Abstract
Introduction:
About one-third of patients with severe ulcerative colitis (UC) do not respond to corticosteroid therapy and receive rescue therapy with infliximab or cyclosporine. Up to 20% of such patients fail to respond to rescue therapy and undergo colectomy.
Objective:
We investigated the outcomes of infliximab and a plant-based diet (PBD) as first-line therapy for severe UC.
Methods:
Patients with severe UC defined by the Truelove and Witts criteria were admitted and given standard induction therapy with infliximab (5.0 mg/kg-7.5 mg/kg) at 0, 2, and 6 weeks. Additionally, they received a PBD. The primary endpoint was remission or colectomy in the induction phase and 1 year after discharge. Secondary endpoints were changes in inflammatory markers in the induction phase and the PBD score at baseline and follow-up. A higher PBD score indicates greater adherence to a PBD.
Results:
Infliximab and PBD as first-line therapy was administered in 17 cases. The remission rate was 76% (13/17), and the colectomy rate was 6% (1/17) in the induction phase. C-reactive protein values and the erythrocyte sedimentation rate significantly decreased at week 6 from 9.42 mg/dL to 0.33 mg/dL and from 59 to 17 mm/h, respectively (p < 0.0001). At 1-year follow-up, the cumulative relapse rate was 25%, and there were no additional colectomy cases. Mean PBD scores of 27.7 at 1 year and 23.8 at 4 years were significantly higher than baseline scores of 8.3 and 9.9, respectively (p < 0.0001 and p = 0.0391).
Conclusion:
This new first-line therapy for severe UC demonstrated a higher remission rate and lower colectomy rate than with the current modality.
Keywords: colectomy, dietary intervention, environmental factors, first-line therapy, infliximab, IPF, lifestyle medicine, plant-based diet, remission, severe ulcerative colitis, vegetarian diet
INTRODUCTION
Newly introduced biologic agents have revolutionized the medical treatment of various conditions, including inflammatory bowel disease (IBD). Antitumor necrosis factor α antibodies (infliximab, adalimumab, and golimumab) and anti-α4β7 antibody (vedolizumab) were introduced for treatment of ulcerative colitis (UC).1-5 They have been shown to effectively induce and maintain remission in outpatients with moderate to severe UC that is unresponsive to corticosteroids, immunosuppressants, or both. Their induction rates of remission are from 19% to 49%.1-5 Infliximab is effective in reducing the colectomy rate at 1 year after therapy.6 The long-term colectomy rate was reported to be reduced in the biologics era (2005-2011) compared with that in the prebiologics era (1998-2004).7
Severe UC develops in 10% to 25% of patients with UC.8-10 It is a potentially life-threatening disease, with a 1% mortality rate.9,11,12 Therefore, treatment requires hospitalization. First-line therapy is intensive intravenous administration of corticosteroids.13 In the prebiologics era, colectomy was indicated if patients were unresponsive to corticosteroids in 3 days, which was approximately one-third of patients.11 Currently, infliximab or cyclosporine is used as rescue second-line treatment of patients who are unresponsive to corticosteroids. This rescue therapy is unsuccessful in approximately 11% to 20% of patients, resulting in colectomy.14 When remission is successfully induced with infliximab, scheduled maintenance therapy with infliximab is recommended.10,14,15 Nevertheless, some cases require colectomy, and colectomy rates increase to 26% to 37% at 1 year.14
Infliximab is indicated for UC treatment in second-line therapy treatment for outpatients with moderate to severe UC and in treatment for inpatients with acute severe UC. In both situations, corticosteroids are used first, and then biologics are indicated when corticosteroids are ineffective.9,10,15 As reported in the literature, 16% to 34% of patients are nonresponders to corticosteroids.11,16-18 Even though corticosteroids are effective in the induction phase, there is a drawback to corticosteroid use in the follow-up period. At 1 year, corticosteroid dependence or surgical intervention occurs in nearly 50% of such patients.17 Because of this critical problem of corticosteroids, we replaced prednisolone (a glucocorticoid) with infliximab in 2010 when infliximab became available to use for UC in Japan. In Crohn disease (CD), early use of infliximab (top-down approach) is reported in the literature. The first-line use of infliximab for severe UC, however, has scarcely been reported.19 Ochsenkühn et al.19 demonstrated that infliximab and corticosteroids were equally effective for patients with severe UC who were not corticosteroid refractory, and the authors indicated that infliximab could be an alternative in patients who cannot receive corticosteroids.
IBD is a polygenic disease triggered by environmental factors.20 Among a variety of environmental factors, a westernized diet (high in fat, animal protein, and sugar; low in dietary fiber) is thought to be the most ubiquitous. A westernized diet, which tends to cause gut microbial dysbiosis followed by changes of microbial metabolites, is proinflammatory. On the contrary, a plant-based diet (PBD), which is low in fat, animal protein, and sugar and high in dietary fiber and which tends to increase microbial diversity, is antiinflammatory.20 PBDs are listed as variations of US Department of Agriculture healthy eating patterns.21 Epidemiologic studies have provided convincing evidence that individuals consuming PBDs experience greater longevity and are less affected by common chronic diseases compared with those eating omnivorous diets.22,23
We designed a semivegetarian diet, which is a type of PBD, as a therapeutic diet for patients with IBD.24 From 2003, we have provided the PBD to all inpatients with IBD at our center. We achieved far better outcomes both in the induction and maintenance phases in patients with CD and for relapse prevention in patients with UC than those previously reported in the literature. On the basis of our recent reports on IBD therapy that replaced westernized diets with a PBD, we recommended PBD for patients with IBD.25
To our knowledge, no previous study has incorporated a PBD in the induction phase of treatment of severe UC. We designed infliximab and PBD as first-line (IPF) therapy not for corticosteroid-refractory patients but for new patients with severe UC without prior intensive intravenous corticosteroid use.13 After induction of remission, patients were followed without scheduled infliximab maintenance therapy. We hypothesized that these modalities could enhance the induction rate in the short term and reduce the relapse rate in the medium term, as we experienced in patients with CD.24,26 The aim of this study was to investigate the remission and colectomy rate in the induction phase and in the medium term with IPF therapy for severe UC.
METHODS
Design and Settings
We designed a prospective single-group, nonrandomized, open-label, uncontrolled trial, which was conducted at 2 hospitals in Akita, Japan (study ID no.: University Hospital Medical Information Network [UMIN] UMIN000019061 and UMIN000020402; registration: www.umin.ac.jp). Both Nakadori General Hospital and Akita City Hospital are tertiary care hospitals in Akita City. The first author (MC) worked for Nakadori General Hospital between 2003 and 2012 and has been working for Akita City Hospital since 2013.
