Abstract
Background
Soy products are associated with many beneficial health consequences, but their effects on the human intestinal microbiome are poorly characterized.
Objectives
To identify the changes in the oral and fecal microbiome in lean and obese participants due to consumption of Q-CAN®, and to assess the expected consequences of these changes based on the published literature.
Methods
Prospective study of lean (10) and obese (9) participants consuming Q-CAN® twice daily for 4 weeks with 8 weeks follow-up. Microbial DNA was extracted from saliva and stool samples, amplified against the V4 region of the 16S ribosomal RNA gene and data analyzed using QIIME 1.9.1 bioinformatics. Four hundred forty-four samples were collected in total, 424 of which were productive and yielded good quality data.
Results
STOOL. In the lean population Bifidobacteria and Blautia show a significant increase while taking Q-CAN®, and there was a trend for this in the obese population. ORAL. There were relatively fewer major changes in the oral microbiome with an increase in the family Veillonellaceae in the lean population while on Q-CAN®.
Conclusion
Q-CAN® consumption induced a number of significant changes in the fecal and oral microbiome. Most notably an increase in the stool microbiome of Bifidobacteria and Blautia, both of which are associated with positive health benefits, and in the saliva an increase in Veillonellaceae.
Trial registration
This trial was registered with Clinicaltrials.gov on January 14th 2016.
ClinicalTrials.gov Identifier: NCT02656056
Supplementary Information
The online version contains supplementary material available at 10.1186/s40795-021-00408-4.
Keywords: Soy, Gut microbiome, Oral microbiome, Commensals, Obesity
Background
Soybeans have long been recognized as sources of high-quality protein and beneficial lipids with several health benefits [1]. Consumption of fermented soybean foods is associated with many health benefits including reduced risks of type 2 diabetes (T2D) and blood pressure [2–4], and improved plasma triglyceride levels [5]. However, the mechanisms through which fermented soy may exert the above effects are unknown. One possibility is that soy nutrients are altering the microbiome of the gastrointestinal system, which is subsequently having beneficial effects. There is a considerable amount of data on the effects of soy nutrients on the microbiome of animals including mice, chickens and pigs [6, 7]. There has been great interest in the role of soy on the human gastrointestinal microbiome but there has been very limited data and using techniques that can produce results biased based on pre-existing assumptions [8–11]. To overcome these limitations, we were interested in obtaining an unbiased data set of the effects of soy products on the human gastrointestinal microbiome using 16S RNA sequencing.
Q-CAN® is a fermented soybean beverage and has been used for over 30 years as a nutritional food supplement to aid in the recovery from a wide range of conditions and is also taken during health. The beneficial effects of Q-CAN® fermented soy may be attributed to the combination of isoflavones such as Genistein and Daidzein, amino acids, trace elements, minerals, bioactive peptides and branched-chain fatty acids. Among the several putative ingredients of Q-CAN®, isoflavones were shown to exert several health benefits on the host via alterations in key bacteria that are associated with a beneficial effect [12]. In line with this, consumption of fermented soy milk was previously shown to increase healthy microbiota, such as Bifidobacteria and Lactobacilli, and to decrease the pathogenic ones, such as Clostridia, in healthy individuals [8, 10]. The possible restoration of the gut microbiome upon fermented soy consumption is of particular significance given that altered gut microbiome has been shown by many studies to contribute to the development and progression of cardiometabolic disorders, such as atherosclerosis, obesity, and T2D [13], NAFLD [14] and cancer [15, 16]. Q-CAN® may restore the gut and oral microbiome and through this mechanism exert its beneficial effects.
Here, we hypothesize that Q-CAN® exerts beneficial effects through reduction of pathogenic bacteria and increase of beneficial ones. Given that obesity is increasing dramatically [17] we examined a lean and an obese population and assessed the consequences of these changes based on the published literature.
Methods
Aim
The aim of the study was to establish the changes induced in the oral and intestinal microbiome by Q-CAN®.
Participants
This study was approved by the Human Investigation Committee of Yale University. Subjects were recruited mostly from the campus of Yale University and had no history of abdominal surgeries (excluding cholecystectomy, appendectomy, hysterectomy, hernia repair), inflammatory bowel disease (e.g., ulcerative colitis, Crohn’s disease), GI bleeding, radiation proctitis or other known poorly controlled medical conditions that could interfere with bowel function, acute or chronic diseases, allergy to soy, soy derivatives, milk protein, alcohol use disorder, anorexia nervosa, autoimmune disease, bulimia, celiac disease, chronic infections and illicit drug use. Other exclusion criteria were major changes in dietary habits in the past 6 months, use of proton pump inhibitors, antibiotics, probiotics, laxatives (chronic use), anticholinergics, or systemic corticosteroid (within 3 months of enrollment) and medicines affected by modest dietary changes (including but not limited to, warfarin, immunosuppressives), pregnancy or history of pregnancy within the past 6 months or intent to get pregnant during study period, use of tobacco (cigarettes, smokeless tobacco, cigars, pipes) within past 30 days. Before executing this study, written informed consent was acquired from all participants. Totally 19 participants participated at the beginning. Participants were advised to maintain their normal life style during the course of the study.
Q-CAN® composition
Q-CAN® contained 8% fermented soy powder in water with 290.12 mg of total soy isoflavones in 240 ml and 3.6% protein. The isoflavones daidzin, genistin, daidzein, and genistein were all present at greater than 25 mg in each bottle. A profile of over 300 herbicides, pesticides and fungicides was negative and no chemical residual solvents tested were above the limit of quantification. Lead, mercury, arsenic, and cadmium were all below the detectable limit (5 to 10 parts per billion). 24-month serial assessment (accelerated and shelf) of Q-CAN® determined that soy isoflavones did not decrease in potency and microbiology analysis revealed no contamination.
Study design
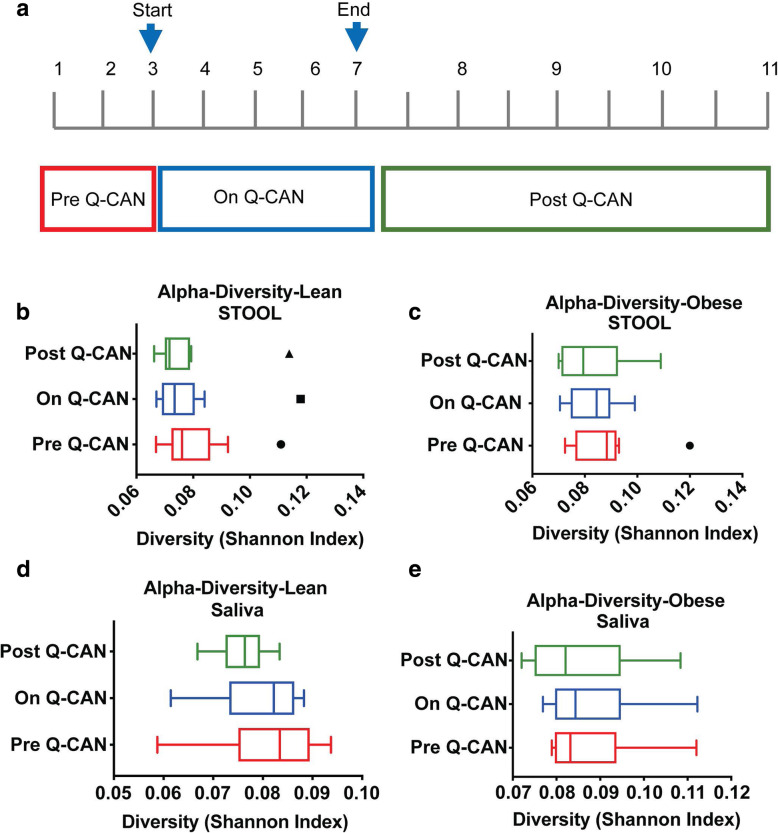
Twenty participants including 10 lean (3 males, 7 females, mean age 32 years, mean BMI 22 Kg/m2 ) and 10 obese individuals (7 males, 3 females, mean age 45 years, mean BMI 34 Kg/m2) were enrolled in this study. The participants performed in total 11 visits (Fig. 1a). The first 3 visits take place with one-week intervals and participants provided stool and saliva without any intervention. After the 3rd visit they started the Q-CAN® consumption (237 ml) twice daily for 4 weeks until visit 7. Every week stool and saliva were collected. At the 7th visit they stopped the Q-CAN® consumption and were monitored for 8 weeks post Q-CAN®. They gave stool and saliva samples every 2 weeks in the post Q-CAN® period. The 8-week follow up was to identify how sustained the changes induced by Q-CAN® were after consumption ceased. Three samples (2 saliva and 1 stool) were collected per visit. Saliva sample 1 was collected first thing in the morning when the participants awoke, and before brushing their teeth, eating or drinking. The stool sample was collected during the day and the saliva sample 2 was collected right after the collection of the stool sample. Saliva sample 1 was used for generating microbiome data.
