Abstract
Background:
We sought to ascertain whether there is an association between prostate cancer (PC)-specific mortality (PCSM) and salvage androgen deprivation therapy (ADT) timing amongst men with short versus long prostate-specific antigen doubling times (PSA-DT)s.
Patients and Methods:
The study cohort was selected from 206 men with localized unfavorable-risk PC who were randomized to radiation therapy (RT) or RT plus 6 months of ADT between 1995 and 2001. Fifty-four men who received salvage ADT for PSA failure after a median follow up of 18.72 years following randomization defined the study cohort. Fine-Gray competing risks regression analyzed whether the timing of salvage ADT was associated with an increased risk of PCSM after adjusting for age, comorbidity, known PC prognostic factors, and previously identified interactions.
Results:
After a median follow-up of 5.68 years (IQR 3.05 – 9.56) following salvage ADT 49 of the 54 men (91%) died, 27 from PC (54% of deaths). Increasing PSA-DT as a continuous covariate (per month increase) was associated with a decreasing risk of PCSM (adjusted hazard ratio [AHR] 0.33, 95% CI 0.13, 0.82; P=0.02). Amongst men with a long PSA-DT (≥6 months), initiating salvage ADT later (PSA>12ng/mL, upper quartile) versus earlier was associated with an increased risk of PCSM (AHR 8.84, 95% CI 1.99–39.27; P=0.004); whereas for men with a short (<6months) PSA-DT (AHR 1.16, 95% CI 0.38–3.54; P=0.79) this was not true.
Conclusion:
Early initiation of salvage ADT for post-RT PSA recurrence in men with a PSA-DT of 6 months or more may reduce the risk of PCSM.
1. Introduction:
Prostate cancer (PC) is the most common non-cutaneous malignancy in men, with 161,360 new cases and 26,730 deaths due to prostate cancer (PC) projected to occur during 2017.1 Though radical prostatectomy (RP) or radiation therapy (RT) with or without androgen deprivation therapy (ADT) are often curative treatments for localized disease,2–5 approximately one-quarter of patients will recur within 10 years following curative-intent therapy.6,7 A rising prostate-specific antigen (PSA) identifies men with biochemical recurrence defined as nadir + 2 following RT, or a detectable and rising following RP.8,9 Although ADT has been a standard treatment for when combined with RT in men with unfavorable (intermediate or high)-risk localized PC,5 recent evidence demonstrates that adding ADT to RT provides a progression-free and overall survival benefit in the biochemically recurrent rising PSA after RP.10,11 However Level 1 evidence is lacking to guide management for patients with a biochemical recurrence following definitive treatment with RT with or without ADT.
Moreover, the optimal timing of ADT initiation after post RT biochemical recurrence remains an open question given the lack of randomized data comparing this approach to surveillance and therefore unknown impact of salvage ADT use on survival. To help guide timing of ADT initiation after biochemical recurrence, National Comprehensive Cancer Network (NCCN) guidelines recommend that patients with a short PSA-DT and an otherwise long life expectancy be encouraged to consider earlier ADT.2,12–15 However, an alternative hypothesis is that patients with favorable risk factors such as long PSA-DT and interval to PSA recurrence and Gleason score 7 or less PC may be the patients who would have the potential for improved cancer-specific outcomes given that the recurrence may be less likely to be castration resistant.
Therefore, the purpose of the current study was to use data from a mature prospective randomized clinical trial that evaluated the use of RT versus RT and ADT as initial treatment in men with unfavorable-risk PC to ascertain whether a significant association existed between an increased risk of PC-specific mortality (PCSM) when salvage ADT was initiated at high versus lower PSA level amongst men with short versus long PSA-DT’s after adjusting for age and known PC prognostic factors and previously identified interactions.12
2. Materials and Methods:
2.1. Patient Population, Treatment and Follow-up
The study cohort was selected from 206 men with localized (1992 American Joint Commission on Cancer tumor category 1b to 2b) unfavorable (intermediate or high)-risk PC16 that underwent central pathology review and who were enrolled in a randomized trial of RT or RT plus 6 months of ADT between December 1, 1995 and April 15, 2001. Information on eligibility criteria and patient characteristics stratified by randomized treatment arms has previously been reported.5 Radiotherapy was delivered using three-dimensional conformal radiotherapy to 70.2Gy in 39 fractions of 1.8Gy per fraction to the prostate and seminal vesicles. Combined androgen blockade included two injections of an LHRH agonist (leuprolide acetate 22.5mg Q3months or goserelin 10.8mg Q3 months) and a nonsteroidal antiandrogen (flutamide 250 mg Q8 hours or bicalutamide 50mg QD, discontinued on day 85 after the second administration of the LHRH agonist). Baseline comorbidity at the time of study enrollment was characterized using the ACE-27 index.17
Prior to PSA failure, patients were followed with PSA, physical exam, and digital rectal exam every 3 months for 2 years then every 6 months until 5 years, and annually thereafter.5 If PSA failure occurred, bone scan and pelvic computed tomography or magnetic resonance imaging was performed. One-hundred and eight patients developed biochemical failure defined as 2ng/mL elevation above the lowest PSA value achieved. The study protocol recommended lifelong salvage ADT or bilateral orchiectomy when PSA levels approached 10ng/mL.18 Ultimately, 54 men (39 and 15 men randomized to RT vs RT and ADT, respectively) received salvage ADT (50) or orchiectomy (4) for PSA failure after a median follow up of 18.72 years following randomization and these men defined the study cohort as shown in the CONSORT diagram (Figure 1). Fifty-four remaining men with PSA failure were not treated with salvage ADT because PSA remained less than 10ng/mL (n=48), because of significant comorbid illness (n=3), or because the patients had a PSA-DT more than 2 years and advanced age >75 (n=3).
