Abstract

The composition of low calorific value synthesis gas varies greatly depending on the raw material and processing technology, which makes the combustion extremely complicated. The three mechanisms of the GRI-Mech 3.0, Li-Model, and FFCM-Mech are used to numerically simulate CH4/CO/H2/N2 air premixed combustion by using ANSYS CHEMKIN-PRO. The numerical simulation is the calculation of laminar flame velocity and adiabatic flame temperature at an initial temperature of 298 K, an equivalence ratio of 0.6–1.4, and an initial pressure of 0.1–0.5 MPa, discussing through thermodynamics and chemical kinetics. The formation of NOX, H, and OH radicals by fuel composition was analyzed. The result shows that the concentrations of H, O, and OH radicals have a positive effect on laminar flame velocity. The combustion reaction of H2 is higher than that of CH4 and CO; with the increase of N2 content, the priority is higher. The thermal diffusivity of flame under different equivalence ratios is affected by inert gas, which affects adiabatic combustion temperature and laminar combustion velocity. In thermal kinetics and chemical kinetics, CH4 has more influence on combustion temperature than CO, while laminar flame velocity is relatively low. Under the change of initial pressure, the laminar combustion flux increases to the initial pressure and the laminar combustion velocity decreases to the increase in pressure. Reactions H + O2 = O + OH, HO2 + H = 2OH, and CH3 + HO2 = OH + CH3O are mainly due to change in the concentration of O, H, and OH radicals.
1. Introduction
Nowadays, the shortage of fossil energy and air pollution are increasingly affecting the living environment of human beings. The world is advocating energy saving and sustainable development.1 It is very important for us to promote the development of renewable energy and establish the energy structure with new energy and renewable energy as the main body in the energy conversion process. Biomass is considered to be one of the most promising renewable sources of energy2 because it is rich in resources and has the ability to absorb naturally released carbon and maintain carbon neutrality.3 In the past, direct combustion was the most important way to use biomass, which was often inefficient and caused serious pollution.4 Most gasified biomass are mixtures of H2, CO, CH4, N2, CO2, and other minor species.5−8 Biomass is the third largest renewable energy source used for electricity (after hydraulic and wind energy).9 In order to meet the growing demand, low calorific value syngas has gradually come into our view. In recent years, a large number of low calorific value natural gas are used in industries and homes. Since low calorific value syngas and conventional fuel exhibit diverse combustion features, the flame features will vary significantly, and with the increase of calorific value, flame instability may become a problem.10
For a long time, a large number of scholars have done a lot of research on hydrocarbon fuels. Biogas, landfill gas, and synthetic gases have also been studied due to the need for fossil energy substitution.11 The methane content of biogas and landfill gas is about 40–60%, and the calorific value is about one-half of the calorific value of CH4, which may be used as an alternative energy source.12 In addition, the synthesis gas containing H2/CO composition has been confirmed in which the flame stability will decrease as the CO ratio increases.13 Numerical simulations and experiments are used to verify the effect of adding diluent gases, such as N2, He, and CO2 in hydrocarbon fuels on flame stability.14 The addition of nitrogen exceeds about 10%, which is hard to demonstrate the flame stability.9
The laminar flame velocity of H2 /CH4 and air plays an important role in explaining the combustion properties of combustible fuels, and it determines the stability of flame combustion and flame burning rate. Plenty of previous studies are about the laminar flame velocity of H2/air mixtures,15−18 laminar burning rate of CH4/air mixtures,19−23 laminar flame velocity of H2/CH4/air mixtures,24−28 and laminar flame velocity of CO/CH4/H2/air mixtures.29−32 Nowadays, there are a few studies on the laminar flame velocity of CH4/CO/H2/N2 and air mixture.
For the study of the combustion features of low heating value gases (LHVGs), Shin et al.11 studied the flame stability restriction and flame length through a coaxial non-premixed jet burner. When the heating value is reduced, the flame stability limit value is significantly reduced. Francisco and Oliveira9 did not expect the effect of coaxial airflow; instead, the heating value of the fuel was reduced by increasing the nitrogen content in methane, and the flame structure was studied.
The purpose of this paper is to analyze the different combustion characteristics of CH4/CO/H2/N2 air premixed flame by using ANSYS CHEMKIN-PRO; Table 1 details the composition of the fuel mixture; the laminar combustion velocity and adiabatic temperature under different equivalence ratios (0.6–1.4) were simulated by using the GRI-Mech 3.0, Li-Model, and FFCM-Mech mechanisms; and the results were discussed and analyzed. The effects of fuel components on the flame structure were discussed from the view of chemical kinetics, including the mole fraction of elements, yield, and net reaction rate of main reactions. The influence of initial pressure and fuel form of laminar combustion features were studied by sensitivity analysis of the initial pressure.
