Abstract
Background
New onset or worsening drug-induced liver injury challenges coinfected patients on antiretroviral therapy (ART) initiation during antituberculosis (TB) treatment.
Methods
Post hoc analysis within a randomized trial, the Starting Antiretroviral Therapy at Three Points in Tuberculosis trial, was conducted. Patients were randomized to initiate ART either early or late during TB treatment or after TB treatment completion. Liver enzymes were measured at baseline, 6-month intervals, and when clinically indicated.
Results
Among 642 patients enrolled, the median age was 34 years (standard deviation, 28–40), and 17.6% had baseline CD4+ cell counts <50 cells/mm3. Overall, 146/472 patients (52, 47, and 47: early, late, and sequential arms) developed new-onset liver injury following TB treatment initiation. The incidence of liver injury post-ART initiation in patients with CD4+ cell counts <200 cells/mm3 and ≥200 cells/ mm3 was 27.4 (95% confidence interval [CI], 18.0–39.8), 19.0 (95% CI, 10.9–30.9), and 18.4 (95% CI, 8.8–33.8) per 100 person-years, and 32.1 (95% CI, 20.1–48.5), 11.8 (95% CI, 4.3–25.7), and 28.2 (95% CI, 13.5–51.9) per 100 person-years in the early, late integrated, and sequential treatment arms, respectively. Severe and life-threatening liver injury occurred in 2, 7, and 3 early, late, and sequential treatment arm patients, respectively. Older age and hepatitis B positivity predicted liver injury.
Conclusions
High incidence rates of liver injury among cotreated human immunodeficiency virus (HIV)–TB coinfected patients were observed. Clinical guidelines and policies must provide guidance on frequency of liver function monitoring for HIV–TB coinfected patients.
Keywords: HIV–TB integration, liver injury, antiretroviral treatment, tuberculosis treatment, South Africa
Post hoc analysis of the Starting Antiretroviral Therapy at Three Points in Tuberculosis trial demonstrates high incidence rates of liver injury among human immunodeficiency virus (HIV)–tuberculosis (TB) coinfected patients receiving concomitant treatment for TB and HIV.
Globally, tuberculosis (TB) remains a leading cause of mortality and morbidity among people living with human immunodeficiency virus (HIV), despite effective treatment for both diseases. South Africa bears a disproportionate burden of disease with 567 per 100 000 cases reported in 2017 [1]. Drug-induced liver injury (DILI) and liver enzyme elevations are commonly occurring complications of cotreatment of TB and HIV. DILI has been known to complicate therapy in 5%–33% of TB-infected patients and can affect 9%–30% of patients living with HIV who receive antiretroviral therapy (ART) [2, 3]. Liver injury often results in interruption of anti-TB or antiretroviral treatment and may exacerbate patient morbidity, resulting in poor treatment outcomes [2]. DILI is usually a diagnosis of exclusion. While a liver biopsy may improve diagnostic accuracy, it is not routinely performed. Multiple clinical and biochemical definitions of DILI exist as defined by the American Thoracic Society, British Thoracic Society, European Respiratory Society, and the World Health Organization (WHO) [2].
Earlier African studies report grade 2 liver injury incidence rates of 19.7 episodes per 100 person-years (95% confidence interval [CI], 16.1–24.0] post-ART initiation, and data from Taiwan describe an incidence rate of 36 cases per 100 person-years [4–8]. An Ethiopian study demonstrated a 10-fold increase in liver toxicity in HIV–TB cotreated patients when compared to patients on TB therapy exclusively [9]. South African in-hospital and 3-month mortality data associated with TB therapy or ART-induced liver injury was shown to be 27% and 35%, respectively [10]. Recently published Controlled Comparison of Two Moxifloxacin Containing Treatment Shortening Regimens in Pulmonary Tuberculosis (REMoxTB) trial data showed that patients who received standard isoniazid-containing TB therapy were at higher risk of liver toxicity compared to those who received a moxifloxacin-based TB regimen [11]. Furthermore, transaminase elevation of ≥3 times the upper limit of normal (ULN) occurred in 15% of patients living with HIV compared to 9% of patients not living with HIV [11].
Limited data exist related to the impact of initiating ART at differing time points of TB therapy on the development and resolution of DILI. An understanding of whether the timing of ART initiation during TB therapy influences the incidence, severity, and resolution of liver injury in HIV–TB cotreated patients will enhance clinical management of this common complication of cotreatment. Our aim in this study was to investigate the incidence, risk factors, and resolution of liver injury among HIV–TB coinfected patients enrolled in a clinical trial designed to determine the optimal time of ART initiation during TB treatment.
