Abstract

Levulinic acid (LA) recently has attracted much attention as a promising biorefinery platform due to its potential to be economical and sustainable. This paper addresses technical, techno-economic, and exergetic analyses of an industrial LA production via acid-catalyzed dehydration. The process was simulated through Aspen Plus, considering a processing capacity of 15,175.60 kg/h of banana empty fruit bunches. The global productivity yield was 25.56%, producing 3883.13 kg/h of LA. The techno-economic analysis evidenced that this process may be an attractive alternative for biomass valorization, considering the obtained financial results. This process’s total production cost was 0.178 $USD per kilogram of biomass and a total annualized cost of $USD 29,163,638.95. Exergy analysis revealed that this process had an irreversibility rate of 1.48 × 105 MJ/h. The pretreatment stage presented the lowest exergetic efficiency. Globally, the exergy efficiency was 53.76%, which is within the reported results for analogous biomass transformation processes.
1. Introduction
Levulinic acid (LA) is an organic compound with formula CH3C(O)CH2CH2CO2H and is identified as a keto-acid (oxo-acid) characterized for having a carboxyl group and a ketone group. This compound is crystalline, soluble in water, and polar organic compounds; it is obtained by biological means from the treatment of cellulose, and it is an essential precursor of biofuels.1 The first LA synthesis was reported in 1840 through sucrose heating with mineral acids at high temperatures. The United States Department of Energy has identified this substance in the top-12 of the most valuable biochemicals currently produced.2 LA is a chemical with several applications for multiple purposes, such as polymer resins, animal feed, fragrance industry, additives, and antifreeze products. It can also be used in biorefinery facilities as an intermediate for production of other chemical substances.3
The acid-catalytic dehydration process is used for 5-hydroxymethyl-furfural (HMF) production, which acts as an intermediate for forming LA and formic acid (FA). The process is developed in a two-stage reactor system: the first is a tubular one and the second a continuous stirred-tank reactor.4 In the first reactor, the biomass is mixed with sulfuric acid under 210–220 °C and 25 bars. The residence time for the first reactor is 12 s, which allows the polysaccharides to be degraded into soluble sugars, such as hexoses, pentoses, and hydroxymethyl-furfural, among others.5 The outlet stream is sent to the second reactor, which operates between 190 and 200 °C and at 14 bar.6 Many investigations have recently demonstrated much attention in studying LA production via biomass transformation due to this substance potential. In this sense, there are promising results for the yield of LA from corn stover as an alternative for biomass waste valorization.7 Rok et al.8 developed multi-scale modeling of transformation technologies for producing HMF, furfural, and LA. A production process for LA (with ethanol and succinic acid) synthesis was generated under a process synthesis and superstructure approach.9 Lab-scale studies have shown successful outcomes for producing LA and other bio-based chemicals from lignocellulosic biomass, considering sustainable engineering principles.10 Girisuta et al.11 studied the reaction kinetics of LA synthesis from biomass via acid catalysis, finding that the maximum obtainable yield is 76% mol using a continuous reactor configuration. The industrial production of LA via lignocellulosic biomass remains a promising alternative in the biorefinery framework. However, some challenges have to be addressed to attain LA production on a large scale.12 These improvements are related to evolving economic- and environmental-friendly technologies to obtain higher efficiencies and design more efficient separation processes.13
Considering the above, the authors are aware of the described limitations of industrial LA production. In this sense, Meramo-Hurtado et al.14 studied the LA production process under environmental and safety assessments, obtaining valuable information about this topology toward its viability toward the implemented analyses. Environmental and safety aspects are not exclusively essential factors in evaluating the process design; other parameters such as economic and exergy/energy are quite relevant regarding the chemical process design.15 Topologies for isopropanol production were synthesized under techno-economic analysis.16 Techno-economic analysis has been a straightforward tool in advancing biorefineries since it shows a prospective or current chemical plant’s behavior considering all costs related to its start-up and operation.17 Zang et al.18 developed a techno-economic assessment of an integrated biorefinery based on biomass transformation to produce furfural, lignin, and ethanol. A fish-waste biorefinery based on anaerobic digestion for biomethane production was modeled and evaluated under techno-economic analysis for studying its profitability at the industrial implementation level.19 A pre-feasibility study based on a techno-economic analysis framework was performed to assess astaxanthin production from H. pluvial Microalgae in phase zero.20 Carjaval et al.21 studied different lignin extraction pathways under economic and environmental analyses, seeking the most cost-effective and environmentally friendly technology.
As mentioned, energy and thermal efficiency is a crucial aspect of biorefinery design under the sustainability framework. Exergy analysis involves evaluating how a plant efficiently uses its current resources (mass and energy) to accomplish the defined task (production).22 In this regard, exergy analysis is directly related to resource conservation and sustainable operation in chemical process design, considering that it provides information about exergy outflows of a system traduced into irreversibilities.23 Previous research has involved addressing exergy analysis for assessing chemical processes toward a sustainability approach. A new topology for producing TiO2 nanoparticles through a Green Chemistry synthesis was evaluated under exergy analysis,24 finding the need to improve separation processes to reach higher efficiencies. An oil sludge gasification process was analyzed via exergetic assessment to assess its potential use in power generation.25 In biorefinery design, applying exergetic analysis to find improvement opportunities and determine real energy usage/production within this type of plant has also been fundamental.26 A biorefinery for simultaneous lactic acid and electricity production from sugarcane was examined under exergy analysis, determining a global efficiency of 52.71%.27 Besides, combined approaches based on techno-economic and exergetic analyses have shown successful results toward measuring the technical and sustainability viability of a chemical design. Energy and financial factors and variables were studied for a multiproduct biorefinery system based on pyrolysis technology for coal utilization.28 Singh et al.29 applied exergy and thermo-economic analysis of a ghee production plant to evaluate this plant’s feasibility and thermal efficiency.
Previously described investigations clearly indicated the relevance of evaluating techno-economic and exergetic factors associated with bioprocesses and biorefineries due to the insights gained through their implementation. It should be noted that research is absent for LA production at an industrial scale, including not reporting information about its possible behavior from thermodynamic and techno-economic perspectives. This study represents the opportunity to analyze the insights associated with exergetic analysis since exergy involves determining the real work generated within process boundaries. The above is still a crucial aspect of the sustainable design approach for advancing novel technologies based on Green Chemistry and the biorefinery framework. It should be mentioned that previous investigations have reported an economic-based assessment for LA production processes. Different routes to produce LA and ethyl levulinate were assessed under financial and environmental analyses considering worldwide conditions.30 Cao Nhien et al.12 studied hybrid purification systems combining extraction and distillation technologies for LA production. A biorefinery for LA production was assessed based on economic assessment and CO2 emission accounting to evaluate its sustainability and profitability.31 No reported studies have addressed the exergetic analysis of LA production to the authors’ best knowledge, neither industrial nor laboratory scales. Even though LA production’s economic aspects have been studied, it is noteworthy that the current gap for this biorefinery platform still requires more research, and other perspectives (such as exergy/energy viewpoints) need to be considered. Thus, this work presents a production topology of LA from lignocellulosic biomass and its economic and exergy assessment results accompanied by technical analysis based on chemical sustainability indicators that evaluate the potential of using food crop wastes in LA production.
2. Results
2.1. Simulation of LA Production
2.1.1. Pretreatment Stage
The presented process comprises three central units for producing LA from banana empty fruit bunches: (1) acid pretreatment, (2) enzymatic hydrolysis/acid-catalyzed dehydration, and (3) purification stages. Stream 1 was the primary feedstock flow. It is presumed that feedstock was previously clean, milled, and crushed for modeling purposes, being available for dilute acid pre-hydrolysis. The flow is directed to a mixing tank with H2SO4 to form the dilute acid solution. The mixture formed is preheated (in HE-1) to 215 °C under 1 atm for reaching optimal temperature conditions before the pretreatment reactor (RX-1). The pretreatment reactor is set to hydrolyze 7% cellulose, 90% hemicellulose, and 5% lignin. This reaction system was modeled as a stoichiometric reactor in the software, taking into account reported chemical equations and fractional conversion yields reported by Luo et al.32 Partial hydrolysis of biomass involves transforming five-carbon starches such as arabinan, mannan, and galactan into reducer sugars; thus, this simulation assumed that these are hydrolyzed considering the same conditions described for hemicellulose.19 The pretreatment reactor’s outlet mass flow (stream 7) is depressurized and sent to a flash separator (FP-1) for removing vast amounts of water and some impurities. This separation unit was set at 140 °C and 1 atm. Within the pretreatment reaction, acetic acid is formed from acetate present in the biomass chemical structure; hence, this substance and sulfuric acid raise the mixture pH. The hydrolysate condensed flow is sent to a washing press filter (FT-1) for solid–liquid separation; then, the filtrate flow is cooled to 40 °C and directed to the next detoxification stage. Figure 1 shows the simulation process flowsheet diagram of the pretreatment unit for LA production.
Figure 1.
