Abstract
The therapeutic potential of mesenchymal stem cells (MSCs) has been heralded by their multipotentiality and immunomodulatory capacity. MSCs migrate toward sites of tissue damage, where specific pro-inflammatory factors ‘license’ their immunosuppressive functions. Recent studies in animal models of ocular surface disease have demonstrated the potential of MSC-derived therapies to limit inflammation and promote tissue repair. Herein, we review the immunoregulatory mechanisms of MSCs, as well as strategies to harness their regenerative function at the cornea. We examine reports of the therapeutic application of MSCs in the setting of ocular surface inflammation; including corneal injury, transplantation, ocular surface autoimmunity and allergy.
1. Introduction
Mesenchymal stem cells (MSCs) are multipotent stromal cells that participate in tissue repair. Notable for their plasticity, the therapeutic potential of these cells to restore the function of damaged or diseased tissues has attracted attention for many years [1]. MSCs can be isolated from a wide variety of tissues including bone marrow, nervous tissue, adipose tissue, amniotic fluid, placenta, and Wharton's jelly of the umbilical cord [2]. Exhibiting a similar spindle-like morphology to fibroblasts, MSCs have a potent capacity for self-renewal, and can be maintained for multiple passages without significantly changing their properties [3]. MSCs can differentiate exclusively into a variety of mesenchymal lineages, including adipocytic, chondrocytic and osteocytic [1]. It has also been demonstrated that particular MSC-like cells isolated from both mice and humans can be induced into cells of neuroectodermal and endodermal lineages, such as neurons, endothelial cells and hepatocytes [4-7]. MSCs have been shown to preferentially migrate to injured or inflammatory tissues when infused intravenously [8,9]. This characteristic is particularly intriguing given that, in addition to tissue repair functions, MSCs have substantial immunoregulatory capacities that facilitate tissue repair and maintain immune homeostasis [2,10,11]. Indeed, through the release of proinflammatory factors, both the innate and adaptive immune responses have been shown to provoke MSC-mediated immunosuppression [10].
This review surveys the therapeutic function of MSCs at the ocular surface. The tissue regenerative and immunomodulatory functions of MSCs are summarized. Following this, studies investigating the therapeutic effects of MSCs in corneal injury, transplantation, ocular surface autoimmunity and allergy are examined.
2. Tissue regenerative and immunomodulatory mechanisms of mesenchymal stem cells
2.1. Tissue regeneration
The important role of MSCs in promoting tissue repair is well established [12]. In response to tissue injury, pro-inflammatory and chemotactic factors are released from stressed and necrotic cells, which increase the recruitment and activation of immune cells [13-15]. The homing of MSCs to the site of injury is promoted by pro-inflammatory mediators released both directly from damaged tissues, as well as by recruited immune cells such as macrophages [16-18]. The factors that govern these processes have not been fully delineated, partly due to the lack of reliable MSC tracing markers [10]. Nevertheless, the homing and tissue-regenerative capacities of these multipotent stromal cells have prompted substantial research investigating their translational potential.
The capacity of MSCs to promote tissue repair in osteogenesis imperfecta, a group of genetic disorders predominantly affecting the bones, has been demonstrated [19]. Furthermore, the therapeutic effect of MSCs has been exhibited in multiple experimental animal models including myocardial infarction [20], lung injury [21], kidney disease [22] and diabetes [23]. Initially, the demonstrated therapeutic efficacy of MSCs was believed to be due to their developmental plasticity. Indeed, the capacity of MSCs to differentiate across germinal boundaries in vitro is well-recognized [24]. However, in a number of these studies low levels of MSC engraftment in vivo was reported. For example, at 3 weeks following an infusion of human MSCs into immunodeficient mice with acute myocardial infarction, Iso et al. failed to detect engrafted donor cells despite observing improved cardiac function [20]. In a study using a rat model of glomerulonephritis, Kunter et al. found that 85–95% of MSCs that localized to the glomeruli on day 6 after disease induction failed to express endothelial, mesangial, or monocyte/macrophage antigens [22]. Collectively, these reports (and others) suggest that the principal mode of action of MSCs in ameliorating tissue damage might be through paracrine modulation of the tissue microenvironment rather than transdifferentiation.
The paracrine factors released by MSCs are diverse, and the tissue repair mechanisms are correspondingly complex. MSCs have been shown to release a variety of growth factors including vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), insulin-like growth factor (IGF)-1, transforming growth factor (TGF)-β, fibroblast growth factor (FGF), angiopoietin-1 and stromal cell-derived factor (SDF)-1 [25]. These trophic factors foster the proliferation of endothelial cells, fibroblasts, macrophages and other tissue-intrinsic progenitor cells, which in turn participate in tissue repair and regeneration [26]. The therapeutic effects of MSCs have been emulated using MSC-conditioned medium, highlighting the importance of paracrine factors [26-29]. As further evidence of this, co-culture of dermal fibroblasts with bone marrow-derived MSCs, without direct cell-cell contact, results in increased proliferation and accelerated migration of dermal fibroblasts in a scratch assay [30]. The demonstration in pre-clinical studies that infusion of MSC-conditioned medium yields comparable improvements in organ function relative to MSC transplantation suggests that specific MSC-associated proteins might be administered for therapeutic purposes rather than whole cell preparations. However, given that MSC-associated factors remain partly unidentified and their potential synergisms undefined, the transplantation of stem cells for their paracrine tissue regenerative effects remains a viable therapeutic option [31].
2.2. Immunomodulation
The finding that mesenchymal stem cells contribute to tissue regeneration through paracrine modulation of the inflammatory microenvironment has provoked intense investigation of the cellular and molecular mechanisms by which MSCs interact with the immune system [32,33]. There is evidence that the immunosuppressive capacity of MSCs is not constitutive, but rather is ‘licensed’ by inflammatory cytokines [34,35]. This important finding reconciled two apparently conflicting findings regarding the efficacy of MSCs in treating graft versus host disease (GvHD). Some earlier reports had demonstrated MSC-mediated inhibition of the immune response in vivo in steroid-resistant, severe, acute GvHD; yet others did not observe improvements in rescue from GvHD despite showing in vitro suppression of lymphocyte proliferation by MSCs [34,36,37]. According to the paradigm proposed by Ren et al., MSCs home to inflamed microenvironments where, upon arrival, their immunosuppressive function is induced [34]. Thus, the MSC immunoregulatory function is understood as tunable; it is amplified in response to high concentrations of specific inflammatory cytokines, and dampened in their absence. Given that the local cytokine milieu varies considerably during disease progression, the immunoregulatory role of MSCs may fluctuate accordingly.
MSCs inhibit the function of a variety of cells including macrophages, neutrophils, natural killer (NK) cells, dendritic cells (DCs), B lymphocytes and T lymphocytes (Fig. 1) [32]. For example, both in vivo and in vitro, MSCs have been shown to induce IL-10-secreting monocytes and/or macrophages [38]. MSCs are known to inhibit IL-2-induced NK cell proliferation, and have been shown to alter the phenotype of NK cells and suppress NK cell cytokine secretion both via cell-to-cell contact and with soluble factors (e.g. TGF-β and prostaglandin E2 [PGE2]) [39,40]. MSCs have been demonstrated to suppress monocyte differentiation into DCs, and restrict the maturation of DCs by limiting the expression of MHCII, CD1-α, CD40, CD80 and CD86 [41]. The inhibition of T cell proliferation by MSCs is well-established [2,32,33]. In particular, MSCs have been shown to suppress the differentiation of CD4+ T cells into the proinflammatory Th1 and Th17 lineages, and promote the generation of CD4+CD25+Foxp3+ regulatory T cells (Tregs) [42]. Expansion of Tregs also occurs due to MSC-triggered T-cell apoptosis, which results in the release of TGF-β from macrophages thus driving Treg proliferation [43]. Given the diverse immunoregulatory properties of MSCs, and their strong translational potential for the treatment of inflammatory disorders, delineating the mechanisms by which MSCs modulate the immune response is a subject of lively research.
