Abstract
An in vitro study was conducted to assess the impact of organochlorine pesticides (OCPs) on cellular growth, morphology, cell viability, biofilm-formation activity, and growth-regulating substances of a soil bacterium. Phosphate-solubilizing EAM 35 isolated from rhizosphere soil was molecularly identified as Enterobacter cloacae (accession number MT672578.1). Strain EAM 35 tolerated varying levels of OCPs, viz., benzene hexachloride (BHC), chlorpyrifos (CP), dieldrin (DE), and endosulfan (ES). The toxicity of OCPs to strain EAM 35 was displayed in a concentration-dependent manner. Among the OCPs, ES at a concentration of 200 μM showed a higher toxicity, where it maximally reduced the bacterial synthesis of indole-3-acetic acid (IAA), salicylic acid (SA), and 2,3-dihydroxy-benzoic acid (DHBA) by 73% (p ≤ 0.001), 85% (p ≤ 0.005), and 83% (p ≤ 0.001), respectively, over the control. While comparing the toxicity of OCPs to P-solubilizing activity of E. cloacae after 10 days of growth, the toxicity pattern followed the order ES (mean value = 82.6 μg mL–1) > CP (mean value = 93.2 μg mL–1) > DE (mean value = 113.6 μg mL–1) > BHC (mean value = 127 μg mL–1). Furthermore, OCP-induced surface morphological distortion in E. cloacae EAM 35 was observed as gaps, pits on both cellular facets, and fragmented and disorganized cell structure under a scanning electron microscope (SEM). The membrane-compromised cells increased as the concentrations of OC pesticides increased from 25 to 200 μM. Additionally, microbial counts (log10 CFU/mL) were also affected after pesticide exposure and decreased with increasing concentrations. While assessing the impact of OCPs on inhibition (%) of log10 CFU/mL, 150, 175, and 200 μM concentrations of ES completely reduced the growth of E. cloacae. Similarly, while comparing the toxicity of higher concentrations of OCPs to bacterial growth, sensitivity followed the order ES > DE > CP > BHC. In addition, the biofilm-formation ability of strain EAM 35 was inhibited in a pesticide-dose-dependent manner, and it was statistically (p ≤ 0.05, p ≤ 0.005, and p ≤ 0.001) significant. Conclusively, the present study clearly suggests that before applying pesticides to soil, their recommended dose should carefully be monitored.
Introduction
Pesticides including insecticides are foreseeable tools of agronomic practices used to protect the crops from damaging effect of various pests.1−3 After application, these chemicals reach the soils and negatively interact with soil microorganisms.4 The irregular and indiscriminate use of such chemical pesticides leads to the destruction of physicochemical processes of soil, microbial structure,5 physiology, and enzymatic activity.6 Soil microorganisms are an important biological component of the soil ecosystem and have a vital role in the fertility of soil through decomposition of organic matter and nutrient cycling.7 Also, soil microbes inhabiting various environments enhance the yield and growth of crops by synthesizing numerous growth-regulating substances like phytohormones,8,9 siderophores,10,11 1-amino-cyclopropane-1-carboxylate deaminase,12 ammonia,13,14 etc. These pesticides when reach soil adversely affect the physiology and morphology of soil microbes.15 In this regard, several workers have reported the negative/toxic effect of pesticides on soil microbes and their associated activities. For instance, Kumar et al.16 have reported that organophosphate pesticides like acephate, phorate, monocrotophos, glyphosate, etc. severely affect the growth, physiology, and siderophore production ability of characterized soil microbes. Similarly, in another studies, different groups of pesticides interrupted the metabolic pathways, leading to reduction/impairment in synthesis of growth-regulating substances, damaged surface structure, cell permeability, and inhibition of P-solubilization of numerous soil bacteria, viz., Pseudomonas sp.,17Azotobacter sp.,18Enterobacter sp.,19Azotobacter vinelandii,20 and Burkholderia cepacia.21
Organochlorine pesticides (OCPs), which belong to a chlorinated hydrocarbon derivative group, are semisynthetic pesticides and are semivolatile by nature.22,23 These OCPs are vastly used as chemical protectants in the area of agriculture.24 The long-term application of OCPs and their persistence in the natural soil system are the main causes of environmental pollution. The haphazard use of such chemicals may negatively affect the soil microbial population including phosphate-solubilizing bacteria.25 The negative effects of OCPs on molecularly characterized beneficial bacterial strains including PSB are reported, which have been confirmed by various studies. For example, Singh and Singh26 in a similar study observed that the increasing concentrations of OCPs, for example, lindane, decreased the growth-regulating substances of a soil beneficial isolate Microbacterium sp. Likewise, the cumulative concentrations of endosulfan (ES) decreased the indole-3-acetic acid (IAA)- and ammonia-producing ability of two pesticide-tolerant strains Delftia lacustris IITISM30 and Klebsiella aerogenes IITISM42.27 Additionally, an in vitro study conducted by Tripti et al.28 confirmed the toxicity of some commercially available OCPs, viz., chlorpyrifos (CP), phorate, and endosulfan toward a soil isolate Burkholderia sp. L2 recovered from pesticide-polluted rhizosphere of Lycopersicum esculentum.
Keeping in view the problem of organochlorine pesticide toxicity to soil microbes, the present study was intended to (i) isolate and molecularly characterize (using 16S rRNA analysis) the Enterobacter cloacae strain EAM 35, (ii) determine the minimum inhibitory concentration (MIC) of four OCPs toward recovered isolates, (iii) assess the impact of different concentrations of OCPs on P-solubilization, indole-3-acetic acid, and siderophores synthesized by strain EAM 35, (iv) evaluate the effect of OCPs on the surface morphology and cell permeability of E. cloacae, (v) assess the impact of pesticides on growth behavior and CFU counts of E. cloacae, and (vi) determine the effect of various concentrations of OCPs on the biofilm-formation ability of E. cloacae.
Results and Discussion
Biochemical Characterization and Molecular Identification of Bacteria
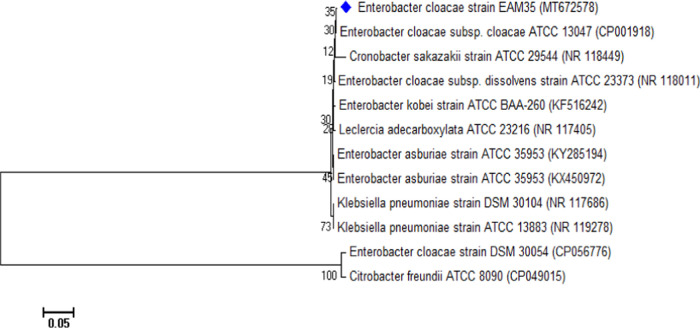
The microbiological and biochemical features of chosen soil isolates varied significantly (Table 1). The Gram staining and microscopic observation confirmed that isolates are Gram-negative short rods. The isolate EAM 35 exhibited variable reactions toward various biochemical tests (Table 1). The 16S rRNA sequences were obtained from Macrogen and data available in the NCBI data bank and LPSN web portal, and isolate EAM 35 showed the maximum base sequence similarity (100.00%) to type strains E. cloacae DSM 30054T (Accession number CP056776.1) and E. cloacae ATCC 13047T (accession number CP001918.1) (maximum base sequence similarity > 99.26%) (Figure 1). On the basis of maximum relatedness to type strains, isolate EAM 35 was identified as E. cloacae (Accession number MT672578.1). Therefore, there could be minimal differences in E. cloacae isolated in this study and reported elsewhere in other studies. Other workers have also recovered the different species of Enterobacter from various soil habitats and characterized them by 16S rRNA partial gene sequence analysis and other state-of-the-art tools.29−32
Table 1. Microbiological and Biochemical Features of Enterobacter cloacae Strain EAM 35.
| features | E. cloacae EAM 35 |
|---|---|
| Morphological Characteristics | |
| Gram’s reaction | –ve |
| configuration | round |
| margin | entire |
| surface | smooth |
| pigmentation | white to creamy |
| opacity | translucent |
| shape | short rods |
| Cultural Characteristics | |
| optimum temperature | 28 ± 2 °C |
| Biochemical Reactions | |
| citrate utilization | yes |
| indole reaction | no |
| methyl red | no |
| nitrate reduction | yes |
| oxidase | no |
| Voges–Proskaur | yes |
| Carbohydrate Utilization | |
| dextrose | yes |
| lactose | yes |
| mannitol | no |
| sucrose | yes |
| urea hydrolysis | no |
| starch hydrolysis | yes |
| gelatin hydrolysis | yes |
Figure 1.
Unrooted neighbor-joined phylogenetic tree of E. cloacae EAM 35. The tree was constructed based on 16S rRNA partial gene sequences of selected bacteria (marked with the red square) and closely related phylogenetic species (type cultures) derived using the NCBI BLAST search tool. Sequences were aligned using the Clustal W sequence alignment tool in MEGA 7.0 software.