Figure 1.
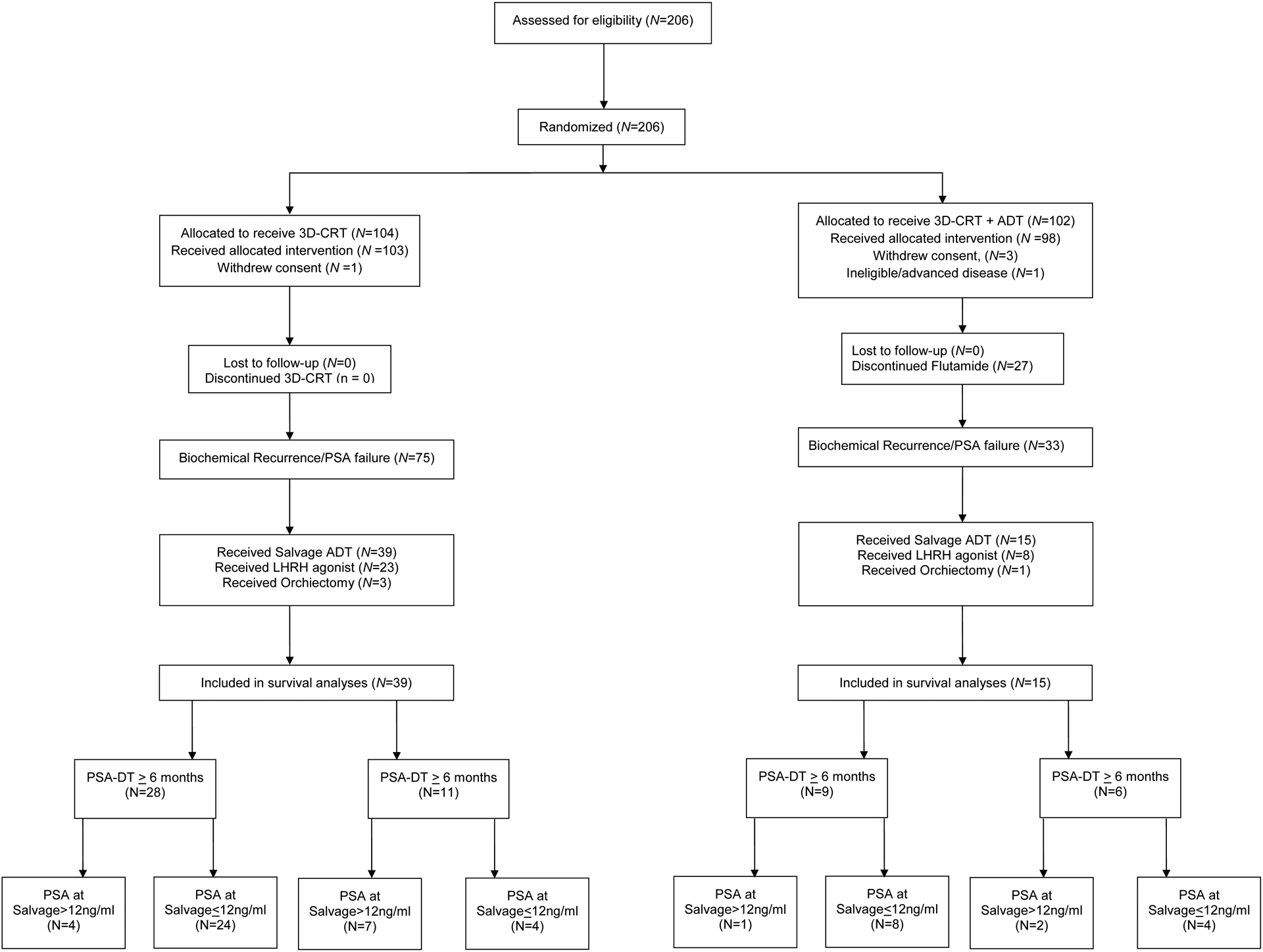
CONSORT diagram.
Time zero for this study commenced at the time of salvage ADT initiation and concluded on the date of death or last follow-up through 9/6/16, with no patients lost to follow-up. Our institutional review board granted permission to perform this study.
2.2. Cause of Death Determination
If a patient became refractory to first-line salvage ADT, second- and third-line ADT was applied usually followed by cytotoxic chemotherapy. Cause of death was determined by the treating oncologist who followed the patient from study entry until death. All cause of death determinations were reviewed and confirmed by the principal investigator of the study (AVD).