METHODS
Study Design Overview
The Starting Antiretroviral Therapy at Three Points in Tuberculosis trial was a randomized, open-label clinical trial undertaken from June 2005 to July 2010. Patients with CD4+ cell counts <500 cells/mm3 were included in the study. All 642 patients initiated standard TB therapy at study enrollment. Both first-episode TB patients and patients with recurrent TB were enrolled. All patients with a first episode of TB were treated with a fixed combination of rifampicin, isoniazid, ethambutol, and pyrazinamide for 2 months (intensive phase), with doses determined according to pretreatment weight, and a subsequent 4 months (continuation phase) of a fixed combination of isoniazid and rifampicin [12]. As per South African TB treatment guidelines at the time, patients with recurrent TB were given additional streptomycin with a longer duration of intensive phase TB treatment. Patients were randomly assigned to 3 groups in a ratio of 1:1:1 in permuted blocks of 6 or 9 with no stratification and were assigned to initiate ART within 4 weeks of TB treatment initiation (hereafter referred to as the early integrated arm), within 4 weeks after completion of the intensive phase of TB treatment (hereafter referred to as the late integrated arm), or within completion of TB therapy (hereafter referred to as the sequential treatment arm).
All patients received standard co-trimoxazole prophylaxis and anti-TB therapy. The standard first-line ART regimen comprised 300 mg/d lamivudine, 250 mg/d didanosine (for patients weighing ≥60 kg), or 400 mg/d (for patients weighing ≥60 kg) and 600 mg/d efavirenz. Detailed methodology has been described elsewhere [13].
Setting and Study Population
The study was conducted at the Centre for the AIDS Programme of Research in South Africa e-Thekwini treatment clinic, which adjoins the Prince Cyril Zulu Communicable Disease Centre, an outpatient TB facility in Durban. Patients were aged ≥18 years, coinfected with HIV and pulmonary TB, and provided informed consent for study participation. Pulmonary TB was confirmed by a positive sputum smear for acid fast bacilli, and HIV infection was confirmed by 2 positive HIV rapid screening tests.
Ethics Statement
The University of KwaZulu-Natal Biomedical Research Ethics Committee and the Medicines Control Council of South Africa approved the study.
Laboratory Methods and Definitions of Liver Injury
Liver enzymes were monitored at 6 monthly intervals postrandomization or as per clinician discretion, and testing was conducted at a local laboratory. Normal range values for males and females used in this analysis were alanine aminotransferase (ALT) 10 U/L–40 U/L and 7 U/L–35 U/L and aspartate aminotransferase (AST) 15 U/L–40 U/L and 13–35 U/L, respectively. Liver injury was graded according to the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events (version 1.0, 28 December 2004). All elevated ALT or AST values were referenced against the ULN threshold standardized by the local reference laboratory. The laboratory was accredited by the South African National Accreditation System and met minimum standards for good laboratory practice. Elevated liver enzymes of grade 1 and higher after the baseline visit were regarded as abnormal and included in the analysis. Patients with baseline abnormal liver enzymes who developed further worsening of liver toxicity were assessed using the same grading scale. Resolution of liver injury was defined as reversion to normal of any grade of liver injury by the end of follow-up.
Statistical Methods
Time at risk of developing liver injury was calculated as time from randomization to the date when abnormal results were detected, using the single random-point method. Time of worsening liver toxicity was calculated as time from baseline to the time point of increasing grade of liver toxicity, using the single random-point method. Time at risk for patients who did not experience liver injury was calculated from randomization to study termination or death, whichever occurred first.
The Fisher exact test was used for analyses of categorical variables. The Wilcoxon rank sum test or unpaired t test was used for analysis of continuous data. Poisson approximation was used to calculate 95% CIs for incidence rates and the F-distribution to calculate 95% CIs for incidence ratios. Incidence of liver injury was analyzed using the Kaplan-Meier curve and the log-rank test. Predictors of incident liver injury were assessed using the univariable and multivariable proportional hazards model. Analysis of predictors of liver injury included randomized arm, age, gender, body mass index (kg/m2, classified as BMI <18.5 or ≥18.5), previous history of TB, previous history of extrapulmonary TB, WHO stage 4, log viral load, and hepatitis B surface antigenemia (HBsAg) status. Statistical analyses were done using SAS Enterprise Guide version 7.1. All statistical tests were conducted at a 5% level of significance.