This protocol was approved by the Ethical Committee of Nakadori General Hospital and by the Ethical Committee of Akita City Hospital (protocol no: 19-2003, 17-2014, 15-2015). Informed consent was obtained from all participants.
Patients
Infliximab, the first biologic agent for treatment of UC in Japan, was approved in 2010. All patients with severe UC were hospitalized for possible IPF therapy between August 2012 and April 2019. Severity was judged using the Truelove and Witts criteria.9,27 There are 6 items in the Truelove and Witts criteria for severe UC27: diarrhea 6 or more times per day, bloody stool, and 4 systemic toxicity signs (pulse rate ≥ 90/min, temperature ≥ 37.5°C, hemoglobin < 10.5 g/dL, and erythrocyte sedimentation rate [ESR] ≥ 30 mm/h). Severe UC was defined as bloody diarrhea 6 or more times per day associated with 1 or more of the systemic signs.27 Exclusion criteria were moderate severity of UC and previous treatment with biologics. A referred severe case already treated by intensive intravenous corticosteroids13 also was excluded. Patients receiving 5-aminosalicylic acid, prednisolone, and azathioprine were included.
Protocol: Infliximab and PBD as first-line (IPF) Therapy
The IPF protocol was the same as that described for CD.26 In summary, oral metronidazole (750 mg/d) was given after hospital admission, and any medication administered before admission was maintained. The following information was ascertained before the start of IPF therapy or simultaneously depending on the condition of the patients: 1) confirmation of diagnosis by pathologic findings; 2) an assessment of severity of disease by morphologic studies (ultrasonography, barium enema study), laboratory data, and clinical observation; 3) exclusion of other infectious colitis by stool culture or rapid membrane enzyme immunoassay for Clostridium difficile (C. Diff Quik Chek Complete, TechLab, Blacksburg, VA); 4) blood test for cytomegalovirus antigenemia28; 5) examinations for tuberculosis or hepatitis B infection29; and 6) presentation of application form, which includes diagnosis of UC and its severity to the city office for public aid.
Infliximab (Remicade, Janssen Biotech, Horsham, PA) was infused at weeks 0, 2, and 6.30 The amount of infliximab was determined by body weight: 200 mg for those with a weight of 40 kg or less, 300 mg for more than 40 to less than or equal to 60 kg (≥ 5 mg/kg to < 7.5 mg/kg), and 400 mg for more than 60 to less than or equal to 80 kg (> 5 mg/kg to < 6.6 mg/kg).
The PBD was initiated on the same day as the infliximab infusion and comprised a lacto-ovo-vegetarian diet with fish once a week and meat once every 2 weeks.24 Whether to give rice gruel or regular rice and the amount of energy (initially 800 kcal/d or 1100 kcal/d) were decided according to each patient’s condition. The energy was gradually increased to a maximum of about 30 kcal/kg of standard body weight.
After about 1 month, metronidazole was switched to 5-aminosalicylic acids. If azathioprine and/or prednisolone were administered by referral physicians, azathioprine was continued throughout the study while prednisolone was adequately tapered off. After the third infusion of infliximab, patients were discharged. Patients were morphologically studied by colonoscopy and/or contrast-enhanced barium enema before discharge. Patients who achieved clinical remission and could not be admitted for the entire induction phase were discharged after the second infliximab infusion and readmitted for the third infusion.26
Follow-up Studies
All patients excluding those who moved away and who underwent colectomy were followed. The medication used was oral 5-aminosalicylic acid. Azathioprine was continued if it had been administered. Scheduled infliximab maintenance therapy was indicated for some patients who achieved incomplete remission and who had been faced with an intractable clinical course. The interval between outpatient visits was 8 weeks.
Food-Frequency Questionnaire and PBD Score
A questionnaire of dietary habits and lifestyle behaviors before onset or relapse of the disease was obtained immediately after admission, as described in a previous report.31 On the basis of the questionnaire, a table was generated that summarized the patient’s current and future recommended lifestyle and dietary habits. This table was given to the patient during hospitalization and was used by the dietitian when giving dietary guidance. The questionnaire was repeated during short-term (≤ 2-y) or long-term (> 2-y) follow-up.31
A PBD score (PBDS) was calculated from the questionnaire. The method for how the PBDS was calculated has been described previously.31 In brief summary, 8 items considered to be preventive factors for IBD (vegetables, fruits, pulses [beans, soybeans, peas, etc], potatoes, rice, miso soup, green tea, and plain yogurt) contributed to a positive score (PBDS+), whereas 8 items considered to be IBD risk factors (meat, minced or processed meat, cheese/butter/margarine, sweets, soft drinks, alcohol, bread, and fish) contributed to a negative score (PBDS−). Scores of 5, 3, and 1 were given according to the frequency of consumption: every day, 3 to 5 times per week, and 1 to 2 times per week, respectively. The PBDS was calculated as the sum of the positive and negative scores and ranged between −40 and +40. A higher PBDS indicated greater adherence to the PBD. The PBDS of a PBD during hospitalization was 35.31
Assessment of Efficacy of IPF Therapy
Short-term Period (Induction Phase)
In the induction phase, the remission rate, colectomy rate, and mortality were assessed. The primary endpoint was clinical remission, defined as the disappearance of bloody stool27 at week 6 after commencement of the first infliximab infusion. Response without remission was defined as improvement without disappearance of bloody stool. Indication for colectomy or switching to another medication because of poor response before completion of the induction therapy was regarded as therapeutic failure. The secondary endpoints were change of C-reactive protein (CRP) level and ESR.
Medium-Term Period (Quiescent Phase)
In the follow-up studies, the remission rate, colectomy rate, relapse rate, and mortality were assessed at 3 months and 1 year after induction phase. A relapse was defined as a flare-up that required more aggressive medical treatment.32-34 A reappearance of streak blood, a small volume of blood, or bloody stool was not counted as a relapse if the blood disappeared or was controlled with previous medication and/or modification of the diet or a lifestyle behavior. The secondary endpoint was change over time in PBDS. Short-term (≤ 2 y after discharge) and long-term (> 2 y) chronological changes in PBDS were studied.
Safety Evaluations
Safety assessments included vital signs, patient symptoms, findings during daily practitioner rounds, physical examination findings, and periodic laboratory data.
Statistical Analysis
Demographic parameters were expressed as mean (standard deviation) and/or median (interquartile range [IQR]), as appropriate. Kaplan-Meier survival analysis was used to calculate the cumulative proportion of patients who had a relapse. To evaluate the effect of treatment on CRP and ESR, differences were analyzed by analysis of variance. If the results of the analysis of variance were statistically significant, the data were analyzed using the post hoc Tukey-Kramer honestly significant difference test. Chronological changes in PBDS+, PBDS−, and scores in identical patients were compared using the paired t-test or Wilcoxon test. A p value of 0.05 or less was considered to indicate a statistically significant difference. Statistical analysis was performed using JMP 8 software (SAS Institute Inc, Cary, NC).
