Summary
Oesophageal and gastro-oesophageal junction (GOJ) neoplasms, and their predisposing conditions, may be encountered by the practicing pathologist both as biopsy samples and as surgical specimens in daily practice. Changes in incidence of oesophageal squamous cell carcinomas (such as a decrease in western countries) and in oesophageal and GOJ adenocarcinomas (such as a sharp increase in western countries) are being reported globally. New modes of treatment have changed our histologic reports as specific aspects must be detailed such as in post endoscopic resections or with regards to post neo-adjuvant therapy tumour regression grades. The main aim of this overview is therefore to provide an up-to-date, easily available and clear diagnostic approach to neoplastic and pre-neoplastic conditions of the oesophagus and GOJ, based on the most recent available guidelines and literature.
Key words: oesophageal dysplasia, Barrett’s dysplasia, oesophageal squamous cell carcinoma, oesophageal adenocarcinoma, tumour regression grade
Introduction
Oesophageal and gastro-oesophageal junction (GOJ) neoplasms, and their predisposing conditions may be encountered by the practicing pathologist both as biopsy samples and as surgical specimens. While oesophageal squamous cell carcinomas are decreasing in incidence in the western world, a sharp increase in oesophageal Barrett’s dysplasia, oesophageal adenocarcinoma and GOJ cancer has been observed due to better recognition and widespread predisposing factors. New modalities of treatment are impacting our daily reporting practice and the pathologist is increasingly being asked to provide reliable diagnoses necessary for optimal management and surveillance.
The main aim of this overview is to provide an up to date, easily available and clear diagnostic approach to neoplastic and pre-neoplastic conditions of the oesophagus and GOJ, based on the most recent available guidelines and literature.
Oesophageal squamous carcinoma
Globally, oesophageal squamous carcinoma (OSCC) accounts for the majority of the cases of oesophageal cancer, although its proportion, relative to oesophageal adenocarcinoma, varies from country to country. Oesophageal squamous dysplasia is the main pre-neoplastic lesion.
PRE-NEOPLASTIC LESION - OESOPHAGEAL SQUAMOUS DYSPLASIA (FIG. 1)
Figure 1.
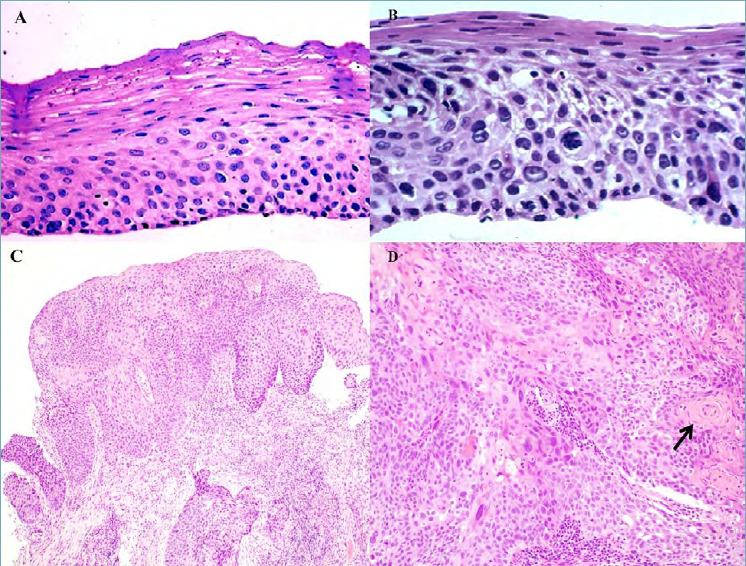
(A) Oesophageal biopsy section showing low grade squamous dysplasia with only mild cytological atypia within the lower half of the epithelial thickness (magnification x40). (B) Oesophageal biopsy section showing high grade squamous dysplasia with severe cytological atypia in more than half of the epithelium (magnification x40). (C) Dysplastic squamous epitelium and invasive squamous cell carcinoma (magnification x10). (D) Invasive squamous cell carcinoma with keratin pearl formation (arrow) (magnification x20).
Oesophageal squamous dysplasia (OSD) (also known as oesophageal squamous intra-epithelial neoplasia) is an unequivocal neoplastic alteration of the oesophageal squamous epithelium, without invasion. Squamous dysplasia can occur anywhere in the oesophagus and it is likely to follow the distribution of squamous cell carcinoma 1.
The diagnosis of precancerous lesions or OSCC in its early phase should be the main goal of screening, considering that advanced OSCC shows poor prognosis (overall survival of 38% at 1 year and 12% at 5 years after diagnosis) 2. Precancerous dysplastic lesions are detectable using endoscopy as they can appear as small, superficial or flat lesions. Though endoscopy is still considered gold standard for the diagnosis of dysplasia and early OSCC, alternative approaches, such as exfoliative cytology, have been suggested 3.
Histologic diagnosis of oesophageal squamous dysplasia
Histologic criteria for OSD were initially described in 1970 4-6 and modified in the 1980s, based on experiences from China 7. The diagnosis of OSD requires the presence of both cytological and architectural atypia. Cytological atypia is characterized by nuclear atypia (enlargement, pleomorphism and hypercromasia), loss of normal cell polarity while architectural atypia is characterized by abnormal epithelial maturation without invasion of epithelial cells through the basement membrane. The two-tiered (low grade versus high grade) histological system is currently recommended by the WHO classification of Tumours Editorial Board 1. Low grade dysplasia is defined as a lesion involving only the lower half of the epithelium, with only mild cytological atypia. High-grade dysplasia is diagnosed when more than half of the epithelium is involved or when severe cytological atypia is present (regardless of the extent of epithelial involvement). High-grade dysplasia includes the group of lesions also termed “carcinoma in situ” in Japan and other parts of Asia 8. Overexpression of p53 and hypermethylation of CDKN2A (P16INK-4a) have been reported in OSD 9.
Differential Diagnoses
Benign and malignant lesions can morphologically mimic squamous dysplasia. These include squamous papilloma, pseudo-epitheliomatous (regenerative hyperplasia) induced by erosions, ulcers, or oesophagitis, multinucleated change in oesophagitis and radiation or chemotherapy effect. Squamous papillomas lack cytologic atypia and show an orderly cellular maturation from the basal layer toward the surface. Pseudo-epitheliomatous hyperplasia may also simulate invasive squamous cell carcinoma. However, neither significant nuclear pleomorphism, nor loss of polarity are present. Distinction of OSD from regenerative or reactive squamous epithelium may sometimes be difficult, especially in biopsy specimens. Regenerative squamous epithelium lacks significant nuclear pleomorphism, overlapping, or crowding. In addition, surface maturation is usually present. Inflammation frequently accompanies reactive squamous epithelium, so, in the presence of inflammation, a diagnosis of OSD should always be made with caution. In practice, when low-grade dysplasia is suspected in biopsy specimens with inflammation, the use of the term “indefinite” for squamous dysplasia, similar to the nomenclature in Barrett’s oesophagus (see later) or inflammatory bowel disease, could be used and follow-up with biopsies is recommended after treatment of the underlying oesophagitis. The term “atypical regenerative hyperplasia” of the oesophagus in endoscopic biopsies has been used to describe lesions that approach squamous cell carcinoma 10. Not uncommonly, the basal layers of regenerative squamous epithelium may undergo increase of nuclear/cytoplasm ratio and simulate dysplasia. After radiotherapy or chemotherapy, squamous epithelium may become atypical and mimic dysplasia or even carcinoma. Atypical cells after therapy do not show an altered nuclear/cytoplasm ratio, but reveal prominent cytoplasmic vacuolization instead. In addition, similar changes may also be observed in stromal cells.
Malignancies that mimic dysplasia include lateral spread of invasive squamous cell carcinoma and verrucous carcinomas 11. Lateral spread of squamous cell carcinoma may mimic dysplasia particularly if the invasive component is not present in the biopsy sample 12. In these cases, nuclear hyperchromasia and pleomorphism are usually more prominent compared with high-grade dysplasia, and a clear demarcation from carcinomatous to non-carcinomatous epithelium is typically present. There are two general types of lateral spread. One shows full-thickness replacement by carcinoma (full-thickness type) and the other reveals only basal epithelium involvement (basal layer-type). In the basal layer-type, involvement may be seen in only one or two layers of the epithelium. Verrucous carcinoma is an extremely rare form of malignancy and shows an exophytic papillary growth pattern 13 with only minor cytologic atypia and a blunt pushing invasive margin which may be difficult to interpret as invasive.
OESOPHAGEAL SQUAMOUS CELL CARCINOMA
Definition
Oesophageal squamous cell carcinoma NOS is a malignant oesophageal epithelial neoplasm displaying squamous cell differentiation characterized by keratinocyte-type cells with intercellular bridges and/or keratinization. It is located most commonly in the middle third of the oesophagus followed by the lower third 14.
