Abstract
Objective
To analyse tracheostomies after intubation for SARS-Cov-2 infection performed by otorhinolaryngologists in 7 university hospitals in the Paris area of France during the month March 24 to April 23, 2020.
Material and methods
A multicentre retrospective observational study included 59 consecutive patients. The main goals were to evaluate the number, characteristics and practical conditions of tracheostomies, and the COVID-19 status of the otorhinolaryngologists. Secondary goals were to analyse tracheostomy time, decannulation rate, immediate postoperative complications and laryngotracheal axis status.
Results
Tracheostomy indications were for ventilatory weaning and extubation failure in 86% and 14% of cases, respectively. The technique was surgical, percutaneous or hybrid in 91.5%, 3.4% and 5.1% of cases, respectively. None of the operators developed symptoms consistent with COVID-19. Postoperative complications occurred in 15% of cases, with no significant difference between surgical and percutaneous/hybrid techniques (P = 0.33), although no complications occurred after percutaneous or hybrid tracheostomies. No procedures or complications resulted in death. The decannulation rate was 74.5% with a mean tracheostomy time of 20 ± 12 days. In 55% of the patients evaluated by flexible endoscopy after decannulation, a laryngeal abnormality was found. On univariate analysis, no clinical features had a significant influence on tracheostomy time, decannulation rate or occurrence of laryngeal lesions.
Conclusion
The main findings of the present retrospective study were: absence of contamination of the surgeons, heterogeneity of practices between centres, a high rate of complications and laryngeal lesions whatever the technique, and the specificities of the patients.
Keywords: SARS-CoV-2, Tracheostomy, Otorhinolaryngology, France
1. Introduction
On January 24, 2020, the first 3 cases of SARS-Cov-2 (severe acute respiratory syndrome coronavirus-2) in France were diagnosed. In hospitals, the subsequent epidemic led to an exponential rise in the number of patients requiring mechanical ventilation. In order to facilitate withdrawal of mechanical ventilation, free up hospital beds and alleviate workload in intensive care, intensivists proposed tracheostomy and in some cases called on ORL surgeons for this, as had been done in the East of France and in Italy a few weeks before [1], [2].
On March 21, 2020, the French society of otorhinolaryngology (SFORL) published recommendations entitled “COVID-19 alert: ORL endoscopy and flexible endoscopy, endonasal surgery, tracheostomy and tracheostomy care” (https://www.sforl.org/wp-content/uploads/2020/03/Alerte-COVID-19-Endoscopies-et-fibroscopies.pdf), followed up on April 14 by guidelines entitled “French consensus on tracheostomy and tracheostomy care during the COVID-19 pandemic” [3]. The main objectives of the present study in this context were to assess the number, characteristics and practical modalities of tracheostomies performed by academic ORL physicians in the Paris area of France in the month following the publication of the guidelines, and the COVID-19 status of the physicians. Secondary endpoints comprised procedure time, types and rate of immediate complications, tracheostomy duration, decannulation rate and laryngotracheal axis status at > 1 month post-tracheostomy.
2. Patients and methods
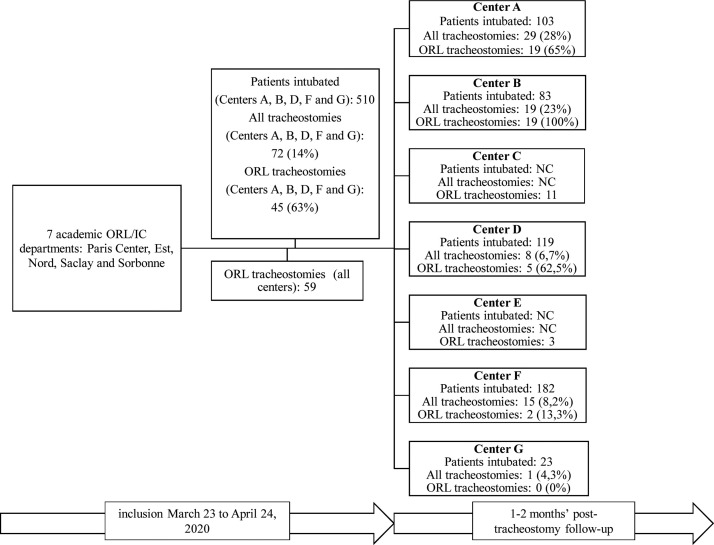
The multicentre retrospective observational study analysed a cohort of 59 patients undergoing tracheostomy after intubation for SARS-Cov-2, performed by ORL surgeons in 7 academic departments in 5 universities in the Paris area of France [Paris Centre, Est, Nord, Saclay and Sorbonne (Fig. 1 )] during the first month (March 23 to April 23, 2020) following publication of the SFORL recommendations (“COVID-19 alert: ORL endoscopy and flexible endoscopy, endonasal surgery, tracheostomy and tracheostomy care”) (https://www.sforl.org/wp-content/uploads/2020/03/Alerte-Covid-19-Endoscopies-et-fibroscopies.pdf).
Fig. 1.
Patients undergoing intubation and tracheostomy per centre. ND: No data.
