Abstract
We report a miniaturized Filter Aided Sample Preparation method (micro-FASP) for low-loss preparation of submicrogram proteomic samples. The method employs a filter with ~0.1 mm2 surface area, reduces the total volume of reagents to < 10 μL, and requires only two sample transfer steps. The method was used to generate 25,883 unique peptides and 3,069 protein groups from 1,000 MCF-7 cells (~100 ng protein content), and 13,367 peptides and 1,895 protein groups were identified from 100 MCF-7 cells (~10 ng protein content). Single blastomeres from Xenopus laevis embryos at the 50-cell stage (~200 ng yolk free protein/blastomere) generated 20,943 unique peptides and 2,597 protein groups; the proteomic profile clearly differentiated left and right blastomeres and provides strong support for models in which this asymmetry is established early in the embryo. The parallel processing of 12 samples demonstrates reproducible label free quantitation of 1-μg protein homogenates.
Graphical Abstract

Bottom-up proteomic analyses can be divided into two tasks. The first task generates tryptic peptides from a protein homogenate, which requires sample lysis, protein solubilization, and tryptic digestion. The second task identifies those peptides by use of chromatographic or electrophoretic separation, tandem mass spectrometry detection, and database searching and other bioinformatic analyses. Dramatic improvements in peptide analysis now allow detection and identification of zeptomole quantities of peptides.1,2 In contrast, relatively large amounts of starting material (>20 μg) are typically required to generate those peptides for analysis.3-11
Conventional methods for sample preparation usually perform reduction, alkylation, and digestion on proteins retained on a solid support. Mann’s Filter Aided Sample Preparation (FASP) method has become a standard approach for sample preparation. FASP performs manipulations on a commercial spin filter and relies on a centrifuge to pump reagents over the sample.6 FASP and other conventional methods are very robust, and produce outstanding performance for samples larger than 10 μg.
Several manuscripts have reported improved sample recovery for application of the conventional FASP method to smaller amounts of sample. For example, addition of carrier substances such as polyethylene glycol (PEG) or dextran to the processed samples improves the peptides yields up to 30% in the low to submicrogram range.12,13 However, trace amounts of low molecular weight polyethylene were observed in the products, which can contaminate both the liquid chromatography column or mass spectrometer.12 Similarly, substitution of 0.2% deoxycholic acid for urea increased sensitivity, recovery, and proteomic coverage for samples,13 but the starting material used in that report was relatively high (>50 μg).
It has been recognized that the number of sample processing steps and relatively large volume reactors employed in conventional methods result in sample losses and introduction of contaminants,14 which limits performance when analyzing low microgram and smaller quantities of proteins. A number of methods have been reported that reduce protein loss and contamination when analyzing nanogram quantities of proteins.14-24 The current state of the art appears to be the nanodroplet processing in one pot for trace samples (nanoPOTS) platform.24 This method improves the processing efficiency and sample recovery by downscaling processing volumes to <200 nL to minimize surface losses. Combined with ultrasensitive LC-MS (a 30 μm i.d. × 70 cm long C18 column with an Orbitrap Fusion Lumos Tribrid MS), nanoPOTS has been used to identify ~1,500 from ~10 and ~3,000 proteins from ~140 cells. By incorporating the match between runs algorithm of MaxQuant, >3000 proteins were consistently identified from as few as 10 cells. However, nanoPOTS requires manipulation of nanoliter amounts of reagent, which requires specialized liquid handling systems that are not widely available. More recently, the nanoPOTS workflow was upscaled to be compatible with conventional pipetting, termed microdroplet processing in one pot for trace samples (μPOTS),14 which could identify ~1800 unique proteins from as few as ~25 cultured HeLa cells.
In this work, we report a miniaturized FASP method (MICRO-FASP) to generate deep proteome profiling from a limited amount of starting material. The method retains all the advantages of the conventional FASP method but minimizes sample loss by radically reducing the surface area of the filter to ~0.1 mm2. The buffer volumes used for both sample washing and elution are less than 3 μL, which can be provided with conventional pipettors. The micro-FASP method addresses the time-consuming nature of conventional FASP by using a high molecular weight cutoff membrane for rapid centrifugal pumping of reagents without degrading performance. Finally, the micro-FASP method employs only two sample transfer steps, reducing contamination and sample loss.
EXPERIMENTAL SECTION
Chemicals and Materials.
Bovine pancreas TPCK-treated trypsin, urea, ammonium bicarbonate (NH4HCO3), dithiothreitol (DTT), iodoacetamide (IAA), cOmplete™ Protease Inhibitor Cocktail and PhosSTOP™ tablets (provided in EASYpacks), Microcon-30kDa Centrifugal Filter Unit with Ultracel-30 membrane (molecular weight cut-off, MWCO: 30 kDa, RC30 kDa) were purchased from Sigma-Aldrich (St. Louis, MO). Ultracel regenerated cellulose (RC) membranes with 100 kDa MWCO (RC100 kDa), Biomax polyethersulfone (PES) membranes with 50 kDa MWCO (PES50 kDa), 100 kDa MWCO (PES100 kDa), 300 kDa MWCO (PES300 kDa), 500 kDa MWCO(PES500 kDa), and Durapore hydrophilic polyvinylidene fluoride (PVDF) membrane with 0.1 μm pore size (HILICPVDF 0.1 μm) were purchased from MilliporeSigma (Burlington, MA). Formic acid (FA) and acetonitrile (ACN) were purchased from Fisher Scientific (Pittsburgh, PA). Methanol was purchased from Honeywell Burdick & Jackson (Wicklow, Ireland). MS compatible human protein extract (Intact K562 human cell lysate) was purchased from VWR (Radnor, PA). Mammalian Cell-PE LBTM buffer for cell lysis was purchased from G-Biosciences (St. Louis, MO). Water was deionized by a Nano Pure system from Thermo Scientific (Marietta, OH).
