Abstract

Organosolv fractionation is a promising approach for the separation of lignocellulosic components in integrated biorefineries where each component can be fully valorized into valuable platform chemicals and biofuels. In this study, microwave-accelerated organosolv fractionation was developed for the modification of lignocellulosic fractionation of rice husk. The fractionation condition was optimized for 1 h with the microwave irradiation at 300 W using a ternary solvent mixture composed of 24%:32%:44% water/ethanol/methyl isobutyl ketone. The effects of mineral acids (HCl, H3PO4, and H2SO4) and heterogeneous acid promoters (HCl, H3PO4, and H2SO4 impregnated over activated carbon) on the efficiency and selectivity of product yields (i.e., glucan, hemicellulose-derived products, and lignin) were also investigated. It was found that the use of H3PO4–activated carbon as the promoter showed superior performance on the fractionation of rice husk components, resulting in 88.8% recovery of cellulose, with 63.8% purity in the solid phase, whereas the recovery of hemicellulose (66.4%) with the lowest formation of furan and 5-hydroxymethyl furfural and lignin (81.0%) without sugar cross-contamination was obtained in the aqueous ethanol phase and organic phase, respectively. In addition, the morphology structure of fractionated rice husk presented 2.6-fold higher surface area (5.4 m2/g) of cellulose-enriched fraction in comparison with the native rice husk (2.1 m2/g), indicating the improvement of enzyme accessibility. Besides, the chemical changes of isolated lignin were also investigated by Fourier-transform infrared spectroscopy. This work gives pieces of information into the efficiencies of the microwave strategy as a climate neighborly elective fractionation method for this serious starting material in the biotreatment facility business.
Introduction
Lignocellulose biomass is commonly recognized as a potential renewable resource for the production of biofuels, raw chemicals, materials, and energy in biorefineries.1,2 Compared to the conventional fossil-based platform industry, biorefineries represent a sustainable alternative because of the carbon-neutral nature of the raw materials, which helps alleviate the emission of greenhouse gases and at the same time provides economic benefits by valorizing abundant underused local agricultural byproducts. Lignocellulosic biomass is composed mainly of three biopolymers: cellulose, hemicellulose, and lignin. Cellulose is the principal component of plant cell walls, which is a linear homopolysaccharide with an empirical formula of (C6H10O5)n comprising (1→4)β-linked-d-glucan, organized into highly crystalline microfibrial structures.3 Hemicellulose is a heterogeneous and highly branched amorphous polysaccharide containing various pentoses, hexoses, and sugar acids that act as a bridge between cellulose microfibers and lignin. The three-dimensional polymer of phenyl-propanoid groups, comprising p-coumaryl, coniferyl, and sinapyl alcohols, shields the polysaccharides from external physical, chemical, and biological attacks.4 This highly organized and complex lignocellulose structure is highly recalcitrant to enzymatic and microbial deconstruction. An efficient pretreatment or fractionation step to increase its digestibility while separating target lignocellulosic constituents for subsequent chemical and bioconversion to target products is thus a prerequisite to developing a feasible biorefinery process.
Rice husk (RH) is a significant byproduct of the rice milling process, taking up approximately 20% of the rice weight,5 with an annual production of 20 million tons. Most of it is used as animal feed or burned in power plants to produce heat and electricity.6 The utilization of RH as a crude material in biorefineries has pulled in research interest in, for instance, changing to bio-oils, such as phenols, phenol subsidiaries, and long-chain aliphatic compounds.7 A few innovations dependent on substance (corrosive, antacid, and oxidation), thermochemical (steam blast, autohydrolysis, and organosolv), and natural cycles have been created for the pretreatment and fractionation of different agrarian squanders, with an accentuation on item yield and selectivity.8 Among these, organosolv is an adaptable cycle that can be applied productively to a wide assortment of biomass structures, including hardwood, softwood, and grass.9−11 Several advantages of organosolv fractionation have been reported, including its capability to reduce the viscosity of a medium, improve penetration into a biomass, and facilitate highly efficient lignin removal.12,13 However, the application of the organosolv process for the pretreatment and fractionation of RH has been limited, with only a few studies using different solvent systems, including ethanol and ionic liquids.14,15
The clean fraction (CF) method has been identified as an efficient one-step separation technology for the three major lignocellulosic components13 In the CF process, the biomass is treated with a ternary solvent mixture comprising water, a short-chain alcohol, and methyl isobutyl ketone (MIBK) in the presence of H2SO4 as a catalyst at a high temperature, followed by a phase separation step, which results in cellulose-enriched solid, hemicellulose-derived products in the aqueous alcohol phase and high-purity lignin in the organic phase. This process was later modified for acid catalysts (mineral acids and solid acid catalysts), solvent systems, and response acceleration methods and a proof of efficient fractionation of hardwood, softwood, and various agricultural wastes like sugarcane bagasse16 and switchgrass.17 However, the use of homogeneous acid catalysts has drawbacks, including the corrosive nature of acid, reusability of the catalyst, product separation, and the need for waste and water treatment. As an issue of concern, alternative heterogeneous solid acids have been recently investigated as catalysts for the separation of lignocellulosic materials, with the key advantage of a less side-chain degradation of the derived products and easy recovery.18,19 Heterogeneous acid catalysts, for example, H3PO4–activated carbons (AC–H3PO4)20 and a series of SO42–/MxOy/Fe3O4/WO3 solid acid catalysts,21 can be applied for various lignocellulosic applications, including biomass fractionation, cellulose hydrolysis,22,23 lignin depolymerization,24 and torrefaction.25 Microwave irradiation generates heating by the direct coupling of microwave energy with the molecules that are present in the reaction mixture as opposed to a slow and inefficient energy transfer when using conventional heating.18−20 The microwave-accelerated CF process has been reported for rice straw, which showed advantages in product yield and selectivity compared to the conventional CF process, suggesting a potent alternative for developing a less energy-intensive process for the efficient fractionation of lignocellulosic materials.