COVID-19 was diagnosed on positive RT-PCR and/or typical chest CT [4]. Cases of acute respiratory distress not involving COVID-19 intubated during the study period were excluded, as were tracheostomies performed exclusively by intensivists without ORL help. Table 1 shows clinical data for the 59 patients.
Table 1.
Characteristics of the 59 patients with tracheostomy for SARS-Cov-2.
| Characteristics | |
|---|---|
| Male, n (%) | 39 (66.1) |
| Age in years, (mean) | 56 ± 12 |
| BMI in kg/m2, (mean) | 31 ± 8 |
| Comorbidities | |
| Obesity (BMI ≥ 30 kg/m2), n (%) | 30 (50.8) |
| High blood pressure, n (%) | 27 (45.8) |
| Diabetes, n (%) | 15 (25.4) |
| Cardiopathy, n (%) | 9 (15.3) |
| Immunosuppression, n (%) | 9 (15.3) |
| Asthma, n (%) | 7 (11.9) |
| Chronic kidney failure, n (%) | 7 (11.9) |
| OSAHS, n (%) | 5 (8.5) |
| Charlson score (mean) | 2 ± 2 |
| Active or former smoker, n (%) | 5 (8.5) |
| Pack-years (mean) | 15 ± 13 |
BMI: body-mass index. OSAHS: obstructive sleep apnea/hypopnea syndrome.
The main goals were to evaluate the number, characteristics and practical conditions of tracheostomies performed by ORL surgeons, and the COVID-19 status of the surgeons. Secondary goals were to analyse procedure time, type and rate of immediate postoperative complications, tracheostomy duration, decannulation rate, and laryngotracheal axis status at > 1 month post-tracheostomy. Minimum follow-up was 1 month post-tracheostomy.
An Excel database (Appendix 1: available on-line via Mendeley) was constructed and made available to each department to collect data on: the patient [age, gender, body-mass index (BMI), comorbidity on the Charlson scale [5]], the tracheostomy (number of days of prior intubation, reason for tracheostomy, type of tracheostomy, surgical modalities, protection of surgeons), immediate postoperative course (associated treatment, intra- and postoperative complications, tracheostomy duration, decannulation rate), and laryngeal morphologic and dynamic status. Statistical analysis screened for correlations between decannulation, tracheostomy duration and impaired laryngeal status on the one hand and the main characteristics set out in Table 1. Analyses used Chi2 and Fisher tests for qualitative variables and non-parametric Mann–Whitney U test for quantitative variables, and Pearson linear correlation, on IBM SPSS Statistics 20 software. The significance threshold was set at P < 0.005 [6], [7]. Surgeon COVID-19 status (SARS-Cov-2 status) was assessed clinically on symptoms compatible with COVID-19 infection, with or without nasopharyngeal sampling for PCR, and with or without serologic analysis, one month after the procedure.
3. Results
3.1. Practical modalities
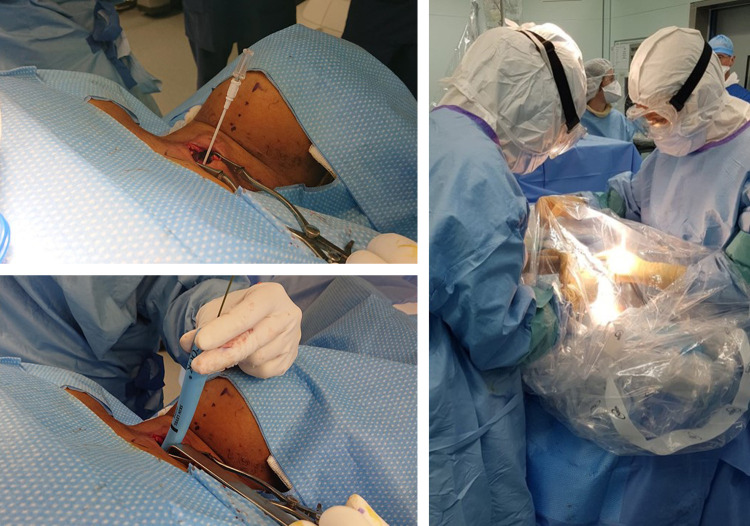
Reasons for tracheostomy were to facilitate withdrawal of ventilation in 86% of cases (51/59) or for failure of a single attempted extubation in 14% (8/59). Critical illness neuromyopathy was observed in 30% of cases (15/59), taking all causes of tracheostomy together. Tracheostomy was performed in theatre in 51% of cases (30/59) and in the intensive care unit in 49% (29/59). Table 2 shows procedure modalities, including technique: surgery, percutaneous endoscopy, or hybrid (Fig. 2 ). In all cases, the procedure was conducted after complete sedation; in surgery, the intubation balloon was pushed beyond the intended tracheal opening, and ventilation was stopped ahead of the incision. In 12% of cases (7/59), a protective suit (“sarcophagus”) was used as a supplementary protective interface (Fig. 3 ). Given the large predominance of surgical tracheostomies (54/59), no statistical analysis of a correlation between technique (surgery, percutaneous endoscopy, hybrid) and clinical characteristics was attempted.
Table 2.