Pesticide Tolerance
The minimum inhibitory concentrations (MICs) of all of the OCPs toward the selected isolates were assessed by growing the bacterial cultures on nutrient medium containing variable doses of pesticides. All of the OCPs showed the variation in terms of MICs toward the selected isolates (Table 2). It was observed that the MIC values of pesticides ranged between 25 and 200 μM. The toxicity of all of the OCPs toward the isolates was observed to follow the order benzene hexachloride (BHC) > chlorpyrifos > dieldrin (DE) > endosulfan. Likewise, the MIC values of different groups of pesticides such as propiconazole, hexaconazole, profenofos, and pretilachlor toward soil microbes was evaluated, and it was observed that 50% of microbial populations were inhibited at the level of 1000 ppm.33 In a similar observation, Suheil and Fehmey34 conducted an in vitro study to determine the minimum inhibitory concentrations (MICs) of three different groups of pesticides, namely, benomyl, super atracedein, and herpicide toward the soil bacterium Azotobacter chroococcum using serial dilutions and incubation periods. They found that after different incubation periods (6, 12, and 18 days), the MIC of pesticides was variable, and it ranged between 0.6 and 1.2 g L–1 and the 10–4 dilution was considered as resistant for concentrations of pesticides.
Table 2. Phosphate-Solubilization Activity of Bacterial Isolates and Minimum Inhibitory Concentrations (MICs) of Organochlorine Pesticides (OCPs)a.
| MIC (μM) |
|||||||
|---|---|---|---|---|---|---|---|
| isolates | solubilization zone (mm) | P-solubilized in liquid broth (μg mL–1) | pH | benzene hexachloride (BHC) | chlorpyrifos (CP) | dieldrin (DE) | endosulfan (ES) |
| EAM 2 | 13 | 132 ± 7.5 | 5.0 | 100 | 25 | 75 | 50 |
| EAM 3 | 15 | 98 ± 3.4 | 4.5 | 100 | 25 | 50 | 75 |
| EAM 5 | 12 | 110 ± 5.5 | 3.9 | 125 | 125 | 50 | 25 |
| EAM 8 | 15 | 145 ± 4.3 | 3.7 | 150 | 125 | 50 | 25 |
| EAM 10 | 16 | 212 ± 7.1 | 4.2 | 150 | 100 | 75 | 50 |
| EAM 11 | 16 | 87 ± 4.2 | 5.2 | 150 | 100 | 50 | 50 |
| EAM 14 | 17 | 92 ± 3.7 | 5.5 | 100 | 125 | 100 | 50 |
| EAM 16 | 15 | 127 ± 6.5 | 6.0 | 100 | 125 | 100 | 50 |
| EAM 20 | 15 | 219 ± 9.2 | 4.0 | 50 | 150 | 100 | 50 |
| EAM 24 | 16 | 139 ± 5.2 | 5.0 | 75 | 100 | 75 | 50 |
| EAM 25 | 13 | 116 ± 00 | 5.0 | 75 | 150 | 75 | 75 |
| EAM 27 | 14 | 215 ±6.2 | 5.0 | 100 | 75 | 50 | 100 |
| EAM 29 | 12 | 187 ± 4.9 | 4.5 | 125 | 100 | 50 | 75 |
| EAM 30 | 17 | 213 ± 8.3 | 4.5 | 150 | 150 | 100 | 75 |
| EAM 32 | 12 | 156 ± 7.2 | 4.0 | 150 | 150 | 100 | 50 |
| EAM 33 | 12 | 118 ± 5.5 | 3.8 | 200 | 100 | 50 | 50 |
| EAM 35 | 19 | 282 ± 12 | 3.9 | 200 | 200 | 150 | 150 |
Each value is a mean (mean ± SD) of three independent replicates.
Indole-3-acetic Acid and Siderophore
E. cloacae strain EAM 35 secreted 127.6 ± 6.5 μg mL–1 indole-3-acetic acids when cultured in Luria–Bertani (LB) broth in the absence of OCPs (controlled condition), which, however, significantly (p ≤ 0.05) reduced with subsequent enhancement in the concentrations of each pesticide. The higher (200 μM) concentrations of each pesticide showed the most robust toxic impact and maximally reduced the synthesis of IAA. For instance, at 200 μM concentrations, each of BHC, CP, DE, and ES, the IAA production was reduced maximally by 55, 42, 53, and 73%, respectively, over the untreated control (Figure 2A). Among all tested OCPs, endosulfan (ES) had the greater toxic effect. While comparing the toxicity of 200 μM concentration, the reduction in IAA production follows the order ES > DE > CP > BHC. The reduction in IAA production at higher concentrations of OCPs could possibly be due to slower growth and altered physiological activity of bacterial cells. Mechanistically, pesticides may affect the metabolism inside the cell by binding to amino and sulfide groups and thus impair the metabolic activity of bacterial cells; therefore, the secretion of phytohormone was reduced. In line with these results, the phytohormone synthesized by Paenibacillus sp. was negatively affected following the exposure to different groups of organochlorine pesticides.35 Similarly, the toxic effects of pesticides on the indole-3-acetic acid-synthesizing ability of soil microbes recovered from various habitats have been reported by other workers.18,36,37
Figure 2.
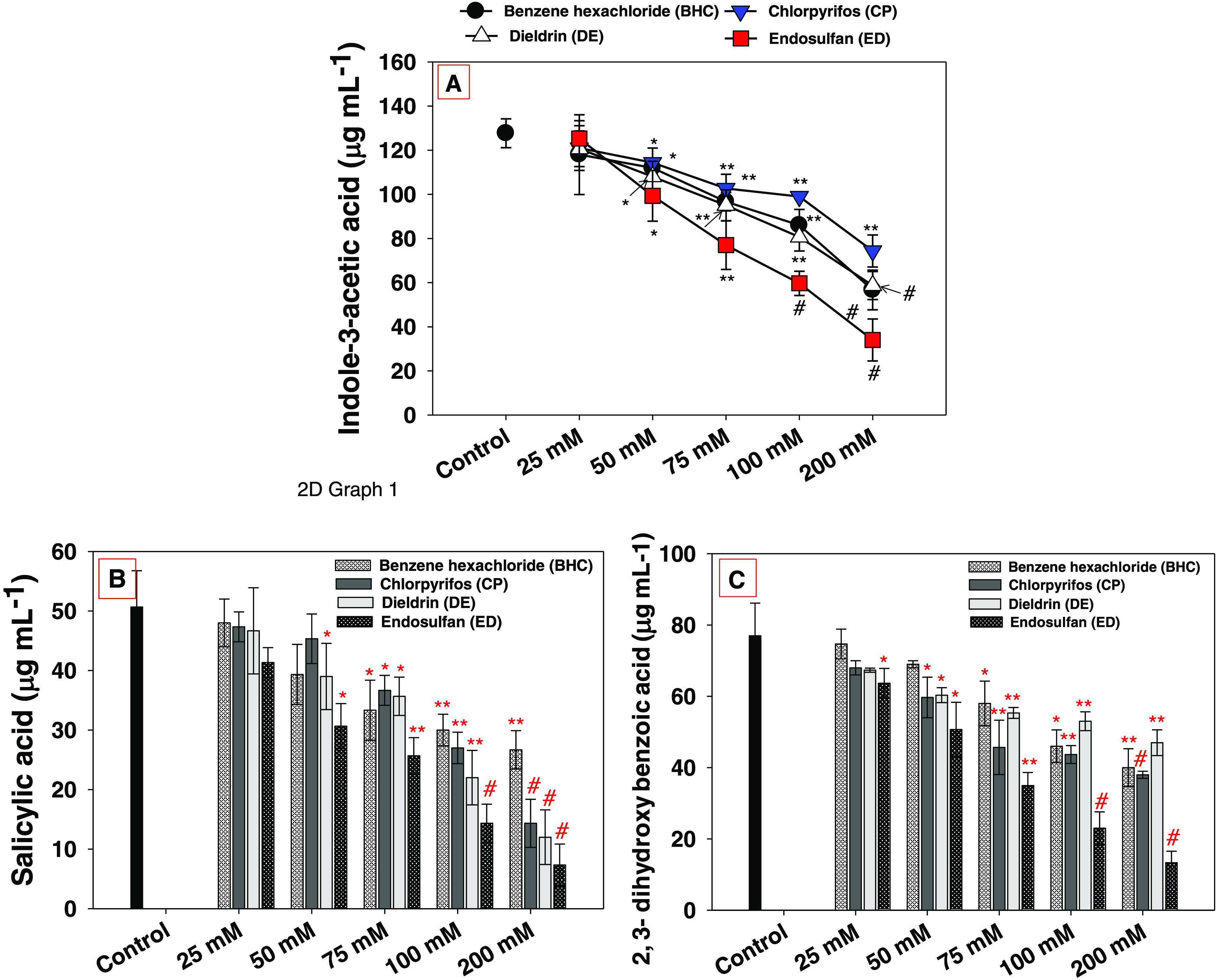
Effect of varying concentrations of organochlorine pesticides on indole-3-acetic acid (A) and siderophore production: salicylic acid (B) and 2,3-dihydroxybenzoic acid (C) synthesized by E. cloacae strain EAM 35. In this figure, the line diagram and histograms represent the mean values of three replicates (n = 3). Corresponding error bars represent the standard deviation (SD) of three replicates (SD, n = 3). The asterisks *, ** and # denote statistical significance at p < 0.05, p < 0.005, and p < 0.001, respectively, computed by Student’s t-test.