In this study, a single-step CF-based organosolv process of RH using a microwave-accelerated process was developed and compared to conventional heating process with the use of various mineral acids or alternative solid acid catalysts. The effects of microwave power, acid concentration, and acid types on the product yield and selectivity were also studied. Besides, the characteristics of solid residues and lignin isolated from the CF process were analyzed by scanning electron microscopy (SEM), X-ray diffraction (XRD), and Fourier transform infrared (FTIR) spectroscopy. This study shows an integration of the microwave-accelerated process and CF-based organosolv fractionation of RH in biorefineries.
Results and Discussion
Comparison between Fractionation Using Traditional Heat and Acceleration by Microwaves
A key method for the use of biomass in an advanced biorefinery process is the fractionation of lignocellulosic biomass with high yield and selectivity. High recalcitrance in the plant biomass structure is the main obstacle to the effective fractionation of the lignocellulosic biomass components.28 Solvo- and hydrothermal treatments effectively relent the recalcitrant structure under a highly pressurized aqueous medium and a high temperature.29 In addition, by directly interfering with the biomass substrate and reaction medium and reducing the intensity of the reaction conditions, microwave irradiation improves the hydrothermal fractionation of the recalcitrant lignocellulosic biomass.
Under microwave irradiation, the dielectric properties of the irradiated material, expressed between the relative permittivity and the dielectric loss, regulate their heating properties. More specifically, it is well known that under microwave irradiation, electrolytes play an important role in the hydrothermal properties of a lignocellulosic biomass. As a result of a sequential reaction, the hydrolysis of monomeric sugars simply creates degraded products. An additional method to improve the dielectric properties of reaction systems is the use of heterogeneous catalysts. Microwave and heterogeneous catalysts, such as palladium-supported charcoal30 and zeolite,31 have recently been documented as coupled. Catalysts based on carbon have been commonly used for lignocellulosic biomass hydrolysis.31−34 Herein, the optimized condition is considered based on the following criteria: cellulose recovery > 80%; lignin removal > 80%; and hemicellulose-derived sugar > 70%, which would be of practical value in CF processes in order to obtain a high cellulose content in solid fraction while still achieving hemicellulose and lignin in aqueous and organic phases.
In the first point, in the absence of an acid catalyst, the fractionation efficiency of the CF-based method using microwave acceleration was tested. The reactions in the ternary water/ethanol/MIBK mixture (24%:32%:44%) were conducted at differing microwave powers from 200 to 450 W compared to traditional heating at 160 °C, which, according to our previous research, is the optimum temperature for the CF-based process. As shown in Figure 1A, glucan yield in the solid fraction obtained from the use of microwave acceleration showed a slightly decreasing trend from 28.4 to 24.8% with an increasing microwave power. Hemicellulose was effectively solubilized from the solid fraction into the aqueous phase (8.0–1.3%), whereas only a slight fraction of lignin was removed (13.5–8.0%) under the experimental conditions (Figure 1B,C). This resulted in a relatively low fractionation efficiency, as reflected in the low selectivity of glucan in the solid phase. The fractionation performance when using conventional heating at 160 °C is comparable to that achieved with a microwave power of 300 W in terms of product yield and selectivity, though with slightly higher 5-hydroxymethyl furfural (HMF) and furfural contents in the aqueous phase. The findings thus suggest the feasibility of using microwave acceleration in the CF-based method as an alternative to traditional heating. The microwave power of 300 W was chosen for subsequent experiments because of the high hemicellulose solubilization in the aqueous phase and few degradation products (HMF and furfural) compared with those at microwave powers of 200 and 450 W.
Figure 1.
Effects of temperature on biomass fractionation. The reaction contained 10% (w/v) RH in water/ethanol/MIBK (24%:32%:44%), treated at different microwave powers (200–450 W) compared with conventional heating (160 °C) for 1 h in the absence of catalyst. (A) Solid fraction; (B) aqueous fraction; (C) organic fraction.
