Abstract
BACKGROUND:
For rectal cancer with unresectable metastases, current practice favors omitting interventions directed at the primary tumor in asymptomatic patients.
OBJECTIVE:
To determine the proportion of patients with primary tumor-related complications, characterize salvage outcomes, and measure survival in metastatic rectal cancer patients who did not undergo upfront intervention for their primary tumor.
DESIGN:
Retrospective analysis.
SETTING:
Comprehensive cancer center.
PATIENTS:
Patients who presented between January 1, 2008, and December 31, 2015, with synchronous stage IV rectal cancer, an unresected primary, and no prior primary tumor-directed intervention.
MAIN OUTCOME MEASURES:
Rate of primary tumor-related complications in the cohort that did not receive any primary tumor directed intervention. The Kaplan-Meier method and Cox regression analysis were used to determine whether complications are associated with survival.
RESULTS:
The cohort comprised 358 patients with median age of 56 years (22–92). Median follow-up was 26 months (range, 1 to 93 months). Among the 168 patients (46.9%) who eventually underwent elective resection of the primary tumor, the surgery was performed with curative intent in 66 patients (18.4%) and preemptive intent in 102 patients (28.5%). Of the 190 patients who did not undergo an upfront or elective intervention for the primary tumor, 68 (35.8%) experienced complications. Nonsurgical intervention for complications was attempted in 34 patients with an overall success rate of 61.8% (21 out of 34). Surgical intervention was performed in 47 patients (including 13 patients for whom nonsurgical intervention failed): diversion in 26 patients and resection in 21 patients. Of those 47 patients, 42 (89.4%) ended up with a colostomy or ileostomy.
LIMITATIONS:
Single center.
CONCLUSION:
A significant proportion of patients with metastatic rectal cancer and untreated primary experience primary tumor-related complications. These patients should be followed closely and preemptive intervention (resection, diversion, or radiation) considered if the primary progresses despite systemic therapy. See Video Abstract at http://links.lww.com/DCR/B400.
Keywords: Complications, Metastasis, Rectal cancer, Salvage, Untreated primary tumor
Abstract
ANTECEDENTES:
Para el cáncer de recto con metástasis no resecables, la práctica actual favorece la omisión de las intervenciones dirigidas al tumor primario en pacientes asintomáticos.
OBJETIVO:
Determinar la proporción de pacientes con complicaciones relacionadas con el tumor primario, caracterizar los resultados de rescate y medir la supervivencia en pacientes con cáncer rectal metastásico que no se sometieron a una intervención inicial para su tumor primario.
DISEÑO:
Análisis retrospectivo.
AJUSTE:
Centro oncológico integral.
PACIENTES:
Pacientes que se presentaron entre el 1 de enero de 2008 y el 31 de diciembre de 2015 con cáncer de recto en estadio IV sincrónico, un tumor primario no resecado y sin intervención previa dirigida al tumor primario.
PRINCIPALES MEDIDAS DE RESULTADO:
Tasa de complicaciones relacionadas con el tumor primario en la cohorte que no recibió ninguna intervención dirigida al tumor primario. Se utilizó el método de Kaplan-Meier y el análisis de regresión de Cox para determinar si las complicaciones están asociadas con la supervivencia.
RESULTADOS:
La cohorte estuvo compuesta por 358 pacientes con una mediana de edad de 56 años (22–92). La mediana de seguimiento fue de 26 meses (rango, 1 a 93 meses). Entre los 168 pacientes (46,9%) que finalmente se sometieron a resección electiva del tumor primario, la cirugía se realizó con intención curativa en 66 pacientes (18,4%) y con intención preventiva en 102 pacientes (28,5%). De los 190 pacientes que no se sometieron a una intervención inicial o electiva para el tumor primario, 68 (35,8%) experimentaron complicaciones. Se intentó una intervención no quirúrgica para las complicaciones en 34 pacientes con una tasa de éxito global del 61,8% (21 de 34). La intervención quirúrgica se realizó en 47 pacientes (incluidos 13 pacientes en los que falló la intervención no quirúrgica): derivación en 26 pacientes y resección en 21 pacientes. De esos 47 pacientes, 42 (89,4%) terminaron con una colostomía o ileostomía.
LIMITACIONES:
Único centro.
