Abstract
Background:
Most clinical and epidemiologic estimates of prenatal cannabis use are based on self-report, and the validity of self-reported cannabis use has not been examined in a large, representative population of pregnant women. We determined the validity of self-reported prenatal cannabis use and predictors of non-disclosure using data from Kaiser Permanente Northern California’s (KPNC) healthcare system with universal prenatal cannabis screening during prenatal care.
Methods:
Validation study using data from 281,025 pregnancies in KPNC among females aged ≥11 who completed a self-administered questionnaire on prenatal cannabis use and a cannabis urine toxicology test from 2009–2017. We calculated sensitivity, specificity, positive predictive value, and negative predictive value of self-reported prenatal cannabis use using urine toxicology testing as the criterion standard, and sensitivity of urine toxicology testing using self-reported use as the criterion standard. We compared sociodemographics of those who disclosed versus did not disclose prenatal cannabis use.
Results:
Urine toxicology testing identified more instances of prenatal cannabis use than self-report (4.9% vs. 2.5%). Sensitivity of self-reported use was low (33.9%). Sensitivity of the toxicology test was higher (65.8%), with greater detection of self-reported daily (83.9%) and weekly (77.4%) than monthly or less use (54.1%). Women with a positive toxicology test who did not disclose use were older, more likely to be Hispanic, with lower income than those who disclosed use.
Conclusions:
Given that many women choose not to disclose prenatal cannabis use, clinicians should educate all prenatal patients about the potential risks and advise them to quit cannabis use during pregnancy.
Keywords: Marijuana, pregnancy, prenatal, sensitivity, THC, urine toxicology test, universal screening
Introduction
To assess maternal use of cannabis during pregnancy, healthcare providers and researchers often rely on patients’ self-reporting because it is more affordable and easier to implement than analysis of drug metabolites (e.g., via testing of urine or hair) (Moeller et al., 2008). Maternal self-report of prenatal cannabis use can provide detailed information about key aspects of use, including frequency and mode of administration. However, pregnant women may be reluctant to self-disclose cannabis use due to social stigma, fear of legal consequences, and discrimination. As a result, self-reported prenatal cannabis use is thought to be underestimated (Metz et al., 2019; Young-Wolff et al., 2017).
In order for healthcare providers and researchers to accurately estimate the prevalence and health risks associated with maternal prenatal cannabis use, it is critical to determine the validity of self-reported use relative to biological sampling. While there is no “gold standard” for assessing prenatal cannabis use, urine testing is a valid and objective method of biological sampling for drug use in research studies and healthcare settings (Moeller et al., 2008). The cannabis metabolite, 11-nor-9-carboxy-delta 9- THC, which is tested for in standard urine samples has a long half-life and is typically detectable in urine for 2–3 days in persons who use occasionally, but may be detectable in urine for 30 days or longer in persons who use more heavily (Niedbala et al., 2001). Initial results from a small study of adult pregnant women with a history of substance use disorders indicate that sensitivity of self-reported prenatal cannabis use compared to urine toxicology testing is low (~58%), although somewhat higher than other types of drugs (Garg et al., 2016). Less is known about the validity of self-reported cannabis use among pregnant women in the general population, as data from large, generalizable samples of pregnant women are lacking.
The primary objective of this study was to determine the validity of self-reported prenatal cannabis use by comparing it to positive urine toxicology testing for cannabis among pregnant women in a large integrated healthcare delivery system with universal screening for prenatal cannabis use. Secondary objectives included examining differences in sociodemographic characteristics of those who self-report versus choose not to disclose cannabis use in pregnancy, identifying the potential for under-detection in toxicology tests using self-reported cannabis use as the criterion standard, and determining whether toxicology under-detection varies by self-reported frequency of use.
