Graphical abstract
Keywords: SARS-CoV-2, Disinfectants, Alcohol, Human, Environment, Hazardous
Highlights
-
•
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in Wuhan city of China in late December 2019.
-
•
The rapid spread of the COVID-19 across the communities shock the entire population, because of no existing therapies.
-
•
Health professionals recommended frequent washing of hands with soap and alcohol-based sanitizers, but these disinfectants has worst effects on human health.
-
•
Precautionary measures should be ensured to protect ourselves and the community from the possible risk associated with disinfectants.
Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in Wuhan city of China in late December 2019 and identified as a novel coronavirus disease 2019 (COVID-19). On January 30, the World Health Organization (WHO) declared the coronavirus outbreak a global public health emergency. The rapid spread of the pathogen across the communities shock the entire population. As no existing therapy were available during the pandemic. Health professionals recommended frequent washing of hands with soap and alcohol-based sanitizers. Disinfectants were extensively sprayed to minimize the possibility of getting COVID-19. Despite the potential benefits of these germicidal agents against COVID-19. Alcohol-based hand sanitizers lead to dry skin, infection, and alcohol poisoning. Children are considered more prone to alcohol poisoning and other major health concern. Precautionary measures should be ensured to protect the community from the possible risk associated with disinfectants.
Introduction
COVID-19 , the novel disease spreads through recent coronavirus 2 (SARS -CoV-2) from Wuhan, China. COVID-19 has been declared as a pandemic by the WHO on March 11, 2020, and till September 2020 the global tally spiked 28 million (Khan et al., 2020). This disease is categorized by acute respiratory disease, pneumonia, dry cough, fever and body pain, and high mortality, especially in the elderly or people with bad health conditions(Lai et al., 2020). Humans are the core cause of the spreading of SARS-CoV-2 from human-to-human. In case, any mild or asymptomatic infected person comes into contact with healthy people (Kratzel et al., 2020). So far, the drugs or vaccines which are available to deal with this new type of coronavirus is not so effective, and the global infection rate is increasing sharply. Under the current circumstances, WHO has proposed preventive measures and a healthy lifestyle with an effective immune system to fight and keep safe from COVID-19(Adams et al., 2008). Adapting to effective hand hygiene is crucial, and one of the best recommendations of WHO is to wash or disinfect your hands frequently with soap or N60% alcohol-based hand sanitizer, respectively(https://www.cdc.gov/). WHO recommends the use of two alcohol-based formulas for hand hygiene in health care to make hands sensitive and reduce the spread and infectivity of coronavirus. These recommendations are based on fast, effective, and broad-spectrum antibacterial activity, and are easy to obtain and consider safe. The alcohol-based hand sanitizer recommended by WHO is mainly composed of ethanol, isopropanol, and different types of hydrogen peroxide portfolio (Kilpatrick et al., 2011). Misuse of these preparations may be toxic to human health and the environment. When these chemicals are released through evaporation, they will have toxic and hazardous effects on the environment(Slaughter et al., 2014). It is recognized that the ingestion of low concentrations of hydrogen peroxide (3% solution) can cause mild gastrointestinal irritation (Moon et al., 2006b), and in a few cases also cause portal vein thrombosis (Sung et al., 2018) and mild mucosal irritation and vomiting (https://www.atsdr.cdc.gov). Accidental or deliberate ingestion of isopropanol can cause severe respiratory or central nervous system depression. The methanol toxicology review issued by the public health department of the United Kingdom shows that methanol is toxic to the mouth and skin, but it is not observed used in hand hygiene products(Mahmood et al., 2020). Ethanol toxicity is also related to respiratory depression, which can cause respiratory arrest, hypothermia, arrhythmia, and possibly cardiac arrest, hypoglycemia, ketoacidosis, and hypotension (Gormley et al., 2012). The main purpose of this review is to emphasize frequent use of hand hygiene products with alcohol-based formulas can cause toxicity and serious health risks to human health and the environment. As a preventive measure for COVID-19, increasing the use of hand wipes is harmful to the environment and harmful to human health. It is recommended to wash your hands with antibacterial soap every once in a while to eliminate possible infections caused by this pandemic.
Methods
The research article on the topic of interest was searched in Google Scholar, Pubmed, Sci-Hub, WHO, Pubchem and the American Association of Poison Control Centers (AAPCC) for systematic analysis of scientific literature. Different terminologies were used as facilitation to search out relevant articles from the search engines and databases. The key words COVID-19, Sar-Cov-2, disinfectants, alcoholism, sanitizers, human and environmental effects of disinfectants, disinfectant effect on wildlife, and several other terminologies were mostly used. The authors searched the reference lists of the included studies to ensure literature coverage. All full text reports were analyzed to know if they met the inclusion criteria. Mostly those article were included in the study which highlighted COVID-19 hazardous effects on humans and wild life. Majority of the papers included in this study have recently been published, although some of the earlier published articles have also been cited in order to clarify the essence of disinfectants, and their effects on living beings because no applicable recent scientific work has been published on the subjects involved.
Feature of coronavirus
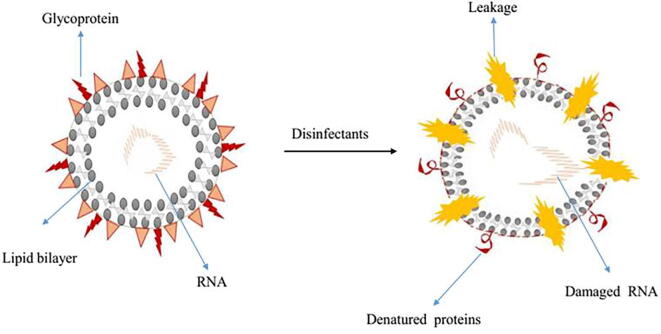
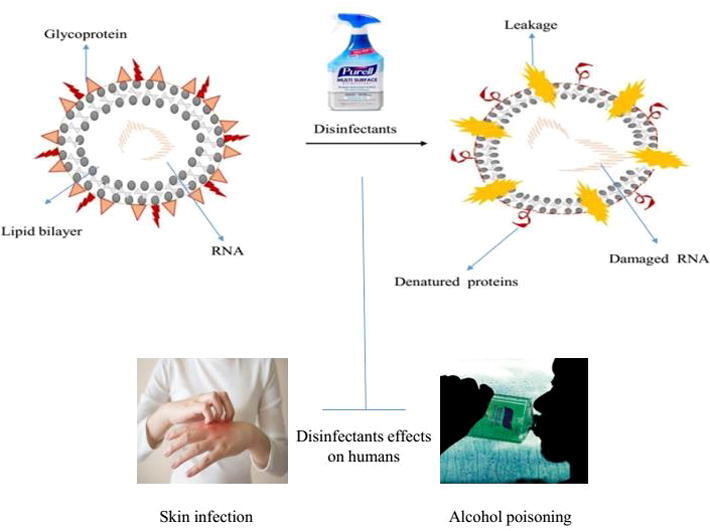
Sars-CoV-2 is an enveloped virus with a fragile outer lipid layer is more sensitive to disinfectants compared to non-enveloped viruses such as poliovirus, rotavirus, and norovirus etcetera (https://cleanroomtechnology.com/). Coronavirus has a spherical shape with an average diameter of 120 nm. Modified proteins that are formed during post-translation modification such as glycoproteins and transmembrane proteins are the constituent of the virus's outer surface envelope(Kilpatrick et al., 2011, Kratzel et al., 2020). These antigenic proteins act as prime mediating agents by attaching to the specific receptors on the host. The potential virus integrates the genetic RNA code and starts replication inside the host cell. The structural integrity of the viral membrane determines the topological and tertiary structure of membrane proteins, therefore, any agents which interfere with the integrity of the enveloped virus discourage the entry of the vial particle, and prevent its infectivity (Fig. 1).
Fig. 1.
Schematic diagram represents how disinfectant interfere cellular integrity of corona viruses.
