Abstract
Myelin plasticity is critical for neurological function, including learning and memory. However, it is unknown whether this plasticity reflects uniform changes across all neuronal subtypes, or whether myelin dynamics vary between neuronal classes to enable fine-tuning of adaptive circuit responses. We performed in vivo two-photon imaging of myelin sheaths along single axons of excitatory callosal neurons and inhibitory parvalbumin+ interneurons in adult mouse visual cortex. We find that both neuron types show homeostatic myelin remodeling under normal vision. However, monocular deprivation results in adaptive myelin remodeling only in parvalbumin+ interneurons. An initial increase in elongating myelin segments is followed by contraction of a separate cohort of segments. This data indicates that distinct classes of neurons individualize remodeling of their myelination profiles to diversify circuit tuning in response to sensory experience.
One Sentence Summary:
Experience-dependent plasticity of myelination profiles in the visual cortex is neuron class-specific.
Myelin is a fundamental cellular structure in the vertebrate nervous system, and its precise formation and regulation are critical for complex neuronal function (1). In vivo imaging studies have shown that myelination in the central nervous system (CNS) continues through adulthood, accompanied by constant remodeling (2, 3). In addition, myelin can be dynamically modulated by neuronal activity and contributes to nervous system plasticity throughout life (4–7). Myelin plasticity in response to experience helps to shape brain structure and function, including learning and memory (6, 8–10). Previous studies of experience-dependent myelin plasticity have demonstrated the importance of myelination by newly-formed oligodendrocytes (3–6, 8–11), but the neuronal substrates and dynamic properties of adaptively-remodeled myelin segments have not yet been addressed. It has been shown that different neuron subtypes have distinct patterns of axonal myelination, and that myelin profiles vary between individual cells within neuronal classes (12–14); furthermore, callosal and subcortical projection tracts differ in the degree of oligodendrogenesis induced by neuronal activation (4). A major unanswered question, therefore, is whether myelin plasticity affects different neuronal populations homogeneously, or whether adaptive remodeling has cell type-specific characteristics that may potentiate circuit tuning, either under normal conditions or driven by experience.
Homeostatic remodeling of pre-existing myelin is neuron class-specific
To study experience-dependent remodeling of myelination profiles and oligodendrocyte dynamics in the adult CNS, we used longitudinal dual-color in vivo two-photon imaging in the binocular area of the primary visual cortex (V1b) both during normal vision and through a period of ocular dominance plasticity induced by monocular deprivation (MD) (15). Since > 90 % of the myelin in layer 2/3 (L2/3) of the neocortex wraps axons of either excitatory neurons (specifically, callosal projection neurons, CPNs) or parvalbumin-expressing GABAergic interneurons (PV-INs) (13), we compared myelin dynamics of these two functionally opposite neuronal classes (Fig. 1, A and B). We used the Tbr2CreERT2; CAGfloxStop-tdTomato mouse line to specifically label L2/3 CPNs (fig. S1, A to E), and the PVCre; CAGfloxStop-tdTomato mouse line to label PV-INs (fig. S1, F and G).
Fig. 1. Pre-existing myelin sheaths on L2/3 PV-INs and CPNs present remodeling in young adult mice.
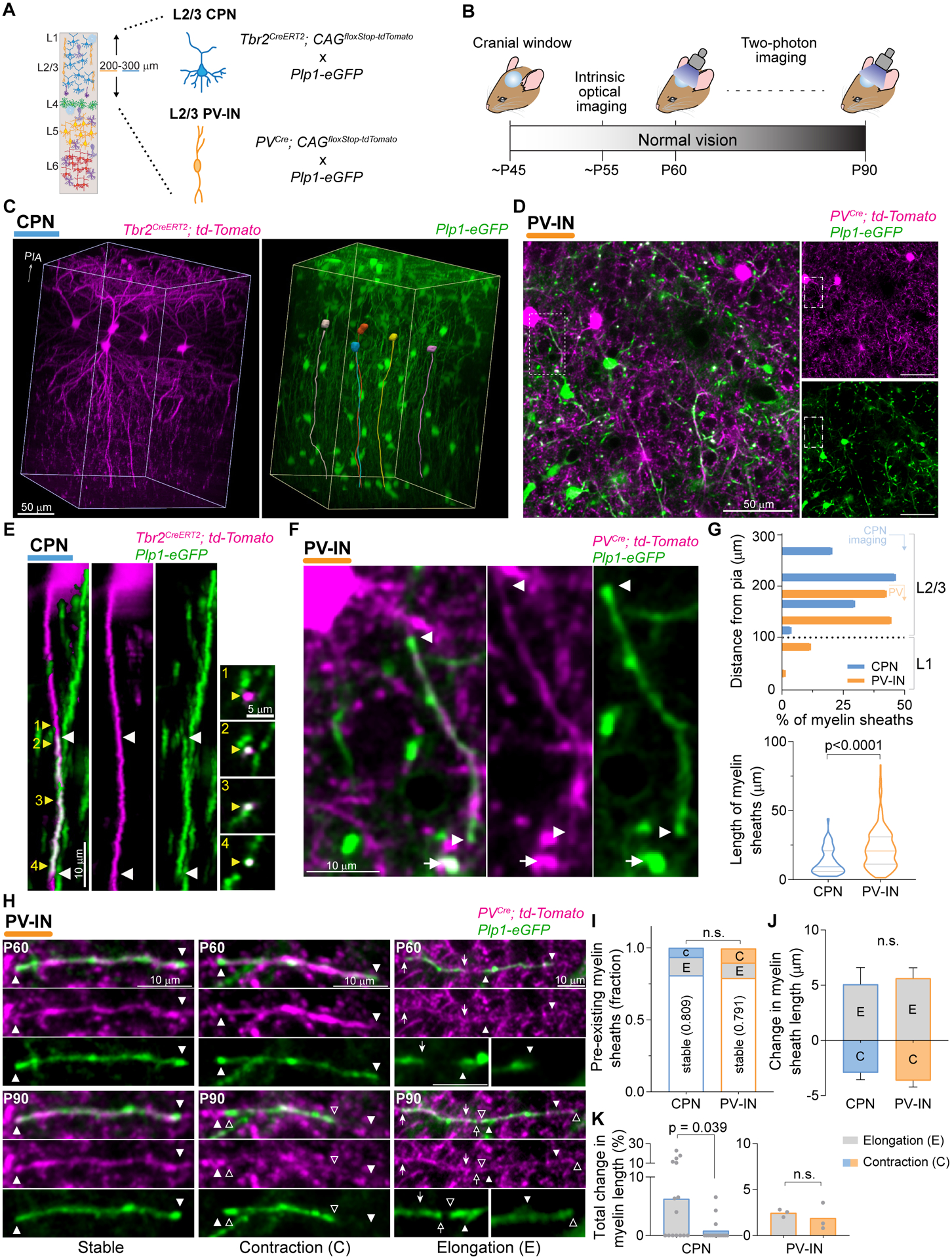
(A) Schematic of cell and myelin labeling strategy. (B) Experimental time course. Cells in V1b were imaged at P60 and P90. (C) Full imaged volume from a Tbr2Cre animal in three-dimensional (3D) perspective, showing CPNs (left) imaged simultaneously with oligodendrocytes and myelin (right). Skeletal reconstruction of cell bodies and primary axons of tdTomato+ CPNs are superimposed on the Plp1-eGFP image (right). (D) Representative single frame (~140 μm below pia) showing L2/3 PV-INs along with oligodendrocytes and myelin sheaths. (E and F) Representative images showing myelin sheaths (white arrowheads and arrows) on single axons of a superficial CPN [(E), 3D image, yellow reconstruction in (C)] and a L2/3 PV-IN [(F), single frame, boxed area in (D)]. Yellow arrowheads indicate location of corresponding cross-section insets. (G) Spatial distribution (top) and length (bottom, violin plot) of myelin sheaths at P90. (H) Maximum z projections (MZP) of myelin sheaths on PV-INs, showing stable (left, 3 frames), contracting (center, 3 frames), and elongating (right, 8 frames) segments. (I) Normalized number of dynamic (elongating and contracting) and stable myelin sheaths. (J) Length change of individual myelin sheaths. (K) Total change in myelin sheath length caused by elongations and contractions of pre-existing sheaths. Data are mean ± s.e.m. For statistics, see table S1. n.s., not significant.