Modalities of the 59 tracheostomies and intra- and postoperative complications.
| Characteristics | |
|---|---|
| Days of prior intubation, (mean) | 17 ± 7 |
| Reason | |
| Ventilation withdrawal, n (%) | 51 (86) |
| Extubation failure, n (%) | 8 (14) |
| Critical illness neuromyopathy, n (%) | 18 (30) |
| Tracheotomy technique | |
| Surgical, n (%) | 54 (91.5) |
| Percutaneous, n (%) | 3 (5.1) |
| Hybrid, n (%) | 2 (3.4) |
| Procedure location | |
| Theatre, n (%) | 30 (51) |
| ICU bedside, n (%) | 29 (49) |
| Surgery time in minutes, (mean) | 27 ± 9 |
| Intraoperative complications | 10 (17) |
| Desaturation, n (%) | 6 (60) |
| Bleeding, n (%) | 4 (40) |
| Postoperative complications, n (%) | 9 (15) |
| Bleeding, n (%) | 4 (44) |
| Infection, n (%) | 4 (44) |
| Leak, n (%) | 1 (12) |
ICU: intensive care unit.
Fig. 2.
Alternative methods and protection used in patients intubated for COVID-19-realyed respiratory distress. Left: Tracheostomy by hybrid technique (Tanaka et al. [32]) using a percutaneous kit under a protective suit (vertical transparent field). Tracheal approach (before opening) performed surgically, then Seldinger technique with guide-wire to dilate the trachea and introduce the cannula. Right: Surgical tracheostomy with protection interface (microscope cover) to minimise aerosolisation during tracheal opening.
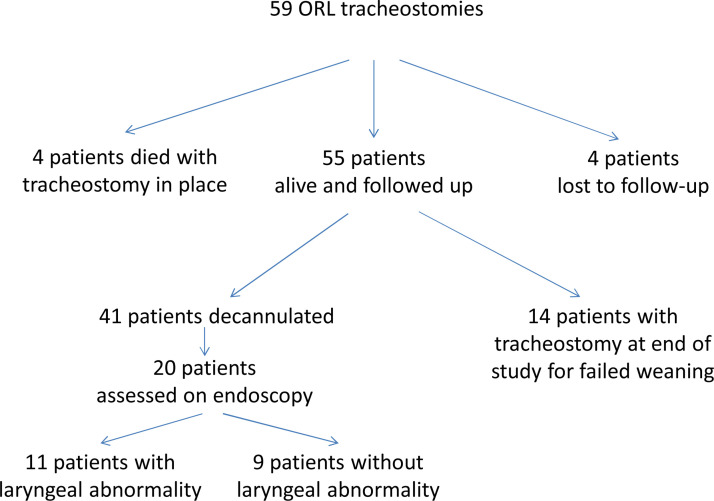
Fig. 3.
Study flowchart and follow-up.
3.2. Operator COVID-19 status
Tracheostomy was performed at a mean 20 ± 6 days after diagnosis. Protective equipment included an FFP2 mask in 95% of cases (56/59); in the other 5% (3/59), a snorkelling mask equipped with an FFP2 filter was used. No operators developed symptoms compatible with COVID-19 infection or were diagnosed as positive on PCR or serology in the month following the procedure.
3.3. Complications
Intraoperative complications occurred in 17% of cases (10/59): hypoxia in 10% (6/59) and bleeding in 7% (4/59), without significant relation to technique (surgical or percutaneous/hybrid) (P = 0.3). Postoperative complications occurred in 15% of cases (9/59): bleeding in 7% (4/59), infection in 7% (4/59) and tracheal orifice leak in 2% (1/59), without significant relation to technique (P = 0.33), although none occurred after percutaneous or hybrid tracheostomy. None of these complications were fatal.
Six patients died (5 with surgical and 1 with percutaneous tracheostomy), including 4 before decannulation; causes of death did not implicate the tracheostomy.
3.4. Decannulation and laryngeal status
Decannulation rate and laryngeal status were assessed in respectively 93.2% (55/59) and 34% of cases (20/59) (Fig. 3). Four of the 8 patients, in whom decannulation was not assessed, died before decannulation and 4 were lost to follow-up. Five of the 39 patients without laryngeal endoscopy died before the assessment could be made, and the other 34 were lost to follow-up (transfer to rehabilitation or other departments).
The decannulation rate was 74.5% (41/55), for a mean tracheostomy duration of 20 ± 12 days. Laryngeal assessment was performed at a mean 69 ± 29 days after intubation and 48 ± 29 days after tracheostomy, and showed abnormalities in 55% of cases (11/20): laryngeal edema in 25% (5/20), unilateral laryngeal immobility in 15% (3/20), hyposensitivity without impaired mobility in 10% (2/20), and granuloma in 5% (1/20).
Univariate analysis found no significant correlations between clinical characteristics (Table 1) and decannulation rate, or between reason for tracheostomy (withdrawal or extubation failure) and decannulation rate (P = 0.095). Pearson linear correlation fond no association between tracheostomy duration and decannulation rate (−0.065; P = 0.653). Univariate analysis found no significant correlation between impaired laryngeal status and decannulation rate (Table 3, Table 4 ).
Table 3.
