Abstract
Fennel (Foeniculum vulgare Miller) is a popular aromatic plant native to the Mediterranean basin and cultivated worldwide that is valued for the nutritional and health benefits of its fruits. Headspace solid-phase microextraction of 12 fennel accessions of cultivated (F. vulgare subsp. vulgare) and wild forms (F. vulgare subsp. piperitum) of different origins was carried out for assessing their volatile distribution. Fifty-four volatiles were identified, with ethers amounting for the major class at ca. 52–99% attributed to the abundance of (E)-anethole and estragole. Several subsp. vulgare accessions proved to be excellent sources of the chief aroma (E)-anethole (95.9–98.4%), whereas high levels of estragole at ca. 72% were observed in subsp. piperitum from Minia and Khartoum and must be considered in the safety assessment of fennel. Other volatile classes were detected including ketones, esters, aldehydes, alcohols, and hydrocarbons (monoterpenes, sesquiterpenes, and diterpenes). Fenchone exceeded 15% of the total volatiles in some fennel specimens, linked to a conspicuous bitter aftertaste. The members of subsp. piperitum were more enriched in monoterpene hydrocarbons with sabinene found exclusively in these, while subsp. vulgare comprised a higher content of ethers. Principle component analysis determined isoterpinolene as a special component in subsp. piperitum. In all specimens from the same group, estragole was the most distinguished volatile compound according to the findings from orthogonal partial least squares-discriminant analysis. The highest estimated estragole levels were detected in subsp. piperitum from Minia at 89.8 mg/g. This comparative study provides the first comprehensive insight into volatile profiling of 12 fennel fruit varieties.
Introduction
Many culinary herbs and spices contain a variety of sensory metabolites that offer a wide range of aromatic, nutritional, and health-promoting effects.1 The carrot family (Apiaceae, Umbelliferae) is one of the largest families of flowering plants, encompassing ca. 450 genera and 3100–3200 species with members mainly distributed in the northern hemisphere,2 and it is well-known for its numerous aromatic species of commercial interest.3 Owing to their essential oil content, many herbaceous Apiaceae species are used in medicine, cosmetics, beverages, and food industry as flavoring and antimicrobial agents.4,5 Phenylpropanoids and terpenes are the major key aroma-imparting compounds in Apiaceae essential oils.6
Fennel (Foeniculum vulgare Miller) is one of the most popular aromatic plants within Apiaceae. The species is native to the Mediterranean basin, where it occurs as two commonly differentiated subspecies, subsp. vulgare and subsp. piperitum. The subspecies piperitum includes mostly wild forms.7 It is therefore considered as the ancestral form of subsp. vulgare, which contains mostly domesticated varieties.8 Fennel cultivars are widely used as flavorings in bread, cheese, ice cream, pickles, pastries, beverages, meat, and fish cuisines.9,10 While F. vulgare subsp. vulgare var. dulce is mainly used as a condiment, var. azoricum is a vegetable plant and var. vulgare is used as a source of (E)-anethole for pharmaceutical purposes.
While the oldest archaeological traces of fennel point to weedy forms in the Near Eastern Neolithic,11 the oldest archaeological indications for its use by humans are from Ancient Egypt’s Middle Kingdom, from 22nd to 18th century BCE,12,13 followed by finds from Carthage.14
Today, fennel is naturalized globally, sometimes even as an invasive weed,15 but mostly in its cultivated varieties. As an economic crop traded globally, fennel is commonly used in household remedies and as a culinary spice in many countries. Its traditional medicinal uses involve the treatment of gastrointestinal and upper respiratory complaints. It is highly recommended for patients suffering from catarrh, bronchitis, and chronic coughs as well as to prevent dyspeptic disorders (colic and flatulence) in nursing infants.16 Fennel fruits have been also used to treat dysmenorrhea, vomiting, kidney stones, and diarrhea.17 Other studies reported hepatoprotective, galactagogue, antimicrobial, anti-inflammatory, diuretic, antispasmodic, analgesic, and antioxidant effects of fennel.16,18
The main phytochemical components responsible for the therapeutic effects of fennel were phenolic and volatile compounds.19 Providing a widely available source of natural antioxidants, the major phenolic compounds present in fennel were flavonoid glucosides (eriocitrin and quercetin-3-O-glucuronide) and hydroxycinnamic acids (chlorogenic and cryptochlorogenic acids).20 In contrast, the prevalent volatile constituents in fennel were (E)-anethole, 1-octen-3-ol, estragole (methyl chavicol), and fenchone.16 Meanwhile, p-cymene, α-pinene, β-pinene, and limonene were detected as minor components.21 Accounting for over 98% of fennel oil, 78 volatile compounds were reported including phenylpropanoids, monoterpene hydrocarbons, and oxygenated monoterpenes.22 Based on the fennel’s phenotype and origin, relative ratios of these compounds vary greatly.23 The essential oil of subsp. piperitum was distinguished by relatively high levels of α-phellandrene, α-pentene, and fenchone, with low estragole and (E)-anethole concentrations.16 Moreover, the essential oil composition, especially monoterpene components, was significantly affected by climatic conditions, namely, rainfall and temperature.24 A recent study revealed the impact of roasting on fennel fruits and identified pyrans, furans, and pyrazines as newly occurring volatile classes upon roasting.25
Metabolomics is a relatively modern approach applied for profiling and/or fingerprinting of the overall chemical composition of plant-derived foods exposed to different conditions.26 For volatile compound profiling, metabolomics mainly uses hyphenated tools such as solid-phase microextraction (SPME) followed by gas chromatography (GC) coupled with mass spectrometry (MS).27 Headspace solid-phase microextraction (HS-SPME) is a very useful rapid and solvent-free technique to extract the less abundant volatiles, allowing their detection without the production of artifacts upon thermal decomposition.28
This study aimed to compare the aroma profiles of different fennel varieties collected at various localities to determine the metabolite heterogeneity among these varieties using multivariate data analyses and consequently for QC applications involving the detection of the authenticity and/or adulteration of fennel samples. Another goal was to determine the estragole levels in these accessions considering their relatively high toxicity.
Results and Discussion
Different varieties of fennel as indicated in Table 1 were subjected to comprehensive volatile profiling. Multivariate data analysis was further employed to help recognize the specimen markers and classification in an untargeted manner.
Table 1. Origin of the Different F. Mill. (Fennel) Fruit Accessions Used in the Analysisa,b.
| taxon and variety | origin | vendor and batch number/botanical garden and index seminum | internal sample code |
|---|---|---|---|
| F. vulgare Mill. subsp. piperitum (Ucria) Cout. | |||
| ND | Egypt, Fayoum | P-01 | |
| ND | Egypt, Minia | P-02 | |
| ND | Sudan, Khartoum | P-03 | |
| ND | ND | P-04 | |
| F. vulgare Mill. subsp. vulgare | |||
| var. azoricum (Mill.) Thell. “Deutscher Gemüsefenchel” | Austria | Arche Noah (FN014, 02–17) | V-A1 |
| var. azoricum (Mill.) Thell. “Fino” | Austria | Reinsaat (Fe11 DBO 90) | V-A2 |
| var. dulce (Mill.) P.FournThell. | Italy | Drogheria & Alimentari (L07315N) | V-D1 |
| var. dulce (Mill.) P.FournThell. | Austria | Kotányi (L 170314 1012) | V-D2 |
| var. vulgare | Austria | Kottas Pharma (W15205845) | V-V1 |
| ND | Germany | HOH (IS 1990, No. 1431) | V-01 |
| ND | Austria | Sonnentor (SOW15111904F04) | V-02 |
| ND | India | P&B Foods (1G1211216) | V-03 |
ND: not determined.
HOH: Hohenheimer Gärten (botanical garden and herbarium at the University of Hohenheim, Stuttgart, Germany).