Principles of environmental cleaning and disinfection
The first step in the disinfection process is cleaning which helps to remove pathogens and effectively reduce their loads on contaminated surfaces. Organic matters (blood, secretions, and excretions), dirt, debris can be removed/reduced by using water, soap (or a neutral detergent), and other mechanical actions like brushing and scrubbing but can’t kill all type of microorganisms(Adams et al., 2008). Organic matter can inhibit direct surface contact of a disinfectant, inactivate several disinfectants' germicidal characteristics or mode of action. Chemical disinfectants, such as chlorine or alcohol, should then be used after washing to destroy any residual microorganisms. A sufficient disinfectant solution should be used to allow surfaces to remain wet and untreated long enough for the disinfectant to inactivate pathogens. Spraying or fogging of certain chemicals, such as formaldehyde, chlorine-based agents or quaternary ammonium compounds, is not recommended for COVID-19, due to adverse health effects (Schyllert et al., 2016, Zock et al., 2007). It would result in eye and skin irritation, bronchospasm due to inhalation, and gastrointestinal effects such as nausea and vomiting (Organization, 2020, Schyllert et al., 2016). Spraying tunnel, cabinet, or chamber with disinfectants is also not suggested under any circumstances, due to physical and psychological threats. Moreover, it would not reduce an infected person’s ability to spread the virus through droplets or contact. Fumigation of outdoor spaces, such as streets, sidewalks, unpaved walkways, or marketplaces, is also not useful to eradicate the COVID-19 virus or other pathogens because disinfectant immediately become inactivated by dirt and debris. Even in the absence of organic matter, chemical spraying is unlikely to adequately cover all surfaces for the duration of the required contact time needed to inactivate pathogens. It is still important to reduce the potential for COVID-19 virus contamination in non– healthcare settings and to reduce the risk of fomite transmission in the hospital setting to any environment outside of the hospital. High-touch surfaces as, door and window handle, kitchen and food preparation areas, countertops, bathroom surfaces, toilets and taps, touchscreen personal devices, personal computer keyboards, and work surfaces should be identified for priority disinfection. The disinfectant and its concentration should be carefully selected to avoid damaging surfaces and to minimize toxic effects on house members or users of public spaces. The environmental cleaning techniques and cleaning principles should be followed as surfaces should always be cleaned with soap or detergent to remove organic matter first, followed by disinfection. In non-healthcare settings, a recommended concentration of 0.1% (1000 ppm sodium hypochlorite) may be used(Bennett et al., 2014). Alternatively, alcohol with 70%-90% concentration may be used for surface disinfection. Some countries have approved no-touch technologies e.g. vaporized hydrogen peroxide and devices using UV irradiation in health-care settings (Weber et al., 2016). Notably, these technologies supplement cannot replace the need for manual cleaning procedures. Before the use of no-touch disinfection technology, environmental surfaces must be cleaned manually first by brushing or scrubbing to remove organic matter (Rutala and Weber, 2013). Various non-touch disinfection methods used in hospitals against highly resistant organism, such as aerosolized hydrogen peroxide, H2O2 vapor, ultraviolet C radiation, pulsed xenon, and gaseous ozone, but no data were found in the search for SARS-CoV-2 procedures (Scarano et al., 2020). It is assumed that UV-C flow germicidal lamps and devices with HEPA filters have additional useful methods of air disinfection when following CDC guidelines. In the clinical dental setting, devices that use plasma disinfection and hydrogen peroxide fogging may also be applied. While ozone is no longer the first-choice option for air decontamination in enclosed medical spaces due to its toxicity. Finally, photocatalytic disinfection is a fascinating disinfection process, though its functional advantages are yet to be explored (Tysiąc-Miśta et al., 2021).
Personal safety when preparing and using disinfectants
Working in places where suspected or confirmed COVID-19 patients are present, or screening, triage, and clinical consultations are carried out, cleaners should wear PPE includes uniforms with long-sleeves, closed work shoes, gowns and/or impermeable aprons, rubber gloves, medical mask, and eye protection (preferably face shield)(Organization, 2020). Avoid combining disinfectants, both during preparation and usage, and perform and do it in well-ventilated areas. In non– healthcare settings, where disinfectants are being prepared and used, the minimum recommended PPE is rubber gloves, impermeable aprons, and closed shoes (Yates et al., 2017). Eye protection and medical masks may also be needed to protect against chemicals in use or if there is a risk of splashing. Cleaning should go from the least soiled (cleanest) to the most soiled (dirtiest) areas, and from the higher to the lower stages. Use a new clean cloth for those areas which are considered to be at high risk of COVID-19 virus contamination. Soiled clothes should be properly reprocessed after every usage and the frequency of cloth changes should be given with an SOP. Equipment used in isolation areas for COVID-19 patients should be color-coded and separated from other’s equipment. Discard the detergent or disinfectant solutions after using with suspected/confirmed patient’s areas. It is preferable to prepare a fresh solution for each change in the cleaning. Buckets should be cleaned with detergent, rinsed, dried, and kept completely inverted to drain (Organization, 2019). Physicians must use a mask for safety in the event of a pandemic involving an airborne-transmissible agent. To understand if there are any points of concern for the health of the doctors, it is important to determine the flow of air through the respirator. However, if masks are not suitably used, the use of protective face masks (PFMs) will not be successful. Because of airflow resistance and discomfort associated with facial heat build-up, particularly in hot and humid weather, many individuals use a PFM without complying with safety regulations (Scarano et al., 2020).It is also very vital to recognize that the entry routes for SARS-CoV-2 are the airways- oral cavity, nostrils, and eye conjunctiva. In addition, ACE2 receptor cells are strongly expressed by both the salivary gland duct epithelium and respiratory tract cells. Therefore, when operating close to the oral cavity of the patient, the risk of contracting the virus is high. Extra care and personal protection equipment’s should be enforced in order to prevent this. In order not to infect themselves and also their patients, all practitioners during this time have to take preventive steps. To do this, the proposed equipment would cover the whole body as well as the nose, eyes and mouth. Mouth protection is considered to be a highly efficient mask by N95, while filtering masks are common with FFP2 (filtering face piece 2) or FFP3 (filtering face piece 3). Glasses and face masks should be used for the safety of the eyes. It is necessary to insure body protection by waterproof, long-sleeved medical gowns and disposable head caps as well as hand protection by sterile surgical gloves that should cover the gown cuffs (Bordea et al., 2020).
Disinfectants and their effect on virus Chlorine-based products
Hypochlorite products contain liquid (sodium hypochlorite), solid or powder (calcium hypochlorite) formula (Table.1). These formulas are dissolved in water to produce an unseparated dilute chlorine solution hypochlorous acid (HOCl) has an antimicrobial activity complex. Hypochlorite shows extensive antibacterial activity, effective for several common pathogens in various concentrations. Hypochlorite is effective against rotavirus at a certain concentration of 0.05% (500 ppm), but the higher concentration of 0.5% is needed for highly resistant pathogens of (5000 ppm) in some medical settings, such as C. auris and C. difficile (Köhler et al., 2018, Pereira, 2015). It is recommended to use 0.1% (1000 ppm) in the following situations: if COVID-19 is in a conservative concentration that will inactivate the majority of other pathogens present in the health care setting. But for blood and body fluids with large amounts of leakage (that is, about 10 ml or more) a recommended concentration of 0.5% (5000 ppm) should be used (Morawska et al., 2020). In the presence of organic matter, hypochlorite is quickly inactivated; therefore, no matter what concentration is used, it’s important to clean the surface first thoroughly with soap and water or detergent with mechanical action (such as scrubbing) Or friction. High concentrations of chlorine can cause metal corrosion and skin or mucous membrane irritation, In addition to the potential side effects related to the smell of chlorine is not suitable for vulnerable people, such as asthma patients (Pereira et al., 2015). The different concentration level commercial sodium hypochlorite products are readily available for use in various settings. In Europe and North America, chlorine concentration changes in commercially available products between 4% and 6% (Kampf et al., 2020). The concentration may also be based on compliance with national regulations and manufacturer's regulations. To reach the required concentration, it is necessary to prepare sodium hypochlorite by diluting the alkaline aqueous solution with a certain percentage of clean, non-turbid water to achieve the final required concentration (Yates et al., 2017). Chlorine can decompose promptly in solution, depending on the source of chlorine and environmental conditions for example ambient temperature or ultraviolet radiation. Chlorine solution should be stored in an opaque container and kept well ventilated, covered area without direct sunlight (Rutala et al., 1998). Chlorine solution is much stable at high pH (greater than9), but a disinfectant performance at lower pH values (<8), of chlorine, is stronger. Chlorine solution of 0.5% and 0.05% have proven to be stable more than 30 days at temperature 25–35 °C when pH is higher than 9. However, the lower pH chlorine solution has a much shorter shelf life (Iqbal et al., 2016).Therefore, the ideal chlorine solution should be prepared fresh every day. If this is not possible then chlorine solution must be used for a few days, and it should be tested daily to make sure the chlorine maintains concentration. Several tests can be used to measure chlorine strength, including chemical titration, chemical spectroscopy or colorimetry, color wheel, and test strips, in order of decreasing precision (Lantagne et al., 2018).
Table 1.
List of recommended disinfectants during pandemic.
| Active ingredient | Structure | Target viruses | Application | Contact time | Formulation type |
|---|---|---|---|---|---|
| Chlorine | Coronavirus | Spray, CF, ULV, moping and wiping | 4 min | Dilutable | |
| Sodium Hypochlorite |  |
Coronavirus | Spray, CF, ULV, moping and wiping | 2 min | Dilutable |
| Ethanol |  |
Coronavirus | Spray,CF, ULV, moping and wiping | 2 min | Ready-To-Use |
| Isopropanol | 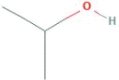 |
Coronavirus | Spray, Cold F, Foaming etc | 30 sec | Ready-To-Use |
| Hydrogen Peroxide (Peroxyacetic Acid) |
 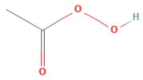
|
Coronavirus | Wiping, spray and moping | 10 min | Dilutable |
| Quaternary ammonia | 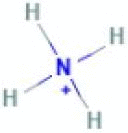 |
Coronavirus | Spray, fogging, Foaming | 10 min | Dilutable |
| Formaldehyde & glutaraldehyde |
|
Coronavirus | Spray, CF, ULV, moping & wiping | 2 min | Ready-To-Use |
| Idophores |  |
Coronavirus | Spray, CF, ULV, moping & wiping | 2 min | Dilutable |
Alcohols
Ethyl alcohol (ethanol) and isopropyl alcohol (isopropanol are utilizing as a disinfectant against viruses, bacteria, and fungi. The biocidal activity of these disinfectants depends on concentration and affinity. The optimal concentration of antibacterial activity is 60–80% of alcohol(Al-Sayah, 2020). Ethanol has more promising results against hydrophilic viruses than isopropanol. Coronaviruses, human immunodeficiency virus (HIV), and rotavirus are largely prone to ethanol than isopropanol. Isopropanol is more active against fat-soluble viruses such as poliovirus and hepatitis A virus (HAV) (Iqbal et al., 2016, Lantagne et al., 2018, McDonnell and Russell, 1999, Warnes et al., 2015) . Ethanol and isopropanol have70-90% of biocidal activity against coronavirus in 30 s(Kampf et al., 2020, Warnes et al., 2015). It is believed that alcohol disintegrates RNA, interfere membrane integrity and cause denaturation of viral proteins. The amphoteric nature of these disinfectants disintegrates the tertiary structure of proteins, causing the breakdown of the intramolecular hydrogen bonds within the structure.