Fig. 1.
a Depiction of the time-frame of Q-CAN® consumption or withdrawal. b-d Shannon Diversity Index of intestinal or oral microbiome is not altered upon Q-CAN® consumption or withdrawal in lean or obese people. The results are the average of 3 visits in pre Q-CAN® group, 4 visits in on Q-CAN® group and 4 visits in post Q-CAN® group for each participant. Obese (n = 9 participants), Lean (n = 10 participants). The data are presented as Tukey box plots showing the median values
Sample collection and microbiome analysis
The saliva and stool samples were kept frozen at -80 °C until their DNA isolation. For saliva samples collection the OMNI gene-oral 501 tube was used and for stool the OMNI gene GUT OMR-200 tube. About 1 g of stool was collected from each subject in each of the 11 visits. DNA was extracted from all the samples (both saliva and stool samples from all 11 visits) according to the manufacturer instructions of the OM-501 and OMR-200 kits (DNA Genotek). All purified DNA samples were quantified via nanodrop and/or Qubit measurements. Acceptable Qubit value was > 20 ng/μl. Extracted microbial DNA from saliva and stool samples was amplified against the V4 region of the 16S ribosomal RNA gene. Raw DNA sequencing data was analyzed with the QIIME 1.9.1 bioinformatics pipeline. Samples producing > 5000 reads were considered for analysis, and the cutoff abundance was 0.01%. Statistical validation was performed using the SAS software package to calculate Least Squares Means and group difference of LSM. Four hundred forty samples were collected in total, 424 of which were productive and yielded good quality data.
The Shannon Diversity Index was calculated based on the following formula:
where:
H = the Shannon diversity index.
Pi = fraction of the entire population made up of species i.
S = numbers of species encountered.
∑ = sum from species 1 to species S.
To calculate the index, the number of individuals of species found in our samples were divided by the total number of individuals of all species. This is the Pi. Afterwards, the Pi was multiplied with its natural log (P1 * ln P1). At the end, the sum of all the - (Pi * ln Pi) products is the value H (Shannon Diversity Index).
Statistical analysis
All data are presented as the mean ± SEM. P < 0.05 was the level of significance. Repeated measure analyses was done on outcomes at each level, taking into account the correlation on observations occurred among the same patient. Treatment stage, gender and age were entered as fixed effects. Unstructured covariance was used. Analyses was done on each BMI level. The relative abundance of bacteria that were present at 50% of participants was also presented at the level of phylum, family, genus and species as a heat maps with hierarchical clustering using Qlucore.
Results
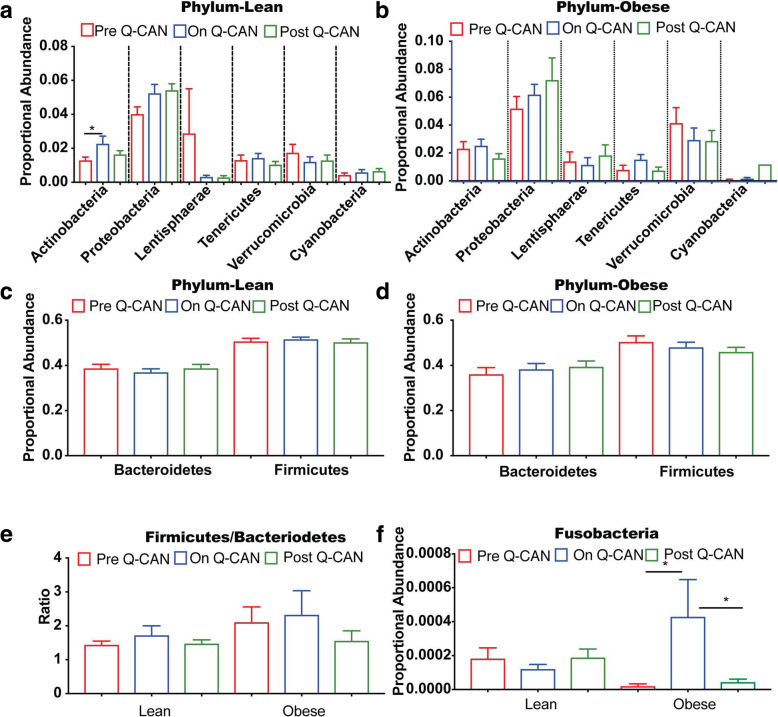
Saliva and stool were collected from healthy, lean or obese individuals before Q-CAN® consumption (Pre Q-CAN® group), during Q-CAN® consumption (On Q-CAN® group) and after the cessation of Q-CAN® consumption (Post Q-CAN® group) (Fig. 1a). Q-CAN® consumption had no effect on alpha diversity of stool or saliva bacteria species in both obese and lean participants (Fig. 1b-e). Consumption of fermented soy product significantly increased stool Actinobacteria phylum populations in lean but not obese participants (Fig. 2a and b). The highest abundant phylum populations (Firmicutes and Bacteriodetes) and their ratio were not affected by Q-CAN® consumption or withdrawal (Fig. 2c-e). On the other hand, in the low abundant populations, Q-CAN® significantly increased Fusobacteria in obese participants (Fig. 2f) that returned to pre-QCAN levels upon its withdrawal. There were no other changes at the phylum level Supplementary (Figure S1).
Fig. 2.
Intestinal microbiome analysis at the level of Phylum. a-f Bacteria at Phylum level in both lean and obese shows that only Actinobacteria and Fusobacteria are altered upon Q-CAN® consumption. e The ratio of Firmicutes/Bacteroidetes has a trend for increase in obese compared to lean ones. The results are the average of 3 visits in pre Q-CAN® group, 4 visits in on Q-CAN® group and 4 visits in post Q-CAN® group for each participant. Obese (n = 9 participants), Lean (n = 10 participants). The data are presented as median with SEM, *p < 0.05
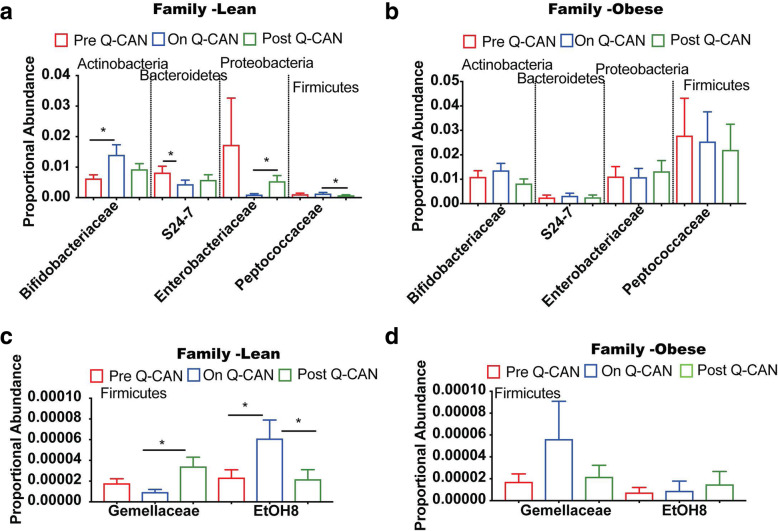
At the level of Family, QCAN consumption in the lean group was associated with decreased abundance of S24–7, Enterobacteriaceae and Gemellaceae families (Fig. 3a, c). The decrease of the S24–7 family was retained upon Q-CAN® withdrawal but not in Enterobacteriaceae and Gemellaceae families (Fig. 3a, c) that were significantly increased upon Q-CAN® withdrawal. Q-CAN® had opposite effect on the Gemellaceae family in obese group compared to lean ones (Fig. 3a, d). Several other changes were also observed in non-identified bacteria in lean and obese upon QCAN consumption or withdrawal (Supplementary Figure 2; heat map).
