Abstract
We investigated the plasma toxicokinetic behavior of free (parent) and total (parent and conjugated forms) of bisphenol S (BPS) and bisphenol AF (BPAF) in plasma of adult male rats and mice following exposure via feed for 7 d to BPS (338, 1125, and 3375 ppm) or BPAF (338, 1125, and 3750 ppm).
In rats, the exposure concentration-normalized maximum concentration (Cmax/D (ng/mL)/(ppm)) and area under the concentration time curve (AUC/D (h*ng/mL)/(ppm)) for free was higher for BPS (Cmax/D: 0.476–1.02; AUC/D: 3.58–8.26) than for BPAF (Cmax/D: 0.017–0.037; AUC/D:0.196–0.436).
In mice, the difference in systemic exposure parameters between free BPS (Cmax/D: 0.376–0.459; AUC/D: 1.52–2.54) and free BPAF (Cmax/D: 0.111–0.165; AUC/D:0.846–1.09) was marginal.
Elimination half-lives for free analytes (4.41 to 10.4 h) were comparable between species and analogues.
When systemic exposure to free analyte was compared between species, in rats, BPS exposure was slightly higher but BPAF exposure was much lower than in mice.
BPS and BPAF were highly conjugated; total BPS AUC values (rats ≥ 18-fold, mice ≥ 17-fold) and BPAF (rats ≥ 127-fold, mice ≥ 16-fold) were higher than corresponding free values.
Data demonstrated that there are analogue and species differences in the kinetics of BPS and BPAF.
Keywords: bisphenol AF, bisphenol S, toxicokinetics, rat, mouse, oral exposure
Introduction
Bisphenol S (4,4’-sulfonyldiphenol, BPS) and bisphenol AF (2,2-bis(4-hydroxyphenyl)perfluoropropane, BPAF) (Figure 1) are structural analogues of bisphenol A (2,2-bis(4-hydroxyphenyl)propane) and are used in a number of applications. BPS is a component of polyether sulfone, a thermoplastic. Polyether sulfone is used in a variety of consumer products and applications, including but not limited to, developer in thermal paper, wash fastening agent in cleaning products, and in microwaveable dishes (Liao et al., 2012c; Rochester and Bolden, 2015). In addition, BPS is used as a chemical intermediate in the preparation of fire retardants, electroplating chemicals and colorant, and as a modifier for leather, fiber, and epoxy curing agents (EPA, 2014). BPAF is used as a crosslinking agent in the synthesis of certain fluoroelastomers (fluorocarbon-based synthetic rubber) and as a monomer in the synthesis of specialty polymers (DuPont, 2006; Halocarbon, 2008; NTP, 2008).
Figure 1.
Structure of A) bisphenol S and B) bisphenol AF.
BPS and BPAF have been detected in personal care products, food stuffs and/or indoor house dust (Liao et al., 2012a; Liao et al., 2012b; Liao et al., 2012c; Liao et al., 2012d; Liao et al., 2012e; Liao and Kannan, 2013). In addition, there is growing evidence of exposure to these analogues, primarily BPS, in the general population based on urinary biomarkers (Liao et al., 2012a; Liao et al., 2012b; Yang et al., 2014; Ye et al., 2015; Thayer et al., 2016; Lehmler et al., 2018; Philips et al., 2018; Wan et al., 2018a; Wan et al., 2018b; Ghayda et al., 2019; Frederiksen et al., 2020; Zhang et al., 2020) although in an EU biomonitoring study of 144 adults urinary BPAF was below the limit of detection (LOD) of 0.02 μg/mL (Husoy et al., 2019). BPAF has been reported in human breast milk, plasma, and serum including maternal and fetal pairs (Jin et al., 2018; Song et al., 2019; Jin et al., 2020; Li et al., 2020; Pan et al., 2020; Zhang et al., 2020).
Lack of adequate data to assess potential adverse human health effects from exposure to BPS and BPAF led the National Toxicology Program (NTP) to study these compounds following exposure of rodents via feed (NTP, 2020a; NTP, 2020b). We have previously reported the absorption, distribution, metabolism, excretion and toxicokinetic (TK) data for BPS and BPAF in rats and mice following a single gavage administration (Waidyanatha et al., 2015; Waidyanatha et al., 2018; Waidyanatha et al., 2019; Waidyanatha et al., 2020). This work compares the TK behavior of BPS and BPAF following repeated exposures via feed in rats and mice in support of the toxicology studies conducted via feed exposure.
Materials and Methods
Chemicals and reagents:
Bisphenol S (BPS) (lot IF20140175) was procured from Ivy Fine Chemicals (Cherry Hills, NJ). Bisphenol AF (BPAF) (CASRN 1478–61-1; lot # 20100425) was obtained from 3B Pharmchem International (Wuhan Co., Ltd., China). The chemical identity was confirmed by infrared spectroscopy, 1H and 13C nuclear magnetic resonance spectroscopy, mass spectrometry, and elemental analysis. The purity was determined using high-performance liquid chromatography (HPLC) with ultraviolet detection (UV) at 210 nm. The purity of BPS and BPAF lots were > 99%. [2H8]BPS and [13C12]BPAF used as internal standards were obtained from Toronto Research Chemicals (Toronto, Canada) and Cambridge Isotope Laboratories, Inc. (Tewksbury, MA), respectively. Male Sprague Dawley rat plasma to be used for matrix calibration curve preparation was obtained from Bio IVT (Westbury, NY). β-glucuronidase from Helix pomatia (at least 100,000 units/g and 7500 units/g for β-glucuronidase and sulfatase activity, respectively) was obtained from Sigma-Aldrich (St. Louis, MO). All other chemicals and reagents were procured from commercial sources.
Animals and maintenance:
BPS and BPAF studies were conducted at Mispro Biotech Services (RTP, NC) and MRIGlobal (Kansas City, MO), respectively, and were approved by the respective Institutional Animal Care and Use Committees. Animals were individually housed in facilities that are fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Animal procedures were in accordance with the “Guide for the Care and Use of Laboratory Animals” (NRC, 2011). Male Hsd:Sprague Dawley® SD® (HSD) rats were obtained from Envigo (Hasslett, MI or Somerset, NJ). Male B6C3F1/N mice were obtained from Taconic Farms (Germantown, NY). The animals were quarantined for at least one week before they were randomized into dosing groups and used in a study. Mice and rats had ad libitum access to certified, irradiated NTP 2000 feed (Ziegler Bros, Inc., Gardners, PA) and certified, irradiated, Advanced Protocol Verified Casein Diet 1 IF, (5K96, PMI Nutrition International, St. Louis, MO), respectively. Feed formulations of BPS and BPAF were prepared in certified, irradiated NTP 2000 for mouse studies (Ziegler Brothers Inc., Gardners, PA) and in 5K96 diet for rat studies (PMI Nutrition International, St. Louis, MO) to be consistent with the NTP toxicology studies (NTP, 2020a; NTP, 2020b). Feed was used within 180 days of milling. All animals had access to city tap water. Environmental conditions included: room temperature 71–75 °F, relative humidity 31–49% and a 12-h light/dark cycle. In BPS studies, male rats (284–336 g) and male mice (22.5–29.9 g) were 9–10 weeks old at the time of dosing. In BPAF studies, male rats (272–308 g) and male mice (21.2–27.6 g) were 8–9 weeks old at the time of dosing.
Study design and exposure:
Exposure concentrations used in BPAF studies were 338, 1125, and 3750 ppm. These were selected after taking into consideration the oral gavage doses used in prior ADME and TK studies (34–340 mg/kg) (Waidyanatha et al., 2015; Waidyanatha et al., 2018; Waidyanatha et al., 2019) and feed exposure concentrations used in NTP toxicology studies for BPAF (338, 1125, and 3750 ppm (NTP, 2020a). Concentration used BPS studies were 338, 1125, and 3375 ppm and were selected after taking into consideration gavage doses used in prior ADME and TK (Waidyanatha et al., 2018; Waidyanatha et al., 2020) studies as well as to be within a similar range as with BPAF feed study for direct comparison.
Feed formulations of BPS and BPAF were prepared in certified, irradiated NTP 2000 for mouse studies (Ziegler Brothers Inc., Gardners, PA) and in 5K96 diet for rat studies (PMI Nutrition International, St. Louis, MO) to be consistent with other NTP studies (Waidyanatha et al., 2015; Waidyanatha et al., 2018; Waidyanatha et al., 2019; NTP, 2020a; NTP, 2020c; Waidyanatha et al., 2020). Dosed-feed formulations were analyzed using validated liquid chromatography methods with ultraviolet detection prior to exposing animals (analytical concentration range: BPS, 3.2–96 μg/mL covering formulation range 400 to 12,000 ppm; BPAF 4–200 μg/mL covering formulation range 200 to 10,000 ppm; r ≥ 0.99; precision determined as relative standard deviation (RSD) ≤ 5%; accuracy determined as relative error (RE) ≤ ±10%). All study formulations were within 10% of the target concentration. Prior to study initiation, the stability of BPS and BPAF in feed formulations stored at refrigerated temperature up to 42 d (RE ≤ ± 10% of day 0 value) and homogeneity (RSD ≤ 5%) were confirmed.