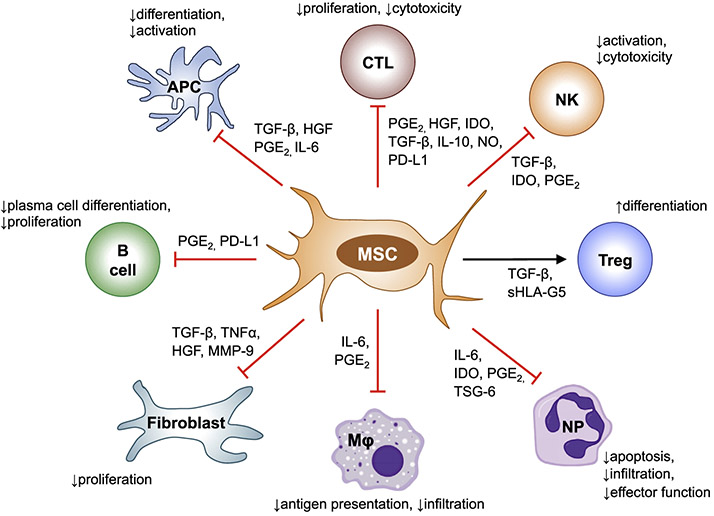
Fig. 1. Immunomodulatory functions of MSCs.
MSCs regulate the immune response via both cell-cell contact and soluble factors. MSCs inhibit the proliferation and function of T cells, limit activation of NK cells, suppress APC differentiation and activation, inhibit B cell proliferation and differentiation into plasma cells, reduce the antigen presenting capacity of macrophages and decrease neutrophil apoptosis, infiltration and effector function. MSC also induce Treg differentiation. Abbreviations: CTL, cytotoxic T cell; NK, NK cell; Treg, regulatory T cell; NP, neutrophil; Mφ, macrophage; APC, antigen-presenting cell; PGE-2, prostaglandin E2; TSG-6, tumor necrosis factor-inducible gene 6 protein; HGF, hepatocyte growth factor; IDO, indoleamine 2,3-dioxygenase; TGF-β, transforming growth factor-β; IL, interleukin; NO, nitric oxide; PD-L1, programmed death ligand 1; SHLA-G5, soluble human leukocyte antigen-G; TNFα, tumor necrosis factor α; MMP-9, matrix metallo-peptidase 9.
A wide variety of secreted factors have been implicated in the immunoregulatory function of MSCs, including tumor necrosis factor-inducible gene 6 protein (TSG-6), PGE2 and interleukin-1 receptor antagonist (IL-1Ra), TGF-β, nitric oxide (NO) and indoleamine 2,3-dioxygenase (IDO) [10,21,38,44-47]. One hypothesis proposes that, having been ‘licensed’ to modulate the immune response by stimulation with IFN-γ and the concomitant presence of proinflammatory cytokines such as IL-1α, IL-1β or TNFα, MSCs secrete the immunomodulatory factors listed above [2,34]. MSCs also express a range of adhesion molecules and chemokines, including intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), CXCR3 ligands and CCR5 ligands [34,48]. These molecules recruit immune cells into close proximity with MSCs, thereby orchestrating a microenvironment in which the effects of local immunosuppressive factors are amplified.
The anti-inflammatory protein TSG-6 has been shown to reduce the inflammatory response and decrease infarct size in a murine model of myocardial infarction [23]. Indeed, Lee et al. demonstrated that knockdown of TSG-6 abrogated the observed therapeutic effects of i.v. injection of MSCs [23]. PGE2 has been demonstrated to suppress T cell proliferation, and is markedly upregulated after co-culture of MSCs with peripheral blood mononuclear cells [49,50]. In a murine model of bleomycin-induced lung injury, MSCs have been reported to exert anti-inflammatory and anti-fibrotic effects via secreted IL-1Ra [21]. The suppression of allergic responses by MSC-derived TGFβ has been demonstrated in a murine model of ragweed-induced asthma [45]. Ren et al. established that the expression of inducible nitric oxide synthase (iNOS) by MSCs was substantially upregulated by exposure to proinflammatory cytokines, and showed that wild-type MSCs, but not iNOS (− / − ) MSCs prevented graft-versus host disease in a murine model [34]. The importance of NO in MSC-mediated immunoregulation was substantiated by the observation that iNOS (−/ −) MSCs have a compromised capacity to reduce inflammation in a murine model of experimental arthritis relative to wild-type MSCs [51]. The enzyme IDO catalyzes the conversion of tryptophan to kynurenine, thereby limiting the proliferation of activated T and NK cells [52]. The expression of IDO by MSCs has been shown to be induced by IFN-γ [53,54]. Interestingly, it has been proposed that the mechanisms by which MSCs exert their immunoregulatory capacity varies among different species, with IDO being the predominant mediator of human-derived MSCs, compared to mouse-derived MSCs which chiefly utilize NO [35].
In light of the ‘tunable’ characteristics of MSCs, and the plethora of immunoregulatory factors that they express, defining the exact biological mechanisms by which MSCs act remains a priority in this research field. The immunomodulatory activity of MSCs is known to be contingent on their origin, their microenvironment and their target cells. With this in mind, we turn our attention to the therapeutic potential of MSCs at the ocular surface – specifically in the context of injury, transplantation, autoimmunity and allergy.
3. Therapeutic potential of mesenchymal stem cells at the ocular surface
Corneal clarity is essential for vision. Excessive ocular surface inflammation results in opacification of the cornea, and is potentially blinding. Thus, immune homeostasis at the cornea constantly balances two opposing objectives – to limit excessive inflammation, whilst protecting the eye against microbial disease. The relatively subdued ocular inflammatory response to foreign antigenic material has led to the eye being defined as an immune-privileged site [55]. The impetus for understanding the role of MSCs at the cornea is based on two translational objectives; firstly, modulating the immune response (thereby limiting the progression of corneal inflammatory disease), and secondly, the regeneration of corneal tissue (both in vivo regeneration and the synthesis of tissue engineered corneal substitutes).
3.1. Wound healing following corneal injury
Ocular trauma is a major cause of corneal blindness, with an estimated 23 million people suffering low vision or blindness due to injuries globally [56]. Loss of corneal clarity results from an inflammatory milieu inciting the differentiation of transparent corneal fibroblasts into opaque myofibroblasts, which in turn produce disorganized extracellular matrix [57]. Given the considerable burden of corneal blindness, the targeted immunoregulatory and tissue regenerative capacities of MSCs have attracted attention as a potential therapeutic (see Table 1). Lan et al. have established the propensity of MSCs to home to damaged corneal tissue [58]. Using thermal cauterization to induce corneal injury in a murine model, the investigators demonstrated that endogenous MSCs are mobilized into blood following corneal injury, and that exogenously administered MSCs home specifically to the injured cornea where they promote tissue regeneration [58]. By tracking intravenously injected ex vivo expanded red Q-dot-labeled or GFP+ bone marrow-derived MSCs with epifluorescence microscopy, MSCs were demonstrated to home to the injured cornea but not to the normal cornea. Importantly, the authors established long-term survival of these labeled MSCs, with MSCs remaining detectable in the cornea at 50 days following injury [58]. In contrast with this observation, Roddy et al. attribute the anti-inflammatory activity of MSCs to the paracrine effect of MSC-derived TSG-6, reporting that less than 10 human MSCs (hMSCs) were observed in injured rat corneas at 1 day or 3 days after i.v. or i.p. administration of 1 × 107 hMSCs in a rat corneal injury model [59]. These conflicting findings may in part be attributed to the potential issues of integration and engraftment when cells are transferred across the xeno-species barrier, with the use of human MSCs in a rat model of corneal injury. Indeed, the specific homing of murine MSCs has been corroborated by Omoto et al. in a murine model of corneal transplantation, in which MSCs were found in abundance at the transplanted cornea and ipsilateral conjunctiva and lymph nodes, but not in contralateral tissues at 3 days post transplantation [60].
Table 1.
