Abstract
The current study tested the hypothesis of whether specific lipids may control angiogenic reactions. Using the chorioallantoic membrane assay of the chick embryo, new vessel formation was analyzed quantitatively by gas chromatography and mass spectrometry as well as bioinformatics tools including an angiogenesis analyzer. Our biochemical experiments showed that a specific lipid composition and stoichiometry determine the angiogenesis microenvironment to accelerate or inhibit vessel formation. Specific lipids of angiogenesis determinants in the vessel area and the non-vessel area were identified as nitrooleic acid, docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), palmitic acid, oleic acid, linoleic acid, linolenic acid, epoxyoleic acid, lysophosphatidylcholine (LPC), cholesterol, 7-ketocholesterol, and docosahexaenoyl lysophosphatidylcholine (DHA-LPC). Vessel formation happens on the surface area of the hydrophilic membrane of the yolk. Our biochemical data demonstrated that angiogenesis was followed in the white lipid complex area to generate more branches, junctions, segments, and extremities. We analyzed lipid fragments in the vessel, non-vessel, and albumen area to show that each area contains a specific lipid composition and stoichiometry. Mass spectrometry data demonstrated that the vessel area has higher concentrations of nitrooleic acid, palmitic acid, stearic acid, LPC, lysophosphatidylethanolamine, cholesterol, oleic acid, linoleic acid, 7-ketocholesterol, and DHA-LPC; however, DHA and EPA were abundant in the hydrophobic non-vessel area. The purpose of vessel formation is to wrap up the yolk area to transport nutrients including specific fatty acids. Besides, angiogenesis requires aqueous albumen shown by distance-dependent vessel formation from albumen and oxygen. Higher concentrations of fatty acids are required for energy and carbon structure from the carbon–carbon bond, membrane building blocks, and amphiphilic detergent to solubilize a hydrophobic environment in the aqueous blood layer. The current study may guide that the uncovered hydrophobic or zwitterionic molecules such as DHA and DHA-LPC may control angiogenesis as antiangiogenic or proangiogenic molecules as potential drug targets for treating uncontrolled angiogenesis-related diseases, including diabetic retinopathy and age-related macular degeneration.
Introduction
Angiogenesis is a multi-step process of new blood vessel sprouting and splitting which is responsible for embryonic development, tissue repair, and tumors.1,2 Vascular networks penetrate every organ and tissue to circulate available nutrients, signaling ions, and oxygen. The development of the blood vascular framework is one of the most initial reactions in embryogenesis for molecular transport. Mesodermal stem cells separate into hemangioblasts during early embryonic development and the activity of hematopoietic cells together with endothelial cells give rise to blood vessels.3 Over the span of further separation, hemangioblasts produce angioblasts, whose accumulation brings the arrangement of blood islands. At that point, a combination of blood and islands brings the essential blood vascular plexus comprising fine vessels shaped by endothelial cells.
Tissue actions of angiogenesis rely on the equalization of many promoting components. The primary signaling reaction that manages multiplication and movement of endothelial cells shaping the premise of any vessel is vascular endothelium growth factors (VEGFs) and its receptors.4,5 The VEGF-dependent signaling network is one of the fundamental mechanism for the early stage of vascular development.
Uncontrolled angiogenesis has been implicated in pathophysiological conditions including cancer, inflammation, diabetes, and neurodegeneration.6−9 The regulation of VEGF in carcinoma cells has been widely examined as an anticancer medicine. However, detailed information of the angiogenic microenvironment at the molecular level is elusive.
Recently, our experiments demonstrated that specific lipid molecules initiate the process of blood vessel formation.10 It is a significant step to understand the initial reactions of angiogenesis, for example, retinopathy of prematurity (ROP) shows abnormal blood vessel growth in the retina of premature infants. The current study tested the hypothesis of whether the composition and stoichiometry of specific lipids may control angiogenic reactions. Our current biochemical study aimed to determine fatty acid identification of angiogenic microenvironment to accelerate or inhibit the vessel formation.
Proangiogenic and antiangiogenic molecules including erythropoietin and lipids exist as natural angiogenic determinants to control the initiation of a blood vessel.11,12 A metabolomics approach including mass spectrometry and database search identified oleic acid, cholesterol, and linoleic acid as angiogenic initiators that control vessel development in a time-dependent and dose-dependent manner. Anthocyanin extracts from Hibiscus sabdariffa are natural products containing a conjugated double bond that regulates proper angiogenesis.13
The current study examined the quantitative identification of hydrophobic molecules in the vessel area, non-vessel area, and albumen of chick embryos to understand their microenvironment of angiogenesis reactions. Gas chromatography–mass spectrometry (GCMS) analysis, database search, and quantitative essay demonstrated that specific lipids that include nitrooleic acid, palmitic acid, oleic acid, linoleic acid, linolenic acid, epoxyoleic acid, lysophosphatidylcholine (LPC, 18:4), cholesterol, 7-ketocholesterol, and docosahexaenoyl lysophosphatidylcholine (DHA-LPC) exist in the vessel area, while docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) were abundant in the non-vessel environment. An unbiased metabolomics approach suggested that lipid composition and stoichiometry may determine the angiogenesis microenvironment which could set as potential therapeutic targets to treat pathological angiogenesis.
Results
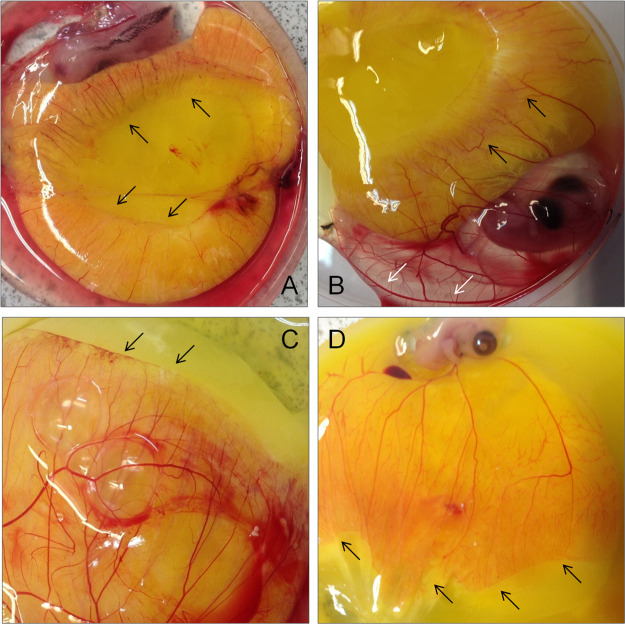
Lipids in the yolk area represent the primary energy and carbon source of nutrients for the chick embryo. Biochemical analysis using a chick chorioallantoic membrane demonstrates that vessel formation increased to wrap up the yolk area in a time-dependent manner in the blood (black arrow, Figure 1A) until residual yolk is completely absorbed. Branches, junctions, and extremities were more abundant in the front line of the vessel-forming area (black arrow, Figure 1B). Surprisingly, angiogenesis happened even in the albumen area as shown in Figure 1B (white arrow). Blood vessels were formed on the thin membrane containing blood, which is also determined by the white lipid complex (Figure 1C,D).