We combined this strategy for cell type-specific neuronal labeling with fluorescent detection of myelinating oligodendrocytes using the Plp1-eGFP transgenic line, allowing the simultaneous imaging of single axons from each neuronal type (tdTomato+), the myelin sheaths wrapping the axons (eGFP+), and the myelinating oligodendrocytes (Fig. 1, C and D, fig. S2A, and movies S1 and S2). We confirmed that the eGFP signal from Plp1-eGFP mice faithfully reflected the presence and length of myelin sheaths by immunohistochemistry against myelin basic protein (MBP) and Cntnap1 (AKA Caspr), and by its spatial colocalization with single axonal branches from either CPNs or PV-INs (Fig. 1, E and F; and fig. S2, B to E). We performed long-term in vivo two-photon imaging of layer 1–3 V1b in young adult animals (postnatal day 60–90 [P60–90]) after the surgical placement of a cranial window and subsequent mapping of V1b using intrinsic optical signal (Fig. 1B).
We first performed two imaging sessions 30 days apart under normal vision to establish baseline changes in myelination. For PV-INs, we traced a total of 292 myelin sheaths [87.6 % of which were in L2/3 (Fig. 1G, top)], which were present at a density of 211 ± 17 myelin sheaths per 10−2 mm3 (n = 3 mice), consistent with the high rate of myelin coverage reported for this type of GABAergic interneuron (13, 14). Contrary to PV-INs, tracing of 47 myelin sheaths on CPNs revealed that most of the CPNs were unmyelinated at P90, with only 20.5 % possessing at least a single myelin sheath (fig. S3, A to I). We also found that the onset of CPN myelination occurs late in development (~P30), and the rate of myelination is strikingly low but persistent throughout adulthood (4.5 % additional myelinated CPNs per month, from P30 to P210; fig. S3, J to L).
We found that in mice with normal visual experience, the average myelin sheath was longer on PV-INs (22.4 ± 0.7 μm) than on CPNs (13.3 ± 1.3 μm) (Fig. 1G, bottom), consistent with previous reports (12, 16). Approximately 20% of the pre-existing myelin sheaths on both PV-INs and CPNs changed in length between P60 and P90 (range approximately −10 μm to +20 μm) (Fig. 1, H and I). For PV-INs, the number of elongation (E) and contraction (C) events was similar (E, 10.6 % vs C, 10.3 %), while pre-existing sheaths on CPNs showed a greater proportion of elongations (E, 12.7 % vs C, 6.4 %) (Fig. 1I). Since the average change in sheath length is similar for elongations and contractions (Fig. 1J), the overall remodeling of pre-existing myelin sheaths on PV-INs was balanced (E, 2.5 ± 0.2 % vs. C, 1.9 ± 0.8 %, of total myelin length; Wilcoxon matched-pairs signed rank test, p = 0.75, n = 3 mice), while there was a net increase in the level of myelination on CPNs (E, 6.3 ± 3.5 % vs. C, 0.9 ± 0.5 %, of total myelin length; Wilcoxon matched-pairs signed rank test, p = 0.039, n = 20 mice) (Fig. 1K). Altogether, these data show that under normal vision remodeling of pre-existing myelin sheaths in the adult neocortex follows neuronal cell-type specific patterns, with L2/3 PV-INs displaying a balanced ratio of elongations and contractions, and CPNs exhibiting shorter myelin sheaths and an overall elongation of segments over time.
De novo generated myelin sheaths are more dynamic
The cortex continues to add new myelin sheaths throughout life (2, 3, 10). To test whether new myelin segments have distinct dynamic characteristics compared to pre-existing segments, we performed longitudinal in vivo two-photon imaging on mice with normal visual experience at one-week intervals between P60 and P90, and analyzed the pre-existing (identified in the first imaging session) and de novo (emerging during the course of the 4-week experiment) myelin sheaths on L2/3 PV-INs and CPNs (Fig. 2A). Along with the longitudinal changes in pre-existing myelin sheaths described in the previous section, we found a high number of new myelin sheaths (~19.7 % of total segments at P90; Fig. 2, B and C), on both CPNs and PV-INs. De novo-generated myelin sheaths had similar lengths as pre-existing sheaths, such that the myelin length distributions across new and pre-existing segments were indistinguishable, for both neuronal populations (Fig. 2D). In PV-INs, new myelin segments were continuously produced, with new segments identified in 15 out of the 16 weekly imaging sessions (Fig. 2, E and F) (the number of new segments in CPNs was not large enough to enable detailed analysis). The integration of new myelinating oligodendrocytes was rare (3 new oligodendrocytes across 4 mice), but in imaging sessions with new myelinating oligodendrocytes we saw an almost five-fold increase in both the rate of de novo generation of myelin segments and the total length of added myelin (Fig. 2F).
Fig. 2. De novo generated myelin sheaths are more plastic than pre-existing sheaths in PV-INs.