Fig. 3.
Intestinal microbiome analysis at the level of Family. A-D) Bacteria at Family level in both lean and obese shows that only Bifidobacteriaea, S24–7 and EtOH8 are altered upon Q-CAN® consumption. The results are the average of 3 visits in pre Q-CAN® group, 4 visits in on Q-CAN® group and 4 visits in post Q-CAN® group for each participant. Obese (n = 9 participants), Lean (n = 10 participants). The data are presented as median with SEM, *p < 0.05
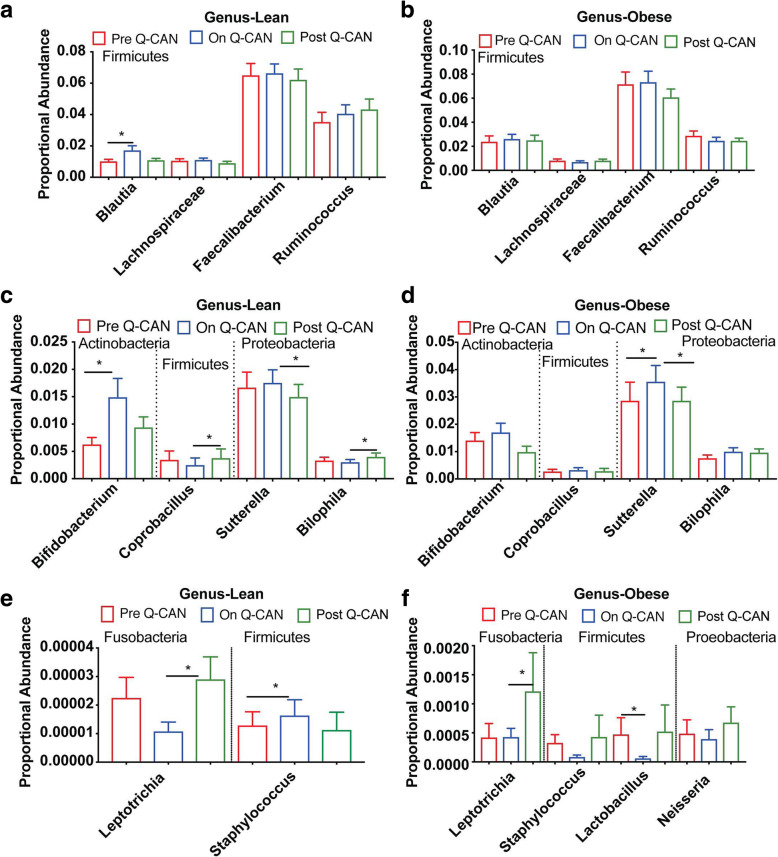
At the genus level, the most abundant bacteria genera were not affected by Q-CAN® consumption. In the less abundant bacteria genera, Q-CAN® consumption increased the levels of Blautia and Bifidobacterium in lean participants (Fig. 4a, c). In obese, only Sutterella (Fig. 4d) was increased significantly upon QCAN consumption, and this was not maintained upon Q-CAN® withdrawal (Fig. 4c, d). All the species that were identified in the different visits are presented also by heat map (Fig. 5; heat map). Each column represents a subject and each row a bacterial taxon. Several other changes were also observed in non-identified genera in lean and obese upon QCAN consumption or withdrawal (Supplementary Figure 3; heat map).
Fig. 4.
Intestinal microbiome analysis at the level of genus. a-f Bacteria distribution at genus level in both lean and obese participants. The levels of Blautia, Bifidobacterium and Staphylococcus genera are altered upon Q-CAN® consumption in lean and the levels of Sutterela and Lactobacillus in obese. The results are the average of 3 visits in pre Q-CAN® group, 4 visits in on Q-CAN® group and 4 visits in post Q-CAN® group for each participant. Obese (n = 9 participants), Lean (n = 10 participants). The data are presented as median with SEM, *p < 0.05
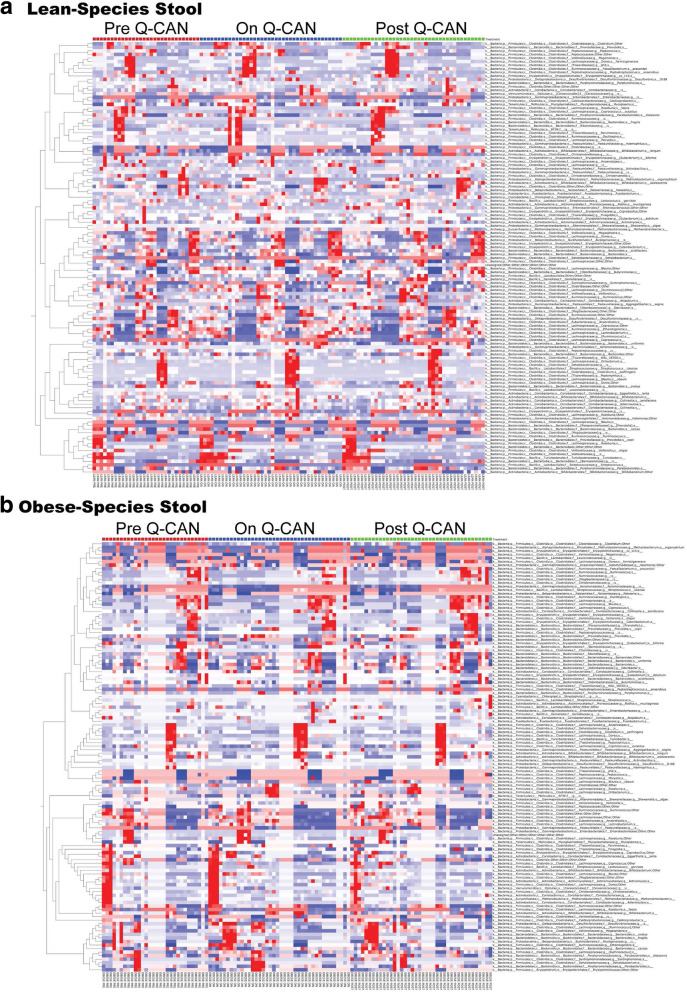
Fig. 5.