CONCLUSIÓN:
Una proporción significativa de pacientes con cáncer de recto metastásico y primario no tratado experimentan complicaciones relacionadas con el tumor primario. Se debe hacer un seguimiento estrecho de estos pacientes y considerar la posibilidad de una intervención preventiva (resección, derivación o radiación) si el tumor primario progresa a pesar de la terapia sistémica. Consulte Video Resumen en http://links.lww.com/DCR/B400. (Traducción—Dr. Gonzalo Hagerman)
INTRODUCTION
The choice of a management strategy for the primary tumor in patients with metastatic colorectal cancer remains contentious. Proponents of resection maintain that it prevents primary-related complications during the subsequent course of treatment and has been associated with improved survival in several meta-analyses.1–4 Opponents of resection point out that the higher survival rates may be attributed to selection bias and that morbidity associated with surgery may delay or preclude systemic therapy. Ongoing randomized trials may provide a definitive answer.5–7
In the modern era of oxaliplatin - or irinotecan-based systemic therapy, the rate of primary tumor-related complications in patients with metastatic colorectal cancer and an untreated primary is estimated to be less than 15%.8,9 However, these figures are derived from mixed cohorts of patients with metastatic colon and rectal cancers, which may not accurately reflect the true incidence of complications associated with an untreated rectal tumor. Colon and rectal tumors differ in several aspects. The anatomic location of the rectum and its proximity to pelvic viscera predispose rectal tumors to unique complications such as rectovaginal or rectovesical fistulae. In addition, nonoperative interventions such as endoscopic stenting are less likely to be tolerated due to symptoms of tenesmus and pain from an indwelling stent in the rectum.10 Emergency surgery for rectal tumor complications is more likely to result in the creation of a stoma, which has implications for the quality of life.11 Another difference is that the extraperitoneal location of rectal tumors makes them amenable to radiotherapy, which can be an option for local control. Because of these important differences, assessments of primary tumor-related complications in patients with metastatic colorectal cancer should consider patients with rectal tumors separately from patients with colon tumors.
The primary tumor is resected less commonly in patients with metastatic rectal cancer than in patients with metastatic colon cancer due to the complexity of the procedure, the necessity of a stoma, and the significant bowel dysfunction associated with rectal resections.12,13 However, only scarce data are available on the rate of primary tumor-related complications specifically in patients with stage IV rectal cancer and an untreated primary.8,9,14 Knowledge of the rates of complications associated with an untreated rectal tumor and of the outcomes of operative or nonoperative salvage management can help guide the clinician’s decision on the management of the primary tumor. Thus, the objectives of our study were to determine the rate of primary-tumor-related complications, characterize salvage outcomes, and measure survival in patients with metastatic rectal cancer who did not undergo an upfront intervention for their primary tumor.
PATIENTS AND METHODS
Database
With approval from the Institutional Review Board of Memorial Sloan Kettering Cancer Center (MSK), patients were identified for inclusion in the study by searching the prospectively maintained MSK institutional database. The database contains patient demographics, histologic diagnoses based on the International Classification of Diseases, initial disease stages based on the classification system of the American Joint Committee on Cancer, inpatient admission and outpatient registration data, operating room procedures, laboratory results, and pharmacy records. The database undergoes continuous cross-platform integration with the MSK Cancer Registry, which reliably captures survival data.
Patients
Using the MSK institutional database, we identified patients who presented at MSK between January 1, 2008, and December 31, 2015, with synchronous, stage IV rectal cancers (less than 15 cm from the anal verge), an intact primary tumor and no prior primary-tumor-directed intervention (resection, diversion, stenting, or radiotherapy). We then excluded patients who underwent an upfront intervention for their primary tumor upon diagnosis at MSK.
Inpatient and outpatient records were reviewed individually by the first author (who at the time of the study was a Clinical Fellow at MSK) to ascertain the presence of an intact primary tumor with no prior primary tumor-directed intervention, the histological diagnosis of rectal adenocarcinoma, the type and duration of the first-line chemotherapy administered, sites of metastatic disease at presentation, curative intent at presentation, and laboratory data (albumin, carcinoembryonic antigen) at presentation. Ambiguities were resolved in consultation with the patients’ attending surgeons.
Patients who subsequently underwent preemptive resection of the primary tumor or resection with curative intent after the commencement of systemic therapy were identified. Preemptive resection was defined as elective resection of the primary tumor prior to the development of complications. Curative intent was defined as the possibility of complete resection of the primary tumor and all metastases with preservation of adequate organ function based on assessment by the multidisciplinary tumor board.
For the remaining patients, the rate of primary tumor-related complications that required nonsurgical (endoluminal stenting, radiation) or surgical intervention was determined, together with the success rate of the interventions. In general, patients who were not offered an upfront intervention were minimally symptomatic at initial consultation with respect to their primary tumor (i.e., no major bleeding, pain, perforation, or impending obstruction).