Oxidizing agents
Hydrogen peroxide and peroxy-acetic acid are peroxide based disinfectants. These disinfectants denature proteins by oxidizing disulfide bonds of proteins and thiol groups(McDonnell and Russell, 1999). Hydrogen peroxide has a virucidal activity at a concentration of 1–3% and inactivates SARS-CoV within one minute. The effectiveness of hydrogen peroxide is even ‘more in the gas phase (Goyal et al., 2014, Herzog et al., 2012). Peracetic acid is more potent and active against a broad spectrum of pathogens at lower concentrations (~0.3%); therefore, it is often used to sterilize medical devices (McDonnell and Russell, 1999). Both peroxy compounds produce hydroxyl free radicals and interfere with different components of the virus, including lipid membranes, proteins, and nucleic acids (Knotzer et al., 2015, Yamaguchi et al., 2016).
Phenol-based-disinfectants
These chemicals are usually based on substitution phenols and bisphenols, where the hydrogen atom in the aromatic ring is substituted by alkyl or halogen (McDonnell and Russell, 1999). Due to the high potency, these compounds have an important role in hospital disinfectants(Addie et al., 2015). Phenol derivatives inactivate hydrophilic viruses at a concentration of 0.5 to 5% in a few minutes i.e. HIV virus. These compounds act by denaturing proteins and membrane disruption, which leads to leakage of components.
Quaternary ammonium compounds
Quaternary ammonium compounds (QACs) are considered effective disinfectants(Rabenau et al., 2005b) . These compounds are based on organic salts, where a cation is an amino group with four organic substituents on the nitrogen atom, and the anion is a halide or sulfate. The variations of substituents on amino groups between combinations of alkyl, aryl, and/or heterocycles provide these compounds a wide range of activities. Generally, one of the substituents is a long alkyl chain, while the other three are smaller in size. This structure promotes the formation of micelles which cause the lysis of the pathogen membrane. A group of QAC family widely used as biocides is alkyl dimethyl ammonium chloride, in which the structural change is related to the length of the alkyl group. It is widely used against coronaviruses at the exposure of less than one minute and a concentration of 1% or even lower (Kampf et al., 2020, Pratelli, 2008, Saknimit et al., 1988).
Formaldehyde and glutaraldehyde
Formaldehyde and glutaraldehyde compounds are being considered as advanced disinfectants for medical and surgical equipment (Rutala and Weber, 2008). However, compared with glutaraldehyde, the use of formaldehyde is restricted because of its strong odor and smoke. It is listed as a possible carcinogen by OSHA (McDonnell and Russell, 1999, Rutala and Weber, 2008, Tarka et al., 2016). These aldehydes can disinfect bacteria and by alkylating their proteins and nucleic acids, they are active against coronaviruses within 2 min of exposure, with a concentration range of 0.5–3%(Kariwa et al., 2006, Rabenau et al., 2005a, Rabenau et al., 2005b)
Iodine-releasing agents
Iodophores is an iodine releasing agent formed by complex iodine and a solubilizer in an aqueous solution because only iodine is unstable in water. For example, povidone-iodine has long been used as an antiseptic for various bacteria on the skin and tissues (Eggers et al., 2018, Kariwa et al., 2006, Wood and Payne, 1998) In addition to damaging nucleic acids, the released elemental iodine can also penetrate membranes and attack the sulfuryl and disulfide bonds of proteins. Research has shown that povidone-iodine can inactivate SARS-CoV in suspension at a concentration of 1% or less in a few (Eggers et al., 2018, Kariwa et al., 2006).
Disinfectants impact on humans and the environment
Currently, there are no effective drugs or vaccines available to contain COVID-19 infection, and the global infection rate has increased dramatically. The WHO proposed preventive measures and a healthy lifestyle to reduce the possibility of contracting COVID-19. The WHO also recommends the use of alcohol-based hand sanitizers for frequent hand washing (Allegranzi and Pittet, 2009)These hand sanitizers are mainly composed of ethanol, isopropanol, and hydrogen peroxide in different combinations. Misuse of these preparations may be toxic to human health and the environment. When these chemicals are released by evaporation, they have known toxicity and harmful effects on the environment. In addition, the chance of frequent use of the hand sanitizer has increased the trend of antimicrobial resistance (Kilpatrick et al., 2011) (Table.2).
Table 2.
Gender wise distribution of disinfectant cases in 2020.
| Male | 10,043 | 45.6% |
|---|---|---|
| Female | 11.762 | 53.4% |
| Unknown | 202 | 0.9% |
Note: Adapted from Disinfectants by American Association of Poison Control Centers (AAPCC), 2020. Retrieved from https://aapcc.org/disinfectants
Impact on human health
Ethanol
Ethanol has been widely used as a disinfectant and consumed orally as an alcoholic beverage. Due to the lack of sufficient research, its potential to cause skin cancer through skin absorption is unclear (Lachenmeier and Toxicology, 2008). However, ingestion or skin contact with ethanol- based hand sanitizers is associated with minimal systemic toxicity (https://proceedings.med.ucla.edu/) (Fig. 2). Different people show different responses and tolerance levels to ethanol, which makes it difficult to determine the degree of toxicity of ethanol- based hand wipes. Skin contact with ethanol can cause skin and eye irritation and allergies, while prolonged contact can cause skin dryness or crack, and redness or itching (Table.5) (https://www.nj.gov/). A German study reported that regular use of ethanol can cause skin irritation or contact dermatitis (Lachenmeier and Toxicology, 2008). A research report published in 2018 stated that continuous use of ethanol-based hand sanitizer affects the concentration of uric acid glyoxyuronic acid, and the production level was observed to be higher than under normal conditions (without using ethanol hand sanitizer (Salomone et al., 2018). The use of hydrogen peroxide as disinfectant agent is supported by some studies due to its virucidal potential(Ionescu et al., 2020). While other highlighted the toxic affect associated with it (Colares et al., 2019). The hydroegen peroxide applicability of 0.5% during mouth wash might be helpful to decrease the viral load and reduced the possibility of getting COVID-19 from asymptomatic carrier during dental care. The use of disinfectant is also dose dependent as 3% hydrogen peroide have been noticed for its side affects (da Mota Santana et al., 2021).Ethanol toxicity is also related to respiratory depression, leading to respiratory arrest hypothermia, arrhythmia and possibly cardiac arrest, hypoglycemia, ketoacidosis, and hypotension (Gormley et al., 2012). An ethanol level of 300 mg/dl in the serum may cause an increased risk of respiratory depression and arrest, while an ethanol level of ≥ 500 mg/dl may cause cardiac arrest and death. (Tõnisson et al., 2013)). Ethanol exposure may be related to acute liver damage, myoglobinuria, hypokalemia, hypomagnesemia, hypocalcemia, hypophosphatemia(Wilson et al., 2015) and hydrodiuresis (Bouthoorn et al., 2011). At the end of the debate, frequent and prolonged use of ethanol-based hand sanitizer may be harmful to health. If someone uses ethanol-based hand rubs countless times a day for several months, skin absorption can cause poisoning as a result of COVID-19 preventive measures. As various reports reflect, negligence or improper handling due to the ingestion of such products can lead to serious health problems (Bouthoorn et al., 2011, Gormley et al., 2012, Salomone et al., 2018).
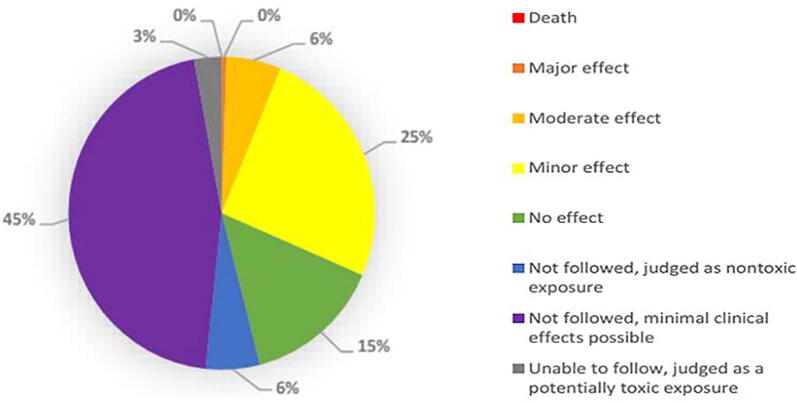
Fig. 2.
Medical outcome of disinfectant cases from 1Jan 2020 to 13 Sep 2020. Confirmed non-exposure and unrelated effect cases are not included in this pie chart. Single and multiple exposure cases included; additional NPDS data is required to correlate cases with outcomes. Note: Adapted from Disinfectants by American Association of Poison Control Centers (AAPCC), 2020. Retrieved from https://aapcc.org/disinfectants.
Table 5.