Table 1. Fuel for this Simulation.
| fuel mixture (vol %) |
|||||||
|---|---|---|---|---|---|---|---|
| flame | XCH4 | XCO | XH2 | XN2 | fuel mixture density (kg/m3) | air–fuel ratio | LHV MJ/Nm3 |
| F1 | 10 | 0 | 10 | 80 | 0.977 | 1.19 | 4.27 |
| F2 | 15 | 0 | 10 | 75 | 0.953 | 1.67 | 5.92 |
| F3 | 20 | 0 | 10 | 70 | 0.929 | 2.14 | 7.56 |
| F4 | 10 | 10 | 10 | 70 | 0.977 | 1.43 | 5.43 |
| F5 | 0 | 15 | 10 | 75 | 1.026 | 0.60 | 2.72 |
| F6 | 0 | 20 | 10 | 70 | 1.026 | 0.71 | 3.30 |
2. Numerical Calculation
The characteristics of CH4/CO/H2/N2/air laminar flame were calculated by CHEMKIN-PRO. One-dimensional, premixed, laminar flame model with the PREMIX code33 and gas-phase equilibrium program EQUIL34 was used to calculate the laminar flame propagation velocity and adiabatic temperature. In this calculation, three combustion kinetic mechanisms of GRI-Mech 3.0,35 Li-Model,36 and FFCM-Mech37 are used. The detailed reaction mechanism of the GRI-Mech 3.0 includes 325 reactions and 53 substances; the detailed reaction mechanism of Li-Model includes 84 reactions and 21 substances; and the detailed reaction mechanism of FFCM-Mech includes 291 reactions and 38 substances. Figure 1 shows a comparison with the previous literature,2 which shows that the results are in good agreement with different mechanisms. In view of the current calculation, the Soret effect and multicomponent transmission model are considered in the simulation. The calculation domain is set to −2 to 10 cm, and the GRAD and CURV values are set to 0.02 to meet the boundary conditions of the free flame propagation and no thermal diffusion. The number of grids is set to 900, which is used to determine the flame velocity prediction for complete convergence.
Figure 1.

Laminar flame speed of H2 (40%)/CO (40%)/CH4 (20%)/air mixtures.
3. Results and Discussion
3.1. Influence of Different Fuel Forms on Adiabatic Temperature and Laminar Combustion Speed
Figure 2 shows the variation distribution of premixed CH4/CO/H2/N2/ air adiabatic temperature and laminar combustion velocity under different fuel components simulated by CHEMKIN-PRO. The thermal diffusivity of different fuel components was calculated by STANJAN.38 It can be seen from Figure 2a that the thermal diffusivity of all simulated fuel mixtures increases monotonously at the equivalence ratio (0.6–1.4). As the proportion of CH4 gas increases, the thermal diffusivity of the flame decreases significantly, while the influence of CO is significantly greater than CH4; when the proportion of CO gas decreases, the thermal diffusion coefficient increases significantly. In conclusion, CH4 fuel suppresses thermal diffusivity, and CO fuel increases thermal diffusivity. In addition, in Figure 2b, the adiabatic flame temperature increases significantly at the equivalent ratio of 0.6–1.0, reaches the peak at the equivalent ratio of 1.0, and decreases significantly at the equivalent ratio of 1.0–1.4. Compared with CO gas, CH4 gas has a higher adiabatic flame temperature; the difference is about 200 K. Although the thermal diffusivity of the F6 fuel component is higher than the F1 fuel component, the adiabatic combustion temperature of the F6 fuel component is greater than that of the F1 fuel component. The combustion speed is slightly different due to the fuel composition. As shown in Figure 2c, the F3 fuel reaches the maximum when the equivalent ratio is 1.0, and the SL of the F4 flame increases significantly compared with the F3 flame. Second, the peak value of SL will move to a rich state with the increase of CO content, and the laminar flame velocity significantly increases compared