Abstract
Precise manipulation of gene expression with temporal and spatial control is essential for functional analysis and determining cell lineage relationships in complex biological systems. The Cre-loxP system is commonly used for gene manipulation at desired times and places. However, specificity is dependent on the availability of tissue- or cell-specific regulatory elements used in combination with Cre. Here we present CreLite, an optogenetically-controlled Cre system using red light in developing zebrafish embryos. Cre activity is disabled by splitting Cre and fusing with the Arabidopsis thaliana red light-inducible binding partners, PhyB and PIF6. Upon red light illumination, the PhyB-CreC and PIF6-CreN fusion proteins come together in the presence of the cofactor phycocyanobilin (PCB) to restore Cre activity. Red light exposure of zebrafish embryos harboring a Cre-dependent multi-color fluorescent protein reporter injected with CreLite mRNAs and PCB resulted in Cre activity as measured by the generation of multi-spectral cell labeling in several different tissues. Our data show that CreLite can be used for gene manipulations in whole embryos or small groups of cells at different developmental stages, and suggests CreLite may also be useful for temporal and spatial control of gene expression in cell culture, ex vivo organ culture, and other animal models.
INTRODUCTION
The study of gene function and cellular lineage relationships in rapidly changing environments such as the developing embryo, regeneration of new tissue after injury and changes during carcinogenesis requires accurate spatial and temporal control of genome manipulation. Cyclic recombinase (Cre) originates from bacteriophage P1 and has been applied to specifically target defined loxP sites to manipulate the genome at a specific time and place 1,2. The versatility of the Cre/loxP system has been broadly applied for conditional control of genome manipulation, yet specificity is currently dependent on the availability of tissue- or cell-specific regulatory elements used in combination with Cre or drug-inducible Cre systems. Cell or tissue types without known regulatory elements at desired stage of development or regeneration, or those sensitive to drug treatment, will be difficult to study.
Optogenetic approaches have recently emerged as a new methodology that allows both temporal and spatial control of biological processes in a precise manner. Phytochrome-like proteins and their binding partners found in the plant Arabidopsis thaliana and bacterium Rhodopseudomonos palustris have provided a wide spectrum of light-inducible protein binding pairs for possible optogenetic use, including blue light (Cry2 and CIB), red light (PhyB and PIF), and near-infrared light (BphP1 and PpsR2) 3–5. Among these light-inducible binding pairs, blue-light based systems have wide applications in cell culture or superficial tissues. However, the red-light induced PhyB and PIF, is preferable as a light sensor for optogenetic manipulation in light-sensitive embryonic tissue or deep tissues due to the low photo-toxicity and great tissue penetrance of red light. As a result, we designed a new red-light inducible Cre/loxP system, we call the CreLite system, to provide precise spatial and temporal control of genetic manipulation in living tissues.
The CreLite system consists of two parts, the light sensing module and the recombinase module. The light-sensing module is the red-light inducible binding pair, PhyB and PIF6. In Arabidopsis, PhyB binds to PIF6 in the presence of its prosthetic group, phytochromobilin (PΦB), in response to red light (~650 nm). These proteins then act as co-transcriptional activators of target genes. PhyB and PIF family members have been successfully engineered to create light-controlled gene expression systems 5–9. In the recombinase module, Cre is split into N- and C-terminal regions that separately lack Cre activity. However, when these regions are physically brought together, Cre activity is restored 10. Therefore, we created fusion proteins of PhyB and PIF6 with the split Cre complementation system, PhyBCreC and PFI6CreN. Notably, the requirement of prosthetic group PΦB in this system provides more control of this system. Practically, PΦB is usually substituted by its analog phycocyanbilin (PCB) due to its broad availability. In the absence of PCB and red light, PhyBCreC and PIF6CreN will not bind each other and thus there will be no Cre activity. However, in the presence of PCB and red light, PhyBCreC and PIF6CreN will bind each other, leading to Cre activity.
We tested the function of the CreLite system in zebrafish. Red-light exposure of transgenic zebrafish embryos harboring a multi-color fluorescent Cre reporter (ubi:zebrabow) and injected with CreLite mRNAs and PCB resulted in Cre activity and the generation of multi-spectral cell labeling in various tissues, including eyes, skeletal muscle and epithelium. Our results also show that CreLite can be used for activation of whole-embryos or small groups of cells at different times throughout development. All the associated plasmids for CreLite have been deposited in publically available databases and provide new tools for precise temporal and spatial control of gene expression in cell culture, ex vivo organ culture, and animal models for developmental biology studies.