Xenopus laevis embryo culture and single blastomere collection.
All animal procedures were performed according to the protocols approved by The University of Notre Dame Institutional Animal Care and Use Facility. Female Xenopus laevis were induced by injection with 700 units of human chorionic 12 hours prior to spawning. Testes were removed from anesthetized males at the time of spawning. Eggs and minced testes were combined in 1/3 X MMR (Mark’s Modified Ringers) for a 20-minute fertilization and dejellied in 2% cysteine for 4 minutes. Embryos were kept in 1/3 X MMR at 23 °C throughout development. Collection of individual blastomeres (cells) occurred at stage 6 (32-cell stage) and stage 6.5 (50-cell stage) via microdissection. Embryo dissociated and blastomere collection were performed using visualization with a microscope. Briefly, embryos were placed in a calcium magnesium free media. The vitelline membranes were removed using a pair of forceps. Individual cells were cut and isolated from whole embryos using an eyebrow-hair knife and loop. All samples were flash frozen in liquid nitrogen and stored at −80 °C until cell lysis and protein extraction.
Sample preparation with the miniaturized FASP method (MICRO-FASP) based method.
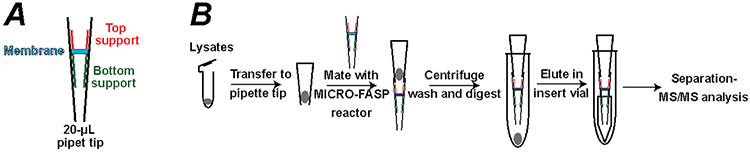
The MICRO-FASP microreactor is fabricated in a 20-μL pipette tip, Figure 1A. A short length (~0.5 mm) was cut from its tip and used as the bottom support, which was pushed into the tip with 18-gauge blunt needle. Then, a small piece of 30 kDa MWCO ultrafiltration membrane taken from a Microcon-30kDa centrifugal filter unit was cut using a blunt 18-gauge needle and pushed into the pipette tip until it was in tight contact with the bottom support. The first step was repeated to put another support into the tip; this second support was pushed with the blunt needle to ensure tight contact with the top of the ultrafiltration membrane. The MICRO-FASP reactor was trimmed to the ~10 μL position.
Figure 1.
(A) Construction of MICRO-FASP reactor. (B) Schematic workflow for sample preparation by the MICRO-FASP method.
2 μL of lysis buffer (Mammalian Cell-PE LBTM buffer) with protease inhibitor was added into MCF-7 or Xenopus single blastomere sample in a small PCR tube, followed by cell lysis via sonication with a Branson Sonifier 250 (VWR Scientific, Batavia, IL) for 5 min on ice. Cell lysates were reduced with 1 μL of 50 mM of DTT at 37 °C for 1 hour, followed by alkylation with 2 μL of 50 mM of IAA at room temperature for 30 minutes. Then cell lysates were aspirated into the pipette tip, then connected to the MICRO-FASP microreactor. Sample was transferred onto the filter with centrifugation at 6,000x g. Then 5 μL of 20 mM NH4HCO3 was loaded onto the microreactor for sample washing. 5 μL of a trypsin solution (trypsin to protein ratio at 1:100) was loaded onto the microreactor at 200x for 20 seconds. The top of the microreactor was sealed with a bottom piece of a PCR tube or Para film, put in a 1.5 mL centrifuge tube, and incubated in a 37 °C water bath overnight. Digests were eluted into autosampler vials of the LC-ESI-MS/MS system for single-shot proteomic analysis.
Sample preparation with the conventional FASP method.
Sample preparation using the original FASP method was performed with minor changes.6 Briefly, the cell lysate was reduced and alkylated in its tube. A 1 μg aliquot was pipetted onto the moistened filter. After centrifugation at 13,000x g for 30 min, the filter was washed with triplicate 100 μL aliquots of 20 mM NH4HCO3. 40 μL of the trypsin solution was loaded onto the filter and centrifuged at 600x g for 1 min. The filter was incubated in a 37 °C water bath overnight. The digest was eluted by centrifugation at 13 000x g for 30 min, followed by triplicate elutions with 40 μL of 0.1% FA in water. The eluate was lyophilized and redissolved in 0.1%FA for LC-ESI-MS/MS analysis.
UPLC-ESI-MS/MS analysis.
An ACQUITY UPLC M-Class system (Waters, Milford, MA, USA) with an ACQUITY UPLC M-Class Peptide BEH C18 column (Waters, 75 μm × 150 mm, 1.7 μm, 300 Å) was coupled to a Q Exactive HF mass spectrometer (Thermo Fisher Scientific) for peptide separation and identification; details of its operation are presented below. Mobile phase A (0.1%FA in water) and mobile phase B (0.1% FA in ACN) were used for gradient separation. Peptides were automatically loaded onto the C18 column and flushed with 2% mobile phase B for 12 min at a flow rate of 0.5 μL/min, then followed by the 2-hour gradient: 12–102 min, 2–30% B; 102–106 min, 30–88% B; 106–110 min, 88% B; 110–111 min, 88-2% B; 111–120 min, 2% B. For samples prepared from different numbers of MCF-7 cells, a 4-hour gradient was used: 12–222 min, 2–30% B; 222–226 min, 30–88% B; 226–230 min, 88% B; 230–231 min, 88-2% B; 231–240 min, 2% B. The eluted peptides from the C18 column were pumped through a capillary tip for electrospray.