Male rats (n=15/exposure group/compound) and mice (n=30/exposure group/compound) were exposed, in individual cages, ad libitum to dosed feed at 338, 1125, and 3375 ppm for BPS or 338, 1125, and 3750 ppm for BPAF for 7 days. Animal body weights and food consumption data were collected daily. Dosed feed was removed on the morning of the day representing the end of 7th 24-h exposure period when the lights were turned on and the control feed was provided through the 24 h blood collection period immediately following it. Blood samples were collected at various timepoints up to 24 h with all timepoints relative to the time lights were turned on. Two blood samples were collected from each rat while mice were sampled only once. In BPS studies, blood samples were collected from 3 animals per timepoint (except for some time points where n=4: rat 3375 ppm group, mouse 1125 and 3375 ppm groups) at 0, 1, 2, 4, 6, 9, 12, 16, 20, and 24 h. The first blood sample collection in rats was by tail venipuncture (ca. 250 μL). In BPAF studies, blood samples were collected from 3 animals per timepoint at 0, 1, 2, 4, 7, 10, 13, 16, 19, and 24 h. The first blood collection in rats was obtained via retro-orbital sinus (ca. 350 μL) under CO2:O2 (70:30). Terminal rat and all mouse sampling was by cardiac puncture following CO2 euthanasia. The actual times for blood collection were recorded at each time point. Immediately following collection, blood was dispensed into tubes containing K3EDTA, mixed by inversion and placed on ice. Plasma was prepared by centrifugation within 1 h of blood collection. Immediately following terminal blood collection, each animal was humanely terminated via exsanguination. All samples were stored at ≤−70 °C.
Quantitation of free and total BPS:
Samples from BPS studies were analyzed for free and total BPS using a validated analytical method as described previously (Silinski et al., 2020; Waidyanatha et al., 2020). Briefly, two stock solutions of BPS were prepared in methanol at 100 μg/mL and further diluted in the same solvent to generate spiking solutions (25 to 5000 ng/mL), using alternate stocks. A stock solution of (2H8)BPS to be used as the internal standard was prepared in methanol at 2250 ng/mL. The deconjugating enzyme solution containing β-glucuronidase and sulfatase for total BPS determination was prepared in 1M ammonium acetate buffer (pH 5) to yield a final solution of at least 2 units/μL β-glucuronidase and 0.4 units/μL sulfatase. Seven-point matrix calibration curves (5 to 1000 ng/mL), QC samples (10 and 500 ng/mL), matrix blanks, and study samples were prepared as below.
To a 50 μL aliquot of plasma, 10 μL of methanol or appropriate BPS spiking solution (matrix calibration standards and QC samples only), and 10 μL of internal standard solution was added. To all samples, 25 μL of 1 M ammonium acetate buffer (for free analysis) or deconjugating enzyme solution (total analysis) was added. Samples were briefly mixed and either stored at ~ 5 °C for 2 h (for free analysis) or incubated at ~ 37 °C for 2 h (total analysis) (Note: 2 h digestion was demonstrated to be sufficient for deconjugation of BPS conjugates). Matrix blanks and study samples were prepared similarly except no BPS was added. To each sample, 300 μL of acetonitrile was added, mixed briefly, and centrifuged at 4 °C for 10 min at ~20,000 g. The supernatant was analyzed by liquid chromatography- tandem mass spectrometry.
A linear regression with 1/X2 weighing was used to relate analyte to internal standard peak area response ratio of analyte to internal standard and concentration of BPS in plasma. The concentration of free and total BPS was calculated using response ratio, the regression equation, initial sample volume, and dilution when applicable. Study samples that exceeded the matrix calibration range were diluted into the validated concentration range using respective control matrix. Each study sample set was bracketed by method blanks, matrix calibration standards, and QC samples. Data from study samples were considered valid if: the matrix calibration curve was linear (r ≥0.99); at least 75% of matrix standards were within 15% of nominal values (except at the limit of quantitation (LOQ) where it was 20%); at least 67% of the QC samples were within 15% of nominal values. All QCs were within 15% of nominal value. The LOQ for both free and total analyses was 5 ng/mL. The LOD, estimated as the standard error (SE) of the LOQ was 1.15 ng/mL for free BPS and 0.862 ng/mL for total BPS. All concentrations above LOD were reported.
Quantitation of free and total BPAF:
Samples were analyzed for free and total BPAF using a validated analytical method as described previously (Waidyanatha et al., 2019). Briefly, two stock solutions of were prepared in methanol at 750 and 1000 μg/mL and further diluted in the same solvent to generate spiking solutions (28 to 1000 ng/mL), using alternate stocks. A stock solution of (13C12)BPAF to be used as the internal standard was prepared in acetonitrile at 600 ng/mL. Eight-point matrix calibration curves (2.8 to 100 ng/mL), QC samples (5 and 80 ng/mL), matrix blanks, and study samples were prepared as below.
For determination of total BPAF, to 50 μL plasma, 10 μL of 0.9% sodium chloride, 5 μL of (13C12)BPAF solution, 25 μL of 200 mM sodium acetate buffer (pH 5), and 5 μL of appropriate BPAF spiking solutions (matrix calibration standards and QC samples only) were added along with 10 μL of β-glucuronidase and sulfatase solution (100,000 units/mL and 7500 units/mL for β-glucuronidase and sulfatase activity, respectively) and samples were incubated at ~ 37°C overnight. For the determination of free BPAF, 50 μL aliquots of plasma were transferred to vials with 5 μL of (13C12)BPAF solution and 5 μL of appropriate spiking solutions (matrix calibration standards and QC samples only). To all samples (free and total measurements), acetonitrile (145 μL for free and 245 μL for total) was added and samples were vortexed for 30 sec and centrifuged at 16,000g for 5 min. The supernatant was analyzed by liquid chromatography- tandem mass spectrometry.
A linear regression with 1/X weighting was used to relate peak area response ratio of analyte to internal standard and concentration of BPAF in plasma. The concentration of free and total BPAF were calculated using response ratio, the regression equation, initial sample volume, and dilution when applicable. Study samples that exceeded the matrix calibration range were diluted into the validated concentration range using control plasma which has been demonstrated to provide accurate data during the method validation (Waidyanatha et al., 2019).
Each study sample set was bracketed by method blanks, matrix calibration standards, and QC samples. Data from study samples were considered valid if: the matrix calibration curve was linear (r ≥0.99); at least 75% of matrix standards were within 15% of nominal values (except at the LOQ where it was 20%); at least 67% of the QC samples were within 15% of nominal values. All QCs were within 15% of nominal value. The LOQ and LOD were 2.8 ng/mL and 0.8 ng/mL.
Toxicokinetic analysis.
All plasma concentrations above LOD were used in TK analysis. Where actual blood collection times were >10% from nominal, the mean of the actual collection time for each group per time point was used. Due to the nature of the study design where the exposure was via feed for 7 days and the samples were collected at timed intervals after the final day of exposure, the mean plasma concentration versus time data were evaluated using noncompartmental analysis (NCA) with uniform weighting using 0–24 h data to allow for direct comparisons between the two compounds and speceis (Phoenix WinNonlin, Version 6.4, Certara, Princeton, NJ). Model 200 was used for extravascular administration. The following parameters were reported: the maximum concentration (Cmax); area under the concentration time curve (AUC); lambda half-life (elimination half-life).
Results
Data are available in the Chemical Effects in Biological Systems (CEBS) database:
https://doi.org/10.22427/NTP-DATA-002–03263-0011–0000-8
https://doi.org/10.22427/NTP-DATA-002–03311-0008–0000-8
Body weights and food consumption following exposure to BPS.
All animals survived until the end of the study. Animals were weighed during acclimation and then daily in the morning during the study. The overall mean daily body weight gain for all rats assigned to the study was 3.7 ± 2.6 g during the pre-study period when the animals were offered control feed. During the study, the group mean daily body weight gain was 3.7 ± 4.4 g, 3.7 ± 2.7 g, and 2.6 ± 5.9 g for rats in the 338, 1125, and 3375 ppm exposure groups, respectively. Mean body weight gain was reduced in the animals in the high exposure group compared with the pre-study and the other two exposure groups which is consistent with the decreased feed consumption noted for this group (see below). For mice the overall mean daily body weight gain was 0.11 ± 0.48 g during the pre-study period when the animals were on control feed. In mice the group mean daily body weight gain was 0.1 ± 0.4 g, 0.1 ± 0.3 g, and 0.1 ± 0.3 g for the 338, 1125, and 3375 ppm groups, respectively, demonstrating that there is no difference in mouse body weight gain between different exposure concentrations.