Figure 1.
Vessel formation happened on the surface of the aqueous membrane (yolk area). Vessel formation was initiated on the yolk and continues until the residual yolk was completely absorbed [black arrow, (A)]. (B) More vessels, branches, junctions, and extremities were formed at the end of the yolk region. (C) Vessel formation stopped when it reached the non-vessel area as seen by the arrows. (D) A larger vessel area was observed at the end of the vessel area with more segmented and branched.
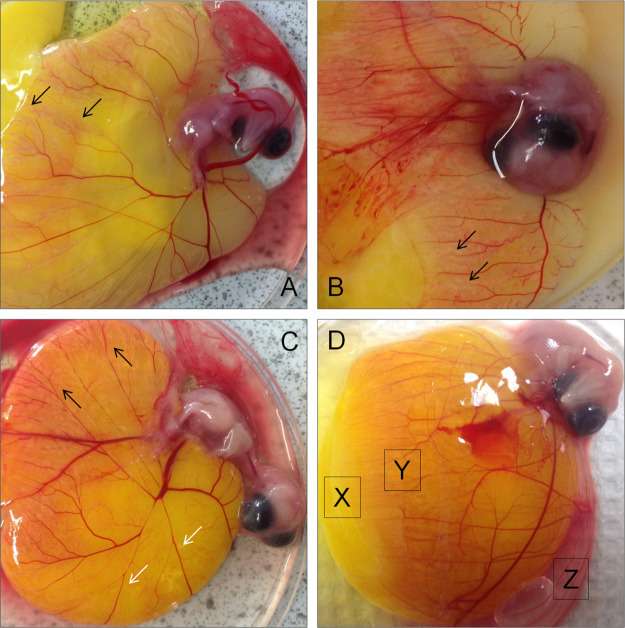
The hydrophobic tail of the lipid bilayer extends forming more branches which are engulfed gradually as the chick develops as shown in Figure 2. The albumen area (egg white) contains 90% water. The remaining 10% is rich in proteins, vitamins, fatty acids, and minerals supplying nutrition to the embryo for the necessary protection. The angiogenesis analyzer indicated that more branches and segments were generated in the aqueous area of the blood inside of the vessel-forming area (Figure 2A). Time-dependent analysis of the vessel formation in their environment showed that white lipid complex, including oleic acid, cholesterol, and linoleic acid led the vessel formation as an angiogenic reaction guide. Morphology analysis demonstrated that the blood vessel followed the white lipid complex area (Figure 2B). Vessel formations were distinguished based on the microenvironment of blood, albumen, oxygen, and carotenoids (Figure 2C). Lipid composition and stoichiometry were further analyzed based on the non-vessel area (X), vessel area (Y), and albumen (Z) (Figures 2D, 4, Supporting Information Figures S1–S3).
Figure 2.
Vessel formation was continued in the white lipid complex area. (A) Many short blood vessel branches were observed at the white lipid complex as shown by the arrows. (B) Decreased branches and junctions were observed on the complex. (C) Vessel junctions and branches became more visible and dispersed. (D) Three distinct areas of X (non-vessel area), Y (vessel starting point), and Z (albumen) were analyzed by GCMS to identify hydrophobic molecules.
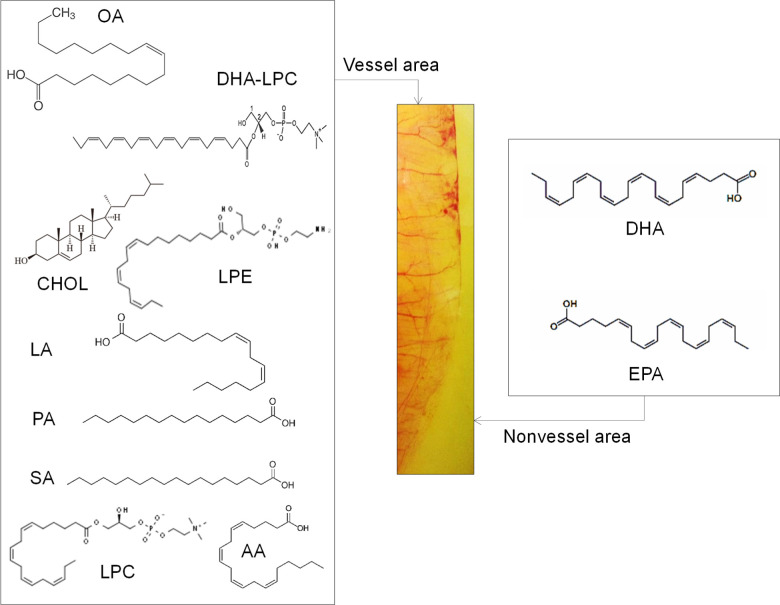
Figure 4.
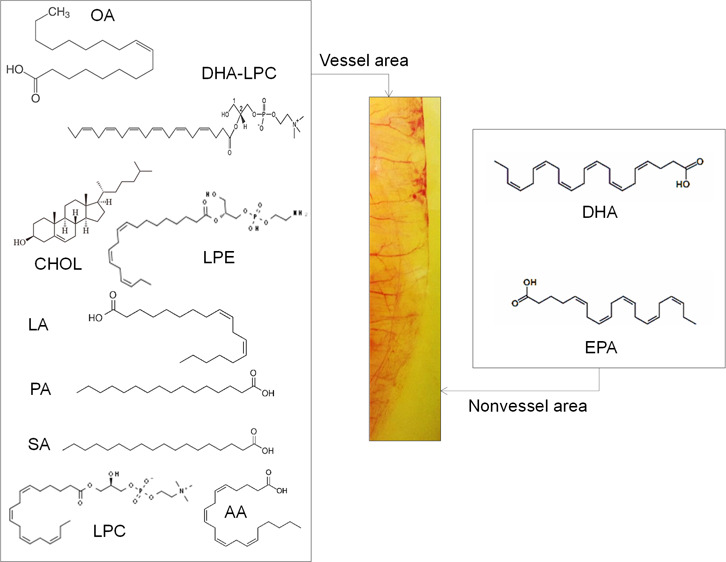
Structural summary of the lipids in the vessel area (left) and the non-vessel area (right) that determine the angiogenesis microenvironment (Supporting Information S1–S5).