Effects of Homogeneous and Heterogeneous Acid Catalysts
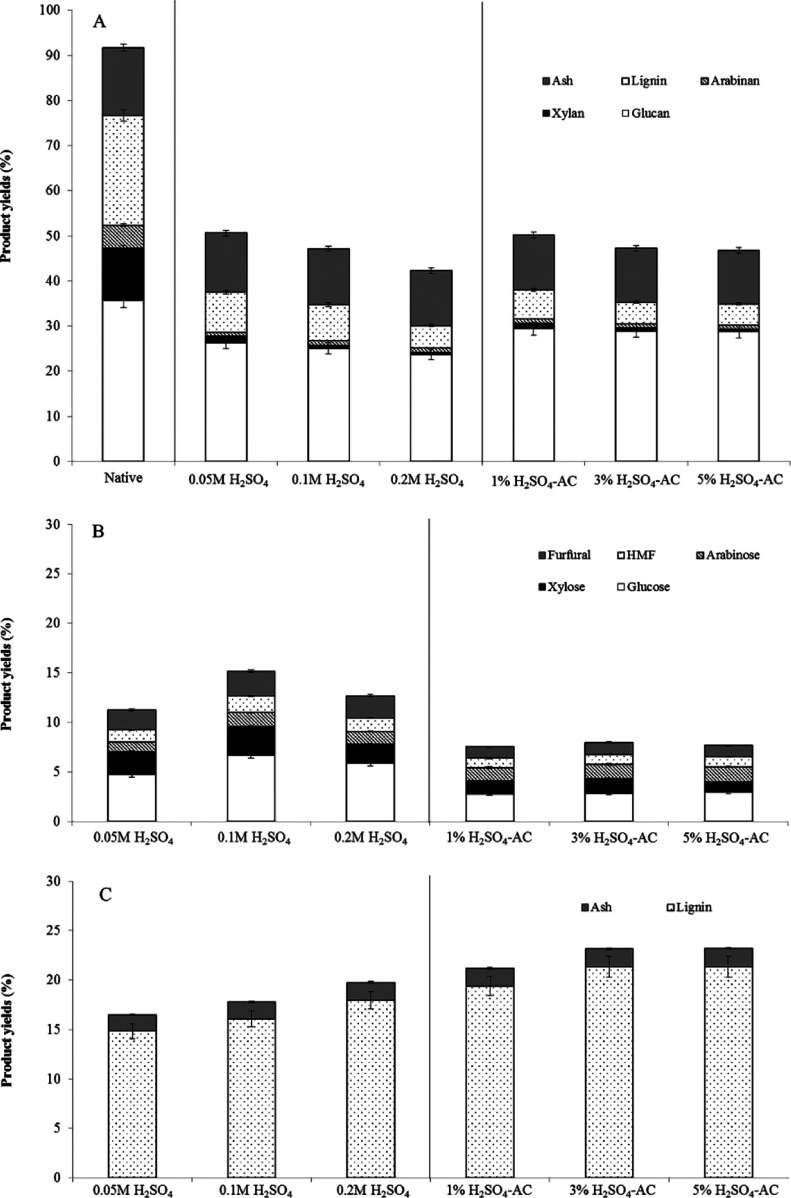
In this study, the effects of H2SO4 as a conventional homogeneous acid catalyst with varying concentrations (0.05–0.2 M) and of solid H2SO4–AC as a heterogeneous acid catalyst (1–5% w/w) in the microwave-accelerated CF-based process using the microwave power at 300 W were compared (Figure 2A–C). The addition of H2SO4 and its derived solid catalyst resulted in a marked improvement in the fractionation efficiency, leading to an effective separation of each lignocellulosic fraction. From the point of view of reaction and mass transfer efficacy, the addition of homogeneous promoters in fractionation is desirable and demonstrates significant benefits in the separation of cellulose and lignin.
Figure 2.
Effects of H2SO4 and H2SO4–AC concentration on biomass fractionation. The reaction contained 10% (w/v) RH in water/ethanol/MIBK (24%:32%:44%), treated at different microwave powers (200–450 W) compared with conventional heating (160 °C) for 1 h in the presence of varying concentrations of H2SO4. (A) Solid fraction; (B) aqueous fraction; (C) organic fraction.
Nevertheless, these homogeneous compounds result in the degradation of the reactor and machinery and isolation of materials and reduce the reusability of homogeneous catalysis and waste effluent treatment.35 Owing to the difficulties of distinguishing homogeneous catalysts from product solutions, many catalytic devices have not been commercialized.36 As shown in Figure 2A, a slight decrease in glucan yield (26.3–23.7%) in the solid fraction was achieved with an increasing H2SO4 concentration (p > 0.05), though with a higher product selectivity for glucan (52.0–57.5%). This result correlates with the higher efficiency of hemicellulose solubilization to the aqueous phase (3.2–3.3%) (p > 0.05) and lignin removal to the organic phase (14.8–17.9%) (p > 0.05). However, the results showed no significant effect on the glucan yield in the solid phase, hemicellulose solubilization in the aqueous phase, and, under laboratory conditions, lignin solubilization in the organic phase. The use of a solid catalyst led to a higher fractionation performance, leading to a higher glucan yield (28.8–29.5%) (p > 0.05) and selectivity (58.7–62.0%) in the solid phase, whereas the solid acid catalyst loading showed no significant effect on the glucan yield under the experimental conditions (p > 0.05). This result was correlated with the high yield (8.6–9.1%) (p > 0.05) and selectivity (62.2–63.0%) of the hemicellulose-derived products, that is, xylan and arabinan, in the aqueous fractions with a lower accumulation of the sugar degradation products, that is, HMF and furfural, compared to those when using the liquid catalyst. The use of H2SO4–AC also resulted in a higher lignin removal efficiency than that when using H2SO4, with an increasing trend with a higher catalyst loading, with the highest lignin yield and selectivity being 21.4 and 92.2%, respectively, and with a solid acid catalyst loading of 3% H2SO4–AC.
Recently, a number of advantages on the use of heterogeneous promoters for enhancing lignocellulosic biomass fractionation have been reported. For example, hemicellulose degradation and enzymatic saccharification can be improved,37 especially during acid or hydrothermal pretreatments,38 which are less corrosive, thus reducing the need for expensive construction materials, including more complete conversion, higher recyclability of solutions, and limited degradation of sugars in the presence of small amounts of toxic compounds.39,40 The H2SO4 concentrations of 0.05 M H2SO4 and 3% H2SO4–AC were chosen for subsequent experiments as they yielded the highest cellulose content in the solid, in the aqueous phase, less oxidation materials, and in the organic phase, the largest lignin yield.