RESULTS AND DISCUSSION
Design and functional test of the light inducible Cre (CreLite) system in zebrafish embryos
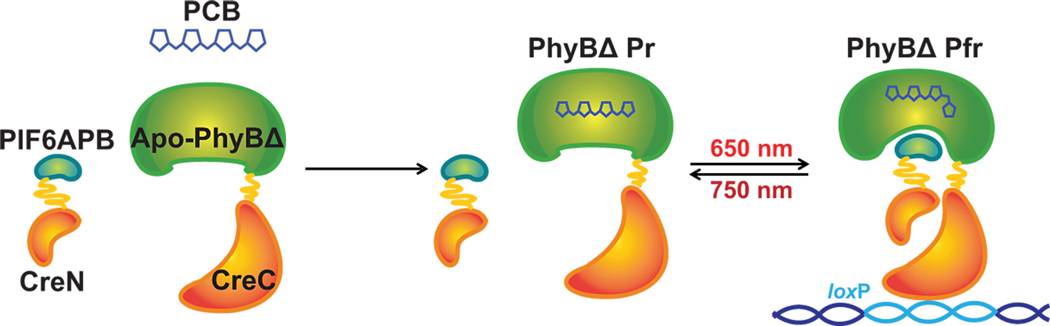
To create a light inducible Cre system, we split Cre into two fragments (N-terminus Cre and C terminus Cre) and fused them with the red-light inducible dimer, PhyB and PIF6 (Figure 1.). In this system, the split Cre should not show activity until exposed to red-light (around 650 nm) with PCB. Specifically, the synthetic fusion proteins consist of the photo-sensory module of phytochrome B (1–621 aa; NTE-PAS-GAF-PHY) 7 and the C-terminus Cre (60–343 aa), and the active protein binding (ABP) domain of PIF6 (1–100 aa) and N-terminus Cre (19–59 aa) 10, referred to as PhyBΔCreC and PIF6CreN (Figure 1). Both of the two fusion proteins have an N-terminal SV40 nuclear localization sequence (NLS) to ensure nuclear translocation. The term “CreLite” is used to represent PhyBΔCreC and PIF6CreN together.
Figure 1. The CreLite system.
The light inducible protein binding pair, truncated Phytochrome B (PhyBΔ) and the active protein binding domain of Phytochrome B interacting factor 6 (PIF6APB) are fused with C-terminus Cre (CreC) and N-terminus Cre (CreN), respectively. A cofactor analog, phycocyanobilin (PCB), is required for PhyBΔ to be functional. Once exposed to red light (λmax = 650 nm), the PhyBΔ and PIF6APB bind each other and bring the two halves of Cre together, resuming its recombinase activity.
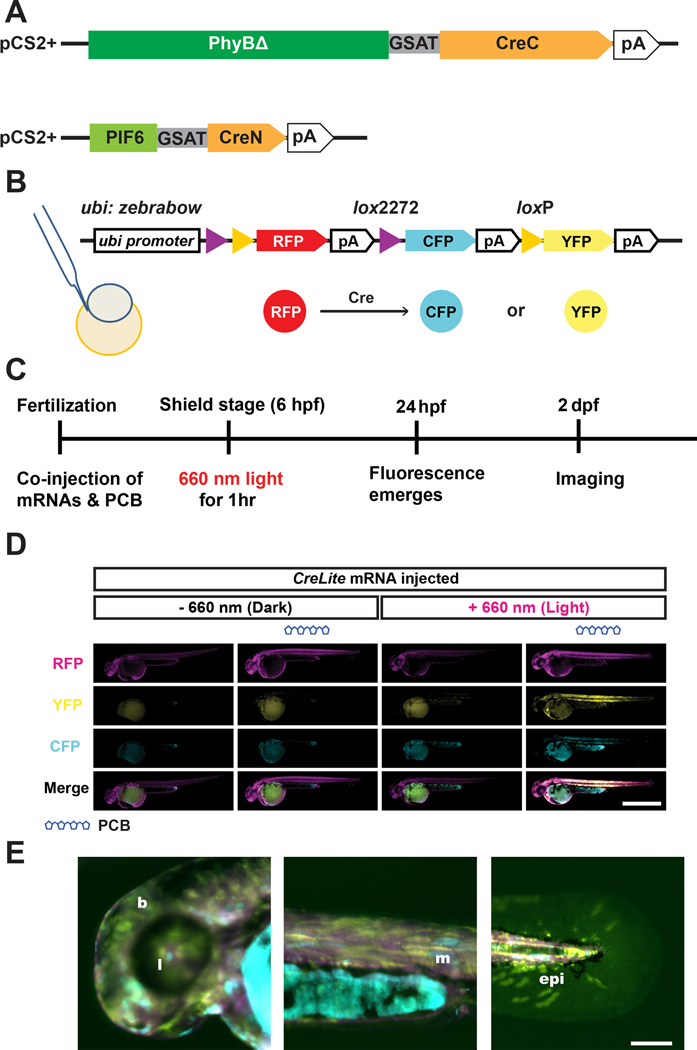
To test the function of CreLite in zebrafish, we injected in vitro transcribed 300 ng/μL PhyBΔCreC and 200 ng/μL PIF6APBCreN mRNAs along with 1.4 μM PCB into zebrafish embryos Tg(ubi:zebrabow-S)a13211, referred as ubi:zebrabow, at the one cell stage (Figure 2A–B). The ubi:zebrabow zebrafish ubiquitously expresses red fluorescent protein (RFP) but upon Cre-mediated recombination can express yellow fluorescent protein (YFP) or cyan fluorescent protein (CFP). Red-light exposure at ~6 hpf (hours post fertilization) of ubi:zebrabow injected with CreLite mRNAs and PCB resulted in Cre activity and generated a diverse color profiles in various tissues, including the lens of the eye, skeletal muscle and epidermis. We observed 56% of larvae harbored cells with fluorescence conversion from RFP to CFP or YFP (n = 89). Conversion of the fluorescent cells was mosaic, and varied among embryos, likely due to the uneven distribution of the injected components and variance in the protein expression 12. In contrast, injection mixtures lacking either the CreLite mRNAs or PCB, as well as those lacking exposure to red-light, showed little to no fluorescence conversion in 48 hpf embryos (Figure 2D). The resulting Cre reporter expression was found in both deep and superficial tissues, including the epithelia, eyes, muscle, or even the blood vessels (Figure 2E), suggesting the CreLite system could be used for conversion of diverse cell types. Further, we observed very little phototoxicity. The abnormality rate of red-light exposed embryos (9.32%; n = 195) is not significantly different from the abnormality rate of non-exposed embryos (9.73%; n = 129). The low conversion rate (20%) in the non-exposed embryos (Figure 3E) is likely due to exposure of ambient light or stochastic dimerization when high concentrations of CreLite proteins exist in cells.