Mass Spectrometer Operating Parameters.
A Q Exactive HF mass spectrometer (Thermo Scientific) was used in this work. The mass spectrometer was programmed in data-dependent mode. The S-lens RF level was set at 50, and heated capillary at 300 °C. Full scan resolution was set to 60,000 at m/z 200. Full scan target was 3.00E+06. Mass range was set to m/z 350-1,650. Maximum injection time 30 ms. Target value for fragment scans was set at 1.00E+05. Maximum injection time 45 ms. A fixed first mass of 100 was used. Loop count 15. Isolation window was 1.4 m/z. Normalized collision energy was set at 28.
Database searching.
Database searching of the raw files was performed in MaxQuant (version 1.6.1.0.).25 Matching between runs was enabled only in searches of samples of different cell numbers and single blastomeres with match and alignment time windows of 0.7 and 20 minutes, respectively. Label-free quantification was selected for the data required quantification analysis. The false discovery rate (FDR) was determined by using a target-decoy search strategy. On the peptide level, the corresponding FDR on peptide level was less than 1%. On the protein level, protein grouping was enabled. The copy number values were calculated using the Proteomic ruler in Perseus downloaded from the Perseus Plugin Store (http://www.coxdocs.org/doku.php?id=perseus:user:plugins:store).26
RESULTS AND DISCUSSION
Optimization of MICRO-FASP method.
Most sample preparation methods employ membranes or chambers with ~1-cm2 surface area. Active sites on those surfaces can capture proteins and peptides, limiting the ability to generate large numbers of identifications from nanogram amounts of cellular lysates.
The MICRO-FASP microreactor described in this manuscript dramatically reduces the dimensions of the reactor with a concomitant decrease in sample loss and contamination. To maximize the sensitivity of the MICRO-FASP method, the experimental conditions including the membrane pore size, the flush volume, and the elution volume were carefully optimized. The numbers of peptide and protein identifications were used as criteria to evaluate sample processing. In initial experiments, 1 μg of K562 cell lysate was loaded onto the MICRO-FASP microreactor, followed by flushing and digestion.
The following membranes were tested in the MICRO-FASP method: RC30 kDa, PES50 kDa, RC100 kDa, PES100 kDa, PES300 kDa, PES500 kDa, and HILICPVDF 0.1 μm, (Supplementary Figure S1). Surprisingly, the membrane with larger molecular weight cutoff (MWCO) retained smaller proteins than in the membrane used in the FASP method (Supplementary Figure 2). We assume that the proteins produced a keystone effect in the limited number of pores on the membrane. However, the keystone effect would disappear after the proteins were digested into much smaller peptides.
As expected, the time required for sample preparation decreased with an increase in the membrane pore size, with a concomitant improvement in sample throughput. Also, low MWCO filters may be clogged by cell debris when a whole cell lysate is loaded onto the reactor without clarification at high centrifugation speed. In this case, we recommend that the sample is subjected to high-speed centrifugation to remove debris before loading onto the MICRO-FASP reactor.
Due to the much smaller size of the filter (~1 mm id, but only less than 0.4 mm id was in contact with the sample due to the area covered by the support) used in the MICRO-FASP reactor compared with the conventional FASP method, the amounts of residual detergents or salts on the membrane are correspondingly reduced, as is the volume of wash buffer required for their removal. The sample was prepared by the MICRO-FASP method using flushing volumes of 2.5, 5, and 10 μL (Supplemental Figure S3). 2.5 μL flushing buffer is sufficient to remove the detergents and salts from the sample.
We also investigated the effect of the elution volume, Supplemental Figure S4. After sample preparation with the MICRO-FASP method, 2.5 μL of elution buffer was used to elute a fraction into a vial marked as E1. Then, the vial was replaced and 1 μL of elution buffer was used to elute a second fraction, marked as E2. The 1-μL elution was repeated three times to produce samples marked as E3, E4, and E5. Before LC-ESI-MS/MS analysis, elution buffer was added to each vial to produce final sample volumes of 5 μL. 3.5 μL of buffer is sufficient to elute the sample from the filter. Since the eluting volume is smaller than the sample loop (5 μL) of the LC system, the eluted sample could be directly used for LC-MS analysis without lyophilization and re-dissolution.
Comparison with the conventional FASP method.
We first investigated two elution buffers in the conventional FASP method for 10 μg of K562 cell lysate. One experiment used the conventional 20 mM NH4HCO3 buffer for elution.6 The other eluted the digest with 0.1% FA while simultaneously quenching trypsin digestion. The 0.1% FA elution buffer generated 5% more peptide identifications and the same number of protein identifications (SI Table S1).
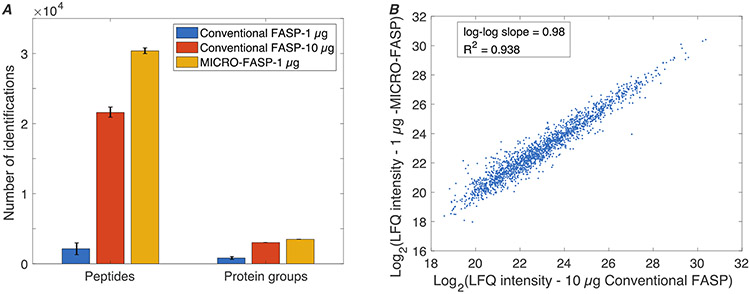
We then compared the conventional FASP method (0.1% FA elution buffer) with the MICRO-FASP protocol for the analysis of 1 μg K562 cell lysate loading amount, Figure 2. We identified 2,142 (n=2, RSD=56%) unique peptides and 821 (n=2, RSD=37%) protein groups from 1 μg of starting material using the conventional FASP method. In contrast, 30,377 (n=2, RSD=3.9%) unique peptides and 3,487 (n=2, RSD=1.4%) protein groups were identified by the MICRO-FASP method from 1 μg of starting material; MICRO-FASP identified over 14 times more peptides and over four times more protein groups than the FASP method for 1 μg of protein.