Disinfection agent effects on people's health.
| Disinfectant Agent | Effects on human health |
|---|---|
| Ethanol | Skin: Dermatitis, allergies, dryness cracks, and redness or itching Eyes: Irritation and allergies Respiratory depression: hypothermia, arrhythmia and possibly cardiac arrest, hypoglycemia, ketoacidosis, hypotension Liver damage: Myoglobinuria, hypokalemia, hypomagnesemia, hypocalcemia, hypophosphatemia, hydrodiuresis |
| Isopropanol | Skin: Irritation, rashes, itching, redness, and dryness Central nervous system (CNS): CNS depression Gastrointestinal track (GIT): Irritate the mucosal lining, gastritis, ketosis Respiratory track (RT): Respiratory depression Cardiac: Increase serum creatinine, myocardial depression, hypotension, hypoglycemia Renal: Rhabdomyolysis, myoglobinuria, and acute renal failure |
| Hydrogen peroxide | Portal vein thrombosis Gastrointestinal problems: vomiting, bloating, gas embolism Skin and mucous membrane irritation |
| Formaldehyde | Breathing difficulties Skin: irritation, itching and dermatitis Renal problems |
| Iodophors | Irritation, itching |
| Glutaraldehyde | Skin: dermatitis, mucous membrane Irritation of the eye, the nose, and the mouth Epistaxis, allergic contact dermatitis, asthma, and rhinitis |
Isopropanol
Isopropyl-based disinfectant poisoning is related to the toxicity of ethanol to some extent, but due to its higher molecular weight, it is more intense than ethanol(Wilson et al., 2015). The toxicity of isopropanol occurs mainly due to the accidental ingestion of the compound, sometimes due to rectal or topical application. 160–240 ml (Ashkar and Miller, 1971), and 250 ml (McBay, 1973), are considered lethal doses of isopropanol. Several studies have reported that isopropanol can cause unconsciousness topical application(McFadden and Haddow, 1969, Vermeulen, 1966, Wise, 1969) . According to the “Material Safety Data Sheet” of Halloa Enterprises, the acute toxicity to the human body will occur at LD50 N 2000 mg/kg (oral), through skin exposure, the acute toxicity will occur at LD50 N 2000 mg/kg, after inhalation LC50 N 5 mg/L. A blood concentration of 1 g/l or higher was found in fatal poisoning (Adelson, 1962). The lethal dose is approximately 250 ml(McBay, 1973). Also the isopropanol is a central nervous system depressant, metabolized into acetone, may cause long-term depression of the central nervous system, reduce respiratory drive and hypotension(Olson et al., 2007, Trummel et al., 1996). Isopropanol can also irritate the mucosal lining of the gastrointestinal tract(Slaughter et al. 2014), gastritis(Olson et al. 2007), cause ketosis (Trummel et al., 1996), hypoglycemia, respiratory depression, and increased serum creatinine. High doses may cause myocardial depression, while long-term use can cause rhabdomyolysis, myoglobinuria, and acute renal failure. Death is related to the ingestion of 100-
200 ml 70% isopropyl solution and plasma concentration ≥ 400 mg/dl(Zaman et al., 2002). According to reports, there was a case of a 43-year-old man suffering from low blood pressure and mental confusion due to the consumption of isopropanol in 2007 (Emadi and Coberly, 2007). Skin absorption Isopropyl alcohol can irritate the skin and ice layer for a long time and frequently exposure causes rash, itching, redness, and dryness(https://www.nj.gov/).
Hydrogen peroxide
The toxicity of hydrogen peroxide depends on its concentration and intake as a general route of exposure (Food and Register, 1988). It has been recognized that the intake of low-concentration hydrogen peroxide (3% solution) has no acute hazard to human health and can cause minor health problems(Moon et al., 2006a). In rare cases, it can cause portal vein thrombosis, gastrointestinal problems, mild mucosal irritation, and vomiting(Adams et al., 2008). According to reports, intestinal dilatation, and exposure to 3% of hydrogen peroxide is related(Moon et al., 2006a, Watt et al., 2004). Hydrogen peroxide causes toxicity through the gas formation and local tissue damage, where it interacts with tissue catalase and decomposes into oxygen and water. The amount of oxygen released is related to the concentration of hydrogen peroxide. 1 ml of 3% hydrogen peroxide is responsible for generating 10 ml of oxygen at standard temperature and pressure, causing bloating and gas embolism. When a large amount of oxygen is produced in a small cavity (such as the stomach), air bubbles may pass through the epithelial space. Due to a large amount of tissue catalase and H2O2 entering the vascular system, gas embolism easily occurs in multiple organ. However, few people who consume 3% H2O2 as a disinfectant will cause death when exposed to high doses(Moon et al., 2006a). According to reports, an 18-month-old child died due to the intake of 8 ounces. Inhalation of 3% H2O2 solution caused by fatal air embolism. Skin contact with 3% hydrogen peroxide can cause mild skin and mucous membrane irritation (https://www.nj.gov/).
Formaldehyde
Formaldehyde is used as a disinfectant and sterilant in both liquid and gaseous states (Rutala and Weber, 2008). Formaldehyde is primarily marketed and used as a water- based solution, known as formalin, which weighs 37% formaldehyde. The aqueous solution is fungicidal, sporicidal, bactericide, tuberculocidal, and virucidal (Emmons and Syphilology, 1933, Protano et al., 2008, Rubbo et al., 1967) OSHA has suggested the handling of formaldehyde as a possible carcinogen and developed a worker’s exposure level for formaldehyde that restricts an average reveals the concentration of 0.75 ppm in an 8-hour time-weighted setting(Blackwell et al., 1981). A second allowable exposure limit in the form of a short-term exposure limit (STEL) of 2 ppm is included in the norm, which is the maximum exposure permitted over a 15-minute duration (De Oliveira Matias and Coelho, 2002). Ingestion of formaldehyde can be lethal, and exposure to low air and skin levels can cause breathing difficulties and skin irritation in the longer term. Such as itching and dermatitis. For these reasons’ workers should have minimal contact with formaldehyde, which restricts their role in the process of sterilization and disinfection (https://www.osha.gov/) While formaldehyde-alcohol is a high-level chemical sterilant, formaldehyde is a disinfectant, its unpleasant fumes, and its stenchy odour even at very low dosages (<1 ppm) restrict the health care usage of formaldehyde. This is why it is a so-called human carcinogen similar to nasal cancer and pulmonary cancer (De Oliveira Matias and Coelho, 2002). Direct exposure to workers is normally restricted when used; however, excessive formaldehyde exposures have been reported in workers of renal transplant units(Blackwell et al., 1981, Milestone and Management, 2017), and students in a gross anatomy laboratory (Control, 1983). Different concentrations of the formaldehyde solution destroy a number of microorganisms. The viruses tested were inactivated with 2% of formalin except for poliovirus that has been inactivated on 8% concentration of formalin in 10 min 724% formaldehyde is a tuberculocidal agent, inactivating 104 M. tuberculosis in 2 min, and in the presence of organic matter 2.5% formaldehyde inactivated about 107 Salmonella Typhi in 10 min (Christensen et al., 1989). Comparatively the sporicidal action of formaldehyde 4% aqueous and 2% glutaraldehyde was slower than that of glutaraldehyde against the spores of B. anthracis (Gorman et al., 1980).
Iodophors
Iodophors are iodine-containing solutions and a solubilizing agent. In this way, the solution slowly releases a small amount of iodine. Iodophors interfere with the various biological entity in the microbial cell. Iodophors penetrate the cell wall and cell membrane of microbes and cause potential disintegration of DNA molecules. Iodophors also bind and denature proteins(Robinson, 2014).The health professional used solutions or tinctures as primary antiseptics on skin or tissue. On the other hand, iodophors have been used both as antiseptics and disinfectants. Iodophor is an iodine-containing agent or carrier which provides a reservoir of iodine to be released on a continuous basis and release small quantities of free iodine to an aqueous solution. Povidone- iodine, a compound of polyvinylpyrrolidone with iodine, is the best known and most commonly used iodophor. The germicidal effectiveness of iodine is maintained by this product and other iodophors but is usually non-containing and relative free of toxicity and irritation (Block, 2001). Many studies have shown that iodophors' antimicrobial efficacy is bactericidal, mycobactericidal, and virucide, but can take longer contact time to destroy certain fungi or bacterial spores in vitro (Berkelman et al., 1982, Favero and preservation, 1991, Lloyd-Evans et al., 1986, Rutala et al., 1991, Terleckyj and Axler, D.A.J.A.a., chemotherapy, , 1987)The virucidal activity of 75–150 ppm available iodine was demonstrated against seven viruses (Protano et al., 2008). Other investigators have questioned the efficacy of iodophors against poliovirus in the presence of organic matter (Christensen et al., 1989)and rotavirus SA-11 in distilled or tap water (Lloyd-Evans et al., 1986).