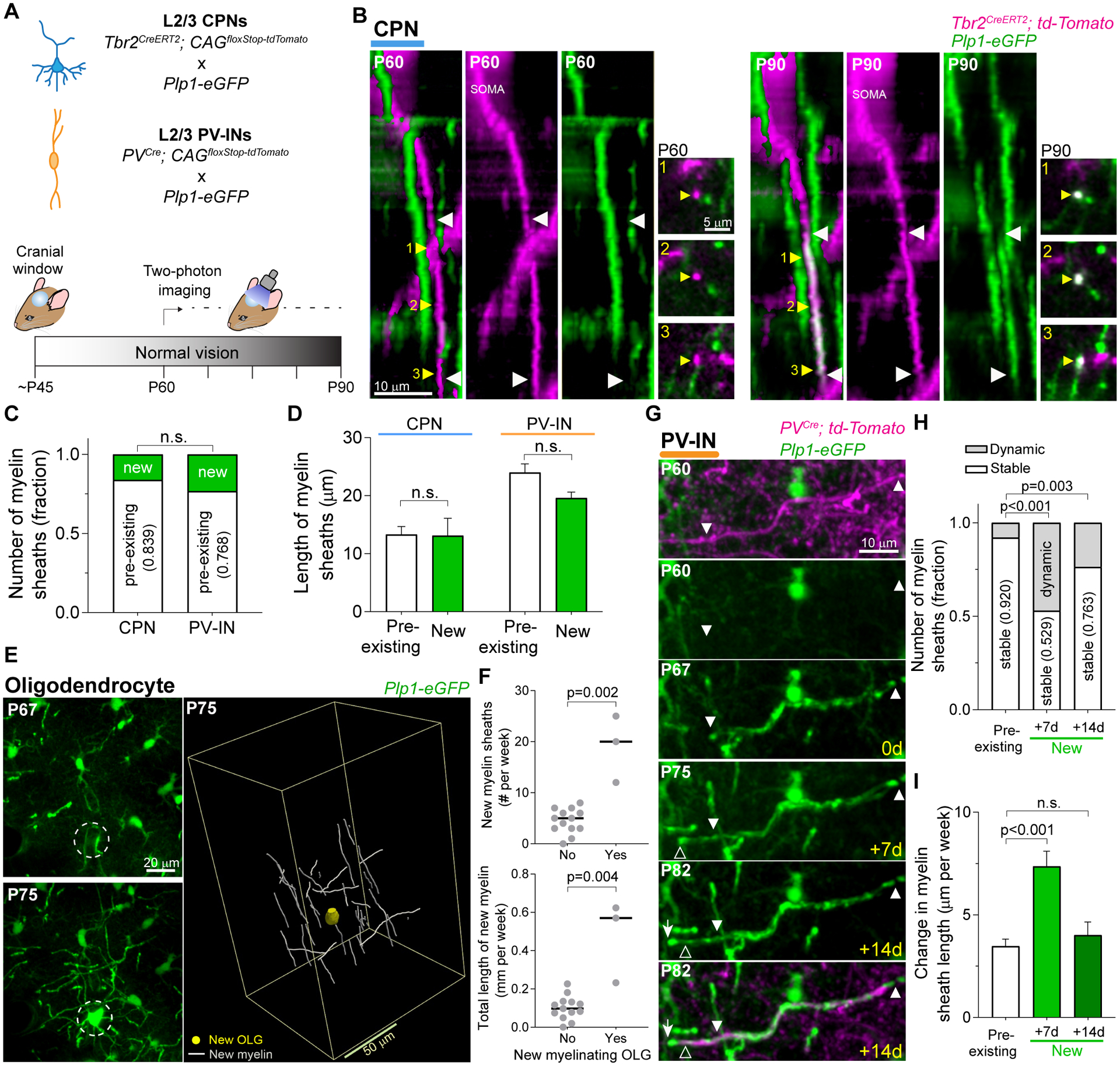
(A) Schematic of cell and myelin labeling strategy (top) and experimental time course (bottom). Cells were imaged weekly between ~P60 and ~P90. (B) Representative 3D images displaying a new myelin sheath (white arrowheads) generated on a single CPN axon. Yellow arrowheads indicate the location of corresponding cross-section insets. (C) Fraction of pre-existing and de novo generated myelin sheaths at P90, using P60 as baseline. (D) Length of individual myelin sheaths at P90. (E) MZP (15 frames) showing the integration of a new myelinating oligodendrocyte (left, circle) and 3D reconstruction of newly generated myelin sheaths on PV-INs, surrounding the cell body of the new oligodendrocyte (OLG; right). (F) Number (top) and total length (bottom) of new myelin sheaths on PV-INs, when a new myelinating oligodendrocyte was present (Yes) or not (No) within the imaged volume. Each dot represents a single imaging session. (G) MZP (14 frames) displaying the remodeling of a de novo generated myelin segment on a PV-IN. Timeline in yellow is relative to the first session in which we detected the new sheath. (H and I) Normalized number of dynamic and stable myelin sheaths (H), and change in sheath length (I). Data represents new segments on PV-INs at timepoints one and two weeks after the session in which they were first identified [see (G)], compared to the pre-existing sheaths. Data are mean ± s.e.m. For statistics, see table S1. n.s., not significant.
We next examined the level of remodeling of new sheaths compared to pre-existing sheaths, by comparing the imaging session in which individual new sheaths were first identified with sessions one and two weeks later (Fig. 2G). Newly-formed oligodendrocytes generate all of their myelin segments within 3 days of their differentiation (17, 18), well within these imaging intervals. While both new and pre-existing sheaths showed elongation and contraction, the new myelin segments exhibited a much larger ratio of elongations to contractions (54 E : 3 C, new sheaths; 31 E : 30 C, pre-existing sheaths). Notably, 47.1 % of the new myelin sheaths displayed changes in length during the first week after they were first detected, while only 8.0 % of pre-existing sheaths were remodeling during the same period (Fig. 2H). The change in length of individual sheaths was also two times larger for new sheaths compared to pre-existing myelin (Fig. 2I). Moreover, the remodeling rate of new sheaths remained higher than that of pre-existing sheaths for at least two weeks after their generation.
Thus, while rarer, new myelin sheaths are more dynamic than pre-existing myelin in PV-INs, and greatly contribute to myelin plasticity in young adult mice.
Experience-dependent myelin remodeling is neuron type-specific
It is unclear whether experience-dependent reconfiguration of neuronal circuits is accompanied by a remodeling of myelination profiles in a homogenous or a cell type-specific fashion. Therefore, we compared the changes in myelin dynamics and the integration rate of new myelinating oligodendrocytes before and during monocular deprivation. MD is a model of sensory deprivation that induces a shift in response properties in both L2/3 inhibitory interneurons and pyramidal neurons in V1b (15, 19), accompanied by remodeling of dendritic and axonal compartments (20, 21). How MD affects myelin plasticity has not been established.
We performed eyelid suture immediately after 14 days of weekly imaging under normal vision (3 sessions, one week apart), followed by two weeks of imaging under MD (imaging at 4d, 7d and 14d of MD), and studied the longitudinal distribution of myelin segments along single axons in V1b (Fig. 3A). We imaged 39 mice for L2/3 CPN (Fig. 3B), and 9 mice for L2/3 PV-INs (Fig. 3F). For each animal, we examined the number of dynamic events and their length changes, for both pre-existing and new myelin segments.