Models used and details of MSC administration in studies of wound healing following corneal injury.
| Author | Model | Tissue source of MSCs | Route of administration | Number of MSCs/animal |
|---|---|---|---|---|
| Lan et al., 2012 [58] | Thermal cauterization (mouse): cautery of half of the cornea and limbus; mechanical debridement of dead epithelium with surgical blade; rinsing with 0.9% NaCl | Murine bone marrow | Intravenous | 5 × 105 cells in 100 μL 0.9% NaCl |
| Roddy et al., 2011 [59] | Chemical injury (rat): absolute ethanol applied to corneal and limbal surface for 30s; rinsed with balanced salt solution; mechanical debridement of corneal and limbal epithelium with surgical blade | Human MSCs (from Center for the Preparation and Distribution of Adult Stem Cells) | Intravenous or intraperitoneal | 1 × 107 cells in 1 mL HBSS |
| Mittal et al., 2016 [61] | Mechanical injury (mouse): removal of 3 mm diameter area of central corneal epithelium and anterior stroma using a hand-held Algerbrush II (Alger Equipment) | Murine bone marrow | Intravenous | 5 × 105 cells in 100 μL normal saline |
| Hertsenberg et al., 2017 [64] | Mechanical injury (mouse): removal of 2 mm diameter area of central corneal epithelium and 10–15 μm of anterior stroma using a hand-held Algerbrush II (Alger Equipment) | Human limbus (from cornea-scleral rims) | Suspended in fibrin gels grafted onto cornea | 5 × 104 cells |
| Oh et al., 2008 [65] | Chemical injury (rat): absolute ethanol applied to corneal and limbal surface for 30s; rinsed with balanced salt solution; mechanical debridement of corneal and limbal epithelium with surgical blade | Rats primary MSC lines (from Millipore) | Topical | 2 × 106 cells in 200 μL media |
| Mittal et al., 2018 [66] | Mechanical injury (mouse): removal of 3 mm diameter area of central corneal epithelium and anterior stroma using a hand-held Algerbrush II (Alger Equipment) | Murine bone marrow | Intravenous | 5 × 105 cells in 100 μL normal saline |
| Amouzegar et al., 2017 [67] | Mechanical injury (mouse): removal of 3 mm diameter area of central corneal epithelium and anterior stroma using a hand-held Algerbrush II (Alger Equipment) | Murine bone marrow | Intravenous | 5 × 105 cells in 100 μL normal saline |
| Basu et al., 2014 [68] | Mechanical injury (mouse): removal of 2 mm diameter area of central corneal epithelium and 10–15 μm of anterior stroma using a hand-held Algerbrush II (Alger Equipment) | Human limbus (from cornea-scleral rims) | Suspended in fibrin gels grafted onto cornea | 5 × 104 cells |
| Yao et al., 2012 [71] | Chemical injury (rat): filter paper soaked in 4 μL NaOH (1 mol/L) applied to cornea for 40s; rinsed with 60 ml 0.9% NaCL for 60 s | Rat bone marrow | Subconjunctival injection | 2 × 106 cells in 100 μL PBS |
| Gu et al., 2009 [72] | Chemical injury (rabbit): filter paper soaked in 1 N NaOH applied to cornea for 30s; rinsed with 0.9% NaCL | Rabbit bone marrow | Suspended in fibrin gels grafted onto cornea | Not specified |
| Jiang et al., 2010 [73] | Chemical injury (rat): filter paper soaked in 0.5 mol/L NaOH placed on the corneal limbus for 20s; rinsed with 0.9% NaCL for 1 min | Rat bone marrow | Seeded onto amniotic membrane; grafted onto cornea | Not specified |
| Ma et al., 2009 [75] | Chemical injury (rat): filter paper soaked in 1 N NaOH applied to cornea for 30s; rinsed with 0.9% NaCL; mechanical debridement of corneal and limbal epithelium with surgical blade | Rat bone marrow | Seeded onto amniotic membrane; grafted onto cornea | Not specified |
| Galindo et al., 2017 [76] | Mechanical injury model of limbal stem cell deficiency (rabbit): either 180° or 360° limbal peritomy by surgical crescent knife | Human adipose tissue | Expanded on amniotic membrane; grafted onto cornea | 2.5 × 105 cells in 250 μL media seeded onto amniotic membrane |
The therapeutic potential of MSCs in corneal injury has been demonstrated by Mittal et al., who established that MSCs have the capacity to restore corneal transparency by secreting high levels of HGF following corneal injury [61]. Using a murine model of mechanical corneal injury, the investigators showed that an inflammatory milieu promotes the secretion of HGF by MSCs, and furthermore demonstrated that the capacity of MSCs to restore corneal transparency is dependent on their HGF expression. These observations are consistent with reports describing the anti-fibrotic and anti-inflammatory capacities of HGF [62,63]. Notably, Mittal et al. demonstrated that topical administration of HGF alone (without MSC administration) restored corneal transparency after injury, thereby identifying an important paracrine mechanism by which MSCs contribute to the repair of damaged ocular tissues [61]. Further studies have highlighted the importance of MSC paracrine pathways at the cornea. Using a murine model of mechanical corneal injury, Hertsenberg et al. demonstrated that application of fibrin gel containing stem cells derived from human corneal stroma reduced the infiltration of CD11b+/Ly6G+ neutrophils and myeloperoxidase expression following tissue debridement [64]. Moreover, the authors demonstrate that knockdown of TSG-6 using small interfering RNA (siRNA) decreased the capacity of stem cells to inhibit neutrophil infiltration and reduce scarring, indicating that paracrine secretion of TSG-6 contributes to the immunomodulatory and anti-fibrotic effects of MSCs [64]. Employing a chemical burn model of corneal injury in rats, Oh et al. reported decreased corneal inflammation and neovascularization in mice treated with either topical MSCs or conditioned media derived from MSC culture [65]. Treated mice exhibited decreased corneal infiltration of CD4+ cells, decreased expression of IL-2 and IFN-γ and increased IL-10, TGF-β1 and IL-6. Notably, although both MSCs and conditioned media derived from MSC culture were effective in reducing corneal inflammation, the effect was amplified in the MSC treatment group, suggesting that cell-cell contact may exert additive effects on the paracrine immunomodulatory effects of MSCs in this setting. A study by Mittal et al. provides further evidence for the importance of cell-cell contact in MSC immunoregulation, as co-culture of MSCs and neutrophils was observed to suppress neutrophil expression of the tissue-damaging enzymes myeloperoxidase and N-elastase, but this phenomenon was not observed when a transwell system was used [66]. Using a murine model of mechanical corneal injury, the investigators demonstrated that treatment of injured mice with MSCs results in decreased myeloperoxidase expression, lower neutrophil frequencies at ocular surface tissues and normalization of corneal tissue architecture compared to untreated injured controls [66]. These results indicate that MSCs suppress neutrophil effector functions by direct cell-cell contact mechanisms during corneal inflammation, thereby limiting tissue damage.
One mechanism by which direct MSC cell-cell contact regulates inflammation following corneal injury has been elucidated by Amouzegar et al. [67]. Using a murine model to examine the immunomodulatory effects of MSCs on myeloid progenitor cells, the investigators demonstrated that mice that received adoptive transfer of MSCs following sterile corneal injury exhibited increased corneal myeloid progenitor cell frequencies and reduced corneal infiltration of inflammatory cells. The authors showed that the inhibitory effect of MSCs on myeloid progenitor differentiation relies on direct cell-cell contact, mediated via interaction of MSC-expressed CD200 and myeloid progenitor-expressed CD200R1 [67].
The therapeutic potential of MSCs to reduce corneal scarring has been further evaluated by Basu et al., who expanded mesenchymal cells from limbal biopsies of human cadaveric cornea-scleral rims (limbal biopsy-derived stromal cells; LBSCs), and engrafted these cells into murine corneas following stromal injury [68]. At the time of mechanical debridement of epithelium and stroma, the investigators applied 50,000 LBSCs to the wounded cornea in a solution of fibrinogen. Although the number of LBSCs in the cornea decreased over time, at 4 weeks, half of the engrafted LBSCs remained in situ. The authors report that LBSCs decreased the formation of light-scattering scar tissue and promoted regeneration of the ablated stroma, thereby demonstrating the potential of autologous stem cell-based approaches for the treatment of corneal opacification [68]. Interestingly, in addition to injury-induced corneal opacification, the capacity of MSCs to restore stromal transparency has also been demonstrated in Lumican-null mice [69]. Lumican-null mice have cloudy corneas due to large collagen fibril aggregates and disorganized stromal architecture [70]. Du et al. demonstrated that injection of 50,000 stem cells isolated from adult human corneal stroma into the corneas of Lumican-null mice restored stromal thickness and haze to levels comparable to wild-type mice [69]. Following injection of human corneal stromal stem cells, the investigators observed Lumican distributed throughout the posterior stroma of Lumican-null mice, and at twelve weeks post-injection regular collagen architecture was observed without fibril aggregates, similar to wild-type noninjected mice [69].
