, cytokines, and biomarkers in autism spectrum disorder. Neurosci. Bull. 2017;33(2):194–204. doi: 10.1007/s12264-017-0103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjorklund G., Saad K., Chirumbolo S., Kern J.K., Geier D.A., Geier M.R., Urbina M.A. Immune dysfunction and neuroinflammation in autism spectrum disorder. Acta Neurobiol. Exp. (Warsz.) 2016;76(4):257–268. doi: 10.21307/ane-2017-025. [DOI] [PubMed] [Google Scholar]
- 13.Zerbo O., Leong A., Barcellos L., Bernal P., Fireman B., Croen L.A. Immune mediated conditions in autism spectrum disorders. Brain Behav. Immun. 2015;46:232–236. doi: 10.1016/j.bbi.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill A.P., Zuckerman K.E., Fombonne E. Obesity and Autism. Pediatrics. 2015;136(6):1051–1061. doi: 10.1542/peds.2015-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park S.Y., Cervesi C., Galling B., Molteni S., Walyzada F., Ameis S.H., Gerhard T., Olfson M., Correll C.U. Antipsychotic use trends in youth with autism spectrum disorder and/or intellectual disability: a meta-analysis. J. Am. Acad. Child Adolesc. Psychiatry. 2016;55(6):456–468.e4. doi: 10.1016/j.jaac.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Vandenbroucke J.P., von Elm E., Altman D.G., Gøtzsche P.C., Mulrow C.D., Pocock S.J., Poole C., Schlesselman J.J., Egger M., STROBE Initiative Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4(10):e297. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams A.D., Benner R.S., Riggs T.W., Chescheir N.C. Use of the STROBE checklist to evaluate the reporting quality of observational research in obstetrics. Obstet. Gynecol. 2018;132(2):507–512. doi: 10.1097/AOG.0000000000002689. [DOI] [PubMed] [Google Scholar]
- 18.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen M., Su T., Chen Y., Hsu J., Huang K., Chang W., Chen T., Bai Y. Comorbidity of allergic and autoimmune diseases in patients with autism spectrum disorder: a nationwide population-based study. Res. Autism Spectr. Disord. 2013;7(2):205–212. doi: 10.1016/j.rasd.2012.08.008. [DOI] [Google Scholar]
- 20.Kohane I.S., McMurry A., Weber G., MacFadden D., Rappaport L., Kunkel L., Bickel J., Wattanasin N., Spence S., Murphy S., Churchill S. The co-morbidity burden of children and young adults with autism spectrum disorders. PLoS One. 2012;7(4):e33224. doi: 10.1371/journal.pone.0033224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Supekar K., Iyer T., Menon V. The influence of sex and age on prevalence rates of comorbid conditions in autism. Autism Res. 2017;10(5):778–789. doi: 10.1002/aur.1741. [DOI] [PubMed] [Google Scholar]
- 22.Brondino N., Fusar-Poli L., Miceli E., Di Stefano M., Damiani S., Rocchetti M., Politi P. Prevalence of medical comorbidities in adults with autism spectrum disorder. J. Gen. Intern. Med. 2019;34(10):1992–1994. doi: 10.1007/s11606-019-05071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shedlock K., Susi A., Gorman G.H., Hisle-Gorman E., Erdie-Lalena C.R., Nylund C.M. Autism spectrum disorders and metabolic complications of obesity. J. Pediatr. 2016;178:183–187.e1. doi: 10.1016/j.jpeds.2016.07.055. [DOI] [PubMed] [Google Scholar]
- 24.Taggart L., Coates V., Truesdale-Kennedy M. Management and quality indicators of diabetes mellitus in people with intellectual disabilities. J. Intellect. Disabil. Res. 2013;57(12):1152–1163. doi: 10.1111/j.1365-2788.2012.01633.x. [DOI] [PubMed] [Google Scholar]
- 25.Akobirshoev I., Mitra M., Dembo R., Lauer E. In-hospital mortality among adults with autism spectrum disorder in the United States: A retrospective analysis of US hospital discharge data. Autism. 2020;24(1):177–189. doi: 10.1177/1362361319855795. [DOI] [PubMed] [Google Scholar]