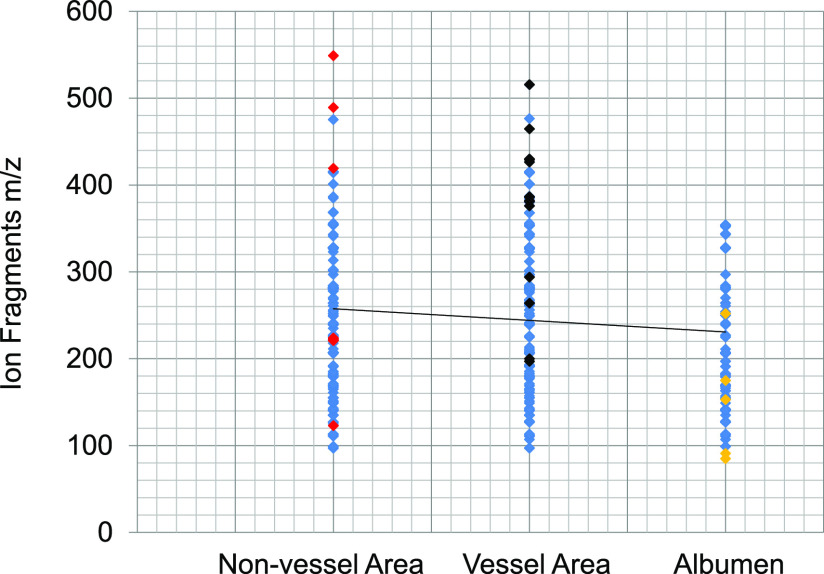
To answer our hypothesis that lipid composition may determine the angiogenesis microenvironment, we analyzed the hydrophobic molecules in the vessel and non-vessel area quantitatively. Lipid composition and fragments were measured using an unbiased metabolomics approach by GCMS in the chick embryo to understand specific molecules in the vessel, non-vessel, and albumen area (Figure 3). Lipid fragments were analyzed by mass to charge (Y-axis) and the relative abundance (X-axis). Area-specific ions were determined by red (non-vessel), black (vessel), and yellow (albumen), suggesting that pro- or antiangiogenic molecules exist in each area.
Figure 3.
GCMS fragments of lipids vs vessel/non-vessel/albumen area were presented. The GCMS ion fragments were shown as the red dot (unique lipids in the vessel area), black (unique non-vessel lipids), and yellow (lipids special to the albumen) while blue represents common fragments in the whole chick embryo (Supporting Information S3–S4).
Relative abundance data from GCMS analysis demonstrated that the vessel area has higher concentrations of oleic acid (oleic acid = 1.7 g/egg in the vessel area: 300–330 mg in the non-vessel area = 44:8%), linoleic acid, cholesterol (200:50 mg = vessel area/non-vessel area = 33.4:8%, Supporting Information Figure S6), nitrooleic acid, palmitic acid, stearic acid, LPC, lysophosphatidylethanolamine (LPE), 7-ketocholesterol, and LPC-DHA. In the non-vessel area, DHA and EPA were abundant as shown by GCMS (Figure 4 and Supporting Information Figures S1–S5).
Mass spectrometry analysis showed that increased levels of DHA (Supporting Information Figure S1, arrow; molecular fragments = 74.1, 185.2, 328, and 354) and EPA (Figure S1, molecular ion = 302.1) exist in the non-vessel area at embryonic day 1 (ED 1, retention time = 17.403 min). However, ion fragments demonstrated that other lipids including nitrooleic acid, linolenic acid, linoleic acid, lauric acid, linoleic acid, palmitic acid, oleic acid, LPC (carbon/double bond = 18:4), stearic acid, cholesterol, nitrooleic acid, LPE (18:3), and arachidonic acid (20:4, molecular ion = 304.4) are abundant in the vessel area at ED 1 (retention time = 25.242 min; Supporting Information Figure S3).
Ion fragments of oleic acid (97.2), linoleic acid (85.3), palmitic/stearic acid (227.3), cholesterol (281.1, 368.4), nitrooctadecenoic acid (327.1), and LPE (20:3, molecular ion = 503.3) were abundant in the vessel area at ED 5 (retention time = 10.245 min; Supporting Information Figure S4). Oleic acid (ion fragments = 111, 165, 207, and 264), linolenic acid (135), palmitic acid (239), nitrooleic acid (297), cholesterol (387), 7-ketocholesterol (401), and DHA-LPC (414) were the major lipid components in the vessel area (ED 5, retention time = 13.857 min; Supporting Information Figure S5).
Discussion
The current biochemical study aimed to determine the angiogenic molecules in the microenvironment to accelerate or inhibit vessel formation. An unbiased metabolomics approach demonstrated that lipid composition and stoichiometry may control the angiogenesis microenvironment, which may set as potential therapeutic targets to treat pathological angiogenesis, including age-related macular degeneration (AMD) and diabetic retinopathy (DR).14,15
Higher concentrations of fatty acids are utilized for the energy and building blocks from the carbon–carbon bonds, membranes, and amphiphilic detergents to solubilize a hydrophobic environment in the aqueous blood layer.16−22 The current angiogenic analysis suggests that specific angiogenic components including 7-ketocholesterol in the vessel area are considered as the potential proangiogenic molecules. It is reported that 7-ketocholesterol is a potent inducer of VEGF in vivo.23 DHA is an omega-3 polyunsaturated fatty acid with 22 carbons and 6 double bonds (22:6). DHA is of particular interest due to its highly unsaturated structure as the most unsaturated lipid in our body and is mostly concentrated in the nervous sytem forming cell membranes, providing a great fluidity to cell structure.24,25
The blood barrier is composed of capillary endothelial cells connected with tight junctions, together with perivascular elements including astrocytic end-foot processes, pericytes, and perivascular neurons. The endothelial cells give the blood barrier highly selective permeability due to its specialized tight junctions. DHA binds to specific proteins that include lipoproteins and albumin. Lipoproteins can transport DHA when they are esterified within triacylglycerols, phospholipids, or cholesteryl esters.26,27 Albumin can bind to DHA under its non-esterified form or esterified within a lysophosphatidylcholine (DHA-LPC).28−30
DHA or DHA-LPC could be hydrolyzed from lipoproteins by lipases before the entry into endothelial cells. It is generally agreed that non-esterified or esterified polyunsaturated fatty acids (PUFAs) in LPC are detached from albumin when getting to the endothelial cells. One pathway considered to be the major entry of DHA within the blood barrier is passive diffusion of non-esterified DHA through the membrane of endothelial cells.30−40
Facilitated transport of non-esterified DHA into the endothelial cells may involve FAT/CD36 (fatty acid translocase/cluster of differentiation 36).24,31,41,42 In human brain microvessel endothelial cells, FAT/CD36 showed a prominent role in the transport of fatty acids across the monolayer. However, PUFAs such as DHA incorporation into brain phospholipids were unaltered in CD36–/– mice. Regarding DHA esterified in LPC, recent studies demonstrated the critical role of Mfsd2a (major facilitator superfamily domain-containing protein 2A), a protein expressed in blood–brain barrier (BBB) endothelium. These studies identified Mfsd2a as a major transporter for DHA only when this fatty acid is esterified to LPC.31,33−35,38,43,44
Mfsd2a-knockout mice showed a significantly reduced DHA level in the brain and a notably reduced level of DHA-LPC uptake. Mfsd2a was also shown to be important for DHA-LPC transport into the retina. From endothelial cells to neural cells, the passive diffusion of non-esterified DHA is also considered as a potential pathway for DHA to enter the brain. DHA and EPA were reported as suppressors of angiogenesis. DHA-LPC promoted HUVEC tube formation at concentrations of 10–50 μM but suppressed tube formation at higher concentrations (100 μM), while our data support the antiangiogenic roles of DHA and EPA. We also observed the presence of these phospholipids as well as other fatty acids in the aqueous vessel area as well as a non-vessel area showing that lipids including cholesterol, oleic acid, palmitic acid, and DHA-LPC could be both proangiogenic and antiangiogenic depending on the concentration and microenvironment based on their molecular interactors. We speculate that DHA-LPC behaves as a zwitterionic detergent to solubilize the insoluble lipid complex in the vessel area as proangiogenic factors.