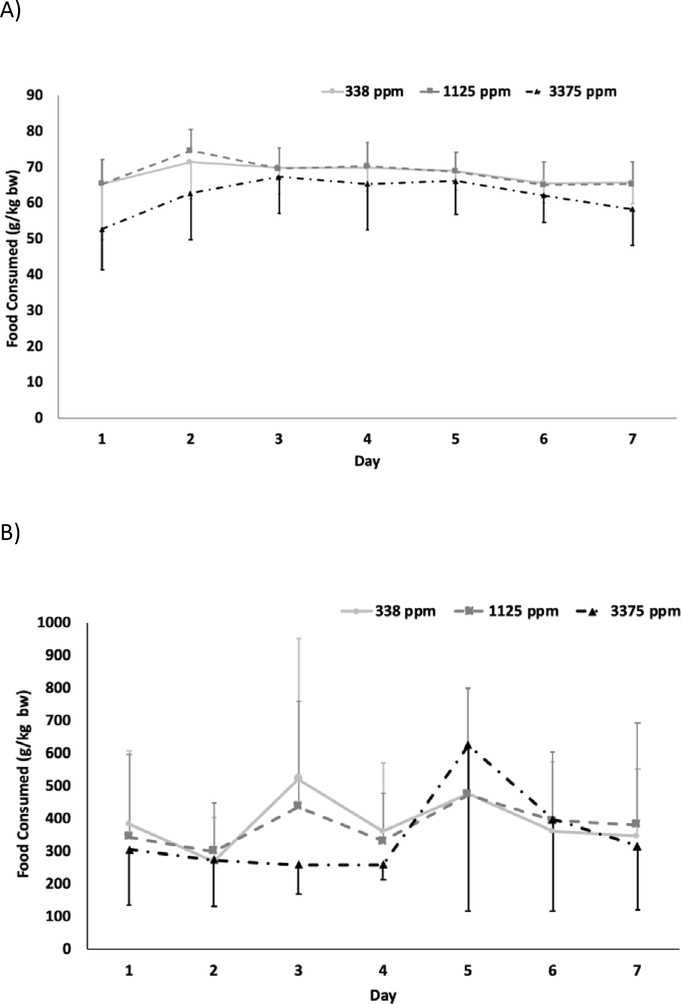
The average daily feed consumption for rats is shown in Figure 2A for each day during the 7 d of exposure. The data show that 3375 ppm exposure groups consumed less feed throughout the exposure period than those in the two lower exposure groups. Using all data from the duration of study, average daily feed consumption values estimated for 338, 1125 and 3375 ppm, respectively, were 68 ± 2.5, 68 ± 3.5, and 62 ± 5.1 g/kg bw/d demonstrating slightly lower average consumption for the highest exposure group. This value (62 ± 5.1 g/kg bw/d) was also lower than the pre-study control feed consumption (71.1 ± 5.86 g/kg bw/d), potentially reflecting a palatability issue at the highest BPS exposure concentration. The average daily dose received (mg BPS/kg bw/day) was calculated over the 7-day exposure period from the feed consumption, animal body weights, and BPS concentration in feed and are given in Table 1 for the 3 exposure groups. The average daily dose increased linearly with the exposure concentration with 23.0 and 76.8 mg/kg bw/day for 338 and 1125 ppm groups, respectively. At 3375 ppm, consistent with lower food consumption observed, the estimated dose was 209 mg/kg bw/day, a 9-fold increase with a 10-fold increase in the exposure concentration (Table 1).
Figure 2.
Daily feed consumption in A) male rats B) male mice during exposure to bisphenol S via feed.
Table 1.
Average daily dose of bisphenol S estimated from feed consumption following exposure of male rats and mice to bisphenol S for seven days via feeda
| BPS consumption (mg BPS/kg bw/day) | ||
|---|---|---|
| Male Rats | Male Mice | |
| 338 ppm | 23.0 ± 0.84 | 131.4 ± 28.6 |
| 1125 ppm | 76.8 ± 3.94 | 428 ± 68.6 |
| 3375 ppm | 209 ± 17.3 | 1176 ± 444 |
Seven-day average ± standard error for 3 animals are given. Dose was estimated using cage food consumption, exposure concentration, and animal body weight.
The average daily feed consumption for each day during 7 d study duration is shown in Figure 2B for mice. A high variability was observed in the amount consumed within each exposure group. This is likely due to the observed behavior of mice in each group removing food from the jars but leaving it unconsumed in the cage. Although there is high variability in the apparent amount consumed within each dose group, there is not much difference in the mean consumption across dose groups (Figure 2B). The average food consumed during study, estimated as an average from 7 d exposure period, were 389 ± 85, 381 ± 61, and 348 ± 131 g/kg bw/d for 338, 1125, and 3375 ppm groups, respectively. These values are all 1.4–1.5 fold higher than the pre-study control feed consumption (255 g/kg bw/d) which is probably due to the high spillage rate, a behavior likely driven by palatability issues. The average daily dose received (mg BPS/kg bw/day) was calculated over 7-day dosing period from the apparent feed consumed, animal body weights, and the BPS concentration in feed and are given in Table 1. Although the calculated average doses increased linearly with the exposure concentration, the values are likely higher than actual BPS dose received by mice due to the documented feed spillage for all mouse groups.
Body weights and food consumption following exposure to BPAF.
All animals survived until the end of the study. Animals were weighed during acclimation and daily during the study. The overall mean daily body weight gain for all rats assigned to the study was 4.7 ± 1.7 g during the pre-study period when the animals were offered control feed. The group mean daily body weight gain was 3.38 ± 3.37 g, 2.12 ± 3.68 g, and −0.4 ± 2.3 for rats in the 338, 1125, and 3750 ppm dose groups, respectively, showing a decrease in body weight gain with increasing exposure concentration. For mice, the overall mean daily body weight gain was 0.1 ± 0.1 g during the pre-study period on the control diet. In mice the group mean daily body weight gain was 0.3 ± 0.5 g, 0.1 ± 0.6 g, and 0.1 ± 0.7 g for the 338, 1125, and 3750 ppm groups, respectively demonstrating that the gain for the mid and high dose are lower than for the low dose.
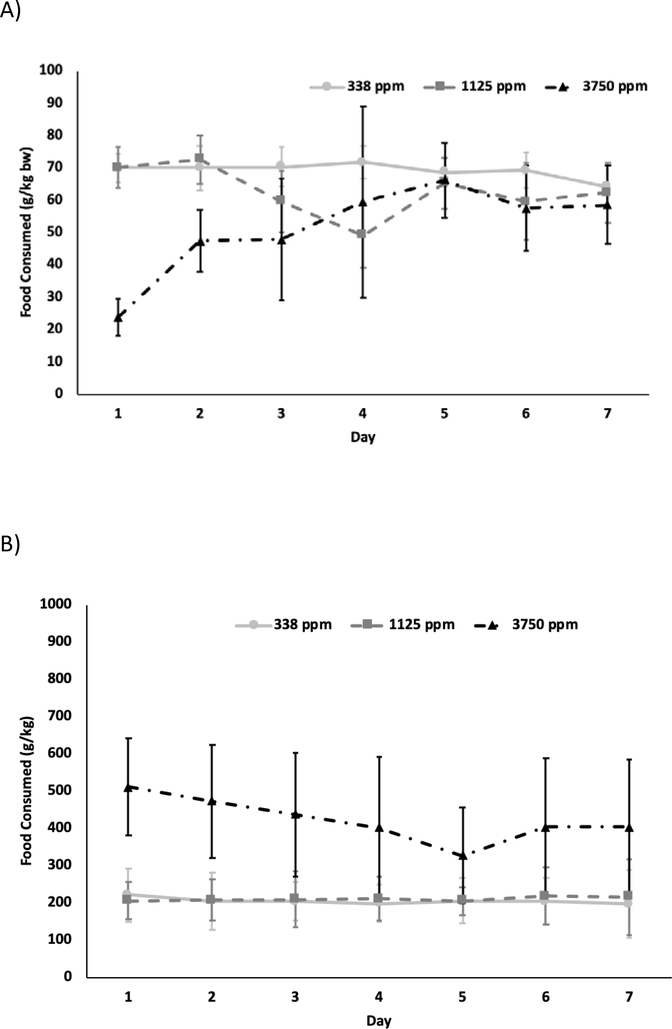
The average daily feed consumption for rats over 7 d study duration is shown in Figure 3A for each day of exposure. After an initial decrease in consumption on Day 1, the animals in the 3750 ppm exposure group acclimated to the dose and consumed a similar amount of feed to the other dose groups. Signs of food spillage by rats were not observed in any exposure group. Average daily food consumption calculated using the seven day consumption data were 69.2 ± 5.8, 61.0 ± 11.7, and 51.5 ± 20.5 g/kg bw/d for 338, 1125 and 3750 ppm, respectively. The average daily dose received (mg BPAF/kg bw/day) was calculated from the feed consumption, body weight, and BPAF concentration in feed and the seven-day average is given in Table 2. The average dose increased less than proportionally with the exposure concentration with 8-fold increase in the dose (23.4–193 mg BPAF/kg b w/d) with 11-fold increase in exposure concentration (338–3750 ppm) (Table 2).