Fig. 3. Experience-dependent remodeling of myelination profiles is neuron class-specific.
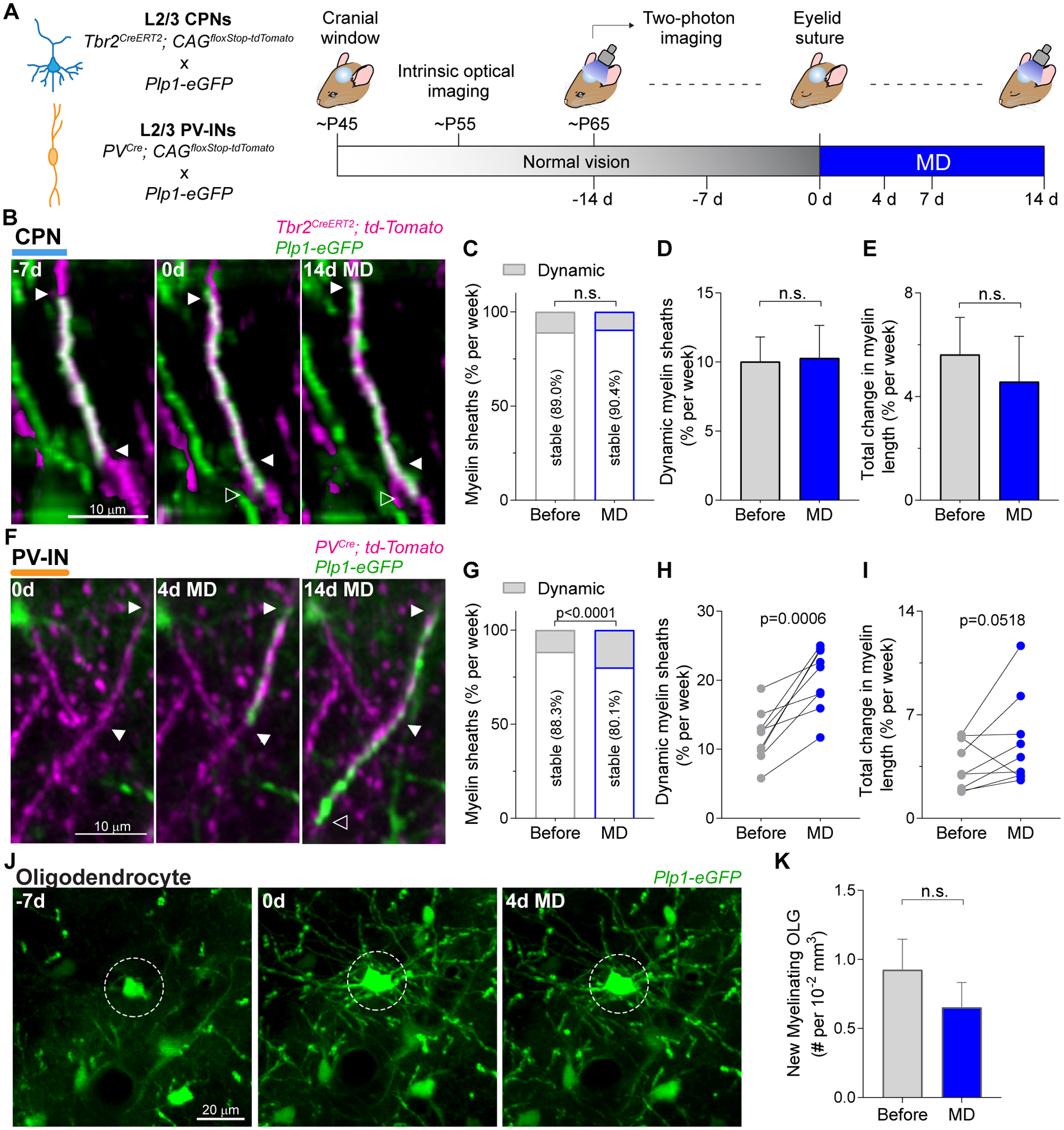
(A) Schematic of cell and myelin labeling strategy (left), and experimental time course (right). Cells in V1b were imaged at each indicated time point. (B) Representative 3D images of an elongating CPN myelin sheath. (C) Fraction of stable and dynamic myelin sheaths on CPNs (combined data from all mice). The data represents the average of two one-week intervals between time points, during the periods before (−14d vs. −7d and −7d vs. 0d) and during MD (0d vs. 7d and 7d vs. 14d). (D) Rate of myelin sheath dynamics for CPNs. Each mouse was analyzed as an independent statistical replicate (n = 39 mice). (E) Rate of change in sheath length elicited by myelin remodeling. (F) MZP (5 frames) of a newly-generated myelin sheath on a PV-IN. (G to I) Analysis of PV-IN myelination (n = 9 mice), corresponding to (C) to (E). (J) MZP (22 frames) of a new myelinating oligodendrocyte (circle, 0 d). The circled cell at −7d is likely an oligodendrocyte progenitor cell. (K) Number of new myelinating oligodendrocytes integrated in the two-week intervals before and during MD (n = 19 mice). Data are mean ± s.e.m. For statistics, see table S1. n.s., not significant.
First, we compared the weekly rate of sheath dynamics on CPNs during the two weeks of normal vision and the subsequent two weeks of MD (Fig. 3, C and D), and we found that the rate of remodeling remained unchanged (before, 10.0 ± 1.8 % per week vs. MD, 10.3 ± 2.4 % per week; paired t-test, p = 0.933; n = 39 mice, 146 sheaths). Similarly, MD did not affect the cumulative change in myelin length caused by the population of dynamic sheaths (% of total length per week: before, 5.6 ± 1.4 % vs. MD, 4.6 ± 1.8 %; paired t-test, p = 0.647; Fig. 3E). We measured the rate of dynamic sheaths and the normalized cumulative change in myelin length for each category of remodeling (elongation, contraction, and de novo generation), and determined that MD did not alter either of these parameters for any of the remodeling categories (fig. S4). These results indicate that MD does not affect the plasticity of myelination profiles in L2/3 CPNs.
Next, we asked whether L2/3 PV-INs had similar myelin dynamics in response to MD as their CPN neighbors (Fig. 3G). In contrast to CPNs, we observed an increase in the rate of dynamic myelin sheaths (before, 11.9 ± 1.3 % per week vs MD, 20.3 ± 1.5 % per week; paired t-test, p = 0.0006; n = 9 mice, 2465 sheaths; Fig. 3H), and in 7 out of 9 mice there was also an increase in the cumulative change in their length (Fig. 3I). This increase in myelin sheath dynamics in PV-INs is exclusive to the binocular visual cortex and is absent in monocular visual cortex (fig. S5), indicating that the changes are associated with the ocular dominance shift induced by MD (fig. S6). These findings demonstrate that sensory experience drives adaptive remodeling of myelination profiles specifically in PV-INs, while CPN myelination shows only a continuation of baseline plasticity.