Figure 2.
Comparison of MICRO-FASP and conventional FASP methods for K562 cell homogenates. (A) The number of identifications for various sample loadings (mean ± SEM; n=2), and (B) correlation of label free quantitation intensities from 1-μg of K562 homogenate using the MICRO-FASP method with the intensities from 10-μg using the conventional FASP method.
We compared the MICRO-FASP method for analysis of 1-μg protein with the performance of the conventional FASP method for analysis of 10-μg of protein. As noted above, the MICRO-FASP method identified 30,477 peptides and 3,487 proteins from 1-μg of protein. In contrast, the conventional FASP method identified only 24,657 ± 557 (n=3) unique peptides and 3,433 ± 28 (n=3) protein groups, Table S1, from 10-μg of starting material. We compared the properties of proteins and peptides identified from 10-μg of starting material for the FASP method and from 1-μg of starting material for our MICRO-FASP method, Supplementary Figure S5 (A-E). There was no obvious bias between them. Finally, label free quantitation (LFQ) was used to compare the MICRO-FASP method’s analysis of 1-μg protein with the conventional FASP method's analysis of 10-μg protein loading, Figure 2(B). The data from the two measurements were highly correlated (R2 > 0.93), which confirms quantitative repeatability.
Preparation of low cell numbers sample by MICRO-FASP method.
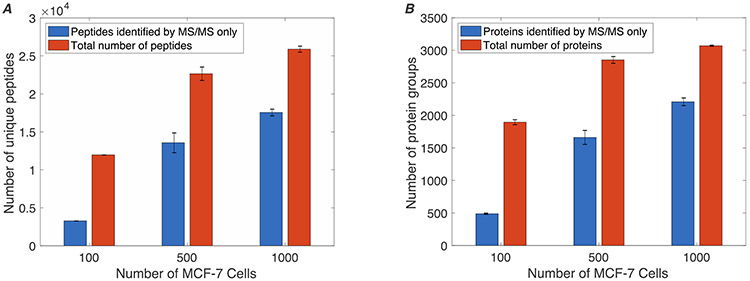
We applied the MICRO-FASP method to process material from 100, 500, and 1,000 MCF7 cells, Figure 3. We identified 13,367 unique peptides from 100 MCF-7 cells using the matching between runs feature in MaxQuant.25 Information on these identified peptides is provided in spreadsheets included in supporting information.
Figure 3.
(A) Number of unique peptides and (B) protein groups identified from different numbers of MCF-7 cells (mean ± SEM; n=2) using MICRO-FASP.
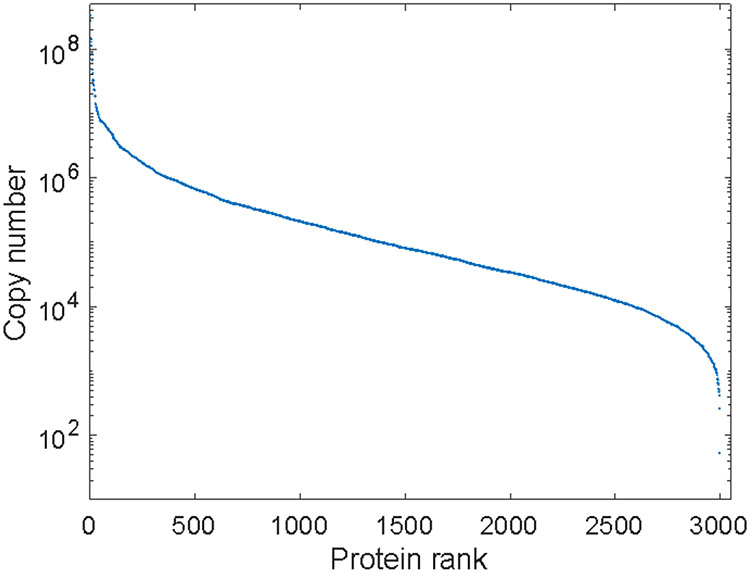
The apparent dynamic range of protein expression was over six orders of magnitude, Figure 4. Median protein expression was 1.1×106 copies per cell, which is approximately 50 times higher than the value determined in an earlier study of the HeLa proteome.15 There are at least two reasons for this difference. First, the signal generated by peptides from low abundance proteins is too low to be detected by the mass spectrometer, which skews the median to higher values. Second, no fractionation step was carried out before our LC-MS analysis; as a result, the ionization efficiency of the peptides from low abundance proteins is suppressed by the high abundance peptides, again hindering the identification of low abundance proteins. We also observed that the median protein expression values increase as the number of cells decreased, indicating that higher abundance proteins are preferentially identified from the limited starting material. The most abundant protein identified from 1000 of MCF-7 cells was histone H2B type 1-C/E/F/G/I at 3.4×108 copies per cell. We observed 370 proteins with more than 106 copies per cell, including histone components, ribosomal proteins, oxidoreductases, cytoskeletal proteins, and transferases, as observed previously.14
Figure 4.
The apparent dynamic range of protein expression from 1000 MCF-7 cells using MICRO-FASP.