RESULTS
Patient Characteristics
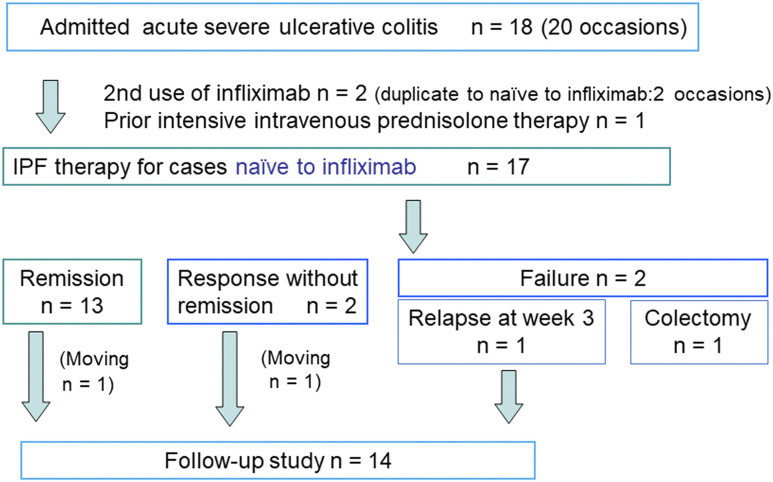
Eighteen patients with severe UC were admitted 20 times; 2 patients were admitted twice and treated with infliximab and PBD twice (Figure 1). One patient was excluded from the study because of prior intensive intravenous corticosteroids treatment13 by a referring physician. Therefore, IPF therapy for 17 patients naive to infliximab was included in this study. The demographics of the 17 patients are presented in Table 1. Mean (standard deviation) age was 43 (20) years. There were 11 initial episode cases, 4 relapsing-remitting cases, and 2 chronic continuous cases. There were 13 cases with extensive colitis and 4 cases with left-sided colitis. The median (IQR) disease duration was 36 (11-103) months. The median (IQR) CRP levels and ESRs were 4.5 (1.5-11.4) mg/dL and 54 (40-66) mm/h, respectively. No case had a positive result on the cytomegalovirus antigenemia test (n = 13) and the immunoassay for C difficile (n = 14; Table 1). Eight patients were discharged after the second infliximab infusion, and 7 of the 8 were readmitted for the third infusion.
Figure 1.
Enrollment of inpatients with severe ulcerative colitis for infliximab and plant-based diet as first-line (IPF) therapy.
Table 1.
Demographic characteristics of 17 patients with severe ulcerative colitis
| Characteristic | Total |
|---|---|
| Male/female sex, no. (%) | 11/6 (65/35) |
| Age, y | |
| Range | 18-78 |
| Mean (SD) | 43 (20) |
| Median (IQR) | 38 (24-62) |
| Clinical type, no. (%) | |
| Initial episode | 11 (65) |
| Relapsing-remitting | 4 (24) |
| Chronic continuous | 2 (12) |
| Extent of ulcerative colitis, no. (%) | |
| Proctitis | 0 (0) |
| Left-sided colitis | 4 (24) |
| Extensive colitis | 13 (76) |
| Disease duration, mo | |
| Range | 1-336 |
| Mean (SD) | 79 (103) |
| Median (IQR) | 36 (11-103) |
| Case referral status, no. (%) | |
| Referred | 9 (53) |
| Nonreferred | 8 (47) |
| Previous status or treatment | |
| Steroid dependent, no. (%) | 2 (12) |
| Previous proctocolectomy, no. (%) | 0 (0) |
| Laboratory test results | |
| C-reactive protein, mg/dL (reference ≤ 0.3 mg/dL) | |
| Mean (SD) | 7.1 (7.2) |
| Median (IQR) | 4.5 (1.5-11.4) |
| Erythrocyte sedimentation rate, mm/h (reference ≤10 mm/h in men, ≤15 mm/h in women) | |
| Mean (SD) | 54 (23) |
| Median (IQR) | 54 (40-66) |
| Positive cytomegalovirus antigenemia, no. (%) | 0/13 (0) |
| Positive immunoassay for Clostridium difficile,a no. (%) | 0/14 (0) |
| Medication during hospitalization, no. (%)b | |
| Oral 5-aminosalicylic acids (5-ASA) | 11 (65) |
| Immunomodulator | 3 (18) |
| Prednisolone (PS) alone or combined with 5-ASA | 2 (12) |
| PS, azathioprine, and 5-ASA | 1 (6) |
| Follow-up period after discharge, y (n = 14) | |
| Mean (SD) | 3.7 (2.8) |
| Median (IQR) | 3.1 (1.3-6.1) |
a C. Diff Quik Chek Complete, TechLab, Blacksburg, VA.
b Percentages do not total to 100 because of rounding.
IQR = interquartile range; SD = standard deviation.
Induction Phase
Primary Endpoint: Remission and Colectomy Rate
The remission rate in the intention-to-treat analyses, colectomy rate, response without remission, and therapeutic failure excluding colectomy was 76% (13/17), 6% (1/17), 12% (2/17), and 6% (1/17), respectively (Table 2 and Figure 1). One patient receiving total parenteral nutrition underwent proctocolectomy 12 days after the first infusion. In this case, intravenous prednisolone (60 mg/d) was initiated on the fourth day after the infusion because of a lack of improvement in symptoms. Prednisolone was also ineffective, and colectomy was indicated. The resected specimen was identified to be infected with cytomegalovirus. Two patients responded without remission. One patient was therapeutic failure who discharged in remission after the second infusion but relapsed 1 week later. This patient was treated with oral prednisolone (40 mg/d), resulting in remission. Seven patients who were discharged after the second infusion and readmitted during the third infusion achieved remission.
Table 2.
Outcomes of IPF therapy for severe ulcerative colitis, number (percentage)
| Outcome | Induction phase | Medium-term period | |
|---|---|---|---|
| At 6 weeks (n = 17) | At 3 months (n = 14) | At 1 year ( n = 12)a | |
| Remission | 13 (76) | 13 (93)b | 9 (75)b |
| Response without remission | 2 (12) | NA | NA |
| Colectomy | 1 (6) | 0 (0) | 0 (0) |
| Failure | 1 (6) | NA | NA |
| Relapse | NA | 0 (0) | 3 (25) |
| Corticosteroid treatment | NA | 1 (7) | 0 (0) |
| Corticosteroid dependence | NA | 0 (0) | 0 (0) |
| Death | 0 (0) | 0 (0) | 0 (0) |
a Two follow-up cases are not included because their follow-up periods were less than 1 y.
b Three cases of scheduled infliximab maintenance therapy in each subgroup are included.