Siderophores (the iron chelators) have low-down molecular weight compounds that are synthesized/released by a number of soil microbes under the Fe dearth situation.38 The siderophore-secreting ability of strain EAM 35 was assessed both in the absence and in the presence of test OCPs. Like IAA, under controlled conditions (in the absence of OCPs), strain EAM 35 produced a substantial amount of phenolate-type siderophores; SA (50.6 ± 6.1 μg mL–1) and 2,3-DHBA (77 ± 9.1 μg mL–1). The siderophore-producing ability of the strain decreased consistently with addition of 25–200 μM concentrations of each organochlorine pesticide. For example, among all of the pesticides, ES showed the maximum inhibitory effect and it reduced SA and 2,3-DHBA by 85 and 82%, respectively, over the untreated control (Figure 2B,C). While comparing the mean values, the synthesis of salicylic acid decreased in the order ES (mean value = 117 μg mL–1) > DE (mean value = 154 μg mL–1) > CP (mean value = 169 μg mL–1) > BHC (mean value = 176 μg mL–1). Similarly, the reduction in 2,3-DHBA synthesis showed the following pattern: ES (mean value = 187 μg mL–1) > CP (mean value = 253 μg mL–1) > DE (mean value = 282 μg mL–1) > BHC (mean value = 287 μg mL–1). The possible mechanism behind the decreased bacterial siderophore is the adsorption of chemical pesticides on the microbial cell surface and therefore it affects the transport of ions. Sometimes respiratory arrest/loss is reported, leading eventually to the death of bacteria while growing under pesticide stress. In the sequence of these events, changes take place in the oxidoreduction level and, depending on the chemical composition and dose of chemical pesticides, the microorganism concerned may be unable to survive and bacterial growth and physiological activity become slower. Therefore, the release of active biomolecules including siderophores by bacterial species was altered. In a similar study, the effects of different groups of organophosphate pesticides, viz., glyphosate, phorate, monocrotophos, acephate, and glyphosate, on the siderophore-producing ability of soil microbes like P. fluorescens, R. leguminosarum, B. brevis, A. vinelandii, and S. typhimurium have been reported.16 In addition, a constant and concentration-dependent decrease in the synthesis of phenolate-type siderophores (SA and 2,3-dihdroxy benzoic acid) released by B. subtilis has been reported when cultured in liquid medium supplemented with different groups of fungicides.39
P-solubilization under Organochlorine Pesticides
Solubilization of the insoluble form of inorganic phosphate (iP) into its soluble form is the characteristic feature of soil microbes inhabiting various environmental conditions.40 The qualitative and quantitative phosphate-solubilization potential of the E. cloaceae strain under variable concentrations of organochlorine pesticides (OCPs) was evaluated by growing the bacterium in liquid PKV medium (Table 3). It was noticed that the quantity of P-solubilization in liquid broth decreases with increasing concentrations of pesticides from 25 to 200 μM. Among the concentrations, the higher doses (200 μM) showed the maximum toxic effect. As an example, at a 200 μM dose and after 2 days of incubation, BHC, CP, DE, and ES decreased the phosphate-solubilization potential of E. cloaceae strain EAM 35 by 77% (decreased from 278 to 62 μg mL–1), 81% (from 278 to 51 μg mL–1), 68% (from 278 to 87 μg mL–1), and 86% (from 278 to 38 μg mL–1), respectively, over the control (278 μg mL–1). While comparing the mean values of OCPs, the maximum negative effect on PSA was observed in the following order: ES (mean value = 82.6 μg mL–1) > CP (mean value = 93.2 μg mL–1) > DE (mean value = 113.6 μg mL–1) > BHC (mean value = 127 μg mL–1). Also, a significant drop in the pH value of inoculated and OCP-treated PKV liquid broth was recorded at different intervals. It has been reported by many workers that the P-solubilization property of soil bacteria is due to the drop in pH, which has been associated with their ability to secrete low-molecular-weight organic acids such as citric acid, oxalic acid, malic acid, acetic acid, succinic acid, maleic acid, gluconic, 2-ketogluconic acid, etc. The changes in pH values of liquid medium under pesticide pressure are consistent with the decline in the P-solubilizing efficiency of strain EAM 35. These changes may occur probably due to the reduction in the secretion of bacterial organic acids or the degraded byproducts of pesticides; truly, both the factors might be involved in uplifting the pH of pesticide-amended liquid medium. Similar to the present finding, the pesticide-concentration-dependent inhibitions in the P-solubilization potential of a soil beneficial isolate A. vinelandii along with the drop in pH of liquid broth have recently been reported.20
Table 3. Effect of Different Concentrations of Organochlorine Pesticides (OCPs) on Phosphate-Solubilizing Activity of Enterobacter cloacae Strain EAM 35 Grown in PKV Medium and Change in pH of Liquid Brotha.
| P-solubilization (μg mL–1) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| organochlorine pesticides | concentrations (μM) | day 2 | pH | day 4 | pH | day 6 | pH | day 8 | pH | day 10 | pH |
| benzene hexachloride (BHC) | 25 | 271 ± 23 | 5.4 ± 0.15 | 278 ± 17 | 5.2 ± 0.12 | 283 ± 18 | 5.1 ± 0.10 | 291 ± 9.8 | 5.0 ± 0.10 | 145 ± 18* | 5.5 ± 0.15 |
| 50 | 245 ± 13 | 5.6 ± 0.10 | 247 ± 12.5 | 5.3 ± 0.10 | 252 ± 14 | 5.4 ± 0.05 | 263 ± 7.8 | 5.4 ± 0.05 | 205 ± 13 | 5.7 ± 0.15 | |
| 75 | 187 ± 10* | 5.7 ± 0.15 | 189 ± 10.5* | 5.5 ± 0.05 | 189 ± 10* | 5.5 ± 0.11 | 193 ± 5.6* | 5.6 ± 0.10 | 132 ± 4.7* | 5.8 ±.10 | |
| 100 | 134 ± 5.0** | 5.7 ± 0.10 | 144 ± 0.0** | 5.8 ± 0.11 | 147 ± 7.1* | 5.5 ± 0.10 | 153 ± 4.0* | 5.8 ± 0.06 | 116 ± 0.0** | 6.0 ± 0.10 | |
| 200 | 62 ± 4.7# | 5.9 ± 0.11 | 64 ± 0.0# | 6.0 ± 0.10 | 67 ± 6.6** | 6.1 ± 0.02 | 69 ± 2.0# | 6.2 ± 0.10 | 37 ± 2.7# | 6.2 ± 0.10 | |
| mean | 179.8 | 5.66 | 184.4 | 5.56 | 187.6 | 5.52 | 193.8 | 5.6 | 127 | 5.84 | |
| chlorpyrifos (CP) | 25 | 245 ± 15 | 5.4 ± 0.10 | 250 ± 12 | 5.3 ± 0.05 | 262 ± 3.4 | 5.3 ± 0.12 | 278 ± 0.0 | 5.4 ± 0.10 | 133 ± 12* | 5.7 ± 0.10 |
| 50 | 235 ± 11 | 5.6 ± 0.15 | 239 ± 16.3 | 5.5 ±0.10 | 244 ± 25 | 5.5 ± 0.05 | 248 ± 8.0 | 5.6 ± 0.10 | 113 ± 11** | 5.8 ± 0.15 | |
| 75 | 174 ± 9.2 | 5.7 ± 0.15 | 176 ± 22* | 5.5 ± 0.10 | 182 ± 11.5* | 5.6 ± 0.06 | 191 ± 5.4* | 5.6 ± 0.10 | 97 ± 8.8** | 5.8 ± 0.12 | |
| 100 | 111 ± 8.4** | 5.7 ± 0.11 | 114 ± 15** | 5.8 ± 0.10 | 119 ± 6.1** | 5.9 ± 0.05 | 125 ± 3.2** | 6.0 ± 0.10 | 85 ± 4.9# | 6.2 ± 0.15 | |
| 200 | 51 ± 5.2# | 5.9 ± 0.10 | 53 ±6.7# | 6.0 ± 0.10 | 56 ± 5.2# | 6.1 ± 0.06 | 61 ± 2.8# | 6.2 ± 0.05 | 38 ± 0.0# | 6.4 ± 0.12 | |
| mean | 163.2 | 5.66 | 166.4 | 5.62 | 172.6 | 5.68 | 180.6 | 5.76 | 93.2 | 5.98 | |
| dieldrin (DE) | 25 | 233 ± 10.3 | 5.4 ± 0.12 | 240 ± 19 | 5.4 ± 0.15 | 245 ± 11 | 5.7 ± 0.01 | 266 ± 7.7 | 5.7 ± 0.11 | 168 ± 0.0* | 5.9 ± 0.11 |
| 50 | 226 ± 7.4 | 5.6 ± 0.10 | 229 ± 16 | 5.7 ± 0.15 | 233 ± 9.8 | 5.8 ± 0.14 | 241 ± 5.6 | 5.8 ± 0.13 | 149 ± 0.0* | 5.9 ± 0.10 | |
| 75 | 203 ± 5.3* | 5.7 ± 0.12 | 206 ± 17* | 5.7 ± 0.10 | 211 ± 6.9* | 5.8 ± 0.15 | 217 ± 7.3* | 5.8 ± 0.06 | 123 ± 7.7** | 6.0 ± 0.12 | |
| 100 | 166 ± 6.2** | 5.7 ± 0.05 | 171 ± 12* | 5.9 ± 0.05 | 181 ± 5.2* | 6.1 ± 0.10 | 183 ± 3.5* | 6.1 ± 0.05 | 86 ± 8.1# | 6.3 ± 0.12 | |
| 200 | 87 ± 2.6# | 5.9 ± 0.01 | 89 ± 8.8** | 6.1 ± 0.11 | 92 ± 2.0# | 6.2 ± 0.10 | 95 ± 0.0** | 6.2 ± 0.05 | 42 ± 2.1# | 6.5 ± 0.11 | |
| mean | 183 | 5.66 | 187 | 5.76 | 192.4 | 5.92 | 200.4 | 5.92 | 113.6 | 6.12 | |
| endosulfan (ES) | 25 | 214 ± 17.1 | 5.4 ± 0.12 | 215 ± 12 | 5.7 ± 0.12 | 219 ± 7.3 | 5.7 ± 0.11 | 222 ± 12 | 5.8 ± 0.10 | 112 ± 16** | 5.9 ± 0.06 |
| 50 | 192 ± 21* | 5.6 ± 0.10 | 192 ± 10* | 5.8 ± 0.13 | 194 ± 4.3* | 5.8 ± 0.13 | 198 ± 17* | 5.9 ± 0.10 | 109 ± 10** | 6.0 ± 0.10 | |
| 75 | 165 ± 13* | 5.7 ± 0.15 | 165 ± 9.2* | 5.8 ± 0.11 | 167 ± 5.0** | 5.8 ± 0.11 | 170 ± 18* | 5.9 ± 0.10 | 89 ± 8.8# | 6.0 ± 0.05 | |
| 100 | 128 ± 9.3** | 5.7 ± 0.06 | 129 ± 7.4** | 6.1 ± 0.05 | 134 ± 3.2** | 6.1 ± 0.05 | 137 ± 10** | 6.2 ± 0.10 | 77 ± 6.1# | 6.4 ± 0.04 | |
| 200 | 38 ± 2.1# | 5.9 ± 0.05 | 40 ± 4.2# | 6.1 ± 0.01 | 40 ± 1.5# | 6.2 ±0.01 | 41 ± 0.0# | 6.2 ± 0.10 | 26 ± 1.0# | 6.6 ±0.10 | |
| mean | 147.4 | 5.66 | 148.2 | 5.9 | 150.8 | 5.92 | 153.6 | 6 | 82.6 | 6.18 | |
| control (without any pesticides) | 0 | 278 ± 23 | 4.0 ± 0.15 | 282 ± 12 | 3.9 ± 0.06 | 292 ± 8.7 | 3.6 ± 0.10 | 311 ± 17 | 3.4 ± 0.10 | 327 ± 14 | 3.4 ± 0.15 |
Each value is a mean (mean ± SD) of three independent replicate. The asterisks “*”, “**” and “#” denote statistical significance at p < 0.05, p < 0.005, and p < 0.001, respectively, computed by Student’s t-test. PKV, Pikovskaya (PVK) broth medium.