Statistical Analysis
Demographic and clinical characteristics were summarized using means, medians, and minimum and maximum values for continuous variables and counts and percentages for categorical variables. Overall survival was calculated from the date of the primary diagnosis until death or the date of last follow-up using the Kaplan-Meier method and Cox regression.
The main aim of the analysis was to determine the rate of primary tumor-related complications in patients with an untreated rectal tumor. The secondary aim was to determine whether the complications are associated with survival. Because complications occurred at different time points after primary diagnosis, the development of complications was included in the Cox regression as a time-dependent variable. In addition, for patients who experienced a complication, we calculated the median time from diagnosis to intervention for the complication and the Kaplan-Meier estimate for median time from intervention to death. All estimates are reported with 95% confidence intervals (CI). All analyses were conducted with the software packages SAS version 9.4 and R version 3.5.0.
RESULTS
Patient Characteristics
Three hundred and ninety-nine patients with metastatic rectal cancer, an intact primary tumor, and no prior primary tumor-directed intervention were identified from the institutional database. Forty-one patients underwent an upfront intervention for the primary tumor, and the remaining 358 patients constituted the study cohort (Figure 1). Median follow-up was 26 months (range, 1 to 93 months).
FIGURE 1.
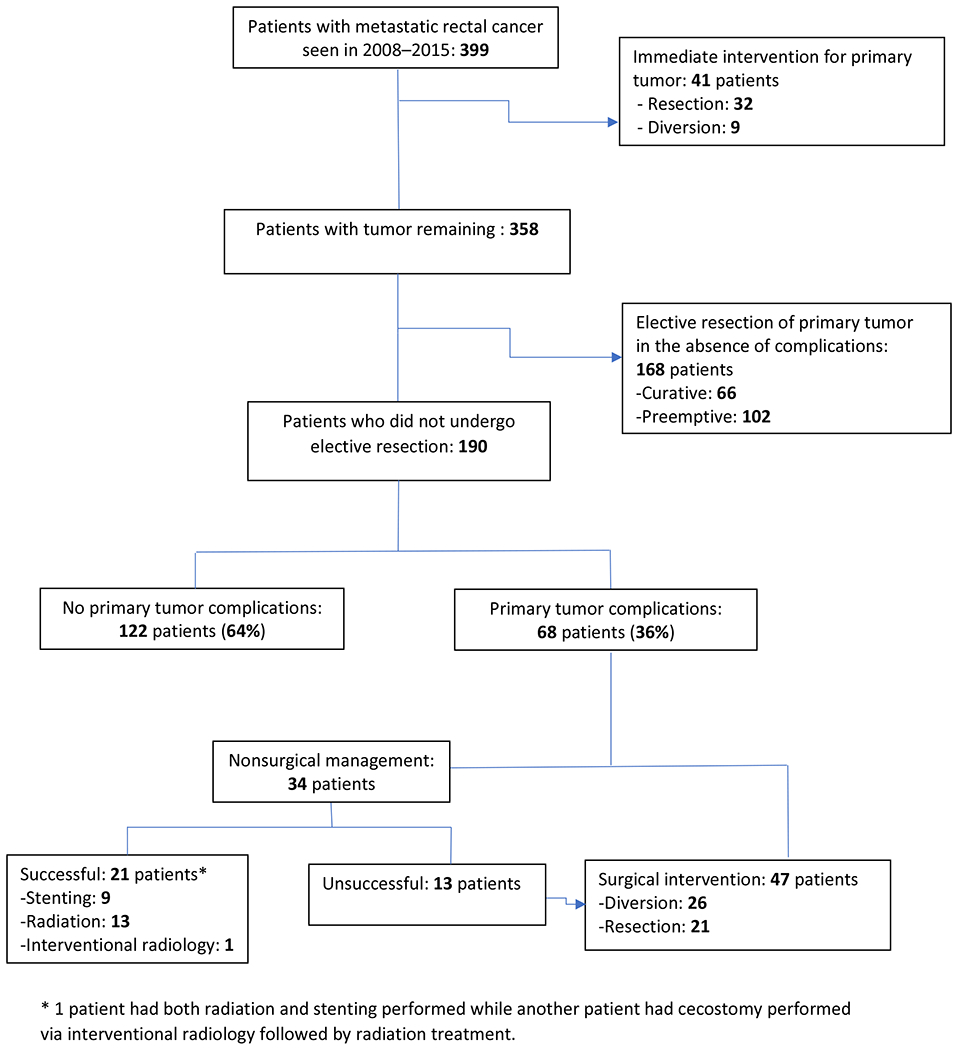
Patients and treatments.
