Abstract
Background
Stimulator of interferon genes (STING) is a key cytosolic receptor for small nucleotides and plays a key role in anticancer and antiviral immunity. Cyclic dinucleotide STING agonists may comprise a novel class of vaccine adjuvants capable of inducing cellular immune responses and protective efficacy against intracellular pathogens.
Methods
We generated a recombinant Bacillus Calmette-Guérin ([BCG] BCG-disA-OE) that overexpresses the endogenous mycobacterial diadenylate cyclase gene and releases high levels of the STING agonist bis-(3’-5’)-cyclic dimeric adenosine monophosphate (c-di-AMP). We used a 24-week guinea pig vaccination-Mycobacterium tuberculosis (M.tb.) challenge model to test the protective efficacy of BCG-disA-OE versus wild-type BCG and measured lung weights, pathology scores, and M.tb. organ colony-forming unit (CFU) counts.
Results
BCG-disA-OE elicited significantly stronger tumor necrosis factor-α, interleukin (IL)-6, IL-1β, interferon (IFN) regulatory factor 3, and IFN-β levels than BCG-wild type (WT) in vitro in murine macrophages. In vivo in guinea pigs, we found that BCG-disA-OE reduced lung weights, pathology scores, and M.tb. CFU counts in lungs by 28% (P < .05), 34%, and 2.0 log10 CFU units (P < .05) compared with BCG-WT, respectively.
Conclusions
We report a strategy of delivering a STING agonist from within live BCG. Overproduction of the STING agonist c-di-AMP significantly enhanced the protective efficacy of BCG against pulmonary and extrapulmonary tuberculosis. Our findings support the development of BCG-vectored STING agonists as a tuberculosis vaccine strategy.
Keywords: Calmette-Guérin (BCG), c-di-AMP, CDNs, Mycobacterium tuberculosis, STING, TB vaccine
We report a strategy of delivering c-di-AMP, a STING agonist, from within live BCG. Overproduction of c-di-AMP significantly enhanced the protective efficacy of BCG against tuberculosis. Our findings support the development of BCG-vectored STING agonists as a TB vaccine strategy.
(See the Editorial Commentary by Kaushal, on pages 1031–2.)
Recent studies have identified a key role for the stimulator of interferon genes (STING)- an intracellular sensor protein in mediating innate immune responses to cellular stress or pathogen infection [1, 2]. The STING is a cytosolic receptor for both pathogen-associated molecular pattern molecules such as cyclic dinucleotides bis-(3’-5’)-cyclic dimeric adenosine monophosphate (c-di-AMP) or bis-(3’-5’)-cyclic dimeric guanosine monophosphate (c-di-GMP) produced by bacteria and for mammalian endogenous danger-associated molecular pattern molecules such as 2’,3’-cyclic GMP-AMP (cGAMP) that is synthesized by cyclic GMP-AMP synthase (cGAS) in response to microbial or self-derived cytosolic double-stranded deoxyribonucleic acid (DNA) [1–4]. The STING activation triggers signal transducer and activator of transcription 6 (STAT-6) and interferon (IFN) regulatory factor (IRF) 3 transcription factor signaling leading to nuclear factor (NF)-κB induction and increased expression of type I IFNs (IFNα/β), proinflammatory genes, and antiviral chemokines. Thus, endogenous and exogenous cyclic dinucleotides (CDNs) are strong Toll-like receptor-independent mediators of innate host defenses [5, 6]. Based on the key role of STING signaling, pharmacological stimulation of the pathway using small molecule STING agonists is currently being tested for enhancement of antitumor immunity and as potential antiviral therapy [5, 7].
Vaccine adjuvant properties of CDNs have been demonstrated in conjunction with tuberculosis (TB) protein subunit vaccines resulting in enhanced cellular immune responses and more durable protective immunity [8]. Van Dis et al [8] showed that intranasal delivery of the CDN-adjuvanted protein vaccine conferred superior protection to that of Bacillus Calmette-Guérin (BCG) and elicited both Th1 and Th17 immune responses. Adjuvant properties of intranasal CDN administration have also been demonstrated with model antigens β-galactosidase or ovalbumin administered to mice and the finding of elevated immunoglobulin G levels and more robust Th1, Th2, and Th17 responses [9]. Among microbial-derived CDNs, c-di-AMP has been shown to stimulate activation and maturation of both macrophages and dendritic cells and to enhance both serologic and cellular immune responses to protein antigens in animal models [10, 11]. Thus, CDN STING agonists have well demonstrated activity in potentiating immune responses to vaccines.
However, efforts to harness small molecule CDNs as vaccine adjuvants may be limited by their rapid clearance and by the fact that as negatively charged molecules, CDNs do not readily cross cell membranes [6]. Hence, there is a need to develop cost-effective formulations of CDNs and related STING agonists that allow for sustained release to the cytosolic compartment of antigen-presenting cells.