- 26.Alabaf S., Gillberg C., Lundström S., Lichtenstein P., Kerekes N., Råstam M., Anckarsäter H. Physical health in children with neurodevelopmental disorders. J. Autism Dev. Disord. 2019;49(1):83–95. doi: 10.1007/s10803-018-3697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davignon M.N., Qian Y., Massolo M., Croen L.A. Psychiatric and Medical Conditions in Transition-Aged Individuals With ASD. Pediatrics. 2018;141(4) Suppl. 4:S335–S345. doi: 10.1542/peds.2016-4300K. [DOI] [PubMed] [Google Scholar]
- 28.Flygare Wallén E., Ljunggren G., Carlsson A.C., Pettersson D., Wändell P. High prevalence of diabetes mellitus, hypertension and obesity among persons with a recorded diagnosis of intellectual disability or autism spectrum disorder. J. Intellect. Disabil. Res. 2018;62(4):269–280. doi: 10.1111/jir.12462. [DOI] [PubMed] [Google Scholar]
- 29.Guinchat V., Cravero C., Diaz L., Périsse D., Xavier J., Amiet C., Gourfinkel-An I., Bodeau N., Wachtel L., Cohen D., Consoli A. Acute behavioral crises in psychiatric inpatients with autism spectrum disorder (ASD): recognition of concomitant medical or non-ASD psychiatric conditions predicts enhanced improvement. Res. Dev. Disabil. 2015;38:242–255. doi: 10.1016/j.ridd.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 30.Gurney J.G., McPheeters M.L., Davis M.M. Parental report of health conditions and health care use among children with and without autism: National Survey of Children’s Health. Arch. Pediatr. Adolesc. Med. 2006;160(8):825–830. doi: 10.1001/archpedi.160.8.825. [DOI] [PubMed] [Google Scholar]
- 31.Jones K.B., Cottle K., Bakian A., Farley M., Bilder D., Coon H., McMahon W.M. A description of medical conditions in adults with autism spectrum disorder: A follow-up of the 1980s Utah/UCLA Autism Epidemiologic Study. Autism. 2016;20(5):551–561. doi: 10.1177/1362361315594798. [DOI] [PubMed] [Google Scholar]
- 32.McDermott S., Moran R., Platt T., Dasari S. Prevalence of diabetes in persons with disabilities in primary care. J. Dev. Phys. Disabil. 2007;19(3):263–271. doi: 10.1007/s10882-007-9058-4. [DOI] [Google Scholar]
- 33.McManus S., Meltzer H., Brugha T., Bebbington P., Jenkins R. Adult Psychiatric Morbidity Survey. 2007 https://files.digital.nhs.uk/publicationimport/pub02xxx/pub02931/adul-psyc-morb-res-hou-sur-eng-2007-rep.pdf
- 34.Tyler C.V., Schramm S.C., Karafa M., Tang A.S., Jain A.K. Chronic disease risks in young adults with autism spectrum disorder: forewarned is forearmed. Am. J. Intellect. Dev. Disabil. 2011;116(5):371–380. doi: 10.1352/1944-7558-116.5.371. [DOI] [PubMed] [Google Scholar]
- 35.Vohra R., Madhavan S., Sambamoorthi U. Comorbidity prevalence, healthcare utilization, and expenditures of Medicaid enrolled adults with autism spectrum disorders. Autism. 2017;21(8):995–1009. doi: 10.1177/1362361316665222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization. The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Geneva: World Health Organization; 1997. [Google Scholar]
- 37.World Health Organization. The ICD-9 classification of mental and behavioural disorders. Geneva: World Health Organization; 1978. [Google Scholar]
- 38.World Health Organization. The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. Geneva: World Health Organization; 1992. [Google Scholar]
- 39.Anckarsäter H., Lundström S., Kollberg L., Kerekes N., Palm C., Carlström E., Långström N., Magnusson P.K., Halldner L., Bölte S., Gillberg C., Gumpert C., Råstam M., Lichtenstein P. The child and adolescent twin study in Sweden (CATSS). Twin Res. Hum. Genet. 2011;14(6):495–508. doi: 10.1375/twin.14.6.495. [DOI] [PubMed] [Google Scholar]
- 40.Hansson S.L., Svanström Röjvall A., Rastam M., Gillberg C., Gillberg C., Anckarsäter H. Psychiatric telephone interview with parents for screening of childhood autism - tics, attention-deficit hyperactivity disorder and other comorbidities (A-TAC): preliminary reliability and validity. Br. J. Psychiatry. 2005;187(3):262–267. doi: 10.1192/bjp.187.3.262. [DOI] [PubMed] [Google Scholar]