Patient and disease characteristics are listed in Table 1. Median age was 56 years (range, 22 to 92 years). The most common site of metastasis at presentation was the liver (305 patients, 85.2%), followed by the lungs (107 patients, 29.9%), retroperitoneal lymph nodes (45 patients, 12.6%), and the peritoneum (20 patients, 5.6%). Metastatic disease was found at a single site in 239 patients (66.8%), two sites in 103 patients (28.8%), and three or more sites in 16 patients (4.5%).
TABLE 1.
Patient and disease characteristics
| Characteristic | No. (%) of patients (n = 358) |
|---|---|
| Median age | 56 (22–92) years |
| Median CEA | 31.2 (1–15,970) ng/ml |
| Median albumin | 4.0 (2.3–5.0) g/dl |
| Sites of metastasis at presentation | |
| Liver | 305 (85.2) |
| Lung | 107 (29.9) |
| Retroperitoneal lymph nodes | 45 (12.6) |
| Peritoneum | 20 (5.6) |
| No. of metastasis sites at presentation | |
| 1 | 239 (66.8) |
| 2 | 103 (28.8) |
| ≥3 | 16 (4.5) |
| First-line chemotherapy | |
| None | 8 (2.2) |
| FOLFOX or CAPEOX | 221 (61.7) |
| FOLFOX or CAPEOX with EGFR inhibitor or bevacizumab | 85 (23.7) |
| FOLFIRI or XELIRI | 10 (2.8) |
| FOLFIRI or XELIRI with EGFR inhibitor or bevacizumab | 14 (3.9) |
| FOLFIRINOX | 4 (1.1) |
| Fluoropyrimidine monotherapy | 16 (4.5) |
Abbreviations: CEA, carcinoembryonic antigen; FOLFOX, fluorouracil-leucovorin-oxaliplatin; CAPEOX, capecitabine-oxaliplatin; FOLFIRI, fluorouracil-leucovorin-irinotecan; XELIRI, capecitabine-irinotecan; FOLFIRINOX, fluorouracil-leucovorin-irinotecan-oxaliplatin
First-Line Systemic Treatment
Fluoropyrimidine-based systemic therapy was administered to 350 (97.8%) of the 358 patients (Table 1). Of the 8 patients who did not receive systemic treatment, 3 declined treatment and 5 were deemed too frail for chemotherapy. For 306 patients (85.5%), the chemotherapy included oxaliplatin (FOLFOX or CAPEOX), and for 24 patients (6.7%), it included irinotecan (FOLFIRI or XELIRI). Fluoropyrimidine monotherapy was administered to 16 patients (4.5%). The remaining 4 patients received a combination of fluoropyrimidine, irinotecan, and oxaliplatin. The median number of cycles of first-line chemotherapy administered was 6 (range, 1–18 cycles).
Primary Tumor-Related Complications
Of the 358 patients with an in situ primary tumor, 168 (46.9%) eventually underwent elective resection of the primary tumor after a median of 6 courses of systemic therapy were administered—with curative intent in 66 patients (18.4%) and preemptive intent in 102 patients (28.5%) (Figure 1). Patients who underwent preemptive primary tumor resection were offered surgery either due to symptoms from the primary (55 patients, 15.4%) or in conjunction with the placement of a hepatic artery infusion pump in instances where the primary was resectable (47 patients, 13.1%). Median intervals from diagnosis to curative resection and preemptive resection were 5.4 months (range, 3–48 months) and 7.2 months (range, 2.7–50 months), respectively (Table 2).
Table 2.
Survival of various groups within study cohort
| Group (n) | Median Time (months) from Diagnosis to Intervention (range) | Median Survival (months) after Intervention (95% CI) |
|---|---|---|
| Curative resection (66) | 5.4 (3–48) | Not reached |
| Preemptive resection (102) | 7.2 (2.7–50) | 49.4 (33.6–58.6) |
| Tumor in situ with complications (68) | 7.7 (0.3–67) | 17.3 (9.4–19.2) |
Of the 190 patients who did not undergo an upfront or elective intervention for the primary tumor, 68 (35.8%) experienced the following complications (Figures 1 and 2): obstruction in 37 patients (19.5%), rectovesical or rectovaginal fistula in 9 patients (4.7%), perforation in 8 patients (4.2%), intractable pain from the primary tumor necessitating hospitalization for pain control in 8 patients (4.2%) and rectal bleeding requiring inpatient admission and transfusion in 6 patients (3.2%). The median interval from diagnosis to the onset of a complication was 7 months (range, 1 to 67 months).