Clinical aspects
Over the past three decades, a consistent decline in the rate of OSCC has been observed in Western Europe while a stable rate of slower decline has been seen in central Europe and conversely, an increase in OSCC incidence has been reported in Eastern Europe. In moderate and lower-risk Western countries, the most important risk factors are the combination of tobacco smoking and excessive alcohol consumption 15; indeed, the decline in OSCC incidence in Western Europe has been mainly attributed to the reduction in alcohol intake and smoking habits 16.
With regards to the possibility of screening for OSCC in moderate and lower-risk countries, it would have to include an excessively large population at risk and therefore seems impractical. Screening is therefore usually proposed to a small subgroup of patients who are at a very high risk of cancer development, such as those with a previous or concomitant diagnosis of head/neck squamous cell carcinoma 17, achalasia (up to 10 times risk) 18, previous radiotherapy for breast cancer, previous caustic injury to the oesophagus and tylosis 19. In practice, no consensus has been reached among world experts with regards to timing of surveillance programmes: practices are still varied, with screening starting within 1 year after diagnosis in some cases, compared to 5 and 10 years in others. Furthermore, surveillance intervals also vary from 2 to 5 years 20.
Typical symptoms include new onset dysphagia, gastrointestinal bleeding, recurrent aspiration or emesis, weight loss and/or loss of appetite.
OSCC is most often found in older men (aged > 60 years) with male to female ratio of 3:1, although this ratio varies considerably across geographical regions 21,22. Apart from alcohol overconsumption and tobacco smoking, particularly when in combination, other risk factors include the consumption of red meat 23 and of very hot beverages 24. Genetic factors are also involved: a pooled analysis of three genome-wide association studies found new susceptibility loci for OSCC 25.
Treatment Options
The main factors affecting choice of treatment are tumour stage and location, histological type, patient’s performance status and comorbidities 26. While surgery (transthoracic oesophagectomy Ivor-Lewis procedure) alone is the treatment of choice in limited disease (cT1-T2 cN0 M0), neo-adjuvant therapy with chemotherapy or chemoradiotherapy has supplemented surgery as standard treatment for locally advanced oesophageal cancer (cT3-T4 or cN1-N3 M0). This is mostly due to difficulty in achieving complete R0 tumour resections with surgery alone, while neo-adjuvant preoperative treatment has been shown to increase R0 resections and survival rates 27,28.
Macroscopic and Microscopic description
Macroscopically, OSCC often presents as an ulcerative mass, especially when advanced. The most useful macroscopic classification is provided by Japan Esophageal Society 29.
Histology shows a typical, invasive squamous carcinoma with both vertical and horizontal growth beyond the basement membrane (Fig. 1). Histological grading is based on the degree of cytological atypia, mitotic activity and presence of keratinization. A three-tiered system is commonly applied:
Grade 1 (well differentiated): shows enlarged cells with abundant eosinophilic cytoplasm and keratin pearl production. Cytological atypia is minimal and the mitotic rate low. The invasive margin is pushing and the cells are well oriented.
Grade 2 (moderately differentiated): has evident cytological atypia and the cells are less ordered. Mitotic figures are easily identified while keratin pearl formation is infrequent.
Grade 3 (poorly differentiated): consists predominantly of basal-like cells forming nests, which may show central necrosis. The tumour nests consist of sheets or pavement-like arrangements of tumour cells with occasional parakeratotic or keratinizing cells.
SUBTYPES OF OESOPHAGEAL SQUAMOUS CELL CARCINOMA
Verrucous squamous cell carcinoma
Most cases are encountered in the lower third of the oesophagus, as a protuberant mass. The tumour comprises differentiated squamous cells with minimal cytological atypia, low mitotic activity and surface papillary projections. recognizes occurs abruptly, with no intervening granular cell layer. The invasive front is pushing and the tumour is slow-growing and metastases are uncommon 30-32.
Spindle cell squamous cell carcinoma
Macroscopically it has a polypoid growth pattern. Histologically, there is a biphasic pattern of neoplastic squamous epithelium and spindle cells. Squamous cells are well to moderately differentiated or may be occasionally carcinoma in situ alone. The spindle cells have high-grade malignancy, which may show osseous, cartilaginous, or skeletal muscle differentiation 33,34. Although these tumours tend to be large, the prognosis is sometimes better than that of OSCC NOS of the same size, due to their intraluminal rather than deeply invasive growth.
Basaloid squamous cell carcinoma
Unlike similar tumours in the oropharynx, this neoplasm is not associated with HPV infection 35. It shows solid or nested growth pattern of basaloid cells, sometimes with comedonecrosis and occasionally with pseudoglandular or cribriform formation. Exclusion of high-grade neuroendocrine carcinoma (NEC) by immunohistochemistry is often required. Areas of squamous cell carcinoma in situ or OSCC NOS are relative common. The tumour is highly aggressive, with a worse prognosis than OSCC NOS, even though differences are not statistically significant 32.
Carcinoma cuniculatum is characterized by prominent surface keratin-filled sinuses lined by bland squamous cells.
Molecular pathways
OSCC develops by stepwise progression from histologically normal squamous mucosa to low-grade dysplasia (intra-epithelial neoplasia) and high-grade dysplasia.
TP53 mutation is a key early driver mutation 36. Genetic changes identified at the intra-epithelial neoplasia stage include aneuploidy, copy-number alterations, changes related to the amplification of genes, such as EGFR, and the silencing of genes, such as CDKN2A, due to promoter hypermethylation 36. Frequently mutated genes are, however, shared at the dysplastic stage and invasive cancer 37. While copy number variations are not significantly increased between dysplastic and malignant cells, it has been suggested that several cancer genes (ATR, MECOM, PIK3CA, BCL6, MYC, and CCND2) are more frequently affected by copy number variations in malignant than dysplastic cells. Many of these commonly mutated genes are shared with other squamous cell carcinomas of the head and neck and of the lung 38,39. It is therefore probable that cancers that arise from similar lineages are more molecularly alike than cells that arise from different lineages within the same organ. This could mean that biomarkers and therapeutic targets for cancer will be shared between tumour types based on cell of origin rather than anatomic location 36.
Molecular subtypes
According to The Cancer Genome Atlas Research network 39, three molecular sub types are identified as follows:
OSCC type 1 recognizes alterations in the NRF2 pathway, which regulates adaptation to oxidative stress. This group also contains a higher frequency of SOX2 and TP63 amplifications. Geographically, it includes 66% of the Asian population.
OSCC type 2 is referred to many of the Eastern European and South American patients. Their tumours are characterized by higher rates of ZNF750 and NOTCH1 mutation, inactivation of the chromatin modulators KDM6A and KDM2D, CDK6 amplification and inactivation of the PIK3CA suppressors PIK3R1 and PTEN.
OSCC type 3 are few and come from North America. Mutations in the cell cycle pathway are absent in this subtype and only 1 patient had a TP53 mutation. They all contained mutations predicted to activate the RTK/RAS/PI3K pathway and 75% contained somatic mutations in chromatin re-modelling.
At present, no molecular tests are required 1.
Oesophageal adenocarcinoma and gastro-oesophageal junction adenocarcinoma
PRE-NEOPLASTIC LESION - BARRETT’S DYSPLASIA (FIG. 2)
Figure 2.
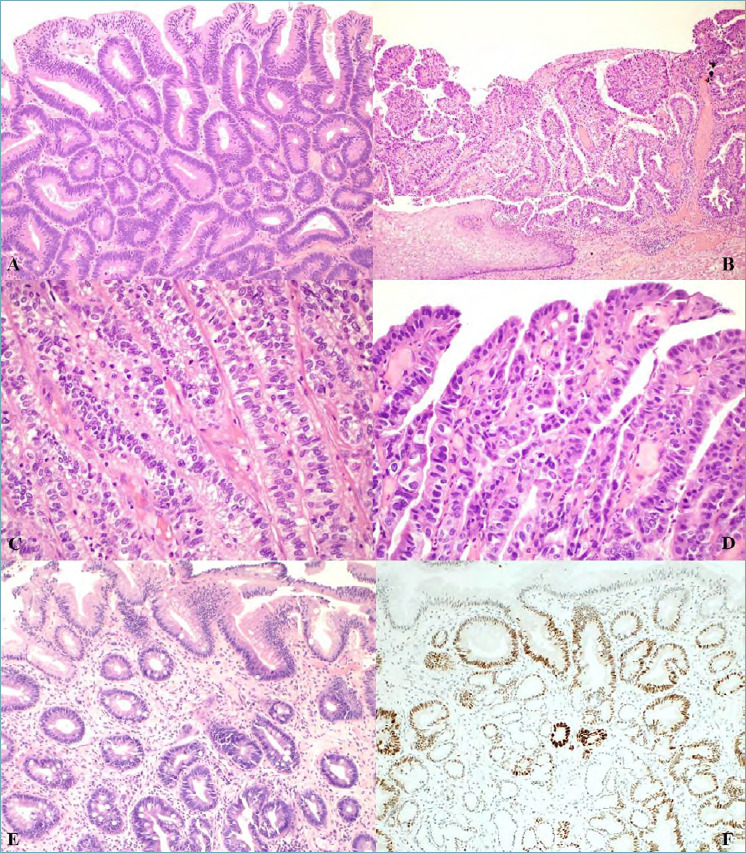
(A) Intestinal type low grade dysplasia in Barrett’s oesophagus showing scanty architectural distortion and mild to moderate cytological atypia; hyperchromatic nuclei with irregular contours, nuclear overlapping and crowding are seen (magnification x20). (B) Intestinal type high grade dysplasia in Barrett’s oesophagus showing both architectural abnormality and severe cytological atypia adjacent to squamous epithelium (magnification x10). (C) Foveolar type low grade dysplasia in Barrett’s oesophagus with closely packed glands with a single layer of columnar cells with no interspersed goblet cells, round/oval basal nuclei with little stratification or pleomorphism and vesicular nuclei (magnification x20). (D) Foveolar type high grade dysplasia with enlarged cells with greater pleomorphism, loss of polarity and increased mitoses (magnification x20). (E) Crypt dysplasia: dysplasia is confined to the crypt with surface epithelium maturation (magnification x20). (F) p53 immunostained section of crypt dysplasia showing p53 nuclear accumulation in the crypt areas of dysplastic epithelium but not in the surface epitheium.
