Abstract
CD8 T cell differentiation is orchestrated by dynamic metabolic changes that direct activation, proliferation, cytotoxic function and epigenetic changes. We report that the BTB-ZF family transcriptional repressor Zbtb20 negatively regulates CD8 T cell metabolism and memory differentiation in mice. Effector and memory CD8 T cells with conditional Zbtb20 deficiency displayed enhanced mitochondrial and glycolytic metabolism, and memory CD8 T cells had enhanced spare respiratory capacity. Furthermore, Zbtb20 deficient CD8 T cells displayed increased flexibility in the use of mitochondrial fuel sources. Phenotypic and transcriptional skewing toward the memory fate was observed during the CD8 T cell response to Listeria monocytogenes. Memory cells mounted larger secondary responses, and conferred better protection following tumor challenge. These data suggest that inactivation of Zbtb20 may offer an approach to enhance metabolic activity and flexibility, and improve memory CD8 T cell differentiation, useful attributes for T cells used in adoptive immunotherapy.
Introduction
Upon infection, small numbers of antigen-specific naïve CD8 T cells differentiate into multiple types of effector and memory cells, which mediate pathogen clearance and provide long-term protective immunity. In an acute infection, which can be effectively cleared by the host, the CD8 T cell response is characterized by three distinct phases: clonal expansion, contraction, and memory (1). As antigen specific CD8 T cells become activated, they undergo extensive clonal expansion to fight the infection. A large number of effector CD8 T cells can be found in infected tissues by the time of pathogen clearance. Those effector CD8 T cells differ in effector cytokine production, migration patterns, pro-apoptotic and anti-apoptotic protein expression, cytokine receptor expression, and proliferation capacity, and thus have varying potential to form memory CD8 T cells (2). The majority of effector CD8 T cells die during the ensuing contraction phase to bring the host immune system back into homeostasis (1), leaving only a small fraction to survive and differentiate into memory CD8 T cells. Certain surface markers have been shown to correlate with distinct CD8 T cell fates. Short-lived effector cells are characterized by low expression of IL-7 receptor α chain (CD127) and high expression of killer cell lectin-like receptor G1 (KLRG-1), whereas memory precursor effector cells are characterized by high expression of CD127 and low expression of KLRG-1 (2–6). The majority of short lived effectors enter apoptosis during the contraction phase, whereas memory precursors are more likely to survive and become memory CD8 T cells (2–6).
Naïve, effector and memory CD8 T cells have distinct metabolic profiles (7). Naïve CD8 T cells are metabolically quiescent and mostly employ mitochondrial respiration to meet their energy demands by breaking down glucose, glutamine and fatty acids (8). These cells only maintain basal nutrient uptake to support their slow homeostatic turnover. Upon activation, CD8 T cells promptly upregulate glycolysis as well as nutrient uptake. Although effector CD8 T cells preferentially use glycolysis to generate ATP, glycolysis and mitochondrial respiration are both upregulated to high levels. Intermediate metabolites from both pathways can be used for biosynthesis of lipid, nucleic acids and proteins (7) which are crucial for growth, proliferation and effector cytokine production. Memory CD8 T cells preferentially utilize mitochondrial respiration for ATP production. They also possess higher mitochondrial mass and larger spare respiratory capacity (SRC) which indicates the ability to generate ATP more quickly than a naïve CD8 T cells (8).
There are many gaps in our knowledge regarding the regulation of immunometabolism, however the roles for some central factors is clear. Signaling through the mammalian target of rapamycin (mTOR) pathway, culminating in activation of the transcription factors HIF-1α and Myc is necessary to enhance glycolysis and glutaminolysis and for effector T cell differentiaton (9, 10). In contrast, AMP-activated protein kinase (AMPK), which promotes the metabolic switch to catabolism when the AMP/ATP ratio is low is required for memory CD8 T cell generation (11, 12). AMPK can also induce mitochondrial fusion and elongation (13–15), which is accompanied by tightly organized cristae allowing more efficient use of the electron transport chain in memory cells. This is in contrast to mitochondria in effector CD8 T cells, which are more fragmented and exhibit looser conformation of the cristae (16).
Zbtb20, also known as HOF or DPZF, belongs to an evolutionarily conserved transcription factor family named broad complex, tramtrack, bric-à-brac and zinc finger (BTB-ZF) family. There are more than 49 BTB-ZF genes in mammalian genomes (17), characterized by one or more C-terminal C2H2 zinc finger DNA binding domain in combination with an N-terminal BTB domain that mediates protein–protein interactions (18). Zinc finger domains bind to specific DNA target sequences in the regulatory regions of target genes, then the BTB domain recruits co-repressor proteins that can lead to remodeling of chromatin and repression of target gene expression (18). Other member of this gene family include Bcl-6, BAZF and PLZF, which play important regulatory roles both in the immune system and in other cell types, regulating cellular differentiation, development, oncogenesis and the maintenance of stem cell pools (18). More importantly, many BTB-ZF proteins, like Bcl-6 and BAZF, are also key factors in the development and function of lymphocytes and myeloid cells. Zbtb20 is widely expressed in neuronal and hematopoietic tissues (19), promotes antibody-secreting B cell longevity and differentiation and is indispensable for maintaining the long-lived plasma cell response (20). In addition, Zbtb20 induces cell survival factors including Bcl-2, Bcl-w, Bcl-x and blocks cell cycle progression in a plasma cell line (20). Global Zbtb20 deficiency causes premature death of mice due to growth retardation and metabolic dysfunction (21). Transcriptional profiling of liver tissue from Zbtb20 KO pups revealed dysregulation of a number of genes related to metabolism and mitochondrial function, including AKT, PGC1α, PDK4, CPT, PI3K and fatty acid synthase (21).
Here we show Zbtb20 deletion after CD8 T cell activation enhanced both glycolytic and mitochondrial metabolism and allowed more flexibility in mitochondrial fuel sources. These CD8 T cells were phenotypically and transcriptionally biased toward memory cells, and mounted enhanced secondary responses. Adoptive transfer experiments showed Zbtb20-deficient memory cells confered superior anti-tumor protection when compared with WT cells. This implies that Zbtb20 is an important negative regulator of CD8 T cell metabolism and memory differentiation, and deletion of this gene may offer a novel immunotherapeutic approach to enhance adoptive T cell therapy.
Materials and Methods
Mice, virus and bacteria.
Zbtb20-GFP mice (MMRRC# 030006-UCD) were obtained from the Knockout Mouse Project (KOMP). Zbtb20-fl/fl mice were generated by Dr. Weiping J. Zhang (Second Military Medical University, China) (22). OT-I mice were originally purchased from Jackson Laboratory (003831). CD45.1 mice were purchased from Jackson Laboratory (002014). GZB-cre mice were kindly provided by Dr. Rafi Ahmed (Emory University). CD45.1 OT-I mice, Zbtb20-GFP CD45.1 OT-I mice and GZB-cre Zbtb20-flox CD45.1 OT-I mice were crossed and bred in-house at Dartmouth College. MHV-68-Ova virus was kindly provided by Dr. Phillip Stevenson (University of Queensland, Australia). LM-actA-Ova was kindly provided by Dr. John Harty (University of Iowa).
Primers.
Primers GCAAGTTGCAGGCACAGCTAGTT and TAGCGGCTGAAGCACTGCA were used to genotype Zbtb20-GFP mice. Primers GZACCGCTGGCAACACCTATCTG and CTCTCCCCTCCTCCCTCTGG were used to genotype Zbtb20-floxed mice. Primers GCATTACCGGTCGATGCAACGAGTGATGAG and GAGTGAACGAACCTGGTCGAAATCAGTGCG were used to genotype GZB-cre mice. Primers CCTGCCTGAACTTTGAAGCTGTT and GCAACTGATGTCACAATCAGATGACC were used for ZBTB20 quantitative fluorescent PCR (QF-PCR).
IL-2/IL-15 in vitro CD8 T cell differentiation.
Total splenocytes were harvested from OT-I mice and GZB-cre Zbtb20-fl/fl OT-I mice, then seeded at 2×106 cells/mL with 10μg/mL SIINFEKL peptide for 48h without exogenous IL-2. Activated cells were further cultured with 100U/ml rhIL- 2 only at 0.5×106 cells/mL or with 50ug/ml rmIL-15 at 106 cells/mL for 7 days. Cultures were split and provided fresh media every 2–3 days.
Seahorse analysis.
Assays were performed according to the manufacturer’s protocols. 150,000 cells were seeded per well for IL-2/IL-15 in vitro differentiated CD8 T cells. 200,000 cells were seeded per well for ex vivo CD8 T cells. 1 μM oligomycin, 1.5 μM FCCP and 0.5 μM R/AA were used for mitochondrial stress assays (Seahorse XF Cell Mito Stress Test Kit; Seahorse Agilent cat:103015-100); 0.5 μM Rotenone/Antimycin A and 50mM 2-Deoxyglucose were used for Glycolytic rate assays (Seahorse XF Glycolytic rate Assay; Seahorse Agilent cat:103344-100).
Ex vivo Seahorse Bioanalyser assays.
Naïve CD8 T cells were harvested from CD45.1 OT-I mice (WT) or GZB-cre Zbtb20-fl/fl CD45.1 OT-I mice (KO) using EasySep mouse naïve CD8 T cell isolation kits (Stemcell Technologies cat:19858A). 50,000 naïve OT-I cells were retro-orbitally injected into B6 recipients, which were then retro-orbitally infected with 106 CFU LM-actA-Ova 1 day later. On D7 and D28 post infection, splenocytes were harvested from recipients, stained with anti-CD45.1-APC antibody then purified with Mojosort mouse anti-APC nanobeads (Biolegend Cat:480072). 200,000 enriched cells (purity greater than 95%) were seeded into each well for Seahorse mitochondrial stress tests and Glycolytic Rate tests. 1 μM oligomycin, 1.5 μM 4-(trifluoromethoxy)phenyl)carbonohydrazonoyl dicyanide (FCCP) and 0.5 μM Rotenone/Antimycin A were used for mitochondria stress assays. 0.5 μM Rotenone/Antimycin A and 50mM 2-deoxyglucose were used for Glycolytic rate assays.
Mitochondrial fuel flexibility assays.
Total splenocytes were harvested from OT-I mice and GZB-cre ZBTB20-f/f OT-I (KO) mice, then activated with SIINFEKL peptide for 48h without exogenous IL-2. Activated cells were further cultured with 50ug/ml rmIL-15 for 7 days. Cultured cells were then analyzed using Seahorse XFe96 Analyzer. Cells were treated with no inhibitors or combinations of different inhibitors that prevented the utilization of different mitochondrial fuel source (etomoxir for long-chain fatty-acid; UK5099 for pyruvate; BPTES for L-glutamine; utilization of short and medium chain fatty acid were not manipulated), followed by a conventional Seahorse Agilent Mito Stress test. The maximal Respiratory Capacity of each condition was normalized to the group without inhibitor treatment. 4 μM Etomoxir, 2 μM UK5099, 3 Μm BPTES, 1 μM oligomycin, 1.5 μM FCCP and 0.5 μM R/AA were used for mitochondrial fuel flexibility assay (Seahorse XF Cell Mito Stress Test Kit; Seahorse Agilent cat:103015-100).
Adoptive transfers.
Naïve CD8 T cells were harvested from CD45.1 OT-I mice (WT) or GZB-cre Zbtb20-fl/fl CD45.1 OT-I mice (KO) and purified using EasySep mouse naïve CD8 T cell isolation kits (Stemcell Technologies cat:19858A). 50,000 naïve OT-I cells were retro-orbitally injected into congenic B6 recipient mice, which were then retro-orbitally infected with 106 CFU LM-actA-Ova 1 day later.
MC38-Ova tumor protection.
Naïve CD8 T cells were harvested from CD45.1 OT-I mice (WT) or GZB-cre Zbtb20-fl/fl CD45.1 OT-I mice (KO) using EasySep mouse naïve CD8 T cell isolation kit (Stemcell Technologies cat:19858A). 50,000 naïve OT-I cells were retro-orbitally injected into B6 recipients, which were then retro-orbitally infected with 106 CFU LM-actA-Ova 1 day later. On D80 post infection, splenocytes were harvested from recipients, stained with anti-CD45.1-APC antibody then purified with Mojosort mouse anti-APC nanobeads (Biolegend Cat:480072). 106 enriched memory OT-I cells were adoptively transferred into MC38-Ova tumor-bearing mice, which were subcutaneously inoculated with 106 MC38-Ova tumor cells 4 days earlier. Tumor areas were measured three times a week.
Confocal microscopy.
Cells were mounted using poly-D-lysine, fixed with 2% glutaraldehyde then quenched with 1mg/mL NaBH4. Cells were then rendered permeable using 0.25% Triton X-100 solution, blocked and stained with polyclonal anti-rabbit TOM20 antibody (abcam ab78547 LOT:GR3199811-2) to label mitochondrial outer membranes, DAPI for nuclear staining. Texas red anti-rabbit IgG (VECTOR TI-1000) was used as a secondary antibody for TOM20 staining. Quantification was performed with Bitplane Imaris software (Oxford Instruments). Outlines were traced manually for each mitochondrion in all images, and Imaris software used to calculate the total mitochondrial volume and surface area for each cell. All microscopy was performed in the Dartmouth Institute for Biomolecular Targeting (BioMT).
ATP detection assay.
Naïve CD8 T cells were purified from spleens of CD45.1 OT-I mice (WT) or GZB-cre Zbtb20-fl/fl CD45.1 OT-I mice (KO) using EasySep mouse naïve CD8 T cell isolation kits (Stemcell Technologies cat:19858A). 50,000 naïve OT-I cells were retro-orbitally injected into congenic recipient mice, which were then retro-orbitally infected with 106 CFU LM-actA-Ova 1 day later. On D7 and D28 post infection, splenocytes were harvested from recipients, stained with anti-CD45.1-APC then purified with Mojosort mouse anti-APC nanobeads (Biolegend Cat:480072). Purified cells (purity greater than 95%) were then analyzed using a luminescence-based ATP detection assay (Cayman Chemical cat:700410).