Glutaraldehyde
Glutaraldehyde is a high-level disinfectant and chemical sterilant, which has been commonly accepted(Lloyd-Evans et al., 1986) . Glutaraldehyde aqueous solutions are acidic and typically are not sporicidal in this state. The solution becomes sporicidal only when the solution is “activated” (made alkaline) by the use of alkalinizing agents at pH 7.5–8.5. Until enabled, due to the polymerization of glutaraldehyde molecules at alkaline pH levels, these solutions have a shelf-life of at least 14 days. The active sites (aldehyde groups) of the molecules of glutaraldehyde that are responsible for its biocidal activity are blocked by this polymerization. The problem of rapid loss of activities (e.g. 28 – 30 days in the use-life) is resolved in new glutaraldehyde formulation (i.e. glutaraldehyde-phenol sodium phenate, acid potentiated glutaraldehyde, alkaline stabilized glutaraldehyde) in the past 30 years, although retaining generally outstanding operation (Miner and Ross, 1991, Raval et al., 2017). However, not only age but also conditions of use such as dilution and organic stress depend upon antimicrobial activity. Literature from manufacturer sources says that the microbicide and anticorrosion properties of neutral and alkaline glutaraldehydes are superior to glutaraldehyde acids and many papers published to support these arguments. (Babb et al., 1980, Collins and Montalbine, 1976, Masferrer, 1977)However, two studies did not indicate a difference in alkaline and glutaraldehyde acid microbicidal activity (Collins and Montalbine, 1976, Rutala et al., 1991). Glutaraldehyde solutions are widely used in health facilities, due to their benefits, including their excellent biocidal properties; organic matter (20% bovine serum) operation, and non-corrosive steps for endoscopic equipment, thermometers, rubbers, or plastics (Food and Administration 2001). Several researchers found that < 2% aquatic glutaraldehyde solutions, pH-buffered to 7.5–8.5, have been effectively destroying sodium bicarbonate vegetative bacteria in < 2 min; M. Bacillus and Clostridium spores in 3 h and tuberculose, fungi and viral disease in < 10 min (Babb et al., 1980, Block, 2001, Tuynman et al., 1997). A glutaraldehyde-phenol / phenate concentrate has been cleared by the FDA as a high-level disinfectant containing 1.12% glutaraldehyde with a concentration of 1.93% phenol/phenate. Other glutaraldehyde sterilants cleared by the FDA that produce 2.4%-3.4% glutaraldehyde are used undiluted. Health workers may be exposed to high glutaraldehyde vapor levels if the equipment is processed in poorly ventilated spaces, where spills occur, glutaraldehyde solutions activated or changed (Leinster et al., 1993). When the baths are open for immersion. Skin irritation or dermatitis and mucous membrane. irritation of the eye, the nose, the mouth, or pulmonary signs may result in acute or chronic exposure(Weber et al., 1998). Glutaraldehyde-exposed health care staff have reported epistaxis, allergic contact dermatitis, asthma, and rhinitis (Beauchamp et al., 1992, Corrado et al., 1986) In order to solve these problems, engineering controls and work practices may include exhaust duct hoods, air-systems exchanging 7 to 15 airs per hour, ductable fumigation hoods with glutaraldehyde vapor absorbers, straightener lids in dip baths, personal protection (i.e. nitrile or butyl rubber gloves, not natural gloves) to minimize skin or mucus membranes(Foliente et al., 2001).
Risk factor for children
Most hand sanitizers are packed in brightly colored bottles and have an attractive smell, such as candy or any food flavor that is very attractive to young children. If a child licks a small amount of disinfectant to taste, he may not get sick, but the taste ingested is more than the risk of alcohol poisoning (Table.3) (https://aapcc.org/track/hand-sanitizer). Compare with adolescence, young children, including babies, are more likely to get sick from alcoholism. The storage of liver glycogen in young children decreases, which increases their sensitivity to hypoglycemia and increases many pharmacokinetic factors, making them more prone to alcoholism. Recent reports have recognized serious problems, including apnea, acidosis, and coma in young children who consume alcohol-based (alcohol) hand sanitizer. The CDC researcher’s publication investigated the data on the exposure of children under the age of 12 to hand sanitizer reported to the National Poison Data System (NPDS) from 2011 to 14. The analysis was carried out by age group (0–5 years old and 6–12 years old). In this age group, approximately 70,669 people have been exposed to hand sanitizers, 92% of whom have been exposed to alcohol-based disinfectants, and the remaining 8% have been exposed to non-alcoholic disinfectants (Santos et al., 2017). Following the COVID-19 outbreak in December 2019, WHO recommended the use of hand sanitizer as a preventive measure against this epidemic, which has led to an exponential increase in the use of alcohol-based hand sanitizer as a hand hygiene. In the first five months of 2020, the AAPCC reported 9504 alcoholism Cases of contact with hand sanitizer in children under 12 years of age and recognized that even a small amount of alcohol can cause alcoholism in children, leading to poisoning, vomiting, and drowsiness. In severe cases, it can also cause respiratory arrest and death(https://aapcc.org/track/hand-sanitizer) .
Table 3.
No of exposure in children (12 years or younger) with hand sanitizer in 2020
| Month | No. of exposure cases |
|---|---|
| January | 1610 |
| February | 1674 |
| March | 2466 |
| April | 1882 |
| May | 1924 |
| June | 1833 |
| July | 2312 |
| August | 2248 |
Note: Adapted from Hand Sanitizer by American Association of Poison Control Centers (AAPCC), 2020. Retrieved from https://aapcc.org/track/hand-sanitizer
Increased risk of other viral diseases
Medical experts have begun to warn that excessive use of alcohol-based hand sanitizers as a preventive measure against the coronavirus will indirectly increase the risk of infection caused by skin diseases. There is a reason for using too much disinfectant for new pneumonia that caused the virus to damage the skin and reduce its ability as a barrier against other harmful viruses. Disinfectants have been widely used all over the world to improve hand hygiene. Excessive use of alcohol-based cleaners will increase skin permeability, deprive the skin of oil and water, and cause skin roughness and irritation. Dry and damaged skin has become a hotbed for many diseases, which can increase the risk of bacterial infection with viruses into the skin (https://english.kyodonews.net/) Research reports indicate that excessive use of disinfectants in some cases may increase the risk of viral outbreaks (Vogel, 2011). Previously published reports show that heavy use of alcohol-based hand sanitizers increases the risks of the norovirus outbreak.
A survey of 160 nursing facilities was conducted to discover the association between the preferential use of alcohol disinfectants and norovirus outbreaks. Of the total number of facilities under investigation, 91 received positive responses during the investigation, of which 73 outbreaks of these, 29 cases were diagnosed with norovirus. Employees in facilities that have experienced norovirus infection are six times more likely to use hand sanitizer than soap and water (Blaney et al., 2011).
Antimicrobial resistance caused by overuse of hand sanitizer
Due to the coronavirus pandemic, scientists, doctors, and the government advise community members on the best hygiene practices and protect them from COVID-19 by using hand sanitizer(https://www.cdc.gov/). Alcohol disinfectants have been used to control many microbial- derived diseases worldwide (Pidot et al., 2018). It has been observed that excessive use of alcohol- based hand sanitizers can lead to drug resistance, which may place more burdens on already struggling healthcare professionals. Repeated exposure of disinfectants, antibiotics, or other genotoxic chemicals to microorganisms can cause them to mutate through natural processes, making them resistant to repeated use of hand sanitizer (https://www.cdc.gov/; Pidot et al., 2018) Between 1997 and 2015, 139 strains of Enterococcus faecalis isolated from hospitals were tested for alcohol tolerance. The results showed that after 2010, Enterococcus faecalis was 10 times more resistant to alcohol than older strains. In the early 2000 s, Australian hospitals began to install more hand sanitizers. These hand sanitizers caused a faster rise in enterococcal infections. Similar results have been observed in other parts of the world due to excessive use of alcohol-based hand sanitizers (Mahmood et al., 2020). According to reports, E. coli and Pseudomonas aeruginosa are respectively resistant to all disinfectants available on the market. Pseudomonas aeruginosa and Cryptococcus have been found to be resistant to daylight hand sanitizers. Almost all gram-negative bacteria are resistant to Cool & Cool, Safeguard, Purell, Fresh up, Insta foam disinfectants(Hayat and Munnawar, 2016).
Toxic impacts on the environment
Ethanol has been widely used in industry and households, and its impact on humans and the environment is still controversial (Pendlington et al., 2001). Aquatic organisms may be directly affected by the leakage of ethanol in water bodies. Many studies have been conducted to evaluate the effects of ethanol on different species reflecting different effects. The New England Interstate Water Pollution Control Commission (NEIWPCC) (Water, 2001) evaluated the data available at the time and established a water quality benchmark for ethanol use. They evaluated Aquatic invertebrates, such as daphnia species, rainbow trout, and blackhead flatfish, have baseline levels of acute and chronic exposures of 564 mg/l, and 63 mg/l, respectively. Later, the ECOTOX database was established on the EPA to determine. In 2001, the NEIWPCC was related to these species.(https://www.nlm.nih.gov/) HSDB found that the octanol/water partition coefficient (Kow) of ethanol is 0.49, which indicates that due to the high expected value, ethanol is unlikely to be bio-concentrated in adipose tissue metabolic rate. The NEIWPCC (Water, 2001), evaluated the effect of ethanol on oxygen depletion after the leakage of ethanol at 55 mg/l, 32 mg/l, and 13 mg/l in small streams, ordinary rivers, and large rivers, respectively. On the other hand, because ethanol evaporates, penetrates, or penetrates into the depths of soil or groundwater and rapidly biodegrades, it is less likely that terrestrial animals are exposed to ethanol leakage. However, it is expected that local microorganisms and other invertebrates may be affected by the leakage (https://www.mass.gov/). According to the U.S. Environmental Protection Agency (USEPA), the 2011 ECOTOX report, wildlife is affected by different percentages of ethanol solutions (Table.4). The impact of baseline ethanol concentration on aquatic organisms and wildlife shows that compared with terrestrial organisms, aquatic organisms face greater risks. The hazards of ingesting ethanol-containing foods are unlikely to occur because ethanol can cause adverse effects due to its high volatility and no accumulation in fatty tissue(Miranda et al., 2011).
Table 4.