Intestinal microbiome analysis at the level of species. a-b Relative abundance of bacterial species is visualized by heat map. Each column represents a subject and each colored row a bacterial taxon. The intensity of the red color represents the highest abundance taxa and the intensity of the blue color the lowest abundance taxa in lean and obese people. The results are the average of 3 visits in pre Q-CAN® group, 4 visits in on Q-CAN® group and 4 visits in post Q-CAN® group for each participant. Obese (n = 9 participants), Lean (n = 10 participants)
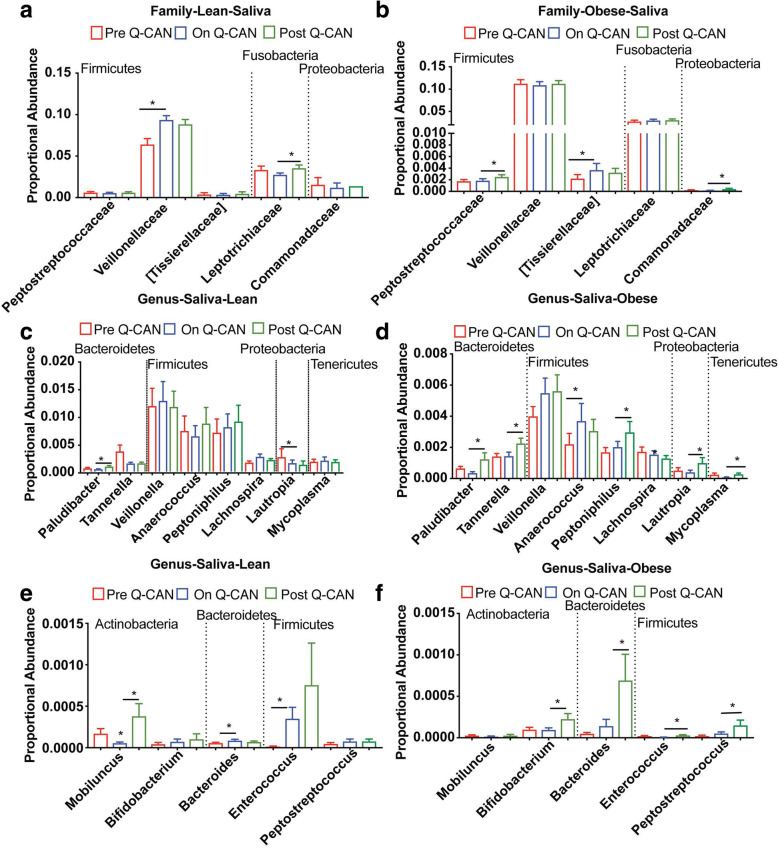
In the saliva samples, fewer changes were observed at the phylum, family and genus level than in stool. Moreover, Q-CAN® consumption affected different oral microbes from the stool ones. At the phylum level there was no effect of Q-CAN® consumption in any of the bacteria in both lean and obese individuals (Supplementary Figure 4; heat map). At the family level, Veillonelaceae (Fig. 6a) was significantly increased during fermented soy consumption in the lean population. In the obese, with the exception of Tisserelaceae that was significantly increased during fermented soy consumption, the Peptrosreptococcaceae and the Commanonadaceae families were not affected during Q-CAN® consumption but were increased in the post Q-CAN® when compared to the on Q-CAN® group (Fig. 6b). All the bacteria at family level that were identified in the different visits are presented also by heat map (Supplementary Figure 5; heat map).
Fig. 6.
Oral microbiome analysis at the level of Family and Genus. a, b Bacteria at Family level in both lean and obese shows increase of Veillonelleae in lean and Tissierallacea in obese upon Q-CAN® consumption. c-f Bacteria distribution at genus level in both lean and obese participants. The results are the average of 3 visits in pre Q-CAN® group, 4 visits in on Q-CAN® group and 4 visits in post Q-CAN® group for each participant. Obese (n = 10 participants), Lean (n = 10 participants). The data are presented as median with SEM, *p < 0.05
At the genus level, several genera were altered upon Q-CAN® consumption in the lean whereas in the obese the most alterations were observed upon Q-CAN® withdrawal. In the lean Lachnospira, Bacteroides and Enterococcus were increased upon Q-CAN® consumption whereas Lautropia and Mobiluncus were decreased (Fig. 6c, e). Plaudibacter was increased only in post Q-CAN® when compared to the on Q-CAN® group in the same population (Fig. 6c). In the obese with the exception of Anaerococcus that was significantly increased during fermented soy consumption, the Plaudibacter, Tennerella, Peptoniphillus, Lautropia, Bifidobacterium, Bacteroides, Enterococcus and Peptosptreptococcus were not affected during Q-CAN® consumption but were increased in the post Q-CAN® when compared to the on Q-CAN® group (Fig. 6d, e). Several other changes were also observed in non-identified genera in lean and obese upon QCAN consumption or withdrawal (Supplementary Figure 6; heat map).
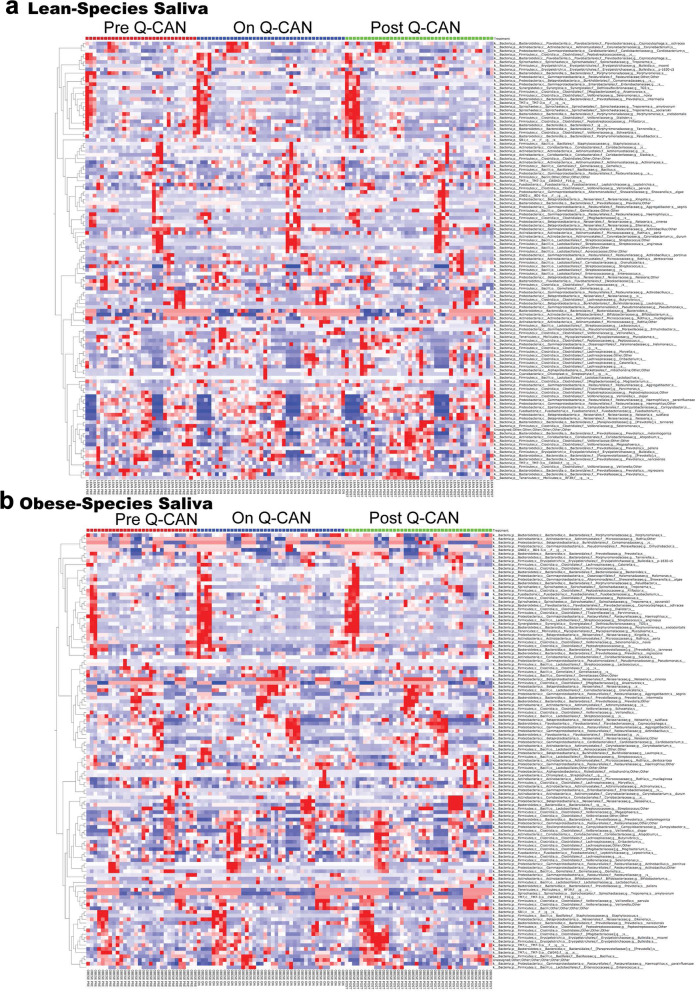
At the level of species, fewer changes were observed and they were only in non-identified bacteria (Fig. 7). All the species that were identified in the different visits are presented also by heat map (Fig. 7; heat map). Each column represents a subject and each row a bacterial taxon.
Fig. 7.
Oral microbiome analysis at the level of Species. a-b Relative abundance of bacterial species is visualized by heat map. Each column represents a subject and each colored row a bacterial taxon. The intensity of the red color represents the highest abundance taxa and the intensity of the blue color the lowest abundance taxa in lean and obese people. The results are the average of 3 visits in pre Q-CAN® group, 4 visits in on Q-CAN® group and 4 visits in post Q-CAN® group for each participant. Obese (n = 10 participants), Lean (n = 10 participants)
All the above changes are summarized in Table 1 where we can see that Q-CAN® consumption, compared to the Pre-QCAN®, group increases several bacteria in lean (n = 8 in stool and n = 1 in saliva) and obese (n = 3 in stool, n = 2 in saliva) participants whereas decreases few of them in lean (n = 2 in stool and n = 1 in saliva) and in obese (n = 2 in stool and n = 0 in saliva) participants. Several bacteria are also altered upon QCAN withdrawal as compared upon QCAN consumption. In the lean 5 bacteria are increased in stool and 5 in saliva whereas 3 bacteria are decreased in stool and 0 in saliva. In the obese 1 bacterium is increased in stool and 13 in saliva whereas 2 bacteria are decreased in stool and 0 in saliva.
Table 1.