Growth Behavior and Log10 CFU/mL Counts under OCP Stress
The growth response of E. cloacae strain EAM 35 to different concentrations of four organochlorine pesticides varied (Figure 3). In the early lag phase, strain EAM 35 grew at a snail’s pace, which, however, increased linearly with increasing growth periods, and thereafter it declined sharply. In the presence of 25 μM concentrations of each pesticide, the bacterial strain survived somehow better. In contrast, among the concentrations of OCPs used, the higher (200 μM) doses had the maximum severe damaging effect on bacterial growth. While comparing the toxicity of higher concentrations (200 μM) of all organochlorine pesticides to bacterial growth, the sensitivity followed the order ES > DE > CP > BHC. The toxic effect of other pesticides as observed in another study while developing the bacterial strain in pesticide-containing liquid broth41 was possibly be due to the better solubility and mobility of such chemical pesticides relative to those observed on solid medium. The growth inhibition as observed in this study could, therefore, be probably due to the transport/uptake of OCP ions across the membranes. Likewise, the inhibitory effect of pesticides on the growth kinetics of soil isolates cultured in nutrient medium added with pesticides has previously been reported.42 The increasing concentration of different groups of pesticides reduced the CFU count of P. fluorescens and P. putida under in vitro conditions.43
Figure 3.
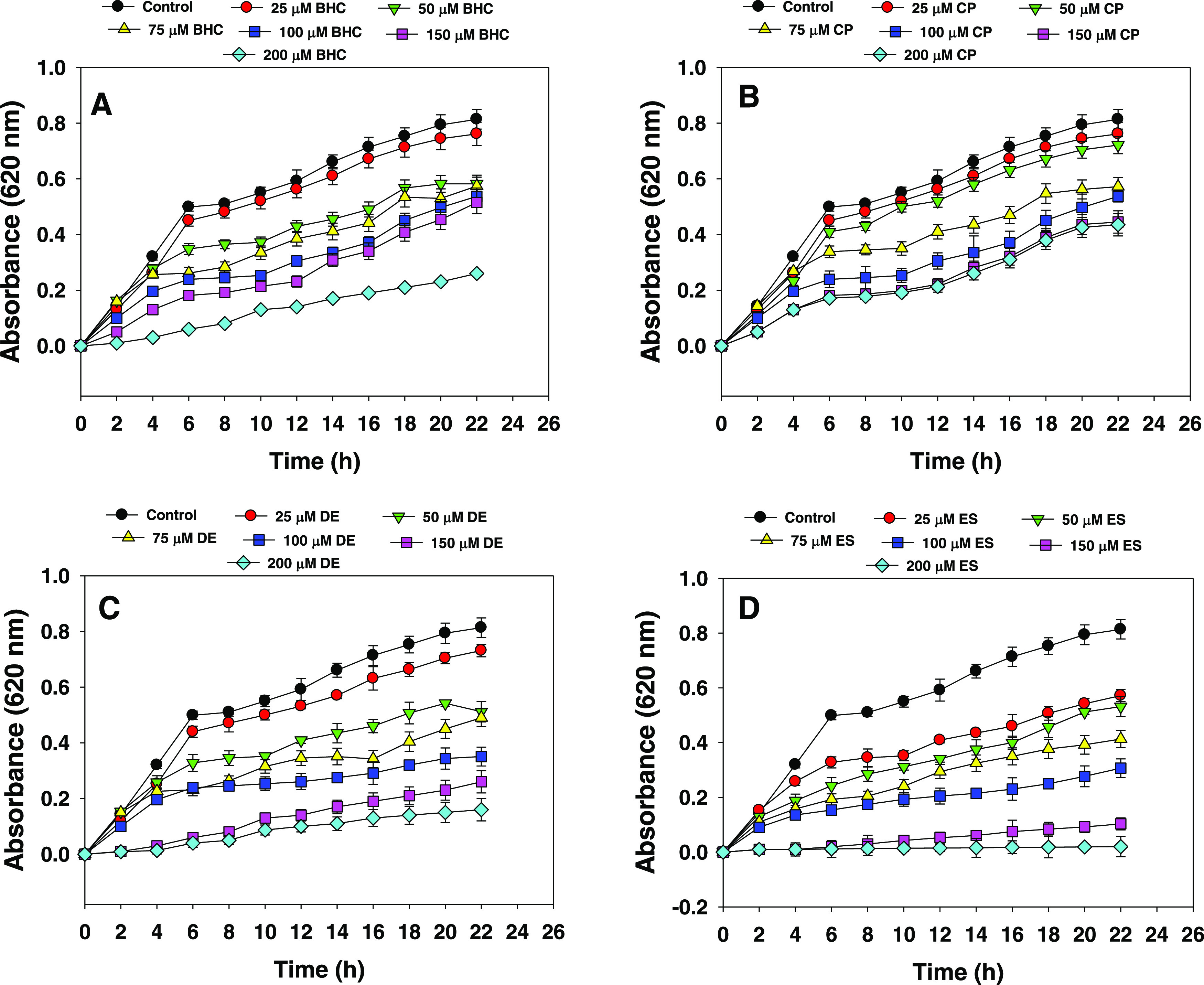
Time- (0–22 h) and concentration (25–200 μM)-dependent growth inhibition of E. cloacae EAM 35. In this figure, panels (A, B, C, and D) represent the growth curves of the bacterium under varying concentrations of benzene hexachloride (A), chlorpyrifos (B), dieldrin (C), and endosulfan (D).
Pesticide-Induced Surface Morphological Distortion
The OCP-induced surface morphological changes in strain EAM 35 were assessed by growing the bacterial cells in liquid broth treated with 200 μM each benzene hexachloride, chlorpyrifos, dieldrin, and endosulfan along with control (without any OCPs). The SEM microscopic images revealed a distorted/ruptured and disordered rod-shaped bacterial cell (Figure 4, panel II B, C, and D). The OCP-treated cells had a large number of gaps, pits on both cellular facets, and fragmented and disorganized cell structure over untreated control cells. In contrast, untreated bacterial cells appeared as smooth and rod-shaped and displayed intact/undamaged surface morphology (Figure 4A). The microscopic examination observed in this finding therefore confirmed the toxic/inhibitory action of OCPs. The obliteration/damage in bacterial cells by OCPs could be attributed to an accumulation of such toxic chemicals in the bacterial membrane, which might have altered the membrane potential, eventually leading to cell death. Similarly, the morphological distortion/alterations in B. cepacia cells have been observed under a scanning electron microscope when bacterial cells were exposed to herbicide glyphosate.21 The pesticide-induced structural deformation causing significant aberration, cracks, and gaps in two soil beneficial Pseudomonas spp.17 has been reported.
Figure 4.