Volatile Components and Their Contribution to the Aroma of Fennel Accessions Via SPME Analysis
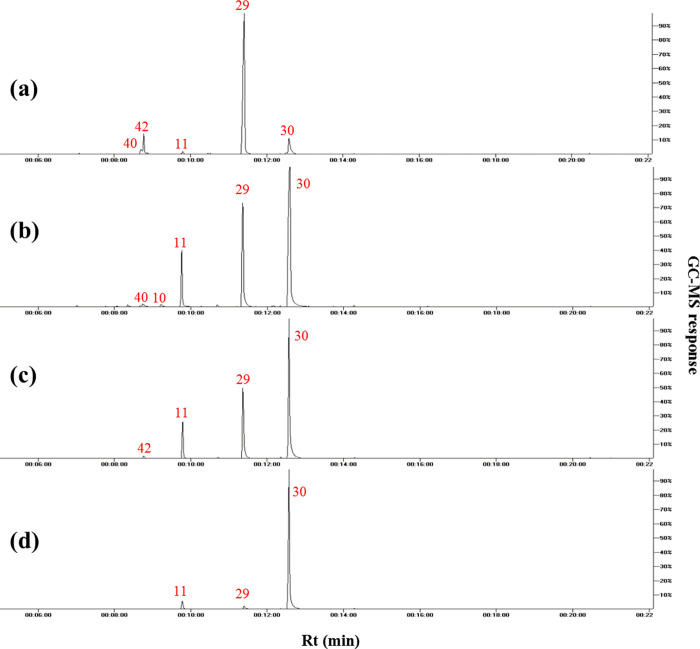
GC–MS analysis of volatiles (Figure 1) preceded by SPME resulted in the detection of 54 peaks belonging to ethers, ketones, esters, aldehydes, alcohols, monoterpenes, sesquiterpenes, and diterpenes as listed in Table 2 with ethers amounting for the major class in all fennel varieties. SPME is a relatively recent technique for aroma extraction without the use of solvents, in addition to its good sensitivity, low temperature used during extraction, and simplicity in revealing the true aroma of fennel fruits.29
Figure 1.
Representative SPME–GC–MS total ion chromatograms of volatiles acquired from (a) P-03, (b) P-04, (c) V-D1, and (d) V-A2. For codes and peak numbers, refer to Tables 1 and 2.
Table 2. Relative Percentage of Volatile Metabolites in F. vulgare Fruit Accessions Analyzed Via SPME–GC–MSa.
| no. | name | Rt(min) | RI | P-01 | P-03 | P-02 | P-04 | V-D1 | V-D2 | V-A2 | V-A1 | V-01 | V-02 | V-V1 | V-03 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| alcohols | |||||||||||||||
| 1 | linalool | 9.921 | 1076 | 0.20 ± 0.19 | - | - | 0.01 ± 0.02 | - | - | - | - | - | - | - | - |
| 2 | 4,8-dimethyl-1,7-nonadien-4-ol | 10.139 | 1090 | 0.07 ± 0.09 | - | - | - | - | - | - | - | - | - | - | - |
| 3 | fenchol | 10.269 | 1098 | 0.14 ± 0.12 | 0.03 ± 0.02 | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.02 ± 0.01 | 0.28 ± 0.27 | 0.03 ± 0.02 | 0.03 ± 0.02 | - | - | - | - |
| 4 | 2,6-dimethyl-2,7-octadiene-1,6-diol | 10.513 | 1115 | 0.35 ± 0.21 | 0.36 ± 0.06 | 0.47 ± 0.10 | 0.02 ± 0.00 | 0.04 ± 0.05 | 0.22 ± 0.26 | 0.04 ± 0.01 | 0.02 ± 0.01 | - | 0.04 ± 0.02 | - | - |
| 5 | (Z)-β-terpineol | 11.153 | 1161 | 0.05 ± 0.03 | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.01 ± 0.01 | - | - | 0.01 ± 0.01 | - | - | - | - | - |
| 6 | (Z)-carveol | 11.828 | 1209 | 0.09 ± 0.02 | 0.09 ± 0.02 | 0.09 ± 0.01 | - | - | - | - | - | - | - | - | - |
| 7 | nerolidol | 15.443 | 1497 | 0.14 ± 0.07 | - | - | - | - | - | - | - | - | - | - | - |
| 8 | 1,14-tetradecanediol | 20.587 | 1794 | 0.07 ± 0.04 | - | 0.01 ± 0.02 | - | - | - | 0.01 ± 0.01 | - | - | - | - | - |
| 9 | isophytol | 21.703 | 1863 | 1.06 ± 0.68 | 0.05 ± 0.01 | 0.07 ± 0.00 | - | - | 0.03 ± 0.04 | 0.12 ± 0.10 | 0.01 ± 0.01 | - | - | - | - |
| total alcohols | - | - | 2.17 | 0.57 | 0.75 | 0.09 | 0.06 | 0.53 | 0.22 | 0.06 | - | 0.04 | - | - | |
| ketones | |||||||||||||||
| 10 | fenchone isomer | 9.633 | 1059 | 0.07 ± 0.10 | - | 0.09 ± 0.13 | 1.58 ± 2.23 | - | 0.22 ± 0.31 | - | - | - | - | - | - |
| 11 | fenchone | 9.781 | 1068 | 4.02 ± 2.89 | 5.91 ± 2.06 | 1.38 ± 0.21 | 15.74 ± 4.24 | 0.51 ± 0.07 | 6.23 ± 2.02 | 16.90 ± 2.54 | 3.20 ± 0.30 | 1.05 ± 0.31 | 1.58 ± 0.35 | 1.93 ± 0.25 | 2.12 ± 0.31 |
| 12 | camphor | 10.711 | 1130 | 0.05 ± 0.04 | 0.08 ± 0.00 | 0.02 ± 0.00 | 0.26 ± 0.06 | - | 0.03 ± 0.02 | 0.17 ± 0.02 | 0.02 ± 0.00 | - | - | - | - |
| 13 | carvone | 12.145 | 1233 | 0.14 ± 0.07 | 0.26 ± 0.08 | 0.27 ± 0.03 | 0.05 ± 0.01 | - | 0.08 ± 0.05 | 0.29 ± 0.05 | 0.93 ± 0.02 | - | - | - | - |
| 14 | jasmone | 13.837 | 1366 | 8.22 ± 4.34 | - | - | - | - | - | - | - | - | - | - | - |
| 15 | anisylacetone | 14.052 | 1382 | 0.15 ± 0.09 | 0.18 ± 0.08 | 0.40 ± 0.07 | - | - | - | 0.25 ± 0.35 | 0.31 ± 0.13 | - | - | 0.06 ± 0.05 | - |
| 16 | 4-methoxypropiophenone | 14.675 | 1434 | 2.32 ± 0.75 | - | - | - | - | - | - | - | - | - | - | - |
| total ketones | - | - | 14.98 | 6.43 | 2.17 | 17.63 | 0.51 | 6.56 | 17.61 | 4.45 | 1.05 | 1.58 | 2.00 | 2.12 | |
| aldehydes | |||||||||||||||
| 17 | p-anisaldehyde | 12.35 | 1248 | 1.43 ± 0.28 | 0.08 ± 0.02 | 0.19 ± 0.11 | 0.25 ± 0.08 | 0.32 ± 0.10 | 0.67 ± 0.26 | 0.67 ± 0.19 | 0.25 ± 0.03 | 0.04 ± 0.02 | 0.90 ± 0.96 | 0.09 ± 0.08 | 0.12 ± 0.10 |
| 18 | methoxycinnamaldehyde | 16.547 | 1573 | 0.27 ± 0.09 | - | - | - | - | - | - | - | - | - | - | - |
| total aldehydes | - | - | 1.70 | 0.08 | 0.19 | 0.25 | 0.32 | 0.67 | 0.67 | 0.25 | 0.04 | 0.90 | 0.09 | 0.12 | |
| esters | |||||||||||||||
| 19 | benzyl acetate | 10.936 | 1145 | 1.35 ± 0.88 | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.01 ± 0.01 | - | - | - | - | - | - | - | - |
| 20 | sabinyl acetate | 11.488 | 1189 | 0.02 ± 0.00 | 3.47 ± 4.86 | 0.02 ± 0.01 | 0.14 ± 0.14 | - | - | 0.02 ± 0.01 | - | - | - | - | - |
| 21 | fenchyl acetate | 11.758 | 1203 | 0.21 ± 0.03 | 0.22 ± 0.01 | 0.16 ± 0.05 | 0.13 ± 0.04 | 0.04 ± 0.03 | 0.29 ± 0.33 | 0.15 ± 0.06 | 0.06 ± 0.01 | - | 0.01 ± 0.01 | - | 0.01 ± 0.01 |
| 22 | bornyl formate | 12.488 | 1257 | 0.98 ± 0.90 | 1.01 ± 0.24 | 1.64 ± 0.36 | 0.54 ± 0.38 | - | 0.80 ± 1.14 | 0.52 ± 0.37 | 0.06 ± 0.03 | - | - | - | - |
| 23 | (Z)-3-hexenyl benzoate | 16.061 | 1541 | 0.60 ± 0.67 | - | - | - | - | - | - | - | - | - | - | - |
| 24 | methyl jasmonate | 17.125 | 1611 | 0.13 ± 0.11 | - | - | - | - | - | - | - | - | - | - | - |
| 25 | benzyl benzoate | 19.794 | 1752 | 3.33 ± 3.61 | - | - | - | - | - | - | - | - | - | - | - |
| 26 | methyl ester of palmitic acid | 21.504 | 1850 | 0.40 ± 0.37 | 0.03 ± 0.02 | 0.06 ± 0.02 | - | - | - | - | - | - | - | - | - |
| total esters | - | - | 7.01 | 4.73 | 1.89 | 0.81 | 0.04 | 1.10 | 0.68 | 0.12 | - | 0.01 | - | 0.01 | |
| ethers/oxides | |||||||||||||||
| 27 | cineole | 8.849 | 1008 | 0.36 ± 0.20 | 0.38 ± 0.13 | 0.33 ± 0.10 | 0.12 ± 0.04 | 0.06 ± 0.03 | 0.59 ± 0.80 | 0.10 ± 0.01 | 0.03 ± 0.01 | - | 0.06 ± 0.05 | - | - |
| 28 | limonene oxide | 10.436 | 1110 | 0.28 ± 0.16 | 0.22 ± 0.03 | 0.34 ± 0.05 | 0.06 ± 0.01 | 0.08 ± 0.05 | 0.26 ± 0.28 | 0.10 ± 0.01 | 0.02 ± 0.00 | - | 0.04 ± 0.02 | - | - |
| 29 | estragole | 11.387 | 1177 | 12.31 ± 8.22 | 72.38 ± 1.41 | 71.63 ± 0.59 | 26.81 ± 6.57 | 0.74 ± 0.15 | 2.10 ± 0.54 | 20.98 ± 1.97 | 1.34 ± 0.06 | 0.30 ± 0.02 | 0.68 ± 0.31 | 0.18 ± 0.01 | 0.45 ± 0.02 |
| 30 | (E)-anethole | 12.597 | 1267 | 38.84 ± 6.17 | 0.15 ± 0.02 | 5.04 ± 2.76 | 49.55 ± 11.52 | 97.78 ± 0.63 | 84.35 ± 5.27 | 56.69 ± 1.34 | 92.93 ± 0.15 | 98.43 ± 0.29 | 95.90 ± 1.24 | 97.57 ± 0.24 | 96.94 ± 0.02 |
| 31 | eugenol | 13.596 | 1343 | 0.23 ± 0.13 | - | - | - | - | - | - | - | - | - | - | - |
| total ethers/oxides | - | - | 52.01 | 73.13 | 77.34 | 76.53 | 98.66 | 87.30 | 77.87 | 94.33 | 98.73 | 96.68 | 97.76 | 97.39 | |