RESULTS
Baseline Characteristics
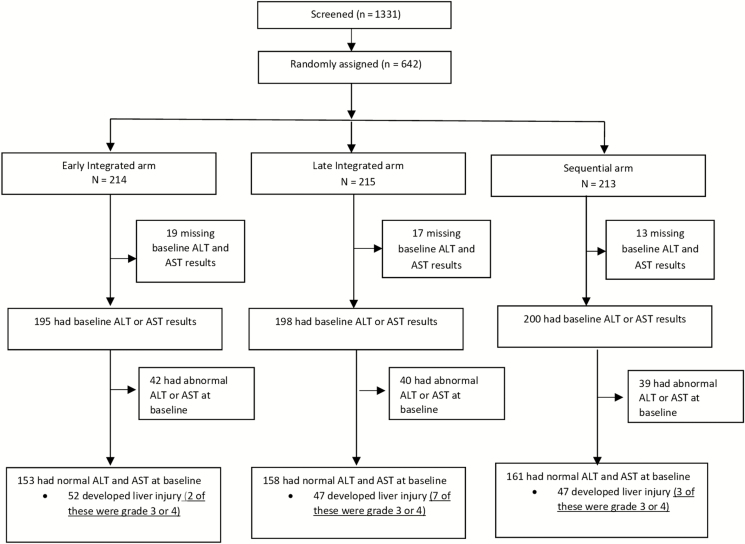
A total of 642 patients were enrolled and randomly assigned to 3 ART treatment arms (Figure 1). Forty-nine patients with missing baseline AST and ALT levels were subsequently excluded from the analysis. Of the 593 patients analyzed, 121 (20.4%) had elevated liver enzymes at baseline, of which 52.1% were male. Among patients with baseline raised transaminases, 34.7% had CD4+ cell counts <50 cells/mm3 compared to 12.9% among those with no liver injury (P < .001), 11.3% vs 7.6% were HBsAg positive (P = .237), and 21.5% vs 35.8% reported a previous history of TB (P = .002; Table 1).
Figure 1.
Study flow diagram. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Table 1.
Baseline Characteristics of Participants in the Starting Antiretroviral Therapy at Three Points in Tuberculosis Trial
| Variable | Early Integrated Arm (n = 214) | Late Integrated Arm (n = 215) | Sequential Arm (n = 213) | Patients With Liver Injury (n = 121) | Patients With No Liver Injury (n = 472) | P Value a |
|---|---|---|---|---|---|---|
| Mean age (SD), y | 34.3 (8.0) | 34.5 (8.7) | 33.9 (8.2) | 34.1 (8.3) | 34.2 (8.2) | .873 |
| Male, n (%) | 97 (45.3) | 112 (52.1) | 110 (51.6) | 63 (52.1) | 230 (48.7) | .542 |
| Body mass index <18.5 kg/m2, n (%)b | 25 (11.7) | 28 (13.0) | 29 (13.6) | 12 (9.9) | 63 (13.1) | .440 |
| History of TB, n (%) | 80 (37.4) | 68 (31.6) | 66 (31.0) | 26 (21.5) | 169 (35.8) | .002 |
| History of extrapulmonary TB, n (%)c | 10 (4.7) | 9 (4.2) | 9 (4.3) | 8 (6.6) | 18 (3.8) | .212 |
| World Health Organization stage 4, n %) | 14 (6.5) | 11 (5.1) | 13 (6.1) | 11 (9.1) | 24 (5.1) | .127 |
| Alcohol use, n (%) | ||||||
| Occasional | 24 (11.8) | 23 (11.0) | 28 (13.7) | 15 (12.8) | 53 (11.6) | .877 |
| Frequent | 6 (2.9) | 9 (4.3) | 9 (4.4) | 4 (3.4) | 20 (4.4) | |
| Patients with CD4+ count <50 cells/mm3, n (%) | 37 (17.3) | 35 (16.3) | 41 (19.2) | 42 (34.7) | 61 (12.9) | <.0001 |
| Median alanine aminotransferase (interquartile range), U/L | 21.0 (13–33) | 19.0 (13–29) | 17.0 (12–28) | … | … | … |
| Mean log10 human immunodeficiency virus RNA (SD), copies/mL d | 5.0 (0.9) | 5.0 (0.9) | 5.1 (0.7) | 5.1(0.9) | 5.0 (0.9) | .616 |
| Hepatitis B surface antigen positive, n (%)e | 15 (8.8) | 15 (8.1) | 13 (7.5) | 12 (11.3) | 30 (7.6) | .237 |
Abbreviations: SD, standard deviation; TB, tuberculosis.
a P value for the comparison of patients with liver enzyme abnormalities to those without.
bFive patients in the sequential treatment group had missing baseline body mass index data, which were not included in the percentage calculation.
cAmong these patients, none had TB of the liver. One patient in the late arm and 3 patients in the sequential arm had missing extrapulmonary TB data, which were not included in the percentage calculation.
dBaseline viral load data were not available for 16 patients in the early arm, 16 in the late arm, and 12 in the sequential arm.
eThe hepatitis B surface antigen status was missing for 25, 12, and 27 patients in early, late, and sequential arm, respectively.