Finally, since new oligodendrocytes account for a substantial fraction of myelin remodeling under normal vision (Fig. 2), we investigated the integration rate of new myelinating oligodendrocytes during MD (Fig. 3J). The initial density of mature oligodendrocytes was 47.1 ± 1.6 cells per 10−2 mm3 (P65, n = 19 mice), and the integration rate of new myelinating oligodendrocytes did not change upon MD (new myelinating oligodendrocytes per 10−2 mm3: before, 0.92 ± 0.22 vs MD, 0.65 ± 0.18; Wilcoxon matched-pairs signed rank test, p = 0.542, n = 19 animals; Fig. 3K), in either L1 or L2/3 (fig. S7, A to D). Furthermore, we found no changes in the proliferation or apoptosis of oligodendrocyte precursor cells upon MD (fig. S7, E to I), nor any elimination of mature oligodendrocytes. These data reveal an unprecedented level of specificity for myelin plasticity, demonstrating that MD-induced adaptive remodeling of myelination profiles is neuron class-specific and is sufficient to achieve circuit reconfiguration without the need for integration of new myelinating oligodendrocytes.
Temporal dynamics of adaptive remodeling of PV+ interneurons
GABAergic neurons in L2/3 of adult mouse V1b respond to MD with changes in their dendritic arbors that first undergo retraction and subsequently elongate (20). Therefore, we asked whether the experience-dependent increase in myelin dynamics in PV-INs also follows a defined spatiotemporal pattern (Fig. 4A). We analyzed myelin dynamics over the time course of MD, and found that PV-INs presented a two-phase sequence of myelin dynamics. Throughout the first week of MD, there was an acute increase in myelination, by more frequent elongation of pre-existing sheaths (three-fold increase in rate) and the generation of new myelin segments (Fig. 4, B and C; and fig. S8, A to D). In the second week (7–14 d MD), the elongation rate returned to baseline levels, accompanied by three-fold increases in contractions and in the cumulative change in length (Fig. 4, B and C). We also observed full elimination of myelin segments upon MD in PV-INs (fig. S9, A and B). This extreme form of contraction was not seen in PV-INs during normal visual experience or in CPNs. Notably, MD also caused the recruitment of pre-existing oligodendrocytes to produce new myelin segments on PV-INs (Fig. 4D, fig. S9C and movie S3). These results show that MD triggers a series of events that progressively modify the myelination profile of PV-INs.
Fig. 4. MD elicits temporally distinct phases of myelin dynamics in PV-INs.
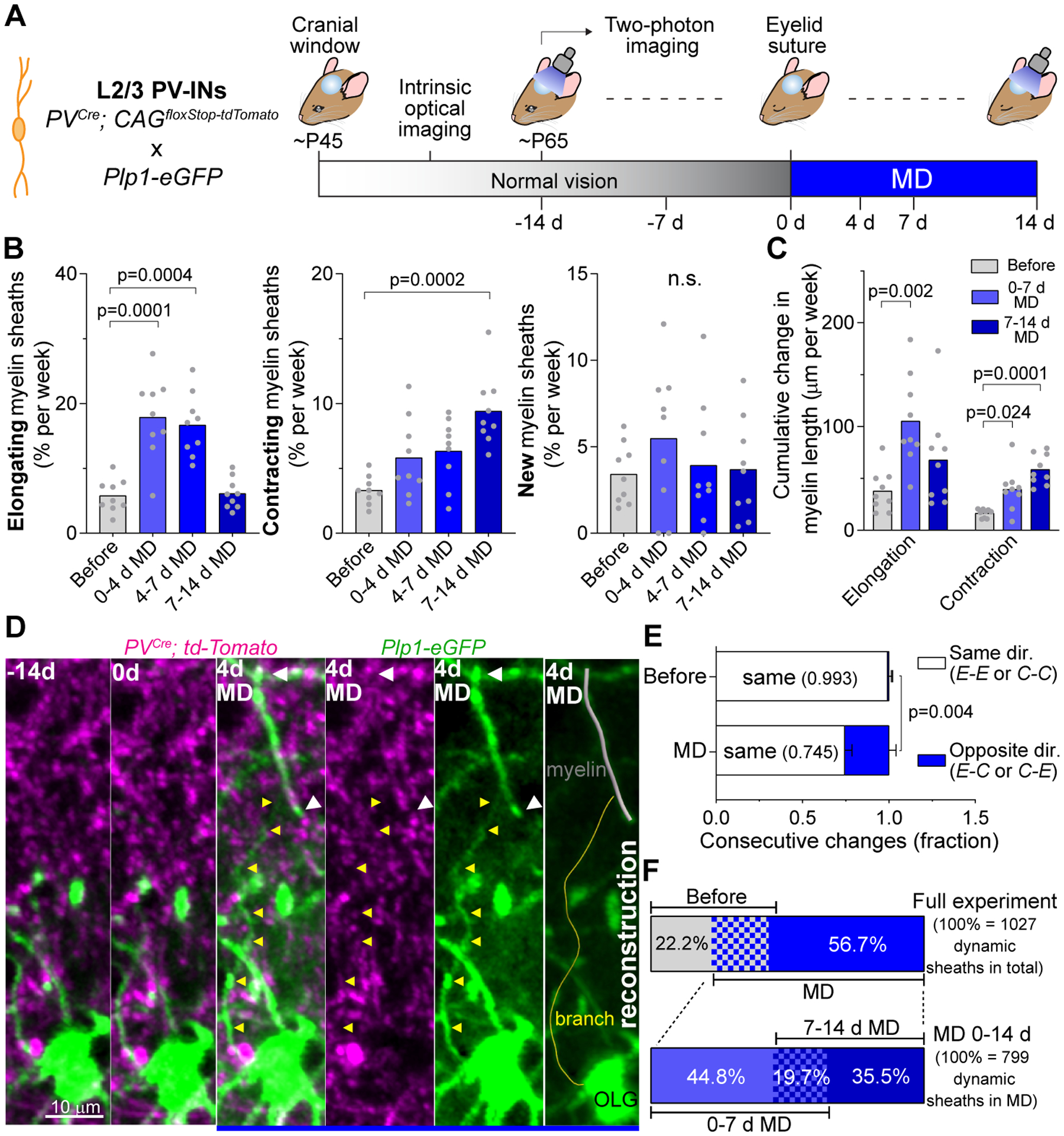
(A) Schematic of PV-IN and myelin labeling strategy (left), and experimental time course (right). (B) Rate of myelin sheath elongation (left), contraction (center), and de novo generation (right) in PV-INs before and during MD. Each dot represents an animal. The data prior to MD (−14 d to 0 d) was combined into a single group (before). (C) Rate of absolute cumulative change in sheath length produced by contractions and elongations. (D) MZP (5 frames) showing a pre-existing oligodendrocyte (OLG) generating a new myelin sheath (white arrowheads) on a PV-IN during MD. Yellow arrowheads indicate the branch that connects the myelin segment to the OLG cell body. A 3D reconstruction is displayed at far right. (E) Fraction of changes in individual sheaths over consecutive imaging sessions classified by the different combinations of change direction: same (elongation/elongation or contraction/contraction) and opposite (elongation/contraction or contraction/elongation). (F) (Top) Percentage of myelin sheaths presenting changes only before MD (gray), only during MD (blue), and in both conditions (gridded, 21.1%). (Bottom) Percentage of myelin sheaths presenting changes only during the first week of MD (lighter blue), only the second week of MD (darker blue), and during both weeks of MD (gridded). Data are mean ± s.e.m. For statistics, see table S1. n.s., not significant.