with the F3 fuel composition condition. From the laminar flame theory,39SL ∝ (αRR)1/2, the laminar flame velocity is positively related to the adiabatic flame temperature (Tad) and thermal diffusivity (α) and is directly related to the reaction rate (RR). In addition, it is worthy of our attention that the three mechanisms of the GRI-Mech 3.0, Li-Model, and FFCM-Mech can predict the laminar flame velocity at different equivalence ratios. In the simulation of F6 fuel components, the peak velocity of the GRI-Mech 3.0 is more inclined to the fuel-rich part, which is slightly different from the other two mechanisms.
Figure 2.
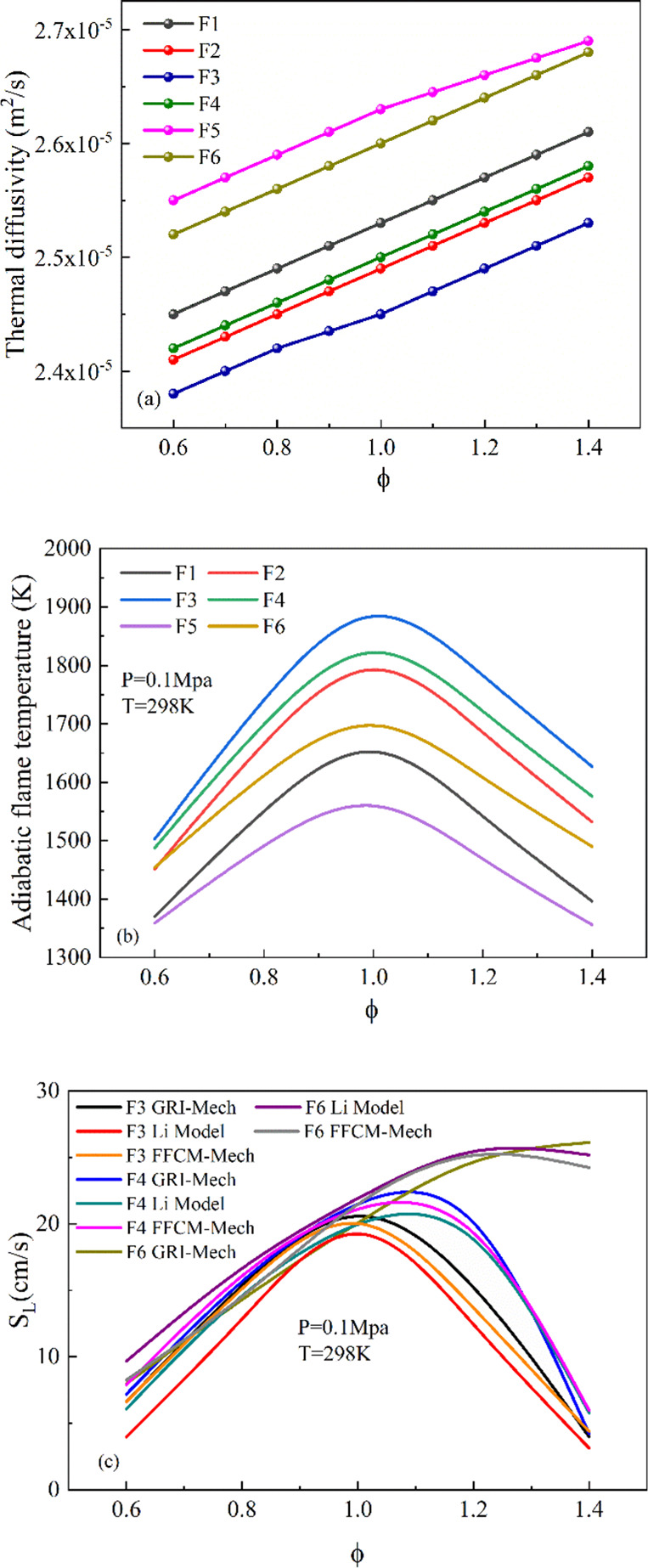
Mechanism validation: (a) Thermal diffusivity and (b) adiabatic flame temperature at different fuel compositions. (c) Laminar flame speed of CH4/CO/H2/N2/air mixtures at different fuel compositions.
It can be known from previous papers that the effect of increasing the proportion of inert gas on the laminar combustion rate is mainly due to the increase in heat capacity and the decrease in flame temperature, thereby changing the thermal performance of hydrocarbon fuels.40 The simulation results of this work are similar to the pure methane in the earlier papers.41 On the one hand, with the increase of the inert gas ratio, the concentration of fuel is diluted, resulting in the decrease of heat release and the reaction rate. Second, it will also affect the thermal diffusion coefficient and oxidation reaction kinetics of the mixture.42,43
3.1.1. Flame Structures
Figure 3 shows the most important basic reaction for calculating the laminar combustion velocity of different fuel components (F3, F4, F6) using the GRI-Mech 3.0, Li-Model, and FFCM-Mech. The combustion occurred mainly in the range of 3.96–4.08 cm. Second, the combustion reaction of H2 is higher than that of CH4 and CO, and with the increase of N2 content, this priority is higher. It can be known in the GRI-Mech 3.0 that there are five main reactions where H2 affects the combustion speed, reactions O + H2 = H + OH (R3), H + O2 + H2O=HO2 + H2O (R35), H + O2 + N2 = HO2 + H2O (R36), H + O2 = O + OH (R38), and OH + CO=H + CO2 (R99). It can be learned from the Li-Model that H2 mainly has four important reactions affecting the combustion speed, reactions H + O2 = O + OH (R1), HO2 + H = 2OH (R15), CO + sOH=CO2 + H (R29), and CH3 + H(+M) = CH4(+M) (R53). In FFCM-Mech, it can be known that H2 affects the combustion rate mainly in four important reactions H + O2 = O + OH (R1), H2O + M = H + OH + M (R15), CO + OH=H + CO2 (R32), and HCO + M = H + CO + M (R35). From these three mechanisms, we can know that reaction R1 is a branched chain reaction that generates a large amount of H and OH radicals. These active radicals (H, O, and OH) have an important effect on the laminar flame velocity.
Figure 3.

Sensitivity analysis for various CH4/CO/H2/N2 air mixtures: (a) GRI-Mech; (b) Li-Model; and (c) FFCM-Mech.
With the change of N2 in the fuel, when CH4 fuel is the main gas, H + O2 = O + OH (R38), it has the most important effect on combustion, and the H + CH3 (+M) = CH4 (+M) (R52) reaction has the highest negative sensitivity coefficient and inhibits flame propagation. At the same time, when CO is used as the main fuel, the OH + CO=H + CO2 (R99) reaction has the highest positive sensitivity coefficient, promoting the flame propagation. Meanwhile, reaction H + HO2 = O2 + H2 (R45) has a large negative sensitivity coefficient, reducing the flame propagation speed. When CH4/CO are used as the main fuel, reaction H + O2 = O + OH (R38) has the maximum positive sensitivity coefficient.