Prevalence and Incidence of Liver Injury
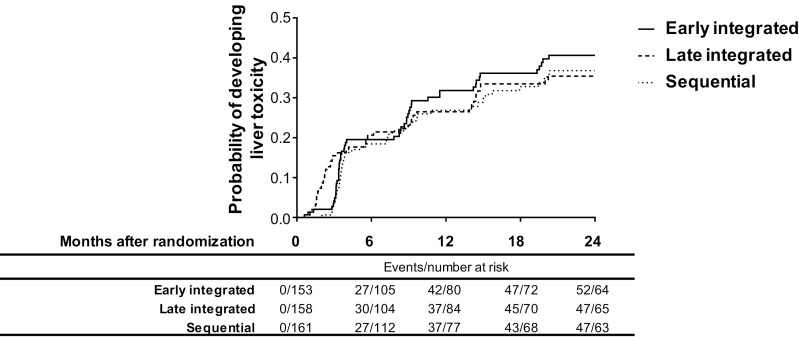
Of the 593 patients analyzed, 121 (20.4%) presented with liver injury at the baseline visit, with 17% (7/42), 30% (12/40), and 21% (8/39) of patients developing worsening of preexisting liver injury in the early integrated, late integrated, and sequential treatment arms, respectively (Figure 1). Among those with normal baseline liver enzymes, 34%, 30%, and 29% developed new onset liver injury during study follow-up in the early integrated, late integrated, and sequential treatment arms, respectively (Figure 1). There was no overall significant difference in the occurrence of liver injury across the 3 treatment arms for the duration of follow-up (Figure 2).
Figure 2.
Kaplan-Meier estimates of cumulative probability of developing liver toxicity by study.
Incidence of Liver Injury Stratified by CD4+ Cell Count Pre- and Post-ART Initiation
In the subset of patients with CD4+ cell counts <200 cells/mm3, the highest incidence rate of pre-ART initiation liver injury (grades 1–4) of 50.3 (95% CI, 28.1–82.9) per 100 person-years occurred in the late integrated treatment arm (Table 2). Overall, similarly high liver injury incidence rates were observed in this subset of patients across all treatment arms, irrespective of whether ART was initiated or not. A similar incident rate was observed when stratified by CD4+ cell counts ≥50 cells/mm3 and <50 cells/mm3 (data not shown)
Table 2.
Incidence Rate of Developing New Liver Injury Pre- and Post-antiretroviral Therapy Initiation, Stratified by CD4+ Cell Count
| Early Integrated Arm (n = 153) | Late Integrated Arm (n = 158) | Sequential Arm (n = 161) | Early Integrated vs Late Integrated | Early Integrated vs Sequential | Late Integrated vs Sequential | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Events | Person-years | Incidence Rate per 100 Person- years (95% CI) | Events | Person- years | Incidence Rate per 100 Person-years (95% CI) | Events | Person- years | Incidence Rate per 100 Person-years (95% CI) | IRR (95% CI); P Value | IRR (95% CI); P Value | IRR (95% CI); P Value | |
| Grades 1 to 4 incident cases | ||||||||||||
| CD4+ cell count <200 cells/mm 3 | ||||||||||||
| Pre-ART liver injury | 3 | 7.58 | 39.6 (8.2–115.7) | 15 | 29.83 | 50.3 (28.1–82.9) | 16 | 53.84 | 29.7 (17.0–48.3) | 0.79 (.23–2.73); .709 | 1.33 (.39–4.56); .650 | 1.69 (.84–3.42); .144 |
| Post- ART liver injury | 27 | 98.62 | 27.4 (18.0–39.8) | 16 | 83.99 | 19.0 (10.9–30.9) | 10 | 54.44 | 18.4 (8.8–33.8) | 1.44 (.78–2.67); .248 | 1.49 (.72–3.08); .281 | 1.04 (.47–2.29); .922 |
| Subtotal | 30 | 106.19 | 28.3 (19.1–40.3) | 31 | 113.82 | 27.2 (18.5–38.7) | 26 | 108.28 | 24.0 (15.7–35.2) | 1.04 (.63–1.72); .878 | 1.18 (.7–2.00); .537 | 1.13 (.67–1.90); .646 |
| CD4+ cell count ≥200 cells/mm 3 | ||||||||||||
| Pre-ART liver injury | 0 | 5.94 | … | 10 | 17.52 | 57.1 (27.4–105.0) | 11 | 39.1 | 28.1 (14.0–50.3) | … | … | 2.03 (.86–4.78); .105 |
| Post- ART liver injury | 22 | 68.61 | 32.1 (20.1–48.5) | 6 | 50.72 | 11.8 (4.3–25.7) | 10 | 35.47 | 28.2 (13.5–51.9) | 2.71 (1.1–6.68); .030 | 1.14 (.54–2.41); .731 | 0.42 (.15–1.16); .093 |
| Subtotal | 22 | 74.56 | 29.5 (18.5–44.7) | 16 | 68.24 | 23.4 (13.4–38.1) | 21 | 74.56 | 28.2 (17.4–43.1) | 1.26 (.66–2.4); .482 | 1.05 (.58–1.91); .873 | 0.83 (.43–1.59); .574 |
| Overall incidence | 52 | 180.75 | 28.8 (21.5–37.7) | 47 | 182.06 | 25.8 (19.0–34.3) | 47 | 182.85 | 25.7 (18.9–34.2) | 1.11 (.75–1.65); .604 | 1.12 (.75–1.66); .573 | 1.00 (.67–1.5); 1.000 |
| Grades 3 and 4 incident cases | ||||||||||||
| Pre- ART liver injury | 2 | 139.96 | 1.4 (.2–5.2) | 5 | 151.13 | 3.3 (1.1–7.7) | 1 | 139.21 | 0.7 (0–4.0) | 0.43 (.08–2.22); .313 | 1.99 (.18–21.95); .574 | 4.61 (.54–39.46); .163 |
| Post- ART liver injury | 0 | 101.65 | … | 2 | 88.02 | 2.3 (.3–8.2) | 2 | 97.64 | 2.0 (.2–7.4) | … | … | 1.11 (.16–7.88); .917 |
| Overall incidence | 2 | 241.61 | 0.8 (.1–3.0) | 7 | 239.16 | 2.9 (1.2–6.0) | 3 | 236.84 | 1.3 (.3–3.7) | 0.28 (.06–1.35); .112 | 0.65 (.11–3.89); .637 | 2.31 (.6–8.93); .225 |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; IRR, incidence rate ratio.