Changes in Stool and Saliva Microbiome associated with Consumption of Q-CAN®
| LEAN On vs Pre |
LEAN Post vs On |
OBESE On vs Pre |
OBESE Post vs On |
|
|---|---|---|---|---|
| STOOL | ||||
| Phylum Level | ||||
| Actinobacteria | Increase* | |||
| Fusobacteria | Increase* | Decrease* | ||
| Family Level | ||||
| Firmicutes;c__Clostridia;o__Clostridiales;f__Peptococcaceae | Decrease* | |||
| Firmicutes;c__Bacilli;o__Gemellales;f__Gemellaceae | Decrease | Increase* | ||
| Firmicutes;c__Clostridia;o__Clostridiales;f__EtOH8 | Increase* | Decrease* | ||
| Bacteroidetes;c__Bacteroidia;o__Bacteroidales;f__S24-7 | Decrease* | |||
| Actinobacteria;c__Actinobacteria;o__Bifidobacteriales;f__Bifidobacteriaceae | Increase* | |||
| Proteobacteria;c__Gammaproteobacteria;o__Enterobacteriales;f__Enterobacteriaceae | Decrease | Incerase * | ||
| Genus Level | ||||
| Firmicutes;c__Clostridia;o__Clostridiales;f__Lachnospiraceae;g__Blautia | Increase* | Decrease | ||
| Firmicutes;c__Erysipelotrichi;o__Erysipelotrichales;f__Erysipelotrichaceae;g__Coprobacillus | Increase* | |||
| Firmicutes;c__Bacilli;o__Lactobacillales;f__Lactobacillaceae;g__Lactobacillus | Decrease* | Increase | ||
| Actinobacteria;c__Actinobacteria;o__Bifidobacteriales;f__Bifidobacteriaceae;g__Bifidobacterium | Increase* | Decrease | ||
| Proteobacteria;c__Betaproteobacteria;o__Burkholderiales;f__Alcalige0ceae;g__Sutterella | Decrease* | Increase* | Decrease* | |
| Proteobacteria;c__Deltaproteobacteria;o__Desulfovibrio0les;f__Desulfovibrio0ceae;g__Bilophila | Increase* | |||
| Fusobacteria;c__Fusobacteriia;o__Fusobacteriales;f__Leptotrichiaceae;g__Leptotrichia | Decrease | Increase* | Increase* | |
| Species Level | ||||
| Bifidobacterium species | Increase* | Decrease | ||
| Blautia species | Increase* | Decrease | ||
| Acidaminococcus species | Increase* | Decrease | ||
| Gemellaceae species | Decrease* | Increase* | ||
| Parabacteroidetes species | Decrease* | Increase * | ||
| Comamonadaceae species | Increase * | Decrease | ||
| SALIVA | ||||
| Family Level | ||||
| Firmicutes;c__Clostridia;o__Clostridiales;f__Veillonellaceae | Increase* | |||
| Firmicutes;c__Clostridia;o__Clostridiales;f__Peptostreptococcaceae | Increase * | |||
| Firmicutes;c__Clostridia;o__Clostridiales;f__[Tissierellaceae] | Increase * | |||
| Firmicutes;c__Clostridia;o__Clostridiales;f__Ruminococcaceae | Increase * | |||
| Proteobacteria;c__Alphaproteobacteria;o__Rhizobiales;f__Methylobacteriaceae | Increase * | |||
| Proteobacteria;c__Betaproteobacteria;o__Burkholderiales;f__Comamonadaceae | Increase * | |||
| Fusobacteria;c__Fusobacteriia;o__Fusobacteriales;f__Leptotrichiaceae | Increase * | |||
| Genus Level | ||||
| Firmicutes;c__Bacilli;o__Lactobacillales;f__Enterococcaceae;g__Enterococcus | Increase * | |||
| Firmicutes;c__Clostridia;o__Clostridiales;f__[Tissierellaceae];g__Anaerococcus | Increase * | |||
| Firmicutes;c__Clostridia;o__Clostridiales;f__[Tissierellaceae];g__Peptoniphilus | Increase * | |||
| Firmicutes;c__Clostridia;o__Clostridiales;f__Peptostreptococcaceae;g__Peptostreptococcus | Increase * | |||
| Firmicutes;c__Clostridia;o__Clostridiales;f__Lachnospiraceae;g__Lachnospira | Increase* | |||
| Bacteroidetes;c__Bacteroidia;o__Bacteroidales;f__Bacteroidaceae;g__Bacteroides | Increase* | Increase * | ||
| Bacteroidetes;c__Bacteroidia;o__Bacteroidales;f__Porphyromonadaceae;g__Paludibacter | Incerase * | |||
| Bacteroidetes;c__Bacteroidia;o__Bacteroidales;f__Bacteroidaceae;g__Bacteroides | Increase * | |||
| Bacteroidetes;c__Bacteroidia;o__Bacteroidales;f__Porphyromonadaceae;g__Paludibacter | Increase * | |||
| Bacteroidetes;c__Bacteroidia;o__Bacteroidales;f__Porphyromonadaceae;g__Tannerella | Increase * | |||
| Actinobacteria;c__Actinobacteria;o__Bifidobacteriales;f__Bifidobacteriaceae;g__Bifidobacterium | Increase * | |||
| Actinobacteria;c__Actinobacteria;o__Actinomycetales;f__Actinomycetaceae;g__Mobiluncus | Decrease | Increase* | ||
| Proteobacteria;c__Betaproteobacteria;o__Neisseriales;f__Neisseriaceae;g__Eikenella | Decrease* | Increase * | ||
| Proteobacteria;c__Betaproteobacteria;o__Burkholderiales;f__Burkholderiaceae;g__Lautropia | Increase * | |||
| Proteobacteria;c__Gammaproteobacteria;o__Pseudomonadales;f__Pseudomonadaceae;g__Pseudomonas | Increase * | |||
| Tenericutes;c__Mollicutes;o__Acholeplasmatales;f__Acholeplasmataceae;g__Acholeplasma | Increase * | |||
* denotes statistical significant
Discussion
Fermented soy consumption has been shown to have a number of health benefits, however, the mechanisms through which fermented soy products exert these effects are totally unknown. Here we found that fermented soy beverage Q-CAN® alters the microbiome in lean, and healthy obese individuals, with a number of the changes occurring in a direction expected to improve overall health. In detail, we found that there was no effect on alpha diversity in the microbiome in the stool or saliva. There was alpha diversity however: 1) While taking Q-CAN®, at phylum level, in the stool of lean individuals there was an increase in Actinobacteria, and in obese individuals there was an increase in Fusobacteria (Fig. 2), 2) While taking Q-CAN®, at the family level in the stool of lean individuals, there was an increase in Bifidobacteriacea and EtOH8, and a decrease in S24–7 (Fig. 3), and 3) While taking Q-CAN®, at a genus level in the stool of lean individuals, there was an increase in Blautia, Bifidobacterium and Staphylococcus (Fig. 4).