| monoterpene hydrocarbons | |||||||||||||||
| 32 | unknown | 6.84 | 893 | 0.05 ± 0.06 | 0.12 ± 0.16 | 0.17 ± 0.10 | 0.03 ± 0.04 | - | - | - | - | - | - | - | - |
| 33 | α-thujene | 6.963 | 899 | 0.02 ± 0.02 | 0.18 ± 0.11 | 0.19 ± 0.13 | 0.07 ± 0.05 | - | - | - | - | - | - | - | - |
| 34 | α-pinene | 7.071 | 905 | 0.55 ± 0.27 | 0.79 ± 0.24 | 0.32 ± 0.16 | 0.26 ± 0.09 | - | - | 0.04 ± 0.02 | - | - | - | - | - |
| 35 | sabinene | 7.789 | 946 | 0.10 ± 0.07 | 0.15 ± 0.05 | 0.11 ± 0.05 | 0.07 ± 0.04 | - | - | - | - | - | - | - | - |
| 36 | β-pinene | 7.889 | 952 | 0.07 ± 0.03 | 0.05 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.01 | 0.01 ± 0.01 | 0.26 ± 0.20 | 0.03 ± 0.02 | 0.04 ± 0.01 | - | - | - | - |
| 37 | myrcene | 8.072 | 962 | 0.09 ± 0.01 | 0.12 ± 0.06 | 0.15 ± 0.07 | 0.33 ± 0.17 | 0.04 ± 0.03 | 0.60 ± 0.65 | 0.19 ± 0.04 | 0.05 ± 0.02 | - | - | - | - |
| 38 | α-phellandrene | 8.381 | 980 | 2.85 ± 4.03 | - | 1.52 ± 2.14 | - | - | - | - | - | - | - | - | - |
| 39 | limonene isomer | 8.461 | 987 | 0.24 ± 0.33 | - | 0.84 ± 1.18 | - | - | - | - | - | - | - | - | - |
| 40 | isoterpinolene | 8.675 | 997 | 4.17 ± 2.96 | 5.88 ± 2.63 | 3.04 ± 2.28 | 0.87 ± 0.46 | - | 0.20 ± 0.28 | 0.44 ± 0.55 | - | - | - | - | - |
| 41 | p-cymene | 8.698 | 998 | 0.39 ± 0.27 | 0.31 ± 0.09 | 0.20 ± 0.08 | 0.96 ± 0.89 | - | 0.04 ± 0.06 | 0.06 ± 0.01 | - | - | - | - | - |
| 42 | limonene | 8.765 | 1003 | 3.73 ± 2.64 | 6.90 ± 0.45 | 10.13 ± 5.60 | 0.83 ± 0.40 | - | 0.27 ± 0.24 | 1.14 ± 0.28 | 0.19 ± 0.05 | - | - | - | - |
| 43 | τ-terpinene | 9.239 | 1033 | 0.14 ± 0.10 | 0.23 ± 0.13 | 0.07 ± 0.03 | 0.73 ± 0.36 | - | - | 0.08 ± 0.03 | - | - | - | - | - |
| 44 | β-terpinene | 9.553 | 1053 | 4.02 ± 5.68 | - | 0.25 ± 0.35 | - | - | 0.59 ± 0.83 | - | - | - | - | - | - |
| 45 | isoterpinolene isomer | 9.667 | 1060 | - | - | - | 0.12 ± 0.07 | - | - | - | - | - | - | - | - |
| 46 | fenchene | 10.339 | 1104 | 0.29 ± 0.28 | 0.06 ± 0.02 | 0.06 ± 0.02 | 0.05 ± 0.01 | 0.08 ± 0.05 | 1.08 ± 1.46 | 0.10 ± 0.05 | 0.02 ± 0.00 | - | 0.04 ± 0.02 | - | - |
| total monoterpene hydrocarbons | - | - | 16.70 | 14.77 | 17.08 | 4.34 | 0.13 | 3.04 | 2.08 | 0.31 | - | 0.04 | - | - | |
| sesquiterpene hydrocarbons | |||||||||||||||
| 47 | bergamotene | 14.282 | 1402 | 0.13 ± 0.10 | 0.08 ± 0.02 | 0.14 ± 0.07 | 0.25 ± 0.04 | 0.29 ± 0.11 | 0.58 ± 0.24 | 0.43 ± 0.13 | 0.22 ± 0.02 | 0.13 ± 0.05 | 0.75 ± 0.75 | 0.13 ± 0.08 | 0.33 ± 0.21 |
| 48 | β-farnesene | 14.296 | 1410 | 0.03 ± 0.01 | - | 0.01 ± 0.01 | 0.03 ± 0.03 | - | - | - | 0.02 ± 0.01 | - | - | - | - |
| 49 | α-farnesene | 14.842 | 1448 | 0.06 ± 0.07 | - | - | - | - | - | - | 0.09 ± 0.01 | - | - | - | - |
| 50 | α-curcumene | 14.887 | 1452 | 0.06 ± 0.05 | 0.05 ± 0.01 | 0.10 ± 0.02 | - | - | - | 0.02 ± 0.02 | 0.01 ± 0.02 | - | - | - | - |
| 51 | α-farnesene isomer | 14.989 | 1460 | 0.44 ± 0.52 | 0.03 ± 0.01 | 0.07 ± 0.04 | 0.04 ± 0.03 | - | 0.22 ± 0.13 | 0.04 ± 0.02 | 0.08 ± 0.04 | 0.04 ± 0.01 | - | 0.02 ± 0.02 | 0.02 ± 0.03 |
| 52 | unknown | 20.758 | 1803 | 0.78 ± 0.99 | 0.02 ± 0.01 | 0.05 ± 0.04 | - | - | - | 0.03 ± 0.02 | - | - | - | - | - |
| total sesquiterpene hydrocarbons | - | - | 1.51 | 0.18 | 0.36 | 0.32 | 0.29 | 0.80 | 0.52 | 0.42 | 0.16 | 0.75 | 0.15 | 0.36 | |
| diterpene hydrocarbons | |||||||||||||||
| 53 | neophytadiene isomer | 20.44 | 1786 | 2.91 ± 3.81 | 0.07 ± 0.04 | 0.15 ± 0.07 | 0.02 ± 0.01 | - | 0.01 ± 0.01 | 0.30 ± 0.12 | 0.05 ± 0.04 | - | - | - | - |
| 54 | neophytadiene | 20.985 | 1818 | 1.02 ± 1.28 | 0.03 ± 0.01 | 0.07 ± 0.03 | - | - | - | 0.04 ± 0.03 | - | - | - | - | - |
| total diterpene hydrocarbons | - | - | 3.93 | 0.10 | 0.22 | 0.02 | - | 0.01 | 0.34 | 0.05 | - | - | - | - | |
Ethers/Oxides
Ethers/oxides were the most abundant class in all fennel varieties amounting for ca. 52.0–98.7% confirming their antioxidant and antimicrobial effects that make them as valuable food ingredients.30 The results were in agreement with a previous study where ethers reached 76%.31 Reaching 98.4% of the total volatiles in V-01, (E)-anethole (30) was the main volatile component in all fennel specimens except for subsp. piperitum from Minia and Khartoum (P-02 and P-03) where estragole (29) prevailed at 71.6–72.4%. (E)-anethole and its isomer estragole are phenylpropanoid derivatives widely distributed in other plants. (E)-anethole (30) constitutes the main volatile component in Illicium anisatum (star anise) and Pimpinella anisum (anise), while estragole (29) is abundant in Ocimum basilicum (sweet basil) and Artemisia dracunculus (tarragon).32 (E)-Anethole (30) is responsible for the sweet, distinct, aniselike flavor characterizing fennel fruits leading to its incorporation in cosmetics and food industry as a perfume and flavoring agent, respectively.33 Conversely, estragole (29) has no significant impact on the overall aroma of fennel albeit estragole closely related alkenylbenzenes, that is, methyleugenol and safrole are recognized as carcinogens (IARC class 2B) that led the European Union (EU) to limit the use of estragole in beverages to 10 mg/kg.21 Aside from its deep flavor, (E)-anethole (30) is reported to exhibit a broad range of pharmacological effects, namely, neuroprotective, immunomodulatory, antidiabetic, and anti-inflammatory effects.34 In contrast, estragole (29) has recently attracted attention for being genotoxic and a hepatocarcinogen.35 These effects are attributed to the sulfuric ester of 1′-hydroxyestragole, a metabolite of estragole, forming an adduct with DNA.36 Acknowledging these hazards, the European Medicine Agency recommended that administration of estragole should be minimized for nursing mothers, infants, and pregnant women.37 In addition, although no authority has prevented the use of estragole-containing herbs, the EU Commission prohibited estragole as a food additive.35 Therefore, the elevated levels of estragole (29) in P-03 and P-02 have to be considered in the assessment of whether these wild fennel varieties could represent hazards to consumers’ health. Eugenol (31), a natural spicy fragrance characterizing clove buds and cinnamon barks,38 was detected exclusively in P-01 samples suggestive to be a characterizing component for that accession.