We observed 25,676 unique peptide identifications when analyzing 1,000 MCF-7 cells with the MICRO-FASP method. This number of IDs should be compared with identification of ~10,000 unique peptides reported using the iST method for analysis of 1,000 HeLa cells and 15,384 unique peptides using the SP3 method also for analysis of 1,000 HeLa cells.15-16 Sample preparation by the MICRO-FASP method could be done within an hour (including loading, washing, and eluting the sample), which is comparable with SP3 and iST method.
Preparation of single blastomere samples by the MICRO-FASP method.
Individual blastomeres produce a geometric progression in sample size during development because the protein content per blastomere roughly divides by two with each cell division.27 The MICRO-FASP method was applied to a set of single blastomeres that were dissected from 32-cell and 50-cell stage Xenopus laevis embryos. For this experiment, we used a reactor with a HILICPVDF 0.1 μm membrane because a large amount of cell debris was observed in the lysates. Six blastomeres were dissected from two embryos (three blastomeres from each embryo) at the 32-cell stage and nine blastomeres were dissected from three embryos (three blastomeres from each embryo) at the ~50-cell stage. The identification results are summarized in Table 1. The numbers of identifications are significantly larger than recently reported using microsampling,28,29 and are the deepest proteome coverage to date for single blastomeres. This reactor reduced the sample preparation time to less than 1 h, and did not prevent identification of very small proteins, as shown in Supplementary Figure S6. Information on peptides identified in this study is supplied in supporting information.
Table 1.
Identification results from single Xenopus laevis blastomeres at 50-cell stage of development
| Blastomere ID | Protein groups |
Peptides | |
|---|---|---|---|
| Embryo-1 | 1 | 2,563 | 20,266 |
| 2 | 2,597 | 20,754 | |
| 3 | 2,299 | 16,582 | |
| Embryo-2 | 4 | 2,544 | 19,311 |
| 5 | 2,581 | 19,576 | |
| 6 | 2,446 | 18,826 | |
| Embryo-3 | 7 | 2,487 | 18,129 |
| 8 | 2,539 | 19,322 | |
| 9 | 2,471 | 16,499 |
67%-72% of the protein groups (1,631 protein groups) were consistently detected in the nine blastomeres. Label-free quantification intensity was used to compare the proteomic profile of the single blastomeres. High Pearson correlations (r = 0.95-0.97) were found between the blastomeres from the same embryo (Supplementary Figure S7), which confirmed quantitative repeatability. However, lower Pearson correlations (r = 0.81-0.89) were found between the blastomeres taken from the different embryos, which suggests that there is embryo-to-embryo heterogeneity in protein expression. For example, the Pearson correlation coefficients between blastomere-4 (from embryo-2) and blastomere-7, blastomere-8, and blastomere-9 (from embryo-3) are 0.85, 0.89, and 0.81, respectively.
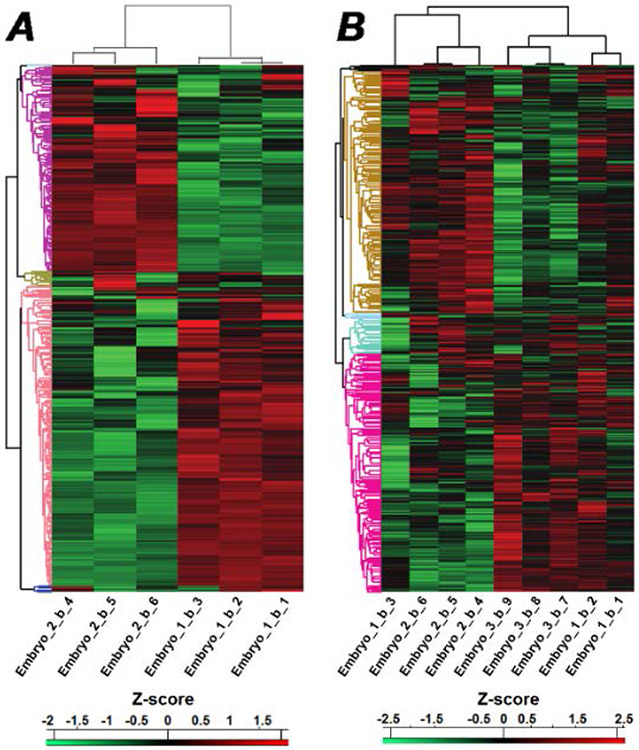
Protein samples were prepared from single blastomeres randomly taken from the animal hemisphere of 32- and 50-cell embryos. Strikingly, protein expression analysis revealed that the blastomeres could be placed into one of two distinct groups based upon these profiles (Figure 5). The profile plots for two main clusters from 32- and 50-cell embryos are shown in Supplementary Figure S8. Gene sets associated with each of the clusters are also provided in the supporting information. The patterns suggest that the protein distribution may reflect one of the cardinal axes of the embryo. Several proteins and mRNA are asymmetrically distributed along the animal-vegetal axis of the Xenopus egg, which becomes the anterior-posterior axis during gastrulation.30 The first cell cleavage of the egg engenders left-right asymmetry, while the position of the dorsal-ventral axis is established during the second round of cell division. It appears possible that the distinct profiles of protein expression we detect could be related to one of the latter two axes.
Figure 5.
(A) Heatmap of protein expression across six blastomeres taken from 32-cell embryos. (B) Heatmap of protein expression across nine blastomeres taken from 50-cell embryos.