FIGURE 2.
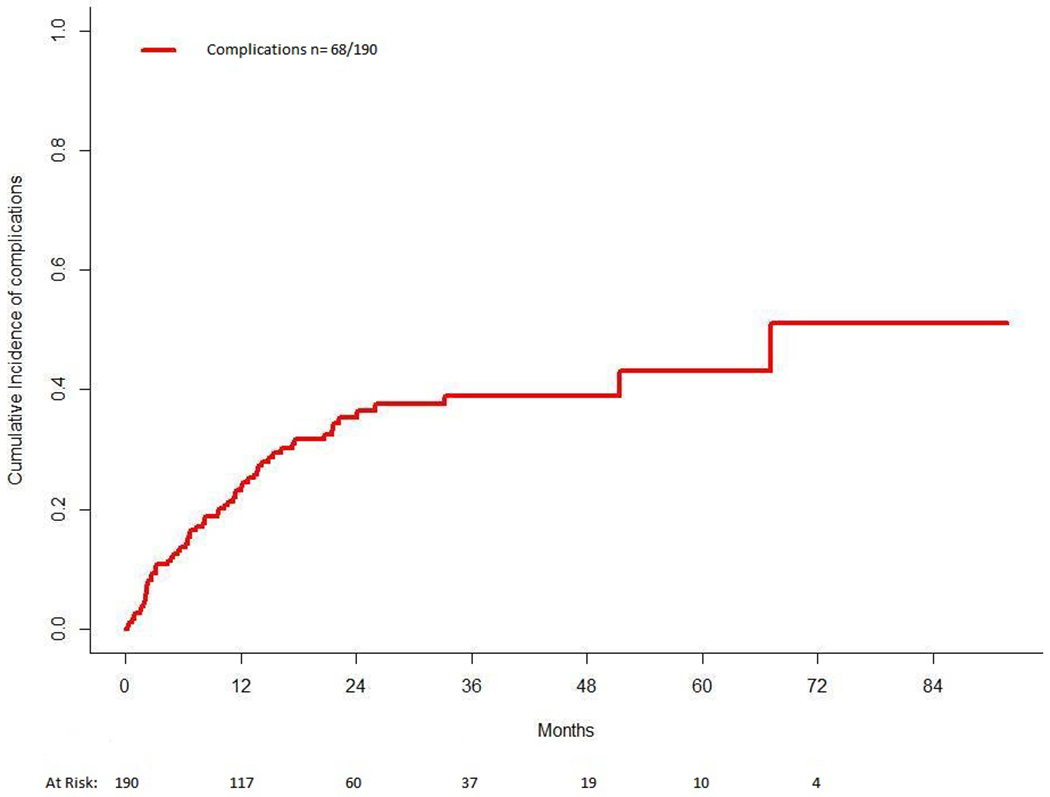
Incidence of complications among the study cohort.
Nonsurgical Intervention
Nonsurgical intervention was attempted in 34 (17.9%) of the 190 patients with an untreated primary tumor: endoscopic stenting in 21 patients (11%), radiation in 13 patients (6.8%), and a decompressive cecostomy via interventional radiology in 1 patient (1 patient initially received radiation for obstructive symptoms before eventually undergoing endoscopic stenting and is included in both groups). Of the 21 endoscopic stents attempted, 9 failed to achieve long-term decompression (42.9%) and 3 resulted in a stent-induced perforation (14.3%). The overall success rate of nonsurgical interventions was 61.8% (21 of 34 patients) (Figure 1). The 13 patients for whom nonsurgical intervention did not succeed (38.2%) proceeded to surgery.
Surgical Intervention
Surgical intervention was performed in 47 (24.7%) of the 190 patients with an untreated primary tumor (including the 13 patients for whom nonsurgical intervention did not succeed). Diversion was utilized in 26 patients and resection in 21 patients. Of those 47 patients, 42 (89.4%) ended up with either a colostomy (80.8%) or an ileostomy (8.5%).
Survival
Median overall survival for the entire cohort of 358 patients was 36.6 months (95% CI, 31.8–40.8 months). The median overall survival times (after intervention) for patients who underwent an elective curative resection, patients who underwent an elective preemptive resection, and patients who underwent an intervention for primary tumor-related complications are listed in Table 2. Median overall survival for the 122 patients who did not experience primary tumor-related complications was 23 months (range, 20.8 to 38.9 months). When included as a time-varying covariate in a Cox regression model, primary tumor-related complications were negatively associated with survival (hazard ratio [HR], 2.60; 95% CI, 1.85–3.67) (Table 3). The other variables associated with overall survival were albumin < 3 g/dL (HR, 1.78; 95% CI, 1.01–3.12), 2 or more metastasis sites (HR, 1.53; 95% CI, 1.13–2.06), and resectable disease at presentation (HR, 0.34; 95% CI, 0.22–0.53) (Table 3).