Materials and Methods
Study Setting and Population
Kaiser Permanente Northern California (KPNC) is a nonprofit multispecialty healthcare delivery system serving more than 4 million diverse patients representative of the Northern California population (Gordon, 2013, 2015; Selby et al., 2005; Terhune, 2013). Pregnant women in KPNC are universally screened for prenatal cannabis use by both self-report (via a self-administered questionnaire) and a urine toxicology test to which they provide written consent. The primary study sample comprised KPNC pregnant women who were screened for self-reported cannabis use during pregnancy between 2009 and 2017 and had a urine toxicology test for cannabis ± two weeks from the date they completed the self-reported screening questionnaire. Screenings are typically done on the same day at the first prenatal visit (at ~8 weeks gestation). Subsamples used for sensitivity analyses included pregnancies with the urine toxicology test on the same day as the self-reported screening questionnaire, and the subset of pregnancies with the self-reported screening questionnaire and urine toxicology test at least 6 weeks after patients’ last menstrual period. The KPNC institutional review board approved and this study with waiver of consent.
Measures
Prenatal cannabis use was based on: 1) self-reported cannabis use during pregnancy on the self-administered screening questionnaire (none, monthly or less, weekly, daily), and 2) a universal toxicology test for cannabis within ±2 weeks of the self-administered questionnaire. Screening tests were performed on a Beckman Coulter AU680 chemistry analyzer using the Emit II Plus Cannabinoid Assay from Siemens with a cutoff of 45ng/mL. Confirmatory testing for the presence of the cannabis metabolite, 11-nor-9-carboxy-delta 9- THC, was performed by liquid chromatography-tandem mass spectrometry for all positive immunoassay results. The confirmation test methodology was LC-MS/MS on a triple quadrupole system with a cutoff for positivity of 15ng/mL.
Data on patients’ age and self-reported race/ethnicity were extracted from the electronic health record (EHR). Median household income information was obtained from census data based on members’ geocoded addresses and reflect members’ neighborhoods, not individual data. We categorized this variable into quartiles.
Statistical Analysis
To validate self-reported prenatal cannabis use we computed sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) using urine toxicology results as the criterion standard. Sensitivity was calculated as the proportion of patients with a positive urine toxicology test for cannabis who self-reported prenatal cannabis use. Specificity was calculated as the proportion of patients with a negative urine toxicology test who self-reported no prenatal cannabis use. PPV was calculated as the proportion of patients who self-reported prenatal cannabis use who had a positive urine toxicology test. NPV was calculated as the proportion of patients who self-reported no prenatal cannabis use who had a negative urine toxicology test. We also examined the sensitivity of urine toxicology test results by self-reported prenatal cannabis use and use frequency.
To determine whether results would change if the self-reported screening questionnaire was done on the same day as the urine toxicology test, we re-ran analyses using the subset of patients who received both screenings on the same day. In addition, because the THC metabolite, 11-nor-9-carboxy-delta 9- THC, is detectable up to ~30 days after last use, we re-ran analyses with the subset of patients who were screened for prenatal substance use at least six weeks after the last menstrual period (i.e., approximately four weeks after conception), to reduce the likelihood that the toxicology test would detect pre-conception cannabis use.
In secondary analyses, we characterized sub-groups at risk for misclassification of self-reported prenatal cannabis use by comparing sociodemographic characteristics of women who chose not to disclose prenatal cannabis use but tested positive via urine toxicology (i.e., not identified by self-report) with those of women who self-reported prenatal cannabis use regardless of toxicology results (i.e., identified by self-report). We tested for statistical significance of differences by fitting multilevel logistic regression models to account for correlation in sociodemographic characteristics of women with multiple pregnancies. Analyses were conducted using SAS 9.4. P-values <.05 (2-sided) were considered statistically significant.
Results
Of 367,314 pregnancies, 1,996 (0.54%) missing data on date of last menstrual period, 1,582 (0.43%) missing data on self-reported frequency of prenatal cannabis use, 40,401 (11.0%) missing a toxicology test, and 42,310 (11.5%) with a urine toxicology test more than ±2 weeks of the self-administered questionnaire were excluded. To evaluate possible selection bias, we compared the demographic characteristics of women who were included versus excluded due to missing urine toxicology data or a toxicology test more than ±2 weeks of the self-administered questionnaire, and the samples were nearly identical, indicating minimal to no selection bias (Table A.1 in the Supplement).