Our previous studies revealed that Mycobacterium tuberculosis (M.tb.), an intracellular pathogen, expresses a di-adenylate cyclase gene, disA (Rv3586), whose gene product synthesizes and secretes c-di-AMP into the host cell cytosol [12]. We observed that bacterial-derived c-di-AMP contributes to increased autophagy and proinflammatory cytokine production (IFNβ and tumor necrosis factor [TNF]-α) during M.tb. infection in a STING-dependent and cGAS-independent manner. We also showed that an M.tb. strain engineered to overexpress c-di-AMP (M.tb.-disA-OE) was significantly attenuated in a mouse model. Moreover, release of c-di-AMP into the macrophage cytosol was found to be independent of the bacterial ESX-1 secretion system (absent in BCG) because both the M.tb.-disA-OE and the analogous BCG-disA-OE strains showed significant and comparable increased IRF3 and IFN-β induction compared with their corresponding parental strains, M.tb.-wild type (WT) and BCG-WT [12]. In a separate study, we showed that mutation of the cdnP gene (Rv2837c) encoding a CDN phosphodiesterase (CdnP) that hydrolyzes c-di-AMP results in higher levels of c-di-AMP accumulation and that the cdnP mutant strain—secreting excess c-di-AMP—is attenuated for virulence similar to the M.tb.-disA-OE strain, which also releases excess c-di-AMP [13].
Hence, both M.tb. and BCG produce the STING agonist c-di-AMP endogenously, and bacterial-derived c-di-AMP gains access to the host cell cytosol and possibly to adjacent cells through connexins forming gap junctions [14]. Bacterial-derived c-di-AMP, a STING-agonist, contributes to increased autophagy and IFN-β production, and recombinant M.tb. and BCGs strains, which overexpress the disA gene, elicit higher levels of proinflammatory cytokine production and autophagy. Based on these results, we evaluated the protective efficacy of the BCG-disA-OE strain versus BCG-WT as a TB vaccine in the well validated guinea pig model. We hypothesized that this approach toward a boosted BCG strain may offer benefit in light of the failures of subunit and viral vaccines used in prime-boost studies with BCG [15] and the recent demonstration of added TB protection with BCG vaccination during adolescence [16].
METHODS
Animals
All procedures involving live animals were performed in agreement with the protocols approved by the Institutional Animal Care and Use Committee at the Johns Hopkins University School of Medicine. Pathogen-free female outbred guinea pigs (300 grams) and C57BL/6 mice were purchased from Charles River Laboratories (North Wilmington, MA). Uninfected guinea pigs were housed under pathogen-free conditions at a biosafety level (BSL) 3 animal facility without cross-ventilation. C57BL/6J mice were housed in a BSL2 animal facility at the School of Medicine, Johns Hopkins University. Animals were given free access to water and standard mouse or guinea pig chow, respectively. The general behavior and appearance were monitored by veterinary specialists.
Bacterial Strains and Cell Culture
Details of all bacterial strains are provided in Supplementary Table 1. BCG Pasteur was a gift from Frank Collins (US Food and Drug Administration), and M.tb. H37Rv and CDC1551 were obtained from American Type Culture Collection. Frozen vials of bacterial strains were revived and subsequently subcultured in 7H9 Middlebrook liquid medium (B271310; Fisher Scientific) supplemented with oleic acid-albumin-dextrose-catalase (B11886; Fisher Scientific), 0.5% glycerol (G5516; Sigma), and 0.05% Tween-80 (BP338; Fisher Scientific) in a BSL3 facility. Murine bone marrow was isolated from 4- to 6-week-old female C57BL/6J mice. Approximately 108 cells were stored in cryopreservation media made of 10% dimethyl sulfoxide (D2650; Sigma) in heat-inactivated fetal bovine serum ([FBS] 10082-147; Fisher Scientific) overnight at −80°C followed by transfer to deep cryopreservation in liquid nitrogen. For differentiation of bone marrow cells into primary macrophages, bone marrow cells were differentiated for 7 days in presence of Roswell Park Memorial Institute (RPMI) medium-GlutaMAX (61870-036; Fisher Scientific) supplemented with 10% heat-inactivated FBS and antibiotics (penicillin-streptomycin solution) (15140-122; Fisher Scientific) and 30% (vol/vol) L929 conditioned media. Mouse fibroblast L929 cells (ATCC CCL-1) were maintained in RPMI 1640 medium supplemented with 10% FBS and antibiotics.
Overexpression of MT3692 in BCG
High-molecular-weight-genomic DNA was isolated using the CTAB (cetyl trimethylammonium bromide) method from log-phase cultures of M.tb.-CDC 1551. Using gene-specific primers (Supplementary Table 2), pSD5hsp60.MT3692 (F) and pSD5hsp60.MT3692 (R), the disA (MT3692) gene of M.tb, was polymerase chain reaction (PCR) amplified from M.tb.-derived genomic DNA. Gene amplicons were cloned into mycobacterial shuttle vector pSD5-hsp60 (Supplementary Table 1) at the NdeI and MluI restriction sites. The clone (pSD5-hsp60-MT3692) was confirmed by insert release and sequence analyses. The construct pSD5-hsp60-MT3692 was subsequently used to transform BCG. Recombinant clones (BCG-disA-OE) were selected against kanamycin (25 µg/mL) and further conformed using colony PCR using kanamycin-specific primers (Supplementary Table 2). MT3692 overexpression phenotype of BCG-disA-OE strain was further confirmed by messenger ribonucleic acid (mRNA) expression using quantitative real-time PCR (qRT-PCR).