DHA is highly concentrated in the retina and the brain; however, it is not synthesized de novo in situ and should be obtained from the environment.37,39,45,46 Egg yolk exists as an energy source as well as a carbon reservoir including DHA; the solubility change from LPC to DHA-LPC conversion may assist its transport to the vessel area. DHA is localized in the hydrophobic non-vessel area, whereas DHA-LPC is more abundant in the aqueous vessel area due to the zwitterionic property of the choline structure. It is of interest that the vessel area and the non-vessel area control DHA concentration by interconversion of DHA-LPC and DHA.
DHA levels are decreased in apoptotic neurological disorders, including Alzheimer’s and Parkinson’s diseases. The precursor of DHA, alpha-linolenic acid (ALA, 18:3 ω-3), is not synthesized de novo and is poorly converted into DHA in mammals. However, mechanisms of DHA entry into the brain and the retina are not fully identified. Highly unsaturated DHA has the potential role of oxidation by lipoxygenases to produce diverse oxylipins that regulate several biological processes in the brain. The BBB or blood–retinal barrier provides effective sealing of the neuronal compartment from the other tissue. The BBB is composed of brain capillary endothelial cells connected with tight junctions with perivascular elements including astrocytic end-foot processes, pericytes, and perivascular neurons. The endothelial cells give the BBB highly selective permeability due to its specialized tight junctions, providing neuronal system immune-privileged environment allowing only small molecules including O2, CO2, glucose, and hydrophobic hormones into the brain. DHA binds to specific proteins within the plasma, including lipoproteins and albumin. Lipoproteins can transport DHA and other PUFAs when they are esterified with triacylglycerols, phospholipids, or cholesteryl esters.
Albumin also binds with DHA under its non-esterified form or esterified within a LPC. The mechanism of DHA import into the brain has been a controversy for years.33,36,40,47,48 Circulating lipoproteins bind to their receptors and hydrolyzed intracellularly after transcytosis with lipases present inside the endothelial cells. DHA or DHA-LPC may be hydrolyzed from lipoproteins by lipases before the entry into endothelial cells. It is agreed that non-esterified or esterified PUFAs in LPC are detached from albumin when entering the endothelial cells. One pathway of the major entry of DHA within the BBB is passive diffusion of non-esterified DHA through the membrane of endothelial cells.
Active pathways of DHA incorporation into the brain include members of the FABP (fatty acid-binding protein) family. Isoforms of the FABP family are expressed in specific tissues according to the rates of fatty acid uptake. FABPs bind DHA, which is subsequently transported across the membrane of endothelial cells into the neural cells through the fatty acid transport protein (FATP)-dependent mechanism. LPC is associated with albumin, which indicates that albumin has at least as much affinity to DHA-LPC as non-esterified DHA, providing a significant delivery pathway of DHA to the brain.
The metabolic origin of DHA-LPC is likely produced from DHA-containing PC such as hydrolysis of DHA-PC by liver phospholipase A1. An additional source of DHA-LPC is through the action of endothelial lipase that cleaves preferentially the sn-1 position of DHA-containing phospholipids. DHA esterified in LPC at the sn-2 position migrates easily from this position to the sn-1 position because of the higher reactivity of its primary alcohol. It is of interest that DHA and DHA-LPC could be translocated based on their solubility and hydrophobicity.49−51
Conclusions
The current study determined whether hydrophobic molecules may regulate angiogenic reactions. The chorioallantoic membrane assay of the chick embryo demonstrated that new vessel formation was accelerated where specific lipids including oleic acid, cholesterol, linoleic acid, and DHA-LPC are increased. Biochemical experiments showed that lipid composition and stoichiometry are different in the vascular microenvironment to accelerate or inhibit the vessel formation. Vessel formation happens on the surface area of the hydrophilic membrane of the yolk. Our data demonstrated that angiogenesis was followed in the white lipid complex area to generate more branches, junctions, segments, and extremities. We analyzed lipid fragments in the vessel, non-vessel, and the albumen area to show that each area contains a different lipid composition and stoichiometry. Mass spectrometry data demonstrated that the vessel area has higher concentrations of nitrooleic acid, palmitic acid, stearic acid, LPC, LPE, cholesterol, oleic acid, linoleic acid, 7-ketocholesterol, and DHA-LPC; however, the hydrophobic non-vessel area contains increased levels of DHA and EPA. Especially, oleic acid (5.5:1) and cholesterol (4.2:1) showed specific stoichiometry in the vessel area vs the non-vessel area. The purpose of vessel formation is to transport nutrients and oxygen from the yolk area. Our data suggest that higher concentrations of fatty acids are needed for energy from the carbon–carbon bond, membrane building blocks, and amphiphilic detergent to solubilize a hydrophobic environment in the aqueous blood layer. The current study may guide that the uncovered hydrophobic or zwitterionic molecules such as DHA and DHA-LPC may control angiogenesis as antiangiogenic or proangiogenic molecules as potential drug targets for treating uncontrolled angiogenesis-related diseases, including DR and AMD.