Ketones
Ketones were the second dominant class of volatiles after ethers/oxides in all varieties of subsp. vulgare in addition to P-04 from subsp. piperitum. Ketones existed at comparable levels in P-04 and V-A2 at ca. 17.6% but were found at much lower levels in V-D1 at 0.5%, suggestive of the impact of origin and agricultural conditions, that is, soil, temperature, and water on fennel aroma. Our finding was in accordance with a previous study where ketones reached 19%.39 Fenchone (11) was the only ketone detected in all fennel accessions and was reported to be another major volatile constituent of fennel reaching 16.9 and 15.7% in V-A2 and P-04, respectively. Accounting for the bitter aftertaste of fennel, fenchone (11) is used as a food flavor due to its camphorlike odor,40 aside from its wound healing, acaricidal, and antifungal effects.41 The European Pharmacopoeia stated that the volatile oil of bitter fennel encompasses at least 15% of fenchone.42 Jasmone (14) and 4-methoxypropiophenone (16) were present exclusively in subsp. piperitum from Fayoum (P-01) at 8.2 and 2.3%, respectively, suggestive to be distinguishing components for that accession. 4-Methoxypropiophenone has a fruity, acetonelike flavor,43 while jasmone (14) is used widely in the manufacturing of perfumes owing to its warm fruity smell.44
Mono-/Sesqui-/Diterpene Hydrocarbons
Compared to ketones, monoterpene hydrocarbons were the second abundant class after ethers/oxides in subsp. piperitum from Egypt and Sudan (P-01, P-02, and P-03) at ca. 14.8–17.1% and to a lesser extent in P-04 in addition to V-D2 and V-A2, two different varieties from subsp. vulgare as indicated in Table 1 (2.1–4.3%), and reached almost 0% in the rest of the accessions. This was in accordance with a previous study where hydrocarbons reached 7%.39 The major identified monoterpenes included limonene (39, 42) and isoterpinolene (40, 45) detected at their highest levels in P-02 (10.1%) and P-03 (5.9%), respectively. Limonene (39, 42), a major constituent of citrus fruits, is added to many food products for its lemonlike flavor aside from its protective effect against multimodal intestinal inflammation.45 Comparable quantities of isoterpinolene (40, 45) and its precursor α-pinene (34)(46) were detected, accounting for the positive correlation between their levels in the studied samples. α-Phellandrene (38) was detected only in P-01 and P-02 at 2.9 and 1.5%, respectively, with a minty, herbal, spicy note,47 suggestive to be a distinguishing component for these fennel accessions.
P-01, a subsp. piperitum from Fayoum, encompassed the highest levels of sesqui- and diterpene hydrocarbons at 1.5 and 3.9%, respectively. Neophytadiene (53, 54) was the only detected diterpene. This branched hydrocarbon is a predominant component in tobacco leaves48 and exhibits a myriad of effects, that is, antioxidant, anti-inflammatory, antimicrobial, and analgesic effects.49
Alcohols/Aldehydes/Esters
Contributing to the antioxidant and antimicrobial effects of various essential oils,50 alcohols were most abundant in P-01 at 2.1% as exemplified by isophytol (9). Isophytol (9) is used as a flavoring agent due to its fine floral balsamic smell.51 Aldehydes included less diverse number of volatiles than alcohols with methoxycinnamaldehyde (18) detected exclusively in P-01. In contrast, p-anisaldehyde (17) was present in all fennel accessions and had been previously reported as a component of fennel.31p-Anisaldehyde (17) has a strong, sweet, aniselike fragrance and is formed from (E)-anethole (30) upon oxidation.52 Esters were more abundant in subsp. piperitum from Egypt and Sudan (P-01, P-02, and P-03) ranging from 1.9 to 7.0% compared to the other specimens. Sabinyl acetate (20) was present at a much higher level in P-03 (3.5%) concurrent with the highest levels of its precursor sabinene (35). Sabinyl acetate (20) detected also in V-A2 (a ssp. vulgare var. azoricum) has a fresh spicy, fruity note, while fenchyl acetate (21) is characterized by a camphoraceous, herbal flavor.53 Having a balsamic, herbal aroma, benzyl benzoate (25) was found only in subsp. piperitum from Fayoum P-01 (Table 1) at 3.3%. Benzyl acetate (19) was detected at higher levels (1.4%) in P-01 with a floral, fresh sweet note.54
Multivariate Data Analysis of Fennel Fruit Aroma Profile
Recognizing the considerable number of detected peaks in 12 fennel accessions, each comprising of three replicates, we tried to categorize them holistically using chemometric tools. Multivariate data analyses including principal component analysis (PCA) and hierarchical clustering analysis (HCA) as unsupervised pattern recognition models were applied to the volatile metabolite GC–MS abundance data set (Figure 2). The PCA score plot (Figure 2a) showed 54% of the total variance among the samples prescribed by PC1 and PC2. It also revealed good segregation of the two clusters, subsp. piperitum from Khartoum and Minia (P-03 and P-02), at the right side of PC1, in addition to a part from subsp. piperitum from Fayoum P-01 samples. Conversely, all other specimens including subsp. vulgare were positioned collectively at the left side of PC1 albeit distributed along PC2. Examination of the corresponding loading plot (Figure 2b) revealed that estragole (29) was the discriminatory volatile component for P-02, rationalizing for its allocation at the far side of PC1 as shown in Figure 2a. In contrast, limonene (42), isoterpinolene (40), and bornyl formate (22) were identified as markers for subsp. piperitum from Egypt and Sudan (P-01, P-02, and P-03) and confirmed their enrichment in monoterpene hydrocarbons. Oxygenated monoterpenes, that is, ketones were represented by fenchone (11) in P-04 and V-A2, whereas (E)-anethole (30) was more enriched in V-V1, ssp. vulgare var. vulgare, and V-02 leading to higher levels of ethers/oxides. Similar findings with consistent clustering of both subsp. piperitum samples from Khartoum and Minia (P-03 and P-02) at one subgroup were observed, whereas overlapping of the other samples was observed in the HCA-derived dendrogram at the other subgroup (Figure 2c).