2.3. Statistical Methods
2.3.1. Distribution and comparison of the clinical characteristics at the time of randomization stratified by PSA doubling time and level at the time of salvage ADT
Descriptive statistics characterized the distribution of clinical characteristics for the 54 men in the study cohort, stratified by PSA-DT (≥6 versus <6months) and level (>12ng/mL versus ≤12 ng/mL) at the time of salvage ADT. PSA-DT was stratified by ≥6 versus <6 months since PSA-DT less than 6 months is a well-established poor prognostic factor associated with a high risk of subsequent distant metastases and death due to PC.18–21 PSA at salvage ADT was stratified by >12ng/mL versus ≤12 ng/mL since 12.05 ng/mL represented the upper quartile and we sought to determine whether a delay in the initiation of salvage ADT using PSA level as a surrogate for timing of ADT was associated with a higher risk of PCSM amongst men with short (<6 months) or long (≥6 months) DTs. The Wilcoxon rank sum test22 and the Fisher Exact test23 were used to compared the distribution of the continuous covariates and categorical covariates, respectively. The log rank test24 was used to compare the median survival times following salvage ADT in years. These comparisons were made across subgroups defined by the pre-specified PSA-DT and level cutpoints (i.e. 6 months and 3rd quartile of 12 ng/ml respectively) and the results are shown in Table 1.
Table 1.
Distribution and comparison of the clinical characteristics at the time of randomization and median survival following salvage androgen deprivation therapy stratified by PSA doubling time and level at the time of salvage ADT.
| Clinical Characteristics | PSA Doubling Time ≥ 6 months (n = 37) | PSA Doubling Time < 6months (n = 17) | ||||
|---|---|---|---|---|---|---|
| PSA at Salvage (ng/mL) | PSA at Salvage (ng/mL) | |||||
| PSA >12 ng/ml (n = 5) |
PSA≤12 ng/ml (n = 32) |
P-value | PSA >12 ng/ml (n = 9) |
PSA≤12 ng/ml (n = 8) |
P-value | |
| Median Age in years (IQR) at Salvage | 83.26 (78.76, 83.98) | 76.61 (71.27, 80.23) | 0.09 | 71.52 (69.49, 73.77) | 76.37 (75.22, 80.03) | 0.09 |
| Median interval in months to PSA Failure (IQR) | 43.89 (19.25, 71.13) | 37.75 (24.92, 53.42) | 0.91 | 14.32 (8.71, 21.52) | 23.16 (19.37, 58.78) | 0.11 |
| Median survival after Salvage ADT in years (IQR) | 4.94 (1.92, 5.87) | 6.78 (5.13, 12.45) | 0.02* | 3.23 (2.07, 5.20) | 3.10 (2.87, 7.29) | 0.29* |
| AJCC clinical Tumor category | ||||||
| T1, No. (%) | 2 (40%) | 14 (43.75%) | 1.00 | 2 (22.22%) | 2 (25%) | 1.00 |
| T2, No. (%) | 3 (60%) | 18 (56.25%) | 7 (77.78%) | 6 (75%) | ||
| Highest Gleason Score | ||||||
| ≤ 6, No. (%) | 2 (40%) | 9 (28.13%) | 0.24 | 0 (0%) | 0 (0%) | 0.62 |
| 7, No. (%) | 1 (20%) | 18 (56.25%) | 5 (55.56%) | 6 (75%) | ||
| 8–10, No. (%) | 2 (40%) | 5 (15.63%) | 4 (44.44%) | 2 (25%) | ||
| Randomly Assigned Intervention | ||||||
| RT alone, No. (%) | 4 (80%) | 24 (75%) | 1.00 | 7 (77.78%) | 4 (50%) | 0.33 |
| RT + ADT, No. (%) | 1 (20%) | 8 (25%) | 2 (22.22%) | 4 (50%) | ||
| ACE-27 defined Comorbidity | ||||||
| No/minimal | 3 (60%) | 24 (75%) | 0.60 | 9 (100%) | 7 (87.5%) | 0.47 |
| Moderate/severe | 2 (40%) | 8 (25%) | 0 (0%) | 1 (12.5%) | ||
Abbreviations: ADT (Androgen Deprivation Therapy), ACE (Adult Comorbidity Evaluation-27), AJCC American Joint Commission on Cancer, IQR (Interquartile Range), PSA (Prostate-Specific Antigen), RT (Radiation Therapy)
Log-Rank Test P-values
2.3.2. Univariable and multivariable competing risks regressions
Univariable and multivariable Fine-Gray competing risks regression analyses25 were performed to ascertain whether the timing of salvage ADT was significantly associated with an increased risk of PCSM adjusting for age, comorbidity and known PC prognostic factors as well as previously identified interactions. Specifically, we included age, interval to PSA failure (continuous), PSA-DT (continuous), PSA at time of salvage ADT (continuous), highest Gleason score (≤6 [referent] versus 7 versus 8–10), clinical tumor category (T1 [referent] versus T2), randomly assigned treatment arm (RT [referent] versus RT plus ADT), and comorbidity status (no/minimal [referent] versus moderate/severe) in the primary model. The PSA-DT and level were log transformed to ensure that the followed a normal distribution. A comorbidity × ADT interaction term was also included as it was identified as a significant interaction in the randomized trial.5 In a second model we categorize the PSA-DT split at 6 months and PSA level at the time of salvage ADT split at 12 ng/mL in order to generate a testable hypothesis regarding the risk of PCSM and early versus delayed initiation of salvage ADT amongst men with short versus long PSA-DTs. We also include an interaction term between PSA-DT and PSA level at time of salvage ADT to ascertain whether an increase in the risk of PCSM could exist in men with long but not short PSA-DT when salvage ADT was initiated at a high versus lower level of PSA. Unadjusted and adjusted hazard ratios (HR) and associated 95% confidence interval (CI) with associated P-values were calculated for each covariate.