Left-right patterning transpires through a series of developmental steps. In Xenopus, the expression of the transcription factor foxji during gastrulation determines the formation of the left-right organizer (LRO).31 Cilia of the central LRO promote leftward flow of extracellular fluids that direct nodal signaling in the left lateral plane mesoderm during neurulation. While all of these steps occur after zygotic transcription has begun at mid-blastula stage, there is evidence that essential events for left-right patterning occur within the first few rounds of cell division following fertilization.32-34 A prime candidate for determining these early upstream steps is a proton translocating ATPase that is asymmetrically distributed as early as the 2-cell stage.35 Inhibition of the H+-V-ATPase results in heterotaxia that can be directly related to ectopic Nodal expression. We detect distinct levels of H+-V-ATPase (atp6v1) in blastomeres from 32- and 50-cell embryos consistent with immunohistochemical assays showing biased deposition of the protein on the right side of 4- and 8-cell embryos. The proteomic profile demonstrates that left and right blastomeres can be differentiated, and provides strong support for models in which this asymmetry is established early in the embryo. In the case of Xenopus, this patterning precedes zygotic transcription and the expression of well-known downstream determinants such as nodal, lefty, and pitx2. Our results are in accord with a recent metabolomic study of single blastomeres that detected left-right asymmetry in 8-cell Xenopus embryos.36
High throughput MICRO-FASP method.
We developed a multiplexed version of the MICRO-FASP method using polypropylene plates (2 mL, 96 deep wells) as supports. Because the maximum speed of our centrifuge (Microcentrifuge 5430R, with Keypad and 2 x MTP/PCR rotor) is 2204×g, MICRO-FASP reactors with PES300 kDa membrane were used for higher throughput sample preparation.
We evaluated the performance of the multiplexed MICRO-FASP method using Xenopus laevis lysate as the starting material. Twelve samples, each containing 1 μg of lysate, were processed in parallel. 16,942 (n=12, RSD=1.3%) unique peptides and 2701 (n=12, RSD=0.5%) protein groups were identified. LFQ intensity was used to compare the correlation between the replicates, Supplementary Figure S9. The data from the twelve measurements were highly correlated (R2 > 0.99), which confirms the reproducibility of the high throughput MICRO-FASP method.
CONCLUSIONS
The MICRO-FASP method allowed processing of the total cell lysate from a small number of cells, which addresses the long-standing problem of quantitative reproducibility and precision in the conventional FASP method when processing sub-microgram amounts of starting material. The identification of more than 3,000 proteins in single runs using only 1,000 mammalian cells as starting material opens up interesting applications for ultrasensitive proteomics. We applied the MICRO-FASP method in processing single blastomeres from Xenopus laevis embryos, and we identified approximate 2,600 protein groups and over 20,000 peptides, which is the deepest proteome coverage to date from single blastomeres from the 50-cell stage embryo. We observed the protein expression differences between blastomeres, particularly from blastomeres from different embryos. Moreover, the identification results could be further improved by using more sensitive separation technique such as capillary zone electrophoresis,37-41 and an advanced mass spectrometer with faster scan speed.41
Supplementary Material
ACKNOWLEDGMENT
We thank Yini Zhu from Prof. Xin Lu’s group at University of Notre Dame for providing the MCF-7 cell samples. We also thank Dr. William Boggess and Dr. Matthew Champion in the Notre Dame Mass Spectrometry and Proteomics Facility for their help with this project. This work was funded by the National Institutes of Health (Grant R35GM136334).
Footnotes
Supporting Information
Supporting Information is available free of charge on the ACS Publications website. Additional supporting information describing effects of membrane type, flushing volume, and elution volume on the MICRO-FASP method, molecular weight distributions obtained from different membranes, comparison of conventional FASP and MICRO-FASP methods, molecular weight distribution of the proteins identified from different blastomeres from Embryo-1 at 50-cell stage, multi scatter plot based on LFQ intensity from nine single blastomere samples taken from ~50-cell embryos, the profile plots for Clusters from 32- and 50-cell embryos, multi scatter plot based on LFQ intensity from twelve samples prepared by high throughput MICRO-FASP method, identification numbers from different elution buffers in FASP analysis of 10 μg K562 lysate (PDF).
Additional supporting tables present peptides and protein groups lists for 100 to 1000 cells, peptides and protein groups lists for single blastomeres, gene sets associated with each of the clusters_32cell, gene sets associated with each of the clusters_50cell, high throughput Xenopus peptides, high throughput Xenopus proteinGroups (Excel spreadsheets).