Quantitative Real-Time Polymerase Chain Reaction
Late log-phase BCG culture pellets were bead beaten using Zirconium beads (KT03961-1-102.BK; Berlin Technologies) before performing total RNA isolation. TRIzol reagent (15596026; Fisher Scientific) was used for RNA isolation from both bacterial and mammalian cells. Quantification of mRNA, amplification, and quantification of complementary DNA (cDNA) was carried out using SYBR Fast green double-stranded DNA binding dye (4085612; Applied Biosystems) and ABI StepOnePlus Real-Time PCR System (Applied Biosystems). Amplification of sigH and mouse beta actin was used as internal controls for BCG and mouse bone marrow-derived macrophages (BMDMs), respectively. Melt curve analyses confirmed formation of desired and specific PCR product. Experiments were performed in triplicate using 3 independent biological samples, and results were analyzed and presented using the 2−∆∆CT method. Details of National Center for Biotechnology Information (NCBI) gene identifiers and primer sequences are mentioned (Supplementary Table 2).
Macrophage Infection, Interferon Regulatory Factor 3 Activation Assay, and Cytokine Enzyme-Linked Immunosorbent Assays
Infection assays were performed in resting mouse BMDMs in 24-well plates in triplicates. In brief, early log-phase cultures of BCG strains were washed, diluted appropriately to predefined concentrations using macrophage infection media (Dulbecco’s modified Eagle’s medium [DMEM] with 10% FBS), and deposited on the monolayer of cells at a precalibrated multiplicity of infection (1:20). Infection was allowed to continue for 5 hours, after which uninfected extracellular bacilli were removed by repeated washing using Dulbecco’s phosphate-buffered saline (DPBS). This time point was considered 0 hour, and the cells were incubated for the desired number of hours until the end points were met. To access accurate bacterial counts of infection and internalized bacterial numbers, serial dilutions of the bacterial suspension and 0.025% sodium dodecyl sulfate-lysed macrophage were plated on 7H11 agar plates. RAW-Blue ISG (InvivoGen) reporter cells, derived from the murine RAW 264.7 macrophage cell line by stably integrating an IRF-inducible secreted embryonic alkaline phosphatase (SEAP) reporter construct, were used for IRF activation assay. The cells were infected with WT and BCG-disA-OE strains. Cells were incubated for 18 hours in fresh macrophage infection media (DMEM with 10% FBS), and culture supernatants were harvested for determination of IRF activation by a SEAP colorimetric assay using QUANTI-Blue reagent (InvivoGen). After macrophage infection, culture supernatants were isolated, filtered, and immediately frozen at −80°C for cytokine quantification. Mouse DuoSet ELISA kits for TNF (DY410), interleukin (IL)-6 (DY206-05), and IL-1β (DY201-05) were used for cytokine quantification. The absolute concentrations were determined by referring to a standard curve and expressed as picogram per milliliter. For quantification, experiments were performed in triplicates.
Guinea Pig Immunization and Determination of Protective Efficacy
To test the prophylactic potential of BCG-disA-OE as a vaccine candidate, guinea pigs (n = 12 per group) were immunized intradermally using 105 colony-forming units (CFU)/100 µL of WT parental BCG Pasteur or BCG-disA-OE strains. Guinea pigs were sham immunized with saline. Animals were challenged with ~100 CFU of M.tb. H37Rv strain by the aerosol route 6 weeks (Supplementary Figure 1) after primary immunization. Lungs from one set of infected animals were harvested, and homogenates were plated on day 1 after to check for established implantation. Infected animals from each group were euthanized 14 and 18 weeks later to determine the protective efficacy of the BCG-disA-OE. Gross-pathological features and bacillary burden in lungs and spleen of sham and BCG-immunized guinea pigs after M.tb. challenge were measured as described previously [17]. Supplementary Figure 3 shows the details of experimental plan and different animal groups used in the study, and Supplementary Table 1 depicts the bacterial strains and plasmid used in the study.
Statistical Analyses
All data were represented as Mean ± SEM and differences between individual test groups were analyzed by unpaired Student’s t-test, unless specified in the legend. All statistical analysis were performed using GraphPad Prism, version 5.01. P < .05 were considered statistically significant.
RESULTS
Induction of Type I Interferon Responses by BCG-disA-OE
As described by Dey et al [12], BCG-disA-OE is a recombinant derivative of BCG Pasteur, which overexpresses the endogenous mycobacterial diadenylate cyclase gene disA (Rv3586). The disA genes of M.tb. and BCG are 100% identical at the nucleotide level. disA catalyzes the conversion of 2 adenosine triphosphate molecules to c-di-AMP. The overexpression construct was generated by fusing the disA gene to the strong mycobacterial promoter hsp60 within the episomal mycobacterial overexpression vector pSD5-hsp60 (Supplementary Figure 1a–c). Gene expression profiling by real-time PCR showed ~50-fold upregulation of disA expression in BCG-disA-OE compared with the parental strain (Supplementary Figure 1d).