2.3.3. Estimates of PCSM stratified by PSA doubling time and level at the time of salvage ADT
For the purposes of illustration cumulative incidence (CI) plots26 for PCSM were generated stratified by PSA-DT (≥6 versus <6months) and level (>12ng/mL versus ≤12 ng/mL) at the time of salvage ADT. These estimates were compared across subgroups using Gray’s k-mean P-value.25 The median follow-up for the study was 5.68 years and as such 5-year point estimates of PCSM with associated 95% CIs were calculated and reported stratified by subgroup.
All P-values are two-sided, with a P-value <0.025 considered significant after Bonferroni corrections (n=2 PSA-DT groups) were made for multiple comparisons.27 All analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC), except for the PCSM estimates and Gray’s k-mean P-value which was calculated using R (version 3.2.3, Auckland, New Zealand).
3. Results:
3.1. Distribution and comparison of the clinical characteristics at the time of randomization stratified by PSA doubling time and level at the time of salvage ADT
There was no significant difference in the distribution of patients, cancer, and treatment characteristics amongst men with a PSA-DT <6 months versus ≥6 months who started salvage ADT at a PSA level that exceeded the 3rd quartile (PSA>12ng/mL) versus those who did not (Table 1). However, there was a significantly shorter median survival (4.94 versus 6.78 years; P = 0.02) in men with a long PSA-DT (6 months or more) and a PSA level >12 ng/ml at the time of salvage ADT, as compared to ≤12ng/ml.
3.2. Univariable and multivariable competing risks regressions
After a median follow-up of 5.68 years (IQR 3.05 – 9.56), 49/54 died (91%), with 27 deaths due to prostate cancer (accounting for 54% of deaths). PSA-DT when evaluated as a continuous covariate (per month increase) was significantly associated with a decreasing risk of PCSM as the value of PSA-DT increased (Table 2, footnote). Moreover, amongst men with a long PSA-DT (≥6 months), initiating salvage ADT later (at a PSA >12ng/mL) as opposed to earlier, was associated with an increased risk of PCSM (AHR 8.84, 95% CI 1.99, 39.27; P=0.004). However, this was not seen for men with a short (<6months) PSA-DT (AHR 1.16, 95% CI 0.38, 3.54; P=0.79). As a result, the interaction term between PSA-DT and PSA at salvage was significant in the multivariable analysis (Pinteraction =0.05).
Table 2.
Univariable and multivariable competing risks analyses defined hazard ratios for prostate cancer-specific mortality for each clinical characteristic.
| Clinical Characteristics | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| No. Men | No. PC Deaths | HR (95% CI) | P-value | AHR (95% CI) | P-value | |
| PSA DT × PSA level at Salvage ADT | 54 | 27 | 0.63 (0.10, 3.84) | 0.62 | 0.13 (0.02, 1.03) | 0.05 |
| *PSA Doubling Time ≥ 6 months | ||||||
| PSA ≤ 12 ng/mL | 32 | 10 | 1.00 (referent) | 1.00 (referent) | ||
| PSA > 12 ng/mL | 5 | 3 | 4.26 (1.53, 11.86) | 0.006 | 8.84 (1.99, 39.27) | 0.004 |
| *PSA Doubling Time < 6 months | ||||||
| PSA ≤ 12 ng/mL | 8 | 6 | 1.00 (referent) | 1.00 (referent) | ||
| PSA > 12 ng/mL | 9 | 8 | 1.85 (0.57, 6.01) | 0.30 | 1.16 (0.38, 3.54) | 0.79 |
| Age, years | 54 | 27 | 0.95 (0.89, 1.004) | 0.07 | 0.98 (0.90, 1.07) | 0.65 |
| Interval to PSA failure, months | 54 | 27 | 0.96 (0.94, 0.99) | 0.003 | 0.97 (0.94, 1.003) | 0.08 |
| AJCC clinical tumor category | ||||||
| T1 | 20 | 8 | 1.00 (referent) | 1.00 (referent) | ||
| T2 | 34 | 19 | 1.71 (0.77, 3.80) | 0.19 | 1.33 (0.52, 3.37) | 0.55 |
| Highest Gleason Score | ||||||
| ≤ 6 | 11 | 2 | 1.00 (referent) | 1.00 (referent) | ||
| 7 | 30 | 17 | 4.03 (1.02, 15.89) | 0.046 | 3.19 (0.84, 12.12) | 0.09 |
| 8–10 | 13 | 8 | 5.43 (1.19, 24.64) | 0.03 | 4.39 (0.83, 23.34) | 0.08 |
| ADT × Comorbidity | 54 | 27 | 2.21 (0.15, 32.26) | 0.56 | 3.88 (0.24, 63.20) | 0.34 |
| No/minimal ACE-27 defined Comorbidity | ||||||
| RT | 31 | 19 | 1.00 (referent) | 1.00 (referent) | ||
| RT + ADT | 12 | 4 | 0.48 (0.16, 1.44) | 0.19 | 0.35 (0.06, 1.97) | 0.24 |
| Moderate/severe ACE-27 defined Comorbidity | ||||||
| RT | 8 | 3 | 1.00 (referent) | 1.00 (referent) | ||
| RT+ADT | 3 | 1 | 1.05 (0.09, 12.14) | 0.97 | 1.37 (0.19, 9.88) | 0.75 |
Abbreviations: ACE (Adult Comorbidity Evaluation-27), ADT (Androgen Deprivation Therapy), AHR (Adjusted Hazard Ratio), AJCC American Joint Commission on Cancer, CI (Confidence Interval), HR (Hazard Ratio), IQR (Interquartile Range), No. (Number), PC (Prostate Cancer), PSA (Prostate-Specific Antigen), RT (Radiation Therapy)
When PSA DT (per month increase) and level (ng/ml), both after log transformation, at time of salvage ADT were treated as continuous covariates and without an interaction term the respective AHR [95% CI]; p-value are 0.33 [0.13, 0.82]; 0.02 and 1.27 [0.69, 2.32]; 0.44, respectively.