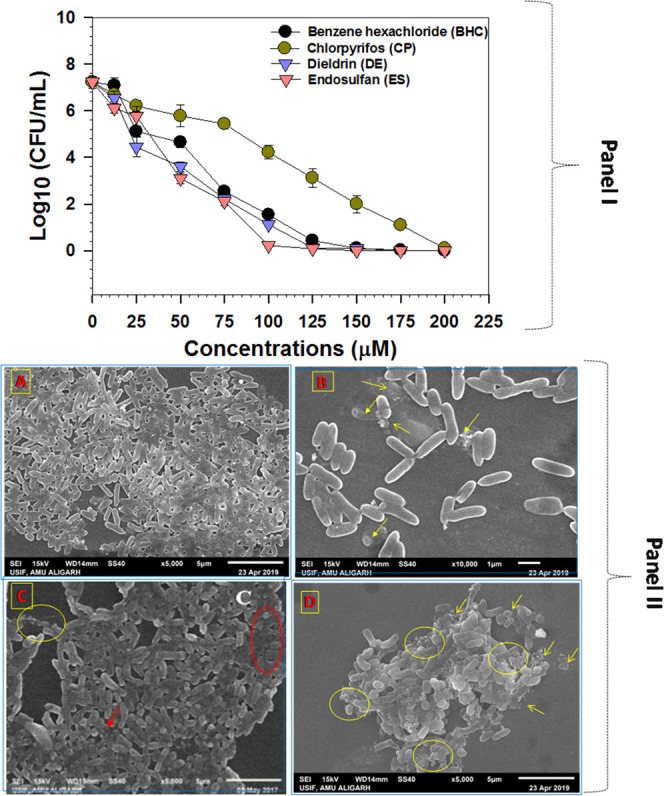
Scanning electron microscopy images of E. cloacae strain EAM 35 cultured in NB without (A) and with 150 μM concentrations of benzene hexachloride (B), chlorpyrifos (C), and endosulfan (D). The red and yellow circles and arrows represent the damage/distortion in the surface morphology of bacterium after the exposure of OCPs.
Membrane Permeability under Pesticide Stress
In this study, the extent of cellular injury caused by OCPs to the cells of E. cloaceae EAM 35 was evaluated in terms of cellular membrane permeability deliberated by staining the bacterial cells with a fluorescently labeled dye, propidium iodide (PI). For the assessment, strain EAM 35 was cultured in a medium supplemented with an increasing dosage (25–200 μM) of test OCPs, stained with red-emitting red fluorescence DNA-bound dye PI (excited at 532 nm), and images were recorded under a confocal microscope. CLSM examination of OCP-treated cells of E. cloaceae EAM 35 revealed the increasing number of dead/injured cells observed in the form of red short rods (Figure 5B–E) compared to untreated control cells (Figure 5A). Cells of bacterial membranes act as selectively permeable barriers for a variety of molecules; however, if permeability increases, cells may take up excessive quantities of materials present in their surroundings.44 Therefore, toxic chemicals including pesticides increase access to intracellular locations and put forth the damaging effect generally in the form of generation of reactive oxygen species. These DNA-bound dyes were not taken up by metabolically active cells. However, they can easily bind to the nucleic acids of membrane-compromised cells. Recently, Khan et al.17 have reported increasing number of dead cells of Pseudomonas sp. after exposure to increasing concentrations of fungicides (hexaconazole and carbendazim).
Figure 5.
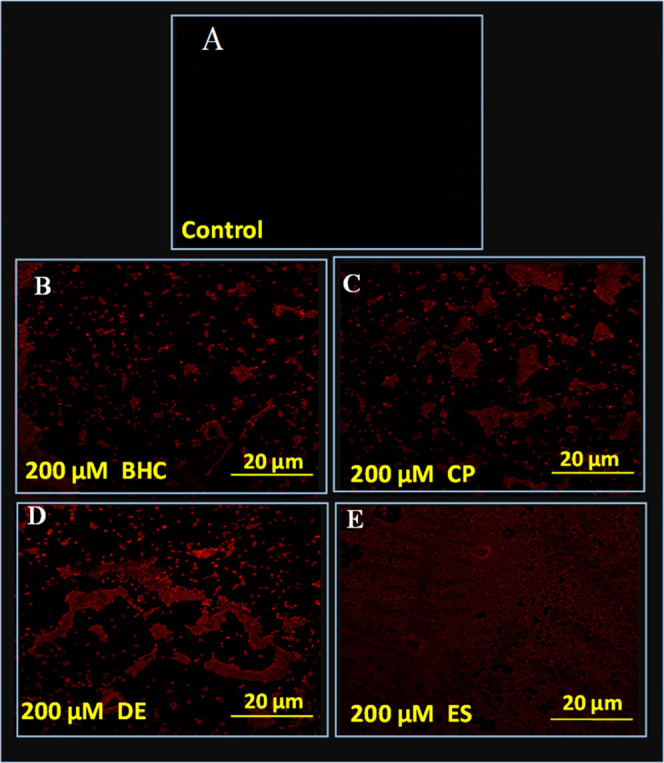
PI-stained confocal laser scanning microscopy images of E. cloacae strain EAM 35: panel (A) shows untreated control cells with no red rod-shaped cells, whereas panels (B, C, D, and E) represent the red rods treated with 200 μM each benzene hexachloride, chlorpyrifos, dieldrin, and endosulfan. In these images, red rods depict membrane-compromised cells.
Biofilm Inhibition
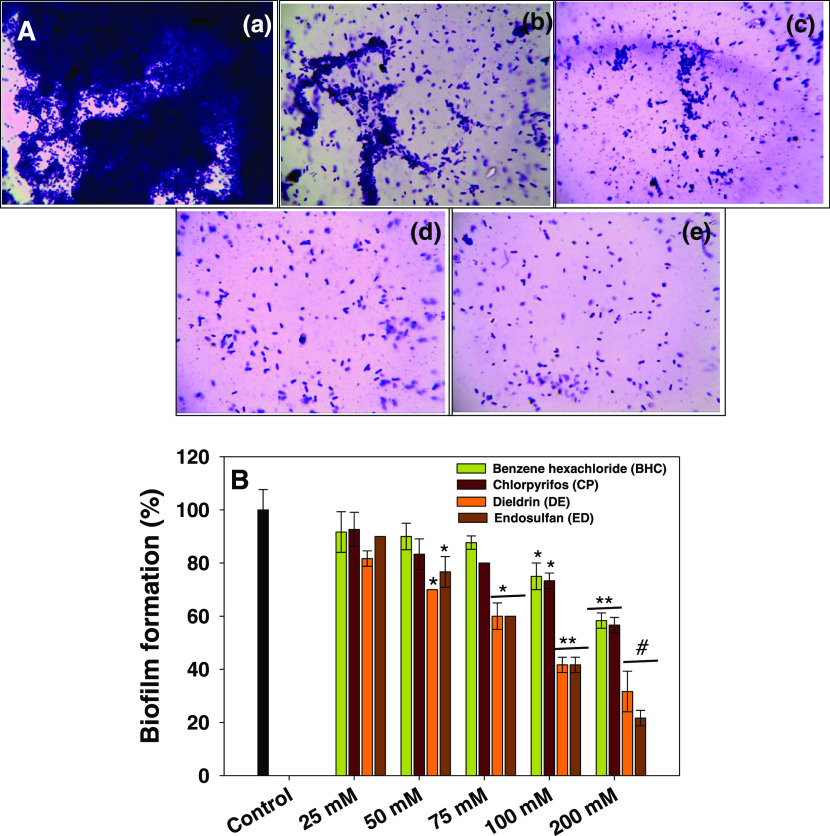
In this finding, the effect of different OCPs on percent biofilm formation in E. cloacae strain EAM 35 was observed in a dose-related manner. The inhibition of biofilm formation by BHC, CP, DE, and ES (Figure 6 panels b, c, d, and e) against strain EAM 35 was statistically (p ≤ 0.05, p ≤ 0.005 and p ≤ 0.001) significant. The higher concentrations of OCPs had the maximum negative impact on the biofilm-formation ability of strain EAM 35. For instance, at 200 μM concentrations of each BHC, CP, DE, and ES, the biofilm-formation ability of strain EAM 35 was greatly reduced by 42, 44, 69, and 79%, respectively, over the untreated control (Figure 6A). The biofilms formed by bacterial species are a complex mixture of glycocalyx matrices that are produced by the cross-talk between quorum sensing molecules that may also facilitate the development of other virulence factors (pyocyanin, rhamnolipids, motility, etc.).45 In this study, the test pesticides inhibited the formation of bacterial biofilms. The disruption/inhibition could possibly be due to the cellular damage/injury in the bacterial cell membrane, altering the permeability, which leads to the leakage/outflow of intracellular content that prevents the synthesis of extrapolymeric substances and other virulence factors.46,47 Also, the inhibitory effect of OCPs on the bacterial biofilm could be attributed to the malfunctioning/damage of water channels throughout the biofilm, which are present for the transportation of nutrients. Furthermore, the chemical pesticides may directly diffuse/disrupt the layer of EPS and exert an antimicrobial action. Likewise, in another studies, pesticides and other chemical compounds inhibited the bacterial biofilms formed by A. chroococcum, B. thuringiensis, P. mosselii, and S. meliloti under in vitro conditions.48
Figure 6.