Univariate analysis of predictive factors for decannulation in the 55 patients with these data.
| Patient characteristics | Decannulated n = 41 | Not decannulated n = 14 | P |
|---|---|---|---|
| Male/female | 28/13 | 8/6 | 0.449 |
| Age in years (mean) | 55 ± 13 | 56 ± 15 | 0.915 |
| BMI in kg/m2 (mean) | 33 ± 9 | 29 ± 8 | 0.272 |
| Comorbidities | |||
| Obesity (BMI ≥ 30): yes/no | 21/20 | 5/9 | 0.316 |
| High blood pressure: yes/no | 20/21 | 5/9 | 0.397 |
| Diabetes: yes/no | 11/30 | 3/11 | 0.689 |
| Cardiopathy: yes/no | 6/35 | 2/12 | 0.975 |
| Immunosuppression: yes/no | 5/36 | 4/10 | 0.153 |
| Asthma: yes/no | 4/37 | 1/9 | 0.981 |
| Chronic kidney failure: yes/no | 3/38 | 4/10 | 0.039 |
| OSAHS: yes/no | 4/37 | 1/13 | 0.769 |
| Charlson score (mean) | 2 ± 2 | 1 ± 1 | 0.438 |
| Active or former smoker: yes/no | 4/37 | 1/13 | 0.751 |
BMI: body-mass index. OSAHS: obstructive sleep apnea/hypopnea syndrome. Significance threshold: P < 0.005.
Table 4.
Univariate analysis of predictive factors for laryngeal lesion > 1 month post-tracheostomy (edema, hyposensitivity, granulations and unilateral laryngeal immobility) in the 20 patients with laryngeal endoscopy.
| Patient characteristics | Laryngeal sequelae n = 11 | No laryngeal sequelae n = 9 | P |
|---|---|---|---|
| Male/female | 7/4 | 5/4 | 0.714 |
| Age in years (mean) | 55 ± 14 | 61 ± 13 | 0.295 |
| BMI in kg/m2 (mean) | 31 ± 12 | 35 ± 6 | 0.062 |
| Comorbidities | |||
| Obesity (BMI ≥ 30): yes/no | 4/7 | 7/2 | 0.064 |
| High blood pressure: yes/no | 5/6 | 5/4 | 0.653 |
| Diabetes: yes/no | 3/8 | 2/7 | 0.796 |
| Cardiopathy: yes/no | 2/9 | 3/6 | 0.795 |
| Immunosuppression: yes/no | 0/11 | 3/6 | 0.038 |
| Asthma: yes/no | 1/11 | 0/9 | 0.353 |
| Chronic kidney failure: yes/no | 0/11 | 0/9 | 0.795 |
| OSAHS: yes/no | 1/11 | 0/9 | 0.754 |
| Charlson score (mean) | 2 ± 1 | 2 ± 1 | 0.780 |
| Active or former smoker: yes/no | 0/11 | 2/9 | 0.099 |
BMI: body-mass index. OSAHS: obstructive sleep apnea/hypopnea syndrome. Significance threshold: P < 0.005.
Univariate analysis found no significant correlations between clinical characteristics (Table 5 ) and tracheostomy duration. Pearson linear correlation found no association between tracheostomy duration and BMI (0.206, P = 0.075) or Charlson score (−0.075, P = 0.647); tracheostomy duration, however, showed a tendency to increase with age (0.329, P = 0.031).
Table 5.
Univariate analysis of predictive factors for tracheostomy duration (days) in the 41 decannulated 41 patients.
| Patient characteristics | Mean tracheostomy duration (days) | P |
|---|---|---|
| Male/female | 18 ± 10/25 ± 15 | 0.201 |
| Comorbidities | ||
| Obesity (BMI ≥ 30): yes/no | 19 ± 9/22 ± 15 | 0.718 |
| High blood pressure: yes/no | 20 ± 9/20 ± 14 | 0.612 |
| Diabetes: yes/no | 21 ± 13/17 ± 10 | 0.664 |
| Cardiopathy: yes/no | 18 ± 14/20 ± 12 | 0.693 |
| Immunosuppression: yes/no | 15 ± 8/21 ± 13 | 0.831 |
| Asthma: yes/no | 16 ± 5/21 ± 13 | 0.958 |
| Chronic kidney failure: yes/no | 13 ± 1/21 ± 12 | 0.748 |
| OSAHS: yes/no | 21 ± 11/20 ± 12 | 0.951 |
| Active or former smoker: yes/no | 19 ± 9/20 ± 12 | 0.227 |
BMI: body-mass index. OSAHS: obstructive sleep apnea/hypopnea syndrome. Significance threshold: P < 0.005.