Simulation process flowsheet for the pretreatment unit in the LA production process.
The above involved adding ion exchange (IO-1) and overliming (RX-2) units to reduce the acidity. IO-1 is set to operate in a continuous configuration at 40 °C, employing ammonia to regenerate the resin (Amberlyst A20 weak base resin).17 Through this operation, about 90% of acetic acid and 100% of sulfuric acid are rejected. Otherwise, a two-step procedure conducted the overliming operation; the flow is first re-acidified (AC-1) using sulfuric acid, and then it is neutralized with lime. This operation stabilized the mixture pH, and it also precipitated gypsum (CaSO4 × 2H2O) as a product of the neutralization reaction. A hydro-cyclone (HC-1) unit separated solid and liquid streams, obtaining a rich-xylose stream and gypsum with other impurities. It is worth mentioning that the pretreatment stage allowed separating cellulose (stream 13) and xylose (stream 23). The relevance of this operation also relies on separating nondesired substances for avoiding inhibitory effects to form glucose in an enzymic hydrolysis reaction and a further decrease of overall productivity yield.20
2.1.2. Acid-Catalyzed Reaction Stage
Stream 13, with most of the cellulose content, is directed to the hydrolysis reactor (RX-3) for glucose production. This stream is first cooled (HE-5) to 30 °C and filtered (FT-2) to remove impurities; then, it is directed to the hydrolysis reactor (RX-3). The cellulose is mixed with the cellulase enzyme that is assumed to be a group of co-enzymes composed of endoglucanases for polymer particle size reduction, exoglucanases for chemical hydrolysis, and b-glucosidase to enhance the six-carbon sugar production.21 The enzymatic reaction is developed at 45 °C and 1.66 atm, with a fractional conversion yield of 0.90 kg glucose/kg cellulose. In this case, the reaction system was simulated as a stoichiometric reactor model.33 As the reaction demands significant amounts of water, the system is configured with recycling units (RE-1 and RE-2) to reduce water consumption and increase the production yield.
The rich-xylose stream is sent to a separation stage (SP-2) to remove the remaining solids and other impurities. The purified pentose flow (stream 41) is mixed with the produced glucose in the enzymatic reactor and sulfuric acid (acid catalyst). This step involved preparing the reactive solution for developing the acid-catalyzed reaction performed over a two-reactor stage. The first one converted glucose into hydroxymethyl-furfural through a plug-flow reactor. This reactor operated (RX-4) at 210 °C and 24.67 atm. The second reactor configuration was a back-mix reactor (RX-5) operating at 180 °C and 14.1 atm. The overall reaction system involved synthesizing HMF as an intermediate in the first reactor; this substance is subsequently dehydrated to produce LA and formic acid. Through a side reaction, furfural is produced from xylose.22,23Figure 2 displays the simulation process flowsheet diagram of the enzymatic hydrolysis and acid-catalyzed dehydration unit for LA production.
Figure 2.
Simulation process flowsheet for an acid-catalyzed dehydration unit in the LA production process.
This reaction system’s fractional production yield is about 80% for glucose to produce LA, while 50% of xylose is transformed into furfural. It should be mentioned that the acid-catalyzed mechanism was simulated using stoichiometric reactor models, taking into account reported literature on industrial production34 and experimental results.11
2.1.3. Purification Stage
The acid-catalyzed dehydration system developed here delivered an outlet mass flow of 9333.11 kg/h, where LA was obtained at 42.25 % wt, which still is a very poor concentration. Formic acid is available with 16.76% wt,and furfural with 6.2% wt. Consequently, the product flow is sent to the purification stage to extract LA under analytical degree. The separation unit comprised two double-effect distillation columns; the first tower (DT-1) removed formic acid and furfural from the flow, taking advantage of these substances’ volatility. This column was simulated as a RadFrac that is the rigorous modeling option available in Aspen Plus to model distillation operations. The distillate flow (stream 51) with most water and formic acid content (with a mass flow rate of 5180 kg/h) is sent to a chiller (HE-8) to make it adequate for discharge disposal or wastewater treatment. It is worth mentioning that formic acid remains with a concentration close to 30% wt, so extracting this substance at a commercial purity degree requires additional(s) separation processes. On the other side, LA is extracted from the second column’s (DT-2) bottoms (Stream 55) at 98.5 % wt. The product is then chilled (HE-10) to 28 °C to be already available for storage and distribution. This modeled large-scale plant produces 3983.13 kg/h of LA, representing a global production yield of about 30%. Figure 3 shows the simulation process flowsheet diagram of the purification unit for LA production.
Figure 3.
Simulation process flowsheet for the purification unit in the LA production process.
This LA production plant simulation provided several quantitative data, showing much information about how this process would behave at a large-scale design baseline. This fact is fundamental since this product is one of the most promising biorefinery platforms, and there is still inferior information about it for industrial application. Table 1 summarizes technical information, operating variables, and reactions related to the LA production process.
Table 1. Summary of Operating Variables for the LA Production Process.
| unit | operating variable | Value | reference | |
|---|---|---|---|---|
| acid pretreatment | temperature (°C) | 190 | (35) | |
| pressure (atm) | 13 | (17) | ||
| % solids | 0.42 | (35) | ||
| acid concentration (% w/w) | 0.0011 | (35) | ||
| water mass flow (kg/h) | 8480.36 | estimated | ||
| acid mass flow (kg/h) | 100.6 | estimated | ||
| NH3 concentration (% w/w) | 1.1 | (17) | ||
| NH3 mass flow rate (kg/h) | 3.8 | estimated | ||
| reactions | (xylan)n + nH2O → nxylose | (35) | ||
| (xylan)n → nfurfural + 2H2O | ||||
| (glucan)m + mH2O → mglucose | ||||
| acetate → acetic acid | ||||
| lignin → soluble lignin | ||||
| H2SO4 + Ca(OH)2 → CaSO4·2H2O | ||||
| enzymatic hydrolysis | reactor temperature (°C) | 45 | (33) | |
| reactor pressure (atm) | 1.66 | (17) | ||
| water supply in the reactor | 22.90 | estimated | ||
| reactions | (glucan)m + mH2O → mglucose | (32) | ||
| acid-catalyzed dehydration | temperature reactor 1 (°C) | 210 | (14) | |
| temperature reactor 2 (°C) | 180 | |||
| pressure reactor 1 (atm) | 24.67 | |||
| pressure reactor 2 (atm) | 14.10 | |||
| % moisture in the final reactor | 28.76% | estimated | ||
| reactions | glucose → 3H2O + HMF | (5) | ||
| xylose → furfural + 3H2O | ||||
| 2H2O + HMF → LA + FA | ||||
| purification | equilibrium stages tower 1 | 10 | estimated | |
| reflux ratio tower 1 (mass) | 1.5 | |||
| distillate rate tower 2 (kg/h) | 5180 | |||
| equilibrium stages tower 2 | 10 | |||
| reflux ratio tower 2 | 2.5 | |||
| distillate rate tower 2 (kg/h) | 170 |
2.2. Technical Analysis
As mentioned, the LA production process simulation generated several data about this process, making available information about extended mass/energy balances, production yield, energy consumption, and water usage, property estimation, among other parameters. These data are essential to develop technical analyses to preview how this process performs under efficiency, economic, and energy parameters. Table 2 reports process variables about resource usage obtained through process simulation for the LA production process.
Table 2. Results of Technical Variables for Resource Usage in the LA Production Process.
| variable | unit | Description | value |
|---|---|---|---|
| feedstock flow | kg/h | total mass flow of a banana empty fruit bunch that entered the process | 15,175.60 |
| product flow | kg/h | total mass flow of LA that left the process | 3883.13 |
| total water supply | m3/h | total volume flow of freshwater supplied within the process | 8670.00 |
| total mass input | kg/h | total mass inlet flow including water, reagents, and raw material | 21,175.6 |
| total energy usage | MJ/h | total energy consumed throughout the process (both cooling and heating) | 146,340.67 |
Data given in Table 2 allowed evaluating technical indicators for assessing the LA production topology. It is noteworthy that only estimating the technical parameters does not provide sufficient data to make decisions, changes, and adequately assess a chemical process. Therefore, the obtained outcomes from such metrics need to be benchmarked to establish the global performance based on the defined targets (please check the defined target values for this process in the Methodology section). Table 3 reports the results for technical indicators for evaluating the LA production process.