Impact of different concentrations (25–200 μM) of organochlorine pesticides on biofilm formation (%) by E. cloacae strain EAM 35 after 24 h of incubation. In the figure (A), panel (a) represents the untreated control, whereas panels (b, c, d, and e) depict the biofilm inhibition after the exposure to 200 μM each BHC, CP, DE, and ED, respectively. In figure (B), the histograms represent the mean value of biofilm percentage over the untreated control (100%), while error bars show standard deviation (SD, n = 3) with a significance of *p ≤ 0.05, **p ≤ 0.005, and #p ≤ 0.001.
Conclusions
The varying levels of OC pesticides, viz., benzene hexachloride, chlorpyrifos, dieldrin, and endosulfan exhibited a visible toxicity to the E. cloacae EAM 35 strain. The inhibitory potential of OCPs was apparent through the decline in plant-growth-promoting (PGP) traits, growth kinetics, and CFU counts. Also, reduced P-solubilization along with a drop in the pH of liquid medium and inhibition of biofilm-formation ability of the bacterial strain was clearly observed with increasing concentrations of each OC pesticide. In addition, the distorted surface morphology and membrane disintegration were proved by a set of microscopic observations. Furthermore, pesticides altered the metabolic pathways, leading to synthesis of indole-3-acetic acid and siderophore (SA and 2,3-DHBA). Conclusively, the present finding requires the attention of the workers and suggests that before applying the pesticides in agronomic practices, their recommended field doses should be clearly known and carefully monitored.
Experimental Section
Isolation and Biochemical Characterization of Bacterial Isolates
Soil samples were collected from pesticide-polluted rhizospheres of chili (Capsicum annuum) and tomato (L. esculentum). Soil samples were serially (10–1–10–7) diluted, and 100 μL of soil suspensions was spread-plated on Pikovskaya (PVK) agar plates added with 5.0 g L–1 tricalcium phosphate (TCP). The PKV plates were incubated at 28 ± 2 °C for 4–5 days until the zone of solubilization appeared around the bacterial colonies. A total of 40 phosphate-solubilizing isolates were recovered and maintained on the same medium. Furthermore, the morphological and biochemical identification of all of the isolates was done using standard protocols.49
Determination of Minimum Inhibitory Concentration (MIC) and Molecular Identification of Selected Isolates
The MIC of all recovered strains (N = 40) was determined by growing them on nutrient agar (NA) plates supplemented with variable (12.5, 25, 50, 75, 100, 125, 150, 175, 200, 225, 250, and 300 μM) concentrations of all OCPs, viz., benzene hexachloride (BHC), chlorpyrifos (CP), dieldrin (DE), and endosulfan (ES) (Table 4). The freshly grown bacterial cells were spot-inoculated on OCP-added NA plates and incubated at 28 ± 2 °C for 2–3 days. After incubation, the growth of bacteria was checked and MIC was determined. MIC is generally defined as the minimum concentration at which no visible growth of bacterial cultures occurs.
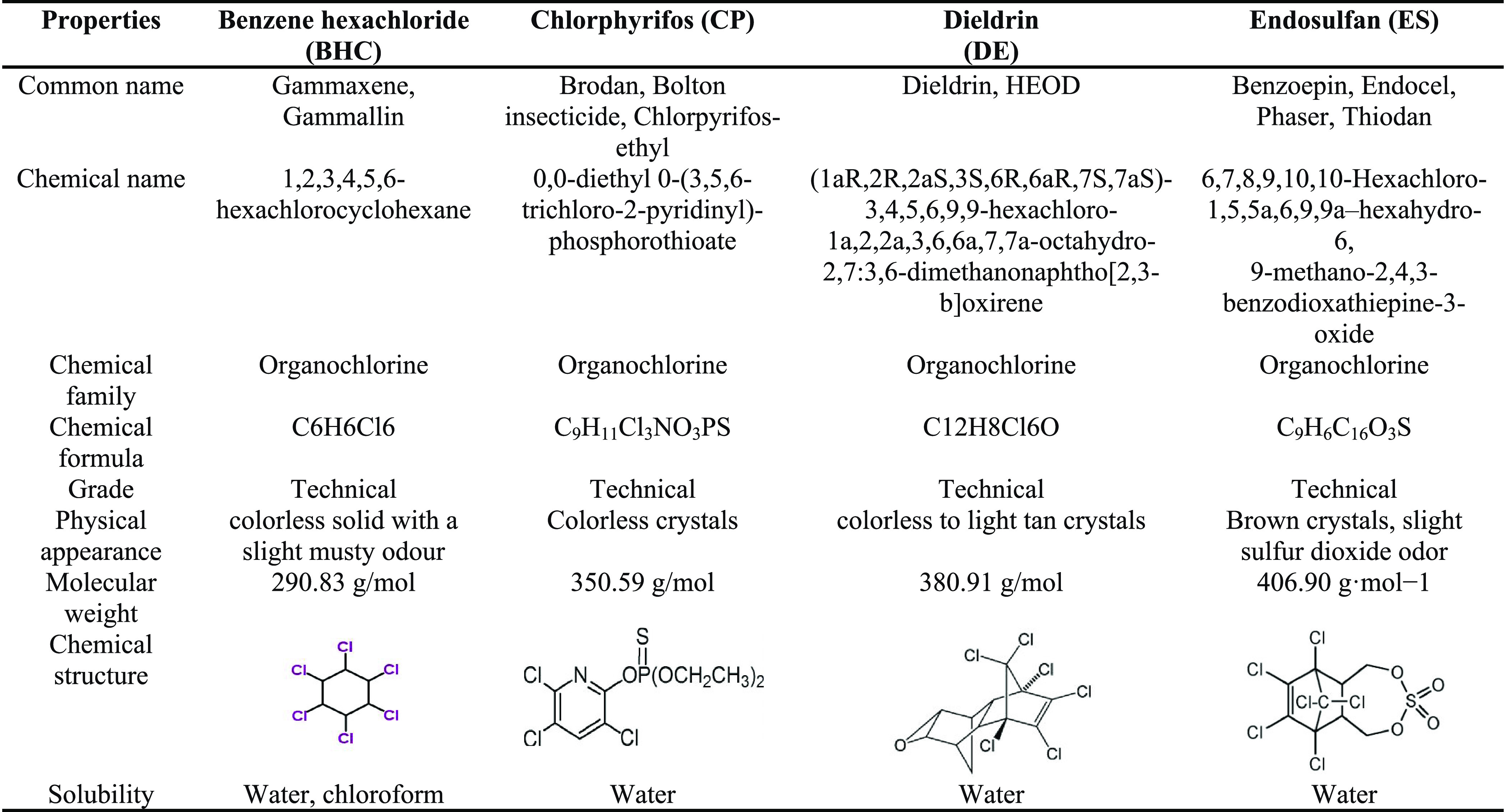
Table 4. Chemical Properties of Organochlorine Pesticides (OCPs) Used in the Study.
Among the 40 P-solubilizing isolates, strain EAM 35 showed the greatest halo (zone of solubilization) (19 mm) around its colonies on PKV plates. Therefore, we decided to identify the strain at the species level using 16S rRNA partial gene sequence analysis (for detailed description of DNA isolation, gel extraction, purification, and PCR, see Supporting Information Section S4.2). For this, strain EAM 35 was sent to Macrogen Inc., Seoul, South Korea (commercially available), for further analysis. The sequences received from Macrogen were analyzed using the BLASTn program available online and matched with the data accessible from the data bank at the NCBI (http://www.ncbi.nlm.nih.gov/BLAST) to find the similarities with known taxonomic information. The nucleotide sequences were deposited in the GenBank sequence database for accession number. Afterward, the phylogenetic tree was constructed.
Effect of OCPs on PGP Traits of E. cloacae EAM 35
Indole-3-acetic Acid (IAA) Activity
The effect of organochlorine pesticides on the IAA-producing ability of E. cloacae EAM 35 was evaluated by growing it in 25–200 μM OCP-treated Luria–Bertani (LB) broth.50 (For detailed description of spectrophotometric determination of IAA, see Supporting Information Section S4.2.1).
Siderophore Production
The siderophores (iron chelating compounds) [salicylic acid (SA) and 2,3-dihydroxybenzoic acid (DHBA)] synthesized by bacterial isolate EAM 35 were measured by growing them in chrome azurol S (CAS) agar (Himedia, India) and liquid Modi medium supplemented with various concentrations of organochlorine pesticides following the method of Alexander and Zuberer51 and Reeves et al.,52 respectively.
Phosphate Solubilization
To assess the impact of OCPs on the P-solubilizing potential of strain EAM 35, bacterial cells were cultured in Pikovskaya (PKV) broth treated with different concentrations of OCPs. For the quantitative measurement, 20–200 μM concentrations each of BHC, CP, DE, and ES were added individually to 100 mL of PKV broth, inoculated with 1 mL of bacterial culture (108 cells mL–1), and incubated at 28 ± 2 °C with intermittent shaking (at 120 rpm). The available P was measured in the bacterial supernatant on 2nd, 4th, 6th, 8th, and 10th days after incubation. The change in pH values of liquid PKV following P-solubilization was also recorded.20
Surface Morphology Assessment of OCP-Treated Bacteria
The OCP-induced changes in the surface morphology of the bacterium were assessed following the protocol as previously described by Shahid and Khan.39 For the assessment, cells of strain EAM 35 were grown in NB medium supplemented with 100 μM concentrations each of BHC, CP, DE, and ES at 28 ± 2 °C for 24 h (see Supporting Information Section S4.3 for cell harvesting and sample preparation for scanning electron microscopy).