The final sample included 281,025 pregnancies with self-report and urine toxicology testing data. Of those, 2.5% self-reported any prenatal cannabis use (self-reported frequency of use: 0.5% daily, 0.7% weekly, and 1.4% monthly or less) and 4.9% had a positive urine toxicology test. Overall, 94.2% were cannabis use negative on both self-report and urine toxicology, 3.3% were negative on self-report but positive on urine toxicology, 0.9% were positive on self-report but negative on urine toxicology, and 1.7% were positive on both self-report and urine toxicology. The correspondence of self-reported prenatal cannabis use frequency and urine toxicology test results are provided in Table A.2 in the Supplement. The median gestational age at self-reported screening was 8.9 weeks (IQR = 3.29).
We validated self-reported prenatal cannabis use using the urine toxicology test as the criterion standard. We found that sensitivity was very low (33.9%) and the positive predictive value of self-reported prenatal cannabis use was moderate (65.8%). The low sensitivity indicates that among people who tested positive for cannabis on the urine toxicology test, only 33.9% were correctly identified through self-report. Specificity (99.1%) and negative predictive value (96.7%) were excellent (Figure 1).
Figure 1.
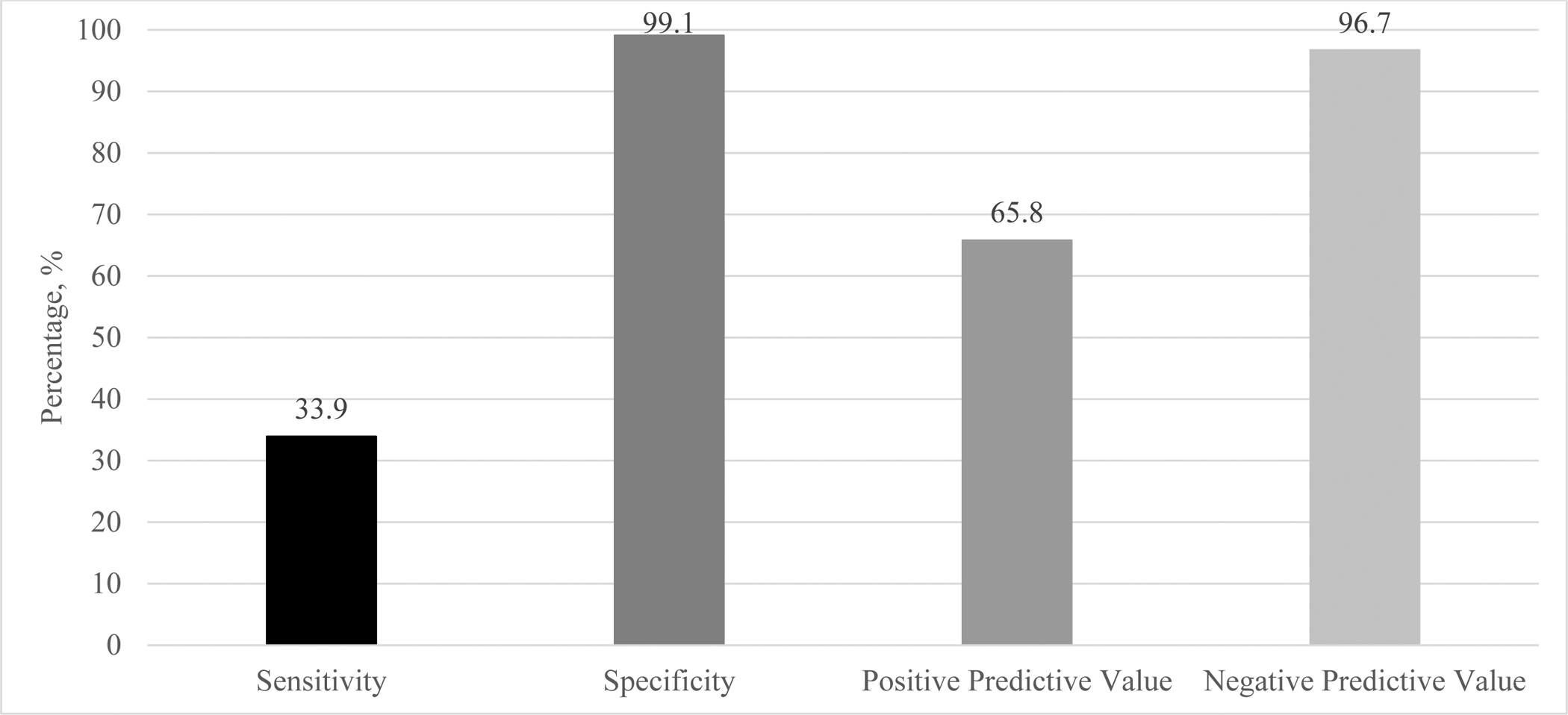
Validity of Self-Reported Prenatal Cannabis Use Versus Urine Toxicology Results Among 281,025 Pregnancies in Kaiser Permanente Northern California, 2009–2017
Notes. The sample included women screened for prenatal cannabis use by self-report with a urine toxicology test within ± two weeks. Sensitivity was calculated as the proportion of patients with a positive urine toxicology test for cannabis who self-reported prenatal cannabis use. Specificity was calculated as the proportion of patients with a negative urine toxicology test who self-reported no prenatal cannabis use. Positive predictive value was calculated as the proportion of patients who self-reported prenatal cannabis use who had a positive urine toxicology test. Negative predictive value was calculated as the proportion of patients who self-reported no prenatal cannabis use who had a negative urine toxicology test.