Table 3. Results of Technical Variables for Resource Usage in the LA Production Process.
| variable | unit | Description | value |
|---|---|---|---|
| productivity yield | % | it indicates the overall production efficiency of the plant | 25.59% |
| fractional water consumption | m3/kg | it indicates the volume of water used to generate products | 0.0022 |
| total freshwater cost | $/h | it shows the cost of freshwater supply on a time basis | 2.26 |
| renewable material index | % | it indicates the percentage of renewable material about total mass inlet flow | 71.67% |
| total energy cost | $/h | it shows the energy cost on a time basis | 251.71 |
| Specific energy intensity | MJ/kg | it indicates the energy consumed in the process per kilogram of product | 37.69 |
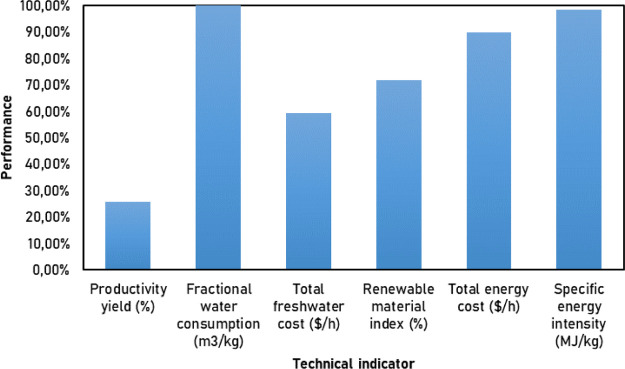
Table 3 shows that this process had excellent water management since the fractional water consumption was low, indicating that vast amounts of this resource are not needed to operate this plant. Besides, this process’s overall production yield was close to 26% for biomass to LA transformation. This result is within the expected values (even higher) related to these bioprocesses and biorefineries. Luo et al.32 reported a global yield for ethanol production of 10.79%, and Meramo-Hurtado et al.15 obtained 12.86% for biobutanol production. The obtained renewable material index (71.67%) was a relatively good value, indicating that this process can be considered as a renewable resource-based topology. Otherwise, the energy consumed within the process was moderately high, and it might show some red flags related to the energy performance. These indicators were normalized to determine their performance considering the reference parameters (please check the Methodology section). Figure 4 displays the performance results of the evaluated indicators for this process.
Figure 4.
Performance results of the evaluated technical indicators of the LA production process.
Figure 4 confirms the water management results of this production process, showing outstanding Fractional water consumption performance. The normalization also showed that contrary to what was anticipated, energy management was also pretty good in this plant with excellent outcomes from both total energy cost and specific energy intensity. These results may indicate that the process does not demand vast amounts of energy and utilities as projected (for this type of process36). This outcome is an insightful finding since bio-based processes are meant to be sustainable to handle their natural and fresh resources. The results of the process’s exergy and techno-economic analyses are next discussed to check in detail the data given by process simulation and outcomes first obtained from these indicators.
2.3. Model Validation
Model validation was made to demonstrate how accurate the developed simulation was and its real industrial context representativeness. Therefore, this task was developed based on two approaches: (i) comparing process results with reported information from both lab and industrial processes and (ii) calculating property estimation accuracy for LA. Table 4 summarizes the validation comparison for approach (i).
Table 4. Summary of Results from This Simulation Compared with Other Reported Studies Based on Approach (i).
Table 4 evidences that the simulation developed here is within the reported values for both experimental and industrial LA production. In this sense, the LA production yield was moderately higher than the compared value. It is worth highlighting that the referenced process uses rice waste for producing LA, while this process employed banana empty fruit bunches. Higher cellulose in this raw material may be the reason for this higher productivity. The pretreatment yield was measured considering cellulose and xylose production from biomass since these are the products to be extracted through the pretreatment stage. A third evaluated variable was acid dehydration yield, measured as the efficiency of producing LA (in kilograms) per mass of reacted glucose. This simulation obtained a value for this variable equal to 0.61, a very close value according to the results reported by Runge and Zhang.5 On the other hand, the second approach for analyzing the modeling results was developed comparing the reported properties for LA (the main product). Table 5 summarizes the validation comparison for approach (ii).
Table 5. Summary of Results from This Simulation Compared with Other Reported Studies Based on Approach (Ii).
The results in Table 5 evidence that this simulation showed reliable outcomes based on property estimation for LA. All three validated properties (heat capacity, density, and molecular weight) show very high accuracies (>97.80%), confirming that this simulation reflects results very close to a real representation of industrial production. This finding is also enhanced by the results described according to Table 4.
2.4. Techno-Economic Analysis
Performing techno-economic assessment involved searching data in process design books, literature, industrial reports, research papers, and calculation using Microsoft Excel worksheets. This topology’s purchasing equipment cost (PEC) was estimated using Aspen Economic Analyzer, finding that this variable corresponded to a total cost of $USD 11,732,000. Meanwhile, the utility cost for the production capacity evaluated in this study corresponded to 50.00 $/t of biomass. Table 6 summarizes the LA production process’s economic variables associated with equipment and installation costs, and process efficiency, among others.
Table 6. Economic Variables Associated with Equipment Cost and Process Efficiency for LA Production Topology.
| variable | value |
|---|---|
| equipment cost F.O.B ($USD) | 11,732,000.00 |
| PEC ($USD) | 12,905,200.00 |
| installation cost ($USD) | 1,173,200.00 |
| utilities ($/t raw material) | 50 |
| flowrate main raw material (t/y) | 146,640.00 |
| process efficiency (%) | 26 |
| cost of raw materials ($/trm) | 20.00 |
| flowrate of the main product (t/yr) | 37,525.18 |
| periods of operation | 15 |
| process units in the plant | 14 |
The delivered PEC is 1.1 times Free on board (FOB) equipment price ($USD 11,732,000), so this variable was $USD 12,905,200. Both direct and indirect production cost estimations were developed based on the delivered purchasing equipment cost. Table 7 shows the IFCI and DFCI calculations for this case study.
Table 7. Calculation of IFCI and DFCI for the LA Production Process.
| calculation of FCI | % deliv. equip. cost | total ($USD/y) |
|---|---|---|
| delivered PEC | 1.1 times FOB price | 12,905,200.00 |
| purchased equipment (installation) | 20% | 2,581,040.00 |
| instrumentation (installed) | 8% | 1,032,416.00 |
| piping (installed) | 20% | 2,581,040.00 |
| electrical (installed) | 13% | 1,677,676.00 |
| buildings (including services) | 40% | 5,162,080.00 |
| services facilities (installed) | 30% | 3,871,560.00 |
| total DFCI | 29,811,012.00 | |
| Land | 10% | 1,290,520.00 |
| yard improvements | 40% | 5,162,080.00 |
| engineering and supervision | 32% | 4,129,664.00 |
| equipment (R + D) | 10% | 1,290,520.00 |
| construction expenses | 34% | 4,387,768.00 |
| legal expenses | 1% | 129,052.00 |
| contractors’ fee | 7% | 2,086,770.84 |
| contingency | 30% | 3,871,560.00 |
| total IFCI | 22,347,934.84 |
The fixed capital investment (FCI) is obtained by adding the DFCI and IFCI, corresponding to $USD 52,158,946.84. The working capital is estimated by 60% of FCI, while the start-up cost is 10% of FCI. Thus, the LA production process presented a WC of $USD 31,295,368.10 and SUC of $USD 5,215,894.68. Now, it was determined that the TCI of this project was $USD 88,670,209.63. Therefore, if the plant lifetime is 15 years and the salvage value is 10% of FCI ($USD 5,086,842.68), this project has an AFC of $USD 3,138,140.28.
Once the TCI was determined, we proceeded to estimate the operating costs of the plant. An average base salary for operators was set to 30 $USD/h. It was assumed that 1.5 operators operate each central processing unit (14 stages in this design), working 40 h/week and 13 monthly payments in a year (an extra payment is added considering extra bonuses). Therefore, the annual labor cost for this plant was $USD 327,600. DPC calculation includes the labor cost, feedstock and reagents costs, operating supplies, and maintenance. Table 8 summarizes the corresponding costs for the direct production cost.
Table 8. Calculation of Direct Production Cost for the LA Production Process.
| variable | % of variable | total ($USD/y) | |
|---|---|---|---|
| a. raw materials | estimated | 2,932,800.00 | |
| b. utilities (U) | estimated | 7,396,521.60 | |
| c. maintenance and repairs (MR) | 5% | FCI | 2,607,947.34 |
| d. operating supplies | 15% | MR | 391,192.10 |
| e. operating labor (OL) | estimated | 327,600.00 | |
| f. direct supervision and clerical labor | 15% | OL | 49,140.00 |
| g. laboratory charges | 10% | OL | 32,760.00 |
| h. patents and royalties | 1% | FCI | 521,589.47 |
| direct production cost (DPC) | 14,259,550.51 | ||
Now, the FCH was estimated based on FCI cost. In this sense, it includes calculating depreciation (D) (7% FCI), local taxes (3% FCI), insurance (1% FCI), and interest/rent (1% FCI). The fixed charges for this project were $USD 6,364,288. Otherwise, the plant overhead is estimated as 60% of the labor costs, obtaining $USD 196,560. Consequently, the total manufacturing cost (TMC) is given by eq 1.
| 1 |
The TMC for this project was 20,820,399 $USD/y and considering the processing capacity, the TMC on a raw material basis corresponded to 0.16 $USD/kg of feedstock or 156.61 $USD/t. The general expenses were estimated by 25% of TMC, obtaining for this project a GE of 5.205.100 $USD/y. Since both GE and TMC were already determined, the plant’s total production cost (or annualized operating cost) was estimated, resulting in an annualized cost of $USD 26,025,499. As both capital investment and operating costs were determined, the total annualized cost (TAC) was calculated, obtaining an annualized cost of 29,163,639 $USD/y.