Dysplasia in Barrett’s Oesophagus (BO) is an unequivocal neoplastic transformation of the epithelial cells confined within the basement membrane of the metaplastic columnar epithelium within which it arises and requires exclusion of regenerative lesions. Dysplasia is endoscopically associated with flat, raised or depressed lesions and is defined by combined architectural abnormalities and cytological atypia identifiable on standard haematoxylin and eosin-stained sections 40,41. Dysplastic glands show architectural changes (gland fusion and budding, variability in gland size, cribriform pattern) and cytological alterations (hyperchromatic and elongated nuclei, nuclear stratification) causing an increasing morphological deviation from the metaplastic phenotype. Cytological and architectural abnormalities involve the entire length of glands and typically cellular maturation toward the surface of the mucosa is missing. Dysplasia confined to the crypt but with surface maturation is also reported 42,43.
Pathologists should report dysplasia as fitting into one of four categories (namely negative for dysplasia, indefinite for dysplasia, low grade dysplasia and high grade dysplasia) 44. The rationale for this tiered approach is to stratify patients into categories of increasing risk for development of, or concurrent presence of, oesophageal adenocarcinoma (OAC) 45.
Negative for dysplasia (NEG)
This diagnosis represents two different situations: a) columnar epithelium with no cell atypia; b) reactive (hyperplastic/regenerative) changes.
Indefinite for dysplasia (IND) - this term only applies to cases where the pathologist cannot decide with certainty whether the lesion is hyperplastic/regenerative or neoplastic in nature. This may be due to inadequate biopsy sampling (e.g. poorly oriented biopsies that do not enable full thickness assessment) or to the presence of cytological atypia and/or structural alterations of doubtful interpretation. It is a “provisional” diagnosis that must be followed by short-term resampling and/or second opinion. The presence of erosion and neutrophilic infiltrate requires additional caution in diagnosing dysplasia.
Low grade dysplasia (LGD) - this is characterized by scanty architectural distortion and mild to moderate cytological atypia. Hyperchromatic nuclei with irregular contours, nuclear overlapping and crowding and dystrophic goblet cells may be present.
High grade dysplasia (HGD) - this is characterized by both architectural abnormality and severe cytological atypia. Aberrant architectural features include glandular crowding, branching or budding glands, villiform, cribriform, micropapillary, or cystically dilated crypt patterns. Cytological features include complete loss of cell polarity, rounded enlarged nuclei with irregular-thickened nuclear membranes and conspicuous nucleoli. Typical and atypical mitotic figures are readily identified at all levels within the glands, as well as on the luminal surface.
The risk of progression to OAC in patients with BO increases with male gender, current tobacco smoking, visceral obesity 46 and Caucasian origin 47. A longer length of BO has been associated with a higher cancer risk. A prospective cohort study 48 has shown that the risk of OAC increases linearly with the length of BO, with a higher risk of developing cancer in long segment BO (> 3 cm) than in short segment BO. A recent model for the calculation of risk of progression of BO, is based on several weighted factors (male gender, cigarette smoking, BO length and confirmed low grade dysplasia) and identifies a risk pyramid varying from 0.13 to 2.1% patient-years increase in progression (high grade dysplasia and OAC) 49. A meta-analysis of 57 studies, shows that the pooled annual incidence of OAC is 0.33% (95% CI 0.28-0.38%) 50 while when considering patients with short segment BO incidence falls to 0.19%. With regards to dysplasia to cancer risk, patients with LGD have a pooled annual incidence rate of 1.7% for HGD/EAC 51, in patients with HGD the annual incidence rate of OAC is 7% 52.
It has long been recognised that there is inter- and intra-observer variability in the diagnosis of Barrett’s dysplasia and this particularly affects LGD and the indefinite category 53-55. A recent study 56 has evaluated the agreement across the entire diagnostic BO spectrum (NEG, IND, LGD, HGD). Excellent concordance (> 70%) has been demonstrated for non-dysplastic BO and HGD, while concordance was intermediate for LGD (46%) and poor for indefinite for dysplasia (23.5%). Ancillary tests (e.g. p53, AMACR and Ki-67 stains) have been advocated to aid the diagnosis of dysplasia. At present, conventional haematoxylin and eosin examination remains the gold standard even though the use of p53 stain is suggested 57,58. Studies have shown that the prediction of progression of oesophageal dysplasia is improved if at least two expert pathologists agree on the diagnosis and increases further when a greater number of pathologists concur 53. In day-to-day diagnostic practice, a diagnosis of dysplasia in the setting of BO requires confirmation by a second pathologist, preferably with a special interest in gastrointestinal pathology. Considering that major inconsistencies are found when diagnosing the indefinite category, the extension of consensus reporting to this category appears advised.
Types of dysplasia
The two main types of dysplasia, which may also be found admixed, are type 1 intestinal (adenomatous) dysplasia and type 2 foveolar or non-intestinal, gastric-type dysplasia.
Intestinal dysplasia is the more widely recognised type and is characterized by the presence of intestinal type columnar cells as well as goblet cells (which may however be mucous depleted) and enterocytes. Foveolar or non-intestinal, gastric dysplasia, on the other hand, shows closely packed glands with a single layer of columnar cells with few or no interspersed goblet cells, round/oval basal nuclei with little stratification or pleomorphism and vesicular nuclei with prominent nucleoli. High-grade foveolar dysplasia shows cells to be enlarged with greater pleomorphism, loss of polarity and increased mitoses 59. Foveolar dysplasia typically expresses gastric type MUC5AC while it is negative for intestinal type markers, such as MUC2, CDX2 and villin, which are typically expressed in intestinal type dysplasia 60. Initial data suggest that foveolar dysplasia may be associated with a high risk of progression 61.
Adenocarcinoma of the Oesophagus and Gastro-Oesophageal Junction
Definition
Adenocarcinoma of the oesophagus (OAC) and GOJ adenocarcinoma (GOJ-AC) are malignant epithelial tumours which show a tubular, papillary, solid with mucin production, mucinous or, less frequently, poorly cohesive patterns of growth.
Clinical aspects
OAC and GOJ-AC are the most rapidly increasing cancer types in the western world including North America, western Europe and Australasia 62. Risk factors include age (mean age 66 years), male gender, Caucasian ethnicity, gastro-oesophageal reflux disease and BO, obesity, smoking and dietary factors. Unfortunately, late stage cancers still harbour a relatively poor prognosis with survival rates falling sharply from 1 year (approximately 50%) to 5 years (15%) 63.
Nearly all cancers (95%) are found in the distal oesophagus/GOJ even if infrequently, adenocarcinoma can be found in the middle/upper third of the oesophagus either arising from an inlet patch area (ectopic gastric oxyntic type mucosa) 64 or from submucosal oesophageal glands.
Most frequent symptoms include dysphagia, anorexia with weight loss, dyspepsia and retrosternal pain. Diagnosis is based on endoscopic biopsy of the lesion while enlarged peri-oesophageal nodes may be amenable to endoscopic ultra-sound cytologic or microhistologic sampling.
Surgery is no longer the only option as endoscopic dissection techniques permit wide dissections of mucosa or submucosa when early and endoscopically identified lesions are present.
Macroscopic description
OAC and GOJ-AC are often found in advanced stages as polypoid/fungating, ulcerated or diffusely invasive lesions leading to strictures. Early stage cancers may be found during endoscopic surveillance and appear as slightly elevated, flat or depressed lesions with possible nodular areas.
Critical problems in distinction between distal oesophageal, GOJ and proximal gastric cancer arise due to the absence of reliable anatomic landmarks, which may be even less readily identified due to complicating BO. In consideration of this, a macroscopic classification – the Siewert classification 65 – aids the pathologist in describing the site of origin of the tumour. The Siewert classification identifies three macroscopic types (Fig. 3):
Figure 3.