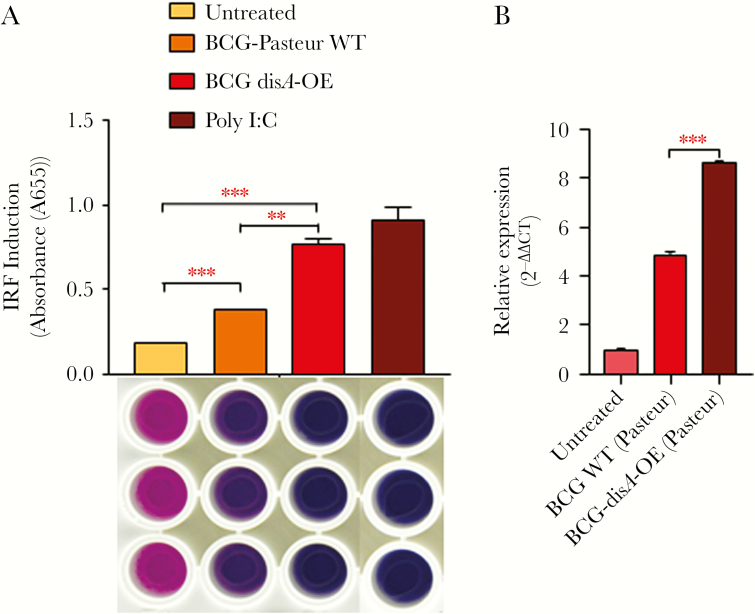
We evaluated whether strong disA expression would result in c-di-AMP-mediated IRF3 activation and consequent elevations in IFN-β levels in mouse macrophages. Raw-Blue reporter macrophage cells when infected with BCG-disA-OE strains showed a significant 2-fold induction of IRF3 compared with that observed with the WT parental strain BCG-WT (Figure 1A). These results indicate that heightened levels of c-di-AMP release from BCG-disA-OE produces significant activation of the STING/IRF3 axis, in keeping with our previous findings [12]. When primary murine BMDMs were infected with BCG-disA-OE and BCG-WT, we observed a 2-fold induction of Ifnb using qPCR (P < .001) (Figure 1B) during an early temporal window, again confirming our previous study [12].
Figure 1.
BCG-disA-OE overexpressing bis-(3’-5’)-cyclic dimeric adenosine monophosphate (c-di-AMP) gives more potent interferon (IFN) regulatory factor (IRF) 3 and type I IFN stimulation than bacillus Calmette-Guérin (BCG)-wild-type (WT): (A) effect of disA overexpression on activation of IRF pathway measured by IRF- secreted embryonic alkaline phosphatase (SEAP) QUANTI Blue reporter assay. RAW-Blue IFN-stimulated gene (ISG) cells were challenged with WT and BCG-disA-OE strains at a multiplicity of infection (MOI) of 1:20 for 5 hours to establish the infection. Uninfected bacteria were washed out using ice-cold Dulbecco’s phosphate-buffered saline (DPBS), and the RAW-Blue ISG cells were subsequently incubated for another 18–24 hours. The culture supernatants of infected RAW-Blue ISG cells were assayed for IRF activation. The image below the IRF-activation graph represents QUANTI Blue assay plate and sample wells; treatment parameters for column of wells correspond to those defined for the bars above aligned with the wells. The graphical points represent the mean of 3 independent experiments ± standard error of mean (SEM). Student’s t test (***, P < .001; **, P < .01). (B) Differential expression of IFN-β: mouse bone marrow-derived macrophages were challenged with WT and BCG-disA-OE strains at an MOI of 1:20 for 5 hours to establish the infection. Uninfected bacteria were washed using ice-cold DPBS, and cells were subsequently incubated for another 6 hours. Expression levels of messenger ribonucleic acid (mRNA) were measured using a SYBR Green-based quantitative real-time polymerase chain reaction (PCR). Basal level of transcript (mRNA) in untreated macrophages was used for data normalization and hence to access relative expression. β-actin was used as an internal control. Data analysis was performed using the 2−∆∆CT method. The graphical points represent mean of 3 independent experiments ± SEM. Mean comparison between BCG WT and BCG-disA OE were performed by Student’s t test (***, P < .001).
Macrophage Activation by c-di-AMP Overexpressing BCG (BCG-disA-OE)
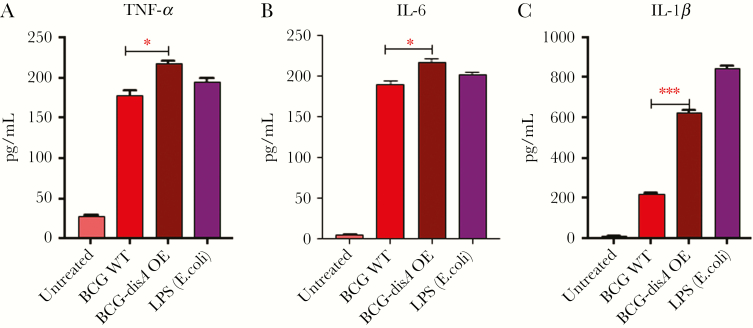
Although the binding affinity of c-di-AMP for STING is weaker than that for cGAMP, ligation of c-di-AMP with STING appears sufficient to induce coactivation of transcription factors other than IRF3 and hence induction of proinflammatory cytokines [18, 19]. We previously showed that disA-OE strains of M.tb. induce strong proinflammatory cytokines, such as TNF-α, IL-6, and IL-1β, suggesting macrophage activation and a complex link between c-di-AMP-based STING activation and induction of proinflammatory cytokines and other IFN-stimulated genes [12]. Bone marrow-derived primary murine macrophages infected with BCG-disA-OE showed significant increased levels of TNF-α, IL-6, and IL-1β in culture supernatants compared with uninfected or BCG-WT-infected controls (Figure 2). These results reveal a robust macrophage activation phenotype in response to c-di-AMP overproducing BCG with increased levels of TNF-α, IL-1β, and IL-6 that is more pronounced than that seen with BCG-WT.