Effects of Acid Types
From the characterizations, the specific surface area of the catalysts was in the range of 798–853 m2/g, whereas the acid site densities were 732.3, 873.1, and 1057.7 μmol/g for HCl–, H2SO4–, and H3PO4–ACs, respectively. In microwave irradiation, carbon-based catalysts are also efficient because carbon is suddenly heated by microwave irradiation. Matsumoto et al.41 used AC as a pretreatment microwave sensitizer for corn starch. In addition, the application of sulfate to the AC support has been documented to increase the catalytic activity of maize starch hydrolysis by reducing the reaction temperature needed.
In this step, the effects of the acid types (i.e., HCl, H3PO4, and H2SO4 and HCl–, H3PO4–, H2SO4–ACs) on the microwave-accelerated CF-based fractionation of RH were studied. It was found that the fractionation performance in the presence of HCl, H3PO4, and H2SO4 was in the same range at a fixed acid concentration of 0.05 M, of which the product yields in the solid, aqueous, and solvent fractions were almost identical, with no significant difference (Figure 3A–C). However, the use of H3PO4 led to significantly (p ≤ 0.05) lower formation of sugar degradation byproducts in the aqueous phase and high-level extracted lignin in the organic phase with a less cross-contamination of ash than for the other acids under these experimental conditions.
Figure 3.
Effects of acid type on biomass fractionation. The reaction contained 10% (w/v) RH in water/ethanol/MIBK (24%:32%:44%), treated at different microwave powers (200–450 W) compared with conventional heating (160 °C) for 1 h with 0.05 M of different mineral acids. (A) Solid fraction; (B) aqueous fraction; (C) organic fraction.
In contrast to the heterogeneous acid catalysts, HCl–, H3PO4–, and H2SO4–AC showed differences in their performance on biomass fractionation, of which HCl–AC presented the lowest fractionation performance at the fixed catalyst loading of 3% w/w. Overall, H3PO4–AC and H2SO4–AC exhibited better performance in the CF-based process compared to the homogeneous acids in terms of product yield and selectivity under this experimental condition. Compared to H2SO4–AC, H3PO4–AC showed better efficiency in terms of biomass fractionation, leading to the highest yield (29.5%) and selectivity (63.8%) for glucan in the solid phase. This also led to a higher yield (66.4%) and selectivity (58.5%) for hemicellulose-derived products in the aqueous phase with lower inhibitory byproduct formation and the highest lignin yield (81.0%) and selectivity (89.0%) in the organic fraction. A comparison of the outputs of this study with the recent researches based on pretreatment technology in the presence of heterogeneous catalysts is shown in Table S1. These results reveal that the use of H3PO4–AC greatly improves the fractionation performance in terms of high product yield and low degradation of monosaccharides. This is because H3PO4–AC has a higher specific surface area and acid density than H2SO4–AC, resulting in more reacting particles between the acid sites and biomass on the surface. However, H2SO4 is a relatively strong acid compared with H3PO4; therefore, it might result in the conversion of more cellulose and hemicellulose to glucan and xylan and subsequent conversion to HMF and furfural. It also shows several favorable characteristics over homogeneous acid promoters, for example, high selectivity separation effectiveness, low toxicity and corrosiveness of equipment, and ease of recovery and reuse.42,43
Structural Analysis of Residues of Fractionated Solids and Derived Lignin
The solid-phase microstructure obtained from the fractionation was compared by SEM with native RH, as shown in Figure 4. The native sample provided a normal and intact surface, indicating the plant material’s highly ordered intact structure (Figure 4A). The surface structures of the biomass of the fractionated solid residues showed destruction of the surface and internal structure with cavities and cracks. The removal of hemicellulose and alteration of the surface lignin is indicated by this observation. For the Brunauer–Emmett–Teller (BET) surface area measurement, raw and pretreated biomass samples were analyzed (Table 1).
Figure 4.
Scanning electron micrographs of native RH and solid residues from the fractionation of RH. (A) Native RH; (B) solid residues from the fractionation of RH.
Table 1. BET Surface Area and Crystallinity Index of Untreated and Pretreated RH at Optimal Conditionsa.
| surface
area (m2/g) |
degree
of crystallinity (%) |
|||
|---|---|---|---|---|
| biomass | native | pretreated | native | pretreated |
| rice husk | 2.1 | 5.4 | 70.1 | 82.3 |
Optimal pretreatment conditions: 10% (w/v) RH in water/ethanol/MIBK (24%:32%:44%) using 3% H3PO4–AC for 1 h at a microwave power of 300 W.
Pretreated solids showed a higher surface area than native RH. This corresponded to a 2.6-fold increase in the surface area of the cellulose-enriched solid fraction (5.4 m2/g) compared to the native biomass (2.1 m2/g). XRD research also revealed an improvement in the crystallinity of the solid phase (3.82%) relative to the native biomass (1.70%), indicating a successful removal of the amorphous hemicellulose and lignin from the raw material. One of the most significant factors influencing the effectiveness of enzymatic digestibility is the accessible surface area.
The internal surface area of RH increases after fractionation relative to the native feedstock because of (i) fragmentation and crack growth and (ii) elimination of lignin and hemicellulose, reducing shielding effects and opening up additional pores. In addition, biomass crystallinity is also known to be an essential factor affecting enzyme digestibility efficiency. Lignin from the fractionation process performed using 3% w/w H3PO4 was extracted from the organic phase using evaporation. For the organosolv lignin and the isolated lignin isolated in this analysis in the 400–4000 cm–1 region, similar FTIR spectra were seen (Figure 5). An FTIR profile composed of aromatic phenylpropane skeleton vibrations (1632, 1515, and 1429 cm–1),44 aromatic and aliphatic hydroxyl groups (3400 cm–1),44 aliphatic C–H bonds (2919 cm–1), vibrations of the aromatic methyl group (1428 cm–1), and ether bridges (1200 and 1061 cm–1)45 are seen by the isolated lignin. The results of the acid hydrolysis of lignin caused by acetic acid released from the hemicellulose of the raw material were phenolic hydroxyl groups (1372 cm–1) and nonconjugated carbonyl groups (1720 cm–1).45 The spectrum also revealed other signals that can be related to syringyl (S) and guaiacyl (G) groups in addition to these general bands: syringyl ring respiration with C–O stretching (1330 cm–1), syringyl aromatic C–H type in plane deformations (1118 cm–1), syringyl and guaiacyl ring respiration with C–O stretching (1218 cm–1), and guaiacyl (1265, 1125, 855, and 810 cm–1) units.46,47
Figure 5.