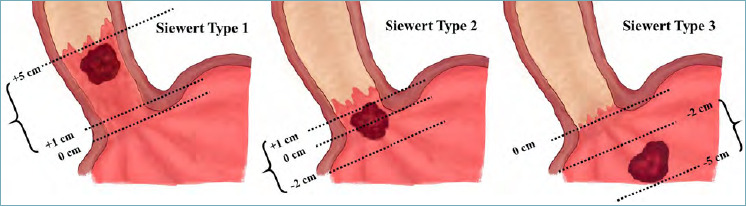
Schematic representation of the Siewert macroscopic classification of gastro-oesophageal junction tumours65. Siewert Type I: Adenocarcinoma of the distal oesophagus. The tumour centre is located 1-5 cm above the gastric cardia. Siewert Type II: Adenocarcinoma of the GOJ/cardia. The tumour centre is located 1 cm above or 2 cm below the gastric cardia. Siewert Type III: Adenocarcinoma of the subcardial stomach. The tumour centre is located 2-5 cm below the gastric cardia. In the figure, 0 cm represents the gastric cardia.
Type I: Adenocarcinoma of the distal oesophagus. The tumour centre is located 1-5 cm above the gastric cardia.
Type II: Adenocarcinoma of the GOJ/cardia. The tumour centre is located 1 cm above or 2 cm below the gastric cardia.
Type III: Adenocarcinoma of the subcardial stomach. The tumour centre is located 2-5 cm below the gastric cardia.
This distinction is a fundamental moment during gross description as in the VIIIth edition of the TNM staging manual, Siewert types I and II are staged using the TNM for the oesophagus/GOJ while Siewert III cancers are staged with gastric cancers 66.
Microscopic description
OAC/GOJ-AC show various patterns which are often found mixed; most frequently observed growth patterns include glandular/tubular, papillary, mucinous or signet ring cell features (Figs. 4, 5).
Figure 4.
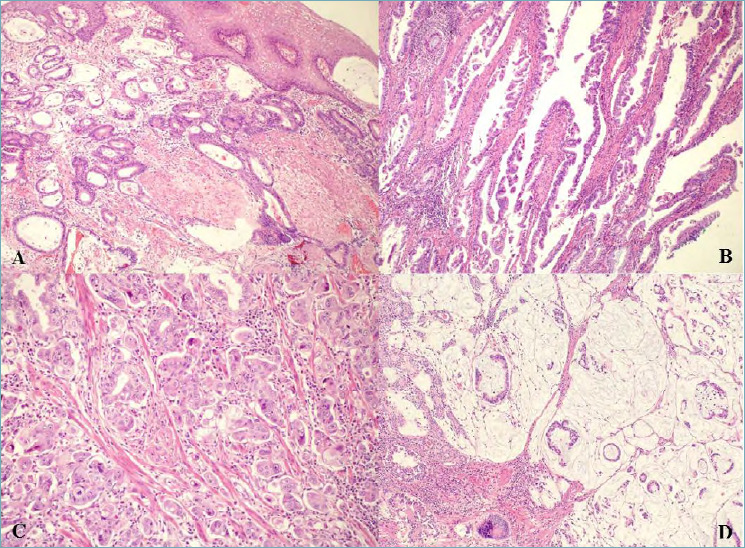
(A) Invasive oesophageal adenocarcinoma, tubular/glandular type which invades and undermines squamous epithelium (magnification x10). (B) Invasive oesophageal/GOJ adenocarcinoma, papillary type (magnification x10). (C) Invasive oesophageal/GOJ adenocarcinoma with micropapillary features (magnification x20). (D) Invasive oesophageal/GOJ adenocarcinoma, mucinous type (magnification x10).
Figure 5.
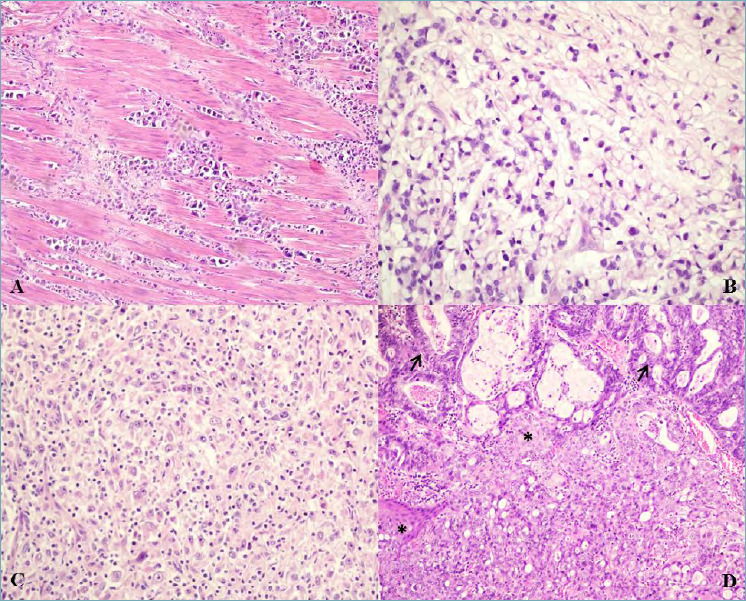
(A) Invasive oesophageal/GOJ adenocarcinoma, poorly cohesive non signet ring cell type (magnification x40). (B) Invasive oesophageal adenocarcinoma, poorly cohesive signet ring cell type (magnification x40). (C) Invasive oesophageal/GOJ adenocarcinoma, undifferentiated carcinoma with lymphoepithelioma-like carcinoma features including a syncytial pattern and prominent lymphocyte infiltration (magnification x40). (D) Invasive oesophageal/GOJ adenocarcinoma, adenosquamous type with squamous areas (asterisks) and glandular areas with mucin production (arrows) (magnification x20).
The glandular/tubular growth pattern is characterized by irregular, fused neoplastic glands lined by a single layer of atypical cells with evidence of mucin production (alcian blue PAS positive) while the papillary pattern shows structured papillae or, rarely, micropapillae.
The signet-ring cell pattern, characterized by the presence of non-cohesive cells containing intracellular mucin which pushes the nucleus to the periphery of the cell, has been reported in approximately 7% of OAC/GOJ-AC, though percentages in the literature range between 0.46% and 26%. The main reason for these marked differences are the diverse criteria for reporting the signet ring cell pattern (some studies consider only tumours with > 50% signet ring cells, others consider any percentage of signet ring cells, while other still consider signet ring cells in mucin pools as well – see next paragraph). Signet ring cell tumours have been shown to behave more aggressively and show worse response to neo-adjuvant treatment 67. Though still little is unknown concerning their biology and optimal treatment strategy, the percentage of signet ring cells within the tumour should be mentioned in the pathology report when they are identified. Signet ring cell carcinoma, in its pure form is extremely rare in the oesophagus.
The mucinous pattern is rare and characterized by abundant pools of extracellular mucin in which carcinoma cells are present; of note is that floating neoplastic cells may show intracellular mucin vacuoles (signet ring cell features), but these should not be classified as signet ring cell cancers.
Poorly cohesive non signet ring pattern in OAC/GOJ-AC is morphologically similar to poorly cohesive carcinomas of the stomach, though much less frequent.
Early stage adenocarcinoma
Intramucosal adenocarcinoma (pT1a) is defined as an adenocarcinoma which invades the lamina propria or the muscolaris mucosae but does not extend into the submucosa. These early lesions will mostly be managed by conservative endoscopic dissections 68 considering the low risk of nodal or distant metastases (< 2%) and the high risk of surgery 69. Sampling protocols are beyond the scope of this review, although the entire endoscopically resected specimen must be sampled after inking of margins. Features which distinguish intramucosal carcinoma from high grade dysplasia include tubules or small aggregates of compact back to back glands, single cells, cribriform patterns or solid sheets. Signet ring cells in intramucosal OAC are extremely rare.
The management of adenocarcinoma with submucosal invasion (pT1b) is less clear cut. Indeed pT1b metastatic risk may be as high as 45% 70 however in a subset of pT1b cancers with low risk features, endoscopic resection may be sufficient (especially in patients who are at high risk for surgery). Low-risk features on endoscopic resections include: < 500-μm invasion in the submucosa (sm1), well/moderately differentiated tumours, absence of lympho-vascular invasion and clear resection margins 71.
Difficulties in distinguishing between pT1a and pT1b cancers may be due to duplication of the muscularis mucosae, which may be seen in endoscopic resections with BO 72.
TUMOUR REGRESSION GRADING SYSTEMS FOR OESOPHAGEAL CANCER
Neo-adjuvant treatment is performed in advanced OSCC and OAC and modifications seen at histology comprise residual fibrous areas with dispersed neoplastic glands or cells showing cytoplasmic modifications as well as mucinous changes 73.