REFERENCES
- (1).Li S; Plouffe BD; Belov AM; Ray S; Wang X; Murthy SK; Karger BL; Ivanov AR An Integrated Platform for Isolation, Processing, and Mass Spectrometry-based Proteomic Profiling of Rare Cells in Whole Blood. Mol. Cell. Proteomics 2015, 14, 1672–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Amenson-Lamar EA; Sun L; Zhang Z; Bohn PW; Dovichi NJ Detection of 1 Zmol Injection of Angiotensin Using Capillary Zone Electrophoresis Coupled to a Q-Exactive HF Mass Spectrometer With an Electrokinetically Pumped Sheath-Flow Electrospray Interface. Talanta 2019, 204, 70–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Medzihradszky KF In Mass Spectrometry: Modified Proteins and Glycoconjugates in Methods in Enzymology; Burlingame AL, Ed.; Academic Press: New York, 2005; pp 50–65. [DOI] [PubMed] [Google Scholar]
- (4).Chen EI; McClatchy D; Park SK; Yates JR III. Comparisons of Mass Spectrometry Compatible Surfactants for Global Analysis of the Mammalian Brain Proteome. Anal. Chem 2008, 80, 8694–8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Nagaraj N; Lu A; Mann M; Wiśniewski JR Detergent-based but Gel-Free Method Allows Identification of Several Hundred Membrane Proteins in Single LC-MS Runs. J. Proteome Res 2008, 7, 5028–5032. [DOI] [PubMed] [Google Scholar]
- (6).Wisniewski JR; Zougman A; Nagaraj N; Mann M Universal Sample Preparation Method for Proteome Analysis Universal Sample Preparation Method for Proteome Analysis. Nat. Methods 2009, 6, 359–362. [DOI] [PubMed] [Google Scholar]
- (7).Altelaar AFM; Heck AJR Trends in Ultrasensitive Proteomics. Curr. Opin. Chem. Biol 2012, 16, 206–213. [DOI] [PubMed] [Google Scholar]
- (8).Ong TH; Kissick DJ; Jansson ET; Comi TJ; Romanova EV; Rubakhin SS; Sweedler JV Classification of Large Cellular Populations and Discovery of Rare Cells Using Single Cell Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry. Anal. Chem 2015, 87, 7036–7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).de Vargas Roditi L; Claassen M Computational and Experimental Single Cell Biology Techniques for the Definition of Cell Type Heterogeneity, Interplay and Intracellular Dynamics. Curr. Opin. Biotechnol 2015, 34, 9–15. [DOI] [PubMed] [Google Scholar]
- (10).Lu Y; Xue Q; Eisele MR; Sulistijo ES; Brower K; Han L; Amir E.-a. D.; Pe’er D; Miller-Jensen K; Fan R Highly multiplexed profiling of single-cell effector functions reveals deep functional heterogeneity in response to pathogenic ligands. Proc. Natl. Acad. Sci 2015, 112, E607–E615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Sielaff M; Kuharev J; Bohn T; Hahlbrock J; Bopp T; Tenzer S; Distler U Evaluation of FASP, SP3, and iST Protocols for Proteomic Sample Preparation in the Low Microgram Range. J. Proteome Res 2017, 16, 4060–4072. [DOI] [PubMed] [Google Scholar]
- (12).Wiśniewski JR; Ostasiewicz P; Mann M High Recovery FASP Applied to the Proteomic Analysis of Microdissected Formalin Fixed Paraffin Embedded Cancer Tissues Retrieves Known Colon Cancer Markers. J. Proteome Res 2011, 10, 3040–3049. [DOI] [PubMed] [Google Scholar]
- (13).Erde J; Loo RRO; Loo JA Enhanced FASP (eFASP) to Increase Proteome Coverage and Sample Recovery for Quantitative Proteomic Experiments. J. Proteome Res 2014, 13, 1885–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Xu K; Liang Y; Piehowski PD; Dou M; Schwarz KC; Zhao R; Sontag RL; Moore RJ; Zhu Y; Kelly RT Benchtop-compatible Sample Processing Workflow for Proteome Profiling of < 100 Mammalian Cells. Anal. Bioanal. Chem 2019, 411, 4587–4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Kulak NA; Pichler G; Paron I; Nagaraj N; Mann M Minimal, Encapsulated Proteomic-Sample Processing Applied to Copy-Number Estimation in Eukaryotic Cells. Nat. Methods 2014, 11, 319–324. [DOI] [PubMed] [Google Scholar]
- (16).Hughes CS; Foehr S; Garfield DA; Furlong EE; Steinmetz LM; Krijgsveld J Ultrasensitive Proteome Analysis Using Paramagnetic Bead Technology. Mol. Syst. Biol 2014, 10, 757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Hughes CS; Moggridge S; Müller T; Sorensen PH; Morin GB; Krijgsveld J Single-pot, Solid-Phase-Enhanced Sample Preparation for Proteomics Experiments. Nat. Protoc 2019, 14, 68–85. [DOI] [PubMed] [Google Scholar]
- (18).Zougman A; Selby PJ; Banks RE Suspension Trapping (STrap) Sample Preparation Method for Bottom-Up Proteomics Analysis. Proteomics 2014, 14, 1006–1010. [DOI] [PubMed] [Google Scholar]
- (19).HaileMariam M; Eguez RV; Singh H; Bekele S; Ameni G; Pieper R; Yu Y S-Trap, an Ultrafast Sample-Preparation Approach for Shotgun Proteomics. J. Proteome Res 2018, 17, 2917–2924. [DOI] [PubMed] [Google Scholar]
- (20).Chen Q; Yan G; Gao M; Zhang X Ultrasensitive Proteome Profiling for 100 Living Cells by Direct Cell Injection, Online Digestion and Nano-LC-MS/MS Analysis. Anal. Chem 2015, 87, 6674–6680. [DOI] [PubMed] [Google Scholar]
- (21).Chen W; Wang S; Adhikari S; Deng Z; Wang L; Chen L; Ke M; Yang P; Tian R Simple and Integrated Spintip-Based Technology Applied for Deep Proteome Profiling. Anal. Chem 2016, 88, 4864–4871. [DOI] [PubMed] [Google Scholar]