Figure 2. CreLite-mediated recombination in zebrafish embryos.
PhyBΔCreC and PIF6CreN gene were subcloned into pCS2+ expression vector for in vitro transcription (A). The resulting CreLite mRNAs, along with the cofactor PCB, were microinjected into ubi:zebrabow zebrafish reporter line (B), which changes its color from red to either cyan and yellow depending on the Cre-mediated recombination event. Injected embryos were exposed to 660 nm red light for an hour at shield stage (C). Negative controls including no PCB and no light exposure were examined (D). Embryos with both PCB and red light exposure show broad recombination in the animal. Fluorescence was analyzed in different tissues throughout the embryos, while signal from the yolk or yolk extension was excluded due to potential autofluorescence; Scale bar = 1 mm. YFP signal of converted animals shows color conversion in various tissues (E), including brain and otic vesicle (b), lens (l), muscle (m), and epithelial (epi) cells; Scale bar = 100 μm.
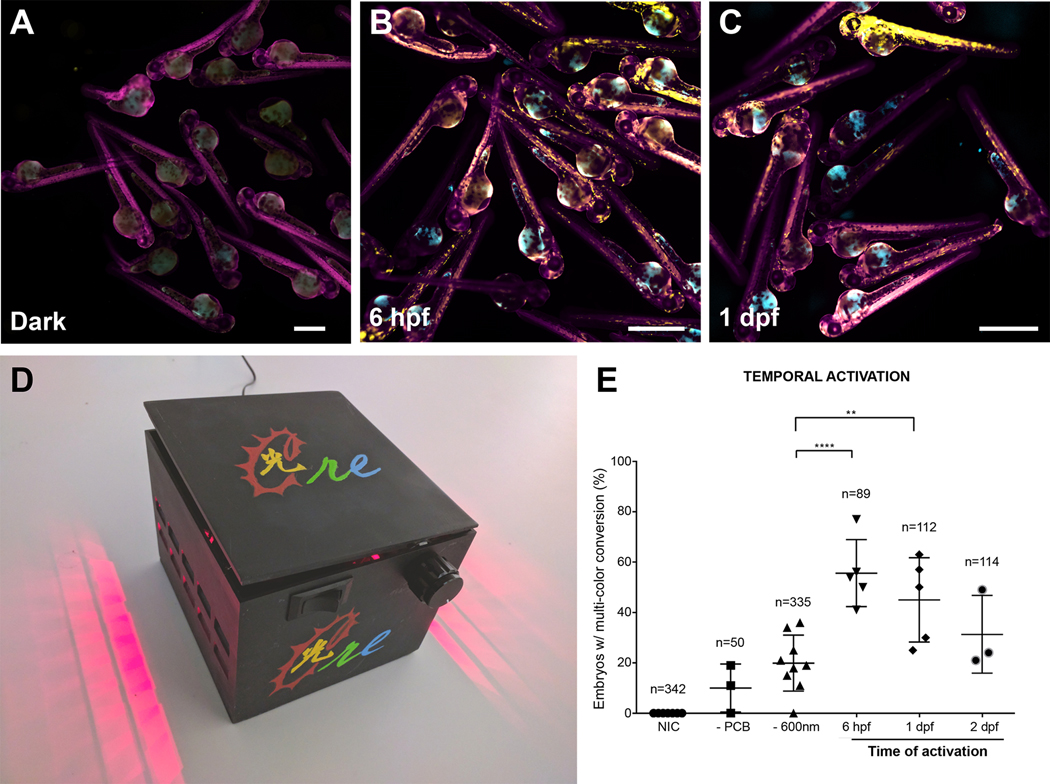
Figure 3. CreLite-mediated recombination at different times in development.
CreLite mRNAs injected ubi:zebrabow embryos were exposed to 660 nm red light in the LED lightbox (D) at different time points (i.e. 6 hpf, 1 dpf) (B and C). Embryos exposed at 6 hpf and 1 dpf were assessed at 2 dpf, while those exposed at 2 dpf were assessed at 3 dpf. Mean number of embryos containing cells that exhibit YFP and/or CFP fluorescence after red light exposure were counted (E). Data are from three independent experiments and error bars represent SD; **** p < 0.0001, ** p < 0.01. Ordinary one-way analysis of variance (ANOVA) with Dunnett’s multiple comparisons test. n = total number of embryos in each group. Each data point represents a clutch of embryos. Data from the negative controls (NIC, -PCB and – 660 nm) was pooled from experiments from the different time points. NIC: non-injected control. Scale bar = 1 mm.