There is now a large amount of data associating changes in the microbiome with physiological changes with impact on health, but very few studies in humans regarding the effects of soy products [18]. Microbiome diversity is considered to be positive and it was reassuring to see that Q-CAN® did not decrease microbiome diversity in the stool or saliva (Fig. 1b-e). The increase in Actinobacteria in lean individuals taking Q-CAN® (Fig. 2a), is of interest as Actinobacteria are one of the four major phyla of gut microbiota and has a crucial role in maintaining gut homeostasis. Actinobacteria are non-motile, multiple branching rods, gram positive, anaerobic bacteria (families: Bifidobacteria, Propionibac-teria and Corynebacteria) [19]. Within this phylum the increase was found to be in the family Bifidobacteria (Fig. 3a). Bifidobacteria have high production of short chain fatty acids (SCFA), and one of the beneficial effects of this is in the maintenance of gut barrier due to the production of butyrate [20]. Bifidobacteria can protect the host from enteropathogenic infections, such as entero-haemorrhagic Eschericl1ia coli and Shigella, and this thought to be due their high production of acetate and the biotransformation of nutrients in the diet [21–23]. This occurs via the fermentation of large polysaccharides, oligosaccarides, unabsorbed sugars and fibers. This results in the release hydrogen, carbon dioxide and SCFAs. It furthermore results in the degradation of proteins, the regulation of lipid metabolism, and the absorption and biosynthesis of vitamin K, iron, calcium and magnesium [24, 25]. Bifidobacteria are also important in the maintenance of a tolerogenic immune environment, and this is thought to be through the stimulation of intrahepatic lymphocytes [26, 27]. This is supported by an increase in gut permeability that leads to the translocation of LPS into the serum when there is a decrease in the number of Bifidobacteria [28]. This provides immune stimulation, and sustains chronic inflammatory conditions, such as insulin resistance, diabetes and liver diseases [29]. In a high fat diet mouse model, administration of Bifidobacterium pseudocatenulatum results in down-regulation of inflammation by reducing the production of inflammatory cytokines and chemokines, especially IL-6 and MCP-1 [30]. Overall Bifidobacteria are seen as improving gut barrier function and reduce the translocation of pro-inflammatory molecules such as lipopolysaccharide into the blood stream [28]. Bifidobacteria and Lactobacilli, are the cornerstone of many probiotic therapeutic approaches. For example a mixture of lyophilized four Lactobacilli and three Bifidobacteria strains has been demonstrated to be effective in several conditions including pouchitis, non-alcoholic steatohepatitis and in the prevention of antibiotic associated diarrhea [31–33]. Bifidobacteria treatment has also been demonstrated to improve symptoms of irritable bowel syndrome [34].
A significant increase in the phylum Actinobacteria and the family Bifidobacteria in the stool was not seen in obese individuals, however there was a trend in that direction (Fig. 2b and 3b). The lack of positive association may be due to the relatively small samples size of ten individuals in each group, and with larger samples sizes a significant increase may be seen. There was a statistically significant increase in the phylum Fusobacteria in the stool of obese individuals but this increase was not followed through at the family (Fusobacteriaceae) level and is of unclear significance. The species Fusobacterium nucleatum has been shown to be associated with colon cancer, although this association has not been universally reproduced [35, 36].
In lean individuals there was also an increase in the family of EtOH8 anaerobic bacteria, (Fig. 3c), but relatively little is known about the biological significance of this and it is difficult to speculate. The uncultured S24–7, a member of the Bacteroidetes family, was reduced in lean individuals while taking Q-CAN® (Fig. 3a). S24–7 are highly anaerobic bacteria that are localized to the gastrointestinal tracts of homeothermic animals and are increasingly being recognized as a numerically predominant member of the gut microbiota but due to the inability to culture them little is known about the nature of their interactions with the host [37].
At the genus level there was an increase in Blautia (family: Lachnospiraceae, order: Clostridiales, class: Clostridia, and phylum: Firmicutes) (Fig. 4a). Higher levels of Blautia have been associated with several positive health features including nutrient assimilation, immunological health, lower amount of visceral fat, reduced risk of graft versus host disease and [38–40], and administration of Blautia has been proposed as a treatment for cancer [41].
In the saliva of lean individuals there was an increase in family Veillonellaceae (phylum Firmicutes, with three genera Veillonella, Acidaminococcus, and Megasphaera) while on Q-CAN® (Fig. 6a). Members of the family Veillonellaceae are of particular interest for their probiotic effects but to date this has been investigated in animal husbandry with trials showing improvement in energy balance and inhibiting colonization by antibiotic resistance strains of bacteria [42, 43]. If it will be interesting to see if such beneficial effects are also found in the future in humans.
In addition to the phylogenetic analysis above it is important to consider analysis at a functional level by addressing changes in genes with a shared function. An example of this is the consideration of bacterial genes whose products are capable of metabolizing estrogens, identified as the estrobolome [44, 45]. A subgroup of estrogens undergoes a first passage in the liver with glucuronization or sulfunation allowing for excretion in bile, urine and feces. These estrogens can be uncombined by enteric bacterial β-glucuronidase and β-glucosidase, determining their resorption in blood circulation. At present the metabolic functions of a minority of bacterial genes has been identified. As this increases the data set presented here will be increasingly valuable and allow for analysis of Q-CAN® induced changes in the functional capacity of the microbiome.
When comparing the lean and obese populations it is clear that Q-CAN® consumption resulted in a greater number of changes in the lean than the obese (Phylum lean 1: obese 1, family lean 3: obese 0, genus lean 3: obese 0). This may be due to the microbiota of obese individuals having less diversity and therefore less opportunity for Q-CAN® to interact with a range of microbes [46–53].
Conclusion
In conclusion, Q-CAN® induced a number of changes in the stool and saliva microbiome. The changes which were most notable and for which we currently have the greatest information on physiological impact are the increase in stool microbiome of lean participants of family Bifidobacteria which are known to have a wide variety of beneficial effects including producing SCFA, reducing intestinal permeability and improving immune function. The increase in Blautia is likewise proposed to have a number of beneficial effects including improved nutrient assimilation and reduced cancer risk. This study has also generated a significant amount of data on bacteria of unclear biological functions and this may be of value as more information is made available on the intestinal microbiome.
Supplementary Information
Additional file 1: Figure S1. Intestinal microbiome analysis at the level of Phylum. A-B) Relative abundance of bacterial is visualized by heat map in both lean and obese. Each column represents a subject and each colored row a bacterial taxon. The intensity of the red color represents the highest abundance taxa and the intensity of the blue color the lowest abundance taxa in lean and obese people. The results are the average of 3 visits in pre Q-CAN® group, 4 visits in on Q-CAN® group and 4 visits in post Q-CAN® group for each participant. Obese (n = 9 participants), Lean (n = 10 participants).
Additional file 2: Figure S2. Intestinal microbiome analysis at the level of Family. A-B) Relative abundance of bacterial is visualized by heat map in both lean and obese. Each column represents a subject and each colored row a bacterial taxon. The intensity of the red color represents the highest abundance taxa and the intensity of the blue colour the lowest abundance taxa in lean and obese people. The results are the average of 3 visits in pre Q-CAN® group, 4 visits in on Q-CAN® group and 4 visits in post Q-CAN® group for each participant. Obese (n = 9 participants), Lean (n = 10 participants).
Additional file 3: Figure S3. Intestinal microbiome analysis at the level of Genus. A-B) Relative abundance of bacterial genera is visualized by heat map in both lean and obese. Each column represents a subject and each colored row a bacterial taxon. The intensity of the red color represents the highest abundance taxa and the intensity of the blue color the lowest abundance taxa in lean and obese people. The results are the average of 3 visits in pre Q-CAN® group, 4 visits in on Q-CAN® group and 4 visits in post Q-CAN® group for each participant. Obese (n = 9 participants), Lean (n = 10 participants).
Additional file 4: Figure S4. Oral microbiome analysis at the level of Phylum. A-B) Relative abundance of bacterial is visualized by heat map in both lean and obese. Each column represents a subject and each colored row a bacterial taxon. The intensity of the red color represents the highest abundance taxa and the intensity of the blue color the lowest abundance taxa in lean and obese people. The results are the average of 3 visits in pre Q-CAN® group, 4 visits in on Q-CAN® group and 4 visits in post Q-CAN® group for each participant. Obese (n = 10 participants), Lean (n = 10 participants).
Additional file 5: Figure S5. Oral microbiome analysis at the level of Family. A-B) Relative abundance of bacterial is visualized by heat map in both lean and obese. Each column represents a subject and each colored row a bacterial taxon. The intensity of the red color represents the highest abundance taxa and the intensity of the blue color the lowest abundance taxa in lean and obese people. The results are the average of 3 visits in pre Q-CAN® group, 4 visits in on Q-CAN® group and 4 visits in post Q-CAN® group for each participant. Obese (n = 10 participants), Lean (n = 10 participants).