Disinfectants effects on wildlife (USEPA ECOTOX Report, 2011)
| Douglas Fir | Applied ethanol concentrations of Seedlings 10% and greater lethal within a week, effects also observed with 5% and 1% solutions |
| Japanese Quail | Ethanol at 2% in drinking water had signi ficant effects on blood, brain weight and growth after 7-day exposure |
| Honeybees | Bees fed solutions of ethanol (5% and greater) showed behavioural effects, and mortality with solutions of 50% ethanol. |
| Little Brown Bat | LD50 of 3.9–4.4 g/kg |
If a large amount of isopropyl alcohol is spilled on the soil, it may be filtered and contaminate the groundwater. Isopropanol has the ability to be oxidized by photochemical substances in the air, which makes it the least persistent in the atmosphere. Due to its rapid biodegradability, it cannot bioaccumulate. Since a large amount of water has a strong ability to consume oxygen in the water body, a large amount of leakage into the water body may cause environmental damage (Gormley et al., 2012). Eventually, it will have an adverse effect on the aquatic biological system. In reported data, trace amounts of propanol were also detected in drinking water samples collected from industrial areas and found to be non-toxic (https://www.atsdr.cdc.gov/). Normal use of isopropyl alcohol will not cause accidental leakage and will not cause any impact on the environment. Isopropanol does not participate in the production of ground-level ozone and photochemical smog-like other volatile organic compounds. According to the American Toxicology and Disease Registry, hydrogen peroxide does not harm the environment due to its rapid reaction with other compounds. It degrades rapidly in water and soil and has no potential to accumulate in the food chain (https://www.atsdr.cdc.gov/)The European (EU) risk assessment of hydrogen peroxide did not find a non-biological half-life in water or soil because it is a short-lived substance in the environment. The estimated half-life in the atmosphere is 24 h. The EU's risk assessment of hydrogen peroxide found short-term toxicity data for fish, invertebrates, and algae from the aquatic environment. The lowest long-term aquatic toxicity test result is a NOEC of 0.1 mg/l for algae. In addition to algae studies, long-term data on zebra mussels are also available. A quantitative risk assessment of aquatic organisms and microorganisms has been carried out. Evaluation proves that no further information or hydrogen peroxide testing is required (https://www.heraproject.com/).
Conclusion
Chemical disinfectants are readily accessible and are useful measures to fight the SARS-CoV virus on the surface or in the water. Some of these disinfectants are common chemicals, like alcohol and hypochlorite solutions, that are affordable, low in toxicity, easy to use and demonstrate excellent bactericidal activity in a short time. In healthcare centers, other more advanced chemicals are used to clean medical equipment and surfaces that are hard to access. Conversely, frequent use of these disinfectants may be fatal. The tendency of ethanol to cause skin cancer by absorption and carcinogenicity of the skin is still under scientific debate and research, despite the lack of recent research. Common hand sanitizers are also linked with minimal system toxicity. Comparable to ethanol, isopropanol has some adverse effects on human health and the environment. Although a small amount of hydrogen peroxide has been documented to be harmless to humans, they have little effect on the environment. Children are at a higher risk of becoming poisoned. In the previously published studies, it's been reported that extensive use of alcohol-based hand sanitizers can develop resistance to antibacterial effects, adding more burden to those who are already dealing with medical staff. Via natural processes, prolonged exposure to disinfectants and antibiotics or other chemical compounds that are genetically toxic to micro-organisms will render them vulnerable to mutations and may withstand the effects of repeated use of hand sanitizer.
Limitation of the review
There is no accuracy at real science, when we explore more with the passage of time, and develop sense to better understand biological phoneomena, precise observation and create more authentic data, we believe that most of the paper cited in the article are recently published, while some of the relevant data are taken from the old articles. Though there must be some articals which were important, might me be missed.
CRediT authorship contribution statement
Zafran Khan: conceptualized the article. Zafran Khan and Dawood Ghafoor wrote original draftt. Asaf Khan, Daniya Ualiyeva and Nasib Zaman helped in writing-review and editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Adams, J., Bartram, J., Chartier, Y., 2008. Essential environmental health standards in health care. World Health Organization.
- D.D. Addie C. Boucraut-Baralon H. Egberink T. Frymus T. Gruffydd-Jones K. Hartmann M.C. Horzinek M.J. Hosie A. Lloret Lutz, H.J.J.o.f.m., surgery, Disinfectant choices in veterinary practices, shelters and households: ABCD guidelines on safe and effective disinfection for feline environments. 17 2015 594 605 [DOI] [PMC free article] [PubMed]
- Adelson L. Fatal intoxication with isopropyl alcohol (rubbing alcohol) Am J Clin Pathol. 1962;38:144–151. doi: 10.1093/ajcp/38.2.144. [DOI] [PubMed] [Google Scholar]
- Al-Sayah M.H. Chemical disinfectants of COVID-19: an overview. J. Water Health. 2020;18:843–848. doi: 10.2166/wh.2020.108. [DOI] [PubMed] [Google Scholar]
- Allegranzi B., Pittet D. Role of hand hygiene in healthcare-associated infection prevention. J. Hosp. Infect. 2009;73:305–315. doi: 10.1016/j.jhin.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Ashkar F.S., Miller R. Hospital ketosis in the alcoholic diabetic: a syndrome due to isopropyl alcohol intoxication. South Med J. 1971;64:1409–1411. doi: 10.1097/00007611-197111000-00028. [DOI] [PubMed] [Google Scholar]
- J. Babb C. Bradley Ayliffe, G.J.J.o.H.I., Sporicidal activity of glutaraldehydes and hypochlorites and other factors influencing their selection for the treatment of medical equipment. 1 1980 63 75 [DOI] [PubMed]
- R.O. Beauchamp M.B.G. St Clair T.R. Fennell D.O. Clarke K.T. Morgan Karl, F.W.J.C.R.i.T., A critical review of the toxicology of glutaraldehyde. 22 1992 143 174 [DOI] [PubMed]
- Bennett J.E., Dolin R., Blaser M.J. Mandell, douglas, and bennett's principles and practice of infectious diseases: 2-volume set. Elsevier Health Sciences. 2014 [Google Scholar]
- R.L. Berkelman B. Holland Anderson, R.J.J.o.c.m., Increased bactericidal activity of dilute preparations of povidone-iodine solutions. 15 1982 635 639 [DOI] [PMC free article] [PubMed]
- Blackwell M., Kang H., Thomas A., Infante P. Formaldehyde: evidence of carcinogenicity. Am Ind Hyg Assoc J. 1981;42(7):A34–A38. [PubMed] [Google Scholar]
- Blaney, D.D., Daly, E.R., Kirkland, K.B., Tongren, J.E., Kelso, P.T., Talbot, E.A.J.A.j.o.i.c., 2011. Use of alcohol-based hand sanitizers as a risk factor for norovirus outbreaks in long-term care facilities in northern New England: December 2006 to March 2007. 39, 296-301. [DOI] [PubMed]
- Block S.S. Lippincott Williams & Wilkins; 2001. Disinfection, sterilization, and preservation. [Google Scholar]
- Bordea I.R., Xhajanka E., Candrea S., Bran S., Onișor F., Inchingolo A.D., Malcangi G., Pham V.H., Inchingolo A.M., Scarano A. Coronavirus (SARS-CoV-2) Pandemic: Future Challenges for Dental Practitioners. Microorganisms. 2020;8:1704. doi: 10.3390/microorganisms8111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouthoorn S.H., Van der Ploeg T., Van Erkel N.E., Van der Lely N. Alcohol intoxication among Dutch adolescents: acute medical complications in the years 2000–2010. Clin. Pediatr. 2011;50:244–251. doi: 10.1177/0009922810388509. [DOI] [PubMed] [Google Scholar]
- R.P. Christensen R.A. Robison D.F. Robinson B.J. Ploeger R.W. Leavitt Bodily, H.L.J.T.J.o.t.A.D.A., Antimicrobial activity of environmental surface disinfectants in the absence and presence of bioburden. 119 1989 493 505 [DOI] [PubMed]
- Colares V.L.P., Lima S.N.L., Sousa N.C.F., Araújo M.C., Pereira D.M.S., Mendes S.J.F., Teixeira S.A., Monteiro C.d.A., Bandeca M.C., Siqueira W.L. Hydrogen peroxide-based products alter inflammatory and tissue damage-related proteins in the gingival crevicular fluid of healthy volunteers: a randomized trial. Sci. Rep. 2019;9:1–11. doi: 10.1038/s41598-019-40006-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- F.M. Collins Montalbine, V.J.J.o.c.m., Mycobactericidal activity of glutaraldehyde solutions. 4 1976 408 412 [DOI] [PMC free article] [PubMed]
- Control, C.f.D., 1983. Formaldehyde exposures in a gross anatomy laboratory--Colorado. 31, 698-700. [PubMed]
- O. Corrado J. Osman Davies, R.J.H.t., Asthma and rhinitis after exposure to glutaraldehyde in endoscopy units. 5 1986 325 328 [DOI] [PubMed]
- da Mota Santana L.A., Andrade Pinho J.N., de Albuquerque H.I.M., de Almeida Souza L.M. Virucidal potential of H(2) O(2) -based spray against SARS-CoV-2 and biosafety in a dental environment. Oral Dis. 2021 doi: 10.1111/odi.13778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- J.C. De Oliveira Matias Coelho, D.A.J.I.J.o.P.R., The integration of the standards systems of quality management, environmental management and occupational health and safety management. 40 2002 3857 3866
- M. Eggers T. Koburger-Janssen M. Eickmann Zorn, J.J.I.d., therapy, In vitro bactericidal and virucidal efficacy of povidone-iodine gargle/mouthwash against respiratory and oral tract pathogens 7 2018 249 259 [DOI] [PMC free article] [PubMed]
- Emadi A., Coberly L. Intoxication of a hospitalized patient with an isopropanol-based hand sanitizer. N. Engl. J. Med. 2007;356:530–531. doi: 10.1056/NEJMc063237. [DOI] [PubMed] [Google Scholar]
- Emmons, C.W.J.A.o.D., Syphilology, Fungicidal action of some common disinfectants on two dermatophytes. 28 1933 15 21
- Favero, M.J.D., sterilization,, preservation, 1991. Chemical disinfection of medical and surgical materials. 617-641.