Figure 2.
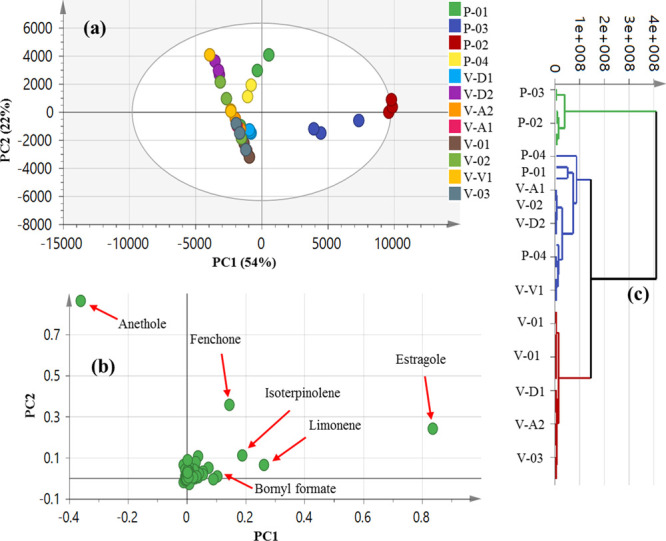
Unsupervised multivariate data of the studied F. vulgare fruit accessions derived from modeling of volatile profiles analyzed via GC–MS (n = 3). (a) PCA score plot of PC1 vs PC2 scores. (b) Respective loading plot for PC1 and PC2 providing their assignments. (c) HCA plot. The metabolome clusters are placed in a two-dimensional space at distinct locations defined by two vectors of principal components PC1 = 54% and PC2 = 22%. For codes, refer to Table 1.
Another PCA model (Figure S1) was adopted to help identify the differences among subsp. vulgare that was clustered in the first PCA model (Figure 2). However, this second model was not successful in discriminating subsp. vulgare of different origins. Subsequently, OPLS-DA (Figure 3) was further employed to model subsp. vulgare against subsp. piperitum accessions to reveal the volatile characteristics of each fennel subspecies with a p value less than 0.001. The clear discrimination between the two subspecies in the OPLS-DA score plot (Figure 3a), in addition to the respective model parameters R2 and Q2 at 0.72 and 0.67, respectively, indicated good model fitness and predictability. The OPLS-DA-derived S-plot (Figure 3b) revealed that estragole (29) was more enriched in subsp. piperitum with no markers for subsp. vulgare. This result suggested that absolute quantification of estragole in subsp. piperitum should now follow to assess the safety of regular use of fennel.
Figure 3.
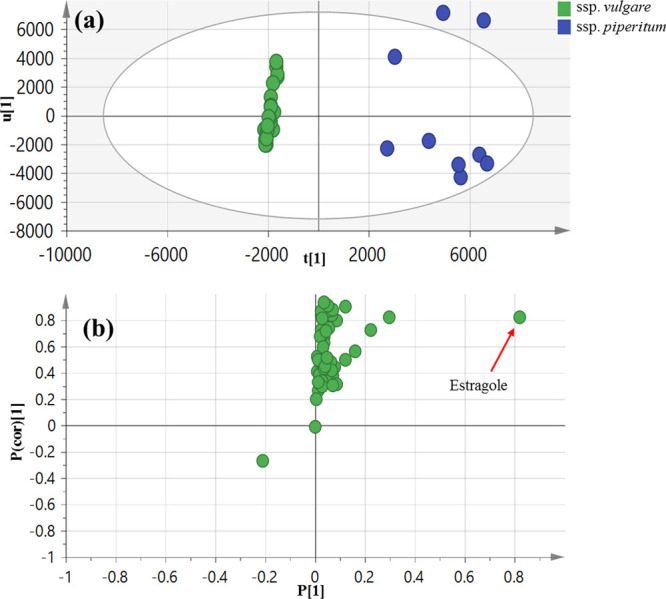
HS-SPME GC–MS-based OPLS-DA score plot (a) derived from modeling volatile metabolites of subsp. vulgare against subsp. piperitum (n = 3). The respective loading S-plots (b) showing the covariance p[1] against the correlation p(cor)[1] of the variables of the discriminating component of the OPLS-DA model. Cutoff values of p < 0.001 were used. Designated variables are highlighted and identifications are discussed in the text.
Absolute Determination of Estragole Levels in Fennel Accessions
Being a potential genotoxic carcinogen, estragole detected at levels higher than 70% in some fennel fruits necessitated its accurate determination.55 The mechanism of its carcinogenicity was previously explained owing to the release of 1′-hydroxyestragole.36 HS-SPME coupled with GC–MS was applied as a simple method for estragole quantification using standard estragole over a concentration range of 1–500 μg/mL. Quantitative differences were observed in estragole levels among fennel fruit accessions (Table 3) ranging from 0.03 to 89.86 mg/g. P-02 subsp. piperitum from Minia contained the highest levels at 89.86 mg/g of estragole indicating higher levels than that usually reported. In general, subsp. piperitum encompassed much higher amounts of estragole at ca. 17.4–89.9 mg/g compared with subsp. vulgare (0.03–4.5 mg/g) as indicated by HCA modeling of fennel accessions based on its estragole level only (Figure S2). Considering that 5 g of P-02 is used to make fennel tea per day, the average estragole exposure for infants would be at ca. 35 mg/kg body weight compared to 0.02 mg/kg reported from another study.56 However, 33 mg/kg body weight of estragole induced hepatocellular carcinoma in female mice exposed for 12 months.57 Further assessment is required for exposure of estragole in sensitive groups including infants, young children, nursing mothers, and pregnant women.