Since all the project cost was already determined, the techno-economic analysis proceeded with estimating economic performance parameters based on the TAC and product sales. It is worth mentioning that this study assumed that all the generated product is completely sold. Table 9 reports profit calculations and economic performance parameters estimated for this case study.
Table 9. Summary of Economic Parameters for the LA Production Process.
| variable | value ($USD/y) |
|---|---|
| gross profit (depreciation not included) (GP) | 68,040,529.51 |
| depreciation (D) | 3,391,228.46 |
| gross profit (depreciation included) (DGP) | 64,649,301.05 |
| profit after taxes (PAT) | 39,436,073.64 |
| discounted non-FCI expenses | –27,154,460.58 |
| LA sales (Revenues) | 93,812,940.00 |
| total production cost | 26,025,498.67 |
The information provided in Table 9 indicates that the process might be a very profitable alternative for LA production, considering the difference between the Revenues and the DPC. This affirmation is supported by the obtained PAT (39.436.073,64 $USD/y), which evidences that this project annually would provide positive cashflows considering taxation and TAC. The analysis presented here is complemented, including the estimation of economic evaluation parameters such as the net present value (NPV), return on investment (ROI), cumulative cash flow (CCF), discounted payback period (DPBP), and economic potentials. In this sense, economic indicators such as NPV, CCF, and DPBP take into account the value of money over time; meanwhile, ROI and Economic Potential indicate the plant’s current potential to recover the initial investment and the capacity of the plant to generate profits. A summary of the results for these economic evaluation indexes is reported in Table 10.
Table 10. Results of Economic Evaluation Indicators for the LA Production Process.
| economic evaluation indicator | Value | |
|---|---|---|
| economic potential 1 [$/y] | 90,880,140.00 | revenues – RM |
| economic potential 2 [$/y] | 83,483,618.40 | revenues – RM-utilities |
| economic potential 3 [$/y] | 67,787,441.33 | revenues – TPC |
| CCF (1/y) | 0.76 | |
| DPBP (y) | 5.22 | |
| ROI (%) | 44.47% | |
| NPV (MM $USD) | 35.97 | |
Economic potentials 1, 2, and 3 confirmed that this design shows positive cash flows considering revenues, raw material costs, utilities, and the TPC. The DPBP of this process was estimated considering the non-discounted FCI (see Table 9), and it is equal to 5.22 years. This is an excellent result because the investment rapidly recovers over time, and comparing with other related processes,32,40,41 this result is auspicious. An analysis of the NPV was developed for the whole plant life. Figure 5 displays the cumulative NPV over the lifetime for the LA production plant.
Figure 5.
Cumulative NPV graphic for the LA production process.
The techno-economic analysis revealed an NPV of $USD 35,970,000, which is also an excellent value, confirming that this project is a profitable alternative. The non-discounted FCI corresponded to approximately −27 MM $USD, recovered at the 5.23 y point (DPBP). This is an excellent value compared with values reported for analogous biorefineries and biomass transformation processes. For example, a biorefinery for butanol production showed a DPBP of 6.75 y,40 while Perez et al.42 reported a payback period of 5.8 y for a biohydrogen synthesis from biomass. The cumulative NPV starts becoming positive forward 10.5 years, so the plant took more than 4 years to generate money free of TAC and initial investment. This behavior confirmed the before-described results regarding the other economic performance parameters that suggest this project is entirely profitable and becomes an attractive alternative for business development.
2.5. Exergy Analysis
The generated data from process simulation (mass/energy balances, property estimation, production yield, among others) were used to develop the exergetic analysis of the LA production process presented here. First, the unknown specific chemical exergies were estimated using the Gibbs free energy of formation and constituent elements’ chemical exergy. The above included estimating this property for LA, formic acid, and HMF. Table 11 shows the Gibbs energy of formation, formula, number of elements, and estimated chemical exergy for the missing components. Otherwise, Table 12 reports the specific chemical exergy of chemical components in the LA production process.
Table 11. Estimated Chemical Exergy of the Missing Components in the LA Production Process.
| chemical | formula | MW (g/mol) | ΔG (kJ/mol) | C | H | O | εch (kJ/mol) | εch (kJ/kg) |
|---|---|---|---|---|---|---|---|---|
| LA | C5H8O3 | 116.11 | –337.94 | 5 | 8 | 3 | 5101.23 | 43,934.46 |
| FA | CH2O2 | 46.03 | –361.40 | 1 | 2 | 2 | 1188.05 | 25,810.34 |
| HMF | C6H6O3 | 126.11 | –368.97 | 6 | 6 | 3 | 4807.89 | 38,124.58 |
Table 12. Specific Chemical Exergy of the Chemical Components in the LA Production Process.
Exergy analysis equations were applied to the LA production process. It should be mentioned that the exergy analysis performed in this paper implicated hierarchizing the whole system into main or central units: (i) pretreatment, (ii) acid dehydration (including enzymatic hydrolysis), and (iii) separation. The irreversibility generation, the exergy of waste, relative irreversibility, and exergetic efficiency were calculated for each section of the process. The results indicated the pretreatment stage’s highest irreversibilities with an exergy flow per product of 28.82 MJ/kg LA. The main results and comparison of exergy efficiency for each processing stage in the LA production process are shown in Figure 6.
Figure 6.
Exergy analysis outcomes per processing unit.
The pretreatment unit obtained an irreversibilities rate according to the literature reported for acid dilute pretreatment technologies compared with results showed by Ojeda et al.,45 which reported an irreversibility generation of 25.4 MJ per kilogram of ethanol produced. This finding may indicate that the pretreatment step in biorefineries and bioprocessing remains a technology that needs improvements in its configuration to reach higher efficiencies. The relative irreversibility was higher for the pretreatment stage (75%), which confirms the above statement, followed by acid-catalyzed dehydration (19%) and separation/extraction unit (6%). The relative irreversibility provides a straightforward way to analyze and compare distinct elements in exergetic assessment even with advantages over the traditional exergy efficiency.46 Decrease of hazardous releases connected with LA production and reduced sugar generation (intermediates for LA synthesis) and resource usage constitute the foremost goal of implementing enzymatic technology. Similarly, exergy analysis gains relevance for accounting materials and waste stream usage.
Acid-dehydration and separation stages showed the highest potential to re-use their waste stream to optimize process performance since these units presented the highest exergy of waste with corresponding rates of 20.99 and 14.31 MJ/kg LA, respectively. The above results might suggest that this process can even be improved if mixing and recycling networks are implemented through mass integration techniques.47 Comparing each processing unit’s exergy efficiency, the separation stage, followed by acid-dehydration, reached the highest values for this parameter (95% and 89%), indicating an excellent performance from energy and thermodynamic viewpoints. Table 13 shows the overall exergy results of the LA production process.
Table 13. Overall Exergy Results of the LA Production Process.
| variable | Exin | Exout | Exirr | Exuti | Exwaste | ex | Rx |
|---|---|---|---|---|---|---|---|
| units | MJ/h | MJ/h | MJ/h | MJ/h | MJ/h | (%) | (MJ/kg LA) |
| LA production | 3.22 × 105 | 1.72 × 105 | 1.48 × 105 | 1.49 × 105 | 1.64 × 105 | 53.76 | 37.33 |
The overall exergy analysis results showed that the total inlet exergy flow of this process was 3.22 × 105 MJ/h, with a generation rate of irreversibilities of 1.48 × 105 MJ/h (most of them generated in the pretreatment unit). This outcome was traduced into a global exergy efficiency of 53.76%. This value is within the expected range for related biorefinery and biomass-based processes. Moreno-Sader et al.48 determined an exergy efficiency of 24% for a bio-oil production via pyrolysis; also, in the case of ethanol production, exergy efficiencies ranging from 49 to 77% were found.43 The total exergy of waste was 1.64 × 105 MJ/h, reflecting the possibility of improving the overall process performance by re-utilizing waste streams that reduce the need for external resources. The inclusion of waste minimization techniques and process intensification might be an alternative for accomplishing the described point.49 Finally, this process’s exergy intensity (Rx) was 37.33 MJ/kg of LA, indicating that this process consumes that exergy rate for producing 1 kg of product. This metric’s performance is observed from comparing this value taking all inlet exergy as consumed by the system. This result corresponded to a maximum exergy intensity of 109.91 MJ/kg of LA. Comparing both current and maximum exergy intensities, it is noticeable that this process forecasts a mid to high exergetic performance, confirming that this design is within good standards to be considered a viable alternative under technical, economic, and energetic perspectives.