Different tumor regression grade (TRG) systems have been proposed for oesophageal cancer 74. The first TRG grading system according to Mandard was indeed originally proposed for oesophageal squamous cell carcinoma 75 and later applied to other cancer types and sites. Since then other systems have been proposed including the JSED system 76, the Chirieac 77, the Swisher 78 and the Schneider 79 systems, amongst others. In these various systems categories vary from 3 to 5 and some systems also take into account nodal response. The choice of which system to use should be discussed between the pathologists, surgeons and oncologists preferably in a multi-disciplinary team setting.
Subtypes of oesophageal adenocarcinoma
While conventional adenocarcinoma of the oesophagus and GOJ have been described in the above paragraph, non-conventional histotypes of oesophageal carcinomas according to the WHO 2019 classification of digestive system tumours1 are rarely seen (Fig. 6). These include: i) adenoid cystic carcinoma; ii) adenosquamous carcinoma; iii) mucoepidermoid carcinoma, and iv) undifferentiated carcinoma (1). Their definitions and clinico-pathologic correlates are briefly described here.
Adenoid cystic carcinoma is defined as a carcinoma composed of two distinct population of cells: epithelial and myoepithelial cells. It accounts for only 0.1% of all oesophageal cancers and is predominantly located in the middle third of the oesophagus, with typical symptoms such as dysphagia and pain being the most common. They are histologically similar to the salivary gland adenoid cystic carcinoma and are characterized by cytokeratin, CEA and CD117/c-KIT positive epithelial cells in a cribriform, tubular or solid architecture. The myoepithelial cells on the other hand show positivity for the myoepithelial markers p63, smooth muscle actin and calponin, as well as for S100. Histological differential diagnoses include the basaloid squamous cell carcinoma, which is negative for smooth muscle actin and S100. Similarly to its salivary gland counterpart, it is often characterized by perineural invasion. The staging of adenoid cystic carcinoma follows that of oesophageal adenocarcinoma. Data on prognosis is scarce and these tumours seem to behave aggressively with the majority of patients having distant metastases at presentation 80.
Adenosquamous carcinoma is defined as a carcinoma with biphasic differentiation, composed of both squamous cell carcinoma and adenocarcinoma, as morphologically distinct components. It is commonly located in the middle third of the oesophagus 81, the male-to-female ratio is about 5 and the median patient age at diagnosis is 60 years. These tumours tend to behave aggressively 82.
Mucoepidermoid carcinoma is defined as a carcinoma composed of typically admixed epidermoid, intermediate and mucin-secreting cells. It accounts for less than 1% of all oesophageal malignancies, and is found more frequently in males with a median age of 58 years. It can be located in the middle or lower oesophagus 83. The epidermoid cells are often arranged in squamous nests with admixed intermediate and mucinous cells. Its differential diagnosis should include adenosquamous carcinoma, which lacks intermediate cells and show more separate components. However, in some cases, the differential diagnosis in almost impossible. The prognosis of mucoepidermoid carcinoma is currently unclear but some evidence of a more aggressive behaviour is available 83.
Undifferentiated carcinoma is a carcinoma without histologic and immunohistochemical evidence of squamous, adenocarcinomatous or neuroendocrine differentiation. The median age of patients is 65 years, with predominance of males. The most common site in the lower oesophagus, often in association with Barrett’s oesophagus 84. Neoplastic cells are undifferentiated, with vesicular or pleomorphic nuclei. Lymphoepithelioma-like carcinoma, which is characterized by syncytial pattern and prominent lymphocyte infiltration, has been regarded as a subtype of undifferentiated carcinoma. Differential diagnoses include non-epithelial tumours, such as melanomas or lymphomas, as well as poorly differentiated epithelial neoplasms (squamous cell carcinomas, adenocarcinomas or neuroendocrine carcinomas). They usually express cytokeratins, while they are negative for p40, synaptophysin and chromogranin-A. The prognosis is remarkably poor.
Immunohistochemical and molecular characteristics OAC and GOJ-AC, similarly to gastric cancer, show a CK7 positive/CK 20 negative phenotype with variable CDX2 expression.
Though numerous studies have provided some insight on the use of immunohistochemistry (MUC2, MUC5AC, MUC 6, CDX2 etc) in the cancerogenic sequence from BO to OAC/GOJ-AC, the use of these markers in routine diagnosis has no prognostic impact and is not advocated. Other markers such as p53, PDL-1 85, p16 and Ki67 86 have been studied as possible prognostic factors both in treatment-naïve, surgically resected, adenocarcinoma and in the neo-adjuvant setting, but these markers are still a long way away from being validated for routine use.
At the present time the only useful predictive evaluations in OAC and GOJ-AC are represented by HER2 status and mismatch repair protein (MMR)/microsatellite instability (MSI) status in relation to possible tailored therapeutic strategies.
HER-2 dysregulation is an early event in oesophageal cancerogenesis 87 and its overexpression/amplification is seen in approximately 15-20% of OAC/GOJ-AC cancers. HER-2 status in gastric and oesophageal cancers utilises a specific immunoscoring system 88 and they often show basolateral/lateral membrane staining 89. From a practical point of view it is important underline that HER-2 expression can be patchy and heterogeneous. This is in particularly important when HER2 evaluation is performed on endoscopic biopsies 90: a minimum set of 5 biopsies has shown to be necessary for reliable HER2 assessment 91.
MMR/MSI status, assessed either via immunostain loss of nuclear MLH1/PMS2 or MSH2/MSH6 protein expression or via MSI, can demonstrate an unstable phenotype in about 5% of cancers 92. This evaluation may prove useful when immunotherapy is considered in the advanced/metastatic setting 93.
The molecular landscape of OAC and GOJ-CA, is increasingly being described as new studies identify diverse molecular subtypes. While gastric molecular subtypes are essentially four (chromosomal instability – CIN – cancers, microsatellite unstable cancers, Epstein Barr Virus positive cancers and genomically stable cancers), oesophageal/GOJ adenocarcinomas are predominantly of CIN type 94 with rare genomically stable cancers at the GOJ. Structural DNA variations and copy number changes are the main events in OAC and rearrangements, amplifications and deletions were shown to be more frequent than point mutations or insertions/deletions 95. The most frequently involved gene is TP53, while less frequently mutated genes, include ARID1A, SMAD4, CDKN2A and ERBB2, amongst others. A better understanding of the molecular alterations in OAC/GOJ-AC will open new paths for targeted treatments 96.
Conclusions
A standardized approach is essential for the histopathologic reporting of oesophageal and gastro-oesophageal junction (GOJ) neoplasms, and their predisposing conditions. Novelties in the diagnostic/interpretation procedures, as well as new modes of treatment, require up to date recommendations for histologic reporting.