TABLE 3.
Cox multivariable model of overall survival
| Parameter | p | Hazard ratio (95% CI) |
|---|---|---|
| Primary tumor-related complicationa | <0.0001 | 2.60 (1.85–3.67) |
| CEA < 200 ng/ml | 0.63 | 0.92 (0.67–1.27) |
| Age < 65 years | 0.067 | 0.75 (0.54–1.02) |
| Albumin < 3 g/dL | 0.045 | 1.78 (1.01–3.12) |
| ≥2 metastasis sites | 0.0055 | 1.53 (1.13–2.06) |
| Resectable disease at presentation | <0.0001 | 0.34 (0.22–0.53) |
Included in the model as a time-varying covariate.
DISCUSSION
In our cohort of patients with metastatic rectal cancer and an untreated primary tumor, most of whom received oxaliplatin - or irinotecan-based chemotherapy, the rate of primary tumor-related complications was 19% (68 of 358 patients). If we exclude the 168 patients who underwent an elective primary tumor resection after systemic therapy was started, the rate of primary tumor-related complications was 36% (68 of 190 patients). Most patients who developed primary tumor-related complications (47 of 68 patients, 69%) required surgical intervention, which resulted in the creation of a stoma in 42 (89%) of 47 patients. In contrast, the stoma creation rate for elective primary tumor resection in patients with metastatic rectal cancer is approximately 15%.15
The rate of primary tumor-related complications in our study cohort appears to be higher than the 10–15% rates reported in previous studies.8,9,16 Several possible factors may account for this difference. Our cohort consisted exclusively of rectal cancer patients, while previous studies also included patients with colon cancer. Rectal primary tumors may carry a higher risk of complications than colon primary tumors, as suggested by a trend observed in several studies (without statistical significance, likely due to limitations in sample size).9,14 Another potential explanation for the higher rate of tumor-related complications may be related to prolonged survival of patients with unresectable metastatic disease due to more effective chemotherapy regimens. This may be compounded further by the reduction in the rates of upfront primary tumor resection in patients with metastatic colorectal cancer.17 In recent studies of patients with metastatic colorectal cancer who mostly received oxaliplatin - or irinotecan-based first-line chemotherapy, the rates of primary tumor-related complications ranged from 20 to 35% compared to 15% in older studies, and in general, median survival was longer or the rate of upfront primary tumor resection was lower (eTable 1).8,9,14,16,18–25 It is hence plausible that primary tumor related complications are dependent on both survival and the rates of upfront primary tumor resection. Clinicians should be cognizant of this and be aware that the true rate of primary tumor related complications in the current era may be higher than previously perceived.
The occurrence of primary tumor-related complications was negatively associated with survival (HR, 2.60; 95% CI, 1.85–3.87) in our cohort. This association persisted in multivariable analysis when confounding variables such as patient age, albumin level, and metastatic burden (carcinoembryonic antigen level, two or more metastasis sites, and resectable disease at presentation) were taken into consideration. These results should however be interpreted with caution as survival was compared between patients who underwent an elective resection in the absence of complications and patients who underwent a surgical or nonsurgical intervention after a complication occurred. A direct comparison of survival between all patients who experienced complications and all patients who did not would not be statistically valid because the need for intervention was unknown at baseline and occurred at different times during follow-up.
There are several limitations to our study. Our study’s retrospective design made it difficult to accurately capture symptom severity and to define complications objectively. While the identification of patients with overt obstruction is usually straightforward, distinguishing between a patient who is severely symptomatic and a patient with an impending obstruction may not be easy. In our study, interventions done on an emergent basis were regarded as complications while patients with symptoms who were treated with elective resection were classified as having undergone pre-emptive resection. This may have led to an over or underestimation of the complication rates reported. The retrospective nature of the study also made it difficult to determine the exact reasons why some patients received upfront surgical treatment for the primary tumor while others did not. A review of the patient charts did reveal that 34 of the 41 patients who had an upfront intervention upon diagnosis had symptoms related to the primary tumor. Another potential limitation is that the findings were obtained at a single comprehensive cancer center and may not be generalizable to all settings. Quality-of-life information, which is particularly pertinent in a cohort of patients with stage IV disease, was also unavailable.