- 41.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. Arlington: American Psychiatric Association; 1994. [Google Scholar]
- 42.Schopler E., Reichler R.J., DeVellis R.F., Daly K. Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS). J. Autism Dev. Disord. 1980;10(1):91–103. doi: 10.1007/BF02408436. [DOI] [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention. National Survey of Children’s Health. https://www.cdc.gov/nchs/slaits/nsch.htm#anchor_1551498833774
- 44.American Psychiatric Association. Diagnostic and statistical manual. 3rd edn. (DSM-III). American Psychiatric Association; Washington: 1980. [Google Scholar]
- 45.American Psychiatric Association. Diagnostic and statistical manual. 4th edn. Text Revision (DSM-IV-TR). American Psychiatric Association; Washington: 2000. [Google Scholar]
- 46.Lord C., Risi S., Lambrecht L., Cook E.H., Jr, Leventhal B.L., DiLavore P.C., Pickles A., Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000;30(3):205–223. doi: 10.1023/A:1005592401947. [DOI] [PubMed] [Google Scholar]
- 47.Freeman S.J., Roberts W., Daneman D. Type 1 diabetes and autism: is there a link? Diabetes Care. 2005;28(4):925–926. doi: 10.2337/diacare.28.4.925. [DOI] [PubMed] [Google Scholar]
- 48.Bertrand J., Mars A., Boyle C., Bove F., Yeargin-Allsopp M., Decoufle P. Prevalence of autism in a United States population: the Brick Township, New Jersey, investigation. Pediatrics. 2001;108(5):1155–1161. doi: 10.1542/peds.108.5.1155. [DOI] [PubMed] [Google Scholar]
- 49.Harjutsalo V., Tuomilehto J. Type 1 diabetes and autism: is there a link? Diabetes Care. 2006;29(2):484–485. doi: 10.2337/diacare.29.02.06.dc05-1552. [DOI] [PubMed] [Google Scholar]
- 50.Iafusco D., Vanelli M., Songini M., Chiari G., Cardella F., Fifi A., Lombardo F., Marinaro A., Melia A., Marsciani A., Vaccà A., Prisco F. Type 1 diabetes and autism association seems to be linked to the incidence of diabetes. Diabetes Care. 2006;29(8):1985–1986. doi: 10.2337/dc06-0842. [DOI] [PubMed] [Google Scholar]
- 51.Lemay J.F., Lanzinger S., Pacaud D., Plener P.L., Fürst-Burger A., Biester T., Hilgard D., Lilienthal E., Galler A., Berger G., Holl R.W., German/Austrian DPV Initiative Metabolic control of type 1 diabetes in youth with autism spectrum disorder: A multicenter Diabetes-Patienten-Verlaufsdokumentation analysis based on 61 749 patients up to 20 years of age. Pediatr. Diabetes. 2018;19(5):930–936. doi: 10.1111/pedi.12676. [DOI] [PubMed] [Google Scholar]
- 52.Stanek K.R., Youngkin E.M., Pyle L.L., Raymond J.K., Driscoll K.A., Majidi S. Prevalence, characteristics, and diabetes management in children with comorbid autism spectrum disorder and type 1 diabetes. Pediatr. Diabetes. 2019;20(5):645–651. doi: 10.1111/pedi.12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bethin K.E., Kanapka L.G., Laffel L.M., Majidi S., Chaytor N.S., MacLeish S., Adams R., Foster N.C., T1D Exchange Clinic Network Autism spectrum disorder in children with Type 1 diabetes. Diabet. Med. 2019;36(10):1282–1286. doi: 10.1111/dme.14069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levitt Katz L.E., Swami S., Abraham M., Murphy K.M., Jawad A.F., McKnight-Menci H., Berkowitz R. Neuropsychiatric disorders at the presentation of type 2 diabetes mellitus in children. Pediatr. Diabetes. 2005;6(2):84–89. doi: 10.1111/j.1399-543X.2005.00105.x. [DOI] [PubMed] [Google Scholar]
- 55.Chen M.H., Lan W.H., Hsu J.W., Huang K.L., Su T.P., Li C.T., Lin W.C., Tsai C.F., Tsai S.J., Lee Y.C., Chen Y.S., Pan T.L., Chang W.H., Chen T.J., Bai Y.M. Risk of Developing Type 2 Diabetes in Adolescents and Young Adults With Autism Spectrum Disorder: A Nationwide Longitudinal Study. Diabetes Care. 2016;39(5):788–793. doi: 10.2337/dc15-1807. [DOI] [PubMed] [Google Scholar]