3.1.2. Chemical Kinetic Structure
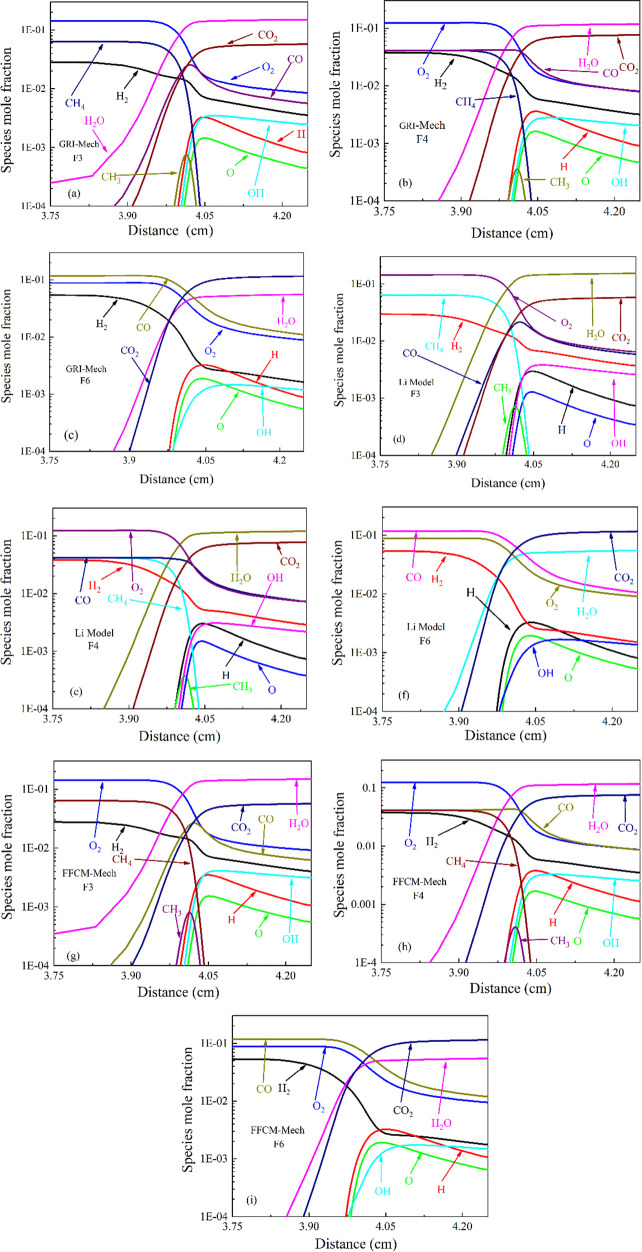
Three different fuel components F3, F4, and F6 were analyzed in detail by the GRI- Mech 3.0, Li -Model and FFCM-Mech mechanisms. Figures 4–6 show the mole fraction, productivity, and net reaction rate of the main basic steps, respectively. For F3 (20% CH4, 10% H2, 70% N2), we can see that in the mechanism of using the GRI-Mech 3.0, Li-Model and FFCM-Mech, the consumption of CH4 mole fraction is later than that of H2, so the formation of H2O is higher than that of any substances. The mole fraction of substances in F3 fuel shows similar change curves in the three mechanisms. In Figure 5a,d,g, we can know that H is consumed first and then generated, the consumption of CO is the latest, and the rate of CO2 generation increases first and then decreases.
Figure 4.
Species mole fraction of CH4/CO/H2/N2 air flames at T = 298 K, P = 0.1 MPa: (a–c) GRI-Mech F3, F4, and F6; (d–f) Li-Model F3, F4, and F6; (g–i) FFCM-Mech F3, F4, and F6.
Figure 6.
Net reaction rate of CH4/CO/H2/N2 air flames at T = 298 K, P = 0.1 MPa: (a–c) GRI-Mech F3, F4, and F6; (d–f) Li-Model F3, F4, and F6; (g–i) FFCM-Mech F3, F4, and F6.
Figure 5.
Production rate of CH4/CO/H2/N2 air flames at T = 298 K, P = 0.1 MPa: (a–c) GRI-Mech F3, F4, and F6; (d–f) Li-Model F3, F4, and F6; (g–i) FFCM-Mech F3, F4, and F6.
In the GRI-MECH 3.0, R38, R97, and R99 play a significant role in F3, generating a large number of O and OH radicals and CO2. The removal of H from CH4 mainly has the following reaction: H + CH4 = CH3 + H2 (R53) and OH + CH4 = CH3 + H2O (R98). Then, CO begins to oxidize, passing OH + CO = H + CO2 (R99). The intermediate product CO is mainly composed of H + CH4 = CH3 + H2 (R53) and O + CH3 ⇒ H + H2 + CO (R284). At the same time, the CH3 can also terminate the free radical chain process through H + CH3(+M) = CH4 + (M).
For the F4 fuel (10% CH4, 10% CO, 10% H2, 70% N2), due to the addition of CO, we can see in Figure 4 that the CO content increases slowly and then decreases, while for the F3 fuel, the CO content rises sharply, and then declines slowly. For the content of CH3-free radicals, the content of the F3 fuel is significantly higher than that of the F4 fuel. In Figure 5b,e,h, we can see that the content of CO generated by the oxidation of CH4 is greater than the consumption of CO. Obviously, the combustion of CH4 precedes CO. In Figure 6e, in the Li-Model mechanism, R1, R3, R29, and R48 play a significant role in the F4 fuel, generating a large number of free radicals of O, H, OH, and CO2. The reaction rate of R29 in the F4 fuel is significantly higher than that of the F3 fuel, thereby increasing the overall reaction rate. Therefore, the F4 fuel has a higher flame laminar flow velocity than the F3 fuel.