Post-ART initiation incidence of liver injury (grades 1–4) in the subset of patients with CD4+ cell counts >200 cells/mm3 was 32.1 (95% CI, 20.1–48.5), 11.8 (95% CI, 4.3–25.7), and 28.2 (95% CI, 13.5–51.9) per 100 person-years of follow-up in the early integrated, late integrated, and sequential treatment arms, respectively (Table 2). Patients in the early integrated arm had a greater than 2-fold incidence rate of liver injury compared to the late integrated arm (incidence rate ratio [IRR], 2.71; 95% CI, 1.1–6.68; P = .030; Table 2). The IRR of the early integrated arm vs the sequential arm was 1.14 (95% CI, 0.54–2.41; P = .731) and the IRR of the late integrated arm vs the sequential arm was 0.42 (95% CI, 0.15–1.16; P = .093; Table 2). Notably fewer events of liver injury were noted pre-ART initiation in this subset of patients.
The highest incidence rates of worsening of preexisting liver injury of 27.4 cases per 100 person-years (95% CI, 14.1–47.8) was observed in the late integrated arm (data not shown). There was no statistically significant difference in the IRR of liver injury between the arms (data not shown). It is noteworthy that the IRR of 0.44 (0.17–1.12; P = .084) comparing occurrence of liver injury between the early and late integrated treatment arms, while not statistically significant, may be of clinical relevance.
Severe Liver Injury and Adverse Events
Severe or life-threatening liver injury (≥ grade 3), including both new onset and worsening events, occurred post-baseline in 2 (8.2 and 10.4 months postenrollment), 7 (interquartile range, 2.1–15.2 months postenrollment), and 3 (8.7, 14.7, and 19.7 months postenrollment) of the early integrated, late integrated, and sequential treatment arm patients, respectively.
Predictors of Liver Injury
Significant predictors of liver injury were age and HBsAg positivity. For every 5-year increase in patient age, the risk of liver injury increased by 21% (hazard ratio [HR], 1.19; 95% CI, 1.05–1.33; P = .005; Table 3). Patients with a positive Hep BSAg at baseline had a 2-fold risk of developing liver injury (HR, 1.79; 95% CI, 1.03–3.13; P = .04; Table 3) compared to HBsAg-negative patients at baseline. Importantly, on reanalysis of the data, including abnormal baseline liver injury cases, alcohol use and male gender were found to be statistically insignificant.
Table 3.