FT-IR spectra of lignin obtained from (A) commercial kraft lignin; (B) microwave heating process at 300 W in the presence of 3% H3PO4–AC.
Conclusions
Under the experimental conditions considered in our research, microwave-assisted fractionation in the presence of H3PO4–AC performs well. The replacement of conventional thermal heat with microwave irradiation can accelerate the fractionation reaction, with the formation of lesser degradation products from monomeric sugars in the aqueous phase. As an acid promoter, the addition of H3PO4–AC led to improved glucan purity along with the increased solubilization of xylose and arabinose into the aqueous-phase and organic-phase lignin yield. This led to the efficient separation of lignocellulosic components from native feedstock. Furthermore, a relatively lower accumulation of degradation products was observed using H3PO4–AC as a promoter than that when using liquid acids, that is, H2SO4 and H3PO4. Along with the trace quantities of ash, high-purity lignin was retrieved in the organic process. Our study suggests that the coupling of microwave-assisted fractionation with H3PO4–AC as a promoter could be a useful technology for the fractionation of lignocellulosic biomass components in the production of chemicals and an integrated biorefinery process.
Experimental Section
Materials
RH was collected from the local rice mill industry in the Khon Kaen Province, Thailand. It was physically processed using a cutting mill (Retsch ZM2000, Haan, Germany) and sieved to retain particles 250–420 μm in diameter. The processed biomass was then used as a starting material for the experimental studies. The RH contained 35.7 wt % cellulose, 21.5 wt % hemicellulose, 24.4 wt % lignin, and 4 wt % ash according to the standard NREL method.26 Analytical-grade organic solvents and chemicals were purchased from major chemical suppliers, Sigma-Aldrich, Merck, and Fluka.
Microwave-Accelerated Fractionation Process
Responses were completed in a 500 mL round-base jar associated with a glass condenser to recuperate the dissipated dissolvable material mounted in an adjustable microwave framework (MW71B, Samsung, Korea) (Figure 6). The commonplace response comprised 1.5 g of RH and 15 mL of a solitary stage dissolvable combination containing water/ethanol/MIBK (24%:32%:44%) in view of volume or different solvents as demonstrated. The response was warmed at 200–450 W for 1 h in the nonattendance or presence of different mineral acids (H2SO4, HCl, and H3PO4) at different fixations (0.05–0.2 M) of the solid acid catalyst. A pulse irradiation of accelerated microwave was controlled to maintain the target temperature (approximate 160 °C) compared to conventional heating. The response was then stopped by squeezing the combination in the water shower. The temperature inside the reactor has been assessed utilizing a thermogun (Welch Allyn ThermoScan Genius 4000 thermometer, Welch Allyn, New York, NY). The strong cellulose-advanced division was isolated by sifting paper utilizing a Buchner channel, washed with 40 mL of MIBK and then with 100 mL of water, and afterward dried at 60 °C. The liquid fraction was combined with the rinsate and placed into a separatory funnel. Water was added to the aqueous/organic fraction until phase separation was achieved. The mixture was stirred and then placed at room temperature for 20 min to complete phase separation. The aqueous phase containing hemicelluloses and soluble products was recovered. The separated organic phase was dried at 105 °C to obtain the lignin.
Figure 6.

Schematic drawing of the modified microwave reactor applied in this study.
Preparation and Characterization of Solid Acid Catalysts
The strong corrosive advertisers, H2SO4, H3PO4, and HCl, on ACs (H2SO4–AC, H3PO4–AC, and HCl–AC) were synthesized by Suriyachai et al.16 The particular surface territories of the advertisers were in the scope of 798–853 m2/g, as indicated by the N2 physisorption procedure, utilizing the BET (Wager) technique, whereas the corrosive site densities were 873.1, 1057.7, and 732.3 μmol/g for H2SO4–, H3PO4–, and HCl–AC. The corrosive site thickness of the advertisers was broken down by the temperature-programmed desorption (TPD) technique [NH3- and CO2-TPD].27
Analytical Methods for Lignocellulose Components and Products
The standard NREL approach was used to evaluate chemical compositions (percent cellulose, hemicellulose, lignin, and ash), in solid, aqueous/organic phases.26 High-performance liquid chromatography (SPD-M10A DAD, Shimadzu, Kyoto), with a refractory index detector using a column Aminex HPX-87H (Bio-Rad, Hercules, CA, USA), was performed with fermentable sugar profiles and inhibiting byproducts (5-hydroxymethylfurfural and furfural) acting as mobile stages at 65 °C, with 5 mM H2SO4 as the mobile phase at a flow rate of 0.5 mL/min. The return of the product is the percentage of the product produced on a dry weight basis based on its value in native RH. The selectivity of the reaction to glucan (G), hemicellulose (H), and lignin (L) is calculated on the basis of eqs 1 and 2.
| 1 |
| 2 |
Physical Analysis of Solid Residues
The microstructure of native RH and solid residues collected during the fractionation process has been examined by means of a JSM-6301F electron scanning microscope (JEOL, Tokyo, Japan). Samples were dried and gold-coated to be analyzed. For the study, an electron beam intensity of 5 kV was used. XRD using an X’Pert PRO diffractometer (PANalytical, Almelo, Netherlands) was performed to calculate the crystallinity of the native and distinct solid fractions. The samples were screened in the range of 2θ = 10–30° at 500 kV and 30 mA with a step size of 0.02°. The crystallinity was based on eq 3.
| 3 |
where I002 is the biomass crystalline (i.e., cellulose) density of 2θ = 22.4 and Iamorphous, the amorphous component peaks (i.e., cellulose, hemicellulose, and lignin) of 2θ = 18.0.