Additional file 6: Figure S6. Oral microbiome analysis at the level of Genus. A-B) Relative abundance of bacterial genera is visualized by heat map in both lean and obese. Each column represents a subject and each colored row a bacterial taxon. The intensity of the red color represents the highest abundance taxa and the intensity of the blue color the lowest abundance taxa in lean and obese people. The results are the average of 3 visits in pre Q-CAN® group, 4 visits in on Q-CAN® group and 4 visits in post Q-CAN® group for each participant. Obese (n = 10 participants), Lean (n = 10 participants)
Acknowledgments
None.
Abbreviations
- T2D
Type 2 diabetes
- SCFA
short chain fatty acids
- LPL
lipoprotein lipase
Authors’ contributions
1. XO, ED and AA designed research (project conception, development of overall research plan, and study oversight). 2. ED, RP, TRW, MRF, conducted research (hands-on conduct of the experiments and data collection). 3. ERS, ED, and EK analyzed data and performed statistical analysis. 4. XO, RP and EK wrote paper. All authors have read and approved the manuscript.
Funding
This project was funded by BESO Biological Research Inc., who also provided Q-CAN®. BESO Biological Research Inc., had no role in the conceptualization, design, execution of the study or in the data analysis and manuscript writing.
Availability of data and materials
Supporting data is available on request to Xinshou Ouyang at xinshou.ouyang@yale.edu
Ethics approval and consent to participate
Approval was obtained from Yale HIC and written consent from each participant.
Consent for publication
Not Applicable.
Competing interests
There are no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Messina M. Soy and health update: evaluation of the clinical and epidemiologic literature. Nutrients. 2016;8(12):754. doi: 10.3390/nu8120754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwon DY, et al. Long-term consumption of fermented soybean-derived Chungkookjang attenuates hepatic insulin resistance in 90% pancreatectomized diabetic rats. Horm Metab Res. 2007;39(10):752–757. doi: 10.1055/s-2007-990287. [DOI] [PubMed] [Google Scholar]
- 3.Kwon DY, et al. Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr Res. 2010;30(1):1–13. doi: 10.1016/j.nutres.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Nozue M, et al. Fermented soy product intake is inversely associated with the development of high blood pressure: the Japan public health center-based prospective study. J Nutr. 2017;147(9):1749–1756. doi: 10.3945/jn.117.250282. [DOI] [PubMed] [Google Scholar]
- 5.Lee Y, et al. PPARgamma2 C1431T polymorphism interacts with the antiobesogenic effects of Kochujang, a Korean fermented, soybean-based red pepper paste, in overweight/obese subjects: a 12-week, double-blind randomized clinical trial. J Med Food. 2017;20(6):610–617. doi: 10.1089/jmf.2016.3911. [DOI] [PubMed] [Google Scholar]
- 6.Lourenco JM, et al. The successional changes in the gut microbiome of pasture-raised chickens fed soy-containing and soy-free diets. Front Sustain Food Syst. 2019;3:1–8. doi: 10.3389/fsufs.2019.00001. [DOI] [Google Scholar]
- 7.Boue S, et al. A novel gastrointestinal microbiome modulator from soy pods reduces absorption of dietary fat in mice. Obesity (Silver Spring) 2016;24(1):87–95. doi: 10.1002/oby.21197. [DOI] [PubMed] [Google Scholar]
- 8.Cheng IC, et al. Effect of fermented soy milk on the intestinal bacterial ecosystem. World J Gastroenterol. 2005;11(8):1225–1227. doi: 10.3748/wjg.v11.i8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-Raudales D, et al. Consumption of different soymilk formulations differentially affects the gut microbiomes of overweight and obese men. Gut Microbes. 2012;3(6):490–500. doi: 10.4161/gmic.21578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoguchi S, et al. Effects of non-fermented and fermented soybean milk intake on faecal microbiota and faecal metabolites in humans. Int J Food Sci Nutr. 2012;63(4):402–410. doi: 10.3109/09637486.2011.630992. [DOI] [PubMed] [Google Scholar]
- 11.Piacentini G, et al. Molecular characterization of intestinal microbiota in infants fed with soymilk. J Pediatr Gastroenterol Nutr. 2010;51(1):71–76. doi: 10.1097/MPG.0b013e3181dc8b02. [DOI] [PubMed] [Google Scholar]
- 12.Vazquez L, et al. Effect of soy isoflavones on growth of representative bacterial species from the human gut. Nutrients. 2017;9(7):727. doi: 10.3390/nu9070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miele L, et al. Impact of gut microbiota on obesity, diabetes, and cardiovascular disease risk. Curr Cardiol Rep. 2015;17(12):120. doi: 10.1007/s11886-015-0671-z. [DOI] [PubMed] [Google Scholar]
- 14.Kolodziejczyk AA, et al. The role of the microbiome in NAFLD and NASH. EMBO Mol Med. 2018;11(2):e9302. doi: 10.15252/emmm.201809302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambalam P, et al. Probiotics, prebiotics and colorectal cancer prevention. Best Pract Res Clin Gastroenterol. 2016;30(1):119–131. doi: 10.1016/j.bpg.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Yu LX, Schwabe RF. The gut microbiome and liver cancer: mechanisms and clinical translation. Nat Rev Gastroenterol Hepatol. 2017;14(9):527–539. doi: 10.1038/nrgastro.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hruby A, Hu FB. The epidemiology of obesity: a big picture. Pharmacoeconomics. 2015;33(7):673–689. doi: 10.1007/s40273-014-0243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loman BR, et al. Chemotherapy-induced neuroinflammation is associated with disrupted colonic and bacterial homeostasis in female mice. Sci Rep. 2019;9(1):16490. doi: 10.1038/s41598-019-52893-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Binda C, et al. Actinobacteria: a relevant minority for the maintenance of gut homeostasis. Dig Liver Dis. 2018;50(5):421–428. doi: 10.1016/j.dld.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Hardy H, et al. Probiotics, prebiotics and immunomodulation of gut mucosal defences: homeostasis and immunopathology. Nutrients. 2013;5(6):1869–1912. doi: 10.3390/nu5061869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukuda S, et al. Acetate-producing bifidobacteria protect the host from enteropathogenic infection via carbohydrate transporters. Gut Microbes. 2012;3(5):449–454. doi: 10.4161/gmic.21214. [DOI] [PubMed] [Google Scholar]
- 22.Scott KP, et al. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol Ecol. 2014;87(1):30–40. doi: 10.1111/1574-6941.12186. [DOI] [PubMed] [Google Scholar]
- 23.Davila AM, et al. Intestinal luminal nitrogen metabolism: role of the gut microbiota and consequences for the host. Pharmacol Res. 2013;68(1):95–107. doi: 10.1016/j.phrs.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Backhed F, Crawford PA. Coordinated regulation of the metabolome and lipidome at the host-microbial interface. Biochim Biophys Acta. 2010;1801(3):240–245. doi: 10.1016/j.bbalip.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Younes H, et al. Effects of two fermentable carbohydrates (inulin and resistant starch) and their combination on calcium and magnesium balance in rats. Br J Nutr. 2001;86(4):479–485. doi: 10.1079/BJN2001430. [DOI] [PubMed] [Google Scholar]
- 26.Sun M, et al. Regulatory immune cells in regulation of intestinal inflammatory response to microbiota. Mucosal Immunol. 2015;8(5):969–978. doi: 10.1038/mi.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarville JL, Caminero A, Verdu EF. Novel perspectives on therapeutic modulation of the gut microbiota. Ther Adv Gastroenterol. 2016;9(4):580–593. doi: 10.1177/1756283X16637819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duca FA, Sakar Y, Covasa M. The modulatory role of high fat feeding on gastrointestinal signals in obesity. J Nutr Biochem. 2013;24(10):1663–1677. doi: 10.1016/j.jnutbio.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Scarpellini E, Tack J. Obesity and metabolic syndrome: an inflammatory condition. Dig Dis. 2012;30(2):148–153. doi: 10.1159/000336664. [DOI] [PubMed] [Google Scholar]
- 30.Cano PG, et al. Bifidobacterium CECT 7765 improves metabolic and immunological alterations associated with obesity in high-fat diet-fed mice. Obesity (Silver Spring) 2013;21(11):2310–2321. doi: 10.1002/oby.20330. [DOI] [PubMed] [Google Scholar]