IPF = infliximab and plant-based diet as first-line (therapy); NA = not applicable.
Secondary Endpoints
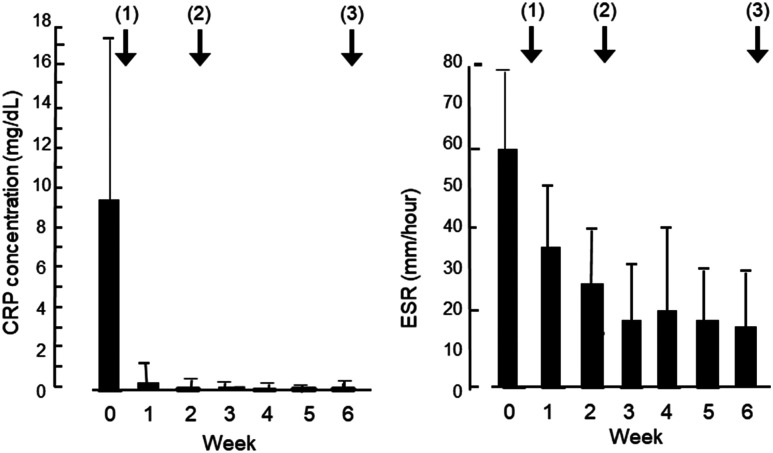
The mean concentration of CRP (reference ≤ 0.3 mg/dL) in 13 cases with clinical remission decreased from 9.42 mg/dL before IPF therapy to 0.61 mg/dL after the first infliximab infusion (p < 0.0001). The CRP concentration normalized (0.11 mg/dL) at week 2 and reached the lowest point (0.04 mg/dL) at week 4 but increased to 0.28 and 0.33 mg/dL at weeks 5 and 6, respectively (Figure 2 and Table 3). The mean ESR (reference ≤ 10 mm/h in men and ≤ 15 mm/h in women) decreased from 59 mm/h before IPF therapy to 17 mm/h at week 6 (p < 0.0001; Figure 2 and Table 3).
Figure 2.
Change in C-reactive protein (CRP) concentration (left) and erythrocyte sedimentation rate (ESR; right) before and after infliximab and plant-based diet as first-line (IPF) therapy in 13 patients with severe ulcerative colitis who achieved clinical remission. Solid bar denotes mean, and error bar shows standard deviation. Arrows with a number in parentheses indicate 3 infliximab infusions at weeks 0, 2, and 6. CRP concentrations and ESRs are presented in Table 3. CRP concentrations and ESRs after IPF therapy were significantly decreased compared with before therapy (analysis of variance, p < 0.0001).
Table 3.
Change of C-reactive protein concentration and erythrocyte sedimentation rate during induction phase after IPF therapy
| Parameter | Weeks after IPF therapy | p value (ANOVA) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | ||
| Number of patients | 13 | 13 | 13 | 10 | 7 | 8 | 13 | |
| CRP, mg/dL, mean (SD)a | 9.42 (8.29) | 0.61 (0.67) | 0.11 (0.17) | 0.09 (0.16) | 0.04 (0.04) | 0.28 (0.54) | 0.33 (0.65) | < 0.0001 |
| Erythrocyte sedimentation rate, mm/h, mean (SD)b | 59 (20) | 37 (13) | 25 (14) | 17 (14) | 26 (21) | 18 (14) | 17 (14) | < 0.0001 |
a Reference range ≤ 0.3 mg/dL.
b Reference range ≤ 10 mm/h for male patients; ≤ 15 mm/h for female patients.
ANOVA = analysis of variance; CRP = C-reactive protein; IPF therapy = infliximab and plant-based diet as the first-line therapy; SD = standard deviation.
One patient among the 13 patients with clinical remission did not undergo morphologic study. Mucosal healing was achieved in 7 of 12 cases (58%). In the other 5 cases, there were still ulcers, although improvement was evident.
Quiescent Phase
Primary Endpoint
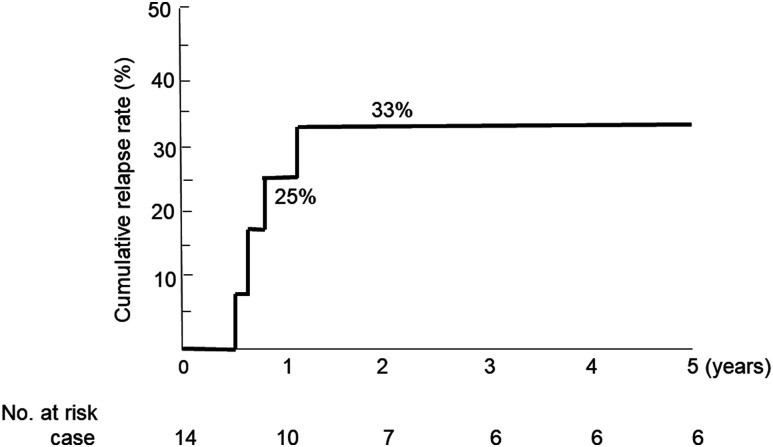
Excluding 1 patient who underwent colectomy and 2 patients who moved away immediately after discharge (1 patient with remission and 1 patient with response without remission), 14 patients were followed (Figure 1). Two patients were followed for less than 1 year, and the remaining 12 patients were followed for more than 1 year. Of these 14 patients, 12 had remission, 1 had a response without remission, and 1 had treatment failure during the induction phase (Figure 1). The median follow-up was 3.1 years (IQR = 1.3-6.1 y; Table 1). Five patients received scheduled infliximab maintenance therapy: 1 patient achieved response without remission, 3 patients achieved clinical remission without endoscopic remission, and 1 patient had severe systemic complication (Takayasu arteritis). One patient with treatment failure was receiving prednisolone at month 3 and achieved remission without prednisolone at 1 year. There was no additional colectomy case through 1-year follow-up after discharge (Table 2). Three patients relapsed at 1 year. All 3 of these patients had achieved clinical remission in the induction phase. The cumulative relapse rate at 1 year of follow-up was 25% (Figure 3). There was no case of corticosteroid dependence at 1 year. There were no deaths during the study (Table 2).
Figure 3.
Cumulative relapse rates during follow-up after discharge for treatment of severe ulcerative colitis (n = 14).