The BET device was used to calculate the total raw material and solid residue surface area using the BELSORP-max TPDpro system (BEL Japan, Tokyo, Japan) fitted with a thermal conductivity detector (semi-diffused structure, four-element W–Re filament). In a PerkinElmer 16PC instrument, FT-IR measurements were done via direct transmission using the KBr pellet technique. About 20 scans ranging from 4000 to 400 cm–1 with a 4 cm–1 resolution were recorded for each wavelength. Prior to each sampling, the background spectra were obtained. Prior to analysis, KBr was oven-dried to remove water intrusion. Sigma-Aldrich’s commercial kraft lignin was used as a reference.
Acknowledgments
This project was financially supported by a research grant (RTA 6280003) from the Thailand Research Fund. S.I. was supported by a Unit of Excellence (UOE63006) from the University of Phayao.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c05575.
Recent literatures on carbon-based catalyst for biomass fractionation (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Raita M.; Denchokepraguy N.; Champreda V.; Laosiripojana N. Effects of alkaline catalysts on acetone-based organosolv pretreatment of rice straw. 3 Biotech 2017, 7, 340. 10.1007/s13205-017-0969-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Osch D. J. G. P.; Kollau L. J. B. M.; van den Bruinhorst A.; Asikainen S.; Rocha M. A. A.; Kroon M. C. Ionic liquids and deep eutectic solvents for lignocellulosic biomass fractionation. Phys. Chem. Chem. Phys. 2017, 19, 2636–2665. 10.1039/c6cp07499e. [DOI] [PubMed] [Google Scholar]
- Balat M. Production of bioethanol from lignocellulosic materials via the biochemical pathway: A review. Energy Convers. Manage. 2011, 52, 858–875. 10.1016/j.enconman.2010.08.013. [DOI] [Google Scholar]
- Pang C. H.; Gaddipatti S.; Tucker G.; Lester E.; Wu T. Relationship between thermal behaviour of lignocellulosic components and properties of biomass. Bioresour. Technol. 2014, 172, 312–320. 10.1016/j.biortech.2014.09.042. [DOI] [PubMed] [Google Scholar]
- Natarajan E.; Nordin A.; Rao A. N. Overview of combustion and gasification of rice husk in fluidized bed reactors. Biomass Bioenergy 1998, 14, 533–546. 10.1016/s0961-9534(97)10060-5. [DOI] [Google Scholar]
- Bazargan A.; Bazargan M.; McKay G. Optimization of rice husk pretreatment for energy production. Renewable Energy 2015, 77, 512–520. 10.1016/j.renene.2014.11.072. [DOI] [Google Scholar]
- Zhou L.; Yang H.; Wu H.; Wang M.; Cheng D. Catalytic pyrolysis of rice husk by mixing with zinc oxide: Characterization of bio-oil and its rheological behavior. Fuel Process. Technol. 2013, 106, 385–391. 10.1016/j.fuproc.2012.09.003. [DOI] [Google Scholar]
- Yoo C. G.; Lee C. W.; Kim T. H. Optimization of two-stage fractionation process for lignocellulosic biomass using response surface methodology (RSM). Biomass Bioenergy 2011, 35, 4901–4909. 10.1016/j.biombioe.2011.10.015. [DOI] [Google Scholar]
- Pan X.; Kadla J. F.; Ehara K.; Gilkes N.; Saddler J. N. Organosolv Ethanol Lignin from Hybrid Poplar as a Radical Scavenger. Relationship between Lignin Structure, Extraction Conditions, and Antioxidant Activity. J. Agric. Food Chem. 2006, 54, 5806–5813. 10.1021/jf0605392. [DOI] [PubMed] [Google Scholar]
- Muñoz C.; Mendonça R.; Baeza J.; Berlin A.; Saddler J.; Freer J. Bioethanol production from bio- organosolv pulps of Pinus radiata and Acacia dealbata. J. Chem. Technol. Biotechnol. 2007, 82, 767–774. 10.1002/jctb.1737. [DOI] [Google Scholar]
- Oliet M.; Gilarranz M.; Domínguez J.; Alonso M.; Rodríguez F. Ethanol-based pulping from Cynara cardunculus L. J. Chem. Technol. Biotechnol. 2005, 80, 746–753. 10.1002/jctb.1217. [DOI] [Google Scholar]
- Pye E. K.; Lora J. H. The alcell process a proven alternative to kraft pulping. Tappi J. 1991, 74, 113. [Google Scholar]
- Kim T. H.; Ryu H. J.; Oh K. K. Improvement of Organosolv Fractionation Performance for Rice Husk through a Low Acid-Catalyzation. Energies 2019, 12, 1800. 10.3390/en12091800. [DOI] [Google Scholar]