Baseline Risk Factors for Incident Liver Injury in Tuberculosis Patients Receiving Antiretroviral Therapy
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| Exposure Variable | HRa | P Value | aHRb | P Value |
| Treatment arm | ||||
| Sequential | Reference | … | Reference | … |
| Early integrated | 1.14 (0.77–1.69) | .526 | 1.19 (0.75–1.89) | .464 |
| Late integrated | 1.04 (0.69–1.56) | .855 | 1.17 (0.73–1.86) | .519 |
| Age (per 5-year increase) | 1.14 (1.04–1.24) | .006 | 1.19 (1.05–1.33) | .005 |
| Gender | ||||
| Female | Reference | … | Reference | … |
| Male | 1.43 (1.03–1.98) | .033 | 1.20 (0.79–1.82) | .399 |
| Body mass index, kg/m2 | ||||
| ≥18.5 | Reference | … | Reference | … |
| <18.5 | 0.90 (0.54–1.52) | .696 | 0.86 (0.49–1.50) | .592 |
| History of TB | ||||
| No | Reference | … | Reference | … |
| Yes | 1.00 (0.71–1.41) | .995 | 1.00 (0.67–1.48) | .996 |
| History of extrapulmonary TB | ||||
| No | Reference | … | Reference | … |
| Yes | 0.85 (0.35–2.07) | .721 | 0.68 (0.06–7.86) | .757 |
| World Health Organization stage | ||||
| 3 | Reference | … | Reference | … |
| 4 | 0.96 (0.45–2.04) | .908 | 0.68 (0.09–5.13) | .713 |
| Alcohol use | ||||
| Never used alcohol | Reference | … | Reference | … |
| History of alcohol use | 1.49 (0.99–2.25) | .059 | 1.34 (0.81–2.21) | .259 |
| CD4+ count (per 50 cells/mm3) | 0.99 (0.93–1.06) | .817 | 0.98 (0.90–1.06) | .538 |
| Hepatitis B surface antigen | ||||
| Negative | Reference | … | Reference | … |
| Positive | 1.79 (1.03–3.13) | .04 | 1.65 (0.88–3.07) | .116 |
Abbreviations: aHR, adjusted hazard ratio; HR, hazard ratio; TB, tuberculosis.
aRefers to hazard ratio (HR) as indicated in the footer.
bRefers to aHR-adjusted hazard ratio as indicated in the footer.
Resolution of Liver Injury
During study follow-up, we observed that liver injury resolved to normal in two-thirds (31 in each study arm) of all patients (Supplementary Table 1). At the end of study follow-up, the sequential treatment arm had the highest number of unresolved worsened liver injury cases. Among patients with severe grades of liver injury, none resolved in the early arm, whereas severe liver injury resolution occurred in 2 late integrated patients and 1 sequential arm patient.
DISCUSSION
Our study demonstrates excessively high incidence rates of liver injury among HIV–TB coinfected patients irrespective of timing of ART in TB therapy. Almost one-third of all patients experienced liver injury during study follow-up. Importantly, the onset of liver injury occurred several weeks to months after ART start. This has important implications as current guidelines for TB, HIV, and HIV–TB cotreatment are silent regarding frequency of liver safety monitoring in coinfected patients receiving concomitant ART during or post-TB treatment completion [14, 15], while also ignoring liver safety monitoring in asymptomatic patients. Prospective evaluation of liver injury in other multicountry randomized trials conducted in coinfected patients receiving early or deferred ART in TB therapy show that while liver injury accounted for the vast majority of reported study-related adverse events, there was no significant difference in occurrence of liver injury by randomized arms [16–18]. A randomized trial investigating safety and efficacy of an efavirenz-based ART regimen in Ethiopia showed no difference in incidence rates or severity of liver injury in HIV–TB coinfected patients initiated at 1 week, 4 weeks, or 8 weeks after TB treatment start [19].
Among post-ART initiation patients with CD4+ cell counts <200 cells/mm3, rates of liver injury were not significantly different between the arms. Patients with CD4+ cell counts ≥200 cells/mm3, however, demonstrated significantly different rates of liver injury post-ART initiation, with the early and sequential treatment arms experiencing more than a 2-fold higher incidence rate of liver injury compared to the late treatment arm. Our findings of higher rates of liver injury among patients receiving concurrent ART and 4-drug TB therapy concur with literature reports that show up to a 10-fold increased risk in liver injury among patients cotreated for HIV and TB [9]. Furthermore, studies have demonstrated that up to 25% of cotreated patients develop liver injury, with approximately 50% developing mild liver injury [7]. Additionally, early hepatic monitoring in the first 2 months of TB therapy was shown to detect about 75% of moderately elevated liver enzymes [11], highlighting the need for close monitoring of liver function tests during intensive TB therapy.
The highest incidence rate of liver injury was observed among pre-ART initiation patients already receiving anti-TB therapy, with CD4+ cell counts <200 cells/mm3, who were randomized to the late treatment arm. This rate was almost 2-fold higher than for sequential treatment arm patients. Isoniazid, rifampicin, and pyrazinamide are all known liver toxic drugs [20], with risk of liver injury known to be 2-fold higher in HIV–TB coinfected patients receiving TB therapy in the absence of ART [21, 22]. We found high levels of liver injury attributable to TB treatment only among ART naive patients. It is noteworthy that other cohorts and randomized studies also show high rates of liver injury prior to ART start in patients receiving anti-TB therapy only [9, 10, 17, 23–25]. Importantly, the frequency of TB treatment–associated liver injury observed in the pre-ART period appeared to increase with longer delays to ART start. Patients with CD4+ cell counts ≥200 mm3 randomized to the early integrated and sequential treatment arms experienced higher rates of liver injury post-ART initiation. This highlights the significant role of TB and ART in the development of liver injury [9, 10]. This finding also demonstrates the direct role of HIV infection in hepatic injury, with almost 80% of individuals living with HIV having abnormal liver function tests [26].