Secondary Endpoints
Mean (standard deviation) baseline PBDS+, PBDS−, and PBDS in 17 patients were 19.9 (7.4), 11.1 (5.3), and 8.8 (7.4), respectively (Table 4). Short-term (≤ 2 y after discharge) and long-term (> 2 y) chronological changes in the PBDS were available in 10 and 8 patients, respectively. For 10 patients, at the median follow-up period of 1 year, respective scores were 30.2 (4.7), 2.5 (3.8), and 27.7 (7.0). These 3 values were significantly better than those at baseline (p < 0.0001, p = 0.0028, and p < 0.0001, respectively; Table 4). In 8 patients, at the median follow-up period of 4 years, PBDS+ was higher than that at baseline. The PBDS− and PBDS were significantly better than those at baseline (p = 0.0167 and p = 0.0391, respectively; Table 4).
Table 4.
Chronological change of plant-based diet score
| Timeframe, n | Follow-up (months) | PBD score+ | p value | PBD score- | p value | PBD score | p Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | ||||
| Baseline, 17 | 19.9 (7.4) | 20.0 (15.0-25.0) | 11.1 (5.3) | 11.0 (7.0-13.5) | 8.8 (7.4) | 8.0 (5.0-13.0) | |||||
| Follow-up | |||||||||||
| Short-term, 10 | 14.3 (6.2) | 12.5 (9.3-19.5) | 30.2 (4.7) | 30.5 (25.0-35.0) | < 0.0001 | 2.5 (3.8) | 0.5 (0-4.8) | 0.0028a | 27.7 (7.0) | 30.0 (22.3-34.3) | < 0.0001 |
| Baseline, 10 | 18.9 (7.6) | 20.5 (12.3-25.0) | 10.6 (5.5) | 11.5 (4.0-14.3) | 8.3 (8.3) | 10.5 (1.5-13.0) | |||||
| Long-term, 8 | 58.4 (31.9) | 48.5 (38.3-64.8) | 26.9 (6.2) | 28.0 (24.8-31.8) | 0.1484a | 3.1 (2.6) | 2.5 (1.3-5.3) | 0.0167 | 23.8 (7.4) | 25.5 (22.3-28.8) | 0.0391a |
| Baseline, 8 | 19.9 (8.4) | 23.5 (10.0-25.0) | 10.0 (4.3) | 11.0 (5.0-13.8) | 9.9 (8.4) | 10.5 (3.8-18.0) | |||||
a p value obtained with paired t-test or Wilcoxon test.
IQR = interquartile range; PBD, plant-based diet; SD = standard deviation.
Safety
In the induction phase, none of the 17 patients experienced infusion reactions to infliximab. Metronidazole was withdrawn in 2 cases: 1 case owing to hand paresthesia and 1 case to gastric distress. In an additional case, 5-aminosalicylic acid was withdrawn because of gastroenteritis. None of the patients experienced an adverse effect, such as gaseous distress, abdominal discomfort, or diarrhea, as a result of the PBD. Scheduled infliximab maintenance therapy was stopped in 2 cases: 13 months later because of an infusion reaction (respiratory distress) and 15 months later because of thrombocytopenia.
DISCUSSION
In this study, IPF therapy for severe UC achieved remission or response without remission in 88% of patients (n = 15 of 17). The colectomy rate was 6% during the induction phase. The cumulative relapse rate at 1 year was 25% without an additional case of colectomy. There were no deaths. There was no case of corticosteroid dependence at 1 year. These outcomes are clearly better than current intensive intravenous corticosteroid therapy or rescue therapy for patients unresponsive to corticosteroids. PBDSs in the short-term and long-term were significantly higher than the baseline PBDSs.
Severe UC is treated on an inpatient basis for intensive care. The first choice of treatment in the current guidelines is intravenous corticosteroids. Efficacy is judged on or around day 3 of corticosteroid therapy. If it is ineffective, either colectomy or rescue medical therapy is planned. The second-line rescue therapy in cases unresponsive to corticosteroids is either infliximab or cyclosporine. If rescue medical therapy is ineffective, colectomy is indicated. If patients respond to infliximab rescue therapy, scheduled infliximab therapy is recommended for maintenance of remission.10,14,15
When we evaluate the efficacy of therapy in severe UC, it is necessary to pay attention to heterogeneity in the degree of severity. There are 6 items in the Truelove and Witts criteria for severe UC27: diarrhea more than 6 times per day, bloody stool, and 4 systemic toxicity signs (tachycardia, fever, anemia, and elevated ESR). The definition of severe differs among reporters in the requirement of systemic signs, from strict requirement of all 4 signs to a liberal requirement of 1 sign.11 Higher numbers of systemic signs translate into higher colectomy rates: 8.5% for 1 sign, 31% for 2 signs, and 48% for 3 or more signs.8 The more common IBD becomes, the easier it is for patients to access physicians and receive early diagnosis and treatment. Physicians will not leave patients untreated. They will take adequate steps before patients deteriorate to the point of fulfilling all 4 criteria in current practice. Therefore, the current definition does not require all 4 systemic signs.8-10 Ungar et al.35 treated moderately severe cases of UC on an outpatient basis and acute severe cases on an inpatient basis. Outcomes of infliximab treatment in patients with UC are related to severity: higher induction rates and lower colectomy rates are seen in moderately severe UC compared with acute severe UC.36 Currently, a CRP concentration of 3.0 mg/dL or greater is commonly used as a surrogate for an ESR at or above 30 mm/h, 1 of the original 4 systemic toxicity signs.8 In our cases, the median CRP level and ESR were 4.5 mg/dL and 54 mm/h, respectively (Table 1).
Both infliximab and cyclosporine have been shown to be effective for rescue treatment in severe patients unresponsive to corticosteroids.9,10 Nonrandomized studies suggested that infliximab was associated with better treatment response and lower risk of colectomy at 12 months.12,37-39 Results of a recent review and meta-analysis showed that, in the case of induction with infliximab, the standard 3 infusions were more effective than a sole infusion.14 There were contradictory results on accelerated infliximab induction therapy, and no superiority of the accelerated therapy to the standard induction therapy was confirmed.14,40,41 It is noteworthy that chronological change in CRP values during the induction phase in patients with CD and those with UC were similar: the lowest value was at 3 to 5 weeks after therapy in CD26 and at 4 weeks in UC. Then, CRP increased at 6 weeks to within the normal range in CD,26 whereas it was above the normal range in UC (Table 3). The third infliximab infusion at week 6 seemed to suppress inflammation again. Although the standard induction schedule for infliximab in UC has been derived from studies in CD,14 3 infusions at weeks 0, 2, and 6 seem adequate.