Table 3. Absolute Determination of Estragole Levels in Different Fennel Varietiesa.
| fennel accession | estragole level mg/g |
|---|---|
| P-01 | 17.36 ± 2.41 |
| P-03 | 54.25 ± 12.57 |
| P-02 | 89.86 ± 1.05 |
| P-04 | 24.59 ± 21.88 |
| V-D1 | 4.50 ± 0.44 |
| V-D2 | 0.63 ± 0.06 |
| V-A2 | 0.32 ± 0.27 |
| V-A1 | 0.05 ± 0.03 |
| V-01 | 0.03 ± 0.03 |
| V-02 | 0.04 ± 0.03 |
| V-V1 | 0.08 ± 0.04 |
| V-03 | 0.04 ± 0.03 |
Conclusions
The present study provides the most comprehensive aroma profiling amongst F. vulgare fruit accessions of various origins. Moreover, the significant discriminatory capacity of subsp. vulgare and subsp. piperitum has been revealed. Within the identified 54 volatiles, ethers, ketones, esters, and monoterpene hydrocarbons were the major classes that mediate the medicinal and culinary attributes of fennel. Ethers expectedly were the most abundant class and their enriched levels can be observed in subsp. vulgare due to their elevated (E)-anethole level. Fenchone, as a source of bitterness in fennel, was detected in at 16.9%, implying a more bitter taste compared to other accessions. In contrast, subsp. vulgare var. dulce from Italy likely exhibited the sweetest taste, being the lowest in fenchone (0.5%) and most enriched in (E)-anethole (97.8%). Subsp. piperitum from Fayoum, Egypt appeared to be the most diverse in volatiles with 53 volatile metabolites with jasmone, 4-methoxy-propiophenone, methoxycinnamaldehyde, 3-hexenyl benzoate, methyl jasmonate, and benzyl benzoate present exclusively in this accession. Among the main identified volatiles, estragole reached maximal levels in mostly F. vulgare Mill. subsp. piperitum accessions with maximal levels in specimens from Khartoum and Minia. Absolute quantification of estragole was determined to enable the estimation of dietary exposures of fennel varieties, with subsp. piperitum encompassing much higher levels of estragole (17.4–89.9 mg/g) compared with subsp. vulgare (0.03–4.5 mg/g) indicating a potential risk for the former subspecies. These results suggest that the volatile biosynthesis of subsp. piperitum is more activated toward the production of estragole compared to subsp. vulgare though such hypothesis needs to be confirmed by analyzing accessions from other origins. The exposure of estragole in infants and young children needs extensive investigation and clear dietary and pharmaceutical regulatory guidelines regarding the nonspecific label information found on most fennel products should be mandated to specify estragole levels in the formulation as in other typical food hazards, that is, trans fat.
Estragole cannot be identified as a chemomarker for piperitum subspecies, owing to its presence in subsp. vulgare as well. Profiling of secondary metabolites in both subspecies, that is, flavonoids should be considered in the future in the search for further more unique markers. Development of simple colorimetric assays specific for estragole detection should also allow for the rapid online screening of fennel fruits at an industrial level.
Our adopted technique can certainly be used as a powerful tool for analyzing fennel from other sources to assess the impact of agricultural practice, storage, growth stage, and seasonal variation on the metabolic makeup. Liquid chromatography coupled to MS (LC–MS) can also be performed in a wider prospect to pinpoint the heterogeneity of metabolites among fennel accessions and more related to their health effects, that is, antioxidants.
Experimental Section
Plant Material
F. vulgare Miller fruits were obtained from different sources with sample information presented in Table 1. After cleaning with water, fruits from each specimen were separately homogenized with a mortar and a pestle under liquid nitrogen and then stored in tight glass containers at −20 °C till further analysis. Vouchers of fennel specimens were deposited at the College of Pharmacy Herbarium, Cairo University, Egypt.
Chemicals and Fibers
For SPME sampling, fibers of polydimethylsiloxane (PDMS) or divinylbenzene–carboxen–polydimethylsiloxane (DVB-CAR-PDMS) of 1 cm length and 50/30 μm were obtained from Supelco (Bellefonte, PA, USA). All standards, chemicals, and solvents were obtained from Sigma-Aldrich (St. Louis, MO, USA).
SPME–GC–MS Volatile Analysis
Volatile analysis using HS-SPME was carried out as reported by Farag et al.58 The finely pulverized fennel fruits, 20 mg spiked with 10 μg of (Z)-3-hexenyl acetate, were accurately weighed in 1.5 mL tightly sealed glass vials with an SPME fiber implanted manually and volatile compounds were extracted at 50 °C for 30 min. Then, the SPME fiber was subsequently removed and placed manually in the injection port of the GC–MS. The analysis of volatile compounds was conducted using an Agilent 5977B GC/MSD equipped with a DB-5 column (30 m length, 0.25 mm i.d., and 0.25 μm film thickness, Supelco) and interfaced with a quadrupole mass spectrometer. The injector and MS interface temperatures were both held at 220 °C. The analysis conditions were as follows: the constant flow of helium in the column was kept at 0.9 mL/min, the oven temperature was held at 40 °C for 3 min, then raised at a rate of 12 °C/min to 180 °C, maintained at 180 °C for 5 min, and finally increased to 240 °C at a rate of 40 °C/min and kept for 5 min. SPME fibers were cleaned for the next analysis by maintaining in the injection port at 220 °C for 2 min. Blank GC–MS runs were performed during sample analyses. For evaluation of biological replicates, under the same conditions mentioned above, three separate samples were analyzed for each fennel specimen. The quadrupole mass spectrometer was operated at 70 eV using the electron impact mode with a scan range of 40–500 m/z. Peak abundance was extracted using a default parameter using the MET-IDEA tool.59 The relative percentile was calculated by dividing each peak area to the total area of the identified peaks multiplied by 100 within each specimen.
Absolute quantification of estragole levels in fruits was carried out using a standard calibration curve recorded under the same SPME conditions with serial dilutions of estragole dissolved in ethanol at 1, 10, 100, and 500 μg/mL. A volume of 2 μl was aliquoted from each concentration level and placed in a 1.5 mL vial and subjected to SPME volatile analysis. Each fennel sample (20 mg) was placed in triplicate inside the same 1.5 mL vial and subjected to the same volatile extraction conditions as standards. The standard calibration curve for estragole showed excellent linearity following the equation y = 18,002 × +195.33 with 0.999 as the correlation coefficient. Limit of quantification and limit of detection were 0.82 and 0.02 μg/mL, respectively.25
Metabolite Identification and Multivariate Data Analyses
Volatile components were identified by comparing their Kovats indices relative to C6–C20 n-alkane series as well as by matching the mass spectra obtained with the NIST and WILEY libraries and with standards when available. Before mass spectral matching, peaks were first deconvoluted using AMDIS software (www.amdis.net), and their abundance data were extracted using the MET-IDEA tool.59 The data table was then normalized to a spiked internal standard before exporting to PCA, HCA, and OPLS-DA using the SIMCA-P version 13.0 software package (Umetrics, Umeå, Sweden). Subsequently, markers were determined by analyzing the S-plot, which revealed covariance (p) and correlation (pcor). All variables were Pareto-scaled and mean-centered. Validation of models was conducted by computing the diagnostic indices, that is, Q2 and R2 values, and by permutation testing.
Acknowledgments
Dr. Mohamed A. Farag thanks the Alexander von Humboldt Foundation, Germany for financial support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c06188.
Unsupervised multivariate data of the studied F. vulgare fruits derived from modeling of volatile profiles of subsp. vulgare analyzed via GC–MS and HCA of estragole alone in fennel varieties (PDF).
The authors declare no competing financial interest.