Other studies of corneal injury have been used to investigate the propensity of MSCs to promote wound healing. Yao et al. have reported that subconjunctival injection of MSCs following corneal alkali burn in a rat model significantly accelerates corneal epithelial repair and reduces corneal infiltration of inflammatory cells [71]. Gu et al. used BrdU-labeled rabbit MSCs suspended in fibrin gels and transplanted onto injured rabbit corneas to evaluate the role of MSCs in corneal epithelial healing [72]. The authors report that, in vivo, BrdU-labeled rabbit MSCs participated in the healing of the injured cornea and expressed cytokeratin 3 (CK3; a protein expressed by corneal epithelium). Jiang et al. have demonstrated that rat bone marrow-derived MSCs induced by co-culture with corneal stromal cells in a transwell system expressed cytokeratin 12, with scanning electron microscopy of these cells showing typical epithelial characteristics [73]. When seeded onto amniotic membrane and grafted onto rat corneas in a model of alkali injury-induced limbal stem cell deficiency, these epithelial-like cells were observed to significantly accelerate corneal epithelial repair and decrease corneal opacity [73]. Recently, Samaeekia et al. have investigated the contribution of MSC-derived exosomes to corneal epithelial wound healing [74]. The authors collected the secretome of MSCs isolated from human cadaver corneas, and employed differential ultracentrifugation to isolate exosomes. Utilizing both an in vitro scratch assay and an in vivo model of mechanical corneal epithelial debridement, MSC-derived exosomes were observed to significantly accelerate wound healing relative to controls [74]. These observations indicate that the bioactive molecules contained in MSC-derived exosomes play an important role in promoting corneal epithelial wound repair, yet further work is required to determine their constituent factors and respective mechanisms of action.
Various groups of investigators have suggested that the therapeutic effect of MSCs at the injured ocular surface may primarily be due to their suppressing inflammation and angiogenesis rather than epithelial transdifferentiation [75,76]. After growth and expansion on amniotic membrane, Ma et al. transplanted human MSCs onto chemically-injured rat corneas by the suturing amniotic membranes in place [75]. The authors report that transplantation of MSCs successfully reconstructed the injured cornea, but immunofluorescent studies revealed that cytokeratin 3 was not expressed in rat epithelium transplanted with MSCs. This finding implies that the therapeutic effect of MSCs may not be due to MSC transdifferentiation into epithelial cells. Rather, the authors found that expression of CD45, interleukin 2 and matrix metalloproteinase-2 was significantly decreased in eyes transplanted with MSCs, suggesting that suppression of inflammation and inflammation-related angiogenesis may partially account for the observed reconstruction of MSC-treated ocular surfaces [75]. Indeed, there is growing consensus that rather than differentiating into corneal epithelial cells, MSCs foster a microenvironment (by reducing neovascularization and inflammation) that upregulates the proliferation and differentiation of resident stem cells [75-78]. It is important to note that CK3 is not highly specific for differentiated corneal epithelium, and furthermore that MSCs themselves have been shown to express CK3 in normal culture conditions [79,80]. Therefore, it is possible that the scarce studies [72,81] that report expression of CK3 by transplanted MSCs in corneal epithelium in vivo may not be observing true transdifferentiation. This paradigm is in agreement with findings from Galindo et al., who investigated the therapeutic effect of human adipose tissue-derived MSCs (hAT-MSCs) in a rabbit model of limbal stem cell deficiency (LSCD) [76]. In this model, a surgical limbal peritomy (either 180° [partial] or 360° [total]) was performed to remove the limbal niche. The authors report that hAT-MSCs transplanted to the ocular surface of rabbits following peritomy migrated to inflamed tissues; with decreased inflammation, reduced corneal neovascularization and diminished corneal opacification observed [76]. Galindo et al. did not observe evidence of transdifferentiation of MSCs, but propose that hAT-MSCs promote the recovery of the corneal epithelium by secreting factors that facilitate the proliferation and differentiation of residual limbal epithelial stem cells [76].
3.2. Inhibition of angiogenesis following corneal injury
Eslani et al. have examined the antiangiogenic properties of cornea-derived mesenchymal stem cells (cMSCs) both in vitro and using a mouse model of corneal injury [82]. The investigators demonstrated that cMSCs derived from a variety of sources (both human cadavers and wild-type mice) expressed high levels of antiangiogenic factors (notably soluble fms-like tyrosine kinase-1 [sFLT-1] and pigment epithelium-derived growth factor [PEDF]) and low levels of the angiogenic factor VEGF-A. In murine ocular injury experiments involving total corneal epithelial debridement using an Algerbrush, cMSCs embedded in fibrin gel were shown to limit corneal neovascularization when applied to mouse corneas following injury. Notably, this effect was significantly reduced when either sFLT-1 or PEDF were removed from the secretome [82]. In a subsequent paper employing the same corneal injury model, the group demonstrated that cMSCs can modulate the phenotype and angiogenic function of macrophages [83]. In vitro, the investigators observed that cMSCs simultaneously induce macrophage apoptosis and promote an immunophenotype (CD14hiCD16hiCD163hiCD206hi) with significantly decreased angiogenic function [83]. Macrophages cocultured with cMSCs were shown to express higher levels of anti-angiogenic and anti-inflammatory factors, as compared to control. Moreover, when these cocultured macrophages were administered topically to injured mouse corneas via fibrin gels, significantly less neovascularization was observed [83]. Taken together, these studies describe both direct and indirect (via macrophages) antiangiogenic functions of cornea-derived MSCs, and implicate sFLT-1 and PEDF as critical factors mediating these phenomena [82,83].
3.3. Corneal transplantation
Globally, over 180,000 corneal transplants are performed each year, making corneal transplantation the most frequent form of tissue grafting [84]. In low-risk corneal transplants, in which host beds are non-vascularized and uninflamed, 5-year graft survival rates surpass 90% [85]. However, in patients with a history of graft rejection, or with vascularized and inflamed host beds, more than 50% of grafts fail [86,87]. In view of these high rejection rates, there is a substantial clinical need for novel immunomodulatory approaches to promote corneal allograft survival.
Omoto et al. have investigated the function of systemically injected MSCs in corneal transplantation [60]. The investigators generated MSCs from the bone marrow of wild-type BALB/c or GFP+ C57BL/6 mice, and intravenously injected these cells into allograft recipients at 3 h following surgery. GFP+ MSCs were identified in the transplanted cornea and ipsilateral conjunctiva and lymph nodes, but were not present in the contralateral (ungrafted) tissues [60]. Treatment with MSCs limited allosensitization, with significantly reduced frequencies of mature MHCII+CD11c+ APCs in the draining lymph nodes relative to control recipients. A concomitant reduction in IFN-γ+ Th1 effector cells was observed in the MSC-injected allograft recipients compared to the control group. Finally, MSC-injected allograft recipients exhibited prolonged allograft survival relative to control allograft recipients [60]. As with the studies of corneal injury described previously, controversy exists regarding the homing of MSCs to the graft site. Oh et al. used human MSCs (in contrast with mouse MSCs used by Omoto et al.) in a murine model of corneal transplantation [60,88]. In their study, the investigators corroborate the prolongation of corneal allograft survival following intravenous infusion of MSCs. However, Oh et al. report that MSCs do not home to the graft site but are trapped in the lungs, and exert their immunomodulatory effects through expression of TSG-6 [88]. As with the previous discussion of corneal injury, these incompatible accounts may be related to the cross-species transplantation of human MSCs.
The capacity of MSCs to promote corneal allograft survival has been further substantiated by Jia et al., who investigated how systemic administration of MSCs modulates the immune response in a rat model of corneal transplantation [89]. The authors report that in addition to suppressing the Th1 pro-inflammatory response, treatment with MSCs significantly upregulated Tregs with increased frequencies of lymph node and splenic CD4+CD25+Foxp3+ Tregs and augmented Foxp3 mRNA expression compared to vehicle-treated controls [89]. These alterations to the effector and regulatory arms of the immune response corresponded to prolonged graft survival time in rats that received postoperative injection of MSCs relative to controls. The expansion of splenic CD4+Foxp3+ regulatory T cells in MSC-treated animals in a rat model of corneal transplantation has also been reported by Treacy et al. [90]. In their study, the investigators evaluated the ability of MSCs from three different sources to promote allograft survival. A fully allogeneic transplantation model was used, with Lewis rat recipients and Dark Agouti donors. Recipient rats were treated intravenously with MSCs from Lewis rats (syn), Dark Agouti rats (allo) or Wistar Furth (third party). Corneal allograft survival was prolonged in allo-MSC treated and third-party MSC treated allograft recipients, with 90% and 80% survival respectively at 30 days following transplantation. In comparison, 80% of grafts in untreated recipients were rejected, and intriguingly, 100% of grafts in syn-MSC treated allograft recipients were rejected. In addition to expanded splenic CD4+Foxp3+ Tregs in the allo- and third-party MSC treated animals, the authors report diminished corneal infiltration of natural killer T cells in these groups. Collectively, these studies suggest that MSCs are a viable strategy for promoting corneal allograft survival (see Table 2).