Figures and tables
References
- 1.WHO Classification of Tumours of the Digestive System. 5th ed. Geneva: World Health Organization Classification of Tumours. WHO Classification of Tumours Editorial Board. Lyon: IARC press; 2019. [Google Scholar]
- 2.RARECARENet Information Network on Rare Cancers. Accessed: 15 Sept 2019. Available from http://rarecarenet.eu/index.php
- 3.Pan Q-J, Roth MJ, Guo H-Q, et al. Cytologic detection of esophageal squamous cell carcinoma and its precursor lesions using balloon samplers and liquid-based cytologyin asymptomatic adults in Llinxian, China. Acta Cytol 2008;52:14-23. https://doi.org/10.1159/000325430 10.1159/000325430 [DOI] [PubMed] [Google Scholar]
- 4.Ismail-Beigi F, Horton PF, Pope CE. Histological consequences of gastroesophageal reflux in man. Gastroenterol 1970;58:163-74. [PubMed] [Google Scholar]
- 5.Weinstein WM, Bogoch ER, Bowes KL. The normal human esophageal mucosa : a histological rappraisal. Gastroenterol 1975;68:40-4. [PubMed] [Google Scholar]
- 6.Taylor PR, Abnet CC, Dawsey SM. Squamous dysplasia-the precursor lesion for esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 2013;22:540-52. https://doi.org/10.1158/1055-9965.EPI-12-1347 10.1158/1055-9965.EPI-12-1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawsey SM, Lewin KJ, Liu FS, et al. Esophageal morphology from Linxian, China. Squamous histologic findings in 754 patients. Cancer 1994;73:2027-37. https://doi.org/10.1002/1097-0142(19940415)73:8<2027::aid-cncr2820730803>3.0.co;2-3 [DOI] [PubMed] [Google Scholar]
- 8.Wang LD, Yanh HH, Fan ZM, et al. Cytological screening and 15 years’ follow-up (1986-2001) for early esophageal squamous cell carcinoma and precancerous lesions in a high-risk population in Anyang Country, Henan Province, Northern China. Cancer Detect Prev 2005;29:317-22. https://doi.org/10.1016/j.cdp.2005.06.004 10.1016/j.cdp.2005.06.004 [DOI] [PubMed] [Google Scholar]
- 9.Savant D, Zhang Q, Yang Z. Squamous Neoplasia in the Esophagus. Arch Pathol Lab Med 2020. Epub ahead of print. https://doi.org/10.5858/arpa.2020-0058-RA 10.5858/arpa.2020-0058-RA [DOI] [PubMed] [Google Scholar]
- 10.Arista-Nasr J, Rivera I, Martinez-Benitez B, et al. Atypical regenerative hyperplasia of the esophagus in endoscopy biopsy: a mimicker of squamous esophageal carcinoma. Arch Pathol Lab Med 2005;129:899-904. https://doi.org/10.1043/1543-2165(2005)129[899:ARHOTE]2.0.CO;2 10.1043/1543-2165(2005)129[899:ARHOTE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 11.Shimizu M, Ban S, Odze RD. Squamous dysplasia and other precursor lesions related to esophageal squamous cell carcinoma. Gastroenterol Clin N Am 2007;36:797-811. https://doi.org/10.1016/j.gtc.2007.08.005 10.1016/j.gtc.2007.08.005 [DOI] [PubMed] [Google Scholar]
- 12.Soga J, Tanaka O, Sasaki K, et al. Superficial spreading carcinoma of the esophagus. Cancer 1982;50:1641-5. https://doi.org/10.1002/1097-0142(19821015)50:8<1641::aid-cncr2820500830>3.0.co;2-9 [DOI] [PubMed] [Google Scholar]
- 13.Biemond P, ten Kate FJ, Van Blankenstein M. Esophageal verrucous carcinoma: histologically a low-grade malignancy but clinically a fatal disease. J Clin Gastroenterol 1991;13:102-7. [PubMed] [Google Scholar]
- 14.Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med 2014;371:2499-2509. https://doi.org/10.1056/NEJMra1314530 10.1056/NEJMra1314530 [DOI] [PubMed] [Google Scholar]
- 15.Rossini AR, Hashimoto CL, Iriya K, et al. Dietary habits, ethanol and tobacco consumption as predictive factors in the development of esophageal carcinoma in patients with head and neck neoplasms. Dis Esophagus 2008;21:316-21. https://doi.org/10.1111/j.1442-2050.2007.00769.x 10.1111/j.1442-2050.2007.00769.x [DOI] [PubMed] [Google Scholar]
- 16.Saftoiu A, Hassan C, Areia M., et al. Role of gastrointestinal endoscopy in the screening of digestive tract cancers in Europe : European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2020;52:293-304. https://doi.org/10.1055/a-1104-5245 10.1055/a-1104-5245 [DOI] [PubMed] [Google Scholar]
- 17.Muto M, Hironaka S, Nakane M., et al. Association of multiple Lugol-voiding lesions with synchronous and metachronous esophageal squamous cell carcinoma in patients with head and neck cancer. Gastrointest Endosc 2002;56:517-21. https://doi.org/10.1067/mge.2002.128104 10.1067/mge.2002.128104 [DOI] [PubMed] [Google Scholar]
- 18.Zendehedel K, Nyrèn O, Edberg A, et al. Risk of esophageal adenocarcinoma in achalasia patients, a retrospective cohort study in Sweden. Am J Gastroenterol 2011;106:57-61. https://doi.org/10.1038/ajg.2010.449 10.1038/ajg.2010.449 [DOI] [PubMed] [Google Scholar]
- 19.Blaydon DC, Etheridge SL, Risk JM, et al. RHBDF2 mutations are associated with tylosis, a familial esophageal cancer syndrome. Am J Hum Genet 2012;90:340-6. https://doi.org/10.1016/j.ajhg.2011.12.008 10.1016/j.ajhg.2011.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravi K, Geno DM, Katzka DA. Esophageal cancer screening in achalasia: is there a consensus? Dis Esophagus 2015;28:299-304. https://doi.org/10.1111/dote.12196 10.1111/dote.12196 [DOI] [PubMed] [Google Scholar]
- 21.Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Dicker D, et al. The Global Burden of Cancer 2013. Jama Oncol 2015;1:505-27. https://doi.org/10.1001/jamaoncol.2015.0735 10.1001/jamaoncol.2015.0735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie SH, Lagergen J. A global assessment of the male predominance in esophageal adenocarcinoma. Oncotarget 2016;7:38876-83. https://doi.org/10.18632/oncotarget.9113 10.18632/oncotarget.9113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qu X, Ben Q, Jiang Y. Consumption of red and processed meat and risk for esophageal squamous cell carcinoma based on a meta-analysis. Ann Epidemiol 2013;23:762-70. https://doi.org/10.1016/j.annepidem.2013.09.003 10.1016/j.annepidem.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Tong Y, Yang C, et al. Consumption of hot beverages and foods and the risk of esophageal cancer: a meta-analysis of observational studies. BMC Cancer 2015;15:449. https://doi.org/10.1186/s12885-015-1185-1 10.1186/s12885-015-1185-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu C, Wang Z, Song X, et al. Joint analysis of three genome-wide association studies of esophageal squamous cell carcinoma in Chinese populations. Nat Genet 2014;46:1001-6. https://doi.org/10.1038/ng.3064 10.1038/ng.3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lordick F, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v50-7. https://doi.org/10.1093/annonc/mdw329 10.1093/annonc/mdw329 [DOI] [PubMed] [Google Scholar]
- 27.Van Hagen P, Hulshof MC, Van Lanschot JJ, et al. Preoperative chemioradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. https://doi.org/10.1056/NEJMoa1112088 10.1056/NEJMoa1112088 [DOI] [PubMed] [Google Scholar]
- 28.Shapiro J, Van Lanschot JJ, Hulshof MC, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. https://doi.org/10.1016/S1470-2045(15)00040-6 10.1016/S1470-2045(15)00040-6 [DOI] [PubMed] [Google Scholar]
- 29.Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part I. Esophagus 2017;14:37-65. https://doi.org/10.1007/s10388-016-0556-2 10.1007/s10388-016-0556-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osborn NK, Keate RF, Trastek VF, et al. Verrucous carcinoma of the esophagus: clinicopathophysiologic features and treatment of a rare entity. Dig Dis Sci 2003;48: 465-74. https://doi.org/10.1023/a:1022572229285 10.1023/a:1022572229285 [DOI] [PubMed] [Google Scholar]
- 31.Al-Shoha M, Nadeem U, George N, et al. Verrucous carcinoma of the esophagus-remains a diagnostic enigma. Am J Gastroenterol 2018;113:919-21. https://doi.org/10.1038/s41395-018-0065-0 10.1038/s41395-018-0065-0 [DOI] [PubMed] [Google Scholar]
- 32.Tripathi M, Swanson PE. Rare tumors of esophageal squamous mucosa. Ann N Y Acad Sci 2016;1381:122-32. https://doi.org/10.1111/nyas.13108 10.1111/nyas.13108 [DOI] [PubMed] [Google Scholar]
- 33.Lyomasa S, Kato H, Tachimori Y, et al. Carcinosarcoma of the esophagus: a twenty-case study. Jpn Clin Oncol 1990;20:99-106. [PubMed] [Google Scholar]
- 34.Raza MA, Mazzara PF. Sarcomatoid carcinoma of esophagus. Arch Pathol Lab Med 2011;135:945-8. https://doi.org/10.1043/2010-0074-RSR.1 10.1043/2010-0074-RSR.1 [DOI] [PubMed] [Google Scholar]
- 35.Bellizzi AM, Woodford RL, Moskauk CA, et al. Basaloid squamous cell carcinoma of the esophagus: assessment of high-risk human papillomavirusand realted molecular markers. Am J Surg Pathol 2009;33:1608-14. https://doi.org/10.1097/PAS.0b013e3181b46fd4 10.1097/PAS.0b013e3181b46fd4 [DOI] [PubMed] [Google Scholar]