Stochastic dimerization based on light-inducible dimers (LID), such as CRY-CIBN, BphP1-PpsR2, or GAVPO, may occur when using optogenetic tools. The percentage of stochastic events in the CreLite system is relatively comparable to the ~30% observed without light exposure in a previous study 13. The combination of PCB and truncated PhyB (1 – 650 a.a. or shorter variant) can create a more stable Pfr form that is less sensitive to far-red induced dissociation, i.e. thermal reversion 14. However, in vivo studies in zebrafish show successful reversion in epithelial cells with the short variant of PhyB (NTE-PAS-GAF-PHY) 7. As a result, we use the short variant of PhyB (1–621 aa; PhyBΔ) in the CreLite system for the following experiments in zebrafish.
Induced reporter gene expression at different developmental stages and at specific regions of interest
The CreLite system is designed to allow precise spatial and temporal control of gene expression, and therefore, we first examined induction of Cre activity at different time points after injection and exposure to red light. The ubi:zebrabow embryos were injected with CreLite mRNAs with PCB were exposed to red light for an hour at different stages of development, 6 hpf, 1 day post fertilization (dpf), and 2 dpf, and the fluorescence conversion (from RFP to CFP or YFP) was assessed at 1 day post exposure, (Figure 3 A–E). Exposure to red light at both 6 hpf and 1 dpf showed a high percent of embryos with fluorescence conversion of distinct cells in individual embryos (~50%). The percent of embryos with fluorescence conversion of distinct cells in individual embryos was reduced at 2 dpf (33%). This may be due to the gradual depletion of the injected mRNA as the embryos developed.
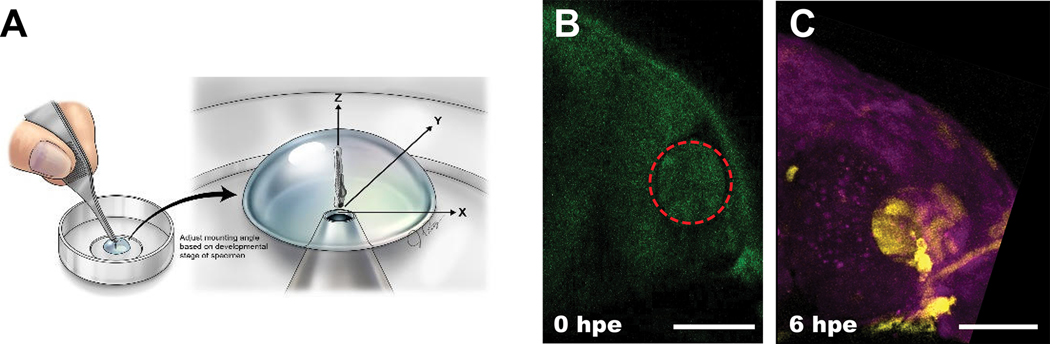
To test if the CreLite system can be controlled and activated at specific regions of interest in zebrafish, we performed activation on a region of interest (ROI) within the developing eye. We mounted the embryo with the anterior end facing the coverglass and objective, and a 50 μm diameter circle covering a portion of the eye was exposed to red light using a 640 nm laser at 5 mW/mm2 for 5 minutes (Figure 4A–B). The YFP signal at 6 hours post exposure (hpe) within the activated region suggests Cre activity was induced successfully at the region of interest, with little impact on surrounding tissues (Figure 4C). We found successful regional activation of the Cre reporter in 12% (n = 17 from 2 independent experiments) of all the exposed embryos. The distribution of the key components (PhyBΔCreC mRNA, PIF6CreN mRNA, and PCB) are likely not homogenous in the embryos, and therefore, and a lower conversion rate compared to the whole embryo is expected given the co-existence of these components may not always be achieved in the same cell. In a recent study, a comparable mosaic activation has also been shown 13. As a result, we suggest precise temporal and spatial control using CreLite is achievable. Of note, although we have observed stochastic activations in the control groups (Figure 3E), these numbers may not apply to the regional activation experiment since they represent the total number of embryos with mosaic distribution of YFP/CFP cells. In the future, generating a transgenic line carrying CreLite fusion protein genes will help to achieve more even distribution of the CreLite components in embryos and further improve the activation rate after light exposure.
Figure 4. Spatial and temporal recombination via CreLite.
Regional locations of the developing eye were activated by exposure to red-light at 1 dpf. CreLite mRNA and PCB injected ubi:zebrabow embryos were mounted rostrally (A) in LMP agarose. Regions of interest (red circle in B) were exposed to 640 nm laser (0 hpe). The color conversion was examined at 6 hpe (C); Scale bar = 50 μm.
Conclusions
Precise spatial and temporal manipulation of gene expression is key for studies of development and tissue regeneration in a wide variety of organisms. Classical methods such as using beads soaked with proteins such as signaling ligands like fibroblast growth factors or small molecules like retinoic acid allows functional studies 15. Yet, these methods are also limited to the studies of secreted molecules and physically placing the soaked beads in the tissue may introduce mechanical disruption or even damage the tissue, leading to undesired side effects. Optogenetic tools have facilitated better control of the expression of their gene of interest, including CRY-CIBN based PA-Cre and LACE systems 16–18, LOV based TAEL system 13,19–21, FKF/GI based system 22 and PhyB-PIF based system 6,9,23,24. Most of these tools achieve light-inducible gene expression by using viral trans-activator VP16 or VP64, which requires continuous light activation when prolonged gene expression is desired. Here, we successfully demonstrate a transient red-light inducible Cre/loxP system that allows light inducible genome manipulation and constant gene expression after induction. In combination with loxP alleles (e.g. loxP-stop-loxP, brainbow, MADM or FLEX), it is also possible to achieve switching of multiple genes with precise regional light induction for clonal analysis, lineage tracing, or to interrogate carcinogenesis using our system.