Table 2.
Models used and details of MSC administration in studies of corneal transplantation.
| Author | Model | Tissue source of MSCs | Route of administration | Number of MSCs/animal |
|---|---|---|---|---|
| Omoto et al., 2014 [60] | Orthotopic allogeneic corneal transplantation (mouse): 2 mm diameter corneal cup from donor (C57BL/6) grafted onto 1.5 mm diameter prepared host bed (BALB/c) using 8 interrupted nylon 11-0 sutures | Murine bone marrow (BALB/c) | Intravenous | 106 cells in 100 μL 0.9% NaCl |
| Oh et al., 2012 [88] | Orthotopic allogeneic corneal transplantation (mouse): 2 mm diameter corneal cup from donor (C57BL/6) grafted onto 1.5 mm diameter prepared host bed (BALB/c using 8 interrupted nylon 11-0 sutures | Human MSCs (from Center for the Preparation and Distribution of Adult Stem Cells) | Intravenous | 106 cells in 100 μL HBSS |
| Jia et al., 2012 [89] | Orthotopic allogeneic corneal transplantation (rat): 3.5 mm diameter corneal cup from donor (Wistar) grafted onto a 3 mm diameter prepared host bed (Lewis) using 8 interrupted nylon 10-0 sutures | Rat bone marrow (Wistar) | Intravenous | 5 × 106 cells in 1 ml PBS |
| Treacy et al., 2014 [90] | Orthotopic allogeneic corneal transplantation (rat): 3 mm diameter corneal cup from donor (Dark Agouti) grafted onto a 2.5 mm diameter prepared host bed (Lewis) using 8–10 interrupted nylon 10-0 sutures | Rat bone marrow (Lewis, Dark Agouti or Wistar rats) | Intravenous | PBS 106 cells in 1 ml PBS |
3.4. Dry eye disease
Dry eye disease (DED) is a highly prevalent disorder of the ocular surface, with more than 16 million US adults estimated to have been diagnosed [91]. DED may occur in isolation, or in association with systemic autoimmune disease such as Sjögren's syndrome. Although the pathogenesis of DED is not fully understood, it has been established that inflammation and the failure of ocular surface immunoregulatory mechanisms are critically implicated in disease progression [92,93]. Noting the capacity of MSCs to promote tissue repair by curbing inflammation, investigators have recently studied the therapeutic potential of MSCs in the setting of DED.
Using a murine model of DED induced by intraorbital injection of concanavalin A, Lee et al. demonstrated that concurrent periorbital injection of 105 human or murine bone marrow-derived MSCs into the periorbital space results in significantly decreased corneal epitheliopathy relative to control-treated mice [94]. Furthermore, mice treated with periorbital MSCs exhibited reduced infiltration of CD4+ T cells into the intraorbital gland and ocular surface, and decreased levels of the pro-inflammatory T-cell-derived cytokines IL-2 and IFN-γ [94]. In contrast with reports detailing MSC-mediated expansion of Tregs, the investigators did not observe an effect of the administration of periorbital MSCs on Treg frequencies [42,43,89,90]. Consistent with the integrity of the corneal epithelia, Lee et al. observed that mice treated with periorbital MSCs had significantly greater aqueous tear production and increased numbers of conjunctival goblet cells relative to controls [94]. Improved tear production by MSC-treated mice has also been reported by Aluri et al. in a NOD (NOR/LtJ) mouse model of Sjögren's syndrome dry eye [95]. In this study, NOD mice were randomized to receive intraperitoneal injection of 106 murine bone marrow-derived MSCs or sterile phosphate-buffered saline (PBS). Over the ensuing 4 weeks, MSC-treated mice exhibited increased tear production compared to both baseline and PBS control. Following sacrifice of the mice at 4 weeks, lacrimal gland examination with hematoxylin and eosin demonstrated no difference in the number of lymphocytic foci between the two groups of mice. However, a significant decrease (> 40%) in the size of the foci was observed in MSC-treated mice compared to control animals [95]. The investigators also report augmented mRNA expression of the water channel aquaporin 5 in the MSC-treated group relative to control in lacrimal glands. Although the immunoregulatory mechanisms of MSCs in DED have not been clearly delineated, preliminary reports suggest that MSCs may protect against autoimmune ocular surface inflammation.
3.5. Allergic conjunctivitis
Ocular allergy is estimated to affect 15–20% of the US population [96]. In acute disease, ocular surface inflammation produces symptoms of itching, epiphora, lid and conjunctival edema as well as erythema [97]. Chronic inflammation compounds these symptoms, with severe discomfort and remodeling of the ocular surface. Although effective in dampening the inflammatory response, corticosteroids are associated with a plethora of side-effects including cataracts, glaucoma and corneal melt. Given the burden of this common condition, there is a marked clinical need for novel therapeutic approaches to the treatment of allergic conjunctivitis.
Ocular surface inflammation in allergic conjunctivitis results from the cross-linkage of membrane-bound IgE in response to allergen exposure, which provokes mast cell degranulation and the release of copious allergic and inflammatory factors [97]. Using a murine short ragweed pollen-induced model of experimental allergic conjunctivitis, Su et al. demonstrated that topical administration of culture medium from TNF-α-stimulated, murine bone marrow-derived MSCs (MSC-CMT) was effective in decreasing IgE production, histamine release, enrichment and activation of mast cells as well as conjunctival vascular hyperpermeability relative to PBS control [98]. Importantly, treatment with MSC-CMT reduced clinical inflammation (scored by slit lamp assessment of conjunctival edema, lid swelling tearing and conjunctival redness) compared to control. Histological examination of conjunctival tissue harvested 14 days following immunization exhibited reduced infiltration of inflammatory cells and eosinophil accumulation in the MSC-CMT treatment group relative to the control group. Moreover, analysis of conjunctival tissue using real-time PCR and ELISA revealed heightened expression of the inflammatory cytokines TNF-α and IL-4 in mice with experimental allergic conjunctivitis relative to naïve control, but this effect was diminished by treatment with MSC-CMT. The investigators report that treatment with MSC-CMT significantly decreased the expression of nuclear factor κB (NF-κB), an important regulator of ocular surface inflammation. Finally, Su et al. describe the abrogation of the therapeutic effects of MSC-CMT in experimental allergic conjunctivitis when MSCs were pretreated with COX2 small interfering RNA, suggesting that MSC-CMT inhibits experimental allergic conjunctivitis through COX-2 dependent mechanisms [98]. Further work is required to define the exact mechanisms by which TNF-α-stimulated, bone marrow-derived mesenchymal stem cells exert their antiallergic effects, but the work of Su et al. provides compelling evidence that MSCs have therapeutic value in this setting.
3.6. Lysosomal storage disorders
Mucopolysaccharidosis VII (MPS VII) is a rare, congenital lysosomal storage disorder resulting from a deficiency in the enzyme β-glucuronidase that results in the accumulation of glycosaminoglycans in various tissues throughout the body, including the cornea where it manifests clinically as opacification [99]. Using a mouse model of MPS VII, Coulson-Thomas et al. have demonstrated that human umbilical MSCs delivered via intrastromal injection into the cornea upregulate extracellular glycosaminoglycan turnover and promote the catabolism of accumulated glycosaminoglycan products by keratocytes. 104 umbilical MSCs were injected into the anterior corneal stroma, pre-labeled with DiI to permit visualization of the cells using a fluorescent stereo microscope [99]. The investigators showed via in vivo confocal microscopy that injection of human umbilical MSCs restored the morphology of keratocytes and endothelial cells, and improved corneal transparency [99]. Importantly, umbilical MSCs were observed to be present in the corneal stroma at one month post-injection, with the adoption of a keratocyte-like morphology and expression of a keratocyte cell marker by MSCs [100]. This study suggests that MSCs may provide a viable therapeutic approach to reduce corneal clouding in congenital metabolic disorders.