3.3. Estimates of PCSM stratified by PSA doubling time and level at the time of salvage ADT
As shown in Figure 2A, CI estimates of PCSM were significantly higher (K P-value 0.014) amongst men with a PSA-DT≥ 6months who started salvage ADT at a PSA >12 ng/mL as opposed to a PSA ≤ 12 ng/mL. However, these estimates were not significantly different (K P-value=0.16) amongst men with a PSA-DT <6months as shown in Figure 2B. Specifically, among men with a PSA-DT≥6months the 5-year CI point estimates with 95% CIs of PCSM were 40.0%, (2.58, 79.48) versus 6.25%, (1.07, 18.37) among men who started salvage ADT at a PSA >12 ng/mL as opposed to a PSA ≤12 ng/mL. In comparison, among men with a PSA-DT <6months, the 5-year CI point estimates with 95% CIs of PCSM were 55.56%, (17.47, 82.03) versus 50.0%, (12.05, 79.69) when salvage ADT was imitated at a PSA >12 ng/mL as opposed to a PSA ≤12 ng/mL.
Figure 2A.
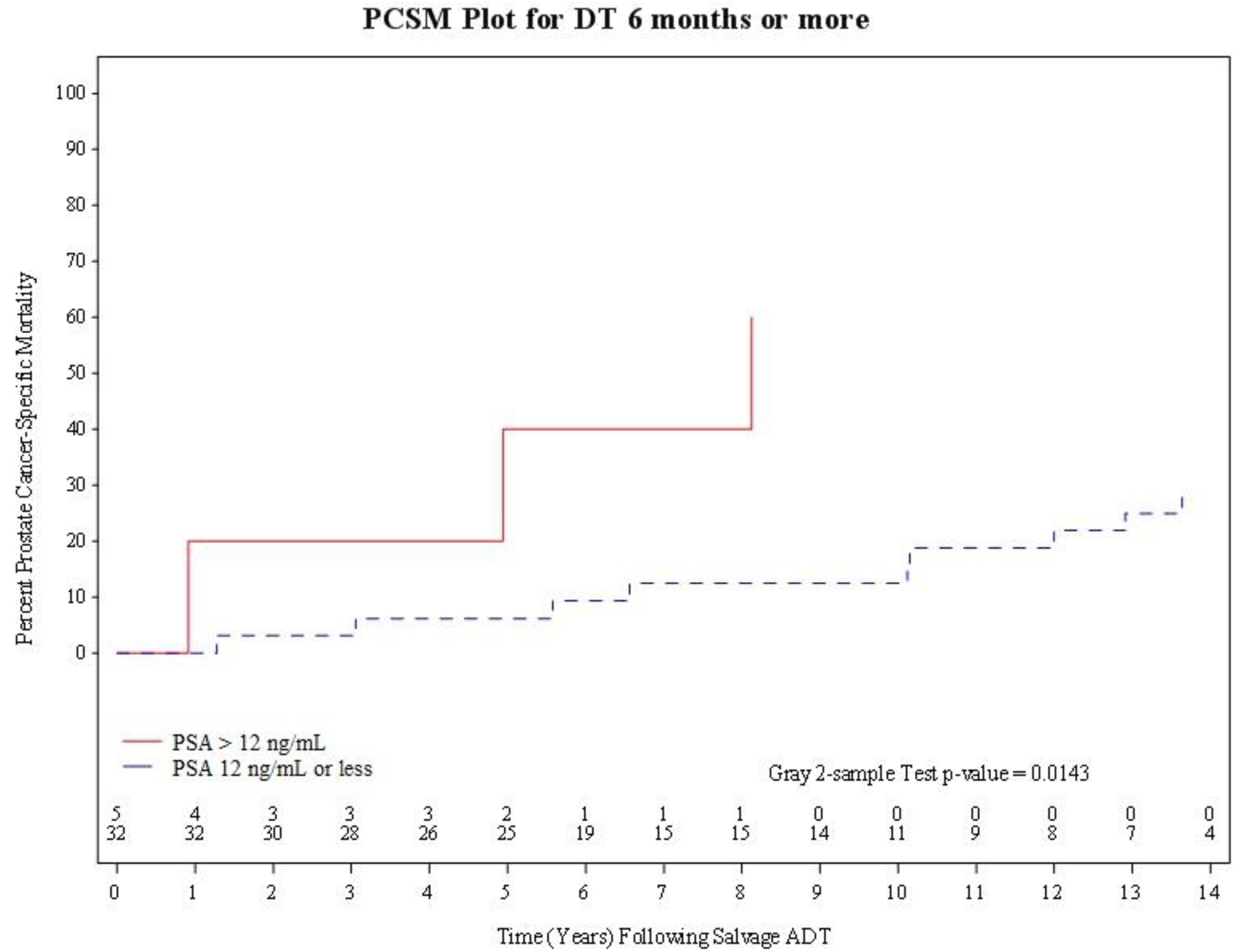
PCSM plot for PSA doubling-time of 6 months or more, stratified by PSA at time of initiation of salvage ADT (PSA> 12ng/nL versus PSA 12 ng/mL or less).