- (22).Tian R; Wang S; Elisma F; Li L; Zhou H; Wang L; Figeys D Rare Cell Proteomic Reactor Applied to Stable Isotope Labeling by Amino Acids in Cell Culture (SILAC)-based Quantitative Proteomics Study of Human Embryonic Stem Cell Differentiation. Mol. Cell. Proteomics 2011, 10, M110.000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Chen W; Adhikari S; Chen L; Lin L; Li H; Luo S; Yang P; Tian R 3D-SISPROT: A Simple and Integrated Spintip-Based Protein Digestion and Three-Dimensional Peptide Fractionation Technology for Deep Proteome Profiling. J. Chromatogr. A 2017, 1498, 207–214. [DOI] [PubMed] [Google Scholar]
- (24).Zhu Y; Piehowski PD; Zhao R; Chen J; Shen Y; Moore RJ; Shukla AK; Petyuk VA; Campbell-Thompson M; Mathews CE; Smith RD; Qian W-J; Kelly RT Nanodroplet Processing Platform for Deep and Quantitative Proteome Profiling of 10-100 Mammalian Cells. Nat. Commun 2018, 9, 882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Cox J; Mann M MaxQuant Enables High Peptide Identification Rates, Individualized p.p.b.-range Mass Accuracies and Proteome-Wide Protein Quantification. Nat. Biotechnol 2008, 26, 1367–1372. [DOI] [PubMed] [Google Scholar]
- (26).Wiśniewski JR; Hein MY; Cox J; Mann MA "Proteomic Ruler" for Protein Copy Number and Concentration Estimation Without Spike-In Standards. Mol. Cell. Proteomics 2014, 13, 3497–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Sun L; Dubiak KM; Peuchen EH; Zhang Z; Zhu G; Huber PW; Dovichi NJ Single Cell Proteomics Using Frog (Xenopus Laevis) Blastomeres Isolated From Early Stage Embryos, Which Form a Geometric Progression in Protein Content. Anal. Chem 2016, 88, 6653–6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Lombard-Banek C; Moody SA; Manzini MC; Nemes P Microsampling Capillary Electrophoresis Mass Spectrometry Enables Single-Cell Proteomics in Complex Tissues: Developing Cell Clones in Live Xenopus Laevis and Zebrafish Embryos. Anal. Chem 2019, 91, 4797–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Saha-Shah A; Esmaeili M; Sidoli S; Hwang H; Yang J; Klein PS; Garcia BA Single Cell Proteomics by Data-Independent Acquisition To Study Embryonic Asymmetry in Xenopus laevis. Anal. Chem 2019, 91, 8891–8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Sullivan SA; Moore KB; Moody SA In Cell Lineage and Fate Determination, Moody SA, Ed.; Academic Press: San Diego, 1999; pp 297–321. [Google Scholar]
- (31).Blum M; Ott T Mechanical Strain, Novel Genes and Evolutionary Insights: News From the Frog Left-Right Organizer. Curr. Opin. Genet. Dev 2019, 56, 8–14. [DOI] [PubMed] [Google Scholar]
- (32).Namigai EKO; Kenny NJ; Shimeld SM Right Across the Tree of Life: The Evolution of Left-Right Asymmetry in the Bilateria. Genesis 2014, 52, 458–470. [DOI] [PubMed] [Google Scholar]
- (33).Tingler M; Ott T; Tözser J; Kurz S; Getwan M; Tisler M; Schweickert A; Blum M Symmetry Breakage in the Frog Xenopus: Role of Rab11 and the Ventral-Right Blastomere. Genesis 2014, 52, 588–599. [DOI] [PubMed] [Google Scholar]
- (34).Vandenberg LN; Lemire JM; Levin M It's Never Too Early to Get It Right: A Conserved Role for the Cytoskeleton in Left-Right Asymmetry. Commun. Integr. Biol 2013, 6, e27155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Adams DS; Robinson KR; Fukumoto T; Yuan S; Albertson RC; Yelick P; Kuo L; McSweeney M; Levin M Early, H+-V-ATPase-dependent Proton Flux Is Necessary for Consistent Left-Right Patterning of Non-Mammalian Vertebrates. Development 2006, 133, 1657–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Onjiko RM; Morris SE; Moody SA; Nemes P Analyst 2016, 141, 3648–3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Sun L; Bertke MM; Champion MM; Zhu G; Huber PW; Dovichi NJ Sci. Rep 2014, 4, 4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Zhang Z; Yan X; Sun L; Zhu G; Dovichi NJ Detachable Strong Cation Exchange Monolith, Integrated With Capillary Zone Electrophoresis and Coupled With pH Gradient Elution, Produces Improved Sensitivity and Numbers of Peptide Identifications During Bottom-Up Analysis of Complex Proteomes. Anal. Chem 2015, 87, 4572–4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Zhang Z; Sun L; Zhu G; Cox OF; Huber PW; Dovichi NJ Nearly 1000 Protein Identifications From 50 Ng of Xenopus Laevis Zygote Homogenate Using Online Sample Preparation on a Strong Cation Exchange Monolith Based Microreactor Coupled With Capillary Zone Electrophoresis. Anal. Chem 2016, 88, 877–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Zhang Z; Peuchen EH; Dovichi NJ Surface-Confined Aqueous Reversible Addition-Fragmentation Chain Transfer (SCARAFT) Polymerization Method for Preparation of Coated Capillary Leads to Over 10 000 Peptides Identified From 25 Ng HeLa Digest by Using Capillary Zone Electrophoresis-Tandem Mass Spectrometry. Anal. Chem 2017, 89, 6774–6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Zhang Z; Hebert AS; Westphall MS; Qu Y; Coon JJ; Dovichi NJ Production of Over 27 000 Peptide and Nearly 4400 Protein Identifications by Single-Shot Capillary-Zone Electrophoresis-Mass Spectrometry via Combination of a Very-Low-Electroosmosis Coated Capillary, a Third-Generation Electrokinetically-Pumped Sheath-Flow Nanospray Interface, an Orbitrap Fusion Lumos Tribrid Mass Spectrometer, and an Advanced-Peak-Determination Algorithm. Anal. Chem 2018, 90, 12090–12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.