Patients with CD4+ cell counts ≥200 mm3 experienced higher incidence rates of liver injury in the early integrated treatment arm. These findings have important implications in the context of the new WHO HIV test and treat policy in coinfected patients who are administered ART in the absence of liver function monitoring. The number of patients who developed severe liver injury in this study was very small, with no patient requiring treatment interruption and rechallenge. It is likely that our inclusion of clinically stable ambulant patients in this study accounted for the low rates of severe liver injury observed in this cohort.
Males in our cohort were at higher risk of liver injury when data analysis included cases with abnormal baseline liver enzymes. This contrasts literature that reported biological variations in drug pharmacokinetics, including slow drug acetylation in women, that contribute to a higher risk of DILI [27, 28]. It is important to note that concurrent alcohol abuse is more frequent among males in our population, likely contributing to the higher risk of liver injury observed. Other risk factors for liver injury in this cohort were similar to other published findings [2, 4, 6, 29, 30]. Despite high rates of injury in this cohort, irrespective of study arm, most patients had complete and spontaneous resolution of liver injury. Severe or life-threatening liver injury was experienced in only 4% of patients, and 13% of patients exited the study with unresolved liver injury. Approximately 20% of asymptomatic study patients developed elevated transaminases following TB therapy initiation that resolved spontaneously. Immune suppressed patients were less likely to resolve their liver injury.
We acknowledge several limitations to this study. Data on the use of traditional remedies and over-the-counter drugs, which may have contributed to liver injury, were not collected. More frequent monitoring of liver enzymes may have provided a more nuanced understanding of liver enzyme perturbations related to ART and TB drug cotreatment. Liver biopsy, histology, and ultrasonography may have added to our understanding as to the true nature and cause of liver injury; however, this is not standard of care.
CONCLUSIONS
Patients on concurrent HIV and TB therapy demonstrated a high incidence of liver injury, especially when initiating ART during the intensive phase of TB therapy. This occurred in the context of an ambulant, clinically stable, coinfected population who were receiving a liver-friendly ART regimen. Careful, close liver function monitoring in HIV–TB coinfected patients, specifically during initiation of ART early in TB treatment, is necessary for the prompt detection and management of DILI. Clinical guidelines and policies for HIV–TB coinfected patients must address frequency of liver function monitoring, guidelines for further investigation, and up-referral of patients with liver injury that is suitable for the task-shifted model of care routinely provided in HIV–TB endemic settings.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the patients for their participation in this study.
Financial support. This work was supported by the US President’s Emergency Plan for AIDS Relief; the Global Fund to Fight AIDS, Tuberculosis, and Malaria; and the National Institutes of Health Comprehensive International Program of Research on AIDS (UM1AI069469). K. N. and N. P. were supported by the Columbia University–South Africa Fogarty AIDS International Training and Research Program (grant D43 TW000231).
Potential conflicts of interest. All authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global Tuberculosis report. 2018. Geneva, Switzerland: WHO. [Google Scholar]
- 2. Jong E, Conradie F, Black A, Menezes C, John M, Meintjes G. Consensus statement: management of drug-induced liver injury in HIV-positive patients treated for TB: guideline. South Afr J HIV Med 2013; 14:113–9. [Google Scholar]
- 3. Montessori V, Press N, Harris M, Akagi L, Montaner JS. Adverse effects of antiretroviral therapy for HIV infection. CMAJ 2004; 170:229–38. [PMC free article] [PubMed] [Google Scholar]
- 4. Hoffmann CJ, Charalambous S, Thio CL, et al. Hepatotoxicity in an African antiretroviral therapy cohort: the effect of tuberculosis and hepatitis B. AIDS 2007; 21:1301–8. [DOI] [PubMed] [Google Scholar]
- 5. Perriëns JH, St Louis ME, Mukadi YB, et al. Pulmonary tuberculosis in HIV-infected patients in Zaire. A controlled trial of treatment for either 6 or 12 months. N Engl J Med 1995; 332:779–84. [DOI] [PubMed] [Google Scholar]
- 6. Tostmann A, Boeree MJ, Aarnoutse RE, de Lange WC, van der Ven AJ, Dekhuijzen R. Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J Gastroenterol Hepatol 2008; 23:192–202. [DOI] [PubMed] [Google Scholar]