Supplementary Material
References
- Opara E. I. Culinary herbs and spices: what can human studies tell us about their role in the prevention of chronic non-communicable diseases?. J. Sci. Food Agric. 2019, 99, 4511–4517. 10.1002/jsfa.9658. [DOI] [PubMed] [Google Scholar]
- Takhtajan A., Flowering Plants. 2 ed.; Springer: Dordrecht/Heidelberg/London/New York, 2009; 871. [Google Scholar]
- Shawky E.; Abou El Kheir R. M. Rapid discrimination of different Apiaceae species based on HPTLC fingerprints and targeted flavonoids determination using multivariate image analysis. Phytochem. Anal. 2018, 29, 452–462. 10.1002/pca.2749. [DOI] [PubMed] [Google Scholar]
- Sayed-Ahmad B.; Talou T.; Saad Z.; Hijazi A.; Merah O. The Apiaceae: Ethnomedicinal family as source for industrial uses. Ind. Crops Prod. 2017, 109, 661–671. 10.1016/j.indcrop.2017.09.027. [DOI] [Google Scholar]
- Horváth G.; Ács K. Essential oils in the treatment of respiratory tract diseases highlighting their role in bacterial infections and their anti-inflammatory action: a review. Flavour Fragr J. 2015, 30, 331–341. 10.1002/ffj.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hadi M. A. M.; Zhang F.-J.; Wu F.-F.; Zhou C.-H.; Tao J. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200–8229. 10.3390/molecules18078200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferioli F.; Giambanelli E.; D’Antuono L. F. Fennel (Foeniculum vulgare Mill. subsp. piperitum) florets, a traditional culinary spice in Italy: evaluation of phenolics and volatiles in local populations, and comparison with the composition of other plant parts. J. Sci. Food Agric. 2017, 97, 5369–5380. 10.1002/jsfa.8426. [DOI] [PubMed] [Google Scholar]
- Miguel M. G.; Cruz C.; Faleiro L.; Simões M. T.; Figueiredo A. C.; Barroso J. G.; Pedro L. G. Foeniculum vulgare essential oils: chemical composition, antioxidant and antimicrobial activities. Nat. Prod. Commun. 2010, 5, 319–328. [PubMed] [Google Scholar]
- Telci I.; Demirtas I.; Sahin A. Variation in plant properties and essential oil composition of sweet fennel (Foeniculum vulgare Mill.) fruits during stages of maturity. Ind. Crops Prod. 2009, 30, 126–130. 10.1016/j.indcrop.2009.02.010. [DOI] [Google Scholar]
- Hadjichambis A. C. H.; Paraskeva-Hadjichambi D.; Della A.; Elena Giusti M.; De Pasquale C.; Lenzarini C.; Censorii E.; Reyes Gonzales-Tejero M.; Patricia Sanchez-Rojas C.; Ramiro-Gutierrez J. M.; Skoula M.; Johnson C.; Sarpaki A.; Hmamouchi M.; Jorhi S.; El-Demerdash M.; El-Zayat M.; Pieroni A. Wild and semi-domesticated food plant consumption in seven circum-Mediterranean areas. Int. J. Food Sci. Nutr. 2009, 59, 383–414. 10.1080/09637480701566495. [DOI] [PubMed] [Google Scholar]
- Fuller D. Q.; Stevens C.; McClatchie M., Routine activities, tertiary refuse and labor organization: social inferences from everyday archaeobotany. Ancient plants and people. Contemporary trends in archaeobotany .University of Arizona Press,Tucson: 2014, 174–217. [Google Scholar]
- Vartavan C. D.; Amorós V. A., Codex of ancient Egyptian plant remains/Codex des restes végétaux de I’Egypte ancienne. Triade Exploration: London, 2010. [Google Scholar]
- Bard K. A., Encyclopedia of the archaeology of ancient Egypt. Routledge: London, 2005, 10.4324/9780203982839. [DOI] [Google Scholar]
- Van Zeist W.; Bottema S.; Van der Veen M., Diet and vegetation at ancient Carthage: the Archaeobotanical evidence. Groningen Institute of Archaeology: 2001. [Google Scholar]
- Randall R. P., A Global Compendium of Weeds. 2 ed.; Department of Agricture and Food, Western Australia: Perth, 2012; 1119. [Google Scholar]
- He W.; Huang B. A review of chemistry and bioactivities of a medicinal spice: Foeniculum vulgare. J. Med. Plant Res. 2011, 5, 3595–3600. 10.5897/JMPR.9000022. [DOI] [Google Scholar]
- Kooti W.; Moradi M.; Ali-Akbari S.; Sharafi-Ahvazi N.; Asadi-Samani M.; Ashtary-Larky D. Therapeutic and pharmacological potential of Foeniculum vulgare Mill: a review. J. HerbMed Pharmacol. 2015, 4, 1–9. [Google Scholar]
- Shahat A. A.; Ibrahim A. Y.; Hendawy S. F.; Omer E. A.; Hammouda F. M.; Abdel-Rahman F. H.; Saleh M. A. Chemical composition, antimicrobial and antioxidant activities of essential oils from organically cultivated fennel cultivars. Molecules 2011, 16, 1366–1377. 10.3390/molecules16021366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad B. S.; Talou T.; Saad Z.; Hijazi A.; Cerny M.; Kanaan H.; Chokr A.; Merah O. Fennel oil and by-products seed characterization and their potential applications. Ind. Crops Prod. 2018, 111, 92–98. 10.1016/j.indcrop.2017.10.008. [DOI] [Google Scholar]
- Faudale M.; Viladomat F.; Bastida J.; Poli F.; Codina C. Antioxidant activity and phenolic composition of wild, edible, and medicinal fennel from different Mediterranean countries. J. Agric. Food Chem. 2008, 56, 1912–1920. 10.1021/jf073083c. [DOI] [PubMed] [Google Scholar]
- Zeller A.; Rychlik M. Character impact odorants of fennel fruits and fennel tea. J. Agric. Food Chem. 2006, 54, 3686–3692. 10.1021/jf052944j. [DOI] [PubMed] [Google Scholar]
- Napoli E. M.; Curcuruto G.; Ruberto G. Screening the essential oil composition of wild Sicilian fennel. Biochem. Syst. Ecol. 2010, 38, 213–223. 10.1016/j.bse.2010.01.009. [DOI] [Google Scholar]
- Díaz-Maroto M. C.; Pérez-Coello M. S.; Esteban J.; Sanz J. Comparison of the volatile composition of wild fennel samples (Foeniculum vulgare Mill.) from Central Spain. J. Agric. Food Chem. 2006, 54, 6814–6818. 10.1021/jf0609532. [DOI] [PubMed] [Google Scholar]
- Aprotosoaie A. C.; Spac A.; Hancianu M.; Miron A.; Tanasescu V. F.; Dorneanu V.; Stanescu U. The chemical profile of essential oils obtained from fennel fruits (Foeniculum vulgare Mill.). Farmacia 2010, 58, 46–53. [Google Scholar]
- Elmassry M. M.; Kormod L.; Labib R. M.; Farag M. A. Metabolome based volatiles mapping of roasted umbelliferous fruits aroma via HS-SPME GC/MS and peroxide levels analyses. J. Chromatogr., B 2018, 1099, 117–126. 10.1016/j.jchromb.2018.09.022. [DOI] [PubMed] [Google Scholar]
- Serag A.; Baky M. H.; Döll S.; Farag M. A. UHPLC-MS metabolome based classification of umbelliferous fruit taxa: a prospect for phyto-equivalency of its different accessions and in response to roasting. RSC Adv. 2020, 10, 76–85. 10.1039/C9RA07841J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag M.; Fathi D.; Shamma S.; Shawkat M.; Shalabi S.; El Seedi H.; Afifi S. Comparative metabolome classification of desert truffles Terfezia claveryi and Terfezia boudierivia its aroma and nutrients profile. LWT 2021, 142, 111046. 10.1016/j.lwt.2021.111046. [DOI] [Google Scholar]
- Farag M. A.; Wessjohann L. A. Volatiles profiling in medicinal licorice roots using steam distillation and solid-phase microextraction (SPME) coupled to chemometrics. J. Food Sci. 2012, 77, C1179–C1184. 10.1111/j.1750-3841.2012.02927.x. [DOI] [PubMed] [Google Scholar]
- Khalil M. N. A.; Fekry M. I.; Farag M. A. Metabolome based volatiles profiling in 13 date palm fruit varieties from Egypt via SPME GC–MS and chemometrics. Food Chem. 2017, 217, 171–181. 10.1016/j.foodchem.2016.08.089. [DOI] [PubMed] [Google Scholar]
- Valdivieso-Ugarte M.; Gomez-Llorente C.; Plaza-Díaz J.; Gil Á. Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: A systematic review. Nutrients 2019, 11, 2786. 10.3390/nu11112786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Maroto M. C.; Díaz-Maroto Hidalgo I. J.; Sánchez-Palomo E.; Pérez-Coello M. S. Volatile components and key odorants of Fennel (Foeniculum vulgare Mill.) and Thyme (Thymus vulgaris L.) oil extracts obtained by simultaneous distillation– extraction and supercritical fluid extraction. J. Agric. Food Chem. 2005, 53, 5385–5389. 10.1021/jf050340+. [DOI] [PubMed] [Google Scholar]