Determination of Cellular Membrane Injury
To examine the membrane-damaging potential of the test pesticides, the strain EAM 35 was cultured in NB broth. After 12 h of incubation, the growing bacterial cells were treated with 25–200 μM concentrations of each OCP and further incubated for 6 h in shaking conditions (at 120 rpm). The harvesting of cells was done using centrifugation (10 000 rpm, 5 min), and cell pellets were washed at least three times with sterile phosphate buffer saline (PBS) and tagged with a mixture solution of fluorescently labeled DNA-binding dyes, propidium iodide (PI) and acridine orange (AO), at a concentration of 50 μM for 20 min at room temperature. Cells were washed with PBS and visualized on a glass slide under a confocal laser scanning microscope (Leica TCS CLSM; Leica Microsystems, Germany), and numbers of active and dead cells were counted.
Bacterial Growth Kinetics and CFU Counts under Pesticide Stress
The kinetics of bacterial growth was determined by growing strain EAM 35 in NB medium containing 0–200 μM concentrations of BHC, CP, DE, and ES. The pesticide-supplemented and bacterium-inoculated broth was maintained at 28 ± 2 °C on a shaking incubator, growth was measured spectrophotometrically at 620 nm at regular intervals, and the growth curve was plotted.15 For CFU count, 0.1 mL volume of the 24 h grown culture from each exposure concentration was spread-plated on nutrient agar (NA) medium and incubated under the abovementioned growth conditions to count the viable cells. The number of colony forming units (CFU) per mL was converted to log10 CFU/mL and plotted as a function of pesticide concentration. The CFU was calculated as follows
Determination of Biofilm Formation
To assess the biofilm formation by E. cloacae strain EAM 35, the absorbance-based crystal violet (CV) method was followed as described by Khan et al.53 In brief, bacterial strains were exposed to 25–200 μM concentrations each of BHC, CP, DE, and ES.
Statistical Analysis
Data were statistically analyzed using SigmaPlot 12.0 and Minitab17 software packages. Tests included two-way analysis of variance (ANOVA) followed by post hoc least significant difference (LSD). Student’s t-test was used where applicable.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group no. (RG-1439-075). The authors would like to thank the Department of Agricultural Microbiology, Faculty of Agricultural Sciences, A.M.U, Aligarh, for providing research facilities; University Sophisticated Instrument facility (USIF) for providing SEM and CLSM facilities; and Macrogen Seoul, Korea, for providing 16S rRNA gene sequencing analysis.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c05931.
Details for identification of rhizobacterial strains using 16S rRNA gene sequencing, detailed methodology for indole-3-acetic acid (IAA) activity, surface morphology assessment of OCP-treated bacteria, and chemical properties of organochlorine pesticides used in the study. (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Wojciechowska M.; Stepnowski P.; Gołębiowski M. The use of insecticides to control insect pests. Invertebr. Survival J. 2016, 13, 210–220. 10.25431/1824-307X/isj.v13i1.210-220. [DOI] [Google Scholar]
- Shahid M.; Khan M. S.. Fungicide Toxicity to Legumes and Its Microbial Remediation: A Current Perspective. In Pesticides in Crop Production: Physiological and Biochemical Action; Wiley, 2020; pp 15–33. [Google Scholar]
- Shahid M.; Zaidi A.; Khan M. S.; Rizvi A.; Saif S.; Ahmed B.. Recent Advances in Management Strategies of Vegetable Diseases. In Microbial Strategies for Vegetable Production; Springer: Cham, 2017; pp 197–226. [Google Scholar]
- Tripathi S.; Srivastava P.; Devi R. S.; Bhadouria R.. Influence of Synthetic Fertilizers and Pesticides on Soil Health and Soil Microbiology. In Agrochemicals Detection, Treatment and Remediation; Butterworth-Heinemann, 2020; pp 25–54. [Google Scholar]
- Pino-Otín M. R.; Ballestero D.; Navarro E.; Mainar A. M.; Val J. Effects of the insecticide fipronil in freshwater model organisms and microbial and periphyton communities. Sci. Total Environ. 2020, 142820 10.1016/j.scitotenv.2020.142820. [DOI] [PubMed] [Google Scholar]
- Poirier L.; Brun L.; Jacquet P.; Lepolard C.; Armstrong N.; Torre C.; Daudé D.; Ghigo E.; Chabrière E. Enzymatic degradation of organophosphorus insecticides decreases toxicity in planarians and enhances survival. Sci. Rep. 2017, 7, 15194 10.1038/s41598-017-15209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y.; Zheng Q.; Noll L.; Zhang S.; Wanek W. Direct measurement of the in situ decomposition of microbial-derived soil organic matter. Soil Biol. Biochem. 2020, 141, 107660 10.1016/j.soilbio.2019.107660. [DOI] [Google Scholar]
- Talaat N. B. Effective microorganisms: An innovative tool for inducing common bean (Phaseolus vulgaris L.) salt-tolerance by regulating photosynthetic rate and endogenous phytohormones production. Sci. Hortic. 2019, 250, 254–265. 10.1016/j.scienta.2019.02.052. [DOI] [Google Scholar]
- Shahid M.; Khan M. S. Assessment of glyphosate and quizalofop mediated toxicity to greengram [Vigna radiata (L.) Wilczek], stress abatement and growth promotion by herbicide tolerant Bradyrhizobium and Pseudomonas species. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 3001–3016. 10.20546/ijcmas.2017.612.351. [DOI] [Google Scholar]
- Ferreira M. J.; Silva H.; Cunha A. Siderophore-producing rhizobacteria as a promising tool for empowering plants to cope with iron limitation in saline soils: A Review. Pedosphere 2019, 29, 409–420. 10.1016/S1002-0160(19)60810-6. [DOI] [Google Scholar]
- Shahid M.; Ameen F.; Maheshwari H. S.; Ahmed B.; AlNadhari S.; Khan M. S. Colonization of Vigna radiata by a halotolerant bacterium Kosakonia sacchari improves the ionic balance, stressor metabolites, antioxidant status and yield under NaCl stress. Appl. Soil Ecol. 2020, 158, 103809 10.1016/j.apsoil.2020.103809. [DOI] [Google Scholar]
- Sagar A.; Sayyed R. Z.; Ramteke P. W.; Sharma S.; Marraiki N.; Elgorban A. M.; Syed A. ACC deaminase and antioxidant enzymes producing halophilic Enterobacter sp. PR14 promotes the growth of rice and millets under salinity stress. Physiol. Mol. Biol. Plants 2020, 26, 1847–1854. 10.1007/s12298-020-00852-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi A.; Khan M. S.; Rizvi A.; Saif S.; Ahmad B.; Shahid M.. Role of Phosphate-Solubilizing Bacteria in Legume Improvement. In Microbes for Legume Improvement; Springer: Cham, 2017; pp 175–197. [Google Scholar]
- Zaidi A.; Khan M. S.; Ahmad E.; Saif S.; Rizvi A.; Shahid M. Growth stimulation and management of diseases of ornamental plants using phosphate solubilizing microorganisms: current perspective. Acta Physiol. Plant. 2016, 38, 117. 10.1007/s11738-016-2133-7. [DOI] [Google Scholar]
- Shahid M.; Zaidi A.; Saghir Khan M. Modulations in growth, structure, cell viability and antioxidant enzyme of a nodule bacterium Mesorhizobium ciceri induced by pesticides. Environ. Dev. Sustainability 2020, 1–17. [Google Scholar]
- Kumar V.; Singh S.; Upadhyay N. Effects of organophosphate pesticides on siderophore producing soils microorganisms. Biocatal. Agric. Biotechnol. 2019, 21, 101359 10.1016/j.bcab.2019.101359. [DOI] [Google Scholar]
- Khan S.; Shahid M.; Khan M. S.; Syed A.; Bahkali A. H.; Elgorban A. M.; Pichtel J. Fungicide-tolerant plant growth-promoting rhizobacteria mitigate physiological disruption of white radish caused by fungicides used in the field cultivation. Int. J. Environ. Res. Public Health 2020, 17, 7251. 10.3390/ijerph17197251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundi W.; Gosal S. K.; Kaur J. Effect of pesticides on growth kinetics and plant growth promoting activities of biofertilizer. Pharma Innov. J. 2020, 9, 442–446. [Google Scholar]
- Ekram M. A. E.; Sarker I.; Rahi M. S.; Rahman M. A.; Saha A. K.; Reza M. A. Efficacy of soil-borne Enterobacter sp. for carbofuran degradation: HPLC quantitation of degradation rate. J. Basic Microbiol. 2020, 60, 390–399. 10.1002/jobm.201900570. [DOI] [PubMed] [Google Scholar]
- Shahid M.; Zaidi A.; Ehtram A.; Khan M. S. In vitro investigation to explore the toxicity of different groups of pesticides for an agronomically important rhizosphere isolate Azotobacter vinelandii. Pestic. Biochem. Physiol. 2019, 157, 33–44. 10.1016/j.pestbp.2019.03.006. [DOI] [PubMed] [Google Scholar]