For the F6 fuel (20% CO, 10% H2, 70% N2), compared with the F3 fuel, CH4 becomes CO. It is learned in Figure 4c,f,i that H2O is generated later than the F3 fuel, and the content of CO2 is higher than that of the F3 fuel. Compared with the F4 fuel, the F6 fuel consumes H2 faster, and the content of H2O is greater than that of CO2. In Figure 6i, it can be found that compared with the previous two flames, the main chemical composition of the flame has been transferred to CO kinetics. R1, R4, R15, and R32 are the main reactions, generating a large amount of O, H, and OH radicals and H2O and CO2 substances. The net rate of the chemical reaction is much lower than that of the F3 fuel and F4 fuel. From the previous results of the laminar combustion temperature in Figure 2b and the laminar combustion velocity in Figure 2c, it is found that the F6 fuel laminar combustion temperature and the laminar combustion speed are higher than that of the F3 fuel flame and the F4 fuel flame. This is the same as the conclusion of Vagelopoulos et al.44 that the CO added into the fuel mixture will not work until lots of the hydrocarbons are consumed, and its effect is essentially heat.
3.1.3. Influence of Inert Gas on NOX Formation
NOX emissions have a great impact on air. On the one hand, NOX is formed by the nitrogen element in the fuel; on the other hand, it is mainly formed by the oxidation of N2 in air. The effect of inert gas on NOX production in CH4 combustion has been reported in the previous literature.45,46Figure 7 shows the changes in the maximum mole fraction of NO and NO2 for F3, F4, and F6 fuels through the GRI-Mech 3.0 mechanism. As shown in the figure, CO has an obvious inhibitory effect on the formation of NO, but the inhibition of NO2 during the combustion process is not very obvious. However, CH4 has a very obvious effect on the formation of NO and NO2. Since the concentration of NO2 is much lower than NO, the NOX in the whole reaction is related to the content of CH4.
Figure 7.
Effect of different inert gas contents on the maximum mole fraction NO and NO2 (T = 298 K, P = 0.1 MPa, ϕ = 1.0): (a) F3; (b) F4; and (c) F6.
3.2. Impact of Pressure
Figure 8 reveals the impact of using the FFCM mechanism at different initial pressures (0.1, 0.3, and 0.5 MPa) and equivalence ratios on the combustion rate of F4 (10% CH4, 10% CO, 10% H2, 70% N2) fuel laminar flow. We can know that the laminar combustion speed of the flame and the thermal diffusivity in the fuel mixture decrease with the increase of the initial pressure.4 Therefore, when the equivalence ratio ϕ = 1.0, there is a maximum laminar combustion velocity.
Figure 8.

Laminar flame speed of F4 at different initial pressures.
3.2.1. Laminar Combustion Flux
From the study of Law and Sung,47 the laminar combustion flux f0 = ρu SL is the basic modulus of flame propagation. It generally reflects fuel diffusivity, exothermicity, and reactivity. Figure 9 shows the laminar combustion flux of F4 (10% CH4, 10% CO, 10% H2, 70% N2) under different initial pressures and equivalence ratios. From the figure, we can find that the laminar combustion speed of F4 is the largest at 0.1 MPa; but on the contrary, it has a smaller laminar combustion flux. The laminar combustion flux of F4 improves with the increase of premier pressure, which is similar to Law’s48 conclusion. He pointed out that the laminar flow velocity of flame decreases with the increase of initial pressure due to the increase of density. According to the conclusion of Law et al.,47 we know that Sb0 ≈ [(λ/cP)bwb]1/2/ρb. This reveals that laminar flame responses rest with the flame dynamics of the typical reaction rate wb, and the transport processes through the density-weighted transport parameter (λ/cP)b. For us, density is the most crucial factor, which determines the explanation of the character of diffuse transport the same as that of the volume mass flow rate.16
Figure 9.

Laminar burning flux of F4 at different initial pressures.
3.2.2. Sensitivity Analysis
Figure 10 shows the important basic reactions that influence the combustion velocity of laminar flame at diverse premier pressures. With the increase of pressure, the number of molecular and free radical collisions is improved and the reaction rate is accelerated. In the FFCM-Mech, we can find that with the increase of initial pressure, the sensitivity of the reaction R1: H + O2 = O + OH, R17: HO2 + H = 2OH, R105: CH3 + HO2 = OH + CH3O and the coefficient also increase accordingly. However, the negative sensitivity coefficient R15: H2O + M = H + OH + M, R16: HO2 + H = H2 + O2, R97: CH3 + O ⇒ H + H2 + CO also increased, which inhibited the propagation of the laminar flame velocity. In the previous literature,49 termination of the reaction was very important as the pressure increased. If we add H and OH radicals and reduce HO2 radicals and a small amount of H2O molecules, so far, it will promote the entire reaction, and the flame burning rate will be greatly increased.
Figure 10.

Sensitivity analysis of different premier pressures.
3.2.3. Chemical Kinetic Analysis
According to previous research,50,51 it is known that the main influencing factors of the laminar flame velocity have a strong positive correlation with the concentration of free radicals H, OH, and O. Figure 11 shows the radical mole fraction of F4 fuels under the FFCM-Mech at different initial pressures. We found that with the increase of the initial pressure, the concentration of H, O, and OH radicals decreased, which is basically similar to the change of the flame laminar combustion speed.
Figure 11.