- Foliente, R.L., Kovacs, B.J., Aprecio, R.M., Bains, H.J., Kettering, J.D., Chen, Y.K.J.G.e., 2001. Efficacy of high-level disinfectants for reprocessing GI endoscopes in simulated-use testing. 53, 456-462 [DOI] [PubMed]
- Register Food D.A.J.F., Oral health care drug products for over-the-counter human use: tentative final monograph: notice of proposed rulemaking. 53 1988 2436 2461
- Gorman, S., Scott, E.M., Russell, A.J.J.o.A.B., 1980. Antimicrobial activity, uses and mechanism of action of glutaraldehyde. 48, 161-190 [DOI] [PubMed]
- Gormley N.J., Bronstein A.C., Rasimas J.J., Pao M., Wratney A.T., Sun J., Austin H.A., Suffredini A.F. The rising incidence of intentional ingestion of ethanol-containing hand sanitizers. Crit. Care Med. 2012;40:290. doi: 10.1097/CCM.0b013e31822f09c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal S.M., Chander Y., Yezli S., Otter J.A. Evaluating the virucidal efficacy of hydrogen peroxide vapour. J. Hosp. Infect. 2014;86:255–259. doi: 10.1016/j.jhin.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayat, A., Munnawar, F.J.I.J.B.B., 2016. Antibacterial effectiveness of commercially available hand sanitizers. 13, 427-431
- Herzog A.B., Pandey A.K., Reyes-Gastelum D., Gerba C.P., Rose J.B., Hashsham S.A. Evaluation of sample recovery efficiency for bacteriophage P22 on fomites. Appl. Environ. Microbiol. 2012;78:7915–7922. doi: 10.1128/AEM.01370-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu, A.C., Brambilla, E., Manzoli, L., Orsini, G., Gentili, V., Rizzo, R.J.O.d., 2020. Efficacy of personal protective equipment and H2O2‐based spray against coronavirus in dental setting. [DOI] [PMC free article] [PubMed]
- Iqbal Q., Lubeck-Schricker M., Wells E., Wolfe M.K., Lantagne D. Shelf-life of chlorine solutions recommended in Ebola virus disease response. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0156136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariwa H., Fujii N., Takashima I. Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents. Dermatology. 2006;212:119–123. doi: 10.1159/000089211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, Z., Ghafoor, D., Khan, A., Ualiyeva, D., Khan, S.A., Bilal, H., Khan, B., Khan, A., Sajjad, W., 2020. Diagnostic approaches and potential therapeutic options for coronavirus disease (COVID-19). New microbes and new infections, 100770. [DOI] [PMC free article] [PubMed]
- Kilpatrick, C., Allegranzi, B., Pittet, D.J.I.J.o.I.C., 2011. WHO First Global Patient Safety Challenge: Clean Care is Safer Care, Contributing to the training of health-care workers around the globe. 7.
- Knotzer S., Kindermann J., Modrof J., Kreil T.R. Measuring the effectiveness of gaseous virus disinfectants. Biologicals. 2015;43:519–523. doi: 10.1016/j.biologicals.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler A.T., Rodloff A.C., Labahn M., Reinhardt M., Truyen U., Speck S. Efficacy of sodium hypochlorite against multidrug-resistant Gram-negative bacteria. J. Hosp. Infect. 2018;100:e40–e46. doi: 10.1016/j.jhin.2018.07.017. [DOI] [PubMed] [Google Scholar]
- A. Kratzel D. Todt V’kovski, P., Steiner, S., Gultom, M., Thao, T.T.N., Ebert, N., Holwerda, M., Steinmann, J., Niemeyer, D., Inactivation of severe acute respiratory syndrome coronavirus 2 by WHO-recommended hand rub formulations and alcohols Emerging infectious diseases 26 2020 1592 [DOI] [PMC free article] [PubMed]
- Lachenmeier, D.W.J.J.o.O.M., Toxicology, Safety evaluation of topical applications of ethanol on the skin and inside the oral cavity. 3 2008 1 16 [DOI] [PMC free article] [PubMed]
- Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantagne D., Wolfe M., Gallandat K., Opryszko M. Determining the Efficacy, Safety and Suitability of Disinfectants to Prevent Emerging Infectious Disease Transmission. Water. 2018;10:1397. [Google Scholar]
- Leinster, P., Baum, J., Baxter, P.J.O., Medicine, E., 1993. An assessment of exposure to glutaraldehyde in hospitals: typical exposure levels and recommended control measures. 50, 107-111 [DOI] [PMC free article] [PubMed]
- Lloyd-Evans, N., Springthorpe, V.S., Sattar, S.A.J.E., Infection, 1986. Chemical disinfection of human rotavirus-contaminated inanimate surfaces. 97, 163-173 [DOI] [PMC free article] [PubMed]
- Mahmood A., Eqan M., Pervez S., Alghamdi H.A., Tabinda A.B., Yasar A., Brindhadevi K., Pugazhendhi A. COVID-19 and frequent use of hand sanitizers; human health and environmental hazards by exposure pathways. Sci. Total Environ. 2020;742 doi: 10.1016/j.scitotenv.2020.140561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masferrer, R., 1977. COMPARISON OF TWO ACTIVATED GLUTARALDEHYDE SOLUTIONS: CIDEX SOLUTION AND SONACIDE.
- McBay A.J. Toxicological findings in fatal poisonings. Clin. Chem. 1973;19:361–365. [PubMed] [Google Scholar]
- McDonnell G., Russell A.D. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 1999;12:147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden S.W., Haddow J.E. Coma produced by topical application of isopropanol. Pediatrics. 1969;43:622–623. [PubMed] [Google Scholar]
- Milestone, S.N.Y.J.O.I.P., Management, 2017. 1. 1683 Antonie van Leeuwenhoek (Fig. 1.1) developed the microscope and proved the existence of microorganisms 2. 1847 Ignaz Semmelweis, an Hungarian obstetrician, advocated handwashing and fingernail scrubbing for infection prevention. 2.
- Miner, N., Ross, C.J.R.c., 1991. Clinical evaluation of ColdSpor, a glutaraldehyde-phenolic disinfectant. 36, 104-109 [PubMed]
- Miranda, G.R., Raetano, C.G., Silva, E., Daam, M.A., Cerejeira, M.J.J.H., Journal, E.R.A.A.I., 2011. Environmental fate of neonicotinoids and classification of their potential risks to hypogean, epygean, and surface water ecosystems in Brazil. 17, 981-995
- Moon J.M., Chun B.J., Min Y.I. Hemorrhagic gastritis and gas emboli after ingesting 3% hydrogen peroxide. The Journal of emergency medicine. 2006;30:403–406. doi: 10.1016/j.jemermed.2005.05.036. [DOI] [PubMed] [Google Scholar]
- Moon, J.M., Chun, B.J., Min, Y.I.J.T.J.o.e.m., 2006b. Hemorrhagic gastritis and gas emboli after ingesting 3% hydrogen peroxide. 30, 403-406. [DOI] [PubMed]
- Morawska L., Tang J.W., Bahnfleth W., Bluyssen P.M., Boerstra A., Buonanno G., Cao J., Dancer S., Floto A., Franchimon F. How can airborne transmission of COVID-19 indoors be minimised? Environ. Int. 2020;142 doi: 10.1016/j.envint.2020.105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson K.R., Anderson I.B., Benowitz N.L., Blanc P.D., Clark R.F., Kearney T.E., Kim-Katz S.Y., Wu A.H.B. Poisoning & drug overdose. Lange Medical. 2007 Books/McGraw-Hill. [Google Scholar]
- Organization, W.H., 2019. Implementation manual to prevent and control the spread of carbapenem-resistant organisms at the national and health care facility level: interim practical manual supporting implementation of the Guidelines for the prevention and control of carbapenem-resistant Enterobacteriaceae, Acinetobacter baumannii and Pseudomonas aeruginosa in health care facilities. World Health Organization
- Organization W.H. Cleaning and disinfection of environmental surfaces in the context of COVID-19: interim guidance, 15 May 2020. World Health. 2020 Organization. [Google Scholar]
- Pendlington R., Whittle E., Robinson J., Howes D.J.F., Toxicology C. Fate of ethanol topically applied to skin. 2001;39:169–174. doi: 10.1016/s0278-6915(00)00120-4. [DOI] [PubMed] [Google Scholar]
- Pereira S.S.P., Oliveira H.M.d., Turrini R.N.T., Lacerda R.A. Disinfection with sodium hypochlorite in hospital environmental surfaces in the reduction of contamination and infection prevention: a systematic review. Revista da Escola de Enfermagem da USP. 2015;49:0681–0688. doi: 10.1590/S0080-623420150000400020. [DOI] [PubMed] [Google Scholar]
- Pidot, S.J., Gao, W., Buultjens, A.H., Monk, I.R., Guerillot, R., Carter, G.P., Lee, J.Y., Lam, M.M., Grayson, M.L., Ballard, S.A.J.S.t.m., 2018. Increasing tolerance of hospital Enterococcus faecium to handwash alcohols. 10. [DOI] [PubMed]
- Pratelli A. Canine coronavirus inactivation with physical and chemical agents. Vet. J. 2008;177:71–79. doi: 10.1016/j.tvjl.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protano, C., Vitali, M., Raitano, A., Sancin, A., Agolini, G.J.J.p.m.h., 2008. Is there still space for the implementation of antisepsis and disinfection to prevent rotavirus and norovirus gastroenteritis outbreaks. 49, 55-60 [PubMed]
- Rabenau H., Cinatl J., Morgenstern B., Bauer G., Preiser W., Doerr H.J.M.m. immunology Stability and inactivation of SARS. 2005;coronavirus. 194:1–6. doi: 10.1007/s00430-004-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabenau H.F., Kampf G., Cinatl J., Doerr H.W. Efficacy of various disinfectants against SARS coronavirus. J. Hosp. Infect. 2005;61:107–111. doi: 10.1016/j.jhin.2004.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raval, K.V., Chaudhari, R., Khant, S.R., Joglekar, O., Patel, D.J.U.a., 2017. Reprocessing and reuse of urological armamentarium: How correct are we! 9, 117. [DOI] [PMC free article] [PubMed]
- Robinson R.K. Academic press; 2014. Encyclopedia of food microbiology. [Google Scholar]
- Rubbo, S., Gardner, J.F., Webb, R.J.J.o.A.B., 1967. Biocidal activities of glutaraldehyde and related compounds. 30, 78-87 [DOI] [PubMed]
- Rutala W.A., Cole E.C., Thomann C.A., Weber D.J. Stability and bactericidal activity of chlorine solutions. Infect. Control Hosp. Epidemiol. 1998;19:323–327. doi: 10.1086/647822. [DOI] [PubMed] [Google Scholar]
- Rutala, W.A., Cole, E.C., Wannamaker, N.S., Weber, D.J.J.T.A.j.o.m., 1991. Inactivation of Mycobacterium tuberculosis and Mycobacterium bovis by 14 hospital disinfectants. 91, S267-S27 [DOI] [PubMed]
- Rutala, W.A., Weber, D.J., 2008. Guideline for disinfection and sterilization in healthcare facilities, 2008.