Materials and Methods
In Vivo Experiments
We followed the Association for Research in Vision and Ophthalmology (ARVO) statement and the NIH Guide for in vivo study. Avian embryos are not considered live animals under the Public Health Service (PHS) policy. The use of chicken embryos at gestation day 12 and younger does not require an Animal Use Protocol Application from the Institutional Animal Care and Use Committee (IACUC). Chick embryos younger than ED 13 are assumed unable to experience pain. Therefore, ED13 and younger embryos were euthanized by hypothermia, typically conducted by placing the eggs in a −20 °C freezer or <4 °C for 4 h. Embryonic death was confirmed by decapitation, membrane disruption, or maceration. Chick embryos between ED13 and ED17 can experience pain and were euthanized by cervical dislocation.
Chick Embryo Incubation
We used chorioallantoic membrane assay to investigate the vascular morphology and angiogenic molecules using metabolomics approaches including mass spectrometry analysis.52,53 Fertilized eggs of White Leghorn chickens (n = 85) were purchased from Veterinary Research Institute Jos and incubated for 1–10 days at 37.5 °C with 62–72% humidity in a laboratory incubator with continuous rotation. Chick embryos were incubated for each day (ED1–ED10) and harvested in Petri dishes.
Lipid Extraction from Chick Embryo
Each sample (1 g) was collected from the vessel area, vessel beginning area, non-vessel area, and albumen of the chick embryo and extracted using organic solvents including methanol (2 mL), water/phosphate-buffered saline (4 mL), and chloroform (8 mL). The solutions were mixed using a vortex mixer until they become homogeneous. The samples were centrifuged (3500g for 5 min) and the lower phase was collected and evaporated to dryness at room temperature (27 ± 3 °C). For biochemical analysis, the preserved samples were dissolved first in hexane before being used.
GCMS Analysis
GCMS analysis was carried out using an Agilent Technologies gas chromatograph (Agilent 7890A) coupled to a mass spectrometer (Agilent 5975C) and fitted with an autosampler (Agilent 7683B).
With the highest temperature set at 325 °C and the initial temperature at 60 °C, lipid samples were programmed to run for 1 min with 10 °C/min rise to 280 °C for 21 min, making the total run time to 35 min using the capillary column (DB-5MS 30 m × 0.322 mm × 0.25 μm). The autosampler injected the samples using a split ratio of 1:1 into the inlet. Pure helium gas (99.99%) was used as the carrier gas at a flow rate of 1.1 mL/min at 5.154 psi pressure. The National Institute Standard and Technology (NIST 2014 and NIST 2011) database gives a mass spectrum interpretation of the samples. The known compounds in the database were compared to the unknown molecules of the mass spectrum generated from the metabolomics samples.
Angiogenic Analysis of Chick Embryo Chorioallantoic Membrane
Vessel morphology was analyzed quantitatively using 21 angiogenic parameters by bioinformatics tools that include Angiogenesis Analyzer macro-connected ImageJ software.54−58 Angiogenesis Analyzer macro software was installed in the toolsets (Gilles Carpentier, http://339 image.bio.methods.free.fr/ImageJ/Angiogenesis-Analyzer-for-ImageJ). Vessel morphology analysis was carried out by first converting the images into 16-bit and HUVEC phase contrast/fluorescent images. Then, we used the software that includes AutoTube (https://github.com/autotubularity/autotube), AngioTool (http://angiotool.nci.nih.gov), REAVER (https://github.com/bacorliss/public_REAVER), VESSGEN 2D (https://software.nasa.gov/software/ARC-17621-1), and Vessel Analysis (https://imagej.net/Vessel_Analysis) for comparison. Changes in vessel length, extremities, and branches were analyzed as nodes identified as three neighbors, whereas twig was the segment delimited by two junctions and branches. Master junctions are delimiting master segments with an intersection implicated only in branch. The master tree is composed of master segments associated with master junctions delimiting the meshes. Two close master junctions can combine into a unique master junction with the primary fragment was all observed on the vessel morphology analysis.
Statistical Analysis
Two-group comparisons utilized a two-tailed t-test, ANOVA, whereas Tukey/Dunnet tests were employed for multiple comparisons using the Stat View software. Values expressed as the mean of three technically independent experiments with large amounts of biological samples (n = 85). P < 0.05 is considered significant statistically.
Acknowledgments
The current research was supported in part by the Research Assistantship and Teaching Assistantship from the American University of Nigeria and Julia Foundation. The authors are grateful to Joshua Madu, Muhammad Falalu Yahaya, and Emmanuel Alakunle for their excellent technical assistance.
Glossary
Abbreviations
- AA
arachidonic acid
- AMD
age-related macular degeneration
- CAM
chorioallantoic membrane assay
- CHOL
cholesterol
- DHA
docosahexaenoic acid
- DHA-LPC
docosahexaenoyl lysophosphatidylcholine
- DR
diabetic retinopathy
- EOA
epoxyoleic acid
- EPA
eicosapentaenoic acid
- GCMS
gas chromatography–mass spectrometry
- LA
linoleic acid
- LPC
lysophosphatidylcholine
- LPE
lysophosphatidylethanolamine
- NOA
nitrooleic acid
- OA
oleic acid
- PA
palmitic acid
- PC
phosphatidylcholine
- ROP
retinopathy of prematurity
- RPE
retinal pigment epithelium
- SA
stearic acid
- VEGF
vascular endothelial growth factor
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c00196.