Figure 3.
Daily feed consumption in A) male rats B) male mice during exposure to bisphenol AF via feed.
Table 2.
Average daily dose of bisphenol AF estimated from feed consumption following exposure of male rats and mice to bisphenol AF for seven days via feeda
| BPAF consumption (mg BPAF/kg bw/day) | ||
|---|---|---|
| Male Rats | Male Mice | |
| 338 ppm | 23.4 ± 0.80 | 69.4 ± 2.48 |
| 1125 ppm | 70.5 ± 8.74 | 236 ± 5.51 |
| 3750 ppm | 193 ± 52.2 | 1590 ± 225 |
Seven-day average ± standard error for 3 animals are given. Dose was estimated using cage food consumption, exposure concentration, and animal body weight.
In the mouse 3750 ppm group, food spillage was noted on multiple days and in a few instances in the 1125 ppm group. No food spillage was observed in the 338 ppm group. The average daily feed consumption is shown in Figure 3B for each day during the exposure duration. There is high variability in the amount consumed in the 3750 ppm likely reflecting the observed spillage/unconsumed feed observed in the cage. The average feed consumed calculated using the seven day consumption data were 207 ± 68, 211 ± 68, and 424 ± 172 g/kg bw/d for 338, 1125, and 3375 ppm groups, respectively. At 3750 ppm, this value is 1.7- fold higher than the pre-study control food consumption (250 ± 134 g/kg bw/d) which is probably due to the high spillage rate, a behavior likely driven by palatability issues. The average daily dose received (mg BPAF/kg bw/d) was calculated from the food consumed, animal body weights, and the BPAF concentration in feed and the seven day- average is given in Table 2. The average daily dose increased linearly with the exposure concentration with 69.4 and 236 mg/kg b. w/d for 338 and 1125 ppm groups, respectively. However, the dose increased 23-fold when the exposure concentration increased 11-fold; the values are likely higher than actual BPAF dose received due to the documented feed spillage for 3750 ppm mouse group as mentioned above.
Toxicokinetic analysis of BPS in rats.
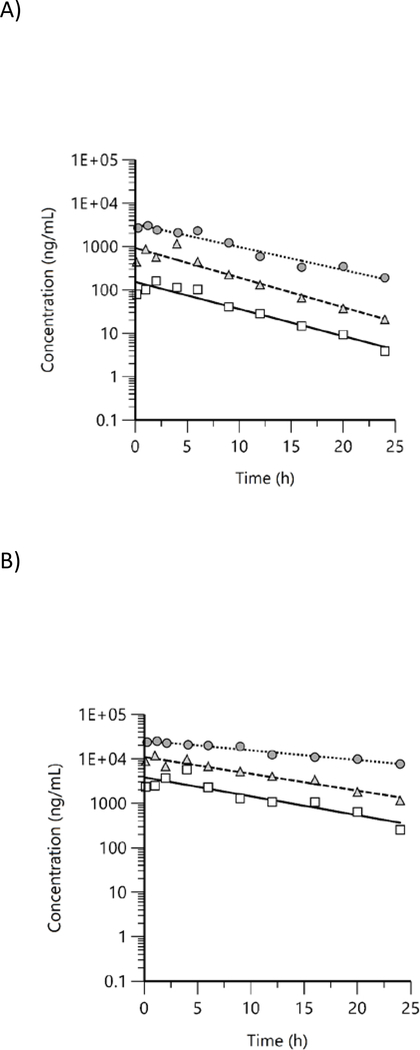
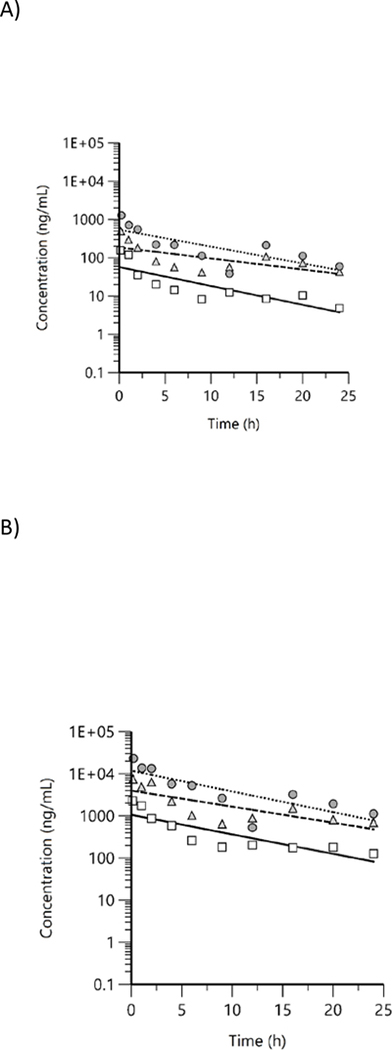
Free and total BPS concentrations were quantified in male rat plasma at 10 timepoints up to 24 h following the last day of exposure and were above LOD in all exposed groups at all timepoints. Concentration versus time plots showed a general decreasing trend with free BPS (Figure 4A) decreasing more rapidly than total BPS (Figure 4B). In addition, signs of enterohepatic recirculation were visible for some groups (e.g., free 1125 ppm group). Mean plasma concentration versus time data were evaluated using NCA and TK parameters estimated are given in Table 3.
Figure 4.
Plasma concentration versus time profiles of A) free and B) total bisphenol S in male rats following exposure to bisphenol S via feed for 7 d. Mean data were analyzed using non-compartmental analysis (squares: 338 ppm, triangles: 1125 ppm, circles: 3375 ppm).
Table 3.
Plasma toxicokinetic parameters of free and total bisphenol S in male rats following exposure to bisphenol S via feed for 7 days.
| Parametera | Exposure concentration (ppm) | ||
|---|---|---|---|
| 338 | 1125 | 3375 | |
| Free | |||
| Cmax (ng/mL) | 161 | 1150 | 3040 |
| Cmax/D (ng/mL)/(ppm) | 0.476 | 1.02 | 0.901 |
| Cmax/D (ng/mL)/(mg/kg)b | 7.00 | 15.0 | 14.5 |
| Elimination half-life (h)c | 4.82 | 4.41 | 5.69 |
| AUC (h*ng/mL) | 1210 | 7030 | 27900 |
| AUC/D (h*ng/mL)/(ppm) | 3.58 | 6.25 | 8.26 |
| AUC/D (h*ng/mL)/(mg/kg)b | 52.6 | 91.5 | 133 |
| Total | |||
| Cmax (ng/mL) | 5730 | 11800 | 24700 |
| Cmax/D (ng/mL)/(ppm) | 17.0 | 10.5 | 7.32 |
| Cmax/D (ng/mL)/(mg/kg) | 249 | 154 | 118 |
| Elimination half-life (h)c | 7.07 | 7.95 | 13.9 |
| AUC (h*ng/mL) | 43700 | 128000 | 510000 |
| AUC/D (h*ng/mL)/(ppm) | 129 | 114 | 151 |
| AUC/D (h*ng/mL)/(mg/kg)b | 1900 | 1667 | 2440 |
| Total/Free | |||
| Cmax (total)/ Cmax (Free) | 36 | 10 | 8 |
| AUC (total)/ AUC (Free) | 36 | 18 | 18 |
Based on non-compartmental analysis. Values from up to 4 animals are given.
Doses estimated from average chemical consumption was used to estimate dose-normalized values.
Lamda half-life is given.
Systemic exposure parameters, Cmax and AUC, for free BPS increased with the exposure concentration. Both the exposure concentration- and dose-normalized values are given in Table 3. The exposure concentration-normalized values for Cmax were 0.476, 1.02, and 0.901 (ng/mL)/(ppm) for 338, 1125, and 3375 ppm, respectively, demonstrating the increase was proportional only in the two highest groups. AUC values increased more than proportionally to the exposure concentration with normalized values of 3.58, 6.25, and 8.26 (h*ng/mL)/(ppm) for 338, 1125, and 3375 ppm, respectively. A similar pattern of behavior was observed for Cmax and AUC based on the dose-normalized values. Plasma elimination half-lives were similar (4.41–5.69 h) regardless of the exposure concentration (Table 3).
Total BPS Cmax and AUC values also increased with the exposure concentration (Table 3). Similar to free BPS, the increase in Cmax was approximately proportional for 1125 and 3375 ppm with exposure concentration-normalized values of 10.5 and 7.32 (ng/mL)/(ppm), respectively. However, for 338 ppm, the value was higher at 17.0 (ng/mL)/(ppm) which was opposite from free BPS where a lower value than 125 and 3375 ppm was observed. Total BPS AUC Increased approximately proportional to the exposure concentration. A similar pattern of behavior for Cmax and AUC was observed based on the dose-normalized values (Table 3). Total BPS plasma elimination half-lives increased with the exposure concentration with 7.07, 7.95, and 13.9 h for 338, 1125, and 3375 ppm, respectively (Table 3).