Cell preparation for single cell RNAseq.
For isolation of CD8 T cells 10 days after infection, single-cell suspensions were generated from four mice per recipient group by macerating spleens through nylon filters. CD8 T cells were enriched from these suspensions using a Stemcell EasySep™ Mouse CD8 T Cell Isolation Kit (#19853). These samples were stained to block Fc receptors then stained with antibodies and live/dead stain (LIVE/DEAD™ Fixable Violet Dead Cell Stain Kit, ThermoFisher # L34955) for 30 minutes on ice shielded from light. The antibodies used for cell surface staining from BioLegend were as follows; PE anti-mouse CD8β Antibody (YTS156.7.7), APC anti-mouse CD45.1 Antibody (A20) and APC anti-rat CD90/mouse CD90.1 (Thy-1.1) Antibody (OX-7). Samples were subsequently washed twice and ~1X106 congenically marked OT-I cells were purified using fluorescence activated cell sorting for each group of recipients. The samples purified in this way from each group of recipients were then suspended in 100μL buffer and labeled with 1μg per sample of the following Total-seq A antibodies from BioLegend: TotalSeq™-A0198 anti-mouse CD127 (A7R34), TotalSeq™-A0250 anti-mouse/human KLRG1 (2F1/KLRG1), TotalSeq™-A0073 anti-mouse/human CD44 (IM7) and TotalSeq™-A0112 anti-mouse CD62L (MEL-14). Samples were labeled for 30 minutes on ice and subsequently washed with 1mL PBS twice.
Single-cell RNA Sequencing.
Single cell RNAseq library preparation were carried out by the Center for Quantitative Biology Single Cell Genomics Core and the Genomics and Molecular Biology Shared Resource at Dartmouth. Droplet-based 3′-end scRNA-seq was performed using the 10x Genomics Chromium platform, and libraries were prepared using the Single Cell v3 3′ Reagent kit according to the manufacturer’s protocol (10x Genomics, CA, USA). Recovery of antibody-DNA tags (ADTs) vfrom single cells (i.e. CITE-seq) was performed by adding 1ul of ADT additive primer (10uM, CCTTGGCACCCGAGAATT*C*C) to the cDNA amplification reaction and following the 10x protocol for separation of the ADT and mRNA-derived cDNA fractions. ADT libraries were further amplified using 1ul SI-PCR primer (10uM, AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGC*T*C) and 1ul Illumina RPI_X index primer, where X represents a unique index sequence per sample. ADT and mRNA libraries were normalized to 4uM and pooled at a 1:9 ratio before loading onto a NextSeq 500 instrument. Libraries were sequenced using 75 cycle kits, with 28bp on read1 and 56bp for read2.
Data Analysis for Single-cell RNA Sequencing.
The Cell Ranger Single-Cell Software Suite (10x Genomics) was used to perform barcode processing and transcript counting after alignment to the mm10 reference genome with default parameters. 7267 cells in the cKO and 10119 cells in the WT were analyzed for 10784 genes. Analysis of the gene-level transcript counts output by Cell Ranger was performed in R (Version 3.5.2) (79) on the merged KO and WT datasets (24) using the Seurat R package (Version 3.1.4) (24, 25). All ribosomal genes and genes with counts in fewer than 25 cells were excluded. Cells with mitochondrial DNA content > 10% (80) or non-zero counts for fewer than 500 genes or more than 3,000 genes were also removed. The filtered gene expression data was normalized using the SCTransform (81) method and subsequent computations were performed on the matrix of corrected counts. Unsupervised clustering was performed using Seurat’s implementation of shared nearest neighbor (SNN) modularity optimization with the resolution parameter set to 0.2 (28). For data visualization, single cell gene expression data were projected onto a reduced dimensional space as computed by the Uniform Manifold Approximation and Projection (UMAP) (27) method for the first 30 principal components of the expression data. The Variance-adjusted Mahalanobis (VAM)(23) method was used to compute cell-specific scores for pathways from Molecular Signature Database collections (MSigDB; Version 7.0) (82) that were filtered to remove pathways with fewer than 5 members or more than 200 members. We identified differentially expressed genes and pathways between KO and WT cells using Wilcoxon rank sum tests applied to either the normalized counts for each gene or the VAM scores for each pathway with p-values adjusted using the Bonferroni method.
Reagents:
EasySep Mouse naïve CD8 T cell isolation kits (Stemcell Technologies cat:19858A); Mojosort mouse anti-APC nanobeads (Biolegend Cat:480072); ATP detection assay kit-luminescence (Cayman Chemical cat:700410); DAPI (Thermo Fisher cat:D1306); Seahorse XF Cell Mito Stress Test Kit (Seahorse Agilent cat:103015-100); 2-DG (Cayman Chemical cat:14325); SIINFEKL peptide (New England peptide Lot:V1355-37/40); recombinant human IL-2 (TECIN cat:Ro23-6019); recombinant murine IL-15 (PeproTech cat:210-15); poly-D-lysine (Millipore Sigma cat:P6407); Glutaraldehyde (Electron Microscopy Science cat:16000); NaBH4 (Alfa Aesar stock#:35788); Triton X-100 (PerkinElmer cat:N9300260).
Antibodies:
violet fluorescent reactive dye (life technologies ref:L34955); CD45.1-BV421 (Biolegend cat:110732); Blimp1-BV421 (BD Bioscience cat:564270); CD8-BV510 (Biolegend cat:100752); CD45.1-BV510 (Biolegend cat:110741); CD45.1-APC (Biolegend cat:110714); CD62L-BV510 (Biolegend cat:104441); CD127-BV510 (Biolegend cat:135033); CD8-BV650 (Biolegend cat:100742); MitoTracker-Green FM (Invitrogen ref:M7514); CD62L-FITC (eBioscience cat:11-0621-85); Thy1.1-A488 (Biolegend cat:202506); Thy1.1-APC (Biolegend cat:202526); TCF1-A488 (cell signaling ref:02/2018); TNFa-FITC (Biolegend cat:506304); MITOsox Red mitochondrial superoxide indicator (Invitrogen ref:M36008); CD45.2-PE (Biolegend cat:109808); CD62L-PE (Biolegend cat:104408); CD127-PE (Biolegend cat:135010); EOMES-PE (invitrogen ref:12-4875-82); IL2-PE (Biolegend cat:503808); Thy1.1-PE (Biolegend cat:202524); TNFa-PE (Biolegend cat:506306); CD8-PerCPcy5.5 (Biolegend cat:100734); CD44-PerCPcy5.5 (Invitrogen ref:45-0441-82); Bcl6-PerCPcy5.5 (BD Pharmingen cat:562198); IFNy-PerCPcy5.5 (Biolegend cat:505822); Thy1.1-PEcy7 (Biolegend cat:202518); KLRG1-PEcy7 (Biolegend cat:138416); CD27-PEcy7 (Biolegend cat:124216); Tbet-PEcy7 (Invitrogen ref:25-5825-82); GZB-PEcy7 (eBioscience ref:25-8898-82); CD25-APC (Biolegend cat:102008); CD44-APC (Biolegend cat:103012); CXCR3-APC (Biolegend cat:126512); IFNy-APC (Biolegend cat:505810); Thy1.1-APC (Invitrogen ref:17-0900-82); p79-APC tetramer (NIH tetramer facility) Bcl2-A647 (Biolegend cat:633510); Bcl6-A647 (BD Pharmingen cat:561525); CD8-APCef780 (eBioscience; REF 47-0081-82); near-IR fluorescent reactive dye (Invitrogen ref:L10119); poly clonal anti-rabbit TOM20 (Abcam ab78547 LOT:GR3199811-2); Texas red anti-rabbit IgG (VECTOR TI-1000): TotalSeq™-A0198 CD127 (BioLegend, cat:135045); TotalSeq™-A0073 CD44 (BioLegend, cat:103045); TotalSeq™-A0112 CD62L (Biolegend cat:104451).
Results
Zbtb20 deficiency negatively regulates glycolytic and mitochondrial metabolism in CD8 T cells.
Previous research has shown Zbtb20 regulates glucose metabolism in liver cells (21), so we tested whether CD8 T cell metabolism was affected. To address how Zbtb20 deletion affected metabolism in effector and memory CD8 T cells, we differentiated OT-I cells in vitro into either terminal effector CD8 T cell (Teff) or central memory CD8 T cell (Tcm). As there was the potential for Zbtb20 to affect naïve CD8 T cell development or function, we elected to use a GZB-cre Zbtb20-fl/fl CD45.1 OT-I transgenic mouse model, where Zbtb20 is deleted in CD8 T cells only after T cell activation. Splenocytes from either GZB-cre Zbtb20-fl/fl CD45.1 OT-I mice (KO) or CD45.1 OT-I mice (WT) were stimulated with peptide in the presence of either IL-2 or IL-15. Consistent with previous reports, culture with IL-2 induced Teff-like cells, which are characterized by high expression of CD25 and low expression of CD62L, and culture with IL-15 induced Tcm-like cells, which express low levels of CD25 and high levels of CD62L (24) (Fig. S1A–B). Cells were then subjected to metabolic analysis to test mitochondrial respiration and glycolytic metabolism using the Seahorse Bioanalyzer.
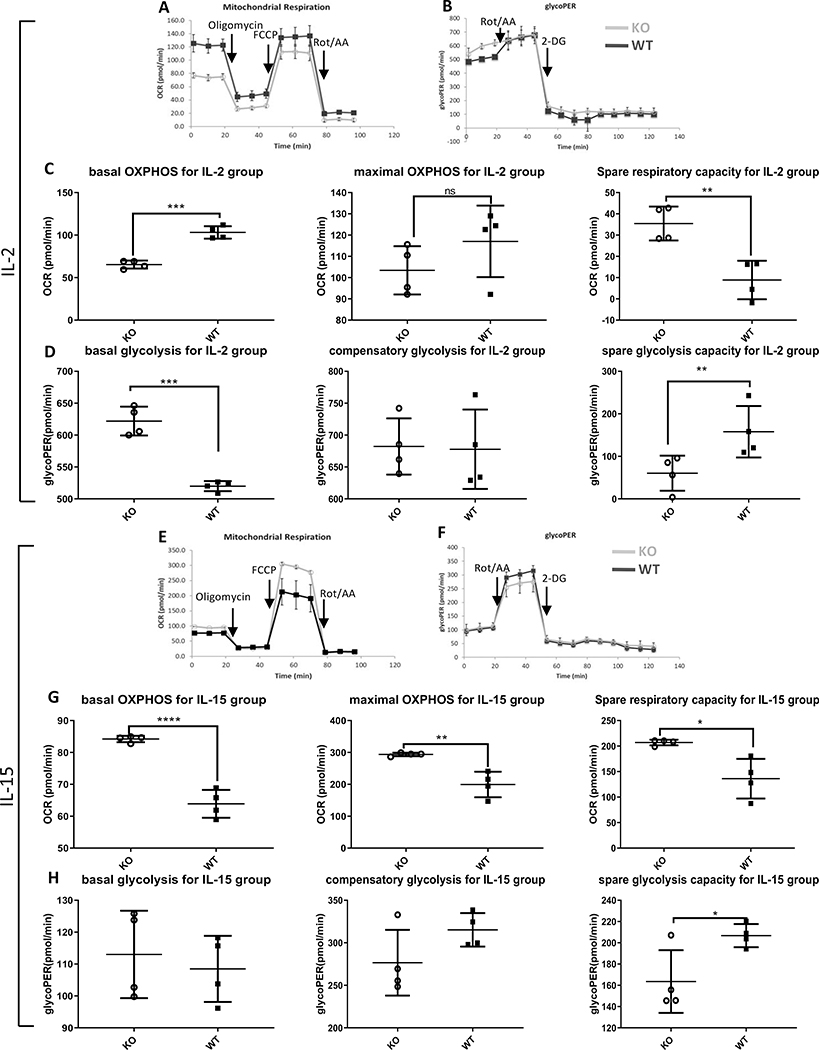
We observed WT Tcm cells had higher spare respiratory capacity (SRC) compared with Teff cells, consistent with previous studies (8) (Fig. 1A, 1E). Zbtb20 KO Teff cells had significantly lower basal mitochondrial respiration, indicated by basal oxygen consumption rate (OCR), compared with WT Teff, but maximal respiration was not different between WT and KO Teff (Fig. 1A, 1C). This resulted in a higher spare respiratory capacity in KO cells. We also interrogated the glycolytic capacity (glycoPER) of KO and WT Teff cells, as effector CD8 T cell are known to heavily depend on glycolysis for production of ATP and effector functions (25). We found that KO Teff displayed higher basal glycolysis compared with WT cells, but maximal glycolytic capacity (compensatory glycolysis) was not different between the groups. This resulted in little spare glycolytic capacity (SGC) in KO Teff, in contrast to WT Teff, which possessed significantly higher SGC (Fig. 1B, 1D). Taken together, our data suggested that in vitro-derived Zbtb20 KO Teff had the same maximal capacity for performing glycolysis as well as mitochondrial respiration as WT Teff. However, under basal conditions KO Teff displayed higher glycolytic activity.
Figure 1. Zbtb20 modulates glycolytic and mitochondrial respiratory capacity in effector and memory CD8 T cells differentiated in vitro.