3.7. Ocular graft-versus-host disease
Graft-versus-host disease (GVHD) is a major cause of morbidity and mortality for patients following allogeneic hematopoietic stem cell transplantation [101,102]. GVHD results from donor-derived T cells recognizing host antigens as foreign, with the resultant immune response promoting inflammation in multiple organs including the mouth, respiratory tract, gastrointestinal tract and skin [101,102]. Approximately 40–60% of patients undergoing allogeneic hematopoietic stem cell transplantation develop ocular GVHD, which causes inflammation of the ocular surface and lacrimal glands, as well as cicatricial scarring of the meibomian glands [103]. Martínez-Carrasco and colleagues have utilized a murine model of GVHD (in which hematopoietic stem cell transplantation is performed between MHC-mismatched mouse strains) to evaluate the therapeutic effect of subconjunctival administration of MSCs on corneal inflammation and squamous metaplasia [104]. The authors reported that GVHD increased infiltration of CD3+ T cells into the cornea, yet this effect was abrogated following treatment with MSCs. Tear osmolarity was also assessed, which revealed increased osmolarity in GVHD mice relative to control, but not in MSC-treated animals [104]. Paired box gene 6 (Pax6) encodes a transcription factor that controls eye morphogenesis, and there is evidence that Pax6 contributes to ocular surface homeostasis postnatally by preventing the initiation and development of keratinizing squamous metaplasia [105,106]. Intriguingly, Martínez-Carrasco and colleagues found that subconjunctival administration of MSCs decreased Pax6 expression in GVHD mice, suggesting that in addition to limiting ocular surface inflammation, MSCs may also inhibit squamous metaplasia in ocular GVHD [104].
4. Concluding remarks, outstanding questions and future directions
MSCs have considerable tissue regenerative and immunomodulatory capacities. Our burgeoning understanding of the ‘tunable’ characteristics of MSCs, and the molecular mechanisms by which they function, continues to highlight the therapeutic potential of these cells. This review has summarized recent studies evaluating MSC-derived therapies in corneal injury, corneal transplantation, dry eye disease, allergic conjunctivitis, congenital metabolic disorders and ocular GVHD.
Further investigation of the precise mechanisms of action and the long-term safety profile of MSCs is crucial if their translational potential is to be realized. Key questions remain unanswered. Firstly, although there is evidence for the therapeutic benefit of MSCs themselves in various ocular surface pathologies, the relative contribution of cell-cell contact vs. paracrine soluble factors in each of these pathologies remains unclear. For example, there is evidence that MSCs suppress differentiation of myeloid progenitor cells into pro-inflammatory CD11b+ mature myeloid immune cells in a contact-dependent manner via CD200-CD200R1 interaction [67], and it has also been reported that MSCs reduce inflammation at the cornea following chemical injury primarily by secretion of TSG-6 [59]. More experimental data are needed to determine the exclusivity of previously reported MSC immunoregulatory mechanisms, and to identify other cell-cell interactions and secreted soluble factors that may be driving the observed phenomena. Another important area of investigation concerns determining the effect of ocular surface inflammation on MSC immunomodulation and tissue repair functions, and specifically the extent to which pro-inflammatory cytokines associated with the adaptive immune response (for instance IL17A in dry eye disease [107]) license the activity of MSCs at the ocular surface. Finally, there is considerable variation in the route of MSC administration employed by researchers in studies of ocular surface inflammation; with some groups delivering MSCs intravenously [58,59,61,66,67], some topically [64,65,73,76], and others via intraperitoneal (IP) injection [59] or subconjunctivally [71]. The comparative therapeutic efficacy of these routes of MSC administration is another question that remains unanswered.
The substantial immunoregulatory and tissue reparative functions of MSCs in the setting of ocular surface disease have been clearly demonstrated. At present the translational potential of MSC-based therapeutics is constrained by the challenges of manufacturing clinical grade products and the considerable expense (both resources and time) of meeting standards for regulatory approval. The future direction of work in this field is likely to involve the standardization of MSC-based therapies employed in studies of ocular surface disease (including cells, supernatants and exosomes), which will facilitate comparison of results between researchers in basic science studies, and will accelerate the translation of MSCs from the bench to the clinic.
Acknowledgments
This work was supported by the National Institutes of Health (EY024602) to S.K.C.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtos.2019.01.006.
References
- [1].Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–7. [DOI] [PubMed] [Google Scholar]
- [2].Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ 2014;21:216–25. 10.1038/cdd.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation 1974;17:331–40. [DOI] [PubMed] [Google Scholar]
- [4].Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol 2000;164:247–56. 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- [5].Fernandes KJL, McKenzie IA, Mill P, Smith KM, Akhavan M, Barnabé-Heider F, et al. A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol 2004;6:1082–93. 10.1038/ncb1181. [DOI] [PubMed] [Google Scholar]
- [6].Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol 2008;180:2581–7. [DOI] [PubMed] [Google Scholar]
- [7].Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002;418:41–9. 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- [8].Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into non-human primates. Blood 2003;101:2999–3001. 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]
- [9].Kawada H, Fujita J, Kinjo K, Matsuzaki Y, Tsuma M, Miyatake H, et al. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood 2004;104:3581–7. 10.1182/blood-2004-04-1488. [DOI] [PubMed] [Google Scholar]
- [10].Shi Y, Su J, Roberts AI, Shou P, Rabson AB, Ren G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol 2012;33:136–43. 10.1016/j.it.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Knaän-Shanzer S. Concise review: the immune status of mesenchymal stem cells and its relevance for therapeutic application. Stem Cell 2014;32:603–8. 10.1002/stem.1568. [DOI] [PubMed] [Google Scholar]
- [12].Dimarino AM, Caplan AI, Bonfield TL. Mesenchymal stem cells in tissue repair. Front Immunol 2013;4:201. 10.3389/fimmu.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zachar L, Bačenková D, Rosocha J. Activation, homing, and role of the mesenchymal stem cells in the inflammatory environment. J Inflamm Res 2016;9:231–40. 10.2147/JIR.S121994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol 2005;6:1182–90. 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- [15].Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol 2007;127:514–25. 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- [16].Krysko DV, Denecker G, Festjens N, Gabriels S, Parthoens E, D'Herde K, et al. Macrophages use different internalization mechanisms to clear apoptotic and necrotic cells. Cell Death Differ 2006;13:2011–22. 10.1038/sj.cdd.4401900. [DOI] [PubMed] [Google Scholar]
- [17].Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol 2007;7:803–15. 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- [18].Van Linthout S, Miteva K, Tschöpe C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc Res 2014;102:258–69. 10.1093/cvr/cvu062. [DOI] [PubMed] [Google Scholar]
- [19].Horwitz EM, Gordon PL, Koo WKK, Marx JC, Neel MD, McNall RY, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci Unit States Am 2002;99:8932–7. 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Iso Y, Spees JL, Serrano C, Bakondi B, Pochampally R, Song Y-H, et al. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem Biophys Res Commun 2007;354:700–6. 10.1016/j.bbrc.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ortiz LA, DuTreil M, Fattman C, Pandey AC, Torres G, Go K, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci Unit States Am 2007;104:11002–7. 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kunter U, Rong S, Djuric Z, Boor P, Muller-Newen G, Yu D, et al. Transplanted mesenchymal stem cells accelerate glomerular healing in experimental glomerulonephritis. J Am Soc Nephrol 2006;17:2202–12. 10.1681/ASN.2005080815. [DOI] [PubMed] [Google Scholar]