Figure 2B.
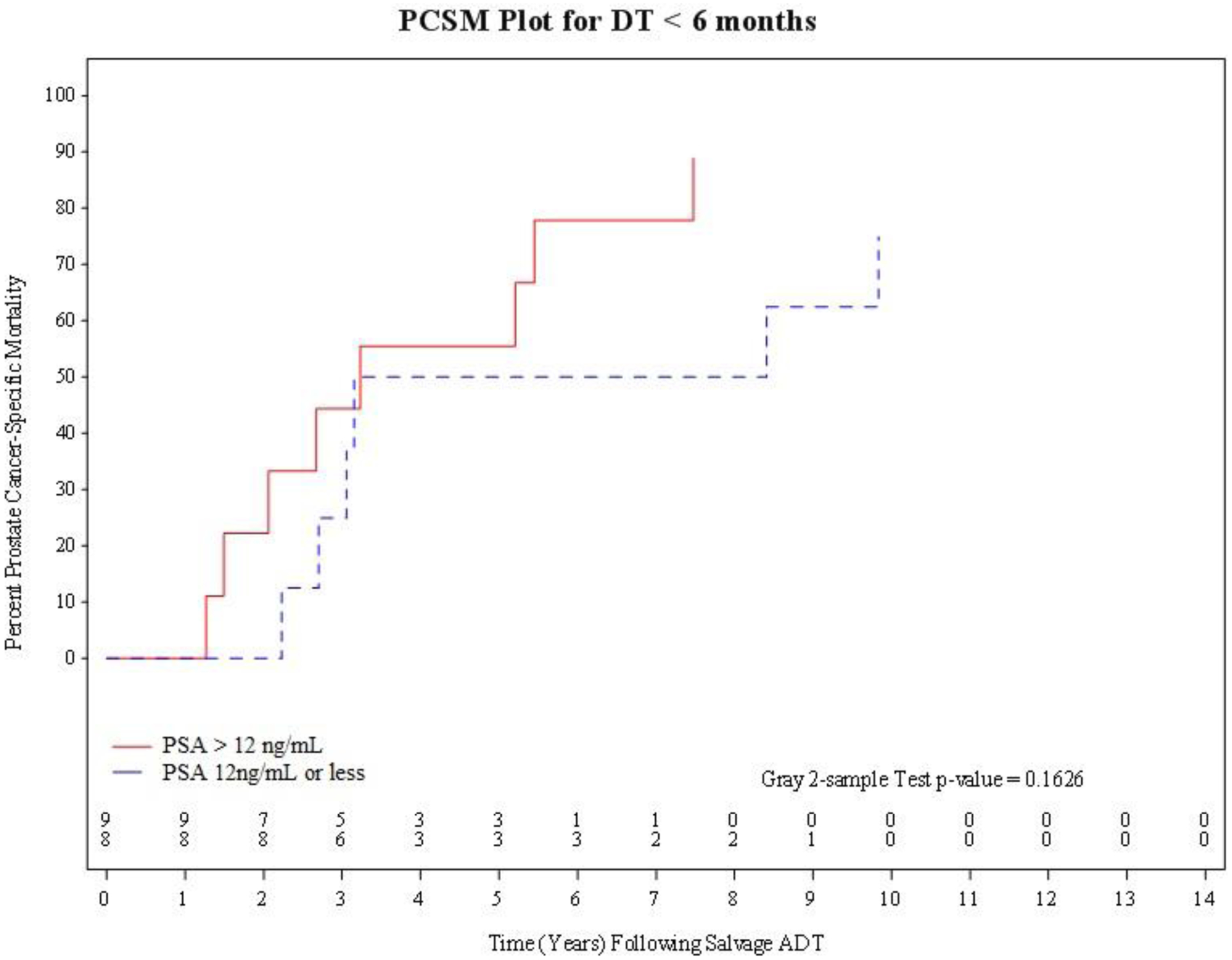
PCSM plot for PSA doubling-time of less than 6 months, stratified by PSA at time of initiation of salvage ADT (PSA> 12ng/nL versus PSA 12 ng/mL or less).