Total splenocytes were harvested from WT OT-I mice and GZB-cre Zbtb20-fl/fl OT-I (KO) mice, then activated with SIINFEKL peptide for 48h without exogenous IL-2. Activated cells were further cultured with 100U/ml rhIL- 2 or 50ug/ml rmIL-15 for 7 days. Cultured cells were then analyzed using Seahorse XFe96 Analyzer. (A) Oxygen consumption profile showing mitochondrial respiration and (B) proton efflux rate profile showing glycolytic metabolism for IL-2 cultured cells. (C) Mitochondrial respiratory capacity of IL-2 cultured cells. (D) Glycolytic capacity of IL-2 cultured cells. (E) Mitochondrial and (F) glycolytic metabolic profiles for IL-15 cultured cells. (G) Mitochondrial respiratory capacity for IL-15 cultured cells. (H) Glycolytic capacity of IL-15 cultured cells. Each group consisted of at least four replicates and each experiment was repeated three times. Each point represents data from an individual mouse. Statistics were performed with Student’s unpaired t-test. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. Representative data from three experiments are shown.
In T cells cultured with IL-15, we found that KO Tcm displayed higher basal mitochondrial respiration, higher maximal respiration as well as higher SRC when compared with WT Tcm (Fig. 1E, 1G). Zbtb20 KO Tcm displayed similar basal glycolysis and compensatory glycolysis but significantly lower SGC compared with WT CD8 T cells (Fig. 1F, 1H).
Collectively, these data show that Zbtb20 deletion increased spare capacity for both glycolysis and mitochondrial respiration in both Teff and Tcm. Interestingly, we also found Zbtb20 deletion played opposite roles regulating basal and maximal mitochondrial respiration in in vitro generated Teff and Tcm, as it decreased those two parameters in Teff but increased them in Tcm compared with WT. Therefore Zbtb20 appears to regulate both glycolysis and mitochondrial respiration in CD8 T cells cultured in vitro.
Zbtb20 deficient memory CD8 T cells have increased mitochondrial mass.
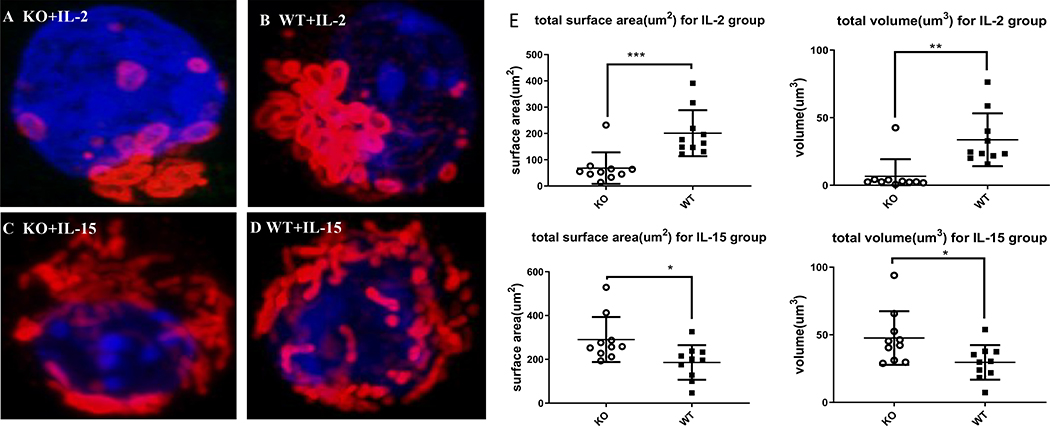
We wished to establish whether enhanced mitochondrial metabolism observed in Zbtb20 deficient Teff or Tcm cells was accompanied by increased mitochondrial content. In vitro generated Teff or Tcm CD8 T cells were fixed then stained to visualize the mitochondrial outer membrane. Examination by confocal microscopy and image analysis was used to quantify mitochondrial surface area and volume. This revealed that Zbtb20 KO Teff had less mitochondrial surface area and volume than WT cells, whereas Zbtb20 KO Tcm had larger mitochondrial surface area and volume than WT cells (Fig. 2A–E).
Figure 2. IL-15 differentiated memory CD8 T cells deficient in Zbtb20 have increased mitochondrial mass.
Cells were cultured as described in Figure 1, then analyzed by confocal microscopy. (A) Representative confocal image of KO OT-I T cells cultured with IL-2, (B) WT OT-I cells cultured with IL-2, (C) KO OT-I cells cultured with IL-15, (D) and WT OT-I cells cultured with IL-15. (E) Quantification of total mitochondrial surface area and volume in IL-2 or IL-15 treated groups. Quantification was determined on 3D reconstructed confocal images using Imaris software. Each point represents a single cell. Statistics were performed with Student’s unpaired t-test. *P<0.05, **P<0.01, ***P<0.001. Combined data from three experiments are shown.
Enhanced glycolysis and mitochondrial respiration in Zbtb20-deficient CD8 T cell responses ex vivo.
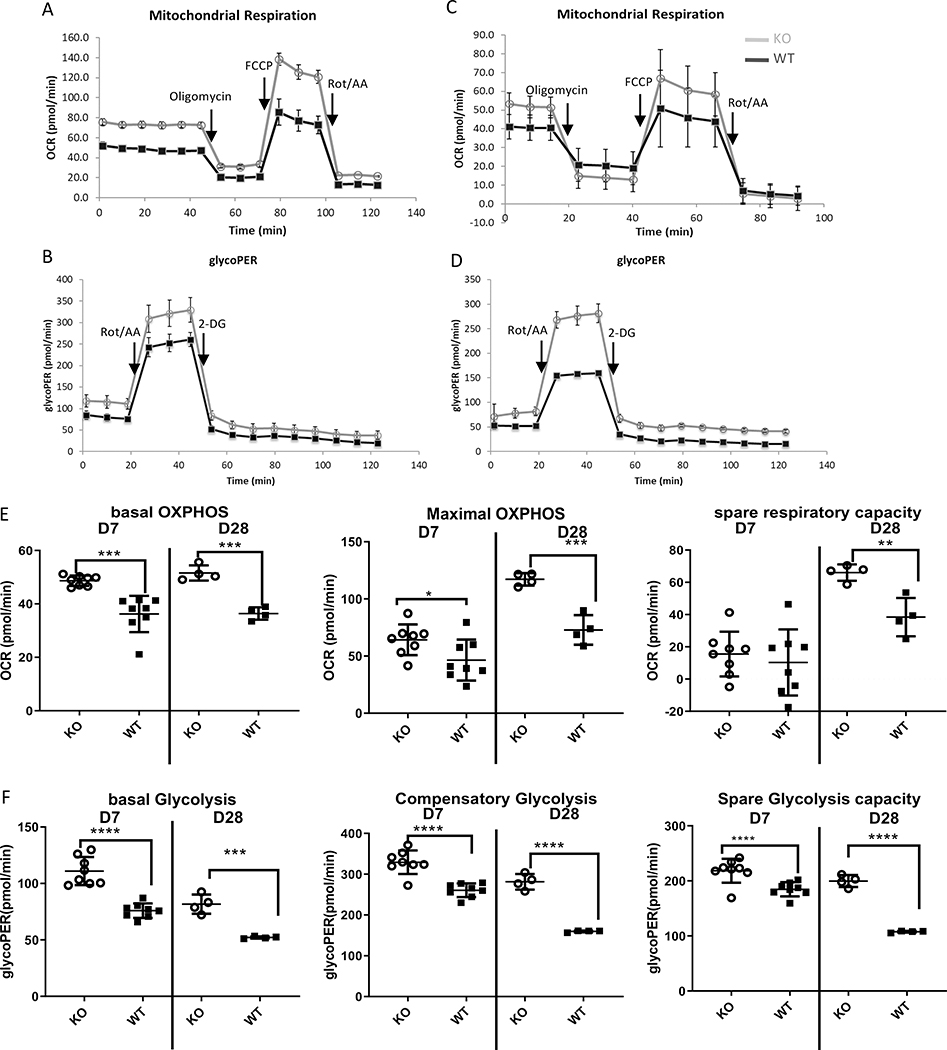
Metabolic changes were observed in Zbtb20-deficient CD8 T cells activated in vitro, so next we tested whether similar changes were present ex vivo. We adoptively transferred either KO or WT OT-I cells into recipient mice subsequently infected with LM-actA-Ova. Splenocytes were harvested at day 7 (effector) or day 28 (memory) post-infection and OT-I cells assayed for mitochondrial respiratory and glycolytic rates. We found that both effector and memory Zbtb20 KO CD8 T cells had higher basal and maximal mitochondrial respiration compared with WT (Fig. 3A, 3C and 3E). Zbtb20 KO memory, but not effector CD8 T cells also had higher spare respiratory capacity compared with WT (Fig. 3A, 3C, 3E). In addition, both effector and memory Zbtb20 KO CD8 T cells exhibited higher basal and maximal glycolysis as well as spare glycolytic capacity (Fig. 3B, 3D, 3F). Therefore, our data indicated that Zbtb20 KO effector and memory CD8 T cells directly taken from infected animals exhibited upregulated mitochondrial metabolism and glycolysis.
Figure 3. Zbtb20 deficient effector and memory CD8 T cells exhibit enhanced mitochondrial respiration and glycolytic capacity ex vivo.
Naïve CD8 T cells were harvested from CD45.1 OT-I mice (WT) or GZB-cre Zbtb20-fl/fl CD45.1 OT-I mice (KO). Naïve OT-I cells were retro-orbitally injected into B6 recipients, which were then retro-orbitally infected with LM-actA-Ova 1 day later. On day 7 or day 28 post-infection, splenocytes were harvested from recipients and OT-I cells were purified then subjected to mitochondrial and glycolytic metabolic analysis. (A) Oxygen consumption profile measuring mitochondrial respiration, (B) proton efflux rate measuring glycolytic metabolism for OT-I cells purified on day 7 post infection. (C) Mitochondrial and (D) glycolytic metabolic profiles for OT-I cells purified on day 28 post infection. (E) Quantitation of mitochondrial respiration in OT-I cells purified on either day 7 or day 28 post-infection. (F) Quantitation of glycolytic metabolism in OT-I cells purified on either day 7 or day 28 post-infection. Each point represents data from an individual mouse. Statistics were performed with Student’s unpaired t-test. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. Representative data from three experiments are shown.
To determine the extent to which these observations extended to other, unrelated infections, we performed similar experiments infecting with murine gammaherpesvirus-68 encoding Ova (MHV-68-Ova). Few changes were observed in the absence of Zbtb20 during the effector response to MHV-68 (d14) (Fig. S2). In contrast, memory (d28) CD8 T cells exhibited an increase in basal oxidative phosphorylation, and elevation in all glycolytic parameters, similar to the changes observed after LM infection. Therefore, while the identity of the infection influences the phenotype, it is clear Zbtb20 regulates both mitochondrial and glycolytic metabolism in two distinct infection models.
Effector and memory CD8 T cells lacking Zbtb20 possess increased ATP content and higher mitochondrial mass.
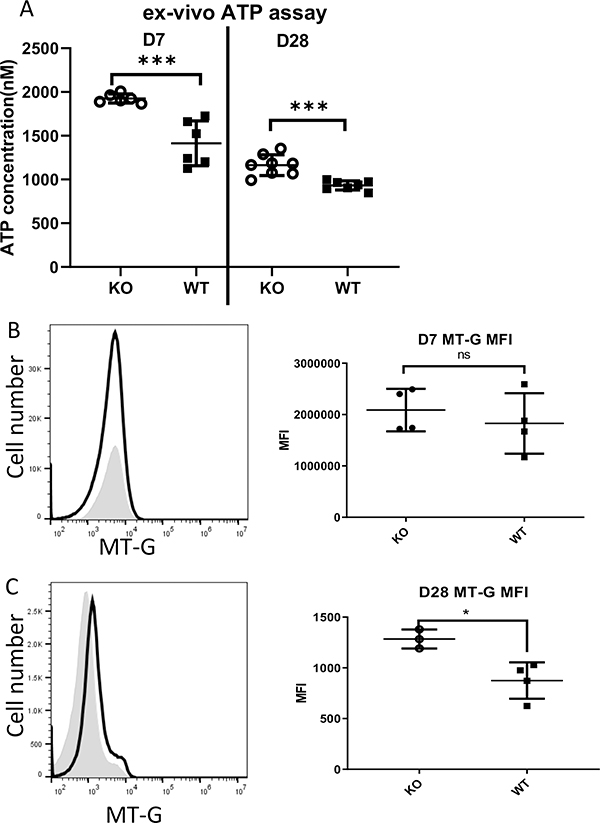
Elevated mitochondrial and glycolytic metabolism observed ex vivo in the absence of Zbtb20 implies these cells produce ATP at a higher rate. Therefore we measured the ATP content in WT and Zbtb20-deficient CD8 T cells. WT or KO OT-I cells purified from recipient mice at d7 or d28 post-infection were used in a luminescence-based ATP detection assay. We found ex vivo purified effector and memory Zbtb20 KO CD8 T cells consistently had higher ATP content than WT cells (Fig. 4A).
Figure 4. Zbtb20 deficient effector and memory CD8 T cells possess a higher ATP concentration and greater mitochondrial mass ex vivo.
Naïve CD8 T cells were harvested from CD45.1 OT-I mice (WT) or GZB-cre Zbtb20-fl/fl CD45.1 OT-I mice (KO). Cells were retro-orbitally injected into B6 recipients, which were then retro-orbitally infected with LM-actA-Ova 1 day later. (A) On day 7 and day 28 post infection, splenocytes were harvested from recipients and OT-I cells were purified by magnetic positive selection then analyzed by an ATP detection assay. On day 7 (B) or day 28 (C) post-infection, splenocytes were harvested from recipients, stained with mito-Tracker Green (MT-G) to quantify total mitochondrial mass then analyzed by flow cytometry. Representative histograms and quantification are shown. Shaded histogram – WT, empty histogram – Zbtb20 KO. Statistics were performed with Student’s unpaired t-test. *P<0.05, ***P<0.001. Data is representative of three experiments.
As our in vitro data indicated higher mitochondrial volume in the absence of Zbtb20 under in vitro conditions that skewed to memory, we measured mitochondrial mass ex vivo. Accurate confocal analysis of these cells was not possible due to the smaller size of effector and memory CD8 T cells directly ex vivo when compared with in vitro generated cells. We therefore measured mitochondrial content by staining with the mitochondrial dye Mitotracker Green. We found that Zbtb20 KO OT-I cells had no significant difference in mitochondrial content at day 7 (Fig. 4B), but higher mitochondrial content at day d28 post-infection (Fig. 4C).