- 56.Xiang A.H., Wang X., Martinez M.P., Walthall J.C., Curry E.S., Page K., Buchanan T.A., Coleman K.J., Getahun D. Association of maternal diabetes with autism in offspring. JAMA. 2015;313(14):1425–1434. doi: 10.1001/jama.2015.2707. [DOI] [PubMed] [Google Scholar]
- 57.Bakalov V.K., Cheng C., Zhou J., Bondy C.A. X-chromosome gene dosage and the risk of diabetes in Turner syndrome. J. Clin. Endocrinol. Metab. 2009;94(9):3289–3296. doi: 10.1210/jc.2009-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Goor J.C., Massa G.G., Hirasing R. Increased incidence and prevalence of diabetes mellitus in Down’s syndrome. Arch. Dis. Child. 1997;77(2):186. doi: 10.1136/adc.77.2.183g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cardol M., Rijken M., van Schrojenstein Lantman-de Valk H. People with mild to moderate intellectual disability talking about their diabetes and how they manage. J. Intellect. Disabil. Res. 2012;56(4):351–360. doi: 10.1111/j.1365-2788.2011.01472.x. [DOI] [PubMed] [Google Scholar]
- 60.Galling B., Roldán A., Nielsen R.E., Nielsen J., Gerhard T., Carbon M., Stubbs B., Vancampfort D., De Hert M., Olfson M., Kahl K.G., Martin A., Guo J.J., Lane H.Y., Sung F.C., Liao C.H., Arango C., Correll C.U. Type 2 diabetes mellitus in youth exposed to antipsychotics: a systematic review and meta-analysis. JAMA Psychiatry. 2016;73(3):247–259. doi: 10.1001/jamapsychiatry.2015.2923. [DOI] [PubMed] [Google Scholar]
- 61.Croonenberghs J., Bosmans E., Deboutte D., Kenis G., Maes M. Activation of the inflammatory response system in autism. Neuropsychobiology. 2002;45(1):1–6. doi: 10.1159/000048665. [DOI] [PubMed] [Google Scholar]
- 62.Grishman E.K., White P.C., Savani R.C. Toll-like receptors, the NLRP3 inflammasome, and interleukin-1β in the development and progression of type 1 diabetes. Pediatr. Res. 2012;71(6):626–632. doi: 10.1038/pr.2012.24. [DOI] [PubMed] [Google Scholar]
- 63.Snell-Bergeon J.K., West N.A., Mayer-Davis E.J., Liese A.D., Marcovina S.M., D’Agostino R.B., Jr, Hamman R.F., Dabelea D. Inflammatory markers are increased in youth with type 1 diabetes: the SEARCH Case-Control study. J. Clin. Endocrinol. Metab. 2010;95(6):2868–2876. doi: 10.1210/jc.2009-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cieślak M., Wojtczak A., Cieślak M. Role of pro-inflammatory cytokines of pancreatic islets and prospects of elaboration of new methods for the diabetes treatment. Acta Biochim. Pol. 2015;62(1):15–21. doi: 10.18388/abp.2014_853. [DOI] [PubMed] [Google Scholar]
- 65.Bazzano A.T., Zeldin A.S., Diab I.R.S., Garro N.M., Allevato N.A., Lehrer D., Team W.P.O., WRC Project Oversight Team The Healthy Lifestyle Change Program: a pilot of a community-based health promotion intervention for adults with developmental disabilities. Am. J. Prev. Med. 2009;37(6) Suppl. 1:S201–S208. doi: 10.1016/j.amepre.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 66.National Health Service. The NHS Long Term Plan. https://www.longtermplan.nhs.uk/wp-content/uploads/2019/08/nhs-long-term-plan-version-1.2.pdf
- 67.Smith K., Shah A., Wright K., Lewis G. The prevalence and costs of psychiatric disorders and learning disabilities. Br. J. Psychiatry. 1995;166(1):9–18. doi: 10.1192/bjp.166.1.9. [DOI] [PubMed] [Google Scholar]
- 68.McVilly K., McGillivray J., Curtis A., Lehmann J., Morrish L., Speight J. Diabetes in people with an intellectual disability: a systematic review of prevalence, incidence and impact. Diabet. Med. 2014;31(8):897–904. doi: 10.1111/dme.12494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s website, along with the published article.