Notably, recent studies have demonstrated a CRY-CIBN based blue-light inducible Cre/loxP system in cell culture and Drosophila melanogaster 4,17,18,25. However, a red-light inducible system has several strengths for geneticists and developmental biologists. The red-light inducible system is intrinsically low photo-toxicity with a high tissue penetrance, as we found YFP or CFP signals in deep tissues (e.g. muscle and heart; Figure 2E) with very few abnormal embryos after prolonged red-light exposure (1 hour at 64 μW/mm2). While a longer exposure of lower energy red-light promoted activation of whole embryos, a short exposure of high-energy red-light was used for regional activation. The red-light induced recombination event is irreversible, and therefore, continuous light-induction is not required in our system. A red-light inducible system frees up more compatible imaging excitation wavelengths 26 and could allow blue light excitation for imaging of widely used GFP-tagged proteins. However, blue and green light have previously been shown to activate phytochrome heterodimerization 12,27, and further studies are required to assess and circumvent activation that may occur during live imaging.
Our current system has the caveat that it currently requires delivery of CreLite mRNA to induce gene expression at desired times and locations, and thus, is limited to early embryonic stages. Alternatively, this attribute allows the CreLite system to be readily used in different genetic backgrounds, in combination with transgenic lines, or even in other species in which introducing mRNA is feasible. The ratio of PhyBΔCreC and PIF6CreN mRNAs, and co-existence with the co-factor PCB, can vary among cells and injected embryos 12, and may require further optimization to achieve a higher conversion rate. Generation of stable lines harboring the PhyBΔCreC-2A-PIF6CreN transgene will help to mitigate these issues and have the added benefit of extending the usage of the CreLite system into adult zebrafish.
The co-factor PCB is required for the CreLite system to function. The delivery of PCB into adult or deep tissues may be a concern, and the additional component may not be ideal for cultured cells or other systems that require minimum manipulation with light. However, the latter issue can be addressed by introducing genes encoding enzymes for PCB synthesis (HO1 and PcyA) in the system, which has been shown previously in a cell culture system 23,28,29. For other ex vivo or in vivo systems, such as mammalian organ culture or later stages of zebrafish development, the cofactor as an additional component in this system is actually beneficial and allows an additional level of control in addition to keeping the experiment in the dark and/or working under green safe light.
As with other optogenetic tools that use a light-induced dimerization concept, we also observed some stochastic activation in our system. For future improvement, the reversible nature of PhyB-PIF6 system allows for application of far-red light (750 nm) to induce dissociation and minimize stochastic events 12. Alternatively, the two halves of dimers could be directed into different compartments in the cell to help minimize the stochastic activation, such as the red-light inducible (nuclear) translocation systems 6,9.
In conclusion, we demonstrate that the CreLite system is a promising red-light inducible Cre/loxP system. Our red-light inducible Cre/loxP system will be useful for control of gene expression in deep tissues and for live-imaging after Cre-mediated induction. This system also opens up the possibilities for more sophisticated genome manipulation that utilizes both red and blue light to dissect complex biological questions. CreLite provides a novel optogenetic tool for precise temporal and spatial control of gene expression in zebrafish embryos that may also be useful in cell culture, ex vivo organ culture, and other animal models for developmental biology studies.
EXPERIMENTAL PROCEDURES
Zebrafish
The zebrafish animal protocol was approved by the Animal Care and Use Committee of the University of Texas MD Anderson Cancer Center. The Tg(ubi:Zebrabow-S)a13211, referred as ubi:zebrabow zebrafish were maintained as both homozygotes and hemizygotes in a 28.5°C fish room with a circulating water system and a regular light/dark cycle of 14 hours light and 10 hours darkness.
Plasmids
The PIF6CreN fragment containing coding sequence (see supplementary information) was synthesized by GeneArt™ Strings™ DNA fragments service (Thermo Fisher Scientific Inc.) and cloned into pSC-B by StrataClone™ Blunt PCR Cloning Kit (Agilent Technologies, La Jolla, CA). To generate pUBC-PIF6iCreN transgene vector, the PIF6CreN fragment was subcloned into cHS2x-UBC-SH-RG-cHS2x 30 at restriction enzyme sites ApaI and XhoI. The PIF6CreN fragment was also amplified by primers SY118 and SY119 (Table S1.) and cloned into expression vector pCS2+ at EcoRI and XhoI sites for in vitro transcription.