GCMS analysis of lipids in the non-vessel area at ED 1; GCMS analysis of lipids in the non-vessel area at ED 3; GCMS analysis of lipids in the vessel area at ED 1; GCMS analysis of lipids in the vessel area at ED 5; GCMS analysis of lipids in the vessel area at ED 5; and cholesterol concentration in the chick embryo (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Falcón B. L.; Hashizume H.; Koumoutsakos P.; Chou J.; Bready J. V.; Coxon A.; Oliner J. D.; McDonald D. M. Contrasting Actions of Selective Inhibitors of Angiopoietin-1 and Angiopoietin-2 on the Normalization of Tumor Blood Vessels. Am. J. Pathol. 2009, 175, 2159–2170. 10.2353/ajpath.2009.090391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri M.; Yousefnia S.; Seyed Forootan F.; Peymani M.; Ghaedi K.; Nasr Esfahani M. H. Diverse Roles of Fatty Acid Binding Proteins (FABPs) in Development and Pathogenesis of Cancers. Gene 2018, 676, 171–183. 10.1016/j.gene.2018.07.035. [DOI] [PubMed] [Google Scholar]
- Poole T. J.; Coffin J. D. Vasculogenesis and Angiogenesis: Two Distinct Morphogenetic Mechanisms Establish Embryonic Vascular Pattern. J. Exp. Zool. 1989, 251, 224–231. 10.1002/jez.1402510210. [DOI] [PubMed] [Google Scholar]
- Roskoski R. Vascular Endothelial Growth Factor (VEGF) Signaling in Tumor Progression. Crit. Rev. Oncol. Hematol. 2007, 62, 179–213. 10.1016/j.critrevonc.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Tammela T.; Enholm B.; Alitalo K.; Paavonen K. The Biology of Vascular Endothelial Growth Factors. Cardiovasc. Res. 2005, 65, 550–563. 10.1016/j.cardiores.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Li Y.; Liu X.; Zhou T.; Kelley M. R.; Edwards P. A.; Gao H.; Qiao X. Suppression of Choroidal Neovascularization Through Inhibition of APE1/Ref-1 Redox Activity. Invest. Ophthalmol. Vis. Sci. 2014, 55, 4461. 10.1167/iovs.14-14451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R. K.; Rogojina A. T.; Chalam K. V. Multiplex Immunoassay Analysis of Biomarkers in Clinically Accessible Quantities of Human Aqueous Humor. Mol. Vis. 2009, 15, 60–69. [PMC free article] [PubMed] [Google Scholar]
- Tomita Y.; Cakir B.; Liu C.-H.; Fu Z.; Huang S.; Cho S. S.; Britton W. R.; Sun Y.; Puder M.; Hellström A.; Talukdar S.; Smith L. E. H. Free Fatty Acid Receptor 4 Activation Protects against Choroidal Neovascularization in Mice. Angiogenesis 2020, 23, 385–394. 10.1007/s10456-020-09717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abokyi S.; To C.-H.; Lam T. T.; Tse D. Y. Central Role of Oxidative Stress in Age-Related Macular Degeneration: Evidence from a Review of the Molecular Mechanisms and Animal Models. Oxid. Med. Cell. Longevity 2020, 2020, 1–19. 10.1155/2020/7901270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson F. P.; Patrick A. T.; Fabunmi T. E.; Yahaya M. F.; Madu J.; He W.; Sripathi S. R.; Tyndall J.; Raji H.; Jee D.; Gutsaeva D. R.; Jahng W. J. Oleic Acid, Cholesterol, and Linoleic Acid as Angiogenesis Initiators. ACS Omega 2020, 5, 20575–20585. 10.1021/acsomega.0c02850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H.; Lee H.; Lamoke F.; Hrushesky W. J. M.; Wood P. A.; Jahng W. J. Neuroprotective Role of Erythropoietin by Antiapoptosis in the Retina. J. Neurosci. Res. 2009, 87, 2365–2374. 10.1002/jnr.22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson F. P.; He W.; Sripathi S. R.; Patrick A. T.; Madu J.; Chung H.; Frost M. C.; Jee D.; Gutsaeva D. R.; Jahng W. J. Dual Switch Mechanism of Erythropoietin as an Antiapoptotic and Pro-Angiogenic Determinant in the Retina. ACS Omega 2020, 5, 21113–21126. 10.1021/acsomega.0c02763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshua M.; Okere C.; Sylvester O. D.; Yahaya M.; Precious O.; Dluya T.; Um J.-Y.; Neksumi M.; Boyd J.; Vincent-Tyndall J.; Choo D.-W.; Gutsaeva D. R.; Jahng W. J. Disruption of Angiogenesis by Anthocyanin-Rich Extracts of Hibiscus Sabdariffa. Int. J. Sci. Eng. Res. 2017, 8, 299–307. 10.14299/ijser.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benny O.; Nakai K.; Yoshimura T.; Bazinet L.; Akula J. D.; Nakao S.; Hafezi-Moghadam A.; Panigrahy D.; Pakneshan P.; D’Amato R. J. Broad Spectrum Antiangiogenic Treatment for Ocular Neovascular Diseases. PLoS One 2010, 5, e12515 10.1371/journal.pone.0012515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello L. P.; Pierce E. A.; Foley E. D.; Takagi H.; Chen H.; Riddle L.; Ferrara N.; King G. L.; Smith L. E. Suppression of Retinal Neovascularization in Vivo by Inhibition of Vascular Endothelial Growth Factor (VEGF) Using Soluble VEGF-Receptor Chimeric Proteins. Proc. Natl. Acad. Sci. 1995, 92, 10457–10461. 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baci D.; Bruno A.; Bassani B.; Tramacere M.; Mortara L.; Albini A.; Noonan D. M. Acetyl-l-Carnitine Is an Anti-Angiogenic Agent Targeting the VEGFR2 and CXCR4 Pathways. Canc. Lett. 2018, 429, 100–116. 10.1016/j.canlet.2018.04.018. [DOI] [PubMed] [Google Scholar]
- Joyal J.-S.; Sun Y.; Gantner M. L.; Shao Z.; Evans L. P.; Saba N.; Fredrick T.; Burnim S.; Kim J. S.; Patel G.; Juan A. M.; Hurst C. G.; Hatton C. J.; Cui Z.; Pierce K. A.; Bherer P.; Aguilar E.; Powner M. B.; Vevis K.; Boisvert M.; Fu Z.; Levy E.; Fruttiger M.; Packard A.; Rezende F. A.; Maranda B.; Sapieha P.; Chen J.; Friedlander M.; Clish C. B.; Smith L. E. H. Retinal Lipid and Glucose Metabolism Dictates Angiogenesis through the Lipid Sensor Ffar1. Nat. Med. 2016, 22, 439–445. 10.1038/nm.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temme E. H.; Mensink R. P.; Hornstra G. Comparison of the Effects of Diets Enriched in Lauric, Palmitic, or Oleic Acids on Serum Lipids and Lipoproteins in Healthy Women and Men. Am. J. Clin. Nutr. 1996, 63, 897–903. 10.1093/ajcn/63.6.897. [DOI] [PubMed] [Google Scholar]