We next asked whether the myelin remodeling induced by MD represented an acceleration of baseline remodeling, or had different dynamics. Under normal vision, consecutive changes of a single myelin sheath length were always in the same direction; i.e., either successive elongations or successive contractions (Fig. 4E). In contrast, during MD, 25.5 ± 1.4 % of successive changes on a single segment were in opposite directions (Fig. 4E), indicating that MD altered the pattern of myelin remodeling. We next asked whether the myelin sheaths remodeled during MD were the same as those affected during normal vision. From the entire population of dynamic sheaths, 56.7 % presented changes exclusively during MD while 22.2 % were dynamic solely under normal vision (Fig. 4F), indicating that MD triggers the remodeling of a different set of sheaths, rather than continuing changes in previously-dynamic sheaths. Moreover, a total of 80.3 % of sheaths dynamic during MD exhibited changes only during the elongation phase (0–7d MD, 44.8 %) or the retraction phase (7–14d MD, 35.5 %), but not both, indicating that the opposite phases affect two different subgroups of myelin segments (Fig. 4F). In particular, 54.0 ± 0.9 % of contracting sheaths during the second week of MD had shown no change until then (vs. 26 ± 5 % of elongating sheaths; fig. S8E).
These results indicate that MD drives the remodeling of myelin profiles in L2/3 PV-INs by recruiting previously-stable myelin segments in two separate phases, first triggering a wave of elongation events, followed by contraction of a different group of myelin sheaths. These novel myelin dynamics distinguish the response to sensory perturbation from baseline myelin plasticity.
MD induces remodeling on PV+ interneuron axons
Since myelin placement relates to the structure and function of axons (1, 22), we interrogated the relationship between myelin plasticity, and changes in axonal morphology and structure (Fig. 5, A). We investigated the impact of sensory experience on the nodes of Ranvier, short unmyelinated segments that are occupied by clusters of ion channels (fig. S2F). It has been shown that changes in myelin structure are accompanied by changes in length of these nodes, which modify action potential propagation along the axon (23). We examined the dynamics of putative nodes of Ranvier in PV-INs (Fig. 5B), defined as short nodes (<5 μm) separating two neighboring myelin sheaths. Surprisingly, we found that putative nodes of Ranvier can be displaced along the axon during MD (14 out of 257 nodes in 9 mice; distance, 2.66 ± 0.25 μm), as a result of the contraction of one sheath and the elongation of another (Fig. 5, C). In contrast, node displacement was rarely seen under normal vision (3 out of 257 nodes). This result suggests that MD induces changes in the longitudinal patterns of PV-IN myelination and an associated increase in the rate of displacement of putative nodes of Ranvier.
Fig. 5. Sensory experience drives changes in the axonal arbor of PV+ interneurons.
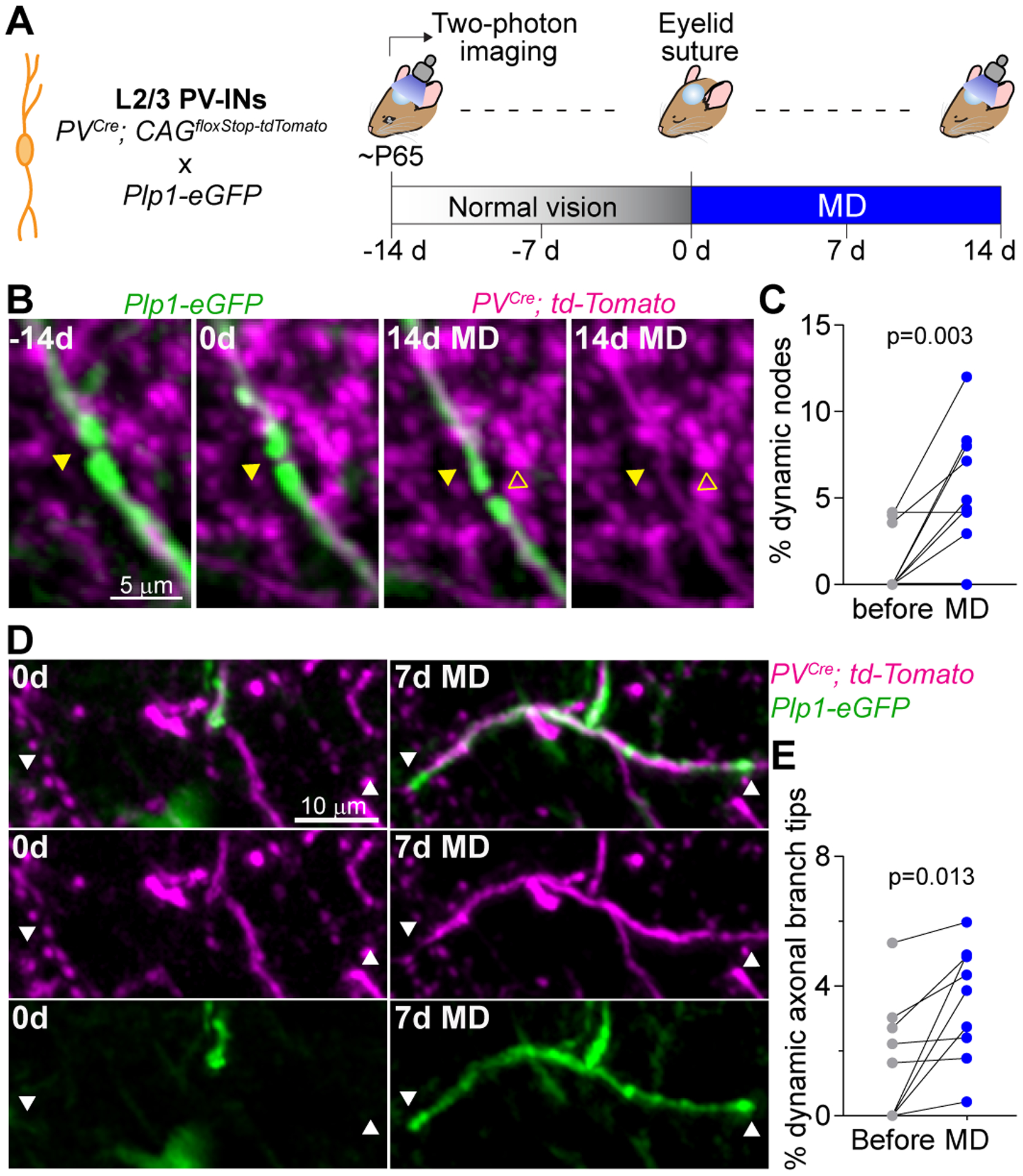
(A) Schematic of fluorescence labeling strategy and experimental time course. (B) Single-frame images showing a putative node of Ranvier (arrowheads) between two neighboring myelin sheaths, and a shift of its location during MD. (C) Number of relocated putative nodes of Ranvier in the two-week intervals before and during MD. (D) MZP (6 frames) showing a new myelin sheath generated on a new axonal branch. (E) Number of dynamic axonal branch tips in the two-week intervals before and during MD. Each dot represents an animal [(C) and (E)]. For statistics, see table S1.