Biologics are used in outpatients with moderate to severe UC and inpatients with severe UC. In both situations, they are indicated in patients refractory to corticosteroids.1-5,8-10 In moderate to severe UC, remission rates are available for 4 biologics (infliximab, adalimumab, golimumab, and vedolizumab), whereas in severe UC only outcomes of infliximab are available. Despite the differences in study participants and in designs between these studies and our study, comparison of the outcomes in our study with those in other studies is merited. Remission rates in the induction phase in patients with moderate to severe UC who are naive to infliximab, adalimumab, vedolizumab, and golimumab are 38.8%, 49%, 19.2%, and 43.9%, respectively, in the literature.1-4 The remission rates were scarcely described when infliximab was used as rescue therapy for severe UC because the main concern was to avoid colectomy.8-10 A systematic review showed that overall colectomy rates at 1 month, 3 months, and 1 year in severe colitis treated with standard infliximab rescue therapy were 10.6%, 16.0%, and 26.2%, respectively.14 In this study, the colectomy rate was 6% at 1 month, which is lower than the 10.6% just cited. In addition, there was no increase in the colectomy rate at 3 and 12 months. This finding indicates that incorporated PBD is beneficial in maintaining remission.
Rates of remission, incomplete remission, and colectomy with an intensive corticosteroid regimen for severe UC were 40% to 58%, 24% to 26%, and 18% to 34%, respectively.11,16,18 Those in this study were 76%, 12%, and 6%, respectively, indicating a higher remission rate and lower colectomy rate (Table 2). At 1-year follow-up, corticosteroid dependence and additional colectomy accounted for approximately 50% of patients treated with corticosteroids.17 There was no case of corticosteroid dependence or additional colectomy in our study (Table 2). Therefore, outcomes in the short term and medium term in IPF therapy could be concluded to be more effective for severe UC than in intensive corticosteroid therapy.
Although the patients with severe UC were different, in most patients without prior corticosteroid treatment in our study vs patients unresponsive to corticosteroids in the other studies,12,14,37 the induction regimen of infliximab and treatment on an inpatient basis were the same in both studies.12,14,37 The difference was the incorporation of a PBD in our study. Approximately 10% to 40% of patients with UC, as well as patients with CD, have been found to be primary nonresponders to infliximab.1,36,41-43 Rates of remission and primary nonresponse are related to each other in reverse fashion. By incorporating a PBD and first-line use of infliximab (IPF therapy) in CD, we showed that all patients achieved remission and that there were no primary nonresponders.26,44,45 In the present study, a high remission rate (76%) and a low rate of primary nonresponders (6%) were observed (Table 2). In our previous studies, relapse prevention effects of PBD were shown in both CD and UC.24,34,46 In this current study, no additional colectomy cases were observed at 1 year after IPF therapy, indicating favorable outcomes in the medium term (Table 2). Although sustained dietary modification is desired, a decrease in PBDS was observed over the long term (median duration = 4 years). Most patients tended to lose their determination once they had been in remission for a few years. However, they still consumed more of the recommended foods and consumed less of the foods that were discouraged compared with baseline (Table 4). Consequently, the PBDS was higher compared with baseline (p = 0.0391). We believe that a PBD and learning about healthy habits during hospitalization contributed to enhance self-management skill in maintaining remission.34,46,47
The described IPF therapy for severe UC has several advantages over an intensive corticosteroid regimen or infliximab alone. The rapid efficacy of infliximab enabled patients to ingest supper on the same day of the treatment in most patients. The standard induction therapy with infliximab alone without immunosuppressive agents is quite safe.26 There is no worry about adverse events associated with the use of corticosteroids.48 Most patients can maintain remission without scheduled infliximab maintenance therapy, which greatly reduces the cost of medical care. A PBD is useful to prevent various common diseases.21-23 When IPF therapy is ineffective, corticosteroids can be used next, as in 2 patients in this study. Infliximab use in patients with UC does not increase the risk of postoperative complications.49
There was 1 colectomy case in this study. This patient was found to be infected with cytomegalovirus in a resected colonic specimen. Although patients are normally screened for cytomegalovirus infection at admission, screening was erroneously not performed in this patient. Cytomegalovirus infection within 3 months before infliximab treatment is shown to be a predictor of nonresponse to infliximab.50
The Ministry of Health, Labour and Welfare of Japan designated UC and CD as intractable diseases. Patients with intractable diseases are provided with public medical aid on registration at the public health office. Therefore, in Japan, physicians can provide therapy with less concern about medical expenses. In addition, biologics are currently approved only for patients unresponsive to conventional medications.1-4,15 Thus, there might be limitations to providing the approach proposed herein in other countries and current practice. Considering various clinical situations, better short-term and medium-term outcomes than those reported in patients with severe UC were demonstrated in the present trial of IPF therapy.
Our study had some limitations. There was no control group, and the sample size was small. We hope that other larger, controlled studies will be conducted to validate our results.
CONCLUSION
Infliximab and a PBD as first-line (IPF) therapy induced remission in 13 (76%) of 17 patients with severe UC at week 6, and colectomy was performed in 1 patient (6%). There were no additional colectomy cases at 1 year. IPF therapy provided better short-term and medium-term outcomes for patients with severe UC than the outcomes reported in the literature.
Acknowledgments
Kathleen Louden, ELS, of Louden Health Communications performed a primary copy edit.
Footnotes
Disclosure Statement: The author(s) have no conflicts of interest to disclose.
Authors’ Contributions: Mitsuro Chiba, MD, PhD, designed and conducted the study and wrote the manuscript. Tsuyotoshi Tsuji, MD, PhD, Satoko Tsuda, MD, and Haruhiko Tozawa, MD, performed the colonoscopy. Hajime Ishii, MD, PhD, Hideo Ohno, MD, Yu Obara, MD, and Masafumi Komatsu, MD, PhD, contributed to the acquisition of cases. Mitsuro Chiba, MD, PhD and Kunio Nakane, MD, PhD, performed the statistical analysis. All authors approved the final version of the manuscript for submission.