Figure 2.
Modulation of proinflammatory cytokines in response to disA overexpression. Differential induction of (A) tumor necrosis factor (TNF)-α, (B) interleukin (IL)-6, and (C) IL-1β in mouse bone marrow-derived macrophages (BMDMs) challenged with wild-type and disA overexpression strains of Mycobacterium bovis BCG Pasteur. The BMDMs were challenged with wild-type and disA OE strains at a multiplicity of infection (MOI) of 1:20 for 5 hours to establish the infection. Uninfected bacteria were washed using ice-cold Dulbecco’s phosphate-buffered saline, and cells were subsequently incubated for another 24 hours. Culture supernatants were assayed by enzyme-linked immunosorbent assay for different cytokines. The graphical points represent the mean of 3 independent experiments ± SEM. Mean comparison between BCG-WT and BCG-disA OE were performed by Student’s t test (*, P < .05; ***, P < .001).
Cyclic Dinucleotide-Adjuvanted Recombinant BCG Offers Better Protection Against Virulent M.tb Challenge in Guinea Pigs
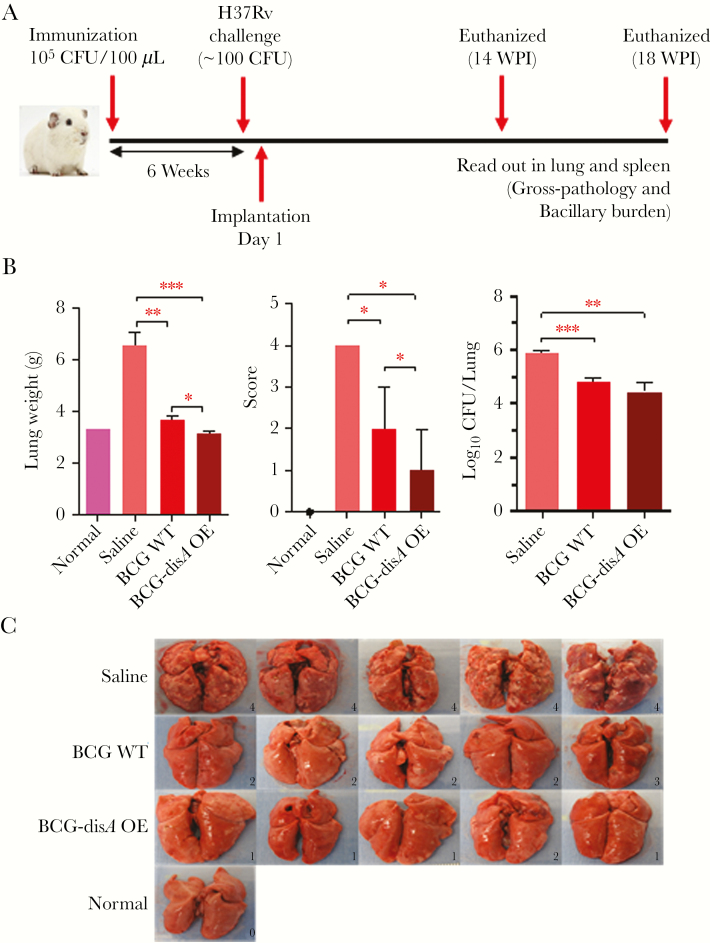
Our in vitro studies accessing macrophage response due to increased levels of c-di-AMP encouraged us to test the vaccine potential of BCG-disA-OE in the guinea pig model of TB infection (Figure 3A). Groups of 12 guinea pigs were vaccinated intradermally with 0.1 mL PBS (sham vaccination), 105 CFU of BCG-disA-OE or BCG-WT, and held for 6 weeks before challenge with aerosol challenge with 102 CFU of M.tb. H37Rv. As described under Methods, lungs from one set of infected animals were obtained on day 1 after challenge to confirm this implantation dose of M.tb. (Supplementary Figure 2). Separate groups of infected animals were euthanized 14 and 18 weeks postchallenge to determine the protective efficacies of BCG-disA-OE and BCG-WT by assessing organ weight, gross pathology, and bacterial loads in lungs and spleen.
Figure 3.