Total BPS Cmax and AUC values, respectively, were ≥ 8-fold and ≥ 18-fold higher than the corresponding free BPS values demonstrating extensive conjugation of BPS following dietary exposure to BPS in male rats (Table 3). The conjugation was higher at the lower concentration, suggesting potential saturation of metabolism at the higher exposure concentrations (Table 3).
Toxicokinetic analysis of BPS in mice.
Free and total BPS concentrations were quantified in male mouse plasma at 10 timepoints up to 24 h following the last day of exposure. In male mice administered BPS in feed at 338 ppm, free BPS concentrations in 2 of 4 samples at the 24 h timepoint were below the LOD. Free and total BPS were above LOD in all other exposed groups at all timepoints. Concentration versus time plots for free (Figure 5A) and total BPS (Figure 5B) showed a general decreasing trend. However, there was some evidence of enterohepatic circulation with periodic increases in concentration beginning ~ 12 h. Plasma concentration versus time data were evaluated with NCA and TK parameters estimated are given in Table 4.
Figure 5.
Plasma concentration versus time profiles of A) free and B) total bisphenol S in male mice following exposure to bisphenol S via feed for 7 d. Mean data were analyzed using non-compartmental analysis (squares: 338 ppm, triangles: 1125 ppm, circles: 3375 ppm).
Table 4.
Plasma toxicokinetic parameters of bisphenol S in male mice following exposure to bisphenol S via feed for 7 days.
| Parametera | Exposure concentration (ppm) | ||
|---|---|---|---|
| 338 | 1125 | 3375 | |
| Free | |||
| Cmax (ng/mL) | 155 | 496 | 1270 |
| Cmax/D (ng/mL)/(ppm) | 0.459 | 0.441 | 0.376 |
| Cmax/D (ng/mL)/(mg/kg)b | 1.18 | 1.16 | 1.08 |
| Elimination half-life (h)c | 6.11 | 10.4 | 6.82 |
| AUC (h*ng/mL) | 514 | 2860 | 5560 |
| AUC/D (h*ng/mL)/(ppm) | 1.52 | 2.54 | 1.65 |
| AUC/D (h*ng/mL)/(mg/kg)b | 3.91 | 6.68 | 4.73 |
| Total | |||
| Cmax (ng/mL) | 2280 | 7460 | 23200 |
| Cmax/D (ng/mL)/(ppm) | 6.75 | 6.63 | 6.87 |
| Cmax/D (ng/mL)/(mg/kg)b | 17.4 | 17.4 | 19.7 |
| Elimination half-life (h)c | 6.51 | 7.89 | 6.11 |
| AUC (h*ng/mL) | 10000 | 48400 | 111000 |
| AUC/D (h*ng/mL)/(ppm) | 29.6 | 43.0 | 32.9 |
| AUC/D (h*ng/mL)/(mg/kg)b | 76.1 | 113 | 94.4 |
| Total/Free | |||
| Cmax (total)/ Cmax (Free) | 15 | 15 | 18 |
| AUC (total)/ AUC (Free) | 19 | 17 | 20 |
Based on non-compartmental analysis. Values from up to 3 animals are given.
Doses estimated from average chemical consumption was used to estimate dose-normalized values.
Lamda half-life is given.
In male mice, Cmax and AUC values of free BPS increased approximately proportional to exposure concentration. The exposure concentration-normalized values of Cmax ranged from 0.376–0.459 (ng/mL)/(ppm) and AUC from 1.52–2.54 (h*ng/mL)/(ppm). The behavior was similar based on the dose of BPS received although it is likely that the dose calculated from feed consumption was higher than actual due to food spillage by mice. Plasma elimination half-lives were 6.11, 10.4, and 6.82 h for 338, 1125, and 3375 ppm, respectively, suggesting no apparent exposure concentration-related pattern in elimination half-life (Table 4).
Total Cmax and AUC also increased with the exposure concentration (Table 4). Similar to free BPS, the increase was approximately proportional to the exposure concentration. Plasma elimination half-lives of total BPS in mice (6.11–7.89 h) were similar to that of free BPS (6.11–10.4 h), with no apparent exposure concentration-related effect (Table 4).
Total BPS Cmax and AUC values were 15- to 18-fold and 17- to 20-fold higher, respectively, than the corresponding free BPS demonstrating extensive conjugation of BPS following dietary exposure to BPS in mice. There was no apparent exposure concentration-related effect (Table 4).
Toxicokinetic analysis of BPAF in rats.
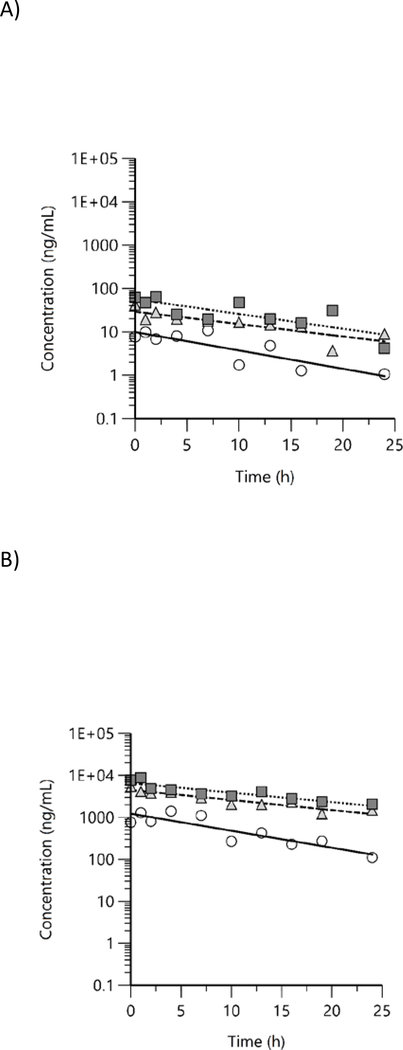
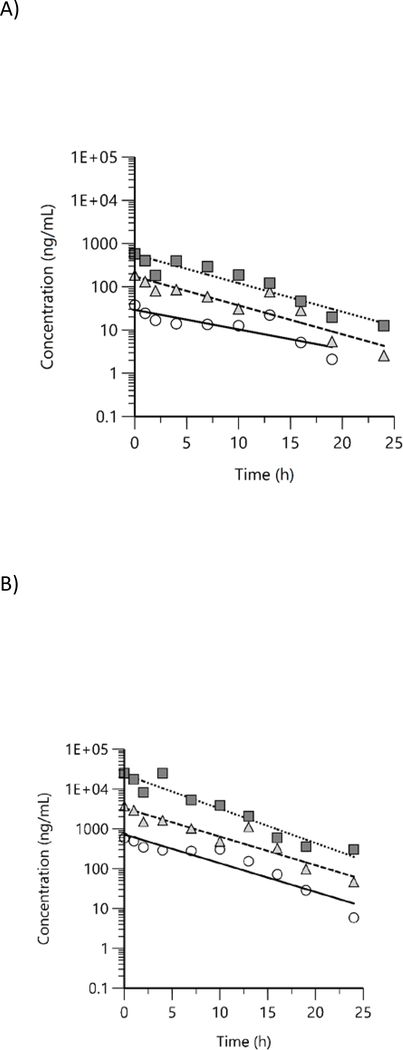
Free and total BPAF concentrations were quantified in male rat plasma at 10 timepoints up to 24 h following the last day of exposure. All values were above LOD except for free BPAF at 19 and 24 h time points in 338 ppm group. Concentration versus time plots for free and total BPAF in rat plasma show a general decreasing trend in both free (Figure 6A) and total BPAF (Figure 6B), however, there was some evidence of enterohepatic circulation with periodic increases in concentration beginning at about 8 h. Plasma concentration versus time data were evaluated with NCA and TK parameters estimated are given in Table 5.
Figure 6.
Plasma concentration versus time profiles of A) free and B) total bisphenol AF in male rats following exposure to bisphenol AF via feed for 7 d. Mean data were analyzed using non-compartmental analysis (squares: 338 ppm, triangles: 1125 ppm, circles: 3750 ppm).
Table 5.