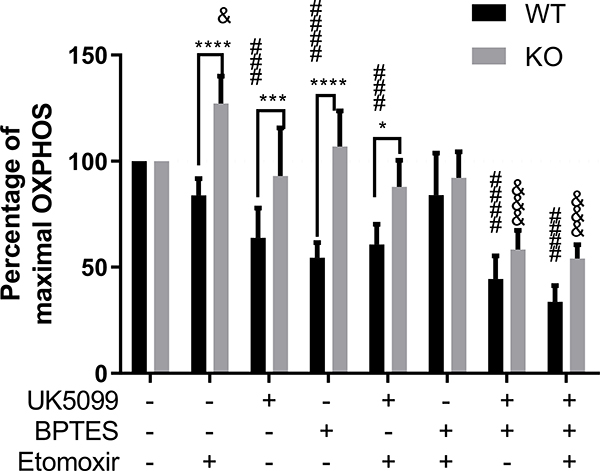
Increased mitochondrial fuel flexibility in the absence of Zbtb20
As we observed increased mitochondrial respiration in the absence of Zbtb20 in memory cells, we tested whether there were differences in the reliance on different mitochondrial fuels. We generated Tcm in vitro by culture with IL-15, then treated with inhibitors of different mitochondrial fuel sources (etomoxir for long-chain fatty-acid; UK5099 for pyruvate; BPTES for L-glutamine), followed by conventional Seahorse mitochondrial stress tests. The maximal respiratory capacity in each condition was normalized to values without inhibitor treatment. We found that WT Tcm cells displayed reduced mitochondrial activity when they could not utilize pyruvate or L-glutamine (Fig. 5). In contrast, ZBTB20 KO Tcm cells only displayed reduced mitochondrial activity when they could not use both pyruvate and L-glutamine. Overall, ZBTB20 KO Tcm cells were more flexible with regard to mitochondrial fuel sources compared with WT Tcm, which suggests that ZBTB20 KO Tcm cells could have survival and/or functional advantages over WT Tcm cells in nutrient-limiting environments.
Figure 5. IL-15 differentiated ZBTB20 KO memory CD8 T cell had increased mitochondrial fuel flexibility.
Splenocytes were harvested from OT-I mice and GZB-cre ZBTB20-f/f OT-I (KO) mice, then activated with SIINFEKL peptide for 48h without exogenous IL-2. Activated cells were further cultured with 50ug/ml rmIL-15 for 7 days. Cultured cells were then analyzed using Seahorse XFe96 Analyzer. Cells were treated with no inhibitor or combinations of different inhibitors that prevented the utilization of different mitochondrial fuel source, followed by a Seahorse Agilent Mito Stress test. The maximal Respiratory Capacity of each condition was normalized to the group without inhibitor treatment. Each group consisted of at least four replicates and each experiment was repeated two times. Statistics were performed using Student’s unpaired t-test. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. & and # symbols are used as equivalents of * symbols, where & indicates statistical significance between inhibitor treated ZBTB20 KO cells vs no inhibitor treated ZBTB20 KO cells, and # indicates statistical significance between inhibitor treated WT cells vs no inhibitor treated WT cells.
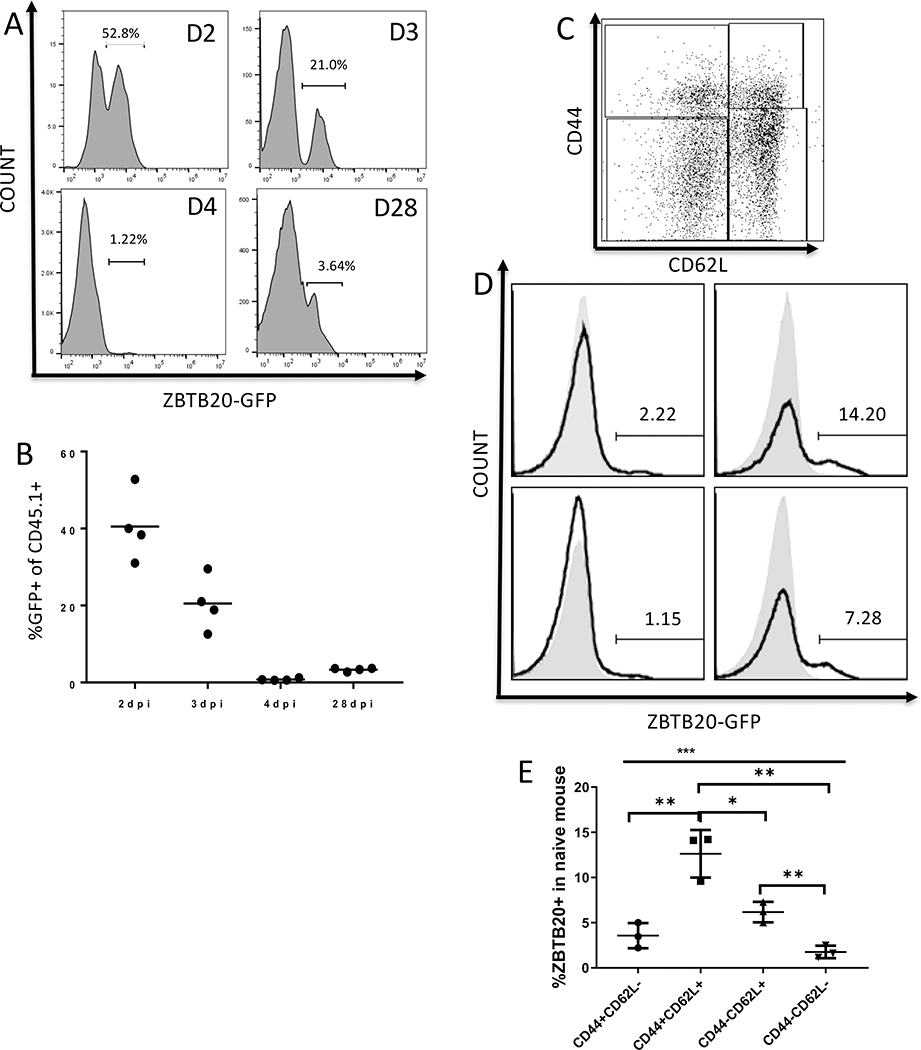
Zbtb20 is induced in activated CD8 T cells.
In order to dissect the expression pattern of Zbtb20 in CD8 T cells, we took advantage of a Zbtb20 reporter mouse strain where GFP is expressed from the Zbtb20 promoter. Naïve Zbtb20-GFP OT-I cells were adoptively transferred into recipient mice, which were then intravenously infected with LM-actA-Ova. Initial experiments showed little GFP expression during the peak of the response, so we focused on early after infection and memory. Splenocytes were harvested from recipient mice on day 2, 3, 4 and 28 post-infection for analysis. We found Zbtb20 was expressed in approximately half of the OT-I population on D2 post infection (Fig 6. A–B). By D3 the proportion of positive cells decreased, and was very low by D4 post infection. However by D28 Zbtb20 reporter was again detectable in a small proportion of cells. To identify T cell populations expressing Zbtb20 during the steady state in the polyclonal T cell repertoire in a naïve mouse, we harvested splenocytes from unmanipulated Zbtb20-GFP mice. We found the phenotype with the highest proportion of Zbtb20 expressing cells was Tcm (defined as CD44+CD62L+) (Fig. 6C–E). Naïve CD8 T cells (defined as CD44-CD62L+) also contained a small proportion of Zbtb20 expressing cells. However, CD44+CD62L− and CD44−CD62L− CD8 T cells contained very low proportions of cells expressing Zbtb20.
Figure 6. Kinetics of Zbtb20 expression in vivo.
Naïve CD8 T cells were purified from CD45.1 OT-I Zbtb20-GFP mice. Cells were retro-orbitally transferred into CD45.2 B6 recipients, which were then retro-orbitally infected with LM-actA-Ova 1 day later. Splenocytes were harvested from recipients and analyzed by flow cytometry. Naïve Zbtb20-GFP mice were used for the naïve time point. (A) Representative histograms for GFP expression at the times indicated after infection and (B) quantification. (C) Representative dot plot for CD44 and CD62L staining in naïve Zbtb20-GFP mice, (D) histograms showing corresponding GFP expression from each quadrant, shaded histogram – B6 negative control, empty histogram – Zbtb20 GFP. (E) Quantification of data shown in (D). Each point represents data from an individual mouse. Each group used at least four mice and each experiment was repeated three times. Statistics were performed with Student’s unpaired t-test. *P<0.05, **P<0.01, ***P<0.001.
To determine the Zbtb20 expression profile among polyclonal virus-specific CD8 T cells, we infected Zbtb20-GFP reporter mice on a B6 background intranasally with MHV-68. Their splenocytes were harvested before infection and on day 10, day 14 and day 28 post infection then stained with an MHC/peptide tetramer folded with the dominant epitope derived from the ORF61 protein. Reporter expression within the tetramer positive population was then measured within populations defined by CD44 and CD62L expression (Fig. S3A–C). Consistent with data from naïve mice, Zbtb20 reporter expression was detected mostly in Tcm (CD44+CD62L+) and naïve (CD44-CD62L+) populations (Fig. S3D). PCR analysis on FACS purified GFP+ and GFP− cells showed GFP faithfully represented Zbtb20 mRNA expression in reporter mice (Fig. S3E).
Given Zbtb20 expression at the early stages of effector differentiation and in a subset of central memory phenotype cells, we tested how Zbtb20 deficiency affected effector and memory differentiation in vivo.
Zbtb20 deletion enhances cytokine production and favors memory precursor differentiation.
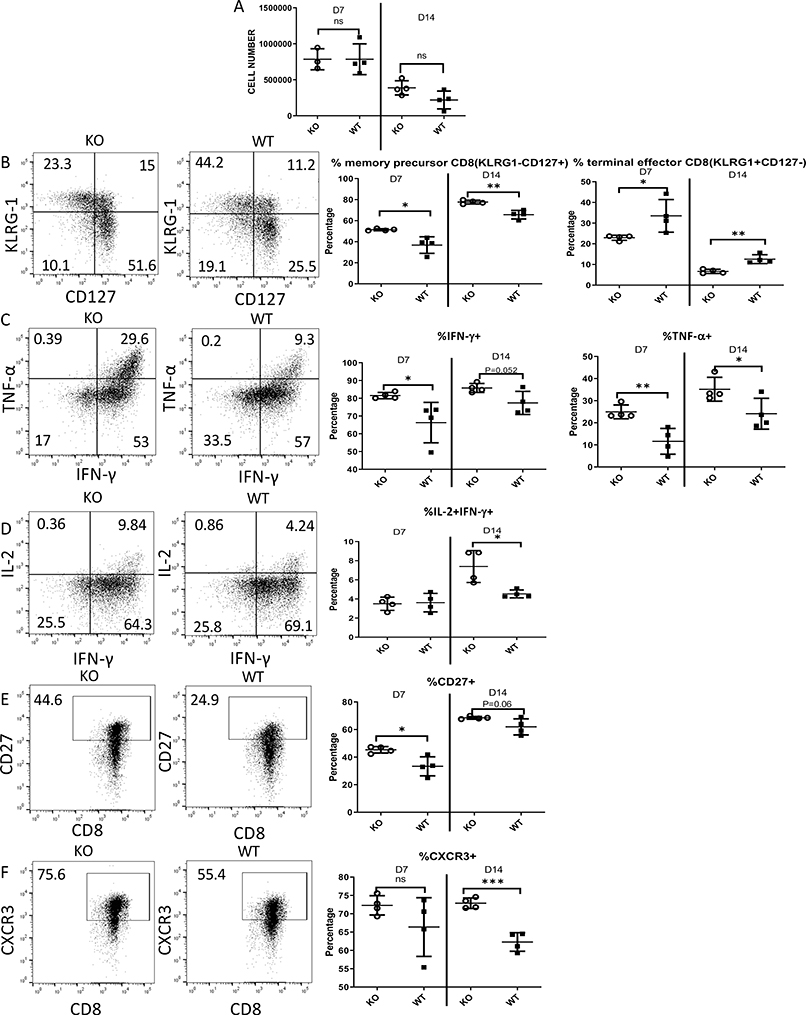
We sought to determine how Zbtb20 deletion affected CD8 T cell clonal expansion, accumulation, function and differentiation. Naïve OT-I cells from KO or WT donor mice were adoptively transferred into recipient mice, which were then intravenously infected with LM-actA-Ova. Splenocytes were harvested for analysis on day 7 or day 14 post-infection. We found the number of transferred OT-I cells recovered from the spleens of recipient to be the same at D7, which measures the peak CD8 T cell response against LM, and D14, which is during the contraction phase (Fig. 7A, gating strategy Fig. S1C). Examining the phenotype of responding OT-I T cells, we found that on both D7 and D14 post-infection, Zbtb20 KO OT-I cells were more skewed towards memory precursors (defined as KLRG-1−CD127+) and less toward terminally differentiated effectors (defined as KLRG-1+CD127−) (Fig. 7B). In addition, cytokine production profiles revealed that a higher proportion of Zbtb20 KO OT-I cells could produce IFN-γ or TNF-α as well as both IL-2 and IFN-γ simultaneously (Fig. 7C–D). Production of IL-2 is a characteristic of memory cells, consistent with memory precursor skewing, and we detected a higher proportion of KO cells expressing CD27, that is preferentially expressed on central memory CD8 T cells (Fig. 7E). We also found a higher proportion of Zbtb20 KO effector CD8 T cell expressed CXCR3 during the contraction phase (Fig. 7F), an important chemokine receptor that drives effector CD8 T cell to sites of inflammation. Taken together, our data suggested that Zbtb20 KO effector CD8 T cells had a phenotype indicative of increased memory potential and enhancements in cytokine production.