Visual manipulations have been shown to induce reorganization of inhibitory neuron axons in adult visual cortex (24). We therefore examined axonal arbor remodeling in PV-INs, focusing on those branches that were myelinated (Fig. 5D, fig. S10A). We found that myelin remodeling overwhelmingly occurred on axonal branches that did not exhibit changes in length during MD (95.4 ± 0.7 % of axons showing myelin remodeling, fig. S10B). This indicates that the experience-dependent remodeling of myelination profiles in PV-INs is not simply a downstream effect of axonal remodeling.
MD also resulted in an increased number of dynamic axonal branch tips in PV-INs (Fig. 5E), as well as a greater cumulative change to axonal length (fig. S10C). In agreement with our findings that MD did not affect CPN myelin plasticity, we did not observe axon remodeling in these neurons (0 out of 68 neurons). Our results indicate that PV-INs show independent increases in both axonal branch remodeling and myelin plasticity in response to MD.
Altogether, the data points at adaptive myelination as part of a coordinated circuit-reconfiguration process, which acts through differential responses by individual neuronal subtypes to perturbations in sensory experience.
Discussion
Prior reports have shown that neuronal activity and experience modulate myelination (4, 7, 11, 17, 25, 26), and that active myelination by newly-formed oligodendrocytes is necessary for learning and memory (8–10). Understanding the dynamic mechanisms that govern myelin plasticity, and how they relate to other mechanisms of neuronal plasticity, remains a fundamental question.
Here we find that sensory deprivation (MD) induces adaptive myelin remodeling in a neuron class-specific manner, highlighting unexpected levels of specificity in neuron-oligodendrocyte interaction. The response to MD is complex, unfolding in an age-dependent and time-specific manner across multiple neuron types (27–29). However, it has been shown that L2/3 interneurons show greater electrophysiological and structural plasticity in response to MD compared to pyramidal cells (19–21, 29). In particular, after MD there is an initial phase of dendritic branch tip retractions in L2/3 inhibitory neurons, followed by a second phase of balanced growth and retraction (20). Here we find that L2/3 PV-INs likewise show a bi-phasic increase in myelin plasticity upon MD compared to CPNs. During the first week after MD there is an increase in PV-IN myelination, while in the second week there is a concerted increase in myelin sheath retraction. However, MD-induced myelin remodeling was primarily restricted to axonal branches that did not change in length, suggesting that myelin plasticity is part of an orchestrated set of circuit reconfiguration processes rather than simply a consequence of axonal remodeling. It would be informative to use electron microscopy to further investigate whether other structural changes, such as myelin thickness and axon caliber, are taking place along with the remodeling of myelination profiles.
Our results demonstrate that MD does not stimulate the integration of new myelinating oligodendrocytes. Rather, it induces remodeling of pre-existing myelin sheaths on PV+ interneurons, including contraction and full elimination of myelin sheaths. Small modifications in myelin sheath structure can be sufficient to substantially impact neural network function (30). We speculate that the reconfiguration of network connectivity that underlies a functional ocular dominance shift likely requires a fine and precise tuning of individual myelination profiles instead of a broad addition of myelin (22, 30, 31). In line with this hypothesis, we observed recruitment of pre-existing oligodendrocytes to generate new myelin sheaths. This finding is in agreement with recent reports that surviving oligodendrocytes can establish new myelin segments in a cuprizone-induced model of oligodendrocyte death and demyelination (17). Our data now indicates that in response to sensory manipulation, new myelin segments are produced by both newly-formed and pre-existing oligodendrocytes.
The data demonstrate cell type-specific dynamics of myelin plasticity, even when the distinct neuronal subpopulations are interconnected within the same circuit, surrounded by a shared environment, and myelinated by a common set of oligodendrocytes. These findings suggest a novel framework for conceptualizing experience-dependent myelin plasticity, where cell type-specific adaptive remodeling of myelination allows different neurons to modify their individual functions and potentiates the tuning capacity of neuronal networks.
Supplementary Material
Acknowledgments:
We thank J.R. Brown, P. Oyler-Castrillo, and former and present members of the Arlotta and Nedivi laboratories for insightful discussions and editing of the manuscript; J. Huang and W. Macklin for sharing the Tbr2CreERT2 and the Plp1-eGFP mouse lines, respectively; J. Boivin for helpful advice on in vivo 2P-imaging; V. Vuong for outstanding technical support and for help with image analysis; and B. Paulsen for help with figure production.
Funding: This work was supported by grants from the Stanley Center for Psychiatric Research, the Broad Institute of MIT and Harvard, the National Institute of Mental Health (U19MH114821 to P.A.), the National Institutes of Health (RO1-EY025437 to E.N.), and the JBP Foundation to E.N. K.M. acknowledges support by the DFG fellowship (MI 2108/1-1).
Footnotes
Competing interests: Authors declare no competing interests.
Data and materials availability: All data is available in the main text or the supplementary materials. Request for materials should be addressed to P.A. (paola_arlotta@harvard.edu) and S.M.Y. (sungminyang@fas.harvard.edu).