Plasma toxicokinetic parameters of bisphenol AF in male rats following exposure to bisphenol AF via feed for 7 d.
| Exposure concentration (ppm) | |||
|---|---|---|---|
| Parametera | 338 | 1125 | 3750 |
| Free | |||
| Cmax (ng/mL) | 10.8 | 41.7 | 64.3 |
| Cmax/D (ng/mL)/(ppm) | 0.032 | 0.037 | 0.017 |
| Cmax/D (ng/mL)/(mg/kg)b | 0.462 | 0.591 | 0.333 |
| Elimination half-life (h)c | 7.10 | 10.5 | 8.83 |
| AUC (h*ng/mL) | 118 | 490 | 735 |
| AUC/D (h*ng/mL)/(ppm) | 0.349 | 0.436 | 0.196 |
| AUC/D (h*ng/mL)/(mg/kg)b | 5.04 | 6.95 | 3.81 |
| Total | |||
| Cmax (ng/mL) | 1400 | 5450 | 8810 |
| Cmax/D (ng/mL)/(ppm) | 4.14 | 4.84 | 2.35 |
| Cmax/D (ng/mL)/(mg/kg)b | 59.8 | 77.3 | 45.7 |
| Elimination half-life (h)c | 7.44 | 12.6 | 13.3 |
| AUC (h*ng/mL) | 15000 | 85700 | 127000 |
| AUC/D (h*ng/mL)/(ppm) | 44.4 | 76.2 | 33.9 |
| AUC/D (h*ng/mL)/(mg/kg)b | 641 | 1216 | 658 |
| Total/Free | |||
| Cmax (total)/ Cmax (Free) | 130 | 131 | 137 |
| AUC (total)/ AUC (Free) | 127 | 175 | 173 |
Based on non-compartmental analysis. Values from up to 3 animals are given.
Doses estimated from average chemical consumption was used to estimate dose-normalized values.
Lamda half-life is given.
Systemic exposure parameters, Cmax and AUC, of free BPAF increased with the exposure concentration although there was an apparent less than exposure concentration-proportional increase at the highest concentration of 3750 ppm. This is likely due to lower food consumption and subsequently the lower chemical consumption estimated in 3750 ppm group (Table 2). The exposure concentration-normalized Cmax values were 0.032, 0.037, and 0.017 (ng/mL)/(ppm), and AUC values were 0.349, 0.436, and 0.196 (h*ng/mL)/(ppm) for 338, 1125, and 3750 ppm, respectively. A similar pattern was observed based on the dose estimated from BPAF consumption where the increase of Cmax and AUC was less-than dose proportional (Table 5). Plasma elimination half-lives were similar between exposure groups (7.10–10.5 h).
Total Cmax and AUC also increased with the exposure concentration. Similar to free BPAF, the increase was less than proportional to the exposure concentration at the highest group with normalized Cmax values of 4.14, 4.84, and 2.35 (ng/mL)/(ppm) and AUC values 44.4, 76.2, and 33.9 (h*ng/mL)/(ppm) for 338, 1125, and 3750 ppm, respectively. Total BPAF elimination half-lives were 7.44, 12.6, and 13.3 h for 338, 1125, and 3375 ppm, respectively, suggesting an increase in half-life with increasing exposure concentration.
Cmax and AUC of total BPAF were much higher than the corresponding free values demonstrating extensive conjugation of BPAF following dietary exposure to BPAF in rats. Total Cmax values were 130- to 137-fold and total AUC values were 127- to 175-fold higher than the corresponding free values (Table 5).
Toxicokinetic analysis of BPAF in mice.
Free and total BPAF concentrations were quantified in male mouse plasma at 10 timepoints up to 24 h following the last day of exposure. Free and total BPAF were above LOD in all exposed groups at all timepoints except in 338 ppm 19 and 24 h timepoints and in 1125 ppm at 24 h timepoint. As with rats, concentration versus time plots for free (Figure 7A) and total BPAF (Figure 7B) show a general decreasing trend in both free and total BPAF concentrations over time, with some evidence of enterohepatic circulation with periodic increases in concentration beginning at about 8 h. Plasma concentration versus time data were evaluated with NCA and TK parameters estimated are given in Table 6.
Figure 7.
Plasma concentration versus time profiles of A) free and B) total bisphenol AF in male mice following exposure to bisphenol AF via feed for 7 d. Mean data were analyzed using non-compartmental analysis (squares: 338 ppm, triangles: 1125 ppm, circles: 3750 ppm).
Table 6.
Plasma toxicokinetic parameters of bisphenol AF in male mice following exposure via feed to bisphenol AF for 7 d.
| Parametera | Exposure concentration (ppm) | ||
|---|---|---|---|
| 338 | 1125 | 3750 | |
| Free | |||
| Cmax (ng/mL) | 37.4 | 186 | 574 |
| Cmax/D (ng/mL)/(ppm) | 0.111 | 0.165 | 0.153 |
| Cmax/D (ng/mL)/(mg/kg)b | 0.539 | 0.788 | 0.361 |
| Elimination half-life (h)c | 6.66 | 4.50 | 4.55 |
| AUC (h*ng/mL) | 286 | 1190 | 4080 |
| AUC/D (h*ng/mL)/(ppm) | 0.846 | 1.06 | 1.09 |
| AUC/D (h*ng/mL)/(mg/kg)b | 4.12 | 5.04 | 2.57 |
| Total | |||
| Cmax (ng/mL) | 586 | 3720 | 25200 |
| Cmax/D (ng/mL)/(ppm) | 1.73 | 3.31 | 6.72 |
| Cmax/D (ng/mL)/(mg/kg)b | 8.44 | 15.8 | 15.9 |
| Elimination half-life (h)c | 4.17 | 4.19 | 3.49 |
| AUC (h*ng/mL) | 4610 | 20800 | 144000 |
| AUC/D (h*ng/mL)/(ppm) | 13.6 | 18.5 | 38.4 |
| AUC/D (h*ng/mL)/(mg/kg)b | 66.4 | 88.1 | 90.6 |
| Total/Free | |||
| Cmax (total)/ Cmax (Free) | 16 | 20 | 44 |
| AUC (total)/ AUC (Free) | 16 | 18 | 35 |
Based on non-compartmental analysis. Values from up to 3 animals are given.
Dose estimated from average chemical consumption was used to estimate dose-normalized values.
Lamda half-life is given.
In male mice, Cmax and AUC for free BPAF increased approximately in proportion to the exposure concentration. Exposure-concentration normalized values of Cmax were 0.111, 0.165, and 0.153 (ng/mL)/(ppm) and AUC values were 0.846, 1.06, and 1.09 for 338, 1125, and 3750 ppm, respectively.
The total BPAF Cmax and AUC increased more than proportional to the exposure concentration with normalized Cmax values of 1.73, 3.31, and 6.72 (ng/mL)/(ppm) and AUC values 13.6, 18.5, and 38.4 (h*ng/mL)/(ppm) for 338, 1125, and 3750 ppm, respectively. Plasma elimination half-lives for free (4.50–6.66 h) and total BPAF (3.49–4.18 h) were similar with no apparent exposure concentration related effect. BPAF was highly conjugated following exposure via feed in mice as evidenced by the 16- to 44-fold and 16- to 35-fold higher total Cmax and AUC, respectively, compared to the corresponding free values (Table 5).
Discussion
There is a strong interest in understanding any adverse human health effects from exposure to the bisphenol analogues due to potential endocrine effects of these analogues. To this end, the NTP is investigating the potential toxicity of BPS and BPAF following exposure of rodents via feed (NTP, 2020a; NTP, 2020b). We previously showed that following a single gavage administration of BPAF or BPS in rodents, these analogues were well-absorbed, rapidly distributed, and underwent extensive first-pass metabolism leading to low bioavailability (Waidyanatha et al., 2015; Waidyanatha et al., 2018; Waidyanatha et al., 2019; Waidyanatha et al., 2020). The current investigation was aimed at generating TK data following repeated exposure via feed to put toxicological findings into greater context and to address any kinetic differences between a single gavage and multiple feed administration of these analogues in rodents.
There are some species similarities and differences in TK behavior of BPS following exposure via feed. In general, free and total BPS Cmax values were similar between rats and mice and was within 2-fold difference. However, there was a distinct species difference based on both free and total AUC. Free BPS AUC values in rats were 2- to 5-fold higher compared to mice. In addition, while the free BPS AUC increased linearly with the exposure concentration in mice, in rats the AUC increased more than proportional to the exposure concentration suggesting saturation of clearance pathways operating at these concentrations. Total BPS AUC values were 3- to 5-fold higher in rats compared to mice. Taken collectively these data demonstrate that rats had a slightly higher systemic exposure to BPS than mice following exposure to similar concentrations via feed. The ratio of Cmax total/free (rats, 8–36; mice, 15–18) and AUC total/free (rats, 18–36; mice, 17–20) (Tables 3 and 4) were within a 2-fold between rats and mice suggesting that the extent of conjugation of BPS was similar between the two species. The plasma elimination half-lives were also within 2-fold between the two species for both free and total BPS.