Secondary analyses found that compared to women who self-reported prenatal cannabis use (regardless of toxicology results), those who did not self-report prenatal cannabis use but were identified as using cannabis by toxicology testing (i.e., those who would have been missed in healthcare systems limited to self-report) were older, more likely to be Hispanic, and lived in neighborhoods with lower median incomes, although the differences were minimal (Table 1).
Table 1.
Sociodemographic Characteristics Among 16,316 Pregnancies in Kaiser Permanente Northern California That Screened Positive for Any Prenatal Cannabis Use, By Self-Report, 2009–2017
| Not Identified as Using Cannabis by Self-Reporta N= 9,169 (56.2%) |
Identified as Using Cannabis by Self-Reportb N = 7,147 (43.8%) |
||
|---|---|---|---|
| N (%) | N (%) | P-valued | |
| Age | <0.0001 | ||
| <18 | 277 (3.0) | 335 (4.7) | |
| 18–24 | 3,504 (38.2) | 2,961 (41.4) | |
| 25–34 | 4,361 (47.6) | 3,118 (43.6) | |
| ≥ 35 | 1,027 (11.2) | 733 (10.3) | |
| Race | <0.0001 | ||
| White | 3,193 (34.8) | 2,592 (36.3) | |
| Black | 1,835 (20.0) | 1,463 (20.5) | |
| Hispanic | 2,935 (32.0) | 1,979 (27.7) | |
| Asian | 194 (2.1) | 247 (3.5) | |
| Other | 1,012 (11.0) | 866 (12.1) | |
| Median Neighborhood Household Incomec | 0.0155 | ||
| <$52,442 | 3,731 (40.7) | 2,783 (38.9) | |
| $52,442-<$71,585 | 2,444 (26.7) | 1,859 (26.0) | |
| $71,585-<$94,083 | 1,775 (19.4) | 1,447 (20.3) | |
| ≥$94,083 | 1,205 (13.1) | 1,034 (14.5) | |
Notes.
Women who do not self-report cannabis use but have a positive toxicology test;
Women who self-report cannabis use and have either a positive or negative toxicology test.
38 women were missing income information.
P-value calculated using multi-level logistic regression to account for the correlation of women included in the dataset more than once due to multiple pregnancies during the study time period.
We also found under-detection of prenatal cannabis use by toxicology tests using self-reported use as the criterion standard. Overall, approximately two-thirds of pregnancies with self-reported prenatal cannabis use were identified by the toxicology test (sensitivity = 65.8%) (Figure 2). To examine whether under-detection by toxicology varied by use frequency, we computed the sensitivity of the urine toxicology by self-reported frequency of prenatal cannabis use (Figure 2). We found greater detection of cannabis use by toxicology testing among women who self-reported more frequent prenatal cannabis use (daily = 83.9%, weekly = 77.4%, monthly or less = 54.1%).