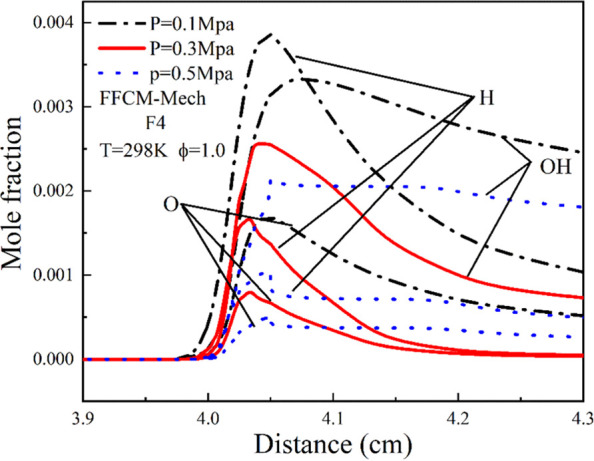
Mole fraction of H, O and OH radicals at different premier pressures.
4. Conclusions
The laminar flame velocity of syngas with a low calorific value at an equivalence ratio of 0.6–1.4 and an initial pressure of 0.1–0.5 MPa was studied by using ANSYS-CHEMKIN, and the effects on thermal diffusivity, formation of NOX, H, and OH radicals were analyzed. The main conclusions are summarized as follows:
(i) Compared with CO, CH4 has a larger adiabatic temperature and a smaller laminar flame velocity. Inert gas affects the development trend of flame thermal diffusion and reduces the adiabatic temperature and laminar combustion velocity.
(ii) The three mechanisms of GRI-Mech 3.0, Li-Model, and FFCM-Mech predict the laminar flame velocity at different equivalence ratios and show good consistency among them. In the F6 fuel, the peak velocity of the GRI-Mech 3.0 is more inclined to the fuel-rich part than that of the Li-Model and FFCM-Mech.
(iii) In the flame structure, the reaction of H2 always takes precedence over CH4 and CO. R1 is a branched reaction that produces a large number of O, H, and OH radicals, which have a positive effect on the laminar flame speed. When CO and CH4 are the main fuels, it is found that there is the same elementary reaction as CH4 as the main fuel. In the mixed gas, CH4 gas plays a major role.
(iv) With the increase of the initial pressure, the laminar combustion speed of the flame and the thermal diffusion coefficient of the fuel mixture decrease accordingly. The increase in pressure increases the number of collisions of active free radicals and speeds up the reaction rate. If we add O, H, and OH radicals, it will promote the entire reaction.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (nos. 51676064 and 51774115).
The authors declare no competing financial interest.
References
- Yao Z.; Deng H.; Dong J.; Wen X.; Zhao W.; Wang F.; Chen G.; Zhang X.; Zhang Q. Effect of the Inclination Angle on Premixed Flame Dynamics in Half-Open Ducts. ACS Omega 2020, 5, 24906–24915. 10.1021/acsomega.0c03667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.; Wen X.; Zhang S.; Wang F.; Zhu Q.; Pan R.; Ji W. Effect of Metal Foam Mesh on Flame Propagation of Biomass-Derived Gas in a Half-Open Duct. ACS Omega 2020, 5, 20643–20652. 10.1021/acsomega.0c03055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A.; Datta A.. Laminar Burning Velocity of Biomass-Derived Fuels and Its Significance in Combustion Devices. In Sustainable Energy Technology and Policies: A Transformational Journey, Volume 2; De S.; Bandyopadhyay S.; Assadi M.; Mukherjee D. A., Eds.; Springer Singapore: Singapore, 2018; pp. 359–378. [Google Scholar]
- Zhou Q.; Cheung C. S.; Leung C. W.; Li X.; Li X.; Huang Z. Effects of fuel composition and initial pressure on laminar flame speed of H2/CO/CH4 bio-syngas. Fuel 2019, 238, 149–158. 10.1016/j.fuel.2018.10.106. [DOI] [Google Scholar]
- Chaudhari S. T.; Bej S. K.; Bakhshi N. N.; Dalai A. K. Steam Gasification of Biomass-Derived Char for the Production of Carbon Monoxide-Rich Synthesis Gas. Energy Fuels 2001, 15, 736–742. 10.1021/ef000278c. [DOI] [Google Scholar]
- Lv P.; Yuan Z.; Wu C.; Ma L.; Chen Y.; Tsubaki N. Bio-syngas production from biomass catalytic gasification. Energy Convers. Manage. 2007, 48, 1132–1139. 10.1016/j.enconman.2006.10.014. [DOI] [Google Scholar]
- Panigrahi S.; Dalai A. K.; Chaudhari S. T.; Bakhshi N. N. Synthesis Gas Production from Steam Gasification of Biomass-Derived Oil. Energy Fuels 2003, 17, 637–642. 10.1021/ef020073z. [DOI] [Google Scholar]
- Wang T.; Chang J.; Lv P. Synthesis Gas Production via Biomass Catalytic Gasification with Addition of Biogas. Energy Fuels 2005, 19, 637–644. 10.1021/ef0400518. [DOI] [Google Scholar]
- Francisco R. W. Jr.; Oliveira A. A. M. Measurement of the adiabatic flame speed and overall activation energy of a methane enriched H2/CO/CO2/N2 low heating value mixture. Int. J. Hydrogen Energy 2020, 45, 29533–29545. 10.1016/j.ijhydene.2020.07.200. [DOI] [Google Scholar]
- Lee K.; Kim J.-M.; Yu B.; Lee C.-E.; Lee S. Effect of various gas compositions on gas interchangeability and combustion characteristics for domestic appliances. J. Mech. Sci. Technol. 2013, 27, 1191–1201. 10.1007/s12206-013-0225-5. [DOI] [Google Scholar]
- Shin C.; Oh Y.; Lee S. Combustion characteristics of coaxial nonpremixed flames for low heating value gases. Energy 2018, 165, 41–52. 10.1016/j.energy.2018.09.096. [DOI] [Google Scholar]
- Amini H. R.; Reinhart D. R. Regional prediction of long-term landfill gas to energy potential. Waste Manage. 2011, 31, 2020–2026. 10.1016/j.wasman.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Hwang J.; Bouvet N.; Sohn K.; Yoon Y. Stability characteristics of non-premixed turbulent jet flames of hydrogen and syngas blends with coaxial air. Int. J. Hydrogen Energy 2013, 38, 5139–5149. 10.1016/j.ijhydene.2013.01.182. [DOI] [Google Scholar]