4. Discussion
The characteristics of the present population (Table 1) agreed with those of other intensive-care cohorts of COVID-19-related respiratory distress: predominance of > 50 year-old males with cardiovascular comorbidity (obesity, high blood pressure, diabetes) [8], [9]. Unexpectedly and unlike prior experience in eastern France and Italy [1], [2], few patients underwent tracheostomy, whether by an ORL or non-ORL operator (Table 2). There may be several reasons for this. Firstly, severity entailed high mortality in intensive care (20–60% depending on the report) [10]. Secondly, the difficulty of withdrawing oxygen support, often beyond 2 weeks, and mechanical ventilation modalities incompatible with tracheostomy (FiO2 ≥ 60%, PEP ≥ 12 mmHg) limited indications [8]. Few indications concerned extubation failure, as early extubation was never attempted while the patient was dependent on oxygen support. Thirdly, intensivists’ habits, concerning tracheostomy for withdrawal of ventilation in respiratory distress of whatever etiology, vary between centres, as seen in the present varied tracheostomy rates. Blot et al. [11], in a retrospective study in 152 French intensive care units, reported wide variations in practice regarding tracheostomy in respiratory distress, with delayed implementation (median, 20 days) and extubation failure as indication in 48% of cases. Finally, geographic variations may explain the low tracheostomy rate in Paris. Over the same inclusion period, Morvan et al. [1], in a retrospective study of tracheostomies performed by military surgeons in Mulhouse in the East of France, reported 18 tracheostomies performed by head and neck surgeons in a total 47 patients intubated in the military hospital. These regional and national disparities may be due the huge rise in intake with restricted intensive care capacity faced by our colleagues in Eastern France and Italy, tracheostomy then offering a means of freeing up beds in intensive care [1], [2]. In the Paris region, the first wave of the epidemic broke 2 weeks later than in Eastern France, and intensive care units had time to adapt their capacity and open a large number of new beds; they were thus never truly submerged.
The total number of tracheostomies, performed by both ORL and non-ORL operators (intensivists or other surgeons), was available for 5 of the 7 centres, showing that 63% (45/72) were performed by ORL surgeons (Fig. 1). In the other 2 centres, the figure could not be determined, due to the exponential increase in the number of intensive care beds at the height of the epidemic. The 37% rate of non-ORL tracheostomies is explained by the autonomy of intensivists, used to performing their own tracheostomies, percutaneously in 75% of cases, as reported in the international study by Vargas et al. [12]. The proportion performed by ORL surgeons varied from 0 to 100% between centres (Fig. 1), certain intensivists calling systematically on ORL colleagues so as to alleviate their own workload, while others did so only in case of foreseeable problems contraindicating percutaneous tracheostomy.
In the present series, tracheostomy was performed at a mean 17 ± 7 days after orotracheal intubation, in line with the 2018 multicentre study in 50 countries by Abe et al. [13] analysing tracheostomy in patients undergoing intubation and ventilation for acute respiratory distress syndrome: median interval of 14 days (range, 7–21 days) between intubation and tracheostomy, and early tracheostomy (before day 7) in 86/309 patients (27.8%). They reported that 13% of patients intubated for acute respiratory distress of whatever cause underwent tracheostomy, to alleviate sedation and facilitate withdrawal of ventilation. The literature data on the benefit of early tracheostomy in shortening intensive-care stay and reducing immediate and long-term mortality are contradictory [14], [15]. Percutaneous tracheostomy is usually performed at the bedside in the intensive care unit, while surgical tracheostomy is performed in theatre [13]. In centres A, C and D (Fig. 1), respectively 45%, 80% and 100% of tracheostomies were performed in the intensive care unit, whatever the technique or patient characteristics, whereas in other centres, tracheostomy was always performed surgically in the operating room. This corresponds to team habits, and was justified by fear of accidental extubation during transfer, with potential oxygen desaturation and virus aerosolisation [15]. Limited theatre access, with a reduced number of operative and recovery rooms, may also explain these differences between centres regarding the procedure site.
In intubation for non-COVID-19 acute respiratory distress, there is no clear consensus as to tracheostomy technique [13]. Percutaneous tracheostomy is quicker, by 13 minutes (95% IC [−19.37; −6.76]; P < 0.0001), with fewer post-procedural complications and tracheostomy site infection according to the meta-analysis by Putensen et al., [16], but with higher risk of per-procedural complications [OR, 4.58 (95% IC [2.21, 9.47]); P < 0.0001], which can be serious or even life-threatening (esophageal perforation, false trajectory, vascular injury, etc.). If performed under fluoroscopic or ultrasound guidance, it can be used in first line in obese patients, as it entails fewer post-procedural complications than surgery in these cases: less bleeding, leakage, infection and pneumopathy [17]. In the present series, the rate of non-severe postoperative complications was only 15%, and exclusively associated with surgical tracheostomy. In COVID-19-related respiratory distress, where there is a majority of obese patients, expert opinion as to technique differs [18]. The percutaneous technique can be performed by intensivists, and ensures good peri-cannula sealing for nursing care, but has the major drawback of increasing the risk of virus aerosolisation during the procedure because of the balloon position above the tracheostomy orifice