Figure 2.
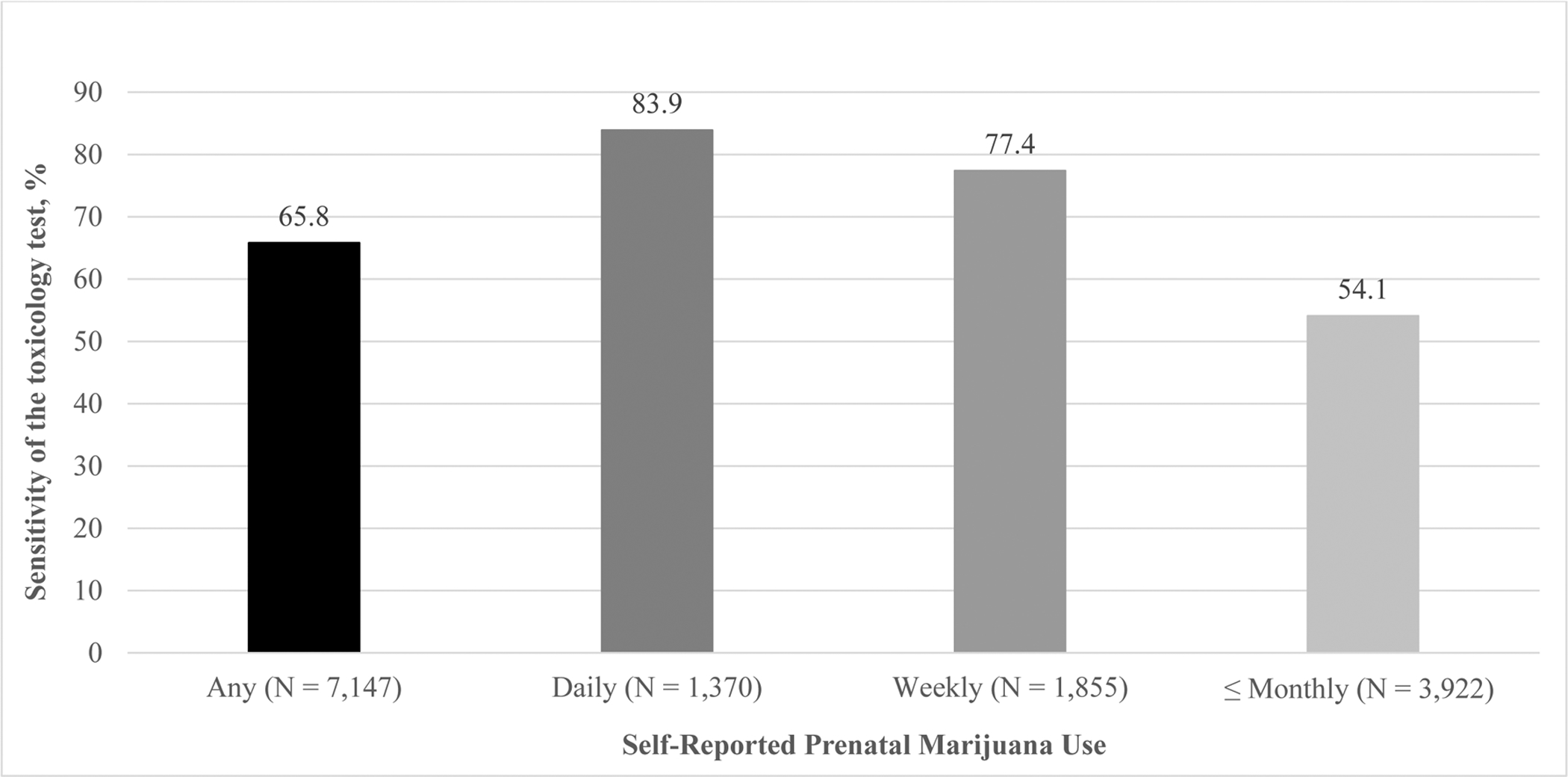
Sensitivity of the Urine Toxicology Test in Detecting Self-Reported Frequency of Prenatal Cannabis Use Among 281,025 Pregnancies in Kaiser Permanente Northern California, 2009–2017
Notes. The sample included women screened for prenatal cannabis use by self-report with a urine toxicology test within ± two weeks. Any prenatal cannabis use includes daily, weekly and monthly or less prenatal cannabis use. Sensitivity was calculated as the proportion of patients who self-reported prenatal cannabis use (any, daily, weekly, monthly or less) who had a positive toxicology test.