Effect on lung weight, gross lung pathology scores, bacterial burden, and gross-morphological features of lungs in guinea pigs 14 weeks postchallenge with M.tb H37Rv after vaccination with BCG-WT or BCG-disA-OE. (A) Time line showing the experimental strategy of BCG immunization and challenge in the guinea pig model of vaccination followed by M.tb. aerosol infection. (B) Lung weights, gross pathology scores, and bacterial burden at 14 weeks post-M.tb challenge. Data are Mean ± SEM except pathological score that is represented as Median ± Range. (C) Images of lungs at necropsy. ***, P < .001, **, P < .01, and *, P < .05; non-parametric Mann-Whitney test. P values were >.05 for CFU comparison between BCG WT and BCG-disA OE, not noted by brackets.
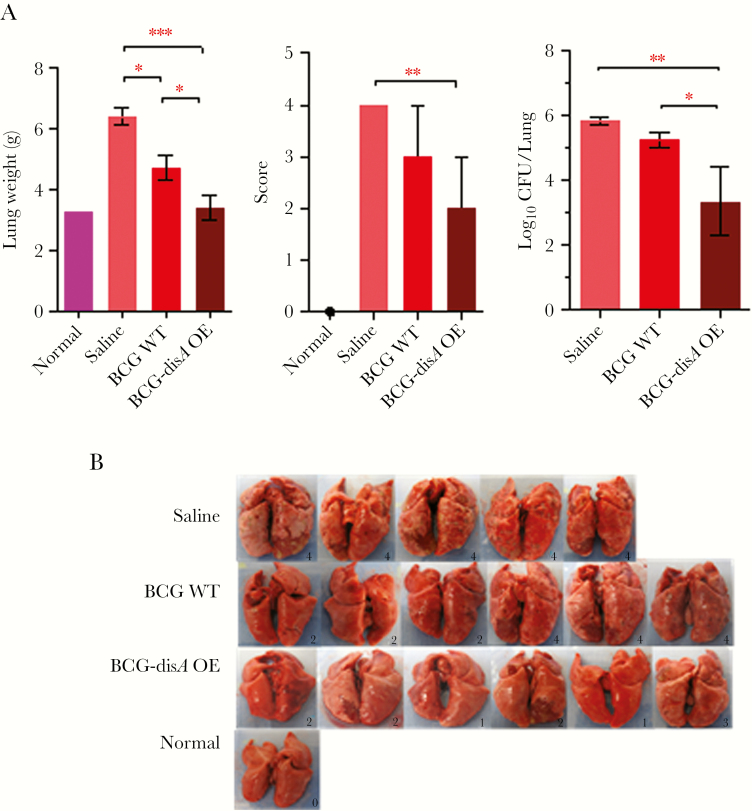
At 14-week postchallenge, both BCG-WT- and BCG-disA-OE-vaccinated animals showed significantly lower lung weight, gross pathology scores, and the bacillary loads in the lungs, relative to saline-treated controls (Figure 3B and C). Vaccination with BCG-disA-OE resulted in the highest reduction in lung gross pathology score, which was even more pronounced at 18 weeks postchallenge (Figure 4A and B). Although the impact of BCG-disA-OE vaccination on lung CFU counts was modest at the 14-week time point, by the 18-week time point lung CFU counts in the BCG-disA-OE vaccinated guinea pigs were 2.0 log10 units lower than in animals vaccinated with BCG-WT. In fact, 2 of 6 guinea pigs in the BCG-disA-OE group had lung CFU counts below the limit of detection, which is ~3–5 bacilli.
Figure 4.
Effect on lung weight, gross lung pathology scores, bacterial burden, and lung gross-morphological features in guinea pigs 18 weeks postchallenge with M.tb H37Rv after vaccination with BCG or BCG-disA-OE. (A) Lung weights, gross pathology scores, and M.tb bacterial burden at 18 weeks post-M.tb challenge. Two guinea pigs in BCG-disA-OE group had lung colony-forming unit (CFU) counts below the limit of our detection. Data are Mean ± SEM except pathological score that is represented as Median ± Range. (B) Images of lungs at necropsy. ***, P < .001, **, P < .01, and *, P < .05; non-parametric Mann-Whitney test. P values were >.05 for score and CFU comparisons between saline and BCG-WT, not noted by brackets.
In addition, vaccination with BCG-disA-OE effectively controlled the growth of M.tb. in the spleen as evident from significant reduction in spleen weights, spleen pathology scores, and spleen bacterial burdens when compared with the sham-immunized animals at both 14 and 18 weeks postinfection (Supplementary Figure 3 and Figure 5, respectively). Although spleen pathology scores were comparable between animals vaccinated with WT and disA overexpressing BCG at 14 weeks postchallenge (Supplementary Figure 3), by 18 weeks after challenge significantly lower spleen pathology scores were observed in the latter group (Figure 5). In addition, there was a trend towards lower spleen CFU in animals vaccinated with the disA-overexpressing strain compared with BCG, especially when examined at 18 weeks postchallenge. Indeed, at 18 weeks postchallenge, 3 of 6 guinea pigs in the BCG-disA-OE group had spleen CFU below the limits of detection. These findings clearly indicate that administration of BCG-disA-OE effectively controlled M.tb. replication in the lungs and its dissemination to the spleen.
Figure 5.