The pCAGEN-PhyBCreC transgene vector was assembled by a four-way ligation. This ligation reaction combines NLS-PhyB (1–908 aa), GSAT linker, and CreC (60–343 aa) into a mammalian expression vector pCAGEN, which was a gift from Connie Cepko (Addgene plasmid # 11160 ; http://n2t.net/addgene:11160 ; RRID:Addgene_11160) 31. PhyB fragment was PCR amplified from pBS-PhyBCre with primers SY096 and SY097. CreC fragment was PCR amplified from pOG321 (Addgene plasmid # 17736) 32 by primers SY094 and SY095. The middle component containing GSAT linker sequence (Part:BBa_K404301, iGEM Registry of Standard Biological parts) between PhyB and CreC was synthesized by GeneArt™ Strings™ DNA fragments service (Thermo Fisher Scientific Inc.) and cloned into pSC-B by StrataClone™ Blunt PCR Cloning Kit (Agilent Technologies, La Jolla, CA). EcoRV, SphI, SexAI, and NotI (from 5’ to 3’) restriction sites were used to link all three fragments together into pCAGEN. The resulting PhyBCreC fragments were also subcloned into pCS2+ for in vitro transcription. We initially constructed the fusion protein with the long form PhyB (1–908 aa; NTE-PAS-GAF-PHY-PAS-PAS) instead of PhyBΔ (1–621 aa; NTE-PAS-GAF-PHY) in our system. However, a previous study has shown limited fusion protein expression in zebrafish when the long form PhyB (which includes two additional PAS domains and plant nuclear localization sequence) is used 7. As a result, we modified our construct and replaced PhyBCreC by PhyBΔCreC in our following experiments. The pCS2+ PhyBΔCreC construct was made by replacing middle part of the sequence of PhyBCreC (between NcoI and NruI) by a 230 bp fragment (Supplementary data) synthesized by GENEWIZ, resulting the short form of PhyB, PhyBΔ (1–621 aa), without PAS-A and PAS-B domains 7.
Zebrafish embryo microinjection
PhyBΔCreC and PIF6CreN mRNAs were in vitro transcribed from pCS2+PIF6CreN (Addgene #131780) and pCS2+ PhyBΔCreC (Addgene #131781) by mMESSAGE mMACHINE® SP6 transcription kit (Ambion). The capped mRNA was purified by alcohol precipitation and resuspended in DEPC-treated water. The microinjection of zebrafish embryos performed as described previously 33,34. Briefly, borosilicate glass capillaries with filaments (OD 1.2mm, ID 0.94, 10cm length; ITEM#: BF120–94-10) were pulled using a Sutter P-97 Pipette Puller to generate the injection needles. The ubi:zebrabow 11 zebrafish embryos were collected in E3 embryo medium and placed into a 10 cm dish with 3% agarose with troughs. The troughs were made by the microinjection mold (Adaptive Science Tools, # TU-1). Under a Leica S6D LED dissecting stereomicroscope, the mRNAs and 1.4 μM of phycocyanobilin (PCB) (SiChem GmbH, Bremen, Germany) in the KCl injection buffer were co-injected into ubi:zebrabow embryos at the 1~4 cell stage at desired concentrations using a pressure-controlled microinjector (WARNER INSTRUMENTS PLI-100A PICO-LITER Injector) and a NARISHIGE micro-manipulator. After injection, the embryos were cultured in E3 embryo medium without methylene blue in the dark at 28.5°C.
Photo-activation with 3D printed light box
Injected embryos along with non-injected control embryos were kept in dark before light exposure. For red light exposure at 1 dpf and 2 dpf, chorions are removed manually and an additional 30 min of soaking in PCB (7 μM) was performed under safe light (with an AT450/50x filter) prior to red light exposure. Red light exposure (64 μW/mm2) of 6 hpf, 1 dpf and 2 dpf zebrafish embryos was performed by using a 3D printed LED light box. The bottom of the illumination box can hold either a 100 mm petri dish or a 6-well plate, allowing large area activation. The color-conversion is assessed at 2 dpf for activation at 6 hpf and 1 dpf and at 3 dpf for activation at 2dpf. For each experiment, the un-injected control is used as a baseline threshold to calibrate the fluorescence display. Quantification of the color conversion events is based on the calibrated images. The box was designed using AutoDesk® 123D® Design software and converted to a printable file with scaffolds by Formlabs Preform print preparation software. The converted file was 3D printed by a Formlabs Form 2 printer with black photopolymer resin (FLGPBK03; Somerville, MA), and then trimmed and polished using sandpaper. Ventilation windows were included in the box design to allow air exchange and heat dissipation while activation. The red light LEDs (LED660N-03; Roithner LaserTechnik GmbH, Vienna, Austria) and 18 Ω metal film resistors (CCF0718R0GKE36; Vishay, Malvern, PA) were soldered together and connected with a potentiometer (RV24BF-10–15R1-A1K-LA; Alpha, Taiwan) with a 0.87”D Black aluminum knob (405–4764; Eagle Plastic Devices) for adjusting the light intensity (0.98 μW/mm2 to 64 μW/mm2). A wall mount AC adaptor (WSU135–0620; Triad Magnetics, Perris, CA) is connected to the circuit through a DC power connector (163–4020; Kobiconn). The light power was measured using an X-Cite® Optical Power Measurement System (Excelitas Technologies Corp.).