- 36.Lin DC, Wang MR, Koeffler HP. Genomic and epigenomic aberrations in esophageal squamous cell carcinoma and implications for patients. Gastroenterology 2018;154:374-89. https://doi.org/10.1053/j.gastro.2017.06.066 10.1053/j.gastro.2017.06.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walker RC, Underwood TJ. Molecular pathways in the development and treatment of oesophageal cancer. Best Pract Res Clin Gastroenterol 2018;36-37:9-15. doi 10.1016/j.bpg.2018.11.013 10.1016/j.bpg.2018.11.013 [DOI] [PubMed] [Google Scholar]
- 38.Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015;517:576-82. https://doi.org/10.1038/nature14129 10.1038/nature14129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012;489:519-25. https://doi.org/10.1038/nature11404 10.1038/nature11404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Odze RD. Diagnosis and grading of dysplasia in Barrett’s oesophagus. J Clin Pathol 2006;59:1029-38. https://doi.org/10.1136/jcp.2005.035337 10.1136/jcp.2005.035337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voltaggio L, Montgomery EA, Lam-Himlin D. A clinical and histopathologic focus on Barrett esophagus and Barrett-related dysplasia. Arch Pathol Lab Med 2011;135:1249-60. https://doi.org/10.5858/arpa.2011-0019-RA 10.5858/arpa.2011-0019-RA [DOI] [PubMed] [Google Scholar]
- 42.Lomo LC, Blount PL, Sanchez CA, et al. Crypt dysplasia with surface maturation: a clinical, pathologic, and molecular study of a Barrett’s esophagus cohort. Am J Surg Pathol 2006;30:423-35. https://doi.org/10.1097/00000478-200604000-00001 10.1097/00000478-200604000-00001 [DOI] [PubMed] [Google Scholar]
- 43.Coco DP, Goldblum JR, Hornick JL, et al. Interobserver variability in the diagnosis of crypt dysplasia in Barret Esophagus. Am J Surg Pathol 2011;35:45-54. https://doi.org/10.1097/PAS.0b013e3181ffdd14 10.1097/PAS.0b013e3181ffdd14 [DOI] [PubMed] [Google Scholar]
- 44.Rugge M, Correa P, Dixon MF, et al. Gastric Dysplasia. The Padova International Classification. Am J Surg Pathol 2000;24:167-76. https://doi.org/10.1097/00000478-200002000-00001 10.1097/00000478-200002000-00001 [DOI] [PubMed] [Google Scholar]
- 45.Anaparthy R, Sharma P. Progression of Barrett oesophagus: role of endoscopic and histological predictors. Nat Rev Gastroenterol Hepatol 2014;11:525-34. https://doi.org/10.1038/nrgastro.2014.69 10.1038/nrgastro.2014.69 [DOI] [PubMed] [Google Scholar]
- 46.Hardikar S, Onstad L, Blount PL, et al. The role of tobacco, alcohol, and obesity in neoplastic progression to esophageal adenocarcinoma: a prospective study of Barrett’s esophagus. PLoS ONE 2013;8:e52192. https://doi.org/10.1371/journal.pone.0052192 10.1371/journal.pone.0052192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corley DA, Kubo A, Levin TR, et al. Race, ethnicity, sex and temporal differences in Barrett’s oesophagus diagnosis: a large community-based study, 1994-2006. Gut 2009;58:182-8. https://doi.org/10.1136/gut.2008.163360 10.1136/gut.2008.163360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anaparthy R, Gaddam S, Kanakadandi V, et al. Association between length of Barrett’s esophagus and risk of high-grade dysplasia or adenocarcinoma in patients without dysplasia. Clin Gastroenterol Hepatol 2013;11:1430-6. https://doi.org/10.1016/j.cgh.2013.05.007 10.1016/j.cgh.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 49.Parasa S, Venalaganti S, Gaddam S, et al. , Development and validation of a model to determine risk of progression of Barret’s Esophagus to Neoplasia. Gastroenterology 2018;154:1282-9. https://doi.org/10.1053/j.gastro.2017.12.009 10.1053/j.gastro.2017.12.009 [DOI] [PubMed] [Google Scholar]
- 50.Desai TK, Krishnan K, Samala N, et al. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett’s oesophagus: a meta-analysis. Gut 2012;61:970-6. https://doi.org/10.1136/gutjnl-2011-300730 10.1136/gutjnl-2011-300730 [DOI] [PubMed] [Google Scholar]
- 51.Singh S, Manickam P, Amin AV, et al. Incidence of esophageal adenocarcinoma in Barrett’s esophagus with low-grade dysplasia: a systematic review and meta-analysis. Gastrointest Endosc 2014;79:897-909. https://doi.org/10.1016/j.gie.2014.01.009 10.1016/j.gie.2014.01.009 [DOI] [PubMed] [Google Scholar]
- 52.Rastogi A, Puli S, El-Serag HB, et al. Incidence of esophageal adenocarcinoma in patients with Barrett’s esophagus and high-grade dysplasia: a meta-analysis. Gastrointest Endosc 2008;67:394-8. https://doi.org/10.1016/j.gie.2007.07.019 10.1016/j.gie.2007.07.019 [DOI] [PubMed] [Google Scholar]
- 53.Curvers WL, Ten Kate FJ, Krishnadath KK, et al. Low-grade dysplasia in Barrett’s esophagus: overdiagnosed and underestimated. Am J Gastroenterol. 2010;105:1523-30. https://doi.org/10.1038/ajg.2010.171 10.1038/ajg.2010.171 [DOI] [PubMed] [Google Scholar]
- 54.Duits LC, Phoa KN, Curvers WL, et al. Barrett’s oesophagus patients with low-grade dysplasia can be accurately risk-stratified after histological review by an expert pathology panel. Gut 2015;64:700-6. https://doi.org/10.1136/gutjnl-2014-307278 10.1136/gutjnl-2014-307278 [DOI] [PubMed] [Google Scholar]
- 55.Horvath B, Singh P, Xie H, et al. Risk for esophageal neoplasia in Barrett’s esophagus patients with mucosal changes indefinite for dysplasia. J Gastroenterol Hepatol 2015;30:262-7. https://doi.org/10.1111/jgh.12696 10.1111/jgh.12696 [DOI] [PubMed] [Google Scholar]
- 56.van der Wel MJ, Coleman HG, Bergman JJGHM, et al. Histopathologist features predictive of diagnostic concordance at expert level among a large international sample of pathologists diagnosing Barrett’s dysplasia using digital pathology. Gut 2020;69:811-22. https://doi.org/10.1136/gutjnl-2019-318985 10.1136/gutjnl-2019-318985 [DOI] [PubMed] [Google Scholar]
- 57.van der Wel MJ, Duits LC, Pouw RE, et al. Improved diagnostic stratification of digitized Barrett’s oesophagus biopsies by p53 immunohistochemical staining. Histopathology 2018;72:1015-23. https://doi.org/10.1111/his.13462 10.1111/his.13462 [DOI] [PubMed] [Google Scholar]
- 58.Tokuyama M, Geisler D, Deitrick C, et al. Use of p53 immunohistochemistry in conjunction with routine histology improves risk stratification of patients with Barrett’s oesophagus during routine clinical care. Histopathology 2020. Online ahead of print. https://doi.org/10.1111/his.14143 10.1111/his.14143 [DOI] [PubMed] [Google Scholar]
- 59.Brown IS, Whiteman DC, Lauwers GY. Foveolar type dysplasia in Barrett esophagus.. Mod Pathol 2010;23:834-43. https://doi.org/10.1038/modpathol.2010.59 10.1038/modpathol.2010.59 [DOI] [PubMed] [Google Scholar]
- 60.Khor TS, Alfaro EE, Ooi EM, et al. Divergent expression of MUC5AC, MUC6, MUC2, CD10, and CDX-2 in dysplasia and intramucosal adenocarcinomas with intestinal and foveolar morphology: is this evidence of distinct gastric and intestinal pathways to carcinogenesis in Barrett Esophagus? Am J Surg Pathol 2012;36:331-42. https://doi.org/10.1097/PAS.0b013e31823d08d6 10.1097/PAS.0b013e31823d08d6 [DOI] [PubMed] [Google Scholar]
- 61.Mahajan D, Bennett AE, Liu X, et al. Grading of Gastric Foveolar-Type Dysplasia in Barrett’s Esophagus. Mod Pathol 2010;23:1-11. https://doi.org/10.1038/modpathol.2009.147 10.1038/modpathol.2009.147 [DOI] [PubMed] [Google Scholar]
- 62.Edgren G, Adami HO, Weiderpass E, et al. A global assessment of the oesophageal adenocarcinoma epidemic. Gut 2013;62:1406-14. https://doi.org/10.1136/gutjnl-2012-302412 10.1136/gutjnl-2012-302412 [DOI] [PubMed] [Google Scholar]
- 63.Hur C, Miller M, Kong CY, et al. Trends in esophageal adenocarcinoma incidence and mortality. Cancer 2013;119:1149-58. https://doi.org/10.1002/cncr.27834. 10.1002/cncr.27834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Orosey M, Amin M, Cappell MS. A 14-Year Study of 398 Esophageal Adenocarcinomas diagnosed among 156,256 EGDS performed at two large hospitals: an inlet patch is proposed as a significant risk factor for proximal esophageal adenocarcinoma. Dig Dis Sci 2018;63:452-65. https://doi.org/10.1007/s10620-017-4878-2 10.1007/s10620-017-4878-2 [DOI] [PubMed] [Google Scholar]
- 65.Siewert JR, Stein HJ. Carcinoma of the cardia: carcinoma of the gastroesophageal junction - classification, pathology and extent of resection. Dis Esophagus 1996;9:173-82 [Google Scholar]