Following exposure of rats and mice to BPAF via feed, mice had higher overall systemic exposure to the parent BPAF than rats. The exposure concentration-normalized free BPAF Cmax values were 4- to 9-fold and AUC were 2- to 6-fold higher in mice than in rats; the difference was higher at the higher exposure concentration likely due to lower chemical consumption observed in rats at the highest exposure concentration of 3750 ppm. The opposite was true for total BPAF with rats having higher levels compared to mice but only at the lower exposure concentration. At the highest exposure concentration, in rats, the total concentrations were either lower (Cmax) or similar (AUC) to mice, the difference again likely due to lower chemical consumption in rats. The ratio of total/free Cmax (rats, 130–137; mice, 16–44) and AUC (rats, 127–175; mice, 16–35) was several fold higher in rats compared to mice demonstrating that the extent of conjugation of BPAF in rats was much higher than in mice. The plasma elimination half-lives for free BPAF were similar between the two species but for total BPAF, half-lives were about 2-to 3-fold longer in rats compared to mice.
When the two analogues are compared, in rats, the systemic exposure to free BPS was higher than BPAF. Exposure concentration normalized Cmax values for free BPS were 15- to 53-fold and AUC values were 10- to 42-fold higher than that for corresponding values of free BPAF although, based on total BPS and BPAF levels, the increase was only marginal (1.5- to 4-fold) (Supplemental Table S1). However, in mice, based on both free and total Cmax and AUC exposure to BPS was similar to BPAF with estimated ratios between 1 and 4 (Supplemental Table S2). Taken collectively, these data demonstrated that the TK behavior of the two analogues were different between rats and mice.
We have previously reported systemic exposure in rats and mice following a single gavage exposure (Waidyanatha et al., 2019; Waidyanatha et al., 2020). Comparison of systemic exposure per unit dose between exposure via gavage and feed to BPS and BPAF in male rats is given in Table 7. A similar comparison could not be conducted for male mice due to uncertainty in the estimated chemical consumption due to observed food spillage. In the current study, following exposure of male rats via feed, the daily dose of BPS received were 23.0, 76.8, and 209 mg/kg for 338, 1125, and 3375 ppm, respectively. These doses are 61–70% of the doses used in the single administration gavage BPS studies in rodents (34, 110, and 340 mg/kg) (Waidyanatha et al., 2020). Using dose normalized Cmax (Cmax/D) and AUC (AUC/D) values following a single gavage administration of BPS (Waidyanatha et al., 2020) and repeated exposure via feed, the ratio of gavage/feed Cmax/D and AUC/D differed by less than 2-fold suggesting similar systemic exposure to parent BPS following gavage and feed exposure in male rats (Table 7).
Table 7.
Comparison of dose-normalized systemic exposure between feed and gavage exposure of bisphenol S and bisphenol AF in male rats
| Bisphenol S | Bisphenol AF | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gavagea | Feedb | Ratioc (Gavage/Feed) | Gavaged | Feedb | Ratioc (Gavage/Feed) | ||||||||||
| Dose | Cmax/De | AUC/Df | Doseg | Cmax/De | AUC/Df | Cmax | AUC | Dose | Cmax/De | AUC/Df | Doseg | Cmax/De | AUC/Df | Cmax | AUC |
| 34 | 9.2 | 79.7 | 23.0 | 7.00 | 52.6 | 1.3 | 1.5 | 34 | 1.79 | 11.7 | 23.4 | 0.462 | 5.04 | 3.9 | 2.3 |
| 110 | 10.4 | 134 | 76.8 | 15.0 | 91.5 | 0.7 | 1.5 | 110 | 1.29 | 11.2 | 70.5 | 0.591 | 6.95 | 2.2 | 1.6 |
| 340 | 9.5 | 184 | 209 | 14.5 | 133 | 0.7 | 1.4 | 340 | 1.62 | 14.2 | 193 | 0.333 | 3.81 | 4.9 | 3.7 |
Data from (Waidyanatha et al., 2020).
Data from the current study.
Gavage/feed ratios based on Cmax and AUC values are shown.
Data from (Waidyanatha et al., 2019).
Unit (ng/mL)/(mg/kg).
Unit (h*ng/mL)/(mg/kg).
Dose in mg/kg b.wt. estimated using feed consumption.
A similar assessment was done for BPAF exposure in male rats (Table 7). The dose of BPAF received following exposure to BPAF via feed at 338, 1125, and 3750 ppm in male rats was 23.4, 70.5, and 193 mg BPAF/kg, respectively. These doses were 57–69% of the single administration gavage doses (34, 110, and 340 mg/kg) used (Waidyanatha et al., 2019). When the dose-normalized systemic exposure (Cmax/D and AUC/D) following gavage (Waidyanatha et al., 2019) was compared with corresponding values generated from feed, the exposure via gavage was approximately 2- to 5-fold and 2- to 4-fold higher, based on Cmax and AUC, respectively, demonstrating a higher exposure of rats to BPAF following a single gavage dose than repeated exposure via feed (Table 7). Although the exact reason is not clear, induction of metabolism following repeated exposure may in part explain the lower systemic exposure of the parent following exposure to BPAF via feed compared to following gavage exposure.
BPAF and BPS have been detected at low levels in human plasma and serum including in the fetal compartment (Jin et al., 2018; Pan et al., 2020; Zhang et al., 2020). The geometric mean (GM) concentrations of total BPAF were 0.013 and 0.097 ng/mL respectively, in maternal and cord serum. For BPS maternal and cord serum total BPS values measured were 0.01 and 0.03 ng/mL, respectively (Zhang et al., 2020). In the study by Pan et al., the GM values reported for free for BPAF were 12.7 and 72.1 pg/g and for BPS were 9.40 and 5.70 pg/g for maternal and cord plasma, respectively (Pan et al., 2020). The exposures may resemble the exposure paradigm similar to the feed exposures described here but at much lower concentrations. Although a direct comparison between rodent and human data are not feasible due to lack of information such as sample collection times in humans and human kinetic data, when the Cmax values for rat and mouse free BPS (rat, 161 ng/mL; mouse, 155 ng/mL) and BPAF (rat, 10.8 ng/mL; mouse, 37.4 ng/mL) for the lowest exposure concentration of 338 ppm were compared to the human data coming from the background exposures (BPS, 9.40 pg/g; BPAF, 12.7 pg/g) (Pan et al., 2020) concentrations in humans were few orders of magnitude lower alluding to much lower environmental exposures to these analogues in humans.
In conclusion, when the systemic exposure data for the two analogues are compared, for a unit exposure concentration, the systemic exposure to free BPS was higher than free BPAF in male rats although in male mice the difference between the two analogues was marginal. Between rats and mice, the systemic exposure to free BPS was only marginally higher in rats, whereas, for free BPAF, mice had higher exposure than rats. Taken collectively, our data demonstrates that there are analogue and species differences in the disposition of BPS and BPAF in male rats and male mice.
Supplementary Material
Acknowledgements
The authors are grateful to Drs. Madelyn Huang and Gabriel Knudsen for their review of this manuscript. This work was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences, Intramural Research project ZIA ES103316-04, and performed for the National Toxicology Program, National Institute of Environmental Health Sciences, National Institutes of Health, U.S. Department of Health and Human Services, under contracts HHSN273201400022C (bisphenol S study conduct and sample analysis; data analysis; RTI International, RTP, NC), (bisphenol AF study conduct and sample analysis; HHSN273201400020C (MRIGlobal, Kansas City, MO) and HHSN273201400027C (bisphenol S formulation; Battelle, Columbus, OH).
Footnotes
Declaration of Interest
The authors report no declarations of interest.
References
- DuPont, 2006. Technical information: Bisphenol AF. Internet address: http://www.dupont.com/polymerspecialties/BPAF_TechSheet.pdf. Last accessed on March, 2020.
- EPA, 2014. Bisphenol A Alternatives in thermal paper. Final Report. January 2014. Available: http://www.epa.gov/sites/production/files/2015-08/documents/bpa_final.pdf.
- Frederiksen H, Nielsen O, Koch HM, Skakkebaek NE, Juul A, Jorgensen N, Andersson AM, 2020. Changes in urinary excretion of phthalates, phthalate substitutes, bisphenols and other polychlorinated and phenolic substances in young Danish men; 2009–2017. Int J Hyg Environ Health 223, 93–105. [DOI] [PubMed] [Google Scholar]
- Ghayda RA, Williams PL, Chavarro JE, Ford JB, Souter I, Calafat AM, Hauser R, Minguez-Alarcon L, 2019. Urinary bisphenol S concentrations: Potential predictors of and associations with semen quality parameters among men attending a fertility center. Environ Int 131, 105050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halocarbon, 2008. Product details. Fluorochemicals: Bisphenol AF. Internet address: http://www.halocarbon.com/fluorochemicals/bisphenol_AFBPAF.shtml. Last accessed on March 9, 2020.