4. Discussion:
We found that among men with long-doubling times (≥6 months), the risk of dying from prostate cancer was significantly greater with initiation of salvage ADT at a PSA level that exceeded the 3rd quartile (PSA >12ng/mL) when compared to salvage ADT initiation at a lower PSA (≤12ng/mL). In contrast, we found that the PSA level at the time of salvage ADT initiation did not significantly impact the risk of PCSM among men with short PSA-DTs (less than 6 months) although not unexpectedly given the shorter PSA-DT the median survival of these men was lower than that for men who PSA-DT was 6 months or more irrespective of what PSA level salvage ADT was initiated as shown in Table 1.
The clinical significance of these observations is that they provide evidence to argue against the unproven assumption that patients with a short PSA-DT are those most likely to benefit from early initiation of salvage ADT. As such, our results raise a question regarding the current national NCCN guideline recommendation encouraging earlier salvage ADT initiation specifically in men with short PSA-DT.2,15,28–30 Instead, consideration of earlier initiation of salvage ADT in men with a long PSA-DT may be appropriate for some. Moreover, for a subgroup of these men with additional favorable prognostic factors (long interval to PSA failure and favorable risk disease) a reduction in the risk of PCSM from salvage local therapy alone may be possible.31 Conversely, men with short PSA-DT may be optimal candidates for enrollment on to randomized control trials evaluating the impact on time to metastasis and survival from adding drugs such as enzalutamide, docetaxel and/or abiraterone that have been shown to improve survival in recurrent, metastatic, and castration resistant prostate cancer to the standard LHRH agonist.32–35
Several points require further discussion. First, our results show an association and not causality between a lower risk of PCSM and initiation of salvage ADT at lower PSA levels in men with long PSA-DT. To date, there is a single randomized trial showing a benefit in survival to early versus delayed salvage ADT (HR 0.55, 95% CI 0.30–1.00, P=0.05) from a group of diverse patients who had PSA relapse after surgery or radiotherapy as initial definitive therapy, or who were medically unfit for definitive therapy.15 Although this study was stratified by PSA-DT of 10 months, there was no report of these results stratified by PSA-DT in the post RT cohort. Remaining evidence is level 2, including data from an observational follow-up study and a systematic review suggesting the benefit of early salvage ADT is limited to men with short PSADT,12,13 whereas data from the CaPSURE database suggested similar outcomes between early and delayed salvage ADT approaches.14 Therefore, proof that the association we observe in the significantly shorter median survival in men with a PSA-DT ≥6 months who started salvage ADT later versus earlier is causal requires prospective validation in a larger study than ours, where only 17 and 37 men were in the short versus long PSADT subgroups, respectively. Second while men with short PSA-DT did not appear to have an increased risk of PCSM whether salvage ADT was started at high versus low PSA levels, this may be a result of the fact that all PSA levels at which salvage ADT was initiated were high. This stems from a fact that a PSA level of 10ng/ml was used in this prospective study to define when salvage ADT should be initiated which was common practice in the late 1990’s into early 2000’s when this study was conducted. It is possible that salvage ADT initiation at lower PSA levels as commonly practiced today could lead to a reduced risk of PCSM even in men with short PSA-DT. However, given that short PSA-DT have been shown to place men at a higher risk for and in some studies a surrogate for prostate cancer-specific and all cause mortality19,20,36, it is likely that such men may already have metastatic castrate resistant prostate cancer in whom conventional salvage ADT with LHRH agonist may not be effective. This supports our proposal to enroll such men onto randomized trials where drugs capable of overcoming castrate resistance34,35,37 such as abiraterone, enzalutamide, or docetaxel are added to the LHRH agonist and the impact on time to metastasis and death is evaluated.
5. Conclusion:
In conclusion, the results of this small cohort of patients sets the stage for further investigation in larger studies as to whether early initiation of salvage ADT at lower absolute PSA levels for biochemical recurrence in the setting of long PSA-DT (≥6 months) reduces the risk of PCSM.