- Chambon C. L.; Chen M.; Fennell P. S.; Hallett J. P. Efficient Fractionation of Lignin- and Ash-Rich Agricultural Residues Following Treatment With a Low-Cost Protic Ionic Liquid. Front. Chem. 2019, 7, 246. 10.3389/fchem.2019.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imman S.; Arnthong J.; Burapatana V.; Champreda V.; Laosiripojana N. Fractionation of rice straw by a single-step solvothermal process: Effects of solvents, acid promoters, and microwave treatment. Renewable Energy 2015, 83, 663–673. 10.1016/j.renene.2015.04.062. [DOI] [Google Scholar]
- Suriyachai N.; Champreda V.; Sakdaronnarong C.; Shotipruk A.; Laosiripojana N. Sequential organosolv fractionation/hydrolysis of sugarcane bagasse: The coupling use of heterogeneous H3PO4-activated carbon as acid promoter and hydrolysis catalyst. Renewable Energy 2017, 113, 1141–1148. 10.1016/j.renene.2017.06.003. [DOI] [Google Scholar]
- Chen Z.; Wan C. Ultrafast fractionation of lignocellulosic biomass by microwave-assisted deep eutectic solvent pretreatment. Bioresour. Technol. 2018, 250, 532–537. 10.1016/j.biortech.2017.11.066. [DOI] [PubMed] [Google Scholar]
- Weerasai K.; Champreda V.; Sakdaronnarong C.; Shotipruk A.; Laosiripojana N. Hydrolysis of eucalyptus wood chips under hot compressed water in the presence of sulfonated carbon-based catalysts. Food Bioprod. Process. 2018, 110, 136–144. 10.1016/j.fbp.2018.05.005. [DOI] [Google Scholar]
- Asawaworarit P.; Daorattanachai P.; Laosiripojana W.; Sakdaronnarong C.; Shotipruk A.; Laosiripojana N. Catalytic depolymerization of organosolv lignin from bagasse by carbonaceous solid acids derived from hydrothermal of lignocellulosic compounds. Chem. Eng. J. 2019, 356, 461–471. 10.1016/j.cej.2018.09.048. [DOI] [Google Scholar]
- Klamrassamee T.; Champreda V.; Reunglek V.; Laosiripojana N. Comparison of homogeneous and heterogeneous acid promoters in single-step aqueous-organosolv fractionation of eucalyptus wood chips. Bioresour. Technol. 2013, 147, 276–284. 10.1016/j.biortech.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Sakdaronnarong C.; Jiratanakittiwat K.; Tangkitthanasakul T.; Laosiripojana N. Ionosolv pretreatment of sugarcane bagasse and rice straw assisted by catalytic hydrothermal and microwave heating for biorefining. Food Bioprod. Process. 2017, 105, 104–116. 10.1016/j.fbp.2017.06.005. [DOI] [Google Scholar]
- Sakdaronnarong C.; Pipathworapoom W.; Vichitsrikamol T.; Sema T.; Posoknistakul P.; Koo-amornpattana W.; Laosiripojana N. Integrative process for a sugarcane bagasse biorefinery to produce glucose, bio-oil and carbon microspheres. Process Saf. Environ. Prot. 2018, 116, 1–13. 10.1016/j.psep.2018.01.006. [DOI] [Google Scholar]
- Intaramas K.; Jonglertjunya W.; Laosiripojana N.; Sakdaronnarong C. Selective conversion of cassava mash to glucose using solid acid catalysts by sequential solid state mixed-milling reaction and thermo-hydrolysis. Energy 2018, 149, 837–847. 10.1016/j.energy.2018.02.073. [DOI] [Google Scholar]
- Panyadee R.; Posoknistakul P.; Jonglertjunya W.; Kim-Lohsoontorn P.; Laosiripojana N.; Matsagar B. M.; Wu K. C.-W.; Sakdaronnarong C. Sequential Fractionation of Palm Empty Fruit Bunch and Microwave-Assisted Depolymerization of Lignin for Producing Monophenolic Compounds. ACS Sustainable Chem. Eng. 2018, 6, 16896–16906. 10.1021/acssuschemeng.8b04246. [DOI] [Google Scholar]
- Sangjan A.; Ngamsiri P.; Klomkliang N.; Wu K. C.-W.; Matsagar B. M.; Ratchahat S.; Liu C.-G.; Laosiripojana N.; Sakdaronnarong C. Effect of microwave-assisted wet torrefaction on liquefaction of biomass from palm oil and sugarcane wastes to bio-oil and carbon nanodots/nanoflakes by hydrothermolysis and solvothermolysis. Renewable Energy 2020, 154, 1204–1217. 10.1016/j.renene.2020.03.070. [DOI] [Google Scholar]
- Sluiter A.; Hames B.; Ruiz R.; Scarlata C.; Sluiter J.; Templeton D.; Crocker D.. Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure; National Renewable Energy Laboratory, 2008; pp 1–16.