Results from sensitivity analyses limiting the sample to women with the screening questionnaire and toxicology test done at least six weeks after their last menstrual period, women with the questionnaire and toxicology test done on the same day, and women with the questionnaire and toxicology test done on the same day and at least six weeks after their last menstrual period were nearly identical (Table A.3 in the Supplement).
Discussion
Results from this large, representative population of pregnant women screened for cannabis use as part of standard prenatal care indicated that the prevalence of prenatal cannabis use was twice as high via urine toxicology testing (4.9%) as by self-report (2.5%). Among women who screened positive for prenatal cannabis use, more than half (56%) were detected by toxicology testing only, 15% were detected by self-report only, and 29% were detected by both methods. The higher prevalence of prenatal cannabis use by biological sampling relative to self-report is consistent with smaller studies that assessed prenatal cannabis use with urine toxicology and umbilical cord testing (Garg et al., 2016; Metz et al., 2019; Young-Wolff et al., 2017).
In our sample, the self-reported screening questionnaire correctly identified only 34% of those who had a positive urine toxicology test. Thus, about two-thirds of women who tested positive for prenatal cannabis use by toxicology testing (66%) would be misclassified as not using based on self-reported data alone. Conversely, the high specificity of self-reported cannabis use suggests that self-reporting no use of cannabis was confirmed by negative toxicology tests in most cases. It is important to note that this study took place while cannabis was legal for medical but not for recreational use in California, and it is unknown how legalization for recreational use in 2018 may impact the sensitivity of self-reported cannabis use during pregnancy.
Although urine toxicology tests were more sensitive than self-reported cannabis use, we also found under-detection of prenatal cannabis use by urine toxicology tests, likely because THC metabolites had already cleared the system. A positive toxicology test correctly identified two-thirds of those who self-reported any prenatal cannabis use, with one-third of women who self-reported use misclassified as not using based on urine toxicology data only. Notably, similar to other smaller studies of biological sampling (Gryczynski et al., 2014; Metz et al., 2019), the urine toxicology test was more likely to correctly identify those who self-reported daily (84%) or weekly (77%) cannabis use versus monthly or less (54%) cannabis use. Conversely, 16% of women who self-reported daily cannabis use, 23% of those who self-reported weekly cannabis use, and 46% of those who self-reported monthly or less use were not identified by urine toxicology tests. These results are important and indicate that maternal urine toxicology tests are not sensitive in detecting infrequent or light use of cannabis during pregnancy. Other methods of biological sampling such as meconium or umbilical cord homogenate may be more accurate, as they detect cannabis use over a longer period of time. However, given evidence that the potency of cannabis products and the frequency of prenatal cannabis use has increased in recent years (ElSohly et al., 2016; Young-Wolff et al., in press), urine toxicology tests may be increasingly sensitive in detecting cannabis use during pregnancy. Taken together, results indicate that neither method of screening is perfect and suggest that a combination of maternal self-report and biochemically verified methods of cannabis detection will likely provide the most accurate estimates of prenatal cannabis use (Konijnenberg, 2015).
Notably, there were significant socio-demographic differences in non-disclosure of prenatal cannabis use: older women, those of Hispanic race/ethnicity, and those with lower median neighborhood incomes were most likely to be misclassified as not using cannabis by self-reported screening. While additional research is needed to understand the factors underlying these differences in willingness to disclose, results suggest that existing estimates of self-reported prenatal cannabis use may be more accurate for certain demographic groups, perhaps due to differences in actual or perceived discrimination, social desirability bias, or general willingness to disclose sensitive information to healthcare providers. Results from future studies may be helpful to better understand which groups of pregnant patients may be more likely to underreport prenatal cannabis use in research studies and healthcare settings limited to self-reported screening.
