Graft-versus-host-disease-associated angiomatosis (GVHD-AA) can cause significant morbidity in patients with chronic cutaneous GVHD and there is no clear treatment.1 Research is needed to elucidate disease pathobiology and inform treatment options but is hampered due to disease rarity. We analyzed 16 GVHD-AA specimens, sclerotic GVHD-non-AA specimens, and healthy specimens for markers that could provide insight into disease pathogenesis and/or identify specific pathways that are directly or indirectly targetable by currently available treatments.
Methods, patient clinical and treatment data, and lesion histology are detailed in Supplemental Material. Similarly to previous reports, GVHD-AA lesions represented vascular proliferations composed of thin-walled vessels. Vessels were not lymphatic in origin (D2-40 negative, data not shown), nor did they result from HHV8 infection (data not shown).
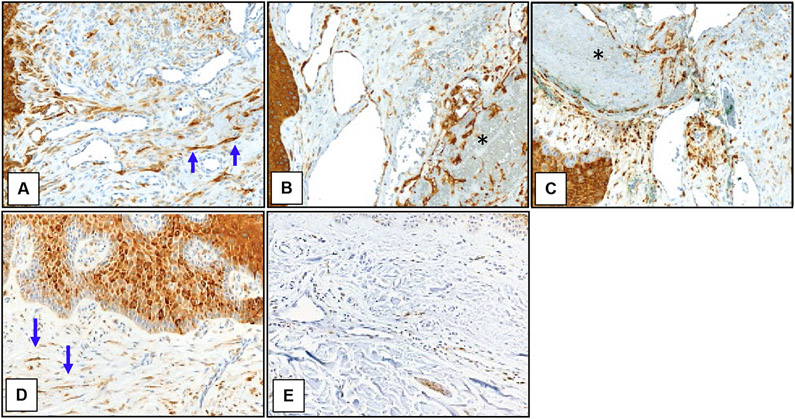
Sirolimus has been proposed as a therapy for GVHD-AA, based on the known activation of the mTORC1 pathway in the endothelium of other vascular lesions.2 mTORC1 signaling was low to absent in the vasculature of GVHD-AA lesions and was comparable to that in the vasculature of healthy control skin, as assessed by pS6 staining (Fig 1). The endothelium of Masson’s tumor-like changes within 3 GVHD-AA lesions stained positively for pS6 despite the remaining GVHD-AA lesion being negative (Fig 1B, C) suggesting that GVHD-AA endothelial cell proliferation employs signaling pathways distinct from other vascular growths. Dermal fibroblasts within both GVHD-AA and sclerotic GVHD-non-AA control skin displayed increased mTORC1 activity (Fig 1A, D). Though a direct benefit of sirolimus against GVHD-AA lesions is rendered questionable by these findings, it is possible that sirolimus may benefit GVHD-AA by treating the underlying sclerotic GVHD. In our small cohort, there was no clear correlation between response to topical or systemic sirolimus and vascular pS6 staining.
Figure 1. mTORC1 is activated in fibroblasts and epidermis but not endothelium in GVHD-AA.
Representative immunohistochemical images of skin samples labeled for pS6 (brown) and counterstained with hematoxylin (blue). (A) GVHD-AA lesion. (B) Intralesional Masson’s tumor from same GVHD-AA lesion as in (A). (C) Intralesional Masson’s tumor from a separate GVHD-AA lesion. (D) Control sclerotic skin adjacent to GVHD-AA lesion. (E) Healthy skin. Blue arrows point to example pS6-expressing fibroblasts. Black asterisks demark Masson’s tumors. Images 200X.
β-blockade is another therapy used in GVHD-AA based on its success in infantile hemangiomas.3 Infantile hemangiomas are reported to express GLUT1.4 In contrast, GVHD-AA vasculature lacked GLUT1 expression (data not shown). This finding suggests that the two diseases are mechanistically distinct, raising the possibility that β-blockade may not be effective in GVHD-AA. Importantly, ß-blocker function in infantile hemangiomas is likely independent of GLUT1,5 so the lack of GLUT1 on GVHD-AA vasculature does not exclude a potential benefit of β-blockade. In our cohort, not all patients treated with ß-blockade experienced benefit, and in those with reported improvement, interpretation was confounded by the combination of other therapies.