Figure 7. Zbtb20 deletion affects effector CD8 T cell differentiation during acute LM infection.
Naïve CD8 T cells were harvested from CD45.1 OT-I mice (WT) or GZB-cre Zbtb20-fl/fl CD45.1 OT-I mice (KO). Naïve OT-I cells were retro-orbitally injected into B6 recipients, which were then retro-orbitally infected with LM-actA-Ova 1 day later. Splenocytes were harvested on day 7 or 14 post-infection and analyzed by flow cytometry. (A-F) All plots were gated on transferred OT-I cells. (A) Total cell counts for transferred OT-I cells from the spleen of each recipient. (B) Representative dot plot showing KLRG-1 and CD127 staining to measure memory precursor cells (KLRG-1-CD127+) and terminal effector cells (KLRG-1+CD127-), and quantification. (C) Representative dot plot showing TNF-α and IFN-γ staining and quantification. (D) Representative dot plot showing IL-2 and IFN-γ staining and quantification. (E) Representative dot plot showing CD27 and CD8 staining and quantification. (F) Representative dot plot showing CXCR3 and CD8 staining and quantification. Each point represents data from an individual mouse. Each group used at least four mice and each experiment was repeated three times. Numbers in dot plot quadrants show percentage of events in the relevant quadrant. Statistics were performed with Student’s unpaired t-tests. *P<0.05, **P<0.01, ***P<0.001.
Zbtb20 deletion affects the memory CD8 T cell phenotype and cytokine production.
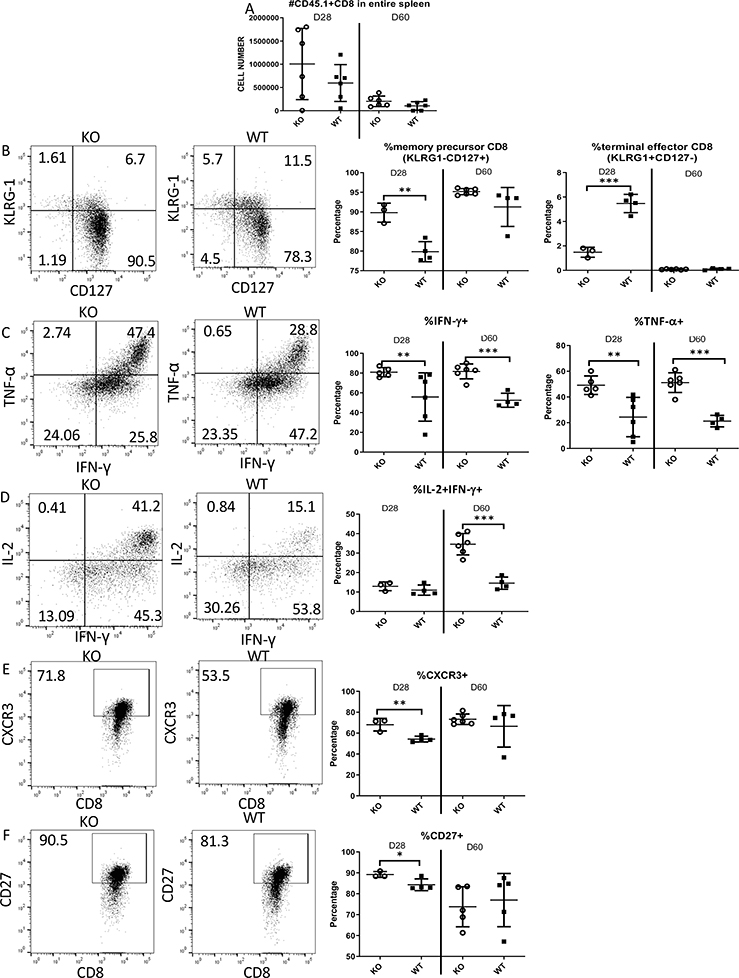
Using the OT-I transfer and LM-actA-Ova infection model described above, we tracked Zbtb20 KO and WT OT-I cells until later times post infection, which allowed us to study the role of Zbtb20 in CD8 T cell memory. On D28 and D60 we found the number of splenic Zbtb20 KO memory OT-I cells to be the same as WT OT-I cells (Fig. 8A). Consistent with earlier times after infection, Zbtb20 KO OT-I cells were more skewed towards memory precursors than effector cells on D28 (Fig. 8B). In addition, a higher proportion of Zbtb20 KO memory OT-I cells could produce IFN-γ or TNF-α (Fig. 8C) as well as both IL-2 and IFN-γ simultaneously (Fig. 8D). Moreover, a larger percentage of Zbtb20 KO memory OT-I cells expressed CXCR3 and CD27 on D28 (Fig. 8E–F). Data summarizing phenotypic marker expression and cytokine production over time after infection is shown in Fig. S1D. Data obtained from memory timepoints therefore showed skewing toward memory CD8 T cells was consistent with earlier times after infection.
Figure 8. Zbtb20 deletion affects CD8 T cell differentiation to memory precursors and effector CD8 T cells.
Samples from the experiment described in Fig. 6 were used to measure cytokine production and memory precursor or effector differentiation on day 28 or day 60 post-infection. (A) Cell counts for transferred OT-I cells from the spleen of each recipient. (B) Representative dot plot showing KLRG-1 and CD127 staining and the percentage of memory precursors (KLRG-1-CD127+) and terminal effector cells (KLRG-1+CD127-) and quantification. (C) Representative dot plot showing TNF-α and IFN-γ staining and quantitation. (D) Representative dot plot showing IL-2 and IFN-γ staining and quantitation. (E) Representative dot plot showing CXCR3 and CD8 staining and quantitation. (F) Representative dot plot showing CD27 and CD8 staining and quantitation. Each point represents data from an individual mouse. Each group consisted of at least four mice and each experiment was repeated three times. Numbers in dot plot quadrants show percentage of events in the relevant quadrant. Statistics were performed with Student’s unpaired t-test. *P<0.05, **P<0.01, ***P<0.001.
Modulation of key transcription factors in the absence of Zbtb20.
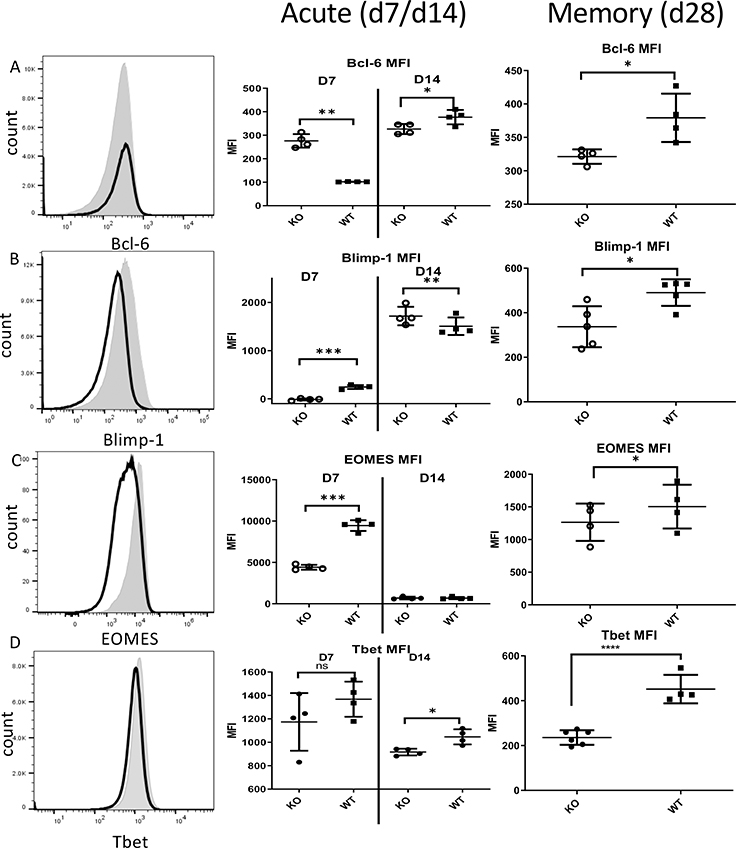
A network of transcription factors tightly orchestrates differentiation of effector and memory CD8 T cells. These regulate the expression of crucial cytokine receptors, pro-apoptotic and anti-apoptotic factors, cellular metabolism and other critical functions. Interrogation of transcription factor expression revealed that Zbtb20 KO effector CD8 T cell expressed higher levels of Bcl-6 and lower levels of Blimp-1 on D7, whereas on D14 KO CD8 T cell expressed lower Bcl-6 and higher Blimp-1 compared with WT (Fig. 9A–B). At the memory timepoint (D28), both Bcl-6 and Blimp-1 were lower in KO CD8 T cells. We found Zbtb20 KO CD8 T cells had lower expression of Eomes, a transcription factor necessary for memory CD8 T cell differentiation, on D7 and D28 post-infection but not on D14 (Fig. 9C). T-bet, a transcription factor important for effector CD8 T cell differentiation, was expressed at a lower level in Zbtb20 KO CD8 T cells at D14 and D28, but did not reach statistical significance at D7 post infection (Fig. 9D). Collectively, our data suggested that Zbtb20 affects expression of several transcription factors important for effector and memory CD8 T cell differentiation. Consistent with these data, we also found Zbtb20 KO memory cells expressed lower levels of Bcl-6, EOMES and T-bet in the MHV-68 infection model (Fig. S4).
Figure 9. Zbtb20 deletion changes expression of key transcription factors in CD8 T cells during acute infection and in memory.
Splenocytes from mice treated as described in Fig. 6 were stained for expression of intranuclear transcription factors on days 7, 14 and 28 post-infection. Representative histograms from D7 are shown, together with quantification for all timepoints for (A) Bcl-6, (B) Blimp-1, (C) EOMES, and (D) T-bet staining. Shaded histogram – WT, empty histogram – Zbtb20 KO. Each point represents data from an individual mouse. Each group comprised at least four mice and each experiment was repeated three times. Statistics were performed using Student’s unpaired t-test. *P<0.05, ****P<0.0001.
Single cell transcriptomic analysis shows enrichment in metabolic and memory pathways in the absence of Zbtb20
Many studies have shown there is substantial heterogeneity in the CD8 T cell response with respect to the potential to differentiate into memory cells. In order to conduct transcriptomic analyses that could capture this heterogeneity, we performed single cell RNAseq analysis on OT-I cells during the primary response. Using the OT-I transfer, LM-actA-Ova infection model described, WT and Zbtb20 KO CD8 T cells were purified, and CITEseq performed with oligonucleotide-labeled antibodies against KLRG-1, CD127 and CD62L, to orient gene expression patterns with known effector/memory markers.
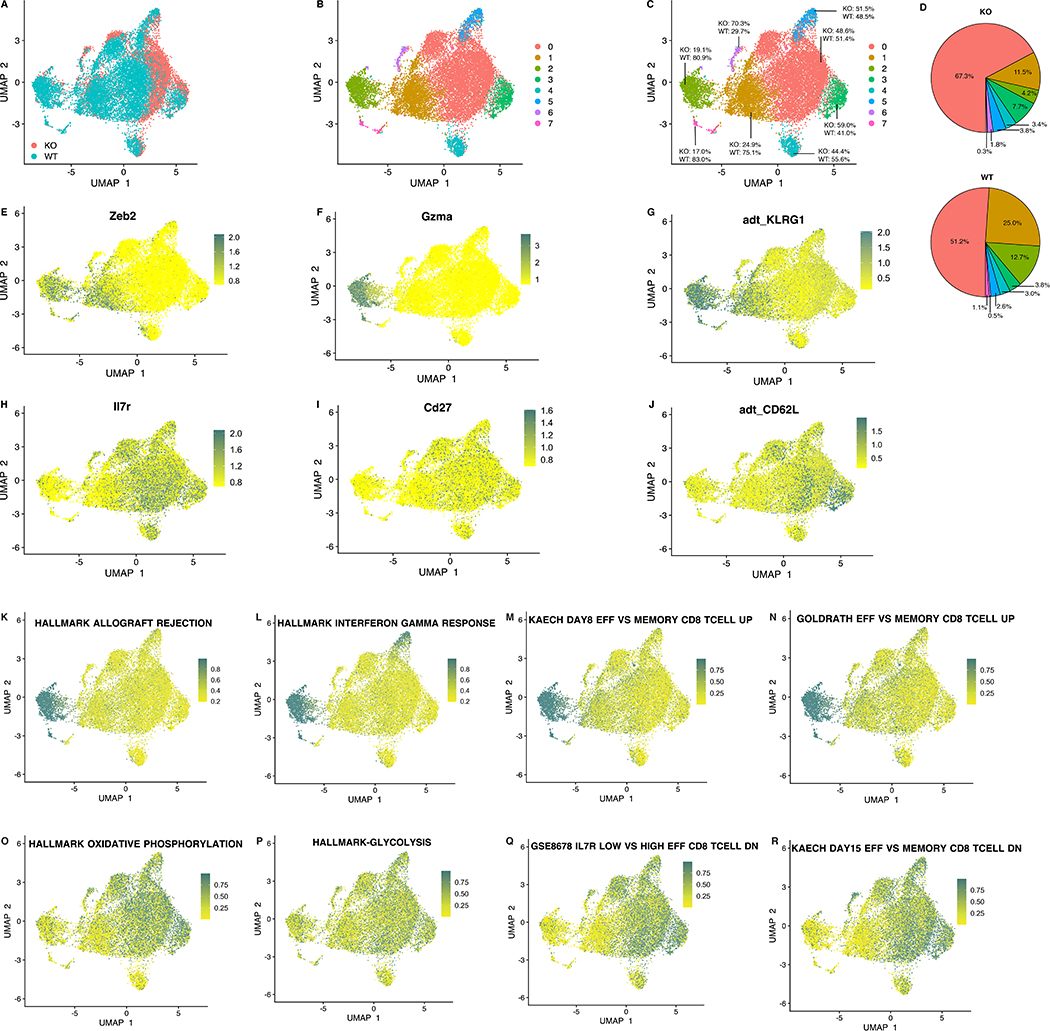
UMAP plots showed some overlaps in clusters occupied by WT and KO cells (Fig. 10A–C), however there were also regions where there was little overlap. In particular a higher proportion of WT cells were in clusters 1 and 2 whereas clusters 0 and 3 were more highly represented in cKO cells (Fig. 10B–C). Analysis of gene representation in these clusters showed that clusters 1 and 2 were enriched for genes and proteins associated with effector T cells (Zeb2, Granzyme A and KLRG-1 staining) (Fig. 10E–G). In contrast, memory associated genes and proteins (IL7r, Cd27 and CD62L staining) were not present in these clusters, and instead seen preferentially in clusters 0, 3, and 5 (Fig. 10H–J), where the majority of KO cells were located. Examination of a wider array of genes expressed in these clusters showed preferential expression of genes associated with effector activity in clusters 1 and 2 (Zeb2, CX3CR1, Klrg1, Gzmb, Gzma) (26–30) (Fig. S5A, left panel). Comparison of genes differentially regulated between WT and KO samples showed KO cells expressed higher levels of Pkm and mt-Nd3, necessary for pyruvate synthesis in glycolysis and mitochondrial NADH dehydrogenase, respectively (Fig. S5A, right panel). An extended list of metabolism-associated genes that were differentially expressed is shown in Fig. S5C.