and of the use of bronchial endoscopy, increasing the risk of operator contamination. In the present study, however, there were no cases of operator contamination despite the predominantly surgical technique. Percutaneous tracheostomy can also be made difficult by certain anatomic variants: short neck, history of cervicotomy, or obesity (which is frequent in COVID-19-related respiratory distress). Surgery has the advantages of being feasible, whatever the anatomy, including obesity, with few intraoperative complications, and those few being mostly benign, and of being performed by the surgery team, thus alleviating the intensivist's workload. It also shows less risk of intraoperative aerosolisation, as the balloon has been pushed down below the orifice. However, the rate of postoperative complications is greater: bleeding, infection, defective sealing with risk of aerosolisation. In the context of COVID-19, the SFORL, exceptionally, recommends the percutaneous technique, to reduce the risk of aerosolisation during ventilation and post-tracheostomy cannula care [3]. The Canadian, Italian and British societies recommend surgery, and other societies make no recommendation [18]. In the present cohort, 91.5% of tracheostomies were surgical, although only 50.8% of patients were obese. This apparent contradiction with regard to French guidelines is due to the habits of head and neck surgeons, who are more used to surgery. The short operating time (27 minutes, far less than the mean 45 minutes reported in the literature [19]) and low rates of intra- and postoperative complications argue for this attitude in experienced operators, as in the present series. The present study is in agreement with McGrath et al. [20] in an international working group recommending using the tracheostomy technique to which the operator is best accustomed, to minimise intra-procedural errors and complications, and thus virus aerosolisation. In the study by Morvan et al. [1], the Mulhouse military head and neck surgeons, in coordination with the intensivists, opted for bedside percutaneous tracheostomy, with only 7 minutes’ procedure time, on condition that the operator was properly trained and worked closely with the intensivists. In the present cohort, percutaneous tracheostomy was associated with no immediate complications, although sample size was insufficient for comparison versus surgery. The almost systematic rate of surgery in the present series, in contradiction to the SFORL guidelines [3], but in line with those of other societies worldwide (no extra risk for either patient or operator), suggest that the SFORL guidelines [3], drawn up urgently on the basis of the “precautionary principle”, may need revising regarding the claimed superiority of percutaneous tracheostomy in the COVID-19 context.
There were few intra- (17%) or post-procedural (15%) complications in the present series, and no major complications or failures, with no significant differences according to technique (Table 2), in agreement with the literature [16]. One major risk of tracheostomy in the COVID-19 context is operator and care team contamination, as the procedure is highly liable to induce aerosolisation [3]. Van Doremalen et al. [21], modelling SARS-CoV-2 aerosolisation by nebulisation, found that the virus remained viable for 3 hours in aerosols. Likewise, Feldman et al. [22], simulating orotracheal intubation, found aerosols on operators’ hair and faces, despite personal protective equipment (PPE). Lastly, Chen et al. [23], in a cohort of 758 care-staff assessed during the 2009 SARS epidemic, reported increased contamination risk in tracheostomy (6 persons infected: OR 4.15 (1.50∼11.50); P < 0.01); this P-value did not reach the 0.005 significance threshold advocated by various statisticians and the European Annals of Otorhinolaryngology Head & Neck Diseases [6], [7], but even so the French Public Health Council (HCSP) (https://www.hcsp.fr/Explore.cgi/avisrapportsdomaine?clefr=830) and the SFORL [3] recommend FFP2 masks during invasive ORL procedures or maneuvers liable to lead to aerosolisation of viral particles. There seems to be a consensus that optimal protection is mandatory in tracheostomy, recognised as an at-risk procedure, for surgeons and/or intensivists and/or anesthetists and all staff present during the procedure: at least an FFP2 or N95 mask, protective glasses with visor or protective suit, overcoat, cap, overshoes and double gloves [2], [18]. Thierry et al. developed a PPE mask by fitting an FFP2 filter onto a snorkelling mask to ensure sealing and air filtration [24]. Foster et al. described a canopy system with air aspiration associated to a filter below the surgical drapes to further reduce aerosolisation risk [25]; this was little used in the present cohort, being difficult to implement. Collaboration with the anesthesia and intensive care teams is primordial to ensure sedation and complete neuromuscular block and total apnea when the trachea is opened [2], [3]. It is also important, if possible, to await SARS-Cov2-negative nasopharyngeal and/or tracheal samples to minimise contamination risk [18]. In the present series, tracheostomy was performed at a mean 20 days after diagnosis of COVID-19: i.e., with lowered or negative viral load in the upper and lower airway. The PPE set accounts for the absence of operator contamination, including in the 8 patients who were PCR-positive at the time of surgery. This raises the issue of the contagiousness of aerosolised viruses. Laccourreye et al. [26], in a cohort of 224 consecutive patients undergoing surgery and/or endoscopy for head and neck cancer in 6 academic departments in the Paris area during the first month of lockdown, found no contamination of surgeons wearing simply an FFP2 mask, including in cases of tracheal opening in COVID-19-positive patients. Thus, a snorkelling mask plus protective suit [24], [27] may be an excess of caution, even in airway surgery.