6. References:
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA: a cancer journal for clinicians. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.NCCN Clinical Practice Guidelines in Oncology. Prostate Cancer 2017. v 2. 2017. Available at: http://www.nccn.org. Accessed 30 March 2017. [Google Scholar]
- 3.Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. The New England journal of medicine. 2011;365(2):107–118. [DOI] [PubMed] [Google Scholar]
- 4.Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. The New England journal of medicine. 2012;367(3):203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Amico AV, Chen MH, Renshaw AA, Loffredo M, Kantoff PW. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA : the journal of the American Medical Association. 2008;299(3):289–295. [DOI] [PubMed] [Google Scholar]
- 6.D’Amico AV, Renshaw AA, Sussman B, Chen MH. Pretreatment PSA velocity and risk of death from prostate cancer following external beam radiation therapy. JAMA : the journal of the American Medical Association. 2005;294(4):440–447. [DOI] [PubMed] [Google Scholar]
- 7.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA : the journal of the American Medical Association. 2005;294(4):433–439. [DOI] [PubMed] [Google Scholar]
- 8.Roach M 3rd, Hanks G, Thames H Jr., et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. International journal of radiation oncology, biology, physics. 2006;65(4):965–974. [DOI] [PubMed] [Google Scholar]
- 9.Moul JW. Prostate specific antigen only progression of prostate cancer. The Journal of urology. 2000;163(6):1632–1642. [PubMed] [Google Scholar]
- 10.Shipley WU, Seiferheld W, Lukka HR, et al. Radiation with or without Antiandrogen Therapy in Recurrent Prostate Cancer. The New England journal of medicine. 2017;376(5):417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrie C, Hasbini A, de Laroche G, et al. Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): a randomised, multicentre, open-label phase 3 trial. The Lancet Oncology. 2016;17(6):747–756. [DOI] [PubMed] [Google Scholar]
- 12.Fu AZ, Tsai HT, Haque R, et al. Mortality and Androgen Deprivation Therapy as Salvage Treatment for Biochemical Recurrence after Primary Therapy for Clinically Localized Prostate Cancer. The Journal of urology. 2017;197(6):1448–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van den Bergh RC, van Casteren NJ, van den Broeck T, et al. Role of Hormonal Treatment in Prostate Cancer Patients with Nonmetastatic Disease Recurrence After Local Curative Treatment: A Systematic Review. European urology. 2016;69(5):802–820. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Albeniz X, Chan JM, Paciorek A, et al. Immediate versus deferred initiation of androgen deprivation therapy in prostate cancer patients with PSA-only relapse. An observational follow-up study. European journal of cancer (Oxford, England : 1990). 2015;51(7):817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duchesne GM, Woo HH, Bassett JK, et al. Timing of androgen-deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01–03 [TOAD]): a randomised, multicentre, non-blinded, phase 3 trial. The Lancet Oncology. 2016;17(6):727–737. [DOI] [PubMed] [Google Scholar]
- 16.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA : the journal of the American Medical Association. 1998;280(11):969–974. [DOI] [PubMed] [Google Scholar]
- 17.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL, Jr. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA : the journal of the American Medical Association. 2004;291(20):2441–2447. [DOI] [PubMed] [Google Scholar]
- 18.Prostate-specific antigen (PSA) best practice policy. American Urological Association (AUA). Oncology (Williston Park, NY). 2013. (update of 2000 publication);14(2):267–272, 277–268, 280 passim. [PubMed] [Google Scholar]
- 19.D’Amico AV, Moul J, Carroll PR, Sun L, Lubeck D, Chen MH. Prostate specific antigen doubling time as a surrogate end point for prostate cancer specific mortality following radical prostatectomy or radiation therapy. The Journal of urology. 2004;172(5 Pt 2):S42–46; discussion S46–47. [DOI] [PubMed] [Google Scholar]
- 20.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA : the journal of the American Medical Association. 1999;281(17):1591–1597. [DOI] [PubMed] [Google Scholar]
- 21.Patel A, Dorey F, Franklin J, deKernion JB. Recurrence patterns after radical retropubic prostatectomy: clinical usefulness of prostate specific antigen doubling times and log slope prostate specific antigen. The Journal of urology. 1997;158(4):1441–1445. [DOI] [PubMed] [Google Scholar]
- 22.Hollander M, and Wolfe DA (1999). Nonparametric Statistical Methods. 2nd ed. New York: John Wiley & Sons. Chapter 4: The Two-Sample Location Problem. Pp. 106–140. [Google Scholar]
- 23.Fisher RA. On the interpretation of X2 from contingency tables, and the calculation of P. J of Royal Statistical Society 1922; 85:87–94. [Google Scholar]
- 24.Bland JM, Altman DG. The logrank test. BMJ (Clinical research ed). 2004;328(7447):1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 26.Gaynor JJ, Feuer EJ, Tan CC, et al. On the use of cause-specific failure and conditional failure probabilities: examples from clinical oncology data. J Am Stat Assoc 1993; 88:400. [Google Scholar]
- 27.Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. BMJ (Clinical research ed). 1995;310(6973):170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Albeniz X, Chan JM, Paciorek AT, et al. Immediate versus deferred initiation of androgen deprivation therapy in prostate cancer patients with PSA-only relapse. J Clin Oncol 32:5s, (suppl; abstr 5003). 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahal BA, Chen MH, Bennett CL, et al. High PSA anxiety and low health literacy skills: drivers of early use of salvage ADT among men with biochemically recurrent prostate cancer after radiotherapy? Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2015;26(7):1390–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreira DM, Banez LL, Presti JC Jr., et al. Predictors of secondary treatment following biochemical recurrence after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital database. BJU international. 2010;105(1):28–33. [DOI] [PubMed] [Google Scholar]
- 31.Lee AK, D’Amico AV. Utility of Prostate-Specific Antigen Kinetics in Addition to Clinical Factors in the Selection of Patients for Salvage Local Therapy. Journal of Clinical Oncology. 2005;23(32):8192–8197. [DOI] [PubMed] [Google Scholar]
- 32.Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. The New England journal of medicine. 2015;373(8):737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.James ND, de Bono JS, Spears MR, et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. The New England journal of medicine. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. The New England journal of medicine. 2011;364(21):1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. The New England journal of medicine. 2012;367(13):1187–1197. [DOI] [PubMed] [Google Scholar]
- 36.Royce TJ, Chen MH, Wu J, et al. Surrogate End Points for All-Cause Mortality in Men With Localized Unfavorable-Risk Prostate Cancer Treated With Radiation Therapy vs Radiation Therapy Plus Androgen Deprivation Therapy: A Secondary Analysis of a Randomized Clinical Trial. JAMA oncology. 2017;3(5):652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. The New England journal of medicine. 2004;351(15):1513–1520. [DOI] [PubMed] [Google Scholar]