- 66.Liu K, Feng F, Chen XZ, et al. Comparison between gastric and esophageal classification system among adenocarcinomas of esophagogastric junction according to AJCC 8th edition: a retrospective observational study from two high-volume institutions in China. Gastric Cancer 2019;22:506-17. https://doi.org/10.1007/s10120-018-0890-2 10.1007/s10120-018-0890-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bleaney CW, Barrow M, Hayes S, et al. The relevance and implications of signet-ring cell adenocarcinoma of the oesophagus. J Clin Pathol 2018;71:201-6. https://doi.org/10.1136/jclinpath-2017-204863 10.1136/jclinpath-2017-204863 [DOI] [PubMed] [Google Scholar]
- 68.Wu J, Pan YM, Wang TT, et al. Endotherapy versus surgery for early neoplasia in Barrett’s esophagus: a meta-analysis. Gastrointest Endosc 2014;79:233-41.e2. https://doi.org/10.1016/j.gie.2013.08.005 10.1016/j.gie.2013.08.005 [DOI] [PubMed] [Google Scholar]
- 69.Sharma P, Shaheen NJ, Katzka D, et al. AGA clinical practice update on endoscopic treatment of Barrett’s esophagus with dysplasia and/or early cancer: expert review. Gastroenterology 2020;158:760-9. https://doi.org/10.1053/j.gastro.2019.09.051 10.1053/j.gastro.2019.09.051 [DOI] [PubMed] [Google Scholar]
- 70.Bollschweiler E, Baldus SE, Schroder W, et al. High rate of lymph-node metastasis in submucosal esophageal squamous-cell carcinomas and adenocarcinomas. Endoscopy 2006;38:149-56. https://doi.org/10.1055/s-2006-924993 10.1055/s-2006-924993 [DOI] [PubMed] [Google Scholar]
- 71.Manner H, May A, Pech O, et al. Early Barrett’s carcinoma with “low-risk” submucosal invasion: long-term results of endoscopic resection with a curative intent. Am J Gastroenterol. 2008,103:2589-97. https://doi.org/10.1111/j.1572-0241.2008.02083.x 10.1111/j.1572-0241.2008.02083.x [DOI] [PubMed] [Google Scholar]
- 72.Abraham SC, Krasinskas AM, Correa AM, et al. Duplication of the muscularis mucosae in Barrett esophagus: an under-recognized feature and its implication for staging of adenocarcinoma. Am J Surg Pathol 2007;31:1719-25. https://doi.org/10.1097/PAS.0b013e318093e3bf. 10.1097/PAS.0b013e318093e3bf [DOI] [PubMed] [Google Scholar]
- 73.Hornick JL, Farraye FA, Odze RD. Prevalence and significance of prominent mucin pools in the esophagus post neoadjuvant chemoradiotherapy for Barrett’s-associated adenocarcinoma. Am J Surg Pathol 2006;30:28-35. https://doi.org/10.1097/01.pas.0000174011.29816.fa 10.1097/01.pas.0000174011.29816.fa [DOI] [PubMed] [Google Scholar]
- 74.Klevebro F, Tsekrekos A, Low D, et al. Relevant issues in tumor regression grading of histopathological response to neoadjuvant treatment in adenocarcinomas of the esophagus and gastroesophageal junction. Dis Esophagus 2020;33:doaa005. https://doi.org/10.1093/dote/doaa005 10.1093/dote/doaa005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mandard AM, Dalibard F, Mandard al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994;73:2680-6. https://doi.org/10.1002/1097-0142(19940601)73:11<2680::aid-cncr2820731105>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 76.Japanese Society for Esophageal Diseases. Guidelines for clinical and pathologic studies on carcinoma of the esophagus, ninth edition: preface, general principles, part I. Esophagus 2004;1:61-88. [Google Scholar]
- 77.Chirieac LR, Swisher SG, Ajani JA, et al. Post-therapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer 2005;103:1347-55. https://doi.org/10.1002/cncr.20916 10.1002/cncr.20916 [DOI] [PubMed] [Google Scholar]
- 78.Swisher SG, Hofstetter W, Wu TT, et al. Proposed revision of the esophageal cancer staging system to accommodate pathologic response following preoperative chemoradiation. Ann Surg 2005;241:810-20. https://doi.org/10.1097/01.sla.0000161983.82345.85 10.1097/01.sla.0000161983.82345.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schneider PM, Baldus SE, Metzger R, et al. Histomorphologic tumor regression and lymph node metastases determine prognosis following neoadjuvant radiochemotherapy for esophageal cancer: implications for response classification. Ann Surg 2005;242:684-92. https://doi.org/10.1097/01.sla.0000186170.38348.7b 10.1097/01.sla.0000186170.38348.7b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sawada G, Moon J, Saito A, et al., A case of adenoid cystic carcinoma of the esophagus. Surg Case Rep, 2015;1:119. https://doi.org/10.1186/s40792-015-0122-5 10.1186/s40792-015-0122-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen SB, Weng HR, Wang G, et al. Primary adenosquamous carcinoma of the esophagus. World J Gastroenterol 2013;19:8382-90. https://doi.org/10.3748/wjg.v19.i45.8382 10.3748/wjg.v19.i45.8382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Evans M, Liu Y, Chen C, et al. Adenosquamous carcinoma of the esophagus: An NCDB-Based Investigation on comparative features and overall survival in a rare tumor. Oncology 2017;93:336-42. https://doi.org/10.1159/000466699 10.1159/000466699 [DOI] [PubMed] [Google Scholar]
- 83.Chen S, Chen Y, Yang J, et al. Primary Mucoepidermoid Carcinoma of the Esophagus J Thorac Oncol 2011;6:1426-31. https://doi.org/10.1097/JTO.0b013e31821cfb96 10.1097/JTO.0b013e31821cfb96 [DOI] [PubMed] [Google Scholar]
- 84.Singhi AD, Seethala RR, Nason K, et al. Undifferentiated Carcinoma of the Esophagus: A Clinicopathological Study of 16 Cases. Hum Path 2015;46:366-75. https://doi.org/10.1016/j.humpath.2014.11.021 10.1016/j.humpath.2014.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Babar L, Kosovec JE, Jahangiri V, et al. Prognostic immune markers for recurrence and survival in locally advanced esophageal adenocarcinoma. Oncotarget 2019;10:4546-55. https://doi.org/10.18632/oncotarget.27052 10.18632/oncotarget.27052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jacobsen F, Hohsar J, Gebauer F, et al. Loss of p16 and high Ki67 labeling index is associated with poor outcome in esophageal carcinoma. Oncotarget 2020;11:1007-16. https://doi.org/10.18632/oncotarget.27507 10.18632/oncotarget.27507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fassan M, Mastracci L, Grillo F, et al. Early HER2 dysregulation in gastric and oesophageal carcinogenesis. Histopathology 2012;61:769-76. https://doi.org/10.1111/j.1365-2559.2012.04272.x 10.1111/j.1365-2559.2012.04272.x [DOI] [PubMed] [Google Scholar]
- 88.Rüschoff J, Dietel M, Baretton G, et al. HER2 diagnostics in gastric cancer-guideline validation and development of standardized immunohistochemical testing. Virchows Arch. 2010;457:299-307. https://doi.org/10.1007/s00428-010-0952-2 10.1007/s00428-010-0952-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grillo F, Fassan M, Sarocchi F, et al. HER2 heterogeneity in gastric/gastroesophageal cancers: from benchside to practice. World J Gastroenterol 2016;22:5879-87. https://doi.org/10.3748/wjg.v22.i26.5879 10.3748/wjg.v22.i26.5879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grillo F, Fassan M, Ceccaroli C, et al. The reliability of endoscopic biopsies in assessing HER2 status in gastric and gastroesophageal junction cancer: a study comparing biopsies with surgical samples. Transl Oncol 2013;6:10-6. https://doi.org/10.1593/tlo.12334 10.1593/tlo.12334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gullo I, Grillo F, Molinaro L, et al. Minimum biopsy set for HER2 evaluation in gastric and gastro-esophageal junction cancer. Endosc Int Open 2015;3:E165-70. https://doi.org/10.1055/s-0034-1391359 10.1055/s-0034-1391359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Farris AB, 3rd, Demicco EG, Le LP, et al. Clinicopathologic and molecular profiles of microsatellite unstable Barrett Esophagus-associated adenocarcinoma. Am J Surg Pathol 2011;35:647-55. https://doi.org/10.1097/PAS.0b013e31820f18a2 10.1097/PAS.0b013e31820f18a2 [DOI] [PubMed] [Google Scholar]
- 93.van Velzen MJM, Derks S, van Grieken NCT, et al. MSI as a predictive factor for treatment outcome of gastroesophageal adenocarcinoma. Cancer Treat Rev 2020;86:102024. https://doi.org/10.1016/j.ctrv.2020.102024 10.1016/j.ctrv.2020.102024 [DOI] [PubMed] [Google Scholar]
- 94.Cancer Genome Atlas Research Network. Integrated genomic characterization of oesophageal carcinoma. Nature 2017;541:169-75. https://doi.org/10.1038/nature20805 10.1038/nature20805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Secrier M, Li X, de Silva N, et al. Mutational signatures in esophageal adenocarcinoma define etiologically distinct subgroups with therapeutic relevance. Nat Genet 2016;48:1131-41. https://doi.org/10.1038/ng.3659 10.1038/ng.3659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hassanabad AF, Chehade R, Breadner D, et al. Esophageal carcinoma: towards targeted therapies. Cell Oncol (Dordr) 2020;43:195-209. https://doi.org/10.1007/s13402-019-00488-2 10.1007/s13402-019-00488-2 [DOI] [PubMed] [Google Scholar]