Figure 10. Gene- and pathway-level single-cell RNA-seq KO and WT comparison.
Mice received naïve OT-I or Zbtb20-deficient OT-I cells and were then infected with LM-actA-Ova. Spleen cells were harvested during the effector response, OT-I cells purified, and CITEseq/RNAseq performed as described. (A) UMAP embeddings of merged KO and WT profiles at day 10 colored by KO and WT status. (B-C) UMAP embeddings colored by expression cluster and displaying distribution of KO and WT cells within each expression cluster. KO and WT cells per cluster are denoted in C as percentages i.e. the number of KO or WT cells divided by the total number of cells in the cluster. (D) The distribution of clusters across all KO cells examined and the distribution of clusters across all WT cells is displayed as pie charts. (E-J) UMAP embeddings displaying expression of effector and memory function genes and the cell surface protein expression of the KLRG1 and CD62L markers. (K-R) UMAP embeddings of merged KO and WT profiles colored by cell-level pathway enrichment scores for gene sets in the Hallmark and C7 pathway collections in the Molecular Signature Database (MSigDB). Activity of pathways enriched in WT cells is displayed in K-N while activity of pathways enriched in KO cells are displayed in O-R.
Pathway level analyses were performed using the novel variance-adjusted Mahalanobis (VAM)(23) method that was recently developed in order to compute cell level gene-set scores visualized in the UMAP plots. Differentially active pathways were also computed using a rank-sum test. Cluster 2 was associated with gene sets previously shown to be upregulated in effector T cells, in addition to gene sets from pro-inflammatory conditions such as allograft rejection and the interferon gamma response (Fig. 10K–N). Gene sets associated with oxidative phosphorylation and glycolysis were preferentially associated with clusters 0 and 3, where the majority of KO cells were located (Fig. 10O–P). A similar pattern of association with clusters 0 and 3 was seen with gene sets previously shown to be downregulated in effector CD8 T cells relative to memory or memory precursor cells (Fig. 10Q–R). An extended list of pathways differentially expressed in the various clusters is shown in Fig. S5B (left panel), and was consistent with effector-associated pathway enrichment in clusters 1 and 2, and memory, glycolysis and mitochondrial metabolism associated pathway enrichment in clusters 0 and 3. Comparison of pathways enriched in KO vs WT samples (Fig. S5B, right panel) showed glycolysis and mitochondrial metabolism associated pathways enriched in KO samples. Pathways upregulated in memory cells when compared with either effector or naïve cells were also enriched KO compared with WT samples. In contrast, effector-associated pathways were enriched in WT samples.
These data clearly confirm our flow cytometric and Seahorse data, showing in the absence of Zbtb20, the CD8 T cell response skews toward the memory phenotype, with enhancement of both glycolytic and mitochondrial metabolism.
Zbtb20 KO memory CD8 T cells mount a more efficient secondary response and confer better anti-tumor immunity.
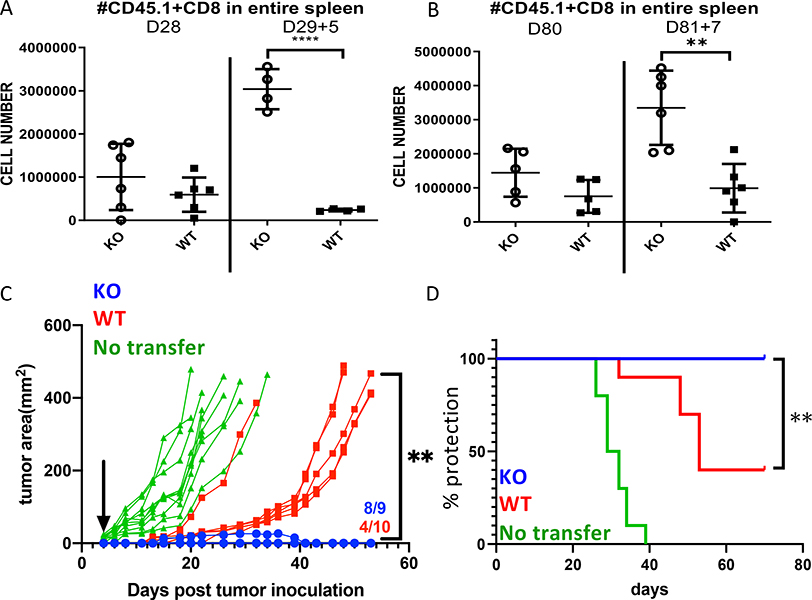
As the previous data indicated the absence of Zbtb20 enhanced differentiation toward memory CD8 T cells, we next tested the capacity of Zbtb20 KO and WT memory CD8 T cells to accumulate following secondary antigenic challenge. Using the same OT-I transfer and LM-actA-Ova infection model, we intravenously challenged groups of recipient mice on D29 or D81 post infection with MHV-68-Ova. Fig. 11A shows numbers of OT-I cells both before and after challenge. The secondary infection was insufficient to induce a detectable recall response from WT memory cells, however Zbtb20 KO memory CD8 T cells expanded robustly upon re-challenge at both timepoints (Fig. 11A–B). Both Zbtb20 KO and WT OT-I cells cleared the MHV-68-Ova to undetectable levels within 5 days after re-challenge.
Figure 11. Zbtb20 deletion enhances the recall response of memory CD8 T cells and increases protection against MC38 tumor.
(A-D) OT-I cells were adoptively transferred and infected as described in Figure 7. On day 29 or day 81 post infection, recipient mice were challenged with MHV-68-Ova retro-orbitally. Splenocytes were harvested after re-challenge for flow cytometric analysis. (A-B) Cell counts for transferred OT-I cells from the entire spleen of recipients challenged on (A) D28 or (B) D80 post-infection. Each point represents data from an individual mouse. Each group comprised at least four mice and each experiment was repeated three times. Statistics were performed with Student’s unpaired t-test. **P<0.01, ****P<0.0001. (C-D) On D80 post infection, splenocytes were harvested from recipient mice and OT-I cells were purified. 106 memory OT-I cells were transferred into MC38-Ova tumor-bearing mice (D4 post subcutaneous inoculation of MC38-Ova) and tumor area was measured. (C) Plot showing tumor growth in individual animals. Arrow represents time of T cell injection. Numbers at bottom right indicate the proportion in each group that did not grow measurable tumor (baseline on graph). (D) Time to endpoint curve, with the endpoint defined as the tumor area exceeding 400 mm2. N=9 for KO group, N=10 for WT and no T cell transfer groups. Data is representative of three replicate experiments. Statistics were performed with 2-way ANOVA (C) or a Mantel-Cox log rank test (D). **P<0.01.
To test whether Zbtb20 KO memory CD8 T cells had enhanced protective capability, we tested their ability to inhibit growth of MC38-Ova tumors. Naïve OT-I cells were transferred into recipient mice that were subsequently infected with LM-actA-Ova. At 80 days post-infection, memory OT-I cells were purified then adoptively transferred into recipient mice bearing subcutaneous MC38-Ova tumors that had been growing for 4 days. In mice that received no CD8 T cells, tumors developed quickly in all mice (Fig. 11C). Tumor growth was significantly slower in recipients of WT memory cells, however the majority of mice developed tumors (Fig. 11D). In contrast, most mice that received Zbtb20 KO memory OT-I cells did not develop measurable tumors, and those that were measurable grew only to a small size then resolved (Fig. 11C–D). Therefore memory CD8 T cells lacking Zbtb20 conferred superior protection against tumors compared with memory cells where Zbtb20 was intact.
Taken together, our data show Zbtb20 restrains mitochondrial metabolism and glycolysis, together with differentiation toward memory precursor cells. This results in memory CD8 T cells with a higher capacity for cytokine production and a more potent ability to mount secondary and anti-tumor responses.
Discussion
Based on phenotypic, functional and metabolic techniques, in conjunction with transcriptional profiling, it is clear that the absence of Zbtb20 skews CD8 T cell differentiation toward the generation of memory. Interestingly, it seems not all KO memory precursor cells survived, as we did not consistently see a larger memory population in KO mice. Bias away from an effector-type profile was particularly evident in our single cell RNAseq analyses, which also showed enrichment for genes sets associated with memory. A particularly novel finding is that both glycolytic and mitochondrial metabolism were enhanced, whereas typically perturbations that promote memory differentiation enhance mitochondrial metabolism at the expense of glycolytic metabolism (31–34).
Previous studies have shown a critical role for Zbtb20 in hippocampal development and the correct development of neuronal layers in the cerebral cortex (35–38). Consistent with this, patients with certain mutations in Zbtb20 develop Primrose syndrome (39), which includes intellectual disability, macrocephaly and increased height and weight (40–45). Detailed study of patients with Primrose syndrome revealed metabolic changes, including reduced glucose tolerance, with prevalence of amino acid and fatty acid catabolism, ketogenesis, and gluconeogenesis (46). This indicates impairment in the normal pathway from glucose to pyruvate and then into the citric acid cycle. Instead, amino acids and fatty acids are converted to glucose and ketone bodies, similar to the processes that occur in diabetes and during prolonged fasting. This indicates Zbtb20 regulates genes associated with glucose and fatty acid metabolism in humans. Consistent with this, data from Zbtb20 knockout mice showed disrupted glucose homeostasis, and dysreglation of genes associated with glucose metabolism in the liver (21). These mice had severe growth defects and decreased survival, not living beyond 12 weeks of age, however restoration of Zbtb20 selectively in the liver markedly improved survival. Later work using liver-specific Zbtb20 deletion showed Zbtb20 regulates genes associated with glycolysis and de novo lipogenesis (47), and beta-cell specific Zbtb20 deletion lead to aberrant glucose metabolism and altered expression of glycolysis-associated genes (48). To our knowledge, the present report is the first to describe a role for Zbtb20 in metabolic control in the immune system. Our single cell RNAseq data suggest several genes central to glycolysis and mitochondrial metabolism are regulated by Zbtb20, and future studies will confirm whether these genes represent direct or indirect targets of Zbtb20.
It is clear that activated and quiescent T cells have distinct bioenergetic and biosynthetic demands (49). Activation, proliferation, epigenetic, cytotoxic functions and differentiation of T cells are directed by dynamic changes of their metabolism (50). These changes are evident both in mitochondrial structure and in the choice of predominantly mitochondrial or glycolytic metabolism used by the T cell. Mitochondria have a highly compartmentalized structure and their morphology can be very dynamic. Mitochondrial morphology is critical for DNA sequestration, reactive oxygen species regulation, oxidative phosphorylation and calcium homeostasis (51). Inter-connected mitochondria are linked to increased demand for ATP or nutrient starvation (13, 14, 52–55), whereas globular and fragmented mitochondria are linked to nutrient excess, lower demand for ATP or severe cellular stress (56, 57). Mitochondria can adapt their morphology under different cellular activation states in T cells, macrophages and mast cells (16, 58, 59). Rapidly proliferating effector CD8 T cells possess globular mitochondria, whereas memory CD8 T cells contain highly inter-connected, tubular mitochondria (16). As memory CD8 T cells rely upon mitochondrial respiration for their energy demands, elongated mitochondria with well-ordered cristae are thought to hold components of the electron transport chain in a more efficient configuration (60). Our data indicate that mitochondria in Zbtb20 KO memory CD8 T cells have a larger volume and surface area compared with wild-type cells, which is consistent with enhanced oxidative phosphorylation observed in these cells. Interestingly, mitochondrial content was lower in Zbtb20 KO in vitro-derived effector CD8 T cells. This is consistent with the observed lower basal and maximal oxidative phosphorylation. Nevertheless KO effector cells did not exhibit impairments in cytokine production or proliferation, presumably due to the enhanced glycolytic metabolism we observed, which provided the necessary ATP and biosynthetic intermediates.