Effect on spleen weight, gross spleen pathology scores, bacterial burden, and spleen gross-morphological features in guinea pigs 18 weeks postchallenge with M.tb H37Rv after vaccination with BCG-WT or BCG-disA-OE. (A) Spleen weights, gross pathology scores, and M.tb colony-forming unit (CFU) counts at 18 weeks post-M.tb challenge. Three guinea pigs in BCG-disA-OE group had spleen CFU counts below the limit of our detection. Data are Mean ± SEM except pathological score that is represented as Median ± Range. (B) Images of spleens at necropsy. Scale bars indicate 1 cm. The number in the box is gross pathological score. ***, P < .001, **, P < .01, and *, P < .05; non-parametric Mann-Whitney test. P values were >.05 for other comparisons not noted by brackets.
DISCUSSION
The cytosolic danger sensor STING has emerged as an important mediator of host innate immune responses. The STING is activated by binding CDNs either secreted by bacteria (such as c-di-AMP or c-di-GMP) or distinct host CDNs (such as cGAMP) generated by host cell receptor after recognition of cytosolic double-stranded DNA [19, 20]. As a relatively new class of immunomodulatory molecules, CDNs exhibit strong potential to promote protective immunity and increase vaccine potency through STING-dependent signaling that involves transcription factors IRF-3, IRF-7, and NF-κB [10]. Therefore, STING agonists are regarded as promising immune adjuvants for promoting immune responses against tumors and infections. However, the efficacy of small molecule STING agonists as vaccine adjuvants may be limited because they are rapidly cleared and may not gain long-lived access to the cytosol for STING activation.
In this study, we tested the hypothesis that a BCG strain overexpressing the STING agonist c-di-AMP might offer sustained intracellular delivery of c-di-AMP and thereby improve the vaccine potential of BCG against TB. We evaluated a c-di-AMP-producing recombinant BCG strain that overexpresses the mycobacterial disA gene [12]. Because BCG survives and replicates intracellularly for several weeks postvaccination, overexpression of disA under the influence of strong constitutive mycobacterial hsp60 promoter allows sustained intracellular exposure of the STING agonist in the cytosol of phagocytic cells. By utilizing BCG as the vector for STING agonist delivery, BCG-disA-OE offers enhanced innate immune activation via the STING pathways in addition to the full antigenic repertoire of BCG.
Complex genomic rearrangements in BCG strains are one of the major contributors of immunological and phenotypic differences that, in turn, contribute to the variability in the degree of protection offered by BCG [21, 22]. A global resurgence of multidrug-resistant TB, human immunodeficiency virus (HIV)-TB coinfection has heightened the need for an improved TB vaccine that provides better protection than that of BCG. In addition, new vaccines must be safe enough to be used in immunocompromised HIV-TB coinfected individuals. Rational modification of live BCG to increase its antigenic repertoire, and with a prior knowledge of attenuation factors and immunity, is critically needed [15, 22, 23, 24]. Although our study did not directly assess the virulence of BCG-disA-OE to that of BCG-WT, based on the finding that M.tb.-disA-OE showed a median time to death in BALB/c mice of 321.5 days compared with 150.5 days for M.tb.-WT after an aerosol infection of 3.5 log10 CFU [12], we anticipate that BCG-disA-OE is a weaker pathogen than BCG-WT.
Inside the host, both CD4+ and CD8+ T cells are essential for protective immunity against TB. Dendritic cells migrating from the alveoli to the draining lymph nodes are crucial for activation of M.tb. antigen-specific CD4+ and CD8+ T cells and contribute to resistance to M.tb. [25]. The requirement for a Th1-like T-cell response for host immunity against M.tb. is clear, and recent vaccine development has sought to stimulate both CD4 and CD8 T-cell responses to produce Th1 cytokines [15]. Hence, elicitation of enduring Th1 responses is a desirable feature of candidate TB vaccines. Not only are STING-activating adjuvants known to elicit antigen-specific Th1 responses, but they also elicit Th17 responses and have been shown to confer improved protection against M.tb. [8]. The recent report suggested that the protective efficacy of protein subunit vaccine adjuvanted with small molecule CDNs was durable for up to 12 weeks after M.tb. challenge in mice, suggesting that a CDN-adjuvanted vaccine can reduce TB progression in mice through T cell-dependent mechanisms [8].
Guinea pigs are highly susceptible to M.tb. infection, and the model provides an important preclinical evaluation of potential vaccine candidates [26]. Immunization with a single dose of BCG-disA-OE resulted in a marked reduction in the gross pathology and bacterial loads in both lungs and spleens of M.tb.-challenged animals when compared with sham treatment or vaccination with BCG alone. Thus, our studies highlight the improved potential of BCG-disA-OE over BCG to impart protection against M.tb. infection and disease dissemination.
CONCLUSIONS
This study is an important step forward towards implementing CDN-based STING agonists as novel vaccine adjuvants into vaccine strategies for TB. Our results provide proof-of-concept data for utilizing BCG as the vector for producing STING agonist(s) naturally from within the intracellular compartment in a sustained fashion. Moreover, our results suggest that the STING agonist overexpressing BCG may offer greater efficacy as a TB vaccine than BCG alone and that this approach may have utility as an immunotherapeutic tool against other diseases including cancer.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Dr. Geetha Srikrishna for editing this manuscript.