3. Conclusions
This work applied technical, economic, and exergetic analyses of industrial production of LA via acid-catalyzed dehydration. The obtained outcomes showed that this process performs with a mid to high level considering technical indicator results. This process’s overall productivity yield was close to 26% with respect to the raw material flow, which is within or even above the reported results for analogous bio-based processes. Technical indicators showed outstanding water management of this process, reflecting a good performance of water resource utilization. The renewability material index obtained in this study indicated that the process employs a high rate of renewable resources (72% of all inlet mass). The above might be an important finding because it demonstrates that this design seeks to better use residual materials to produce a valuable product. The techno-economic assessment allowed estimating capital investment and operating costs for this plant, confirming this design as an attractive alternative for business development under a circular economy framework. This affirmation is supported by the renewability material indicator result and the obtained NPV, DPBP, or CCF. Exergetic analysis was also very insightful, providing much information about energy performance from a thermodynamic viewpoint. It showed that the process had an exergy efficiency within expected values for this type of process and confirmed that even though the process performs moderately well, further inclusion of waste minimization techniques might increase the overall performance. Directions for future works may go toward applying process optimization techniques and process intensification that allow better utilization of waste and energy streams. Application of sustainability footprint metrics might increase this design’s outcomes to get more comprehensive insights regarding industrial development of LA production via acid dehydration.
4. Methodology
A process model of the LA production process was developed using the Aspen Plus software. The simulation data were gathered from lab-scale reported results, industrial reports, and available bibliography associated with bioprocesses and biorefineries.50 The equipment selection and sizing were developed assisted by the simulation results and using the Aspen Economic analyzer software incorporated in Aspen Plus. For this step, experience and reports of bioprocesses were also considered from reports and industrial contracts developed by the National Renewable Energy Laboratory (NREL).51
4.1. Process Simulation and Technical Analysis
Process modeling involves selecting components, thermodynamics, and state equations that fit the problem, establishing production design capacity, among other parameters.52 Aspen Plus software was selected for modeling this production process. Table 14 reports the most important input variables and parameters used to simulate the LA production process. This computer-aided tool has an extensive enterprise database with several chemical substances included with their corresponding physical–chemical properties. Aspen Plus has many features that make this software one of the most potent tools in process engineering and chemical design. However, for emerging technologies that involve new or uncommon chemical components, there can be a possibility that those substances are not included in Aspen Plus databases.
Table 14. Raw Material Composition and Other Key Parameters.
| process variable | design basis |
|---|---|
| banana empty fruit bunch composition56 (% wt) | |
| Glucan | 42 |
| Xylan | 13 |
| Lignin | 12 |
| Ash | 4.7 |
| Acetate | 10 |
| Moisture | 19 |
| thermodynamic model | nonramdon two liquid |
| created or added components in Aspen Plus | cellulose (glucan) |
| hemicellulose (xylan) | |
| Glucose | |
| Xylose | |
| simulation stream class | MIXCISLD |
| ambient reference pressure | 1 atm |
| simulation convergence tolerance | 1 × 104 |
In this sense, this study employed the Aspen Property Estimator subroutine to include the chemical substances not found, typing their needed physical–chemical properties in the software to estimate both temperature-dependent and scalar variables and parameters.53 The National Renewable Energy Laboratory (NREL) reported property sets for including biofuel components in the Aspen Plus Database that are missing for modeling biorefineries and bioprocesses.54 Regarding this simulation, this study selected the thermodynamic model Nonrandom two liquid (NRTL), taking into account its accuracy in estimating both polar and nonpolar mixtures and vapor–liquid equilibria.55
From a global perspective, the LA production presented in this work is shown in Figure 7. Three main stages comprise this topology, each of them with specific physical and chemical operations. The process begins with the pretreatment unit to guarantee breaking the biomass structure to hydrolyze carbohydrates chemically and physically bounded between them. In this case, dilute acid pretreatment was selected, considering its proven effectiveness in extracting xylose, cellulose, and partial lignin solubilization.57 Here, pentoses are directly sent to the acid dehydration process, while nonhydrolyzed cellulose must first pass through the hydrolysis stage to synthesize glucose.
Figure 7.
Hierarchy diagram of the LA production process.
Enzymatic hydrolysis was selected over the thermal-based process since the first one delivered a higher glucose yield (90%)32 than the second one, which can reach a glucose productivity of 80%.58 This stage is crucial because it enhances glucose generation, which is the primary precursor to produce LA. Afterward, xylose and glucose streams are mixed with sulfuric acid, which is the acid catalyst used in this topology. This substance was selected as a catalyst considering the high yield for producing both HMF (80%) and further converting this substance in LA (100%) in the second reactor.59 LA is produced along with formic acid and furfural (from xylose conversion). As the outlet stream contains many substances and the main product, the process continues to the purification stage in which LA is extracted at an analytical purity degree in series of distillation towers.
Technical indicators were included in the first part of the modeling and analysis of this production topology using the process simulation quantitative data. These indicators aim to check how the process performs from efficiency, energy, and economic viewpoints.15Table 15 summarizes the used technical indicators and their reference values.
Table 15. Summary of the Used Indicators with Their formula and Reference Values.
| indicator | Formula | best value | worst value | |
|---|---|---|---|---|
| productivity yield (yi) | 1 | 0 | ||
| fractional water consumption (FWC) | 0 | 2.95 m3/kg | ||
| total freshwater cost (Ci) | Ci = abslute freshwater cost | 0 | all water mass flow under a price of $0.26/m3 | |
| renewable material index (RMI) | 1 | 0 | ||
| total energy cost (Ce) | Ce = absolute energy cost | all energy under coal cost | all energy under electricity cost | |
| specific energy intensity (RSEI) | 0 kJ/kg | 1.95 × 106 kJ/kg |
The method involved here applied the indicators given in Table 15 for obtaining a first overview of how this process is doing concerning the defined goals. The analysis is complemented by normalizing the technical indicators using reference parameters (best and worst values) to determine the process performance and compare the outcomes of the evaluated indicators.60
4.2. Techno-Economic Analysis
The economic analysis developed in this work is based on the 2020 reported United States dollar currency. The economic assumptions included in the analysis are similar to those reported by Meramo et al.61 and Perez et al.,62 considering Colombian taxation and economic policies. The operating and capital costs are estimated based on the data provided by Aspen Plus simulations, Equipment and Sizing estimation made in the Aspen Economic Analyzer, and technical or industrial reports about analogous or related chemical plants. Once the equipment cost is already determined, the method proceeds to estimate the capital investment cost (TCI), including fixed capital investment (FCI), working capital (WC), and start-up cost (SUC). Otherwise, the second step involves estimating the operating cost of the plant that includes the direct production cost (DPC), fixed charges (FCH), plant overhead (POH), and general expenses (GE). It is worth mentioning that calculating the operating costs involves accounting associated feedstock, raw materials, and waste handling cost. The FCH comprises salaries, supervision, maintenance, and property insurance, among others. The following equations are used to estimate the described cost variables for both TCI and OC.
| 2 |
| 3 |
Techno-economic analysis and its variables are regularly used yearly to make comparisons and estimations for an entire period. It is easier to manage economic parameters yearly even if the calculation basis is hourly for mass and energy balances. Thus, we can convert TCI and AOC into their corresponding annualized fixed cost (AFC) and annualized operating cost (AOC), taking into account the plant life periods (N), FCI at initial period zero (FCI0) and FCI salvage value (FCIs). The sum of the AFC and AOC provides the TAC as follows in 4.
| 4 |
| 5 |
| 6 |
It is worth mentioning that the lifetime of the plant is assumed to be 15 years. The location plant would be in South America, in the North Colombia region. This assumption is stated since this plant’s raw material is available and collected in the rural area where productive agricultural activities are carried out, generating the needed amounts of waste to operate the plant. This assumption also simplifies logistics and transportation costs. The variable operating costs are calculated based on the Aspen Plus simulation results, reported cost for utilities within Colombian conditions, and the chemical prices summarized in Table 16.34,63−65
Table 16. Associated Variables and Parameters Used in the Techno-Economic Analysis.
| parameter | Value |
|---|---|
| depreciation | Linear |
| salvage value | 10% FCI |
| plantlife time | 15 years |
| corporative interest rate | 39% |
| construction period | 3 years |
| location | North Colombia region |
| utilities and industrial services | steam, refrigerant, cooling water, electricity |
| components physical state | liquid, solid, and gas |
| material | price ($USD/kg) |
|---|---|
| banana empty fruit bunches | 0.02 |
| sulfuric acid | 0.06 |
| ammonia | 0.51 |
| calcium hydroxide | 0.20 |
| LA | 2.50 |
The second part of the economic analysis involves calculating economic performance indicators, including the NPV, DPBP, % ROI, CCF, and analysis of NPV. These indicators are estimated according to equations given in Table 17. DPBP is calculated based on interpolation of the NPV graphic considering the non-discounted FCI. This metric is essential since it shows a more realistic payback period metric because it considers value money over the lifetime of the plant.66
Table 17. Economic Performance Indicators in the Techno-Economic Assessment.
| indicator | formula | description | |
|---|---|---|---|
| economic potential 1 | this indicator shows the potential net profits by sales discounting the total raw material cost | ||
| economic potential 2 | this indicator is similar to EP1 but includes utility cost (U) | ||
| economic potential 3 | this metric shows the potential net profits discounting the annualized operating cost | ||
| CCF | this indicator is the net cash flow considering sales, annualized operating costs, and total capital investment | ||
| ROI | this metric directly measures the amount of return on a particular investment relative to the initial cost | ||
| NPV | NPV = ∑AFCn(1 + int)−n | it indicates the present value of cash inflows and outflows over the plant lifetime |
4.3. Exergy Analysis
There are two thermodynamics laws that, on their application to any chemical process, generate energetic and exergetic information. The exergy analysis involves gathering data about energy degradation and irreversibility generation during a production process. This is achieved from the second law of thermodynamics; otherwise, it could not be obtained by the limitations of the first law of thermodynamics. Therefore, it could be clearly understood that the second law allows decision-makers and process engineers to find the domains of exergy destruction and improvement areas.29 The first step of the exergy analysis developed in this work involves defining system boundaries and examination sections. A generic process is examined, as displayed in Figure 8.