- Judd J. T.; Baer D. J.; Clevidence B. A.; Kris-Etherton P.; Muesing R. A.; Iwane M. Dietary Cis and Trans Monounsaturated and Saturated FA and Plasma Lipids and Lipoproteins in Men. Lipids 2002, 37, 123–131. 10.1007/s11745-002-0871-9. [DOI] [PubMed] [Google Scholar]
- Jensen L. D. E.; Hansen A. J.; Lundbæk J. A. Regulation of Endothelial Cell Migration by Amphiphiles—Are Changes in Cell Membrane Physical Properties Involved?. Angiogenesis 2007, 10, 13–22. 10.1007/s10456-006-9060-y. [DOI] [PubMed] [Google Scholar]
- Khan W. A.; Blobe G. C.; Hannun Y. A. Activation of Protein Kinase C by Oleic Acid. J. Biol. Chem. 1992, 267, 3605–3612. 10.1016/s0021-9258(19)50567-1. [DOI] [PubMed] [Google Scholar]
- Ye S.; Tan L.; Ma J.; Shi Q.; Li J. Polyunsaturated Docosahexaenoic Acid Suppresses Oxidative Stress Induced Endothelial Cell Calcium Influx by Altering Lipid Composition in Membrane Caveolar Rafts. Prostagl. Leukot. Essent. Fat. Acids 2010, 83, 37–43. 10.1016/j.plefa.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Amaral J.; Lee J. W.; Chou J.; Campos M. M.; Rodríguez I. R. 7-Ketocholesterol Induces Inflammation and Angiogenesis In Vivo: A Novel Rat Model. PLoS One 2013, 8, e56099 10.1371/journal.pone.0056099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillwell W.; Shaikh S. R.; LoCascio D.; Siddiqui R. A.; Seo J.; Chapkin R. S.; Wassall S. R.. Docosahexaenoic Acid. An Influential Membrane-Altering Omega-3 Fatty Acid. In Frontiers in Nutrition Research; Huang J. D., Ed.; Nova Science Publishers, Inc., 2006; pp 1–23. [Google Scholar]
- Xie Y.; Zhou W.; Zhong Z.; Yu H.; Zhang P.; Shen H. Docosahexaenoic Acid Inhibits Bone Remodeling and Vessel Formation in the Osteochondral Unit in a Rat Model. Biomed. Pharmacother. 2019, 114, 108811–108819. 10.1016/j.biopha.2019.108811. [DOI] [PubMed] [Google Scholar]
- Huber B.; Pischetsrieder M. Characterization of Ascorbylated Proteins by Immunochemical Methods. J. Agric. Food Chem. 1998, 46, 3985–3990. 10.1021/jf9803132. [DOI] [Google Scholar]
- Shaikh S. R.; Rockett B. D.; Salameh M.; Carraway K. Docosahexaenoic Acid Modifies the Clustering and Size of Lipid Rafts and the Lateral Organization and Surface Expression of MHC Class I of EL4 Cells. J. Nutr. 2009, 139, 1632–1639. 10.3945/jn.109.108720. [DOI] [PubMed] [Google Scholar]
- Polette A.; Deshayes C.; Chantegrel B.; Croset M.; Armstrong J. M.; Lagarde M. Synthesis of Acetyl, Docosahexaenoyl-Glycerophosphocholine and Its Characterization Using Nuclear Magnetic Resonance. Lipids 1999, 34, 1333–1337. 10.1007/s11745-999-0486-1. [DOI] [PubMed] [Google Scholar]
- Kang J. X.; Leaf A. Protective Effects of Free Polyunsaturated Fatty Acids on Arrhythmias Induced by Lysophosphatidylcholine or Palmitoylcarnitine in Neonatal Rat Cardiac Myocytes. Eur. J. Pharmacol. 1996, 297, 97–106. 10.1016/0014-2999(95)00701-6. [DOI] [PubMed] [Google Scholar]
- Bernoud N.; Fenart L.; Molière P.; Dehouck M.-P.; Lagarde M.; Cecchelli R.; Lecerf J. Preferential Transfer of 2-Docosahexaenoyl-1-Lysophosphatidylcholine Through an In Vitro Blood-Brain Barrier Over Unesterified Docosahexaenoic Acid. J. Neurochem. 1999, 72, 338–345. 10.1046/j.1471-4159.1999.0720338.x. [DOI] [PubMed] [Google Scholar]
- Semba R. D. Perspective: The Potential Role of Circulating Lysophosphatidylcholine in Neuroprotection against Alzheimer Disease. Adv. Nutr. 2020, 11, 760–772. 10.1093/advances/nmaa024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwary S.; Morales J. E.; Kwiatkowski S. C.; Lang F. F.; Rao G.; McCarty J. H. Metastatic Brain Tumors Disrupt the Blood-Brain Barrier and Alter Lipid Metabolism by Inhibiting Expression of the Endothelial Cell Fatty Acid Transporter Mfsd2a. Sci. Rep. 2018, 8, 8267–8280. 10.1038/s41598-018-26636-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L. N.; Ma D.; Shui G.; Wong P.; Cazenave-Gassiot A.; Zhang X.; Wenk M. R.; Goh E. L. K.; Silver D. L. Mfsd2a Is a Transporter for the Essential Omega-3 Fatty Acid Docosahexaenoic Acid. Nature 2014, 509, 503–506. 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- Chan J. P.; Wong B. H.; Chin C. F.; Galam D. L. A.; Foo J. C.; Wong L. C.; Ghosh S.; Wenk M. R.; Cazenave-Gassiot A.; Silver D. L. The Lysolipid Transporter Mfsd2a Regulates Lipogenesis in the Developing Brain. PLoS Biol. 2018, 16, e2006443 10.1371/journal.pbio.2006443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guemez-Gamboa A.; Nguyen L. N.; Yang H.; Zaki M. S.; Kara M.; Ben-Omran T.; Akizu N.; Rosti R. O.; Rosti B.; Scott E.; Schroth J.; Copeland B.; Vaux K. K.; Cazenave-Gassiot A.; Quek D. Q. Y.; Wong B. H.; Tan B. C.; Wenk M. R.; Gunel M.; Gabriel S.; Chi N. C.; Silver D. L.; Gleeson J. G. Inactivating Mutations in MFSD2A, Required for Omega-3 Fatty Acid Transport in Brain, Cause a Lethal Microcephaly Syndrome. Nat. Genet. 2015, 47, 809–813. 10.1038/ng.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugasini D.; Yalagala P. C. R.; Subbaiah P. V. Plasma BDNF Is a More Reliable Biomarker than Erythrocyte Omega-3 Index for the Omega-3 Fatty Acid Enrichment of Brain. Sci. Rep. 2020, 10, 10809–10818. 10.1038/s41598-020-67868-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong B. H.; Silver D. L.. Mfsd2a: A Physiologically Important Lysolipid Transporter in the Brain and Eye. Advances in experimental medicine and biology, 2020; Vol. 1276; pp 223–234. [DOI] [PubMed] [Google Scholar]