Obstetric healthcare providers are well-suited to screen pregnant women for substance use and provide linkages and referrals for substance use counseling. In KPNC, the substance use screening and intervention program, Early Start, is available within obstetrics/gynecology clinics at no cost to pregnant women as part of standard prenatal care (Armstrong et al., 2001). Clinicians in California are not required to alert child protective services or law enforcement agencies when a woman uses substances during pregnancy (National Committee for Quality Assurance, 2018). For these reasons, the healthcare system can universally screen for prenatal cannabis use in a way that is confidential, supportive and non-penalizing, thus increasing receipt of effective and readily available treatment resources. In many states, substance use during pregnancy is considered grounds for child welfare involvement after birth which can lead to termination of parental rights (Child Welfare Information Gateway, 2016), and sensitivity of self-reported prenatal use may be understandably lower in places with less supportive policies. Thus, universal testing for prenatal cannabis use by urine toxicology tests may not be indicated in states with punitive laws due to the potential for unintended public health consequences (e.g., if women who use cannabis choose not to seek or continue with prenatal care) (Roberts and Pies, 2011). Regardless, all healthcare systems should provide universal health education about the potential risks of prenatal cannabis use and advise pregnant women to avoid prenatal cannabis use, as recommended by national guidelines (2017).
This study is limited to pregnant patients in California screened for prenatal cannabis use during standard KPNC prenatal care and results may not be generalizable to other healthcare settings, uninsured populations, or states with different laws regarding prenatal substance use. Our measure of median neighborhood household income was based on census data and may not reflect women of child bearing age. Our self-reported measure of prenatal cannabis use at the first prenatal visit does not differentiate whether use during pregnancy occurred before versus after women learned they were pregnant. The validity of self-reported prenatal cannabis use may change over the course of pregnancy, as many women may have quit use in pregnancy after they realized they were pregnant, and most pregnant women quit using by the third trimester (Forray et al., 2015). Some women could accurately report no prenatal cannabis use but still have positive urine toxicology test results if the drug is still in their system from use prior to conception. However, results from sensitivity analyses that limited the sample to those screened at least 6 weeks after their last menstrual period were very similar, increasing our confidence in study findings.
Patients without a urine toxicology test were excluded from this study which could lead to selection bias. However, because the toxicology tests are part of the standard initial panel of prenatal lab tests ordered for all patients, the provider does not choose who gets a toxicology test and therefore we expect no provider bias. Missing urine toxicology data could be due to a variety of factors, including that the patient declined to give urine or never went to the lab, system issues such as the lab didn’t run the analysis or the urine sample was insufficient (in cases where the patient does not give enough urine, other tests are prioritized before the toxicology test), or if patients transferred from another health plan and therefore the KPNC initial labs were not done. Our analyses indicated minimal to no selection bias, suggesting that the sample of women included was generally representative of the population of all KPNC pregnant women, with minimal differences in demographic characteristics of those excluded due to missing urine toxicology test data.
Conclusions
Results from this study indicate that sensitivity of self-reported prenatal cannabis use during prenatal care is low and misclassification of use by self-report may vary with sociodemographic characteristics. Sensitivity of the urine toxicology test is higher, with greater detection of self-reported daily and weekly use versus monthly or less use. Results provide useful information to inform healthcare providers and researchers who are limited to maternal self-report of prenatal cannabis use. Given that many women chose not to disclose prenatal cannabis use in healthcare settings, it is important that clinicians educate all patients of reproductive age about the potential risks of prenatal cannabis use and advise prenatal patients to avoid using cannabis during pregnancy.
Supplementary Material
Acknowledgements
This study was supported by a NIH NIDA K01 Award (DA043604).
We thank Agatha Hinman for her assistance with manuscript preparation and Dr. Mishka Terplan for his helpful comments on a prior draft of this manuscript.
Footnotes
All authors declare that they have no conflicts of interest.