- Shahid M.; Khan M. S. Glyphosate induced toxicity to chickpea plants and stress alleviation by herbicide tolerant phosphate solubilizing Burkholderia cepacia PSBB1 carrying multifarious plant growth promoting activities. 3 Biotech 2018, 8, 131. 10.1007/s13205-018-1145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraj R.; Megha P.; Sreedev P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100. 10.1515/intox-2016-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meire R. O.; Khairy M.; Targino A. C.; Galvão P. M. A.; Torres J. P. M.; Malm O. Use of passive samplers to detect organochlorine pesticides in air and water at wetland mountain region sites (S-SE Brazil). Chemosphere 2016, 144, 2175–2182. 10.1016/j.chemosphere.2015.10.133. [DOI] [PubMed] [Google Scholar]
- Lupi L.; Bedmar F.; Wunderlin D. A.; Miglioranza K. S. B. Levels of organochlorine pesticides in soils, mesofauna and streamwater from an agricultural watershed in Argentina. Environ. Earth Sci. 2019, 78, 569. 10.1007/s12665-019-8579-3. [DOI] [Google Scholar]
- Aborisade W. T.; Atuanya E. I. Effects of an organochlorine and pyrethroid pesticide formulation on soil’s culturable microbial population. Int. J. Tech. Res. Sci. 2020, 13–24. 10.30780/IJTRS.V05.I01.003. [DOI] [Google Scholar]
- Singh T.; Singh D. K. Rhizospheric Microbacterium sp. P27 showing potential of lindane degradation and plant growth promoting traits. Curr. Microbiol. 2019, 76, 888–895. 10.1007/s00284-019-01703-x. [DOI] [PubMed] [Google Scholar]
- Rani R.; Kumar V.; Gupta P.; Chandra A. Effect of endosulfan tolerant bacterial isolates (Delftia lacustris IITISM30 and Klebsiella aerogenes IITISM42) with Helianthus annuus on remediation of endosulfan from contaminated soil. Ecotoxicol. Environ. Saf. 2019, 168, 315. 10.1016/j.ecoenv.2018.10.059. [DOI] [PubMed] [Google Scholar]
- Tripti T.; Adarsh K.; Kumar V. Effect of commercial pesticides on plant growth-promoting activities of Burkholderia sp. strain L2 isolated from rhizosphere of Lycopersicon esculentum cultivated in agricultural soil. Toxicol. Environ. Chem. 2015, 97, 1180–1189. 10.1080/02772248.2015.1093632. [DOI] [Google Scholar]
- Umar Mustapha M.; Halimoon N.; Wan Johari W. L.; Shokur A.; Yunus M. Enhanced Carbofuran Degradation Using Immobilized and Free Cells of Enterobacter sp. Isolated from Soil. Molecules 2020, 25, 2771. 10.3390/molecules25122771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Xu Z.; Chen Z.; Wang G. Simultaneous degradation of triazophos, methamidophos and carbofuran pesticides in wastewater using an Enterobacter bacterial bioreactor and analysis of toxicity and biosafety. Chemosphere 2020, 261, 128054 10.1016/j.chemosphere.2020.128054. [DOI] [PubMed] [Google Scholar]
- Ramya K.; Vasudevan N. Biodegradation of synthetic pyrethroid pesticides under saline conditions by a novel halotolerant Enterobacter ludwigii. Desalin. Water Treat. 2020, 173, 255–266. 10.5004/dwt.2020.24791. [DOI] [Google Scholar]
- Khalifa A. Y.; Alsyeeh A. M.; Almalki M. A.; Saleh F. A. Characterization of the plant growth promoting bacterium, Enterobacter cloacae MSR1, isolated from roots of non-nodulating Medicago sativa. Saudi. J. Biol. Sci. 2016, 23, 79–86. 10.1016/j.sjbs.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalam A.; Mukherjee A. K. Effects of hexaconazole, propiconazole, profenofos and pretilachlor on soil phosphatase and dehydrogenase activities. Pestic. Res. J. 2002, 14, 337–342. [Google Scholar]
- Suheil F. M.; Fehmey A. H. Determination of minimum inhibitory concentration from chemical pesticides on bacteria Azotobacter numbers with period of incubation diffrant. Diyala Agric. Sci. J. 2009, 1, Ar199–Ar212. [Google Scholar]
- Rani R.; Usmani Z.; Gupta P.; Chandra A.; Das A.; Kumar V. Effects of organochlorine pesticides on plant growth-promoting traits of phosphate-solubilizing rhizobacterium, Paenibacillus sp. IITISM08. Environ. Sci. Pollut. Res. 2018, 25, 5668–5680. 10.1007/s11356-017-0940-z. [DOI] [PubMed] [Google Scholar]
- Shahid M.; Khan M. S. Pesticide-induced alteration in proteins of characterized soil microbiota revealed by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE). J. Proteins Proteom. 2020, 1–9. 10.1007/s42485-020-00028-9. [DOI] [Google Scholar]
- Chennappa G.; Adkar-Purushothama C. R.; Naik M. K.; Suraj U.; Sreenivasa M. Y. Impact of pesticides on PGPR activity of Azotobacter sp. isolated from pesticide flooded paddy soils. Greener J. Biol. Sci. 2014, 4, 117–129. 10.15580/GJAS.2014.4.010314003. [DOI] [Google Scholar]
- Sayyed R. Z.; Chincholkar S. B.; Reddy M. S.; Gangurde N. S.; Patel P. R.. Siderophore Producing PGPR for Crop Nutrition and Phytopathogen Suppression. In Bacteria in Agrobiology: Disease Management; Springer: Berlin, Heidelberg, 2013; pp 449–471. [Google Scholar]
- Shahid M.; Khan M. S. Cellular destruction, phytohormones and growth modulating enzymes production by Bacillus subtilis strain BC8 impacted by fungicides. Pesticide Biochem. Physiol. 2018, 149, 8–19. 10.1016/j.pestbp.2018.05.001. [DOI] [PubMed] [Google Scholar]
- Khan M. S.; Zaidi A.; Ahmad E.. Mechanism of Phosphate Solubilization and Physiological Functions of Phosphate-Solubilizing Microorganisms. In Phosphate Solubilizing Microorganisms; Springer: Cham, 2014; pp 31–62. [Google Scholar]
- Dar M. A.; Kaushik G.; Villarreal-Chiu J. F. Pollution status and bioremediation of chlorpyrifos in environmental matrices by the application of bacterial communities: A review. J. Environ. Manage. 2019, 239, 124–136. 10.1016/j.jenvman.2019.03.048. [DOI] [PubMed] [Google Scholar]
- Castillo J. M.; Casas J.; Romero E. Isolation of an endosulfan-degrading bacterium from a coffee farm soil: Persistence and inhibitory effect on its biological functions. Sci. Total Environ. 2011, 412, 20–27. 10.1016/j.scitotenv.2011.09.062. [DOI] [PubMed] [Google Scholar]
- Reshma P.; Naik M. K.; Esakkimuthu M. Compatibility of fluorescent pseudomonads with different pesticides under in vitro conditions. Int. J. Farm Sci. 2018, 8, 132–136. 10.5958/2250-0499.2018.00122.2. [DOI] [Google Scholar]
- Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebreyohannes G.; Nyerere A.; Bii C.; Sbhatu D. B. Challenges of intervention, treatment, and antibiotic resistance of biofilm-forming microorganisms. Heliyon 2019, 5, e02192 10.1016/j.heliyon.2019.e02192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Jiang Y.; Zhang Y.; Zhang Z.; Yang X.; Ali M. A.; Fox E. M.; Gobius K. S.; Man C. Silver nanoparticles: A novel antibacterial agent for control of Cronobacter sakazakii. J. Dairy Sci. 2018, 101, 10775–10791. 10.3168/jds.2018-15258. [DOI] [PubMed] [Google Scholar]
- Xue Z.; Hessler C. M.; Panmanee W.; Hassett D. J.; Seo Y. Pseudomonas aeruginosa inactivation mechanism is affected by capsular extracellular polymeric substances reactivity with chlorine and monochloramine. FEMS Microbiol. Ecol. 2013, 83, 101–111. 10.1111/j.1574-6941.2012.01453.x. [DOI] [PubMed] [Google Scholar]
- Ahmed B.; Ameen F.; Rizvi A.; Ali K.; Sonbol H.; Zaidi A.; Khan M. S.; Musarrat J. Destruction of cell topography, morphology, membrane, inhibition of respiration, biofilm formation, and bioactive molecule production by nanoparticles of Ag, ZnO, CuO, TiO2, and Al2O3 toward beneficial soil bacteria. ACS Omega 2020, 5, 7861–7876. 10.1021/acsomega.9b04084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt G. J.; Krieg N. R.; Sneath P. H. A.. Gram Negative Aerobic/Microaerophilic Rods and Cocci. In Bergey’s Manual of Determinative Bacteriology, 9th ed.; Williams and Wilkins: Lippincott, Philadelphia, 1994. [Google Scholar]
- Bric J. M.; Bostock R. M.; Silverstone S. E. Rapid in situ assay for indole acetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl. Environ. Microbiol. 1991, 57, 535–538. 10.1128/AEM.57.2.535-538.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander D. B.; Zuberer D. A. Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol. Fert. Soils 1991, 12, 39–45. 10.1007/BF00369386. [DOI] [Google Scholar]
- Reeves M. W.; Pine L.; Neilands J. B.; Balows A. Absence of siderophore activity in Legionella species grown in iron-deficient media. J. Bacteriol. 1983, 154, 324–329. 10.1128/JB.154.1.324-329.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A.; Ameen F.; Khan F.; Al-Arfaj A.; Ahmed B. Fabrication and antibacterial activity of nanoenhanced conjugate of silver (I) oxide with graphene oxide. Mater. Today Commun. 2020, 25, 101667 10.1016/j.mtcomm.2020.101667. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.