Our Seahorse assays clearly showed Zbtb20 deficiency modulates T cell metabolism, however there were some subtle differences observed between in vitro and ex vivo generated effector and memory cells. Basal and maximal glycolysis and oxidative phosphorylation were uniformly increased in ex vivo effector and memory CD8 T cells. While IL-15 generated memory cells also displayed elevated basal and maximal oxidative phosphorylation, glycolytic parameters were similar to wild-type cells. Effector CD8 T cells generated with IL-2 had elevated basal, but not maximal glycolysis, but depressed basal and maximal oxidative phosphorylation. Several factors may be responsible for these discrepancies. CD8 T cells responding to an infection in lymph nodes or the spleen are exposed to a variety of pro-inflammatory mediators, cytokines and activated antigen-presenting cells that are not faithfully replicated by standard in vitro culture conditions. In addition concentrations of key nutrients such as glucose and glutamate are in excess in vitro, and likely more limiting in vivo (61). A recent study found in vitro-derived effector cells operated at their maximal glycolytic capacity, whereas ex vivo-derived cells had larger spare energetic capacity (61). Ex vivo cells also displayed greater oxidative metabolism and switched more easily between mitochondrial and glycolytic pathways. Therefore it is possible the increased metabolic flexibility in Zbtb20 KO cells, possibly in addition to exposure to inflammatory factors present uniquely in vivo, results in the metabolic changes in these cells being better revealed in vivo.
Effector CD8 T cells heavily rely on glycolysis and have high rates of glucose uptake (25), whereas memory CD8 T cells rely on mitochondrial respiration (49). It is clear that the substrate used in the mitochondrial citric acid cycle also influences CD8 T cell function, differentiation and longevity (50). Glutamine metabolism has been reported to be crucial for survival, proliferation and effector function of CD4 T cells upon activation (62). Fatty acid oxidation has been linked to superior mitochondrial capacity and longevity of memory CD8 T cells (63, 64). In addition, instead of obtaining fatty acids from their external environment, memory CD8 T cell synthesize their own triacylglycerol using glucose-derived carbon (64, 65). Concomitantly, memory CD8 T cell also up-regulate expression of the glycerol channel, aquaporin 9, to facilitate the uptake of glycerol required for triacylglycerol synthesis and storage (65). Subsequent studies showed that medium or short chain fatty acids such as acetate also play important roles as mitochondrial fuels in memory CD8 T cells (66–68). Our studies regarding mitochondrial fuel sources show inhibition of glutaminolysis or glycolysis markedly impair mitochondrial respiratory activity in WT CD8 Tcm cells. However Zbtb20 deficient memory CD8 T cells tolerated inhibition of either fuel source without significant diminution of mitochondrial respiration, and only when both pathways were inhibited was there a significant reduction. Availability of glucose and glutamate are limiting in many growing tumors, creating an environment not conducive for protective T cell responses. Limited flexibility with respect to mitochondrial fuel sources may restrict the protective capacity of WT CD8 T cells, and increased flexibility on the part of Zbtb20 deficient memory cells may partially explain their increased protective capacity. Future studies will determine the underlying mechanism for this increased fuel flexibility, and whether it leads to better accumulation and/or effector activity in tumors after T cell infusion.
Spare respiratory capacity is thought to be an important factor contributing to enhanced secondary responses by memory CD8 T cells in response to antigenic rechallenge (63). Therefore it is likely that the larger spare respiratory capacity we observed in Zbtb20-deficient memory CD8 T cells is at least partly responsible for the greater secondary expansion following virus re-challenge. Improved protective capacity from Zbtb20 KO memory cells was demonstrated by superior ability to protect against MC38-Ova tumors. While enhanced expansion of memory cells is no doubt important in this protection, a higher proportion of cells expressing effector cytokines such as IFN-γ and TNF-α, and CXCR3, which may promote homing to the tumor site, may also have contributed to anti-tumor activity.
Our data indicates that Zbtb20 is expressed in the first 2–3 days following CD8 T cell activation, and is important in shaping the phenotypic, metabolic and functional evolution of the anti-microbial response. Expression then declines rapidly, but re-emerges in a small subset of memory CD8 T cells. This may indicate that Zbtb20 exerts its effects during the first few days of the T cell response, then is subsequently active in a defined population of memory cells. Early Zbtb20 activity may exert a sustained effect in part through modulation of the network of other transcription factors critical for T cell differentiation. Blimp-1 suppresses effector CD8 T cell proliferation and drives their terminal differentiation, whereas Bcl-6 promotes proliferation, survival and memory differentiation of CD8 T cells (69). Eomesodermin induces expression of several effector molecules in T cells, such as IFN-γ, perforin and granzyme B (70), but also promotes homeostatic self-renewal of memory cells through inducing expression of the IL-15 receptor (71). Reduced expression of Blimp-1 and Eomes at d7 may contribute to the skewing away from terminally differentiated effector cells and toward memory precursors. Expression of these molecules change during the contraction phase (D14), however this could be a reflection of the altered proportions of effector and memory cells during contraction, as effectors die off and the proportion of memory precursors enlarges. We also observed elevated Bcl-6 expression at day 7, which is consistent with promotion of memory precursor development. However a key function of Bcl-6 is to directly repress genes involved in the glycolysis pathway, including Slc2a1, Slc2a3, Hk2 and Pkm2 (72). As we observed increased glycolytic metabolism in the absence of Zbtb20, the effects of elevated Bcl-6 were likely mitigated by other transcription factors or cofactors.
While most experiments focused on the CD8 T cell response to listeria infection, we also tested the extent to which they extended to a different, unrelated infection. Murine gammaherpesvirus infection is a different class of pathogen (virus vs intracellular bacteria), and unlike listeria, it establishes a persistent infection (73). While we detected changes in T cell metabolism and altered expression of key transcription factors in both infections, there were important differences. Glycolysis was increased in Zbtb20 deficient CD8 T cells in both infections. Basal and maximal mitochondrial respiratory capacity and spare respiratory capacity were all enhanced in knockout memory cells in listeria infection, however these changes were of smaller magnitude in MHV-68 infection. The pattern of expression of Bcl-6, Eomes and T-bet were consistent in memory cells in both infections, however they differed at the acute timepoints. There are a number of factors that may be responsible for these differences, including antigen persistence, engagement of different pattern recognition receptors and cellular tropism. Despite these differences, however, it is clear Zbtb20 affects both immunometabolism and the transcriptional network during CD8 T cell differentiation across infection types.
In conclusion, data presented here identify Zbtb20 as an important regulator of effector and memory CD8 T cell differentiation and metabolism. Given our data showing improved protection from tumors, and the known superiority of memory cells in adoptive T cell therapy, deletion or inhibition of Zbtb20 may provide a promising novel strategy for anti-tumor immunotherapy.
Supplementary Material
Key points:
Zbtb20 is an important regulator of effector and memory CD8 T cell immunometabolism.
Differentiation to a memory precursor phenotype is favored by Zbtb20 deficiency.
CD8 T cells deficient in Zbtb20 confer enhanced protection against tumors.
Acknowledgments:
We thank Dr. Randy Noelle (Dartmouth College) for useful discussions and supplying breeding stocks of Zbtb20-GFP and Zbtb20-fl/fl mice, and Drs Eyal Amiel (University of Vermont) and Juan Cubillos-Ruiz (Weill Cornell Medical School) for discussions regarding analysis of metabolic data. Dr. Mary Jo Turk (Dartmouth College) kindly provided OT-I breeding stocks.
Funding was provided by NIH grants R01 AI122854 (E.J.U.), K01 LM012426 (H.R.F.), P20 GM130454 (H.R.F.). The Center for Quantitative Biology Single Cell Genomics Core and the Genomics and Molecular Biology Shared Resource at Dartmouth is supported by a NCI Cancer Center Support Grant (5P30CA023108-37) and an NIH P20 COBRE grant (1P20GM130454). Imaging studies were performed in the Dartmouth Institute for Biomolecular Targeting (Bio-MT), supported by grant P20 GM113132.
References
- 1.Kaech SM, and Cui W. 2012. Transcriptional control of effector and memory CD8+ T cell differentiation. Nature Reviews Immunology 12: 749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joshi NS, and Kaech SM. 2008. Effector CD8 T Cell Development: A Balancing Act between Memory Cell Potential and Terminal Differentiation. The Journal of Immunology 180: 1309–1315. [DOI] [PubMed] [Google Scholar]
- 3.Remmerswaal EBM, Hombrink P, Nota B, Pircher H, Berge IJM, Lier RAW, and Aalderen MC. 2019. Expression of IL-7Rα and KLRG1 defines functionally distinct CD8 + T-cell populations in humans. European Journal of Immunology 49: 694–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, and Ahmed R. 2008. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. Journal of Experimental Medicine 205: 625–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, and Ahmed R. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nature Immunology 4: 1191–1198. [DOI] [PubMed] [Google Scholar]
- 6.Obar JJ, Jellison ER, Sheridan BS, Blair DA, Pham Q-M, Zickovich JM, and Lefrançois L. 2011. Pathogen-induced inflammatory environment controls effector and memory CD8+ T cell differentiation. Journal of immunology (Baltimore, Md. : 1950) 187: 4967–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearce EL, Poffenberger MC, Chang CH, and Jones RG. 2013. Fueling immunity: Insights into metabolism and lymphocyte function. Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Windt GJW, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, and Pearce EL. 2012. Mitochondrial Respiratory Capacity Is a Critical Regulator of CD8 + T Cell Memory Development. Immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finlay DK, Rosenzweig E, Sinclair LV, Feijoo-Carnero C, Hukelmann JL, Rolf J, Panteleyev AA, Okkenhaug K, and Cantrell DA. 2012. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J Exp Med 209: 2441–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi H, Munger J, and Green DR. 2011. The Transcription Factor Myc Controls Metabolic Reprogramming upon T Lymphocyte Activation. Immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rolf J, Zarrouk M, Finlay DK, Foretz M, Viollet B, and Cantrell DA. 2013. AMPKα1: A glucose sensor that controls CD8 T-cell memory. European Journal of Immunology 43: 889–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He S, Kato K, Jiang J, Wahl DR, Mineishi S, Fisher EM, Murasko DM, Glick GD, and Zhang Y. 2011. Characterization of the Metabolic Phenotype of Rapamycin-Treated CD8+ T Cells with Augmented Ability to Generate Long-Lasting Memory Cells. PLoS ONE 6: e20107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes LC, Di Benedetto G, and Scorrano L. 2011. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nature Cell Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rambold AS, Kostelecky B, Elia N, and Lippincott-Schwartz J. 2011. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proceedings of the National Academy of Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang CR, and Blackstone C. 2007. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. Journal of Biological Chemistry. [DOI] [PubMed] [Google Scholar]
- 16.Buck MDD, O’Sullivan D, Klein Geltink RII, Curtis JDD, Chang CH, Sanin DEE, Qiu J, Kretz O, Braas D, van der Windt GJJW, Chen Q, Huang SCC, O’Neill CMM, Edelson BTT, Pearce EJJ, Sesaki H, Huber TBB, Rambold ASS, and Pearce ELL. 2016. Mitochondrial Dynamics Controls T Cell Fate through Metabolic Programming. Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siggs OM, and Beutler B. 2012. The BTB-ZF transcription factors. Cell Cycle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beaulieu AM, and Sant’Angelo DB. 2011. The BTB-ZF Family of Transcription Factors: Key Regulators of Lineage Commitment and Effector Function Development in the Immune System. The Journal of Immunology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang W, Mi J, Li N, Sui L, Wan T, Zhang J, Chen T, and Cao X. 2001. Identification and characterization of DPZF, a novel human BTB/POZ zinc finger protein sharing homology to BCL-6. Biochemical and Biophysical Research Communications. [DOI] [PubMed] [Google Scholar]