References
- Armstrong MA, Lieberman L, Carpenter DM, et al. Early Start: an obstetric clinic-based, perinatal substance abuse intervention program. Qual Manag Health Care 2001;9(2):6–15. [DOI] [PubMed] [Google Scholar]
- Child Welfare Information Gateway. (2016). Parental drug use as child abuse. Washington, DC: U.S. Department of Health and Human Services, Children’s Bureau. Available at: https://www.childwelfare.gov/topics/systemwide/laws-policies/statutes/drugexposed/. [Google Scholar]
- Committee on Obstetric Practice. Committee opinion no. 722: Marijuana use during pregnancy and lactation. Obstet Gynecol 2017;130(4):e205–e209. [DOI] [PubMed] [Google Scholar]
- ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC. Changes in cannabis potency over the last 2 decades (1995–2014): Analysis of current data in the United States. Biol Psychiatry 2016;79(7):613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forray A, Merry B, Lin H, Ruger JP, Yonkers KA. Perinatal substance use: a prospective evaluation of abstinence and relapse. Drug Alcohol Depend 2015;150:147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg M, Garrison L, Leeman L, et al. Validity of self-reported drug use information among pregnant women. Matern Child Health J 2016;20(1):41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon NP. Characteristics of adult members in Kaiser Permanente’s Northern California Region, as estimated from the 2011 Kaiser Permanente Adult Member Health Survey. Kaiser Permanente California, Oakland CA. Oakland CA, 2013. Available at: https://divisionofresearch.kaiserpermanente.org/projects/memberhealthsurvey/SiteCollectionDocuments/mhs11reg.pdf. Accessed June 27, 2018. [Google Scholar]
- Gordon NP. Similarity of the adult Kaiser Permanente membership in Northern California to the insured and general population in Northen California: statistics from the 2011–2012 California Health Interview Survey. Kaiser Permanente Division of Research, Oakland CA; June, 2015. Available at: https://divisionofresearch.kaiserpermanente.org/projects/memberhealthsurvey/SiteCollectionDocuments/chis_non_kp_2011.pdf. Accessed April 29, 2019. [Google Scholar]
- Gryczynski J, Schwartz RP, Mitchell SG, O’Grady KE, Ondersma SJ. Hair drug testing results and self-reported drug use among primary care patients with moderate-risk illicit drug use. Drug Alcohol Depend 2014;141:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konijnenberg C Methodological issues in assessing the impact of prenatal drug exposure. Subst Abuse 2015;9(Suppl 2):39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz TD, Silver RM, McMillin GA, et al. Prenatal marijuana use by self-report and umbilical cord sampling in a state with marijuana legalization. Obstet Gynecol 2019;133(1):98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc 2008;83(1):66–76. [DOI] [PubMed] [Google Scholar]
- National Committee for Quality Assurance. Initiation and Engagement of Alcohol and Other Drug Abuse or Dependence Treatment (IET). 2018. Available at: http://www.ncqa.org/report-cards/health-plans/state-of-health-care-quality/2017-table-of-contents/alcohol-treatment. Accessed April 18, 2019.
- Niedbala RS, Kardos KW, Fritch DF, et al. Detection of marijuana use by oral fluid and urine analysis following single-dose administration of smoked and oral marijuana. J Anal Toxicol 2001;25(5):289–303. [DOI] [PubMed] [Google Scholar]
- Roberts SC, Pies C. Complex calculations: how drug use during pregnancy becomes a barrier to prenatal care. Matern Child Health J 2011;15(3):333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby JV, Smith DH, Johnson ES, Raebel MA, Friedman GD, McFarland BH. (2005). Kaiser Permanente Medical Care Program. In: Strom BL, ed. Pharmacoepidemiology (4th ed., pp. 241–259). New York: Wiley. [Google Scholar]
- Terhune C Report: Kaiser tops state health insurance market with 40% share. Los Angeles Times; January 29, 2013. Available at: http://articles.latimes.com/2013/jan/29/business/la-fi-mo-health-insure-market-20130129. Accessed April 29, 2019. [Google Scholar]
- Young-Wolff KC, Sarovar V, Tucker LY, et al. Self-reported daily, weekly and monthly marijuana use among women before and during pregnancy, 2009–2017. JAMA Network Open in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Wolff KC, Tucker LY, Alexeeff S, et al. Trends in self-reported and biochemically tested marijuana use among pregnant females in California from 2009–2016. JAMA 2017;318(24):2490–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