- 7. Ikuabe PO, Ebuenyi ID, Harry TC. Limited elevations in antituberculosis drug-induced serum alanine aminotransferase (ALT) levels in a cohort of Nigerians on treatment for pulmonary tuberculosis and HIV infection in Yenagoa. Niger J Med 2015; 24:103–7. [PubMed] [Google Scholar]
- 8. Shu CC, Lee CH, Lee MC, Wang JY, Yu CJ, Lee LN. Hepatotoxicity due to first-line anti-tuberculosis drugs: a five-year experience in a Taiwan medical centre. Int J Tuberc Lung Dis 2013; 17:934–9. [DOI] [PubMed] [Google Scholar]
- 9. Yimer G, Gry M, Amogne W, et al. Evaluation of patterns of liver toxicity in patients on antiretroviral and anti-tuberculosis drugs: a prospective four arm observational study in Ethiopian patients. PLoS One 2014; 9:e94271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schutz C, Ismail Z, Proxenos CJ, et al. Burden of antituberculosis and antiretroviral drug-induced liver injury at a secondary hospital in South Africa. S Afr Med J 2012; 102:507–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tweed CD, Wills GH, Crook AM, et al. Liver toxicity associated with tuberculosis chemotherapy in the REMoxTB study. BMC Med 2018; 16:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abdool Karim SS, Naidoo K, Grobler A, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med 2011; 365:1492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med 2010; 362:697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organization. WHO TB treatment guidelines. 2010. Geneva, Switzerland: WHO. [Google Scholar]
- 15. World Health Organization. Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. 2014. Geneva, Switzerland: WHO. [PubMed] [Google Scholar]
- 16. Mfinanga SG, Kirenga BJ, Chanda DM, et al. Early versus delayed initiation of highly active antiretroviral therapy for HIV-positive adults with newly diagnosed pulmonary tuberculosis (TB-HAART): a prospective, international, randomised, placebo-controlled trial. Lancet Infect Dis 2014; 14:563–71. [DOI] [PubMed] [Google Scholar]
- 17. Havlir DV, Kendall MA, Ive P, et al. ; AIDS Clinical Trials Group Study A5221 Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med 2011; 365:1482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blanc FX, Sok T, Laureillard D, et al. ; CAMELIA (ANRS 1295–CIPRA KH001) Study Team Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med 2011; 365:1471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Amogne W, Aderaye G, Habtewold A, et al. Efficacy and safety of antiretroviral therapy initiated one week after tuberculosis therapy in patients with CD4 counts <200 cells/μL: TB-HAART study, a randomized clinical trial. PLoS One 2015; 10:e0122587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Puri P, Kaur N, Pathania S, Kumar S, Sharma PK, Sashindran VK. Antitubercular therapy induced liver function tests abnormalities in human immunodeficiency virus infected individuals. Med J Armed Forces India 2017; 73:12–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marks DJ, Dheda K, Dawson R, Ainslie G, Miller RF. Adverse events to antituberculosis therapy: influence of HIV and antiretroviral drugs. Int J STD AIDS 2009; 20:339–45. [DOI] [PubMed] [Google Scholar]
- 22. Breen RA, Miller RF, Gorsuch T, et al. Adverse events and treatment interruption in tuberculosis patients with and without HIV co-infection. Thorax 2006; 61:791–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Girardi E, Palmieri F, Cingolani A, et al. Changing clinical presentation and survival in HIV-associated tuberculosis after highly active antiretroviral therapy. J Acquir Immune Defic Syndr (1999). 2001; 26:326–31. [DOI] [PubMed] [Google Scholar]
- 24. Wit FW, Weverling GJ, Weel J, Jurriaans S, Lange JM. Incidence of and risk factors for severe hepatotoxicity associated with antiretroviral combination therapy. J Infect Dis 2002; 186:23–31. [DOI] [PubMed] [Google Scholar]
- 25. Spengler U, Lichterfeld M, Rockstroh JK. Antiretroviral drug toxicity–a challenge for the hepatologist? J Hepatol 2002; 36:283–94. [DOI] [PubMed] [Google Scholar]
- 26. Joshi KS, Shriwastav RR. Highly active antiretroviral therapy and changing spectrum of liver diseases in HIV infected patients. Int J Res Med Sc 2017; 4:3125–9. [Google Scholar]
- 27. Chughlay M, Blockman M, Cohen K. A clinical approach to drug-induced liver injury. Curr Allergy Clin Immunol 2015; 28:252–6. [Google Scholar]
- 28. Shakya R, Rao BS, Shrestha B. Incidence of hepatotoxicity due to antitubercular medicines and assessment of risk factors. Ann Pharmacother 2004; 38:1074–9. [DOI] [PubMed] [Google Scholar]
- 29. Singla R, Sharma SK, Mohan A, et al. Evaluation of risk factors for antituberculosis treatment induced hepatotoxicity. Indian J Med Res 2010; 132:81–6. [PubMed] [Google Scholar]
- 30. Pukenyte E, Lescure FX, Rey D, et al. Incidence of and risk factors for severe liver toxicity in HIV-infected patients on anti-tuberculosis treatment. Int J Tuberc Lung Dis 2007; 11:78–84. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.