References and Notes:
- 1.Baumann N, Pham-Dinh D, Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol. Rev 81, 871–927 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Hill RA, Li AM, Grutzendler J, Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain. Nat. Neurosci 21, 683–695 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes EG, Orthmann-Murphy JL, Langseth AJ, Bergles DE, Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nat. Neurosci 21, 696–706 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, Barres BA, Woo PJ, Vogel H, Monje M, Neuronal Activity Promotes Oligodendrogenesis and Adaptive Myelination in the Mammalian Brain. Science (80-. ). 344, 1252304–1252304 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fields RD, A new mechanism of nervous system plasticity: Activity-dependent myelination. Nat. Rev. Neurosci 16, 756–767 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mount CW, Monje M, Wrapped to Adapt: Experience-Dependent Myelination. Neuron. 95, 743–756 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mensch S, Baraban M, Almeida R, Czopka T, Ausborn J, El Manira A, Lyons DA, Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat. Neurosci 18, 628–630 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKenzie IA, Ohayon D, Li H, De Faria JP, Emery B, Tohyama K, Richardson WD, Motor skill learning requires active central myelination. Science (80-. ). 346, 318–322 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geraghty AC, Gibson EM, Ghanem RA, Greene JJ, Ocampo A, Goldstein AK, Ni L, Yang T, Marton RM, Paşca SP, Greenberg ME, Longo FM, Monje M, Loss of Adaptive Myelination Contributes to Methotrexate Chemotherapy-Related Cognitive Impairment. Neuron. 103, 250–265.e8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan S, Mayoral SR, Choi HS, Chan JR, Kheirbek MA, Preservation of a remote fear memory requires new myelin formation. Nat. Neurosci (2020), doi: 10.1038/s41593-019-0582-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swire M, Kotelevtsev Y, Webb DJ, Lyons DA, Ffrench-Constant C, Endothelin signalling mediates experience-dependent myelination in the CNS. Elife. 8, 1–23 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomassy GS, Berger DR, Chen HH, Kasthuri N, Hayworth KJ, Vercelli A, Seung HS, Lichtman JW, Arlotta P, Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science (80-. ). 344, 319–324 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Micheva KD, Wolman D, Mensh BD, Pax E, Buchanan J, Smith SJ, Bock DD, A large fraction of neocortical myelin ensheathes axons of local inhibitory neurons. Elife. 5, 1–29 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zonouzi M, Berger D, Jokhi V, Kedaigle A, Lichtman J, Arlotta P, Individual Oligodendrocytes Show Bias for Inhibitory Axons in the Neocortex. Cell Rep. 27, 2799–2808.e3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hubel DH, Wiesel TN, The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J. Physiol 206, 419–436 (1970). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stedehouder J, Couey JJ, Brizee D, Hosseini B, Slotman JA, Dirven CMF, Shpak G, Houtsmuller AB, Kushner SA, Fast-spiking Parvalbumin Interneurons are Frequently Myelinated in the Cerebral Cortex of Mice and Humans. Cereb. Cortex 27, 5001–5013 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Bacmeister CM, Barr HJ, Mcclain CR, Thornton MA, Nettles D, Welle CG, Hughes EG, Motor Learning Promotes Remyelination Via New and Surviving Oligodendrocytes. Nat. Neurosci (2020), doi: 10.1038/s41593-020-0637-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Auer F, Vagionitis S, Czopka T, Evidence for Myelin Sheath Remodeling in the CNS Revealed by In Vivo Imaging. Curr. Biol 28, 549–559.e3 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Kameyama K, Sohya K, Ebina T, Fukuda A, Yanagawa Y, Tsumoto T, Difference in binocularity and ocular dominance plasticity between GABAergic and excitatory cortical neurons. J. Neurosci 30, 1551–1559 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen JL, Lin WC, Cha JW, So PT, Kubota Y, Nedivi E, Structural basis for the role of inhibition in facilitating adult brain plasticity. Nat. Neurosci 14, 587–596 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen JL, Villa KL, Cha JW, So PTC, Kubota Y, Nedivi E, Clustered Dynamics of Inhibitory Synapses and Dendritic Spines in the Adult Neocortex. Neuron. 74, 361–373 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ford MC, Alexandrova O, Cossell L, Stange-Marten A, Sinclair J, Kopp-Scheinpflug C, Pecka M, Attwell D, Grothe B, Tuning of Ranvier node and internode properties in myelinated axons to adjust action potential timing. Nat. Commun 6 (2015), doi: 10.1038/ncomms9073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tagoe T, Barker M, Jones A, Allcock N, Hamann M, Auditory nerve perinodal dysmyelination in noise-induced hearing loss. J. Neurosci 34, 2684–2688 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marik SA, Yamahachi H, Meyer zum Alten Borgloh S, Gilbert CD, Large-scale axonal reorganization of inhibitory neurons following retinal lesions. J. Neurosci 34, 1625–1632 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wake H, Lee PR, Fields RD, Control of local protein synthesis and initial events in myelination by action potentials. Science (80-. ). 333, 1647–1651 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makinodan M, Rosen KM, Ito S, Corfas G, A Critical Period for White Mice. Science. 337, 1357–1360 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hengen KB, Lambo ME, VanHooser SD, Katz DB, Turrigiano GG, Firing rate homeostasis in visual cortex of freely behaving rodents. Neuron. 80, 335–342 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hooks BM, Chen C, Circuitry Underlying Experience-Dependent Plasticity in the Mouse Visual System. Neuron. 106, 21–36 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berry KP, Nedivi E, Experience-Dependent Structural Plasticity in the Visual System. Annu. Rev. Vis. Sci 2, 17–35 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seidl AH, Regulation of conduction time along axons. Neuroscience. 276, 126–134 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salami M, Itami C, Tsumoto T, Kimura F, Change of conduction velocity by regional myelination yields constant latency irrespective of distance between thalamus and cortex. Proc. Natl. Acad. Sci. U. S. A 100, 6174–6179 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mallon BS, Elizabeth Shick H, Kidd GJ, Macklin WB, Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. J. Neurosci 22, 876–885 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, Arber S, A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 3, 0878–0890 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matho KS, Huilgol D, Galbavy W, Kim G, He M, An X, Hatfield J, Raudales R, Narasimhan A, Gamache E, Levine J, Genetic dissection of glutamatergic neuron subpopulations and developmental trajectories in the cerebral cortex. bioRxiv, 1–28 (2020). [Google Scholar]
- 35.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H, A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci 13, 133–140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee WCA, Huang H, Feng G, Sanes JR, Brown EN, So PT, Nedivi E, Dynamic remodeling of dendritic arbors in GABAergic interneurons of adult visual cortex. PLoS Biol. 4, 271–280 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villa KL, Berry KP, Subramanian J, Cha JW, Oh WC, Kwon HB, Kubota Y, So PTC, Nedivi E, Inhibitory Synapses Are Repeatedly Assembled and Removed at Persistent Sites In Vivo. Neuron. 89, 756–769 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato M, Stryker MP, Distinctive features of adult ocular dominance plasticity. J. Neurosci 28, 10278–10286 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, MacKlis JD, Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 45, 207–221 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