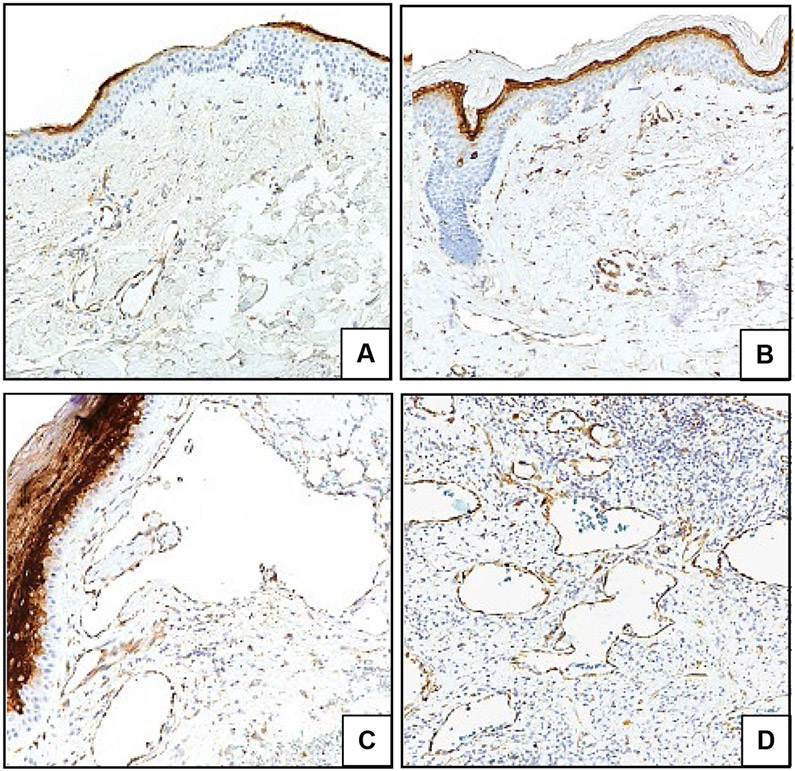
Lastly, systemic and intralesional anti-VEGF therapies are FDA-approved for other diseases6 and VEGF was reportedly elevated in serum of a GVHD-AA patient.7 In our study, VEGF staining in endothelium was variable among GVHD-AA specimens though overall was higher than in control sample vasculature (Fig 2A-D). Expression was also increased within lesional epidermis (Fig 2A, B, C).
Figure 2. VEGF shows variable upregulation in GVHD-AA.
Representative IHC images of skin samples labeled for VEGF (brown) and counterstained with hematoxylin (blue). (A) Healthy skin. (B) Control sclerotic GVHD-non-AA skin. (C, D) Two different GVHD-AA lesions; (C) depicts increased epidermal VEGF expression. Images 200X.
It is reasonable to trial ß-blockers and mTORC1 inhibitors for GVHD-AA, though ideally as monotherapy (if clinically appropriate) and with rigorous recording/reporting of clinical outcomes to better evaluate efficacy. Local anti-VEGF treatment may be worth considering, in particular for larger or recalcitrant lesions shown to have high VEGF expression.
For more detailed results, refer to Supplementary Material at (Link).
Supplementary Material
Acknowledgments
Funding sources: Institutional, NIH DPOD023091
Footnotes
Conflicts of Interest: None declared.
IRB approval status: Reviewed and approved by Partners Healthcare IRB; approval #2014P000923, #2007P002165, #2018P001497
References
- 1.Kaffenberger BH, Zuo RC, Gru A, Plotner AN, Sweeney SA, Devine SM, et al. Graft-versus-host disease-associated angiomatosis: a clinicopathologically distinct entity. J Am Acad Dermatol. 2014;71(4):745–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du W, Gerald D, Perruzzi CA, Rodriguez-Waitkus P, Enayati L, Krishnan B, et al. Vascular tumors have increased p70 S6-kinase activation and are inhibited by topical rapamycin. Lab Invest. 2013;93(10):1115–27. [DOI] [PubMed] [Google Scholar]
- 3.Leaute-Labreze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taieb A Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358(24):2649–51. [DOI] [PubMed] [Google Scholar]
- 4.North PE, Waner M, Mizeracki A, Mihm MC Jr. GLUT1: a newly discovered immunohistochemical marker for juvenile hemangiomas. Hum Pathol. 2000;31(1):11–22. [DOI] [PubMed] [Google Scholar]
- 5.Kum JJ, Khan ZA Mechanisms of propranolol action in infantile hemangioma. Dermatoendocrinol. 2014;6(1):e979699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loges S, Roncal C, Carmeliet P Development of targeted angiogenic medicine. J Thromb Haemost. 2009;7(1):21–33. [DOI] [PubMed] [Google Scholar]
- 7.Adamski H, Le Gall F, Cartron L, Dauriac C, Lancien G, Wechsler J, et al. Eruptive angiomatous lesions associated with graft-versus-host disease. Br J Dermatol. 2003;149(3):667–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.