- Rutala W.A., Weber D.J. Disinfectants used for environmental disinfection and new room decontamination technology. Am. J. Infect. Control. 2013;41:S36–S41. doi: 10.1016/j.ajic.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Saknimit M., Inatsuki I., Sugiyama Y., Yagami K.-I. Virucidal efficacy of physico-chemical treatments against coronaviruses and parvoviruses of laboratory animals. Exp. Anim. 1988;37:341–345. doi: 10.1538/expanim1978.37.3_341. [DOI] [PubMed] [Google Scholar]
- Salomone A., Bozzo A., Di Corcia D., Gerace E., Vincenti M. Occupational exposure to alcohol-based hand sanitizers: the diagnostic role of alcohol biomarkers in hair. J. Anal. Toxicol. 2018;42:157–162. doi: 10.1093/jat/bkx094. [DOI] [PubMed] [Google Scholar]
- Santos, C., Kieszak, S., Wang, A., Law, R., Schier, J., Wolkin, A.J.M.M., report, m.w., 2017. Reported adverse health effects in children from ingestion of alcohol-based hand sanitizers—United States, 2011–2014. 66, 223. [DOI] [PMC free article] [PubMed]
- Scarano A., Inchingolo F., Lorusso F. BioMed research international 2020. 2020. Environmental Disinfection of a Dental Clinic during the Covid-19 Pandemic: A Narrative Insight. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schyllert C., Rönmark E., Andersson M., Hedlund U., Lundbäck B., Hedman L., Lindberg A. Occupational exposure to chemicals drives the increased risk of asthma and rhinitis observed for exposure to vapours, gas, dust and fumes: a cross-sectional population-based study. Occup. Environ. Med. 2016;73:663–669. doi: 10.1136/oemed-2016-103595. [DOI] [PubMed] [Google Scholar]
- Slaughter R., Mason R., Beasley D., Vale J., Schep L.J.C.t. Isopropanol poisoning. 2014;52:470–478. doi: 10.3109/15563650.2014.914527. [DOI] [PubMed] [Google Scholar]
- Sung J., Cossarini F., Palaiodimos L., Benson B., Meholli M. Extra oxygen leads to bubble trouble: portal vein gas embolism from 3% hydrogen peroxide ingestion. Cureus. 2018;10 doi: 10.7759/cureus.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarka P., Kanecki K., Tomasiewicz K. Evaluation of chemical agents intended for surface disinfection with the use of carrier methods. Bactericidal, yeasticidal and sporocidal activity. Postepy Mikrobiologii. 2016;55:99–104. [Google Scholar]
- Terleckyj B., Axler D.A.J.A.a. chemotherapy Quantitative neutralization assay of fungicidal activity of disinfectants. 1987;31:794–798. doi: 10.1128/aac.31.5.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tõnisson M., Tillmann V., Kuudeberg A., Lepik D., Väli M. Acute alcohol intoxication characteristics in children. Alcohol and alcoholism (Oxford, Oxfordshire) 2013;48:390–395. doi: 10.1093/alcalc/agt036. [DOI] [PubMed] [Google Scholar]
- Trummel J., Ford M., Austin P. Ingestion of an unknown alcohol. Ann. Emerg. Med. 1996;27:368–374. doi: 10.1016/s0196-0644(96)70274-3. [DOI] [PubMed] [Google Scholar]
- Tuynman H., Meester H., Meuwissen S. Disinfection of gastrointestinal endoscopes. Procedures in Hepatogastroenterology. Springer. 1997:391–405. [Google Scholar]
- Tysiąc-Miśta M., Dubiel A., Brzoza K., Burek M., Pałkiewicz K. Air disinfection procedures in the dental office during the COVID-19 pandemic. Med. Pr. 2021;72:39–48. doi: 10.13075/mp.5893.01005. [DOI] [PubMed] [Google Scholar]
- Vermeulen R. Isopropyl alcohol and diabetes. Pennsylvania medicine. 1966;69:53–54. [PubMed] [Google Scholar]
- Vogel L. Hand sanitizers may increase norovirus risk. Can Med Assoc. 2011 doi: 10.1503/cmaj.109-3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnes, S.L., Little, Z.R., Keevil, C.W., 2015. Human coronavirus 229E remains infectious on common touch surface materials. MBio 6. [DOI] [PMC free article] [PubMed]
- Water, N.E.I., 2001. HEALTH, ENVIRONMENTAL, AND ECONOMIC IMPACTS OF ADDING ETHANOL TO GASOLINE IN THE NORTHEAST STATES.
- Watt B.E., Proudfoot A.T., Vale J.A. Hydrogen peroxide poisoning. Toxicological reviews. 2004;23:51–57. doi: 10.2165/00139709-200423010-00006. [DOI] [PubMed] [Google Scholar]
- Weber, D., Rutala, W., Rutala, W.J.D., sterilization,, antisepsis in healthcare. Champlain, N.Y.P.P., 1998. Occupational risks associated with the use of selected disinfectants and sterilants. 211-226.
- Weber D.J., Rutala W.A., Anderson D.J., Chen L.F., Sickbert-Bennett E.E., Boyce J.M. Effectiveness of ultraviolet devices and hydrogen peroxide systems for terminal room decontamination: focus on clinical trials. Am. J. Infect. Control. 2016;44:e77–e84. doi: 10.1016/j.ajic.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M.E., Guru P.K., Park J.G. Recurrent lactic acidosis secondary to hand sanitizer ingestion. Indian journal of nephrology. 2015;25:57. doi: 10.4103/0971-4065.135351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise J.R., Jr. Alcohol sponge baths. N Engl J Med. 1969;280:840. doi: 10.1056/NEJM196904102801518. [DOI] [PubMed] [Google Scholar]
- Wood A., Payne D. The action of three antiseptics/disinfectants against enveloped and non-enveloped viruses. J. Hosp. Infect. 1998;38:283–295. doi: 10.1016/S0195-6701(98)90077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y., Shimodo T., Chikamori N., Usuki S., Kanai Y., Endo T., Katsumata K.-I., Terashima C., Ikekita M., Fujishima A. Sporicidal performance induced by photocatalytic production of organic peroxide under visible light irradiation. Sci. Rep. 2016;6:1–7. doi: 10.1038/srep33715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates T., Allen J., Leandre Joseph M., Lantagne D. WASH interventions in disease outbreak response. Oxfam. 2017 [Google Scholar]
- Zaman F., Pervez A., Abreo K. Isopropyl alcohol intoxication: a diagnostic challenge. Am. J. Kidney Dis. 2002;40:e12–11. doi: 10.1053/ajkd.2002.34938. [DOI] [PubMed] [Google Scholar]
- Zock J.-P., Plana E., Jarvis D., Antó J.M., Kromhout H., Kennedy S.M., Künzli N., Villani S., Olivieri M., Torén K. The use of household cleaning sprays and adult asthma: an international longitudinal study. Am. J. Respir. Crit. Care Med. 2007;176:735–741. doi: 10.1164/rccm.200612-1793OC. [DOI] [PMC free article] [PubMed] [Google Scholar]