- Wong B. H.; Chan J. P.; Cazenave-Gassiot A.; Poh R. W.; Foo J. C.; Galam D. L. A.; Ghosh S.; Nguyen L. N.; Barathi V. A.; Yeo S. W.; Luu C. D.; Wenk M. R.; Silver D. L. Mfsd2a Is a Transporter for the Essential ω-3 Fatty Acid Docosahexaenoic Acid (DHA) in Eye and Is Important for Photoreceptor Cell Development. J. Biol. Chem. 2016, 291, 10501–10514. 10.1074/jbc.m116.721340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalagala P. R.; Sugasini D.; Dasarathi S.; Pahan K.; Subbaiah P. V. Dietary Lysophosphatidylcholine-EPA Enriches Both EPA and DHA in the Brain: Potential Treatment for Depression. J. Lipid Res. 2019, 60, 566–578. 10.1194/jlr.m090464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick R. P. Role of Phosphatidylcholine-DHA in Preventing APOE4-Associated Alzheimer’s Disease. FASEB J. 2019, 33, 1554–1564. 10.1096/fj.201801412r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingg J.-M.Water-Soluble Vitamin E—Tocopheryl Phosphate. In Advances in Food and Nutrition Research; Eskin N. A. M., Ed.; Elsevier, 2018; Vol. 83; pp 311–363. [DOI] [PubMed] [Google Scholar]
- Yakubenko V. P.; Byzova T. V. Biological and Pathophysiological Roles of End-Products of DHA Oxidation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 407–415. 10.1016/j.bbalip.2016.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duttaroy A. K.; Basak S. Maternal Dietary Fatty Acids and Their Roles in Human Placental Development. Prostagl. Leukot. Essent. Fat. Acids 2020, 155, 102080. 10.1016/j.plefa.2020.102080. [DOI] [PubMed] [Google Scholar]
- Lobanova E. S.; Schuhmann K.; Finkelstein S.; Lewis T. R.; Cady M. A.; Hao Y.; Keuthan C.; Ash J. D.; Burns M. E.; Shevchenko A.; Arshavsky V. Y. Disrupted Blood-Retina Lysophosphatidylcholine Transport Impairs Photoreceptor Health But Not Visual Signal Transduction. J. Neurosci. 2019, 39, 9689–9701. 10.1523/jneurosci.1142-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverría F.; Valenzuela R.; Catalina Hernandez-Rodas M.; Valenzuela A. Docosahexaenoic Acid (DHA), a Fundamental Fatty Acid for the Brain: New Dietary Sources. Prostagl. Leukot. Essent. Fat. Acids 2017, 124, 1–10. 10.1016/j.plefa.2017.08.001. [DOI] [PubMed] [Google Scholar]
- Bazan N. G. Neuroprotectin D1 (NPD1): A DHA-Derived Mediator That Protects Brain and Retina Against Cell Injury-Induced Oxidative Stress. Brain Pathol. 2006, 15, 159–166. 10.1111/j.1750-3639.2005.tb00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugasini D.; Thomas R.; Yalagala P. C. R.; Tai L. M.; Subbaiah P. V. Dietary Docosahexaenoic Acid (DHA) as Lysophosphatidylcholine, but Not as Free Acid, Enriches Brain DHA and Improves Memory in Adult Mice. Sci. Rep. 2017, 7, 11263. 10.1038/s41598-017-11766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel T.; Quek D. Q. Y.; Wong B. H.; Cazenave-Gassiot A.; Wenk M. R.; Fan H.; Berger I.; Shmueli D.; Shaag A.; Silver D. L.; Elpeleg O.; Edvardson S. Homozygous Mutation in MFSD2A, Encoding a Lysolipid Transporter for Docosahexanoic Acid, Is Associated with Microcephaly and Hypomyelination. Neurogenetics 2018, 19, 227–235. 10.1007/s10048-018-0556-6. [DOI] [PubMed] [Google Scholar]
- Ikeda I.; Sasaki E.; Yasunami H.; Nomiyama S.; Nakayama M.; Sugano M.; Imaizumi K.; Yazawa K. Digestion and Lymphatic Transport of Eicosapentaenoic and Docosahexaenoic Acids given in the Form of Triacylglycerol, Free Acid and Ethyl Ester in Rats. Biochim. Biophys. Acta Lipids Lipid. Metabol. 1995, 1259, 297–304. 10.1016/0005-2760(95)00180-8. [DOI] [PubMed] [Google Scholar]
- Ricaurte L.; Perea-Flores M. D. J.; Martinez A.; Quintanilla-Carvajal M. X. Production of High-Oleic Palm Oil Nanoemulsions by High-Shear Homogenization (Microfluidization). Innovative Food Sci. Emerging Technol. 2016, 35, 75–85. 10.1016/j.ifset.2016.04.004. [DOI] [Google Scholar]
- Naya M.; Imai M. Advantages of Supercritical Carbon Dioxide for Lipid Hydrolysis by Immobilized Lipase with Higher Reaction Rate and Reproducible of Repeated Use. J. Chem. Technol. Biotechnol. 2016, 91, 2620–2630. 10.1002/jctb.4861. [DOI] [Google Scholar]
- Lokman N. a.; Elder A. S. F.; Ricciardelli C.; Oehler M. K. Chick Chorioallantoic Membrane (CAM) Assay as an In Vivo Model to Study the Effect of Newly Identified Molecules on Ovarian Cancer Invasion and Metastasis. Int. J. Mol. Sci. 2012, 13, 9959–9970. 10.3390/ijms13089959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deryugina E. I.; Quigley J. P. Chick Embryo Chorioallantoic Membrane Model Systems to Study and Visualize Human Tumor Cell Metastasis. Histochem. Cell Biol. 2008, 130, 1119–1130. 10.1007/s00418-008-0536-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das N. M.; Hatsell S.; Nannuru K.; Huang L.; Wen X.; Wang L.; Wang L.-H.; Idone V.; Meganck J. A.; Murphy A.; Economides A.; Xie L. In Vivo Quantitative Microcomputed Tomographic Analysis of Vasculature and Organs in a Normal and Diseased Mouse Model. PLoS One 2016, 11, e0150085 10.1371/journal.pone.0150085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macía I.; Graña M.; Paloc C. Knowledge Management in Image-Based Analysis of Blood Vessel Structures. Knowl. Inf. Syst. 2012, 30, 457–491. 10.1007/s10115-010-0377-x. [DOI] [Google Scholar]
- Montoya-Zegarra J. A.; Russo E.; Runge P.; Jadhav M.; Willrodt A.-H.; Stoma S.; Nørrelykke S. F.; Detmar M.; Halin C. AutoTube: A Novel Software for the Automated Morphometric Analysis of Vascular Networks in Tissues. Angiogenesis 2019, 22, 223–236. 10.1007/s10456-018-9652-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onal S.; Uludag G.; Oray M.; Mengi E.; Herbort C. P.; Akman M.; Metin M. M.; Koc Akbay A.; Tugal-Tutkun I. Quantitative Analysis of Structural Alterations in the Choroid of Patients with Active Behçet Uveitis. Retina 2018, 38, 828–840. 10.1097/iae.0000000000001587. [DOI] [PubMed] [Google Scholar]
- Jonathan E.; Enfield J.; Leahy M. J.. Correlation Mapping: Rapid Method for Retrieving Microcirculation Morphology from Optical Coherence Tomography Intensity Images. Dynamics and Fluctuations in Biomedical Photonics VIII; Proceedings of SPIE: Bellingham, WA, USA, 2011; Vol. 7898, p 78980M. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.