- [23].Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci Unit States Am 2006;103:17438–43. 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair–current views. Stem Cell 2007;25:2896–902. 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- [25].da Silva Meirelles L, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev 2009;20:419–27. 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- [26].Chen L, Tredget EE, Wu PYG, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 2008;3. 10.1371/journal.pone.0001886. e1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med 2005;11:367–8. 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- [28].Timmers L, Lim SK, Hoefer IE, Arslan F, Lai RC, van Oorschot AAM, et al. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res 2011;6:206–14. 10.1016/j.scr.2011.01.001. [DOI] [PubMed] [Google Scholar]
- [29].Linero I, Chaparro O. Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. PLoS One 2014;9:e107001 10.1371/journal.pone.0107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Smith AN, Willis E, Chan VT, Muffley LA, Isik FF, Gibran NS, et al. Mesenchymal stem cells induce dermal fibroblast responses to injury. Exp Cell Res 2010;316:48–54. 10.1016/j.yexcr.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gnecchi M, Danieli P, Malpasso G, Ciuffreda MC. Paracrine mechanisms of mesenchymal stem cells in tissue repair. Methods Mol Biol 2016;1416:123–46. 10.1007/978-1-4939-3584-0_7. [DOI] [PubMed] [Google Scholar]
- [32].Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol 2014;15:1009–16. 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- [33].Gao F, Chiu SM, Motan DAL, Zhang Z, Chen L, Ji H-L, et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis 2016;7. 10.1038/cddis.2015.327. e2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008;2:141–50. 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- [35].Ren G, Su J, Zhang L, Zhao X, Ling W, L’huillie A, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cell 2009;27:1954–62. 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- [36].Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 2008;371:1579–86. 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- [37].Sudres M, Norol F, Trenado A, Grégoire S, Charlotte F, Levacher B, et al. Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. J Immunol 2006;176:7761–7. [DOI] [PubMed] [Google Scholar]
- [38].Németh K, Leelahavanichkul A, Yuen PST, Mayer B, Parmelee A, Doi K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E2–dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 2009;15:42–9. 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cell 2006;24:74–85. 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- [40].Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood 2006;107:1484–90. 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- [41].Jiang X-X, Zhang Y, Liu B, Zhang S-X, Wu Y, Yu X-D, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 2005;105:4120–6. 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- [42].Luz-Crawford P, Kurte M, Bravo-Alegría J, Contreras R, Nova-Lamperti E, Tejedor G, et al. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther 2013;4:65. 10.1186/scrt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, et al. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell 2012;10:544–55. 10.1016/j.stem.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 2009;5:54–63. 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nemeth K, Keane-Myers A, Brown JM, Metcalfe DD, Gorham JD, Bundoc VG, et al. Bone marrow stromal cells use TGF-β to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci Unit States Am 2010;107:5652–7. 10.1073/pnas.0910720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].DelaRosa O, Lombardo E, Beraza A, Mancheño-Corvo P, Ramirez C, Menta R, et al. Requirement of IFN-γ-mediated indoleamine 2,3-dioxygenase expression in the modulation of lymphocyte proliferation by human adipose-derived stem cells. Tissue Eng 2009;15:2795–806. 10.1089/ten.TEA.2008.0630. [DOI] [PubMed] [Google Scholar]
- [47].Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood 2009;113:6576–83. 10.1182/blood-2009-02-203943. [DOI] [PubMed] [Google Scholar]
- [48].Ren G, Zhao X, Zhang L, Zhang J, L'Huillier A, Ling W, et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol 2010;184:2321–8. 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Jarvinen L, Badri L, Wettlaufer S, Ohtsuka T, Standiford TJ, Toews GB, et al. Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator. J Immunol 2008;181:4389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation 2003;75:389–97. 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- [51].Bouffi C, Bony C, Courties G, Jorgensen C, Noël D. IL-6-Dependent PGE2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis. PLoS One 2010;5:e14247. 10.1371/journal.pone.0014247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med 2002;196:459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 2004;103:4619–21. 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- [54].Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, et al. Role for interferon-γ in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cell 2006;24:386–98. 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- [55].Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol 2003;3:879–89. 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- [56].Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ 2001;79:214–21. [PMC free article] [PubMed] [Google Scholar]
- [57].Torricelli AAM, Santhanam A, Wu J, Singh V, Wilson SE. The corneal fibrosis response to epithelial–stromal injury. Exp Eye Res 2016;142:110–8. 10.1016/j.exer.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lan Y, Kodati S, Lee HS, Omoto M, Jin Y, Chauhan SK. Kinetics and function of mesenchymal stem cells in corneal injury. Invest Ophthalmol Vis Sci 2012;53:3638–44. 10.1167/iovs.11-9311. [DOI] [PubMed] [Google Scholar]
- [59].Roddy GW, Oh JY, Lee RH, Bartosh TJ, Ylostalo J, Coble K, et al. Action at a distance: systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TNF-α stimulated gene/protein 6. Stem Cell 2011;29:1572–9. 10.1002/stem.708. [DOI] [PubMed] [Google Scholar]
- [60].Omoto M, Katikireddy KR, Rezazadeh A, Dohlman TH, Chauhan SK. Mesenchymal stem cells home to inflamed ocular surface and suppress allosensitization in corneal transplantation. Invest Ophthalmol Vis Sci 2014;55:6631–8. 10.1167/iovs.14-15413. [DOI] [PubMed] [Google Scholar]
- [61].Mittal SK, Omoto M, Amouzegar A, Sahu A, Rezazadeh A, Katikireddy KR, et al. Restoration of corneal transparency by mesenchymal stem cells. Stem Cell Reports 2016;7:583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Herrero-Fresneda I, Torras J, Franquesa M, Vidal A, Cruzado JM, Lloberas N, et al. HGF gene therapy attenuates renal allograft scarring by preventing the profibrotic inflammatory-induced mechanisms. Kidney Int 2006;70:265–74. 10.1038/sj.ki.5001510. [DOI] [PubMed] [Google Scholar]
- [63].Omoto M, Suri K, Amouzegar A, Li M, Katikireddy KR, Mittal SK, et al. Hepatocyte growth factor suppresses inflammation and promotes epithelium repair in corneal injury. Mol Ther 2017;25:1881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hertsenberg AJ, Shojaati G, Funderburgh ML, Mann MM, Du Y, Funderburgh JL. Corneal stromal stem cells reduce corneal scarring by mediating neutrophil infiltration after wounding. PLoS One 2017;12:e0171712. 10.1371/journal.pone.0171712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Oh JY, Kim MK, Shin MS, Lee HJ, Ko JH, Wee WR, et al. The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cell 2008;26:1047–55. 10.1634/stemcells.2007-0737. [DOI] [PubMed] [Google Scholar]
- [66].Mittal SK, Mashaghi A, Amouzegar A, Li M, Foulsham W, Sahu SK, et al. Mesenchymal stromal cells inhibit neutrophil effector functions in a murine model of ocular inflammation. Investig Ophthalmol Vis Sci 2018;59. 10.1167/iovs.17-23067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Amouzegar A, Mittal SK, Sahu A, Sahu SK, Chauhan SK. Mesenchymal stem cells modulate differentiation of myeloid progenitor cells during inflammation. Stem Cell 2017;35:1532–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Basu S, Hertsenberg AJ, Funderburgh ML, Burrow MK, Mann MM, Du Y, et al. Human limbal biopsy-derived stromal stem cells prevent corneal scarring. Sci Transl Med 2014;6. 10.1126/scitranslmed.3009644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Du Y, Carlson EC, Funderburgh ML, Birk DE, Pearlman E, Guo N, et al. Stem cell therapy restores transparency to defective murine corneas. Stem Cell 2009;27:1635–42. 10.1002/stem.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Chakravarti S, Petrol1 WM, Hassell JR, Jester JV, Lass JH, Paul J, et al. Corneal opacity in lumican-null mice: defects in collagen fibril structure and packing in the posterior stroma. Invest Ophthalmol Vis Sci 2000;41:3365–73. [PMC free article] [PubMed] [Google Scholar]