Figure 8.
General overview of exergy analysis of a chemical process.
The exergy analysis begins with determining the chemical exergy of the involved substances. Often these data are available in the literature, but for cases when this is not possible, specific chemical exergy (εch) is determined using the following equation
| 7 |
ΔG0 is the energy Gibbs of formation for the substance, bv is the specific chemical exergy of an element v, and av is the number of atoms of that element.43 According to Figure 8, the general exergy is
| 8 |
Exin is the total inlet exergy, Exout is the total outlet exergy and Exirr represents the irreversibilities. The total inlet exergy is the sum of the mass exergy (Exmass), the exergy of heat streams (Exheat), and exergy of work (Exwork), as follows
| 9 |
Equation 9 is valid, assuming that both kinetic and potential exergy tend to zero.67 The exergy of mass flows comprises the sum of the chemical exergy and physical exergy flows (Exmass = Exch + Exphy). It is worth highlighting that the exergy of utilities is obtained from the exergy of heat streams and the exergy of work (Exuti = Exheat + Exwork). Therefore, eq 9 is transformed into eq 10.
| 10 |
Equation 10 indicates that the total inlet exergy of a system depends on mass balance, energy balance, and consumed work. This means that Exin depends on the process’s available resources. Consequently, a high quantity of Exirr implicates that the system is not favorable using its resources, meaning into a high rate of exergy loss associated with waste streams (Exwaste) or other mechanisms for losing exergy.23Table 18 reports calculation formulas to estimate exergy flows of a system described in eq 10.
Table 18. Calculation Formulas for Exergy flows in a Chemical Process.
| variable | formula | description | |
|---|---|---|---|
| chemical exergy | Exch = εchṁ | εchn and ṁn are the specific chemical exergy and mass flow | |
| physical exergy | Exphy=(H–H0) – T0(S–S0) | (H–H0) is the enthalpy difference, T0 is the environment temperature, and (S–S0) is the entropy difference | |
| exergy of heat | Qh is the duty of heat stream and Th is the available temperature of Qh | ||
| exergy of work | Exwork = Ẇ | Ẇ is a work stream |
The total outlet exergy flow represents (Exout) and comprises the product exergy (usable exergy) (Expro) and exergy of waste streams, as follows in eq 11.
| 11 |
Globally, the performance of a system under exergy analysis is measured through the exergetic efficiency (ex), which indicates how much usable exergy is obtained within process boundaries or a section of it with respect to the total inlet exergy.68
| 12 |
Once exergy efficiency is determined for a whole process or its stages, the analysis involved estimating the relative irreversibility. The concept is understood as a measure of resource degradation based on process irreversibilities. Thus, this metric was estimated as a ratio of irreversibilities of a process stage per total process irreversibilities. This approach identifies those inefficient stages that might be critical and susceptible to be improved or modified.69 In the exergy analysis developed in this work, another metric was included to get a more comprehensive overview regarding the exergetic performance of the system; so the exergy intensity (Rx), as follows in eq 13.15
| 13 |
This metric indicates the net used exergy by the system per mass of product generated during the operation. The exergy intensity is given in MJ/kg of product units. This metric indicates how much exergy flow is consumed by generating 1 kg of the main product. Thus, this indicator may represent a straightforward way to analyze how efficient a system is under the restriction of the second law of thermodynamics. Toward decision-making for analyzing process alternatives, this metric allows designers to easily select the most efficient alternative. The exergy intensity and exergetic efficiency are complementary variables as overall performance metrics in the exergetic analysis of chemical processes.70
Acknowledgments
The authors want to thank the Universidad de Cartagena for supporting the development of this research.
This work was funded by research grants from the University of Cartagena.
The authors declare no competing financial interest.
References
- Chen H.Lignocellulose Biorefinery Product Engineering; Elsevier, 2015. [Google Scholar]
- Ahlkvist J.Catalytic Conversion of Biomass to Biofuels, Umea University, 2010, https://doi.org/10.1039/c004654j. [Google Scholar]
- Frankiewicz A. Overview of 4-Oxopentanoic (Levulinic) Acid Production Methods - an Intermediate in the Biorefinery Process. Chemik 2016, 70, 206–208. [Google Scholar]
- Antonetti C.; Licursi D.; Fulignati S.; Valentini G.; Raspolli Galletti A. New Frontiers in the Catalytic Synthesis of Levulinic Acid: From Sugars to Raw and Waste Biomass as Starting Feedstock. Catalysts 2016, 6, 196. 10.3390/catal6120196. [DOI] [Google Scholar]
- Runge T.; Zhang C. Two-Stage Acid-Catalyzed Conversion of Carbohydrates into Levulinic Acid. Ind. Eng. Chem. Res. 2012, 51, 3265–3270. 10.1021/ie2021619. [DOI] [Google Scholar]
- Maria A.; Galletti R.; Antonetti C.; De Luise V.; Licursi D.; Nassi N.; Nasso D.; Raspolli A.; Antonetti C.; De Luise V.; Licursi D.; Nassi N. Levulinic Acid Production From Waste Biomass. BioResources 2012, 7, 1824–1835. 10.15376/biores.7.2.1824-1835. [DOI] [Google Scholar]
- Alipour S.; Omidvarborna H. High Concentration Levulinic Acid Production from Corn Stover. RSC Adv. 2016, 6, 111616–111621. 10.1039/c6ra23768a. [DOI] [Google Scholar]
- Šivec R.; Grilc M.; Huš M.; Likozar B. Multiscale Modeling of (Hemi)Cellulose Hydrolysis and Cascade Hydrotreatment of 5-Hydroxymethylfurfural, Furfural, and Levulinic Acid. Ind. Eng. Chem. Res. 2019, 58, 16018–16032. 10.1021/acs.iecr.9b00898. [DOI] [Google Scholar]
- Giuliano A.; Cerulli R.; Poletto M.; Raiconi G.; Barletta D. Process Pathways Optimization for a Lignocellulosic Biorefinery Producing Levulinic Acid, Succinic Acid, and Ethanol. Ind. Eng. Chem. Res. 2016, 55, 10699–10717. 10.1021/acs.iecr.6b01454. [DOI] [Google Scholar]
- Yu H.; Xu Y.; Hou J.; Ni Y.; Liu S.; Liu Y.; Yu S.; Nie S.; Wu Q.; Wu C. Efficient Fractionation of Corn Stover for Biorefinery Using a Sustainable Pathway. ACS Sustain. Chem. Eng. 2020, 8, 3454–3464. 10.1021/acssuschemeng.9b07791. [DOI] [Google Scholar]
- Girisuta B.; Janssen L. P. B. M.; Heeres H. J. Kinetic Study on the Acid-Catalyzed Hydrolysis of Cellulose to Levulinic Acid. Ind. Eng. Chem. Res. 2007, 46, 1696–1708. 10.1021/ie061186z. [DOI] [Google Scholar]
- Nhien L. C.; Long N. V. D.; Kim S.; Lee M. Design and Assessment of Hybrid Purification Processes through a Systematic Solvent Screening for the Production of Levulinic Acid from Lignocellulosic Biomass. Ind. Eng. Chem. Res. 2016, 55, 5180–5189. 10.1021/acs.iecr.5b04519. [DOI] [Google Scholar]
- Kang S.; Fu J.; Zhang G. From Lignocellulosic Biomass to Levulinic Acid: A Review on Acid-Catalyzed Hydrolysis. Renew. Sustain. Energy Rev. 2018, 94, 340–362. 10.1016/j.rser.2018.06.016. [DOI] [Google Scholar]
- Meramo-Hurtado S. I.; Ojeda K. A.; Sanchez-Tuiran E. Environmental and Safety Assessments of Industrial Production of Levulinic Acid via Acid-Catalyzed Dehydration. ACS Omega 2019, 4, 22302–22312. 10.1021/acsomega.9b02231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Mercado G. J.; Smith R. L.; Gonzalez M. A. Sustainability Indicators for Chemical Processes: I. Taxonomy. Ind. Eng. Chem. Res. 2012, 51, 2309–2328. 10.1021/ie102116e. [DOI] [Google Scholar]
- Panjapakkul W.; El-Halwagi M. M. Technoeconomic Analysis of Alternative Pathways of Isopropanol Production. ACS Sustain. Chem. Eng. 2018, 6, 10260–10272. 10.1021/acssuschemeng.8b01606. [DOI] [Google Scholar]
- Wooley R.; Ruth M.; Sheehan J.; Majdeski H.; Galvez A.. Lignocellulosic Biomass to Ethanol Process Design and Economics Utilizing Co-Current Dilute Acid Prehydrolysis and Enzymatic Hydrolysis Current and Futuristic Scenarios Lignocellulosic Biomass to Ethanol Process Design and Economics Utilizing Co-Current D. Contract, 1999, No. July, 132.