- Karbasi M.; Wierzba I. Prediction and Validation of Blowout Limits of Co-Flowing Jet Diffusion Flames-Effect of Dilution. J. Energy Resour Technol. 1998, 120, 167–171. 10.1115/1.2795029. [DOI] [Google Scholar]
- Kwon O. C.; Faeth G. M. Flame/stretch interactions of premixed hydrogen-fueled flames: measurements and predictions. Combust. Flame 2001, 124, 590–610. 10.1016/S0010-2180(00)00229-7. [DOI] [Google Scholar]
- Hermanns R. T. E.; Konnov A. A.; Bastiaans R. J. M.; de Goey L. P. H. Laminar Burning Velocities of Diluted Hydrogen–Oxygen–Nitrogen Mixtures. Energy Fuels 2007, 21, 1977–1981. 10.1021/ef060553g. [DOI] [Google Scholar]
- Hu E.; Huang Z.; He J.; Miao H. Experimental and numerical study on laminar burning velocities and flame instabilities of hydrogen–air mixtures at elevated pressures and temperatures. Int. J. Hydrogen Energy 2009, 34, 8741–8755. 10.1016/j.ijhydene.2009.08.044. [DOI] [Google Scholar]
- Xiang L.; Jiang H.; Ren F.; Chu H.; Wang P. Numerical study of the physical and chemical effects of hydrogen addition on laminar premixed combustion characteristics of methane and ethane. Int. J. Hydrogen Energy 2020, 45, 20501–20514. 10.1016/j.ijhydene.2019.11.040. [DOI] [Google Scholar]
- Gu X. J.; Haq M. Z.; Lawes M.; Woolley R. Laminar burning velocity and Markstein lengths of methane–air mixtures. Combust. Flame 2000, 121, 41–58. 10.1016/S0010-2180(99)00142-X. [DOI] [Google Scholar]
- Liao S. Y.; Jiang D. M.; Cheng Q. Determination of laminar burning velocities for natural gas. Fuel 2004, 83, 1247–1250. 10.1016/j.fuel.2003.12.001. [DOI] [Google Scholar]
- Liao S. Y.; Jiang D. M.; Gao J.; Huang Z. H. Measurements of Markstein Numbers and Laminar Burning Velocities for Natural Gas–Air Mixtures. Energy Fuels 2004, 18, 316–326. 10.1021/ef034036z. [DOI] [Google Scholar]
- Hu E.; Li X.; Meng X.; Chen Y.; Cheng Y.; Xie Y.; Huang Z. Laminar flame speeds and ignition delay times of methane–air mixtures at elevated temperatures and pressures. Fuel 2015, 158, 1–10. 10.1016/j.fuel.2015.05.010. [DOI] [Google Scholar]
- Chu H.; Xiang L.; Meng S.; Dong W.; Gu M.; Li Z. Effects of N2 dilution on laminar burning velocity, combustion characteristics and NOx emissions of rich CH4–air premixed flames. Fuel 2021, 284, 119017. 10.1016/j.fuel.2020.119017. [DOI] [Google Scholar]
- Yu G.; Law C. K.; Wu C. K. Laminar flame speeds of hydrocarbon + air mixtures with hydrogen addition. Combust. Flame 1986, 63, 339–347. 10.1016/0010-2180(86)90003-9. [DOI] [Google Scholar]
- Halter F.; Chauveau C.; Djebaïli-Chaumeix N.; Gökalp I. Characterization of the effects of pressure and hydrogen concentration on laminar burning velocities of methane–hydrogen–air mixtures. Proc. Combust. Inst. 2005, 30, 201–208. 10.1016/j.proci.2004.08.195. [DOI] [Google Scholar]
- Huang Z.; Zhang Y.; Zeng K.; Liu B.; Wang Q.; Jiang D. Measurements of laminar burning velocities for natural gas–hydrogen–air mixtures. Combust. Flame 2006, 146, 302–311. 10.1016/j.combustflame.2006.03.003. [DOI] [Google Scholar]
- Law C. Effects of hydrocarbon substitution on atmospheric hydrogen–air flame propagation. Int. J. Hydrogen Energy 2004, 29, 867–879. 10.1016/j.ijhydene.2003.09.012. [DOI] [Google Scholar]
- Li Y.; Bi M.; Li B.; Zhou Y.; Gao W. Effects of hydrogen and initial pressure on flame characteristics and explosion pressure of methane/hydrogen fuels. Fuel 2018, 233, 269–282. 10.1016/j.fuel.2018.06.042. [DOI] [Google Scholar]
- Das A. K.; Kumar K.; Sung C.-J. Laminar flame speeds of moist syngas mixtures. Combust. Flame 2011, 158, 345–353. 10.1016/j.combustflame.2010.09.004. [DOI] [Google Scholar]
- Varghese R. J.; Kolekar H.; Hariharan V.; Kumar S. Effect of CO content on laminar burning velocities of syngas-air premixed flames at elevated temperatures. Fuel 2018, 214, 144–153. 10.1016/j.fuel.2017.10.131. [DOI] [Google Scholar]
- Zhang W.; Gou X.; Kong W.; Chen Z. Laminar flame speeds of lean high-hydrogen syngas at normal and elevated pressures. Fuel 2016, 181, 958–963. 10.1016/j.fuel.2016.05.013. [DOI] [Google Scholar]
- Dong C.; Zhou Q.; Zhao Q.; Zhang Y.; Xu T.; Hui S. Experimental study on the laminar flame speed of hydrogen/carbon monoxide/air mixtures. Fuel 2009, 88, 1858–1863. 10.1016/j.fuel.2009.04.024. [DOI] [Google Scholar]
- Kee R. J.A fortran chemical kinetics package for the analysis of gasphase chemical and plasma kinetics. Sandia Natl. Lab. 1996. [Google Scholar]
- Lutz A. E.; Rupley F. M.; Kee R. J.; Reynolds W. C.; Meeks E.. EQUIL: A CHEMKIN implementation of STANJAN for computing chemical equilibria; Reaction Design Inc.: San Diego, CA, 1998; 6500. [Google Scholar]
- Smith G. P.GRI-Mech 3.0. http://www. me. berkley. edu/gri_mech/ 1999.