- 31.Gionchetti P, et al. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124(5):1202–1209. doi: 10.1016/S0016-5085(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 32.Abu-Shanab A, Quigley EM. The role of the gut microbiota in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2010;7(12):691–701. doi: 10.1038/nrgastro.2010.172. [DOI] [PubMed] [Google Scholar]
- 33.Selinger CP, et al. Probiotic VSL#3 prevents antibiotic-associated diarrhoea in a double-blind, randomized, placebo-controlled clinical trial. J Hosp Infect. 2013;84(2):159–165. doi: 10.1016/j.jhin.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 34.Pinto-Sanchez MI, et al. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology. 2017;153(2):448–459. doi: 10.1053/j.gastro.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Castellarin M, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22(2):299–306. doi: 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sears CL. The who, where and how of fusobacteria and colon cancer. Elife. 2018;7:e28434. doi: 10.7554/eLife.28434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ormerod KL, et al. Genomic characterization of the uncultured Bacteroidales family S24-7 inhabiting the guts of homeothermic animals. Microbiome. 2016;4(1):36. doi: 10.1186/s40168-016-0181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ozato N, et al. Blautia genus associated with visceral fat accumulation in adults 20-76 years of age. NPJ Biofilms Microbiomes. 2019;5:28. doi: 10.1038/s41522-019-0101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jenq RR, et al. Intestinal Blautia is associated with reduced death from graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21(8):1373–1383. doi: 10.1016/j.bbmt.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez I, et al. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J. 2013;7(2):269–280. doi: 10.1038/ismej.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goodman B, et al. Treating cancer using a blautia strain. US Patent Office. 2019;2019 0240268(1):1–45. [Google Scholar]
- 42.Counotte GH, et al. Role of Megasphaera elsdenii in the fermentation of dl-[2-C] lactate in the rumen of dairy cattle. Appl Environ Microbiol. 1981;42(4):649–655. doi: 10.1128/AEM.42.4.649-655.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stanton TB, Humphrey SB. Persistence of antibiotic resistance: evaluation of a probiotic approach using antibiotic-sensitive MEGASPHAERA elsdenii strains to prevent colonization of swine by antibiotic-resistant strains. Appl Environ Microbiol. 2011;77(20):7158–7166. doi: 10.1128/AEM.00647-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Virgili E, et al. Soy intake and breast cancer. J Health Med Inf. 2018;9(316):1–3. [Google Scholar]
- 45.Plottel CS, Blaser MJ. Microbiome and malignancy. Cell Host Microbe. 2011;10(4):324–335. doi: 10.1016/j.chom.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Le Chatelier E, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 47.Peters BA, et al. A taxonomic signature of obesity in a large study of American adults. Sci Rep. 2018;8(1):9749. doi: 10.1038/s41598-018-28126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim EK, et al. Fermented kimchi reduces body weight and improves metabolic parameters in overweight and obese patients. Nutr Res. 2011;31(6):436–443. doi: 10.1016/j.nutres.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 49.Kim MS, et al. Long-term fermented soybean paste improves metabolic parameters associated with non-alcoholic fatty liver disease and insulin resistance in high-fat diet-induced obese mice. Biochem Biophys Res Commun. 2018;495(2):1744–1751. doi: 10.1016/j.bbrc.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 50.Mohd Yusof H, et al. Hepatoprotective effect of fermented soybean (nutrient enriched soybean tempeh) against alcohol-induced liver damage in mice. Evid Based Complement Alternat Med. 2013;2013:274274. doi: 10.1155/2013/274274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song JL, et al. Anti-colitic effects of kanjangs (fermented soy sauce and sesame sauce) in dextran sulfate sodium-induced colitis in mice. J Med Food. 2014;17(9):1027–1035. doi: 10.1089/jmf.2013.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takagi A, Kano M, Kaga C. Possibility of breast cancer prevention: use of soy isoflavones and fermented soy beverage produced using probiotics. Int J Mol Sci. 2015;16(5):10907–10920. doi: 10.3390/ijms160510907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jayachandran M, Xu B. An insight into the health benefits of fermented soy products. Food Chem. 2019;271:362–371. doi: 10.1016/j.foodchem.2018.07.158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Intestinal microbiome analysis at the level of Phylum. A-B) Relative abundance of bacterial is visualized by heat map in both lean and obese. Each column represents a subject and each colored row a bacterial taxon. The intensity of the red color represents the highest abundance taxa and the intensity of the blue color the lowest abundance taxa in lean and obese people. The results are the average of 3 visits in pre Q-CAN® group, 4 visits in on Q-CAN® group and 4 visits in post Q-CAN® group for each participant. Obese (n = 9 participants), Lean (n = 10 participants).
Additional file 2: Figure S2. Intestinal microbiome analysis at the level of Family. A-B) Relative abundance of bacterial is visualized by heat map in both lean and obese. Each column represents a subject and each colored row a bacterial taxon. The intensity of the red color represents the highest abundance taxa and the intensity of the blue colour the lowest abundance taxa in lean and obese people. The results are the average of 3 visits in pre Q-CAN® group, 4 visits in on Q-CAN® group and 4 visits in post Q-CAN® group for each participant. Obese (n = 9 participants), Lean (n = 10 participants).
Additional file 3: Figure S3. Intestinal microbiome analysis at the level of Genus. A-B) Relative abundance of bacterial genera is visualized by heat map in both lean and obese. Each column represents a subject and each colored row a bacterial taxon. The intensity of the red color represents the highest abundance taxa and the intensity of the blue color the lowest abundance taxa in lean and obese people. The results are the average of 3 visits in pre Q-CAN® group, 4 visits in on Q-CAN® group and 4 visits in post Q-CAN® group for each participant. Obese (n = 9 participants), Lean (n = 10 participants).
Additional file 4: Figure S4. Oral microbiome analysis at the level of Phylum. A-B) Relative abundance of bacterial is visualized by heat map in both lean and obese. Each column represents a subject and each colored row a bacterial taxon. The intensity of the red color represents the highest abundance taxa and the intensity of the blue color the lowest abundance taxa in lean and obese people. The results are the average of 3 visits in pre Q-CAN® group, 4 visits in on Q-CAN® group and 4 visits in post Q-CAN® group for each participant. Obese (n = 10 participants), Lean (n = 10 participants).
Additional file 5: Figure S5. Oral microbiome analysis at the level of Family. A-B) Relative abundance of bacterial is visualized by heat map in both lean and obese. Each column represents a subject and each colored row a bacterial taxon. The intensity of the red color represents the highest abundance taxa and the intensity of the blue color the lowest abundance taxa in lean and obese people. The results are the average of 3 visits in pre Q-CAN® group, 4 visits in on Q-CAN® group and 4 visits in post Q-CAN® group for each participant. Obese (n = 10 participants), Lean (n = 10 participants).
Additional file 6: Figure S6. Oral microbiome analysis at the level of Genus. A-B) Relative abundance of bacterial genera is visualized by heat map in both lean and obese. Each column represents a subject and each colored row a bacterial taxon. The intensity of the red color represents the highest abundance taxa and the intensity of the blue color the lowest abundance taxa in lean and obese people. The results are the average of 3 visits in pre Q-CAN® group, 4 visits in on Q-CAN® group and 4 visits in post Q-CAN® group for each participant. Obese (n = 10 participants), Lean (n = 10 participants)
Data Availability Statement
Supporting data is available on request to Xinshou Ouyang at xinshou.ouyang@yale.edu