Regional activation
PCB and CreLite mRNAs injected embryos were kept in dark and manually dechorionated at 1 dpf under a Leica S6E stereo microscope (on transmitted light adjustable mirror base) with a AT450/50x filter (29.5 mm in diameter) placing on the universal optic light guide. After removal of the chorion, embryos were soaked in 7 μM PCB in E3 buffer for 30 min at 28.5°C. Injection of PCB, followed by immersion in PCB, showed the highest activation rate for regional manipulation. The soaked embryos (~10–15 embryos) were then washed with E3 and mounted rostrally on a glass-bottom dish with 0.5% low-melting point agarose. The red-light activation is then done on a Zeiss LSM800 confocal microscope. Under a 20X lens (Zeiss Plan-Apochromat 20x, NA=0.8 WD=0.55), the lens region of the embryo was focus with background fluorescence signal excited by 488 nm laser. A 50 μm circle in diameter was selected for red-light activation under the bleach mode in ZEN software. A 5mW 640 nm laser was used for activation the region for 300 seconds (i.e., 5 min) at 100% laser power. After activation, the embryos were incubate in dark at 28.5°C and then examined by the same confocal microscope at 6 hpe at room temperature.
Phycocyanobilin (PCB) handling
PCB has to be handled with care. Previous study have shown that PCB is prone to oxidation and will change its color from dark blue to brown in cell culture media after 24 hours 24. In addition, methylene blue, a common antifungal additive in zebrafish medium, also a common indicator for redox reaction, may be a potential oxidant to eliminate the activity of PCB. Addition of an anti-oxidant or reducing agents like β-mercapto-ethanol may help to limit the oxidation of PCB in culture. Notably, Buckley et al. 7 has shown that the PCB from cyanobacterium Spirulina extraction without HPLC purification is toxic at the higher dose of PCB (10 μM). Therefore, an HPLC-grade PCB is recommended for use in zebrafish.
Statistical analysis
All the statistics were calculated by Prism 7 or 8 (GraphPad Software Inc.). An ordinary one-way ANOVA was performed and used Dunnett’s multiple comparison test to determine difference between groups.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. David Hawk from the Proteomics and Metabolomics Core of MD Anderson Cancer Center for helping the HPLC purification of Phycocyanobilin. We also thank Dr. Adriana Paulucci in the Microscope core of the Genetics Department at the MD Anderson Cancer Center and Jordan Pietz with MD Anderson Creative Communications group for assistance with the biomedical illustrations. This work was supported by National Institutes of Health (NIH) grant OD19764 and the Ben F. Love Endowment to R.R.B.; by the NIH training grant, HL007676, to J.C.C; and by the Cancer Prevention Institute of Texas, RR140077, National Institutes of General Medical Sciences, GM124043, and the Mark and Linda Quick Basic Science Award to G.T.E.
REFERENCES
- 1.Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. August 28 1997;237(3):752–7. 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- 2.Utomo AR, Nikitin AY, Lee WH. Temporal, spatial, and cell type-specific control of Cre-mediated DNA recombination in transgenic mice. Nat Biotechnol. November 1999;17(11):1091–6. 10.1038/15073. [DOI] [PubMed] [Google Scholar]
- 3.Kaberniuk AA, Shemetov AA, Verkhusha VV. A bacterial phytochrome-based optogenetic system controllable with near-infrared light. Nat Methods. July 2016;13(7):591–7. 10.1038/nmeth.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kennedy MJ, Hughes RM, Peteya LA, Schwartz JW, Ehlers MD, Tucker CL. Rapid blue-light-mediated induction of protein interactions in living cells. Nat Methods. December 2010;7(12):973–5. 10.1038/nmeth.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levskaya A, Weiner OD, Lim WA, Voigt CA. Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature. October 15 2009;461(7266):997–1001. 10.1038/nature08446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beyer HM, Juillot S, Herbst K, et al. Red Light-Regulated Reversible Nuclear Localization of Proteins in Mammalian Cells and Zebrafish. ACS Synth Biol. September 18 2015;4(9):951–8. 10.1021/acssynbio.5b00004. [DOI] [PubMed] [Google Scholar]
- 7.Buckley CE, Moore RE, Reade A, Goldberg AR, Weiner OD, Clarke JDW. Reversible Optogenetic Control of Subcellular Protein Localization in a Live Vertebrate Embryo. Dev Cell. January 11 2016;36(1):117–126. 10.1016/j.devcel.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller K, Engesser R, Metzger S, et al. A red/far-red light-responsive bi-stable toggle switch to control gene expression in mammalian cells. Nucleic Acids Res. April 2013;41(7):e77. 10.1093/nar/gkt002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noda N, Ozawa T. Light-controllable Transcription System by Nucleocytoplasmic Shuttling of a Truncated Phytochrome B. Photochem Photobiol. September 2018;94(5):1071–1076. 10.1111/php.12955. [DOI] [PubMed] [Google Scholar]
- 10.Jullien N, Sampieri F, Enjalbert A, Herman JP. Regulation of Cre recombinase by ligand-induced complementation of inactive fragments. Nucleic Acids Res. November 1 2003;31(21):e131. 10.1093/nar/gng131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan YA, Freundlich T, Weissman TA, et al. Zebrabow: multispectral cell labeling for cell tracing and lineage analysis in zebrafish. Development. July 2013;140(13):2835–46. 10.1242/dev.094631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buckley CE. Optogenetic Control of Subcellular Protein Location and Signaling in Vertebrate Embryos. Methods Mol Biol. 2019;1920:143–162. 10.1007/978-1-4939-9009-2_10. [DOI] [PubMed] [Google Scholar]