- Zang G.; Shah A.; Wan C. Techno-Economic Analysis of an Integrated Biorefinery Strategy Based on One-Pot Biomass Fractionation and Furfural Production. J. Clean. Prod. 2020, 260, 120837. 10.1016/j.jclepro.2020.120837. [DOI] [Google Scholar]
- Kratky L.; Zamazal P. Economic Feasibility and Sensitivity Analysis of Fish Waste Processing Biorefinery. J. Clean. Prod. 2020, 243, 118677. 10.1016/j.jclepro.2019.118677. [DOI] [Google Scholar]
- Morales-Carvajal J.; Villabona-Nuncira R.; Gonzalez-Delgado A. D.; Barajas-Ferreira C.; Barajas-Solano A. Technical-Economic Prefeasibility Study of Astaxanthin Production System from H. Pluvial Microalgae in Colombia. Indian J. Sci. Technol. 2018, 11, 1–8. 10.17485/ijst/2018/v11i34/122627. [DOI] [Google Scholar]
- Carvajal J. C.; Gómez Á.; Cardona C. A. Comparison of Lignin Extraction Processes: Economic and Environmental Assessment. Bioresour. Technol. 2016, 214, 468–476. 10.1016/j.biortech.2016.04.103. [DOI] [PubMed] [Google Scholar]
- Hamdy S.; Moser F.; Morosuk T.; Tsatsaronis G. Exergy-Based and Economic Evaluation of Liquefaction Processes for Cryogenics Energy Storage. Energies 2019, 12, 493. 10.3390/en12030493. [DOI] [Google Scholar]
- Meramo-Hurtado S. I.; González-Delgado Á. D. Aggregate/Weighted Global Sustainability and Exergy Metric for Assessing Emerging Transformation Technologies. ACS Sustain. Chem. Eng. 2020, 8, 16637–16646. 10.1021/acssuschemeng.0c06046. [DOI] [Google Scholar]
- Meramo-Hurtado S.; Moreno-Sader K.; González-Delgado Á. Simulation and Exergy Analysis of TiO2 Nanoparticles Production via Green Chemistry. PeerJ 2019, 1–11, e8113 10.7717/peerj.8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez González A.; Silva Lora E. E.; Escobar Palacio J. C. Syngas Production from Oil Sludge Gasification and Its Potential Use in Power Generation Systems: An Energy and Exergy Analysis. Energy 2019, 169, 1175–1190. 10.1016/j.energy.2018.11.087. [DOI] [Google Scholar]
- Ojeda K.; Sánchez E.; Suarez J.; Avila O.; Quintero V.; El-Halwagi M.; Kafarov V. Application of Computer-Aided Process Engineering and Exergy Analysis to Evaluate Different Routes of Biofuels Production from Lignocellulosic Biomass. Ind. Eng. Chem. Res. 2011, 50, 2768–2772. 10.1021/ie100633g. [DOI] [Google Scholar]
- Farzad S.; Görgens J. F.; Mojarab Soufiyan M.; Tabatabaei M.; Mandegari M.; Aghbashlo M. Exergy Analysis of a Lignocellulosic-Based Biorefinery Annexed to a Sugarcane Mill for Simultaneous Lactic Acid and Electricity Production. Energy 2018, 149, 623–638. 10.1016/j.energy.2018.02.063. [DOI] [Google Scholar]
- Hosseini T.; De Girolamo A.; Zhang L. Energy Evaluation and Techno-Economic Analysis of Low-Rank Coal (Victorian Brown Coal) Utilization for the Production of Multi-Products in a Drying-Pyrolysis Process. Energy Fuels 2018, 32, 3211–3224. 10.1021/acs.energyfuels.7b03840. [DOI] [Google Scholar]
- Singh G.; Singh P. J.; Tyagi V. V.; Barnwal P.; Pandey A. K. Exergy and Thermo-Economic Analysis of Ghee Production Plant in Dairy Industry. Energy 2019, 167, 602–618. 10.1016/j.energy.2018.10.138. [DOI] [Google Scholar]
- Leal Silva J. F.; Grekin R.; Mariano A. P.; Maciel Filho R. Making Levulinic Acid and Ethyl Levulinate Economically Viable: A Worldwide Technoeconomic and Environmental Assessment of Possible Routes. Energy Technol. 2018, 6, 613–639. 10.1002/ente.201700594. [DOI] [Google Scholar]
- Isoni V.; Kumbang D.; Sharratt P. N.; Khoo H. H. Biomass to Levulinic Acid: A Techno-Economic Analysis and Sustainability of Biorefinery Processes in Southeast Asia. J. Environ. Manage. 2018, 214, 267–275. 10.1016/j.jenvman.2018.03.012. [DOI] [PubMed] [Google Scholar]
- Luo L.; van der Voet E.; Huppes G. Biorefining of Lignocellulosic Feedstock - Technical, Economic and Environmental Considerations. Bioresour. Technol. 2010, 101, 5023–5032. 10.1016/j.biortech.2009.12.109. [DOI] [PubMed] [Google Scholar]
- El-Naggar N. E. A.; Deraz S.; Khalil A. Bioethanol Production from Lignocellulosic Feedstocks Based on Enzymatic Hydrolysis: Current Status and Recent Developments. Biotechnology 2014, 13, 1–21. 10.3923/biotech.2014.1.21. [DOI] [Google Scholar]
- Hayes D. J.; Fitzpatrick S.; Hayes M. H. B.; Ross J. R. H. The Biofine Process - Production of Levulinic Acid, Furfural, and Formic Acid from Lignocellulosic Feedstocks. Biorefineries: Ind. Processes Prod. 2008, 1, 139–164. 10.1002/9783527619849.ch7. [DOI] [Google Scholar]
- López-Arenas T.; Rathi P.; Ramírez-Jiménez E.; Sales-Cruz M.; Sales M. Factors Affecting the Acid Pretreatment of Lignocellulosic Biomass: Batch and Continuous Process. Eur. Symp. Comput. Aided Chem. Eng. 2010, 28, 979–984. 10.1016/S1570-7946(10)28164-6. [DOI] [Google Scholar]
- Albarelli J. Q.; Onorati S.; Caliandro P.; Peduzzi E.; Meireles M. A. A.; Marechal F.; Ensinas A. V. Multi-Objective Optimization of a Sugarcane Biorefinery for Integrated Ethanol and Methanol Production. Energy 2017, 138, 1281–1290. 10.1016/j.energy.2015.06.104. [DOI] [Google Scholar]
- Wang L.; Kong F.; Chen H.. Steam Explosion Pretreatment and Saccharification of Lignocellulosic Biomass. In Handbook of Biorefinery Research and Technology; Springer, 2018; pp 1–14. [Google Scholar]
- Nikitin E. D.; Popov A. P.; Bogatishcheva N. S.; Faizullin M. Z. Critical Temperatures and Pressures, Heat Capacities, and Thermal Diffusivities of Levulinic Acid and Four n-Alkyl Levulinates. J. Chem. Thermodyn. 2019, 135, 233–240. 10.1016/j.jct.2019.03.040. [DOI] [Google Scholar]
- Sigma-Aldrich . Levulinic Acid 98 %, 2020, 123-76-2. [Google Scholar]
- Meramo-Hurtado S. I.; González-Delgado Á. D.; Rehmann L.; Quiñones-Bolaños E.; Mehrvar M. Comparison of Biobutanol Production Pathways via Acetone–Butanol–Ethanol Fermentation Using a Sustainability Exergy-Based Metric. ACS Omega 2020, 5, 18710–18730. 10.1021/acsomega.0c01656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada J.; Tamayo J. A.; Cardona C. A. Integrating First, Second, and Third Generation Biorefineries: Incorporating Microalgae into the Sugarcane Biorefinery. Chem. Eng. Sci. 2014, 118, 126–140. 10.1016/j.ces.2014.07.035. [DOI] [Google Scholar]
- Perez D. L.; Luna E. J.; Peralta-ruiz Y. Y.; Perez Zúñiga D. L.; Luna Barrios E. J.; Peralta-ruiz Y. Y.; González-Delgado A. D. Techno-Economic Sensitivity of Bio-Hydrogen Production from Empty Palm Fruit Bunches under Colombian Conditions. Chem. Eng. Trans. 2016, 52, 1117–1122. 10.3303/CET1652187. [DOI] [Google Scholar]
- Ojeda K.; Sanchez E.; Kafarov V. Sustainable Ethanol Production from Lignocellulosic Biomass - Application of Exergy Analysis. Energy 2011, 36, 2119–2128. 10.1016/j.energy.2010.08.017. [DOI] [Google Scholar]