Financial support. This work was funded by National Institutes of Health Grants AI37856 and HL133190 and the Howard Hughes Medical Institute.
Potential conflicts of interest. All authors are coinventors of patent applications related to the technology reported in this manuscript. W. B. holds shares in OncoSTING, LLC. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Li T, Chen ZJ. The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J Exp Med 2018; 215:1287–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Paludan SR, Bowie AG. Immune sensing of DNA. Immunity 2013; 38:870–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burdette DL, Monroe KM, Sotelo-Troha K, et al. . STING is a direct innate immune sensor of cyclic di-GMP. Nature 2011; 478:515–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yin Q, Tian Y, Kabaleeswaran V, et al. . Cyclic di-GMP sensing via the innate immune signaling protein STING. Mol Cell 2012; 46:735–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McCaffary D STING signalling: an emerging common pathway in autoimmunity and cancer. Immunopharmacol Immunotoxicol 2017; 39:253–8. [DOI] [PubMed] [Google Scholar]
- 6. Dubensky TW Jr, Kanne DB, Leong ML. Rationale, progress and development of vaccines utilizing STING-activating cyclic dinucleotide adjuvants. Ther Adv Vaccines 2013; 1:131–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iurescia S, Fioretti D, Rinaldi M. Targeting cytosolic nucleic acid-sensing pathways for cancer immunotherapies. Front Immunol 2018; 9:711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van Dis E, Sogi KM, Rae CS, et al. . STING-activating adjuvants elicit a Th17 immune response and protect against Mycobacterium tuberculosis infection. Cell Rep 2018; 23:1435–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Libanova R, Ebensen T, Schulze K, et al. . The member of the cyclic di-nucleotide family bis-(3’,5’)-cyclic dimeric inosine monophosphate exerts potent activity as mucosal adjuvant. Vaccine 2010; 28:2249–58. [DOI] [PubMed] [Google Scholar]
- 10. Libanova R, Becker PD, Guzmán CA. Cyclic di-nucleotides: new era for small molecules as adjuvants. Microb Biotechnol 2012; 5:168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Škrnjug I, Rueckert C, Libanova R, Lienenklaus S, Weiss S, Guzmán CA. The mucosal adjuvant cyclic di-AMP exerts immune stimulatory effects on dendritic cells and macrophages. PLoS One 2014; 9:e95728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dey B, Dey RJ, Cheung LS, et al. . A bacterial cyclic dinucleotide activates the cytosolic surveillance pathway and mediates innate resistance to tuberculosis. Nat Med 2015; 21:401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dey RJ, Dey B, Zheng Y, et al. . Inhibition of innate immune cytosolic surveillance by an M. tuberculosis phosphodiesterase. Nat Chem Biol 2017; 13:210–7. [DOI] [PubMed] [Google Scholar]
- 14. Ablasser A, Schmid-Burgk JL, Hemmerling I, et al. . Cell intrinsic immunity spreads to bystander cells via the intercellular transfer of cGAMP. Nature 2013; 503:530–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaufmann SH Tuberculosis vaccine development at a divide. Curr Opin Pulm Med 2014; 20:294–300. [DOI] [PubMed] [Google Scholar]
- 16. Nemes E, Geldenhuys H, Rozot V, et al. . Prevention of M. tuberculosis infection with H4:IC31 vaccine or BCG revaccination. N Engl J Med 2018; 379:138–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jain R, Dey B, Dhar N, et al. . Enhanced and enduring protection against tuberculosis by recombinant BCG-Ag85C and its association with modulation of cytokine profile in lung. PLoS One 2008; 3:e3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Diner EJ, Burdette DL, Wilson SC, et al. . The innate immune DNA sensor cGAS produces a noncanonical cyclic dinucleotide that activates human STING. Cell Rep 2013; 3:1355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ablasser A, Goldeck M, Cavlar T, et al. . cGAS produces a 2’-5’-linked cyclic dinucleotide second messenger that activates STING. Nature 2013; 498:380–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Woodward JJ, Iavarone AT, Portnoy DA. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science 2010; 328:1703–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang L, Ru HW, Chen FZ, et al. . Variable virulence and efficacy of BCG vaccine strains in mice and correlation with genome polymorphisms. Mol Ther 2016; 24:398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brosch R, Gordon SV, Garnier T, et al. . Genome plasticity of BCG and impact on vaccine efficacy. Proc Natl Acad Sci U S A 2007; 104:5596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martin C Tuberculosis vaccines: past, present and future. Curr Opin Pulm Med 2006; 12:186–91. [DOI] [PubMed] [Google Scholar]
- 24. Martín C The dream of a vaccine against tuberculosis; new vaccines improving or replacing BCG? Eur Respir J 2005; 26:162–7. [DOI] [PubMed] [Google Scholar]
- 25. Cooper AM Cell-mediated immune responses in tuberculosis. Annu Rev Immunol 2009; 27:393–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Clark S, Hall Y, Williams A. Animal models of tuberculosis: Guinea pigs. Cold Spring Harb Perspect Med 2014; 5:a018572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.