- 13.Reade A, Motta-Mena LB, Gardner KH, Stainier DY, Weiner OD, Woo S. TAEL: a zebrafish-optimized optogenetic gene expression system with fine spatial and temporal control. Development. January 15 2017;144(2):345–355. 10.1242/dev.139238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burgie ES, Bussell AN, Lye SH, et al. Photosensing and Thermosensing by Phytochrome B Require Both Proximal and Distal Allosteric Features within the Dimeric Photoreceptor. Sci Rep. October 20 2017;7(1):13648. 10.1038/s41598-017-14037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crossley PH, Minowada G, MacArthur CA, Martin GR. Roles for FGF8 in the induction, initiation, and maintenance of chick limb development. Cell. January 12 1996;84(1):127–36. 10.1016/s0092-8674(00)80999-x. [DOI] [PubMed] [Google Scholar]
- 16.Polstein LR, Gersbach CA. A light-inducible CRISPR-Cas9 system for control of endogenous gene activation. Nat Chem Biol. March 2015;11(3):198–200. 10.1038/nchembio.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schindler SE, McCall JG, Yan P, et al. Photo-activatable Cre recombinase regulates gene expression in vivo. Sci Rep. September 9 2015;5:13627. 10.1038/srep13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taslimi A, Zoltowski B, Miranda JG, Pathak GP, Hughes RM, Tucker CL. Optimized second-generation CRY2-CIB dimerizers and photoactivatable Cre recombinase. Nat Chem Biol. June 2016;12(6):425–30. 10.1038/nchembio.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X, Wang X, Du Z, Ma Z, Yang Y. Spatiotemporal control of gene expression in mammalian cells and in mice using the LightOn system. Curr Protoc Chem Biol. 2013;5(2):111–29. 10.1002/9780470559277.ch120267. [DOI] [PubMed] [Google Scholar]
- 20.Motta-Mena LB, Reade A, Mallory MJ, et al. An optogenetic gene expression system with rapid activation and deactivation kinetics. Nat Chem Biol. March 2014;10(3):196–202. 10.1038/nchembio.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Chen X, Yang Y. Spatiotemporal control of gene expression by a light-switchable transgene system. Nat Methods. February 12 2012;9(3):266–9. 10.1038/nmeth.1892. [DOI] [PubMed] [Google Scholar]
- 22.Quejada JR, Park SE, Awari DW, et al. Optimized light-inducible transcription in mammalian cells using Flavin Kelch-repeat F-box1/GIGANTEA and CRY2/CIB1. Nucleic Acids Res. November 16 2017;45(20):e172. 10.1093/nar/gkx804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller K, Engesser R, Timmer J, Nagy F, Zurbriggen MD, Weber W. Synthesis of phycocyanobilin in mammalian cells. Chem Commun (Camb). October 11 2013;49(79):8970–2. 10.1039/c3cc45065a. [DOI] [PubMed] [Google Scholar]
- 24.Muller K, Zurbriggen MD, Weber W. Control of gene expression using a red- and far-red light-responsive bi-stable toggle switch. Nat Protoc. March 2014;9(3):622–32. 10.1038/nprot.2014.038. [DOI] [PubMed] [Google Scholar]
- 25.Boulina M, Samarajeewa H, Baker JD, Kim MD, Chiba A. Live imaging of multicolor-labeled cells in Drosophila. Development. April 2013;140(7):1605–13. 10.1242/dev.088930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tischer D, Weiner OD. Illuminating cell signalling with optogenetic tools. Nat Rev Mol Cell Biol. August 2014;15(8):551–8. 10.1038/nrm3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toettcher JE, Gong D, Lim WA, Weiner OD. Light control of plasma membrane recruitment using the Phy-PIF system. Methods Enzymol. 2011;497:409–23. 10.1016/B978-0-12-385075-1.00017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kyriakakis P, Catanho M, Hoffner N, et al. Biosynthesis of Orthogonal Molecules Using Ferredoxin and Ferredoxin-NADP(+) Reductase Systems Enables Genetically Encoded PhyB Optogenetics. ACS Synth Biol. February 16 2018;7(2):706–717. 10.1021/acssynbio.7b00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uda Y, Goto Y, Oda S, Kohchi T, Matsuda M, Aoki K. Efficient synthesis of phycocyanobilin in mammalian cells for optogenetic control of cell signaling. Proc Natl Acad Sci U S A. November 7 2017;114(45):11962–11967. 10.1073/pnas.1707190114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart MD, Jang CW, Hong NW, Austin AP, Behringer RR. Dual fluorescent protein reporters for studying cell behaviors in vivo. Genesis. October 2009;47(10):708–17. 10.1002/dvg.20565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuda T, Cepko CL. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc Natl Acad Sci U S A. January 6 2004;101(1):16–22. 10.1073/pnas.2235688100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Gorman S, Dagenais NA, Qian M, Marchuk Y. Protamine-Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proc Natl Acad Sci U S A. December 23 1997;94(26):14602–7. 10.1073/pnas.94.26.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eisenhoffer GT, Rosenblatt J. Live imaging of cell extrusion from the epidermis of developing zebrafish. J Vis Exp. June 27 2011;(52). 10.3791/2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan S, Sun Z. Microinjection of mRNA and morpholino antisense oligonucleotides in zebrafish embryos. J Vis Exp. May 7 2009;(27). 10.3791/1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.