- 20.Chevrier S, Emslie D, Shi W, Kratina T, Wellard C, Karnowski A, Erikci E, Smyth GK, Chowdhury K, Tarlinton D, and Corcoran LM. 2014. The BTB-ZF transcription factor Zbtb20 is driven by Irf4 to promote plasma cell differentiation and longevity. The Journal of Experimental Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutherland APR, Zhang H, Zhang Y, Michaud M, Xie Z, Patti M-E, Grusby MJ, and Zhang WJ. 2009. Zinc Finger Protein Zbtb20 Is Essential for Postnatal Survival and Glucose Homeostasis. Molecular and Cellular Biology 29: 2804–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie Z, Zhang H, Tsai W, Zhang Y, Du Y, Zhong J, Szpirer C, Zhu M, Cao X, Barton MC, Grusby MJ, and Zhang WJ. 2008. Zinc finger protein ZBTB20 is a key repressor of alpha-fetoprotein gene transcription in liver. Proceedings of the National Academy of Sciences of the United States of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frost HR Variance-adjusted Mahalanobis (VAM): a fast and accurate method for cell-specific gene set scoring. Nucleic Acids Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manjunath N, Shankar P, Wan J, Weninger W, Crowley MA, Hieshima K, Springer TA, Fan X, Shen H, Lieberman J, and von Andrian UH. 2001. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J. Clin. Invest. 108: 871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, June CH, and Thompson CB. 2002. The CD28 signaling pathway regulates glucose metabolism. Immunity. [DOI] [PubMed] [Google Scholar]
- 26.Gerlach C, Moseman EA, Loughhead SM, Alvarez D, Zwijnenburg AJ, Waanders L, Garg R, de la Torre JC, and von Andrian UH. 2016. The Chemokine Receptor CX3CR1 Defines Three Antigen-Experienced CD8 T Cell Subsets with Distinct Roles in Immune Surveillance and Homeostasis. Immunity 45: 1270–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Böttcher JP, Beyer M, Meissner F, Abdullah Z, Sander J, Höchst B, Eickhoff S, Rieckmann JC, Russo C, Bauer T, Flecken T, Giesen D, Engel D, Jung S, Busch DH, Protzer U, Thimme R, Mann M, Kurts C, Schultze JL, Kastenmüller W, and Knolle PA. 2015. Functional classification of memory CD8(+) T cells by CX3CR1 expression. Nat Commun 6: 8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hudson WH, Gensheimer J, Hashimoto M, Wieland A, Valanparambil RM, Li P, Lin J-X, Konieczny BT, Im SJ, Freeman GJ, Leonard WJ, Kissick HT, and Ahmed R. 2019. Proliferating Transitory T Cells with an Effector-like Transcriptional Signature Emerge from PD-1+ Stem-like CD8+ T Cells during Chronic Infection. Immunity 51: 1043–1058.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Omilusik KD, Best JA, Yu B, Goossens S, Weidemann A, Nguyen JV, Seuntjens E, Stryjewska A, Zweier C, Roychoudhuri R, Gattinoni L, Bird LM, Higashi Y, Kondoh H, Huylebroeck D, Haigh J, and Goldrath AW. 2015. Transcriptional repressor ZEB2 promotes terminal differentiation of CD8+ effector and memory T cell populations during infection. J. Exp. Med. 212: 2027–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dominguez CX, Amezquita RA, Guan T, Marshall HD, Joshi NS, Kleinstein SH, and Kaech SM. 2015. The transcription factors ZEB2 and T-bet cooperate to program cytotoxic T cell terminal differentiation in response to LCMV viral infection. J. Exp. Med. 212: 2041–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saibil SD, St Paul M, Laister RC, Garcia-Batres CR, Israni-Winger K, Elford AR, Grimshaw N, Robert-Tissot C, Roy DG, Jones RG, Nguyen LT, and Ohashi PS. 2019. Activation of Peroxisome Proliferator-Activated Receptors α and δ Synergizes with Inflammatory Signals to Enhance Adoptive Cell Therapy. Cancer Res. 79: 445–451. [DOI] [PubMed] [Google Scholar]
- 32.Sukumar M, Liu J, Ji Y, Subramanian M, Crompton JG, Yu Z, Roychoudhuri R, Palmer DC, Muranski P, Karoly ED, Mohney RP, Klebanoff CA, Lal A, Finkel T, Restifo NP, and Gattinoni L. 2013. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J Clin Invest 123: 4479–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hermans D, Gautam S, García-Cañaveras JC, Gromer D, Mitra S, Spolski R, Li P, Christensen S, Nguyen R, Lin J-X, Oh J, Du N, Veenbergen S, Fioravanti J, Ebina-Shibuya R, Bleck C, Neckers LM, Rabinowitz JD, Gattinoni L, and Leonard WJ. 2020. Lactate dehydrogenase inhibition synergizes with IL-21 to promote CD8+ T cell stemness and antitumor immunity. PNAS 117: 6047–6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loschinski R, Böttcher M, Stoll A, Bruns H, Mackensen A, and Mougiakakos D. 2018. IL-21 modulates memory and exhaustion phenotype of T-cells in a fatty acid oxidation-dependent manner. Oncotarget 9: 13125–13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nielsen JV, Nielsen FH, Ismail R, Noraberg J, and Jensen NA. Hippocampus-like corticoneurogenesis induced by two isoforms of the {BTB}-zinc finger gene Zbtb20 in mice. 134: 1133–1140. [DOI] [PubMed] [Google Scholar]
- 36.Tonchev AB, Tuoc TC, Rosenthal EH, Studer M, and Stoykova A. Zbtb20 modulates the sequential generation of neuronal layers in developing cortex. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenthal EH, Tonchev AB, Stoykova A, and Chowdhury K. Regulation of archicortical arealization by the transcription factor Zbtb20. 22: 2144–2156. [DOI] [PubMed] [Google Scholar]
- 38.Xie Z, Ma X, Ji W, Zhou G, Lu Y, Xiang Z, Wang YX, Zhang L, Hu Y, Ding Y-Q, and Zhang WJ. Zbtb20 is essential for the specification of {CA}1 field identity in the developing hippocampus. 107: 6510–6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cordeddu V, Redeker B, Stellacci E, Jongejan A, Fragale A, Bradley TEJ, Anselmi M, Ciolfi A, Cecchetti S, Muto V, Bernardini L, Azage M, Carvalho DR, Espay AJ, Male A, Molin A-M, Posmyk R, Battisti C, Casertano A, Melis D, van Kampen A, Baas F, Mannens MM, Bocchinfuso G, Stella L, Tartaglia M, and Hennekam RC. Mutations in {ZBTB}20 cause Primrose syndrome. 46: 815–817. [DOI] [PubMed] [Google Scholar]
- 40.Primrose DA A slowly progressive degenerative condition characterized by mental deficiency, wasting of limb musculature and bone abnormalities, including ossification of the pinnae. 26 (Pt 2): 101–106. [DOI] [PubMed] [Google Scholar]
- 41.Mathijssen IB, van J, der Velde Hasselt-van, and Hennekam RCM. Testicular cancer in a patient with Primrose syndrome. 49: 127–133. [DOI] [PubMed] [Google Scholar]
- 42.Lindor NM, Hoffman AD, and Primrose DA. A neuropsychiatric disorder associated with dense calcification of the external ears and distal muscle wasting: “Primrose syndrome.” 5: 27–34. [DOI] [PubMed] [Google Scholar]
- 43.Dalal P, Leslie ND, Lindor NM, Gilbert DL, and Espay AJ. Motor tics, stereotypies, and self-flagellation in primrose syndrome. 75: 284–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collacott RA, O’Malley BP, and Young ID. The syndrome of mental handicap, cataracts, muscle wasting and skeletal abnormalities: report of a second case. 30 ( Pt 3): 301–308. [DOI] [PubMed] [Google Scholar]
- 45.Battisti C, Dotti MT, Cerase A, Rufa A, Sicurelli F, Scarpini C, and Federico A. The Primrose syndrome with progressive neurological involvement and cerebral calcification. 249: 1466–1468. [DOI] [PubMed] [Google Scholar]
- 46.Casertano A, Fontana P, Hennekam RC, Tartaglia M, Genesio R, Dieber TB, Ortega L, Nitsch L, and Melis D. Alterations in metabolic patterns have a key role in diagnosis and progression of primrose syndrome. 173: 1896–1902. [DOI] [PubMed] [Google Scholar]
- 47.Liu G, Zhou L, Zhang H, Chen R, Zhang Y, Li L, Lu J-Y, Jiang H, Liu D, Qi S, Jiang Y-M, Yin K, Xie Z, Shi Y, Liu Y, Cao X, Chen Y-X, Zou D, and Zhang WJ. Regulation of hepatic lipogenesis by the zinc finger protein Zbtb20. 8: 14824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Xie Z, Zhou L, Li L, Zhang H, Zhou G, Ma X, Herrera PL, Liu Z, Grusby MJ, and Zhang WJ. The Zinc Finger Protein {ZBTB}20 Regulates Transcription of Fructose-1,6-Bisphosphatase 1 and β Cell Function in Mice. 142: 1571−−1580.e6. [DOI] [PubMed] [Google Scholar]
- 49.Pearce EL 2010. Metabolism in T cell activation and differentiation. Current Opinion in Immunology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dimeloe S, Burgener AV, Grählert J, and Hess C. 2017. T-cell metabolism governing activation, proliferation and differentiation; a modular view. Immunology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vafai SB, and Mootha VK. 2012. Mitochondrial disorders as windows into an ancient organelle. Nature. [DOI] [PubMed] [Google Scholar]
- 52.Mitra K, Wunder C, Roysam B, Lin G, and Lippincott-Schwartz J. 2009. A hyperfused mitochondrial state achieved at G1-S regulates cyclin E buildup and entry into S phase. Proceedings of the National Academy of Sciences of the United States of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rossignol R, Gilkerson R, Aggeler R, Yamagata K, Remington SJ, and Capaldi RA. 2004. Energy Substrate Modulates Mitochondrial Structure and Oxidative Capacity in Cancer Cells. Cancer Research. [DOI] [PubMed] [Google Scholar]
- 54.Tondera D, Grandemange S, Jourdain A, Karbowski M, Mattenberger Y, Herzig S, Da Cruz S, Clerc P, Raschke I, Merkwirth C, Ehses S, Krause F, Chan DC, Alexander C, Bauer C, Youle R, Langer T, and Martinou JC. 2009. SlP-2 is required for stress-induced mitochondrial hyperfusion. EMBO Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rambold AS, Cohen S, and Lippincott-Schwartz J. 2015. Fatty acid trafficking in starved cells: Regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Developmental Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jheng H-F, Tsai P-J, Guo S-M, Kuo L-H, Chang C-S, Su I-J, Chang C-R, and Tsai Y-S. 2012. Mitochondrial Fission Contributes to Mitochondrial Dysfunction and Insulin Resistance in Skeletal Muscle. Molecular and Cellular Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rambold AS, and Pearce EL. 2018. Mitochondrial Dynamics at the Interface of Immune Cell Metabolism and Function. Trends in Immunology. [DOI] [PubMed] [Google Scholar]
- 58.Zhou R, Yazdi AS, Menu P, and Tschopp J. 2011. A role for mitochondria in NLRP3 inflammasome activation. Nature. [DOI] [PubMed] [Google Scholar]
- 59.Zhang B, Alysandratos KD, Angelidou A, Asadi S, Sismanopoulos N, Delivanis DA, Weng Z, Miniati A, Vasiadi M, Katsarou-Katsari A, Miao B, Leeman SE, Kalogeromitros D, and Theoharides TC. 2011. Human mast cell degranulation and preformed TNF secretion require mitochondrial translocation to exocytosis sites: Relevance to atopic dermatitis. Journal of Allergy and Clinical Immunology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cogliati S, Frezza C, Soriano ME, Varanita T, Quintana-Cabrera R, Corrado M, Cipolat S, Costa V, Casarin A, Gomes LC, Perales-Clemente E, Salviati L, Fernandez-Silva P, Enriquez JA, and Scorrano L. 2013. Mitochondrial Cristae Shape Determines Respiratory Chain Supercomplexes Assembly and Respiratory Efficiency. Cell 155: 160–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma EH, Verway MJ, Johnson RM, Roy DG, Steadman M, Hayes S, Williams KS, Sheldon RD, Samborska B, Kosinski PA, Kim H, Griss T, Faubert B, Condotta SA, Krawczyk CM, DeBerardinis RJ, Stewart KM, Richer MJ, Chubukov V, Roddy TP, and Jones RG. 2019. Metabolic Profiling Using Stable Isotope Tracing Reveals Distinct Patterns of Glucose Utilization by Physiologically Activated CD8+ T Cells. Immunity 51: 856–870.e5. [DOI] [PubMed] [Google Scholar]
- 62.Nakaya M, Xiao Y, Zhou X, Chang JH, Chang M, Cheng X, Blonska M, Lin X, and Sun SC. 2014. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van der Windt GJW, Everts B, Chang C-H, Curtis JD, Freitas TC, Amiel E, Pearce EJ, and Pearce EL. 2012. Mitochondrial Respiratory Capacity Is a Critical Regulator of CD8+ T Cell Memory Development. Immunity 36: 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Sullivan D, vanderWindt GWJ, Huang SCC, Curtis JD, Chang CH, Buck MDL, Qiu J, Smith AM, Lam WY, DiPlato LM, Hsu FF, Birnbaum MJ, Pearce EJ, and Pearce EL. 2014. Memory CD8+ T Cells Use Cell-Intrinsic Lipolysis to Support the Metabolic Programming Necessary for Development. Immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cui G, Staron MM, Gray SM, Ho PC, Amezquita RA, Wu J, and Kaech SM. 2015. IL-7-induced glycerol transport and TAG synthesis promotes memory CD8+ T cell longevity. Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raud B, Roy DG, Divakaruni AS, Tarasenko TN, Franke R, Ma EH, Samborska B, Hsieh WY, Wong AH, Stüve P, Arnold-Schrauf C, Guderian M, Lochner M, Rampertaap S, Romito K, Monsale J, Brönstrup M, Bensinger SJ, Murphy AN, McGuire PJ, Jones RG, Sparwasser T, and Berod L. 2018. Etomoxir Actions on Regulatory and Memory T Cells Are Independent of Cpt1a-Mediated Fatty Acid Oxidation. Cell Metab. 28: 504–515.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Balmer ML, Ma EH, Bantug GR, Grählert J, Pfister S, Glatter T, Jauch A, Dimeloe S, Slack E, Dehio P, Krzyzaniak MA, King CG, Burgener A-V, Fischer M, Develioglu L, Belle R, Recher M, Bonilla WV, Macpherson AJ, Hapfelmeier S, Jones RG, and Hess C. 2016. Memory CD8(+) T Cells Require Increased Concentrations of Acetate Induced by Stress for Optimal Function. Immunity 44: 1312–1324. [DOI] [PubMed] [Google Scholar]
- 68.Bachem A, Makhlouf C, Binger KJ, de Souza DP, Tull D, Hochheiser K, Whitney PG, Fernandez-Ruiz D, Dähling S, Kastenmüller W, Jönsson J, Gressier E, Lew AM, Perdomo C, Kupz A, Figgett W, Mackay F, Oleshansky M, Russ BE, Parish IA, Kallies A, McConville MJ, Turner SJ, Gebhardt T, and Bedoui S. 2019. Microbiota-Derived Short-Chain Fatty Acids Promote the Memory Potential of Antigen-Activated CD8+ T Cells. Immunity 51: 285–297.e5. [DOI] [PubMed] [Google Scholar]
- 69.Russ BE, Denton AE, Hatton L, Croom H, Olson MR, and Turner SJ. 2012. Defining the molecular blueprint that drives CD8+T cell differentiation in response to infection. Frontiers in Immunology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao C-A, Shen H, Cereb N, Yang SY, Lindsten T, Rossant J, Hunter CA, and Reiner SL. 2003. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science 302: 1041–1043. [DOI] [PubMed] [Google Scholar]
- 71.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, and Reiner SL. 2005. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nature Immunology 6: 1236–1244. [DOI] [PubMed] [Google Scholar]
- 72.Oestreich KJ, Read KA, Gilbertson SE, Hough KP, McDonald PW, Krishnamoorthy V, and Weinmann AS. 2014. Bcl-6 directly represses the gene program of the glycolysis pathway. Nature Immunology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Obar JJ, Fuse S, Leung EK, Bellfy SC, and Usherwood EJ. 2006. Gammaherpesvirus Persistence Alters Key CD8 T-Cell Memory Characteristics and Enhances Antiviral Protection. J Virol 80: 8303–8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.