- Chareonlimkun A.; Champreda V.; Shotipruk A.; Laosiripojana N. Catalytic conversion of sugarcane bagasse, rice husk and corncob in the presence of TiO2, ZrO2 and mixed-oxide TiO2–ZrO2 under hot compressed water (HCW) condition. Bioresour. Technol. 2010, 101, 4179–4186. 10.1016/j.biortech.2010.01.037. [DOI] [PubMed] [Google Scholar]
- Himmel M. E.; Ding S.-Y.; Johnson D. K.; Adney W. S.; Nimlos M. R.; Brady J. W.; Foust T. D. Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. 10.1126/science.1137016. [DOI] [PubMed] [Google Scholar]
- Mussatto S. I.Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Elsevier, 2016. [Google Scholar]
- Tsubaki S.; Azuma J.-i.. Application of microwave technology for utilization of recalcitrant biomass. Advances in Induction and Microwave Heating of Mineral and Organic Materials; IntechOpen, 2011. [Google Scholar]
- Mochizuki D.; Sasaki R.; Maitani M. M.; Okamoto M.; Suzuki E.; Wada Y. Catalytic reactions enhanced under microwave-induced local thermal non-equilibrium in a core–shell, carbon-filled zeolite@zeolite. J. Catal. 2015, 323, 1–9. 10.1016/j.jcat.2014.12.003. [DOI] [Google Scholar]
- Onda A.; Ochi T.; Yanagisawa K. Selective hydrolysis of cellulose into glucose over solid acid catalysts. Green Chem. 2008, 10, 1033–1037. 10.1039/b808471h. [DOI] [Google Scholar]
- Hara M. Biomass conversion by a solid acid catalyst. Energy Environ. Sci. 2010, 3, 601–607. 10.1039/b922917e. [DOI] [Google Scholar]
- Kobayashi H.; Komanoya T.; Hara K.; Fukuoka A. Water-tolerant mesoporous-carbon-supported ruthenium catalysts for the hydrolysis of cellulose to glucose. ChemSusChem 2010, 3, 440–443. 10.1002/cssc.200900296. [DOI] [PubMed] [Google Scholar]
- Guo F.; Fang Z.; Xu C. C.; Smith R. L. Solid acid mediated hydrolysis of biomass for producing biofuels. Prog. Energy Combust. Sci. 2012, 38, 672–690. 10.1016/j.pecs.2012.04.001. [DOI] [Google Scholar]
- Rinaldi R.; Schüth F. Design of solid catalysts for the conversion of biomass. Energy Environ. Sci. 2009, 2, 610–626. 10.1039/b902668a. [DOI] [Google Scholar]
- Kang K. E.; Park D.-H.; Jeong G.-T. Effects of NH4Cl and MgCl2 on pretreatment and xylan hydrolysis of miscanthus straw. Carbohydr. Polym. 2013, 92, 1321–1326. 10.1016/j.carbpol.2012.10.019. [DOI] [PubMed] [Google Scholar]
- Yu Q.; Zhuang X.; Yuan Z.; Wang Q.; Qi W.; Wang W.; Zhang Y.; Xu J.; Xu H. Two-step liquid hot water pretreatment of Eucalyptus grandis to enhance sugar recovery and enzymatic digestibility of cellulose. Bioresour. Technol. 2010, 101, 4895–4899. 10.1016/j.biortech.2009.11.051. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Lu X.; Zhang S.; Zhang R.; Wang X. Kinetic study for Fe (NO3)3 catalyzed hemicellulose hydrolysis of different corn stover silages. Bioresour. Technol. 2011, 102, 2936–2942. 10.1016/j.biortech.2010.11.076. [DOI] [PubMed] [Google Scholar]
- Monavari S.; Galbe M.; Zacchi G. The influence of ferrous sulfate utilization on the sugar yields from dilute-acid pretreatment of softwood for bioethanol production. Bioresour. Technol. 2011, 102, 1103–1108. 10.1016/j.biortech.2010.08.077. [DOI] [PubMed] [Google Scholar]
- Azuma J.; Tsubaki S.; Sakamoto M.; Yudianti R.; Hermiati E. Refinery of Biomass by Utilization of Specific Effects of Microwave Irradiation. Procedia Chem. 2012, 4, 17–25. 10.1016/j.proche.2012.06.003. [DOI] [Google Scholar]
- Kumar S.; Kothari U.; Kong L.; Lee Y. Y.; Gupta R. B. Hydrothermal pretreatment of switchgrass and corn stover for production of ethanol and carbon microspheres. Biomass Bioenergy 2011, 35, 956–968. 10.1016/j.biombioe.2010.11.023. [DOI] [Google Scholar]
- Olofsson K.; Bertilsson M.; Lidén G. A short review on SSF an interesting process option for ethanol production from lignocellulosic feedstocks. Biotechnol. Biofuels 2008, 1, 7. 10.1186/1754-6834-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozell J. J.; Black S. K.; Myers M.; Cahill D.; Miller W. P.; Park S. Solvent fractionation of renewable woody feedstocks: Organosolv generation of biorefinery process streams for the production of biobased chemicals. Biomass Bioenergy 2011, 35, 4197–4208. 10.1016/j.biombioe.2011.07.006. [DOI] [Google Scholar]
- Min D.-y.; Smith S. W.; Chang H.-m.; Jameel H. Influence of isolation condition on structure of milled wood lignin characterized by quantitative 13C nuclear magnetic resonance spectroscopy. Bioresources 2013, 8, 1790–1800. 10.15376/biores.8.2.1790-1800. [DOI] [Google Scholar]
- Lu S.; Wang Q.; Liang Z.; Wang W.; Liang C.; Wang Z.; Yuan Z.; Lan P.; Qi W. Saccharification of sugarcane bagasse by magnetic carbon-based solid acid pretreatment and enzymatic hydrolysis. Ind. Crops Prod. 2021, 160, 113159. 10.1016/j.indcrop.2020.113159. [DOI] [Google Scholar]
- Qi W.; He C.; Wang Q.; Liu S.; Yu Q.; Wang W.; Leksawasdi N.; Wang C.; Yuan Z. Carbon-Based Solid Acid Pretreatment in Corncob Saccharification: Specific Xylose Production and Efficient Enzymatic Hydrolysis. ACS Sustainable Chem. Eng. 2018, 6, 3640–3648. 10.1021/acssuschemeng.7b03959. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.