- Li J.; Zhao Z.; Kazakov A.; Chaos M.; Dryer F. L.; Scire J. J. Jr. A comprehensive kinetic mechanism for CO, CH2O, and CH3OH combustion. Int. J. Chem. Kinet. 2007, 39, 109–136. 10.1002/kin.20218. [DOI] [Google Scholar]
- Smith G. P.; Tao Y.; Wang H.. Foundational fuel chemistry model version 1.0 (FFCM-1). epub, accessed http://nanoenergy. stanford. edu/ffcm1 July 2016, 26, 2018.
- Dandy D. S.Transport properties & chemical equilibrium calculator; Color. State Univ: 2018. [Google Scholar]
- Kuo K. K., Principles of combustion; 2005.
- Mitu M.; Giurcan V.; Razus D.; Oancea D. Inert gas influence on the laminar burning velocity of methane-air mixtures. J. Hazard. Mater. 2017, 321, 440–448. 10.1016/j.jhazmat.2016.09.033. [DOI] [PubMed] [Google Scholar]
- Liu F.; Guo H.; Smallwood G. J. The chemical effect of CO2 replacement of N2 in air on the burning velocity of CH4 and H2 premixed flames. Combust. Flame 2003, 133, 495–497. 10.1016/S0010-2180(03)00019-1. [DOI] [Google Scholar]
- Glassman I.Combustion; Second Ed.;. Academic Press Inc.: Orlando, FL: United States, 1987. [Google Scholar]
- Law C. K.Combustion physics; Cambridge university press: 2010. [Google Scholar]
- Vagelopoulos C. M.; Egolfopoulos F. N.; Law C. K.. Further considerations on the determination of laminar flame speeds with the counterflow twin-flame technique. In Symposium (International) on Combustion; Elsevier: 1994; 25, 1341–1347, 10.1016/S0082-0784(06)80776-9. [DOI] [Google Scholar]
- Konnov A. A.; Álvarez G. P.; Rybitskaya I. V.; Ruyck J. D. The Effects of Enrichment by Carbon Monoxide on Adiabatic Burning Velocity and Nitric Oxide Formation in Methane Flames. Combust. Sci. Technol. 2008, 181, 117–135. 10.1080/00102200802380173. [DOI] [Google Scholar]
- Lee C. E.; Lee S. R.; Han J. W.; Park J. Numerical study on effect of CO2 addition in flame structure and NOx formation of CH4-air counterflow diffusion flames. Int. J. Energy Res. 2001, 25, 343–354. 10.1002/er.686. [DOI] [Google Scholar]
- Law C. K.; Sung C. J. Structure, aerodynamics, and geometry of premixed flamelets. Prog. Energy Combust. Sci. 2000, 26, 459–505. 10.1016/S0360-1285(00)00018-6. [DOI] [Google Scholar]
- Law C. K. Propagation, Structure, And Limit Phenomena Of Laminar Flames At Elevated Pressures. Combust. Sci. Technol. 2006, 178, 335–360. 10.1080/00102200500290690. [DOI] [Google Scholar]
- Egolfopoulos F. N.; Law C. K. Chain mechanisms in the overall reaction orders in laminar flame propagation. Combust. Flame 1990, 80, 7–16. 10.1016/0010-2180(90)90049-W. [DOI] [Google Scholar]
- Xie Y.; Wang J.; Xu N.; Yu S.; Huang Z. Comparative study on the effect of CO2 and H2O dilution on laminar burning characteristics of CO/H2/air mixtures. Int. J. Hydrogen Energy 2014, 39, 3450–3458. 10.1016/j.ijhydene.2013.12.037. [DOI] [Google Scholar]
- Singh D.; Nishiie T.; Tanvir S.; Qiao L. An experimental and kinetic study of syngas/air combustion at elevated temperatures and the effect of water addition. Fuel 2012, 94, 448–456. 10.1016/j.fuel.2011.11.058. [DOI] [Google Scholar]