- Atkinson R. G. Phenylpropenes: Occurrence, distribution, and biosynthesis in fruit. J. Agric. Food Chem. 2018, 66, 2259–2272. 10.1021/acs.jafc.6b04696. [DOI] [PubMed] [Google Scholar]
- Wang C.; Sun J.; Tao Y.; Fang L.; Zhou J.; Dai M.; Liu M.; Fang Q. Biomass materials derived from anethole: conversion and application. Polym. Chem. 2020, 11, 954–963. 10.1039/C9PY01513B. [DOI] [Google Scholar]
- Aprotosoaie A. C.; Costache I.-I.; Miron A., Anethole and Its Role in Chronic Diseases. In Drug Discovery from Mother Nature, Gupta S. C.; Prasad S.; Aggarwal B. B., Eds. Springer International Publishing: Cham, 2016; 247–267, 10.1007/978-3-319-41342-6_11. [DOI] [PubMed] [Google Scholar]
- Martins C.; Cação R.; Cole K. J.; Phillips D. H.; Laires A.; Rueff J.; Rodrigues A. S. Estragole: a weak direct-acting food-borne genotoxin and potential carcinogen. Mutat. Res., Genet. Toxicol. Environ. Mutagen. 2012, 747, 86–92. 10.1016/j.mrgentox.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Probert P. M.; Palmer J. M.; Alhusainy W.; Amer A. O.; Rietjens I. M.; White S. A.; Jones D. E.; Wright M. C. Progenitor-derived hepatocyte-like (B-13/H) cells metabolise 1′-hydroxyestragole to a genotoxic species via a SULT2B1-dependent mechanism. Toxicol. Lett. 2016, 243, 98–110. 10.1016/j.toxlet.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg S. J. P. L.; Alhusainy W.; Restani P.; Rietjens I. M. Chemical analysis of estragole in fennel based teas and associated safety assessment using the Margin of Exposure (MOE) approach. Food Chem. Toxicol. 2014, 65, 147–154. 10.1016/j.fct.2013.12.035. [DOI] [PubMed] [Google Scholar]
- Kamatou G. P.; Vermaak I.; Viljoen A. M. Eugenol—from the remote Maluku Islands to the international market place: a review of a remarkable and versatile molecule. Molecules 2012, 17, 6953–6981. 10.3390/molecules17066953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschiggerl C.; Bucar F. Volatile fraction of lavender and bitter fennel infusion extracts. Nat. Prod. Commun. 2010, 5, 1934578X1000500 10.1177/1934578X1000500917. [DOI] [PubMed] [Google Scholar]
- Moghtader M. Comparative survey on the essential oil composition from the seeds and flowers of Foeniculum vulgare Mill. from Kerman province. J. Hortic. For. 2013, 5, 37–40. [Google Scholar]
- Keskin I.; Gunal Y.; Ayla S.; Kolbasi B.; Sakul A.; Kilic U.; Gok O.; Koroglu K.; Ozbek H. Effects of Foeniculum vulgare essential oil compounds, fenchone and limonene, on experimental wound healing. Biotechnol. Histochem. 2017, 92, 274–282. 10.1080/10520295.2017.1306882. [DOI] [PubMed] [Google Scholar]
- Hammouda F. M.; Saleh M. A.; Abdel-Azim N. S.; Shams K. A.; Ismail S. I.; Shahat A. A.; Saleh I. A. Evaluation of the essential oil of Foeniculum vulgare Mill (fennel) fruits extracted by three different extraction methods by GC/MS. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 277–279. 10.4314/ajtcam.v11i2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.; Zhang Y.; Yuan X.; Sun E. Determination of volatile compounds of Illicium verum Hook. f. using simultaneous distillation-extraction and solid phase microextraction coupled with gas chromatography-mass spectrometry. Trop J. Pharm. Res. 2015, 14, 1879–1884. 10.4314/tjpr.v14i10.20. [DOI] [Google Scholar]
- Scognamiglio J.; Jones L.; Letizia C. S.; Api A. M. Fragrance material review on cis-jasmone. Food Chem. Toxicol. 2012, 50, S613–S618. 10.1016/j.fct.2012.03.026. [DOI] [PubMed] [Google Scholar]
- d’Alessio P. A.; Ostan R.; Bisson J.-F.; Schulzke J. D.; Ursini M. V.; Béné M. C. Oral administration of d-limonene controls inflammation in rat colitis and displays anti-inflammatory properties as diet supplementation in humans. Life Sci. 2013, 92, 1151–1156. 10.1016/j.lfs.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Siddhardha B.; Kumar M. V.; Murty U.; Ramanjaneyulu G.; Prabhakar S. Biotransformation of α-pinene to terpineol by resting cell suspension of Absidia corulea. Indian J Microbiol. 2012, 52, 292–294. 10.1007/s12088-011-0155-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirovetz L.; Buchbauer G.; Ngassoum M. B.; Parmentier M. Chemical composition and olfactory characterization of essential oils of fruits and seeds of African pear (Dacryodes edulis (G. Don) HJ Lam) from Cameroon. Flavour Fragr J. 2005, 20, 215–218. 10.1002/ffj.1324. [DOI] [Google Scholar]
- Yokoi M.; Shimoda M. Extraction of volatile flavor compounds from tobacco leaf through a low-density polyethylene membrane. J. Chromatogr. Sci. 2017, 55, 373–377. 10.1093/chromsci/bmw178. [DOI] [PubMed] [Google Scholar]
- Dhiman A. Gas chromatography-mass spectroscopy analysis of bioactive compounds in the whole plant parts of ethanolic extract of Asclepias Curassavica L. Int. J. Green Pharm. 2018, 12, 107–114. [Google Scholar]
- Dhifi W.; Bellili S.; Jazi S.; Bahloul N.; Mnif W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. 10.3390/medicines3040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bail S.; Buchbauer G.; Schmidt E.; Wanner J.; Slavchev A.; Stoyanova A.; Denkova Z.; Geissler M.; Jirovetz L. GC-MS-analysis, antimicrobial activities and olfactory evaluation of essential davana (Artemisia pallens Wall. ex DC) oil from India. Nat. Prod. Commun. 2008, 3, 1934578X0800300 10.1177/1934578X0800300705. [DOI] [Google Scholar]
- Su G.-J.; Liu X.-M.; Li W.-G.; Li X.-M.; Chen K.-C.; Yan Y.-X. Biotransformation of anethole to anisaldehyde in aqueous-organic solvents biphasic systems. Fine Chem. 2009, 26, 136–141. [Google Scholar]
- Belhattab R.; Amor L.; Barroso J. G.; Pedro L. G.; Figueiredo A. C. Essential oil from Artemisia herba-alba Asso grown wild in Algeria: Variability assessment and comparison with an updated literature survey. Arab. J. Chem. 2014, 7, 243–251. 10.1016/j.arabjc.2012.04.042. [DOI] [Google Scholar]
- Jirovetz L.; Buchbauer G.; Schweiger T.; Denkova Z.; Slavchev A.; Stoyanova A.; Schmidt E.; Geissler M. Chemical composition, olfactory evaluation and antimicrobial activities of Jasminum grandiflorum L. absolute from India. Nat. Prod. Commun. 2007, 2, 1934578X0700200411. [Google Scholar]
- Ismaiel O. A.; Abdelghani E.; Mousa H.; Eldahmy S. I.; Bayoumy B. Determination of Estragole in Pharmaceutical Products, Herbal Teas and Herbal Extracts Using GC-FID. J. Appl. Pharm. Sci. 2016, 6, 144–150. [Google Scholar]
- Mihats D.; Pilsbacher L.; Gabernig R.; Routil M.; Gutternigg M.; Laenger R. Levels of estragole in fennel teas marketed in Austria and assessment of dietary exposure. Int. J. Food Sci. Nutr. 2017, 68, 569–576. 10.1080/09637486.2016.1262334. [DOI] [PubMed] [Google Scholar]
- Alhusainy W.; Paini A.; Punt A.; Louisse J.; Spenkelink A.; Vervoort J.; Delatour T.; Scholz G.; Schilter B.; Adams T. Identification of nevadensin as an important herb-based constituent inhibiting estragole bioactivation and physiology-based biokinetic modeling of its possible in vivo effect. Toxicol. Appl. Pharmacol. 2010, 245, 179–190. 10.1016/j.taap.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Farag M. A.; Rasheed D. M.; Kamal I. M. Volatiles and primary metabolites profiling in two Hibiscus sabdariffa (roselle) cultivars via headspace SPME-GC-MS and chemometrics. Food Res. Int. 2015, 78, 327–335. 10.1016/j.foodres.2015.09.024. [DOI] [PubMed] [Google Scholar]
- Broeckling C. D.; Reddy I. R.; Duran A. L.; Zhao X.; Sumner L. W. MET-IDEA: data extraction tool for mass spectrometry-based metabolomics. Anal. Chem. 2006, 78, 4334–4341. 10.1021/ac0521596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.