References
- 1.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005 Dec 8;353(23):2462-76. DOI: 10.1056/NEJMoa050516 PMID:16339095 [DOI] [PubMed] [Google Scholar]
- 2.Travis S, Feagan BG, Peyrin-Biroulet L, et al. Effect of adalimumab on clinical outcomes and health-related quality of life among patients with ulcerative colitis in a clinical setting: results from InspirADA. J Crohn’s Colitis 2017 Oct 27;11(11):1317-25. 10.1093/ecco-jcc/jjx093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taxonera C, Rodríguez C, Bertoletti F, et al. ; Collaborators. Clinical outcomes of golimumab as first, second or third anti-TNF agent in patients with moderate-to-severe ulcerative colitis. Inflamm Bowel Dis 2017 Aug;23(8):1394-402. 10.1097/MIB.0000000000001144 PMID:28671873 [DOI] [PubMed] [Google Scholar]
- 4.Feagan BG, Rutgeerts P, Sands BE, et al. GEMINI 1 Study Group. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013 Aug 22;369(8):699-710. 10.1056/NEJMoa1215734 PMID:23964932 [DOI] [PubMed] [Google Scholar]
- 5.Danese S, Fiorino G, Peyrin-Biroulet L, et al. Biological agents for moderately to severely active ulcerative colitis: a systematic review and network meta-analysis. Ann Intern Med 2014 May 20;160(10):704-11. 10.7326/M13-2403 PMID:24842416 [DOI] [PubMed] [Google Scholar]
- 6.Sandborn WJ, Rutgeerts P, Feagan BG, et al. Colectomy rate comparison after treatment of ulcerative colitis with placebo or infliximab. Gastroenterology 2009 Oct;137(4):1250-60. DOI: 10.1053/j.gastro.2009.06.061 PMID:19596014 [DOI] [PubMed] [Google Scholar]
- 7.Abou Khalil M, Boutros M, Nedjar H, et al. Incidence rates and predictors of colectomy for ulcerative colitis in the era of biologics: results from a provincial database. J Gastrointest Surg 2018 Jan;22(1):124-32. 10.1007/s11605-017-3530-y PMID:28808892 [DOI] [PubMed] [Google Scholar]
- 8.Dinesen LC, Walsh AJ, Protic MN, et al. The pattern and outcome of acute severe colitis. J Crohn’s Colitis 2010 Oct;4(4):431-7. 10.1016/j.crohns.2010.02.001 PMID:21122540 [DOI] [PubMed] [Google Scholar]
- 9.McClements D, Probert C. Managing acute severe ulcerative colitis in the hospitalised setting. Frontline Gastroenterol 2015 Oct;6(4):241-5. 10.1136/flgastro-2014-100459 PMID:28839817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leone S, Samhan-Arias A, Ben-Shachar I, et al. ECCO-EFCCA patient guidelines. on ulcerative colitis (UC). Brussels, Belgium: European Federation of Crohn's & Ulcerative Colitis Associations. Accessed April 1, 2019. www.efcca.org/sites/default/files/Ulcerative%20Colitis%20Patient%20Guidelines.pdf. [Google Scholar]
- 11.Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol 2007 Jan;5(1):103-10. 10.1016/j.cgh.2006.09.033 PMID:17142106 [DOI] [PubMed] [Google Scholar]
- 12.Lynch RW, Lowe D, Protheroe A, Driscoll R, Rhodes JM, Arnott ID. Outcomes of rescue therapy in acute severe ulcerative colitis: data from the United Kingdom inflammatory bowel disease audit. Aliment Pharmacol Ther 2013 Oct;38(8):935-45. DOI: 10.1111/apt.12473 PMID:24004000 [DOI] [PubMed] [Google Scholar]
- 13.Truelove SC, Jewell DP. Intensive intravenous regimen for severe attacks of ulcerative colitis. Lancet 1974 Jun 1;1(7866):1067-70. DOI: 10.1016/S0140-6736(74)90552-2 PMID:4135487 [DOI] [PubMed] [Google Scholar]
- 14.Choy MC, Seah D, Faleck DM, et al. Systematic review and meta-analysis: optimal salvage therapy in acute severe ulcerative colitis. Inflamm Bowel Dis 2019 Jun 18;25(7):1169-86. 10.1093/ibd/izy383 PMID:30605549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuoka K, Kobayashi T, Ueno F, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol 2018 Mar;53(3):305-53. 10.1007/s00535-018-1439-1 PMID:29429045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindgren SC, Flood LM, Kilander AF, Löfberg R, Persson TB, Sjödahl RI. Early predictors of glucocorticosteroid treatment failure in severe and moderately severe attacks of ulcerative colitis. Eur J Gastroenterol Hepatol 1998 Oct;10(10):831-5. 10.1097/00042737-199810000-00003 PMID:9831403 [DOI] [PubMed] [Google Scholar]
- 17.Faubion WA Jr, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology 2001 Aug;121(2):255-60. 10.1053/gast.2001.26279 PMID:11487534 [DOI] [PubMed] [Google Scholar]
- 18.Oshitani N, Matsumoto T, Jinno Y, et al. Prediction of short-term outcome for patients with active ulcerative colitis. Dig Dis Sci 2000 May;45(5):982-6. 10.1023/A:1005589428082 PMID:10795764 [DOI] [PubMed] [Google Scholar]
- 19.Ochsenkühn T, Sackmann M, Göke B. Infliximab for acute, not steroid-refractory ulcerative colitis: a randomized pilot study. Eur J Gastroenterol Hepatol 2004 Nov;16(11):1167-71. 10.1097/00042737-200411000-00014 PMID:15489577 [DOI] [PubMed] [Google Scholar]
- 20.Chiba M, Nakane K, Komatsu M. Westernized diet is the most ubiquitous environmental factor in inflammatory bowel disease. Perm J 2019;23:18-107. 10.7812/TPP/18-107 PMID:30624192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dietary Guidelines Advisory Committee . Dietary guidelines for Americans 2015-2020. 8th ed. Washington, DC: US Department of Health and Human Services; 2015. p 35. [Google Scholar]
- 22.Melina V, Craig W, Levin S. Position of the Academy of nutrition and dietetics: vegetarian diets. J Acad Nutr Diet 2016 Dec;116(12):1970-80. 10.1016/j.jand.2016.09.025 PMID:27886704 [DOI] [PubMed] [Google Scholar]
- 23.Orlich MJ, Singh PN, Sabaté J, et al. Vegetarian dietary patterns and mortality in Adventist Health Study 2. JAMA Intern Med 2013 Jul 8;173(13):1230-8. DOI: 10.1001/jamainternmed.2013.6473 PMID:23836264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiba M, Abe T, Tsuda H, et al. Lifestyle-related disease in Crohn’s disease: relapse prevention by a semi-vegetarian diet. World J Gastroenterol 2010 May 28;16(20):2484-95. 10.3748/wjg.v16.i20.2484 PMID:20503448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiba M, Ishii H, Komatsu M. Recommendation of plant-based diets for inflammatory bowel disease. Transl Pediatr 2019 Jan;8(1):23-7. 10.21037/tp.2018.12.02 PMID:30881895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiba M, Tsuji T, Nakane K, et al. Induction with infliximab and plant-based diet as first-line (IPF) therapy for Crohn disease: a single-group trial. Perm J 2017;21:17-009. DOI: 10.7812/TPP/17-009 PMID:29035182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Truelove SC, Witts LJ. Cortisone in ulcerative colitis: final report on a therapeutic trial. BMJ 1955 Oct 29;2(4947):1041-8. 10.1136/bmj.2.4947.1041 PMID:13260656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiba M, Abe T, Tsuda S, Ono I. Cytomegalovirus infection associated with onset of ulcerative colitis. BMC Res Notes 2013 Feb 2;6(1):40. 10.1186/1756-0500-6-40 PMID:23375026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Have M, Oldenburg B, Fidder HH, Belderbos TD, Siersema PD, van Oijen MG. Optimizing screening for tuberculosis and hepatitis B prior to starting tumor necrosis factor-α inhibitors in Crohn’s disease. Dig Dis Sci 2014 Mar;59(3):554-63. 10.1007/s10620-013-2820-9 PMID:23949640 [DOI] [PubMed] [Google Scholar]