- Szargut J. Appendix 1. Standard Chemical Exergy. Thermodyn. Destr. Resour. 2011, 1–8. [Google Scholar]
- Ojeda K.; Sánchez E.; El-Halwagi M.; Kafarov V. Exergy Analysis and Process Integration of Bioethanol Production from Acid Pre-Treated Biomass: Comparison of SHF, SSF and SSCF Pathways. Chem. Eng. J. 2011, 176–177, 195–201. 10.1016/j.cej.2011.06.083. [DOI] [Google Scholar]
- Oyekale J.; Petrollese M.; Heberle F.; Brüggemann D.; Cau G. Exergetic and Integrated Exergoeconomic Assessments of a Hybrid Solar-Biomass Organic Rankine Cycle Cogeneration Plant. Energy Convers. Manage. 2020, 215, 112905. 10.1016/j.enconman.2020.112905. [DOI] [Google Scholar]
- Kermani M.; Périn-Levasseur Z.; Benali M.; Savulescu L.; Maréchal F. A Novel MILP Approach for Simultaneous Optimization of Water and Energy: Application to a Canadian Softwood Kraft Pulping Mill. Comput. Chem. Eng. 2017, 102, 238–257. 10.1016/j.compchemeng.2016.11.043. [DOI] [Google Scholar]
- Moreno-Sader K.; Meramo-Hurtado S. I.; González-Delgado A. D. Computer-Aided Environmental and Exergy Analysis as Decision-Making Tools for Selecting Bio-Oil Feedstocks. Renew. Sustain. Energy Rev. 2019, 112, 42–57. 10.1016/j.rser.2019.05.044. [DOI] [Google Scholar]
- Agarwal A.; Sengupta D.; El-Halwagi M. Sustainable Process Design Approach for On-Purpose Propylene Production and Intensification. ACS Sustain. Chem. Eng. 2018, 6, 2407–2421. 10.1021/acssuschemeng.7b03854. [DOI] [Google Scholar]
- Darkwah K.; Knutson B. L.; Seay J. R. A Perspective on Challenges and Prospects for Applying Process Systems Engineering Tools to Fermentation-Based Biorefineries. ACS Sustain. Chem. Eng. 2018, 6, 2829–2844. 10.1021/acssuschemeng.7b03762. [DOI] [Google Scholar]
- Chen X.; Shekiro J.; Pschorn T.; Sabourin M.; Tucker M. P.; Tao L. Techno-Economic Analysis of the Deacetylation and Disk Refining Process: Characterizing the Effect of Refining Energy and Enzyme Usage on Minimum Sugar Selling Price and Minimum Ethanol Selling Price. Biotechnol. Biofuels 2015, 8, 1–13. 10.1186/s13068-015-0358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meramo-Hurtado S. I.; Sanchez-Tuiran E.; Ponce-Ortega J. M.; El-Halwagi M. M.; Ojeda-Delgado K. A. Synthesis and Sustainability Evaluation of a Lignocellulosic Multifeedstock Biorefinery Considering Technical Performance Indicators. ACS Omega 2020, 5, 9259–9275. 10.1021/acsomega.0c00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meramo-Hurtado S.; Urbina-Suarez N.; González-Delgado Á. Computer-Aided Environmental and Exergy Analyses of a Large-Scale Production of Chitosan Microbeads Modified with TiO2 Nanoparticles. J. Clean. Prod. 2019, 273, 117804. 10.1016/j.jclepro.2019.117804. [DOI] [Google Scholar]
- Wooley R. J.; Putsche V.. Development of an ASPEN PLUS Physical Property Database for Biofuels Components; National Renewable Energy Lab: Victoria, 1996, No. April, 1–38. [Google Scholar]
- Peralta-Ruiz Y.; González-Delgado A. D.; Kafarov V. Evaluation of Alternatives for Microalgae Oil Extraction Based on Exergy Analysis. Appl. Energy 2013, 101, 226–236. 10.1016/j.apenergy.2012.06.065. [DOI] [Google Scholar]
- Igbinadolor R.; Onilude A. Bioprocess Systems Applied for the Production of Bio-Ethanol from Lignocellulosic Biomass of Cocoa Pod Husk (Theobroma Cacao L.) and Other Agricultural Residues: A Review. Afr. J. Biotechnol. 2013, 12, 5375–5388. 10.5897/ajb2013.12890. [DOI] [Google Scholar]
- Liu Q.; Li W.; Ma Q.; An S.; Li M.; Jameel H.; Chang H. M. Pretreatment of Corn Stover for Sugar Production Using a Two-Stage Dilute Acid Followed by Wet-Milling Pretreatment Process. Bioresour. Technol. 2016, 211, 435–442. 10.1016/j.biortech.2016.03.131. [DOI] [PubMed] [Google Scholar]
- Kumar P.; Barrett D. M.; Delwiche M. J.; Stroeve P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. 10.1021/ie801542g. [DOI] [Google Scholar]
- Version D.Levulinic Acid from Lignocellulosic Biomass Buana Girisuta; University Library Groningen, 2017. [Google Scholar]
- Krejčí J.; Stoklasa J. Aggregation in the Analytic Hierarchy Process: Why Weighted Geometric Mean Should Be Used Instead of Weighted Arithmetic Mean. Expert Syst. Appl. 2018, 114, 97–106. 10.1016/j.eswa.2018.06.060. [DOI] [Google Scholar]
- Meramo-Hurtado S.-I.; Gonzalez-Delgado A. D. Application of Techno-Economic and Sensitivity Analyses as Decision-Making Tools for Assessing Emerging Large-Scale Technologies for Production of Chitosan-Based Adsorbents. ACS Omega 2020, 5, 17601–17610. 10.1021/acsomega.0c02064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez J. C. R.; Echeverry L. A. V.; Peralta-Ruiz Y. Y.; González-Delgado A. D. A Techno-Economic Sensitivity Approach for Development of a Palm-Based Biorefineries in Colombia. Chem. Eng. Trans. 2017, 57, 13–18. 10.3303/CET1757003. [DOI] [Google Scholar]
- Herrera-Rodriguez T.; Parejo-Palacio V.; González-Delgado Á. D. Technoeconomic Sensibility Analysis of Industrial Agar Production from Red Algae. Chem. Eng. Trans. 2018, 70, 2029–2034. 10.3303/CET1870339. [DOI] [Google Scholar]
- McAloon A.; Taylor F.; Yee W.; Ibsen K.; Wooley R.. Determining the Cost of Producing Ethanol from Corn Starch and Lignocellulosic Feedstocks; National Renewable Energy Lab, Technical Report 2000, No. October, 1–44.
- Niño-López L.; Cárdenas A. A.; Zambrano R. G. Evaluation of Chemical Pretreatments for Enzymatic Hydrolysis of Lignocellulosic Residues Cassava (Manihot Esculenta Crantz). Rev. Fac. Ing. 2013, 69, 317–326. [Google Scholar]
- El-Halwagi M. M.Sustainable Design Through Process Integration: Fundamentals and Applications to Industrial Pollution Prevention, Resource Conservation, and Profitability Enhancement; Butterworth-Heinemann, 2012. [Google Scholar]
- Zoder M.; Balke J.; Hofmann M.; Tsatsaronis G. Simulation and Exergy Analysis of Energy Conversion Processes Using a Free and Open-Source Framework—Python-Based Object-Oriented Programming for Gas- and Steam Turbine Cycles. Energies 2018, 11, 2609. 10.3390/en11102609. [DOI] [Google Scholar]
- Ghannadzadeh A.; Thery-Hetreux R.; Baudouin O.; Baudet P.; Floquet P.; Joulia X. General Methodology for Exergy Balance in ProSimPlus Process Simulator. Energy 2012, 44, 38–59. 10.1016/j.energy.2012.02.017. [DOI] [Google Scholar]
- Morris D. R. Exergy Analysis and Cumulative Exergy Consumption of Complex Chemical Processes: The Industrial Chlor-Alkali Processes. Chem. Eng. Sci. 1991, 46, 459–465. 10.1016/0009-2509(91)80007-L. [DOI] [Google Scholar]
- Sikdar S. K.; Sengupta D.; Mukherjee R.. Measuring Progress towards Sustainability: A Treatise for Engineers; Springer, 2016. [Google Scholar]