Notwithstanding such potential limitations, this is the largest study to date on primary tumor-related complications in patients with metastatic rectal cancer, with important implications for clinical practice. Our findings indicate that a significant proportion of patients with metastatic rectal cancer experience primary tumor-related complications despite the efficacy of modern chemotherapy. Nonoperative salvage rates for complications are low, and surgical intervention usually results in the creation of a stoma. These findings suggest that patients with an in situ primary should be followed up to assess the tumor’s response to systemic therapy. If the primary tumor progresses despite systemic therapy, a tumor-directed intervention can be offered as an elective option before complications occur. Early initiation of pelvic radiotherapy for local control prior to the onset of symptoms may be an option for rectal tumors that progress despite systemic therapy. Such radiotherapy can be administered using a short-course protocol to minimize interruption of systemic chemotherapy.26
CONCLUSION
In conclusion, despite the efficacy of modern systemic chemotherapy regimens, a significant proportion of patients with metastatic rectal cancer experience primary tumor-related complications that often require surgical intervention and the creation of a stoma. The development of complications was negatively associated with survival. Patients with metastatic rectal cancer and primary in-situ should be followed and pre-emptive intervention (resection, diversion or radiation) considered if the primary progresses despite systemic therapy.
Supplementary Material
Acknowledgments
Funding/Support: NCI grant P30 CA008748
Financial Disclaimers: G. Nash has received an honorarium from Intuitive Surgical. J. J. Smith has received travel support from Intuitive Surgical and has served as a clinical advisor to Guardant Health. J. Garcia-Aguilar has received honoraria from Medtronic, Johnson & Johnson, and Intuitive Surgical. W. J. Tan, S. Patil, J. G. Guillem, P. B. Paty, M. R. Weiser, E. P. Pappou, and I. H. Wei have no relevant financial relationships to disclose.
Footnotes
An abstract of the manuscript will be presented at the 2020 Annual Meeting of the American Society of Colon and Rectal Surgeons, June 6–10, 2020.
References
- 1.Ahmed S, Shahid RK, Leis A, et al. Should noncurative resection of the primary tumour be performed in patients with stage iv colorectal cancer? A systematic review and meta-analysis. Curr Oncol. 2013;20:e420–e441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clancy C, Burke JP, Barry M, Kalady MF, Calvin Coffey J. A meta-analysis to determine the effect of primary tumor resection for stage IV colorectal cancer with unresectable metastases on patient survival. Ann Surg Oncol. 2014;21:3900–3908. [DOI] [PubMed] [Google Scholar]
- 3.Ha GW, Kim JH, Lee MR. Meta-analysis of oncologic effect of primary tumor resection in patients with unresectable stage IV colorectal cancer in the era of modern systemic chemotherapy. Ann Surg Treat Res. 2018;95:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nitsche U, Stöß C, Stecher L, Wilhelm D, Friess H, Ceyhan GO. Meta-analysis of outcomes following resection of the primary tumour in patients presenting with metastatic colorectal cancer. Br J Surg. 2018;105:784–796. [DOI] [PubMed] [Google Scholar]
- 5.Kim CW, Baek JH, Choi GS, et al. The role of primary tumor resection in colorectal cancer patients with asymptomatic, synchronous unresectable metastasis: study protocol for a randomized controlled trial. Trials. 2016;17:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahbari NN, Lordick F, Fink C, et al. ; SYNCHRONOUS trial group. Resection of the primary tumour versus no resection prior to systemic therapy in patients with colon cancer and synchronous unresectable metastases (UICC stage IV): SYNCHRONOUS--a randomised controlled multicentre trial (ISRCTN30964555). BMC Cancer. 2012;12:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.’t Lam-Boer J, Mol L, Verhoef C, et al. The CAIRO4 study: the role of surgery of the primary tumour with few or absent symptoms in patients with synchronous unresectable metastases of colorectal cancer--a randomized phase III study of the Dutch Colorectal Cancer Group (DCCG). BMC Cancer. 2014;14:741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nitzkorski JR, Farma JM, Watson JC, et al. Outcome and natural history of patients with stage IV colorectal cancer receiving chemotherapy without primary tumor resection. Ann Surg Oncol. 2012;19:379–383. [DOI] [PubMed] [Google Scholar]