- 30.Sandborn WJ, Hanauer SB. Infliximab in the treatment of Crohn’s disease: a user’s guide for clinicians. Am J Gastroenterol 2002 Dec;97(12):2962-72. 10.1111/j.1572-0241.2002.07093.x PMID:12492177 [DOI] [PubMed] [Google Scholar]
- 31.Chiba M, Nakane K, Takayama Y, et al. Development and application of a plant-based diet scoring system for Japanese patients with inflammatory bowel disease. Perm J 2016 Fall;20(4):16-9. 10.7812/TPP/16-019 PMID:27768566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Höie O, Wolters F, Riis L, et al. ; European Collaborative Study Group of Inflammatory Bowel Disease (EC-IBD). Ulcerative colitis: patient characteristics may predict 10-yr disease recurrence in a European-wide population-based cohort. Am J Gastroenterol 2007 Aug;102(8):1692-701. 10.1111/j.1572-0241.2007.01265.x PMID:17555460 [DOI] [PubMed] [Google Scholar]
- 33.Solberg IC, Lygren I, Jahnsen J, et al. ; IBSEN Study Group . Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN Study). Scand J Gastroenterol 2009;44(4):431-40. 10.1080/00365520802600961 PMID:19101844 [DOI] [PubMed] [Google Scholar]
- 34.Chiba M, Nakane K, Tsuji T, et al. Relapse prevention in ulcerative colitis by plant-based diet through educational hospitalization: a single-group trial. Perm J 2018;22:17-167. 10.7812/TPP/17-167 PMID:30005726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ungar B, Mazor Y, Weisshof R, et al. Induction infliximab levels among patients with acute severe ulcerative colitis compared with patients with moderately severe ulcerative colitis. Aliment Pharmacol Ther 2016 Jun;43(12):1293-9. 10.1111/apt.13631 PMID:27091119 [DOI] [PubMed] [Google Scholar]
- 36.Seow CH, Newman A, Irwin SP, Steinhart AH, Silverberg MS, Greenberg GR. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut 2010 Jan;59(1):49-54. 10.1136/gut.2009.183095 PMID:19651627 [DOI] [PubMed] [Google Scholar]
- 37.Laharie D, Bourreille A, Branche J, et al. ; Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet 2012 Dec 1;380(9857):1909-15. 10.1016/S0140-6736(12)61084-8 PMID:23063316 [DOI] [PubMed] [Google Scholar]
- 38.Croft A, Walsh A, Doecke J, Cooley R, Howlett M, Radford-Smith G. Outcomes of salvage therapy for steroid-refractory acute severe ulcerative colitis: ciclosporin vs infliximab. Aliment Pharmacol Ther 2013 Aug;38(3):294-302. 10.1111/apt.12375 PMID:23786158 [DOI] [PubMed] [Google Scholar]
- 39.Narula N, Marshall JK, Colombel JF, et al. Systematic review and meta-analysis: infliximab or cyclosporine as rescue therapy in patients with severe ulcerative colitis refractory to steroids. Am J Gastroenterol 2016 Apr;111(4):477-91. 10.1038/ajg.2016.7 PMID:26856754 [DOI] [PubMed] [Google Scholar]
- 40.Gibson DJ, Heetun ZS, Redmond CE, et al. An accelerated infliximab induction regimen reduces the need for early colectomy in patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol 2015 Feb;13(2):330-335.e1. 10.1016/j.cgh.2014.07.041 PMID:25086187 [DOI] [PubMed] [Google Scholar]
- 41.Oussalah A, Evesque L, Laharie D, et al. A multicenter experience with infliximab for ulcerative colitis: outcomes and predictors of response, optimization, colectomy, and hospitalization. Am J Gastroenterol 2010 Dec;105(12):2617-25. 10.1038/ajg.2010.345 PMID:20736936 [DOI] [PubMed] [Google Scholar]
- 42.Papamichael K, Gils A, Rutgeerts P, et al. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis 2015 Jan;21(1):182-97. 10.1097/MIB.0000000000000202 PMID:25222660 [DOI] [PubMed] [Google Scholar]
- 43.Buhl S, Steenholdt C, Rasmussen M, et al. Outcomes after primary infliximab treatment failure in inflammatory bowel disease. Inflamm Bowel Dis 2017 Jul;23(7):1210-7. 10.1097/MIB.0000000000001117 PMID:28445244 [DOI] [PubMed] [Google Scholar]
- 44.Chiba M, Tsuji T, Nakane K, Ishii H, Komatsu M. How to avoid primary nonresponders to infliximab in Crohn’s disease. Inflamm Bowel Dis 2017 Nov;23(11):E55-6. 10.1097/MIB.0000000000001281 PMID:28991860 [DOI] [PubMed] [Google Scholar]
- 45.Chiba M, Tanaka Y, Ono I. Early intestinal obstruction after infliximab therapy in Crohn’s disease. Autops Case Rep 2019 Jan 14;9(1):e2018068. 10.4322/acr.2018.068 PMID:30863735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chiba M, Nakane K, Tsuji T, et al. Relapse prevention by plant-based diet incorporated into induction therapy for ulcerative colitis: a single-group trial. Perm J 2019;23:18-220. DOI: 10.7812/TPP/18-220PMID:31050638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiba M, Nakane K, Komatsu M. Lifestyle medicine in inflammatory bowel disease. Perm J 2018;22:18-062. 10.7812/TPP/18-062 PMID:30028672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waljee AK, Rogers MAM, Lin P, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ 2017 Apr 12;357:j1415. 10.1136/bmj.j1415 PMID:28404617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Z, Wu Q, Wang F, Wu K, Fan D. Meta-analysis: effect of preoperative infliximab use on early postoperative complications in patients with ulcerative colitis undergoing abdominal surgery. Aliment Pharmacol Ther 2012 Nov;36(10):922-8. 10.1111/apt.12060 PMID:23002804 [DOI] [PubMed] [Google Scholar]
- 50.Park SH, Yang SK, Hong SM, et al. Severe disease activity and cytomegalovirus colitis are predictive of a nonresponse to infliximab in patients with ulcerative colitis. Dig Dis Sci 2013 Dec;58(12):3592-9. 10.1007/s10620-013-2828-1 PMID:23979435 [DOI] [PubMed] [Google Scholar]