Orotracheal intubation is liable to induce laryngotracheal lesions, proportionally to intubation time [28]. In a cohort of 775 patients with a median 8 days’ intubation (range, 7.7–8.7 days) for non-COVID-19 respiratory distress, Brodsky et al. [29] found laryngeal lesions in 83% of cases; most were mild, with edema in 70% of cases, but with moderate or severe lesions in 31% and 13% of cases, respectively, notably including unilateral laryngeal immobility in 20% of severe cases. The British association of anaesthetists sounded the alarm concerning a high prevalence of post-intubation laryngeal edema in COVID-19 patients, due not only to intubation as such, but also perhaps to the action of the virus [30]. This could account for the present absence of significant association between patient characteristics, notably including age (known to be a risk factor for laryngotracheal sequelae [28]), and laryngeal lesions (Table 4) or tracheostomy duration (Table 3, Table 5). Li et al. [31] demonstrated the central and peripheral neuroinvasive potential of SARS-CoV-2, inducing critical illness neuromyopathy and laryngeal immobility. These data suggest an etiological role of SARS-CoV-2 in long-term laryngeal lesions, independently of intubation, which, however, also induces such lesions. Moreover, prone positioning with repeated turning, as frequently practiced on patients with COVID-19-related respiratory distress, can cause iterative laryngeal microtraumas when performed during intubation. These hypotheses agree with the present findings that, at 1 or 2 months post-extubation, laryngeal lesions were more frequent than usual following prolonged intubation: 55% of cases, with edema in 25% and unilateral laryngeal immobility in 15%; moreover, this prevalence was probably underestimated, as only 34% of patients underwent laryngotracheal assessment.
Despite its multicentre design, the present study had several biases:
-
•
sample size was less than a hundred, as tracheostomy indications were fewer than expected and tracheostomies performed by intensivists were not included, leading to imbalance between surgical and percutaneous techniques, precluding statistical comparison;
-
•
in 2 of the 7 centres, while the number of tracheostomies performed by head and neck surgeons was known, the total numbers of intubations and of tracheostomies performed by both ORL surgeons and intensivists were not available, due to an exponential increase in the number of intensive care beds at the peak of the epidemic;
-
•
there was considerable loss to follow-up due to transfer to other departments or geographical regions for rehabilitation, without information to the ORL department or the operators.
5. Conclusion
Four main points emerged from this multicentre study in the Paris region of France to analyse the experience of tracheostomy performed by ORL surgeons in COVID-19 patients with prolonged intubation:
-
•
unexpectedly, few tracheostomies were in fact performed by ORL surgeons, and almost all were for ventilation withdrawal and were performed surgically, late in the care process;
-
•
tracheostomy practices varied between centres, but with predominance of surgery, in contradiction to the SFORL recommendations, without major adverse effects whether in terms of severe complications in the patients or of contamination of the operators;
-
•
the rate of laryngotracheal sequelae was high, suggesting that tracheostomy might be performed earlier in future;
-
•
the rate of short-term loss to follow-up (within 2 months) was excessive, arguing for closer teamwork between intensivists and ORL surgeons, and for specific ORL follow-up in a region-wide database for long-term assessment of sequelae and their treatment.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgments
The authors thank the Progrès 2000 association for technical support, and Décathlon for the gift of EasyBreath® masks.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.anorl.2021.03.002.
Online Supplement. Supplementary data
References
- 1.Morvan J.-B., Rivière D., Danguy des Déserts M., Bonfort G., Mathais Q., Pasquier P. Trachéotomie percutanée pour afflux saturant de patients COVID-19 : expérience des ORL militaires déployés à Mulhouse. Eur Ann Otorhinolaryngol Head Neck Dis. 2020 doi: 10.1016/j.aforl.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pichi B., Mazzola F., Bonsembiante A., et al. CORONA-steps for tracheostomy in COVID-19 patients: a staff-safe method for airway management. Oral Oncol. 2020;105:104682. doi: 10.1016/j.oraloncology.2020.104682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultz P., Morvan J.-B., Fakhry N., et al. French consensus regarding precautions during tracheostomy and post-tracheostomy care in the context of COVID-19 pandemic. Eur Ann Otorhinolaryngol Head Neck Dis. 2020;137:167–169. doi: 10.1016/j.anorl.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang Y., Zhang H., Xie J., et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020:200432. doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 6.Ioannidis J.P.A. The proposal to lower P value thresholds to 0.005. JAMA. 2018;319:1429–1430. doi: 10.1001/jama.2018.1536. [DOI] [PubMed] [Google Scholar]
- 7.Laccourreye O., Lisan Q., Bonfils P., et al. Use of P-values and the terms “significant”, “non-significant” and “suggestive” in abstracts in the European Annals of Otorhinolaryngology, Head & Neck Diseases. Eur Ann Otorhinolaryngol Head Neck Dis. 2019;136:469–473. doi: 10.1016/j.anorl.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Grasselli G., Zangrillo A., Zanella A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020 doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arentz M., Yim E., Klaff L., et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020 doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blot F., Melot C., Commission d’epidémiologie et de recherche clinique. Indications, timing, and techniques of tracheostomy in 152 French ICUs. Chest. 2005;127:1347–1352. doi: 10.1378/chest.127.4.1347. [DOI] [PubMed] [Google Scholar]