- [71].Yao L, Li Z, Su W, Li Y, Lin M, Zhang W, et al. Role of mesenchymal stem cells on cornea wound healing induced by acute alkali burn. PLoS One 2012;7:e30842. 10.1371/journal.pone.0030842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gu S, Xing C, Han J, Tso MOM, Hong J. Differentiation of rabbit bone marrow mesenchymal stem cells into corneal epithelial cells in vivo and ex vivo. Mol Vis 2009;15:99–107. [PMC free article] [PubMed] [Google Scholar]
- [73].Jiang T-S, Cai L, Ji W-Y, Hui Y-N, Wang Y-S, Hu D, et al. Reconstruction of the corneal epithelium with induced marrow mesenchymal stem cells in rats. Mol Vis 2010;16:1304–16. [PMC free article] [PubMed] [Google Scholar]
- [74].Samaeekia R, Rabiee B, Putra I, Shen X, Park YJ, Hematti P, et al. Effect of human corneal mesenchymal stromal cell-derived exosomes on corneal epithelial wound healing. Invest Ophthalmol Vis Sci 2018;59:5194–200. 10.1167/iovs.18-24803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Ma Y, Xu Y, Xiao Z, Yang W, Zhang C, Song E, et al. Reconstruction of chemically burned rat corneal surface by bone marrow-derived human mesenchymal stem cells. Stem Cell 2006;24:315–21. 10.1634/stemcells.2005-0046. [DOI] [PubMed] [Google Scholar]
- [76].Galindo S, Herreras JM, López-Paniagua M, Rey E, de la Mata A, Plata-Cordero M, et al. Therapeutic effect of human adipose tissue-derived mesenchymal stem cells in experimental corneal failure due to limbal stem cell niche damage. Stem Cell 2017;35:2160–74. 10.1002/stem.2672. [DOI] [PubMed] [Google Scholar]
- [77].Oh JY, Ko JH, Kim MK, Wee WR. Effects of mesenchymal stem/stromal cells on cultures of corneal epithelial progenitor cells with ethanol injury. Invest Ophthalmol Vis Sci 2014;55:7628–35. 10.1167/iovs.14-15424. [DOI] [PubMed] [Google Scholar]
- [78].Hu N, Zhang Y-Y, Gu H-W, Guan H-J. Effects of bone marrow mesenchymal stem cells on cell proliferation and growth factor expression of limbal epithelial cells in vitro. Ophthalmic Res 2012;48:82–8. 10.1159/000331006. [DOI] [PubMed] [Google Scholar]
- [79].Reinshagen H, Auw-Haedrich C, Sorg RV, Boehringer D, Eberwein P, Schwartzkopff J, et al. Corneal surface reconstruction using adult mesenchymal stem cells in experimental limbal stem cell deficiency in rabbits. Acta Ophthalmol 2011;89:741–8. 10.1111/j.1755-3768.2009.01812.x. [DOI] [PubMed] [Google Scholar]
- [80].Nieto-Miguel T, Galindo S, Reinoso R, Corell A, Martino M, Pérez-Simón JA, et al. In vitro simulation of corneal epithelium microenvironment induces a corneal epithelial-like cell phenotype from human adipose tissue mesenchymal stem cells. Curr Eye Res 2013;38:933–44. 10.3109/02713683.2013.802809. [DOI] [PubMed] [Google Scholar]
- [81].Lin K-J, Loi M-X, Lien G-S, Cheng C-F, Pao H-Y, Chang Y-C, et al. Topical administration of orbital fat-derived stem cells promotes corneal tissue regeneration. Stem Cell Res Ther 2013;4:72. 10.1186/scrt223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Eslani M, Putra I, Shen X, Hamouie J, Afsharkhamseh N, Besharat S, et al. Corneal mesenchymal stromal cells are directly antiangiogenic via PEDF and sFLT-1. Investig Opthalmology Vis Sci 2017;58:5507. 10.1167/iovs.17-22680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Eslani M, Putra I, Shen X, Hamouie J, Tadepalli A, Anwar KN, et al. Cornea-derived mesenchymal stromal cells therapeutically modulate macrophage immunophenotype and angiogenic function. Stem Cell 2018;36:775–84. 10.1002/stem.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Gain P, Jullienne R, He Z, Aldossary M, Acquart S, Cognasse F, et al. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol 2016;134:167. 10.1001/jamaophthalmol.2015.4776. [DOI] [PubMed] [Google Scholar]
- [85].Price FW, Whitson WE, Collins KS, Marks RG. Five-year corneal graft survival. A large, single-center patient cohort. Arch Ophthalmol (Chicago, Ill 1960;111:799–805. 1993. [DOI] [PubMed] [Google Scholar]
- [86].The Collaborative Corneal Transplantation Studies Research Group. Effectiveness of histocompatibility matching in high-risk corneal trasplantation. Arch Ophthalmol 1992;110:1392. 10.1001/archopht.1992.01080220054021. [DOI] [PubMed] [Google Scholar]
- [87].Dana MR, Qian Y, Hamrah P. Twenty-five-year panorama of corneal immunology: emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea 2000;19:625–43. [DOI] [PubMed] [Google Scholar]
- [88].Oh JY, Lee RH, Yu JM, Ko JH, Lee HJ, Ko AY, et al. Intravenous mesenchymal stem cells prevented rejection of allogeneic corneal transplants by aborting the early inflammatory response. Mol Ther 2012;20:2143–52. 10.1038/mt.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Jia Z, Jiao C, Zhao S, Li X, Ren X, Zhang L, et al. Immunomodulatory effects of mesenchymal stem cells in a rat corneal allograft rejection model. Exp Eye Res 2012;102:44–9. 10.1016/j.exer.2012.06.008. [DOI] [PubMed] [Google Scholar]
- [90].Treacy O, O'Flynn L, Ryan AE, Morcos M, Lohan P, Schu S, et al. Mesenchymal stem cell therapy promotes corneal allograft survival in rats by local and systemic immunomodulation. Am J Transplant 2014;14:2023–36. 10.1111/ajt.12828. [DOI] [PubMed] [Google Scholar]
- [91].Farrand KF, Fridman M, Stillman IÖ, Schaumberg DA. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 Years and older. Am J Ophthalmol 2017. 10.1016/j.ajo.2017.06.033. [DOI] [PubMed] [Google Scholar]
- [92].Barabino S, Chen Y, Chauhan S, Dana R. Ocular surface immunity: homeostatic mechanisms and their disruption in dry eye disease. Prog Retin Eye Res 2012;31:271–85. 10.1016/j.preteyeres.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Stevenson W, Chauhan SK, Dana R. Dry eye disease. Arch Ophthalmol 2012;130:90. 10.1001/archophthalmol.2011.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Lee MJ, Ko AY, Ko JH, Lee HJ, Kim MK, Wee WR, et al. Mesenchymal stem/stromal cells protect the ocular surface by suppressing inflammation in an experimental dry eye. Mol Ther 2015;23:139–46. 10.1038/mt.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Aluri HS, Samizadeh M, Edman MC, Hawley DR, Armaos HL, Janga SR, et al. Delivery of bone marrow-derived mesenchymal stem cells improves tear production in a mouse model of sjögren’s syndrome. Stem Cell Int 2017;2017:3134543. 10.1155/2017/3134543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Rosario N, Bielory L. Epidemiology of allergic conjunctivitis. Curr Opin Allergy Clin Immunol 2011;11:471–6. 10.1097/ACI.0b013e32834a9676. [DOI] [PubMed] [Google Scholar]
- [97].Ono SJ, Abelson MB. Allergic conjunctivitis: update on pathophysiology and prospects for future treatment. J Allergy Clin Immunol 2005;115:118–22. 10.1016/j.jaci.2004.10.042. [DOI] [PubMed] [Google Scholar]
- [98].Su W, Wan Q, Huang J, Han L, Chen X, Chen G, et al. Culture medium from TNF-α-stimulated mesenchymal stem cells attenuates allergic conjunctivitis through multiple antiallergic mechanisms. J Allergy Clin Immunol 2015;136:423–32. 10.1016/j.jaci.2014.12.1926. e8. [DOI] [PubMed] [Google Scholar]
- [99].Montaño AM, Lock-Hock N, Steiner RD, Graham BH, Szlago M, Greenstein R, et al. Clinical course of sly syndrome (mucopolysaccharidosis type VII). J Med Genet 2016;53:403–18. 10.1136/jmedgenet-2015-103322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Coulson-Thomas VJ, Coulson-Thomas YM, Gesteira TF, Kao WW-Y. Extrinsic and intrinsic mechanisms by which mesenchymal stem cells suppress the immune system. Ocul Surf 2016;14:121–34. 10.1016/j.jtos.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant 2015;21:389–401. 10.1016/j.bbmt.2014.12.001. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Lee SJ, Flowers MED. Recognizing and managing chronic graft-versus-host disease. Hematology 2008:134–41. 10.1182/asheducation-2008.1.134. 2008. [DOI] [PubMed] [Google Scholar]
- [103].Shikari H, Antin JH, Dana R. Ocular graft-versus-host disease: a review. Surv Ophthalmol 2013;58:233–51. 10.1016/j.survophthal.2012.08.004. [DOI] [PubMed] [Google Scholar]
- [104].Martínez-Carrasco R, Sánchez-Abarca LI, Nieto-Gómez C, García EM, Sánchez-Guijo F, Argüeso P, et al. Subconjunctival injection of mesenchymal stromal cells protects the cornea in an experimental model of GVHD. Ocul Surf 2019. 10.1016/j.jtos.2019.01.001. [DOI] [PubMed] [Google Scholar]
- [105].Chen YT, Chen FYT, Vijmasi T, Stephens DN, Gallup M, McNamara NA. Pax6 downregulation mediates abnormal lineage commitment of the ocular surface epithelium in aqueous-deficient dry eye disease. PLoS One 2013;8:e77286. 10.1371/journal.pone.0077286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].McNamara NA, Gallup M, Porco TC. Establishing PAX6 as a biomarker to detect early loss of ocular phenotype in human patients with Sjögren’s syndrome. Invest Ophthalmol Vis Sci 2014;55:7079–84. 10.1167/iovs.14-14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].De Paiva CS, Chotikavanich S, Pangelinan SB, Pitcher JD, Fang B, Zheng X, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol 2009;2:243–53. 10.1038/mi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]