- 9.Poultsides GA, Servais EL, Saltz LB, et al. Outcome of primary tumor in patients with synchronous stage IV colorectal cancer receiving combination chemotherapy without surgery as initial treatment. J Clin Oncol. 2009;27:3379–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi JH, Lee YJ, Kim ES, et al. Covered self-expandable metal stents are more associated with complications in the management of malignant colorectal obstruction. Surg Endosc. 2013;27:3220–3227. [DOI] [PubMed] [Google Scholar]
- 11.Downing A, Glaser AW, Finan PJ, et al. Functional outcomes and health-related quality of life after curative treatment for rectal cancer: a population-level study in England. Int J Radiat Oncol Biol Phys. 2019;103:1132–1142.. [DOI] [PubMed] [Google Scholar]
- 12.Liang JT, Lai HS, Huang J, Sun CT. Long-term oncologic results of laparoscopic D3 lymphadenectomy with complete mesocolic excision for right-sided colon cancer with clinically positive lymph nodes. Surg Endosc. 2015;29:2394–2401. [DOI] [PubMed] [Google Scholar]
- 13.van der Sijp MP, Bastiaannet E, Mesker WE, et al. Differences between colon and rectal cancer in complications, short-term survival and recurrences. Int J Colorectal Dis. 2016;31:1683–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim DH, Kim B, Choi JH, et al. Tumor characteristics associated with malignant large bowel obstruction in stage IV colorectal cancer patients undergoing chemotherapy. Int J Colorectal Dis. 2016;31:1767–1774. [DOI] [PubMed] [Google Scholar]
- 15.Nash GM, Saltz LB, Kemeny NE, et al. Radical resection of rectal cancer primary tumor provides effective local therapy in patients with stage IV disease. Ann Surg Oncol. 2002;9:954–960. [DOI] [PubMed] [Google Scholar]
- 16.Seo GJ, Park JW, Yoo SB, et al. Intestinal complications after palliative treatment for asymptomatic patients with unresectable stage IV colorectal cancer. J Surg Oncol. 2010;102:94–99. [DOI] [PubMed] [Google Scholar]
- 17.Hu CY, Bailey CE, You YN, et al. Time trend analysis of primary tumor resection for stage IV colorectal cancer: less surgery, improved survival. JAMA Surg. 2015;150:245–251. [DOI] [PubMed] [Google Scholar]
- 18.Kim MS, Chung M, Ahn JB, et al. Clinical significance of primary tumor resection in colorectal cancer patients with synchronous unresectable metastasis. J Surg Oncol. 2014;110:214–221. [DOI] [PubMed] [Google Scholar]
- 19.Miyamoto Y, Watanabe M, Sakamoto Y, et al. Evaluation of the necessity of primary tumor resection for synchronous metastatic colorectal cancer. Surg Today. 2014;44:2287–2292. [DOI] [PubMed] [Google Scholar]
- 20.Yun JA, Park Y, Huh JW, et al. Risk factors for the requirement of surgical or endoscopic interventions during chemotherapy in patients with uncomplicated colorectal cancer and unresectable synchronous metastases. J Surg Oncol. 2014;110:839–844. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Liang L, Yu Y, et al. Primary Tumour resection could improve the survival of unresectable metastatic colorectal cancer patients receiving bevacizumab-containing chemotherapy. Cell Physiol Biochem. 2016;39:1239–1246. [DOI] [PubMed] [Google Scholar]
- 22.Muratore A, Zorzi D, Bouzari H, et al. Asymptomatic colorectal cancer with un-resectable liver metastases: immediate colorectal resection or up-front systemic chemotherapy? Ann Surg Oncol. 2007;14:766–770. [DOI] [PubMed] [Google Scholar]
- 23.Benoist S, Pautrat K, Mitry E, Rougier P, Penna C, Nordlinger B. Treatment strategy for patients with colorectal cancer and synchronous irresectable liver metastases. Br J Surg. 2005;92:1155–1160. [DOI] [PubMed] [Google Scholar]
- 24.Michel P,Roque I,Di Fiore F,et al. Colorectal cancer with non-resectable synchronous metastases: should the primary tumor be resected? Gastroenterol Clin Biol.2004;28434–437. [DOI] [PubMed] [Google Scholar]
- 25.Suárez J, Marín G, Vera R, Oronoz B, Oteiza F, Mata E. Complications from the primary tumour are not related with survival in patients with synchronous stage IV colorectal cancer receiving chemotherapy without primary tumour resection. Int J Colorectal Dis. 2015;30:1357–1363. [DOI] [PubMed] [Google Scholar]
- 26.Picardi V, Deodato F, Guido A, et al. Palliative Short-Course Radiation Therapy in Rectal Cancer: A Phase 2 Study. Int J Radiat Oncol Biol Phys. 2016;95:1184–1190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