- 12.Vargas M., Sutherasan Y., Antonelli M., et al. Tracheostomy procedures in the intensive care unit: an international survey. Crit Care. 2015;19:291. doi: 10.1186/s13054-015-1013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abe T., Madotto F., Pham T., et al. Epidemiology and patterns of tracheostomy practice in patients with acute respiratory distress syndrome in ICUs across 50 countries. Crit Care. 2018;22:195. doi: 10.1186/s13054-018-2126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siempos I.I., Ntaidou T.K., Filippidis F.T., Choi A.M.K. Effect of early versus late or no tracheostomy on mortality and pneumonia of critically ill patients receiving mechanical ventilation: a systematic review and meta-analysis. Lancet Respir Med. 2015;3:150–158. doi: 10.1016/S2213-2600(15)00007-7. [DOI] [PubMed] [Google Scholar]
- 15.Rumbak M.J., Newton M., Truncale T., Schwartz S.W., Adams J.W., Hazard P.B. A prospective, randomised, study comparing early percutaneous dilational tracheostomy to prolonged translaryngeal intubation (delayed tracheostomy) in critically ill medical patients. Crit Care Med. 2004;32:1689–1694. doi: 10.1097/01.ccm.0000134835.05161.b6. [DOI] [PubMed] [Google Scholar]
- 16.Putensen C., Theuerkauf N., Guenther U., Vargas M., Pelosi P. Percutaneous and surgical tracheostomy in critically ill adult patients: a meta-analysis. Crit Care. 2014 doi: 10.1186/s13054-014-0544-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Umbrello M., Fumagalli J., Pesenti A., Chiumello D. Pathophysiology and management of acute respiratory distress syndrome in obese patients. Semin Respir Crit Care Med. 2019;40:40–56. doi: 10.1055/s-0039-1685179. [DOI] [PubMed] [Google Scholar]
- 18.Chiesa-Estomba C.M., Lechien J.R., Calvo-Henríquez C., et al. Systematic review of international guidelines for tracheostomy in COVID-19 patients. Oral Oncol. 2020;108:104844. doi: 10.1016/j.oraloncology.2020.104844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young D., Harrison D.A., Cuthbertson B.H., Rowan K., TracMan collaborators. Effect of early vs. late tracheostomy placement on survival in patients receiving mechanical ventilation: the TracMan randomised trial. JAMA. 2013;309:2121–2129. doi: 10.1001/jama.2013.5154. [DOI] [PubMed] [Google Scholar]
- 20.McGrath B.A., Brenner M.J., Warrillow S.J., et al. Tracheostomy in the COVID-19 era: global and multidisciplinary guidance. Lancet Respir Med. 2020;8:717–725. doi: 10.1016/S2213-2600(20)30230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Doremalen N., Bushmaker T., Morris D.H., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feldman O., Meir M., Shavit D., Idelman R., Shavit I. Exposure to a surrogate measure of contamination from simulated patients by emergency department personnel wearing personal protective equipment. JAMA. 2020;323(20):2091–2093. doi: 10.1001/jama.2020.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen W.-Q., Ling W.-H., Lu C.-Y., Hao Y.-T., Lin Z.-N., Ling L., et al. Which preventive measures might protect health care workers from SARS? BMC Public Health. 2009;9:81. doi: 10.1186/1471-2458-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thierry B., Célérier C., Simon F., Lacroix C., Khonsari R.-H. How and why use the EasyBreath® Decathlon surface snorkelling mask as a personal protective equipment during the COVID-19 pandemic? Eur Ann Otorhinolaryngol Head Neck Dis. 2020 doi: 10.1016/j.anorl.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foster P., Cheung T., Craft P., et al. Novel approach to reduce transmission of COVID-19 during tracheostomy. J Am Coll Surg. 2020 doi: 10.1016/j.jamcollsurg.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laccourreye O., Mirghani H., Evrard D., Bonnefont P., Brugel L., Tankere F., et al. Impact of the first month of COVID-19 lockdown on oncologic surgical activity in the Île-de-France region university hospital otorhinolaryngology departments. Eur Ann Otorhinolaryngol Head Neck Dis. 2020 doi: 10.1016/j.anorl.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adir Y., Segol O., Kompaniets D., et al. COVID-19: minimising risk to healthcare workers during aerosol producing respiratory therapy using an innovative constant flow canopy. Eur Respir J. 2020 doi: 10.1183/13993003.01017-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mota L.A.A., de Cavalho G.B., Brito V.A. Laryngeal complications by orotracheal intubation: literature review. Int Arch Otorhinolaryngol. 2012;16:236–245. doi: 10.7162/S1809-97772012000200014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brodsky M.B., Levy M.J., Jedlanek E., et al. Laryngeal injury and upper airway symptoms after oral endotracheal intubation with mechanical ventilation during critical care: a systematic review. Crit Care Med. 2018;46:2010–2017. doi: 10.1097/CCM.0000000000003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGrath B.A., Wallace S., Goswamy J. Laryngeal oedema associated with COVID-19 complicating airway management. Anaesthesia. 2020;75:972. doi: 10.1111/anae.15092. [DOI] [PubMed] [Google Scholar]
- 31.Li Y.-C., Bai W.-Z., Hashikawa T. The neuroinvasive potential of SARS-CoV-2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020 doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka L., Alexandru M., Jbyeh S., et al. A hybrid approach to tracheostomy in COVID-19 patients ensuring staff safety. Br J Surg. 2020;107:e253–e254. doi: 10.1002/bjs.11705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.