- Husoy T, Andreassen M, Hjertholm H, Carlsen MH, Norberg N, Sprong C, Papadopoulou E, Sakhi AK, Sabaredzovic A, Dirven H, 2019. The Norwegian biomonitoring study from the EU project EuroMix: Levels of phenols and phthalates in 24-hour urine samples and exposure sources from food and personal care products. Environ Int 132, 105103. [DOI] [PubMed] [Google Scholar]
- Jin H, Xie J, Mao L, Zhao M, Bai X, Wen J, Shen T, Wu P, 2020. Bisphenol analogue concentrations in human breast milk and their associations with postnatal infant growth. Environ Pollut 259, 113779. [DOI] [PubMed] [Google Scholar]
- Jin H, Zhu J, Chen Z, Hong Y, Cai Z, 2018. Occurrence and Partitioning of Bisphenol Analogues in Adults’ Blood from China. Environ Sci Technol 52, 812–820. [DOI] [PubMed] [Google Scholar]
- Lehmler HJ, Liu B, Gadogbe M, Bao W, 2018. Exposure to Bisphenol A, Bisphenol F, and Bisphenol S in U.S. Adults and Children: The National Health and Nutrition Examination Survey 2013–2014. ACS Omega 3, 6523–6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Zhuang T, Shi W, Liang Y, Liao C, Song M, Jiang G, 2020. Serum concentration of bisphenol analogues in pregnant women in China. Sci Total Environ 707, 136100. [DOI] [PubMed] [Google Scholar]
- Liao C, Kannan K, 2013. Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure. J Agric Food Chem 61, 4655–4662. [DOI] [PubMed] [Google Scholar]
- Liao C, Liu F, Alomirah H, Loi VD, Mohd MA, Moon HB, Nakata H, Kannan K, 2012a. Bisphenol S in urine from the United States and seven Asian countries: occurrence and human exposures. Environ Sci Technol 46, 6860–6866. [DOI] [PubMed] [Google Scholar]
- Liao C, Liu F, Guo Y, Moon HB, Nakata H, Wu Q, Kannan K, 2012b. Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: implications for human exposure. Environ Sci Technol 46, 9138–9145. [DOI] [PubMed] [Google Scholar]
- Liao C, Liu F, Kannan K, 2012c. Bisphenol s, a new bisphenol analogue, in paper products and currency bills and its association with bisphenol a residues. Environ Sci Technol 46, 6515–6522. [DOI] [PubMed] [Google Scholar]
- Liao C, Liu F, Moon HB, Yamashita N, Yun S, Kannan K, 2012d. Bisphenol analogues in sediments from industrialized areas in the United States, Japan, and Korea: spatial and temporal distributions. Environ Sci Technol 46, 11558–11565. [DOI] [PubMed] [Google Scholar]
- Liao CY, Liu F, Guo Y, Moon HB, Nakata H, Wu Q, Kannan K, 2012e. Occurrence of Eight Bisphenol Analogues in Indoor Dust from the United States and Several Asian Countries: Implications for Human Exposure. Environmental Science & Technology 46, 9138–9145. [DOI] [PubMed] [Google Scholar]
- NRC, 2011. Guide for the Care and Use of Laboratory Animals: Eighth Edition. Washington, DC: The National Academies Press. [Google Scholar]
- NTP, 2008. Chemical information profile for bisphenol AF [CAS No. 1478–61-1]. https://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/bisphenolaf_093008_508.pdf. Last accessed April 01, 2020.
- NTP, 2020a. Testing status of bisphenol AF. https://ntp.niehs.nih.gov/whatwestudy/testpgm/status/ts-08002.html?utm_source=direct&utm_medium=prod&utm_campaign=ntpgolinks&utm_term=ts-08002.
- NTP, 2020b. Testing status of bisphenol S. https://ntp.niehs.nih.gov/whatwestudy/testpgm/status/ts-m940150.html?utm_source=direct&utm_medium=prod&utm_campaign=ntpgolinks&utm_term=ts-m940150.
- NTP, 2020c. Testing status of bisphenol S. https://ntp.niehs.nih.gov/whatwestudy/testpgm/status/ts-m940150.html?utm_source=direct&utm_medium=prod&utm_campaign=ntpgolinks&utm_term=ts-m940150. Last accessed May 25, 2020.
- Pan Y, Deng M, Li J, Du B, Lan S, Liang X, Zeng L, 2020. Occurrence and Maternal Transfer of Multiple Bisphenols, Including an Emerging Derivative with Unexpectedly High Concentrations, in the Human Maternal-Fetal-Placental Unit. Environ Sci Technol 54, 3476–3486. [DOI] [PubMed] [Google Scholar]
- Philips EM, Jaddoe VWV, Asimakopoulos AG, Kannan K, Steegers EAP, Santos S, Trasande L, 2018. Bisphenol and phthalate concentrations and its determinants among pregnant women in a population-based cohort in the Netherlands, 2004–5. Environ Res 161, 562–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester JR, Bolden AL, 2015. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ Health Perspect 123, 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinski MAR, Fletcher BL, Fernando RA, Robinson VG, Waidyanatha S, 2020. Development and validation of an anlytical method for quantitation of bisphenol S in rodent plasma, amniotic fluid and fetuses by UPLC-MS/MS. Anal Bioanal Chem Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SM, Duan YS, Zhang T, Zhang B, Zhao Z, Bai XY, Xie L, He Y, Ouyang JP, Huang XF, Sun HW, 2019. Serum concentrations of bisphenol A and its alternatives in elderly population living around e-waste recycling facilities in China: Associations with fasting blood glucose. Ecotox Environ Safe 169, 822–828. [DOI] [PubMed] [Google Scholar]
- Thayer KA, Taylor KW, Garantziotis S, Schurman SH, Kissling GE, Hunt D, Herbert B, Church R, Jankowich R, Churchwell MI, Scheri RC, Birnbaum LS, Bucher JR, 2016. Bisphenol A, Bisphenol S, and 4-Hydroxyphenyl 4-Isoprooxyphenylsulfone (BPSIP) in Urine and Blood of Cashiers. Environ Health Perspect 124, 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waidyanatha S, Black SR, Aillon K, Collins B, Patel PR, Riordan F, Sutherland V, Robinson VG, Fernando R, Fennell TR, 2019. Toxicokinetics and bioavailability of bisphenol AF following oral administration in rodents: A dose, species, and sex comparison. Toxicol Appl Pharmacol 373, 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waidyanatha S, Black SR, Silinski M, Sutherland V, Fletcher BL, Fernando RA, Fennell TR, 2020. Comparative toxicokinetics of bisphenol S in rats and mice following gavage administration. Toxicol Appl Pharmacol, 115207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waidyanatha S, Black SR, Snyder RW, Yueh YL, Sutherland V, Patel PR, Watson SL, Fennell TR, 2018. Disposition and metabolism of the bisphenol analogue, bisphenol S, in Harlan Sprague Dawley rats and B6C3F1/N mice and in vitro in hepatocytes from rats, mice, and humans. Toxicol Appl Pharmacol 351, 32–45. [DOI] [PubMed] [Google Scholar]
- Waidyanatha S, Mathews JM, Patel PR, Black SR, Snyder RW, Fennell TR, 2015. Disposition of bisphenol AF, a bisphenol A analogue, in hepatocytes in vitro and in male and female Harlan Sprague-Dawley rats and B6C3F1/N mice following oral and intravenous administration. Xenobiotica 45, 811–819. [DOI] [PubMed] [Google Scholar]
- Wan Y, Huo W, Xu S, Zheng T, Zhang B, Li Y, Zhou A, Zhang Y, Hu J, Zhu Y, Chen Z, Lu S, Wu C, Jiang M, Jiang Y, Liu H, Yang X, Xia W, 2018a. Relationship between maternal exposure to bisphenol S and pregnancy duration. Environ Pollut 238, 717–724. [DOI] [PubMed] [Google Scholar]
- Wan Y, Xia W, Yang S, Pan X, He Z, Kannan K, 2018b. Spatial distribution of bisphenol S in surface water and human serum from Yangtze River watershed, China: Implications for exposure through drinking water. Chemosphere 199, 595–602. [DOI] [PubMed] [Google Scholar]
- Yang YJ, Li ZL, Zhang J, Yang Y, Shao B, 2014. Simultaneous determination of bisphenol A, bisphenol AF, tetrachlorobisphenol A, and tetrabromobisphenol A concentrations in water using on-line solid-phase extraction with ultrahigh-pressure liquid chromatography tandem mass spectrometry. Int J Environ an Ch 94, 16–27. [Google Scholar]
- Ye XY, Wong LY, Kramer J, Zhou XL, Jia T, Calafat AM, 2015. Urinary Concentrations of Bisphenol A and Three Other Bisphenols in Convenience Samples of US Adults during 2000–2014. Environmental Science & Technology 49, 11834–11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, He Y, Zhu H, Huang X, Bai X, Kannan K, Zhang T, 2020. Concentrations of bisphenol A and its alternatives in paired maternal-fetal urine, serum and amniotic fluid from an e-waste dismantling area in China. Environ Int 136, 105407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.