Abstract
Several Re(I)pyca conjugates incorporating long aliphatic amines have been synthesized through a one-pot methodology. The compounds have been fully characterized, and seven compounds have been structurally elucidated by single crystal X-ray diffraction. The C14 variant was probed as a potential organometallic IR dye. Large unilamellar vesicles were generated with DOPC and the C14 compound and we observed incorporation of the rhenium complex as observed by FTIR microscopy.
Graphical Abstract

The one pot reaction of Re(CO)3X, pyridine-2-carboxyaldehyde, and primary aliphatic amines results in the formation of lipophilic Re(CO)3 compounds that could be used as IR dyes.
Introduction
The imaging of cells and tissues often requires the use of reagents to either stain specific structural features or to provide contrast between regions.1–7 Most microscopy relies upon the visible region for imaging, however there has been increasing interest in the use of infrared (IR) radiation for both biological and non-biological samples.8–14 With regard to cell or tissue imaging, IR wavelengths can frequently penetrate deeply into tissues, and there are windows where biological samples do not appreciatively absorb, such as the region between 1800 and 2200 cm-1. Molecules that absorb IR radiation in this energy region could potentially be used as IR dyes, providing contrast in biological or materials microscopy.
Several years ago, Zobi and coworkers discussed the potential use of metal carbonyls as IR dyes for biological systems.15–17 These compounds absorb strongly in the 1800 to 2200 cm−1 region and can often be designed for both stability and specific targeting in biological systems. Since the appearance of these reports, a handful of organometallic carbonyl complexes have been used to image various biomolecules by IR microscopy.18–21
In particular, the Re(CO)3 moiety is well suited for these applications, due to the inert nature of the Re(I) ion as well as the ready incorporation of this unit into a variety of structures.22–27 In our recent work, we have designed covalent adducts of this compound using a pyridine-2-carboxyaldehyde (pyca)-primary amine coupling strategy.28–33 This synthetic method, shown in Scheme 1, produces ligand based covalent conjugates between the Re(CO)3 unit and nearly any primary amine, often via one pot conditions. In the present case, we can readily conjugate the Re(CO)3 unit to primary amines with aliphatic chains.
Scheme 1.
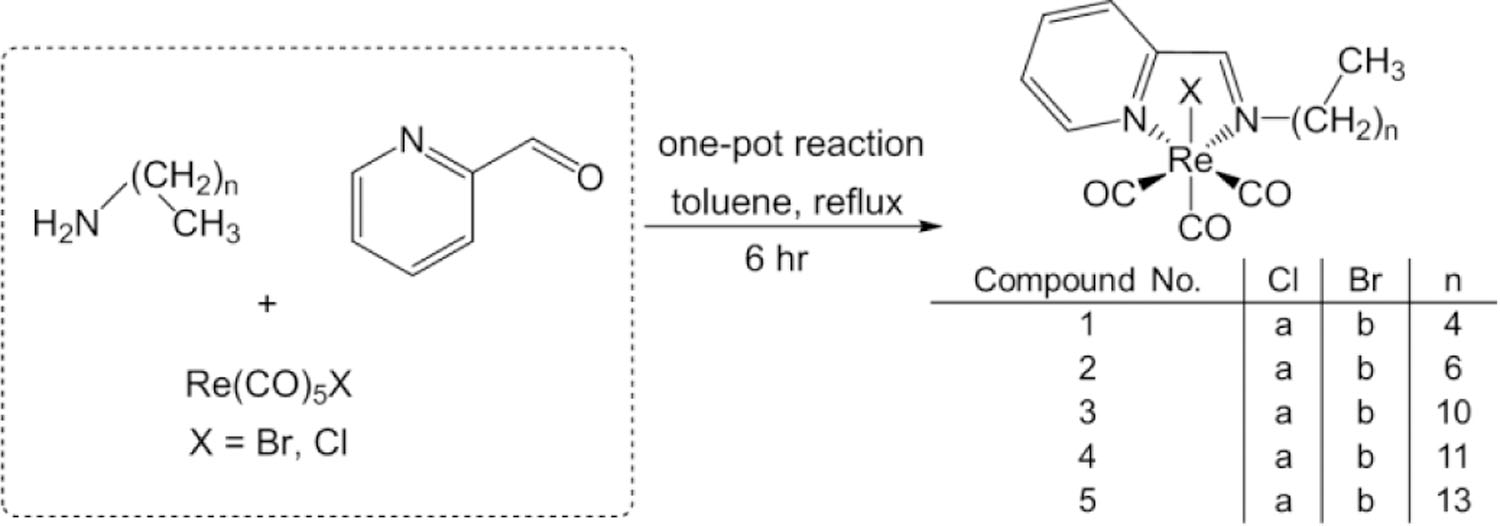
Synthetic route for preparation of the compounds 1a-5a, and 1b-5b.
In this report, we have generated a series of lipophilic Re(CO)3 compounds produced via one pot condensation reactions involving primary amines and the pyca chelate. The resultant compounds have a metal-carbonyl head and a lipophilic alkane tail of varying length, from 5 carbons to 14 carbons. All compounds have been fully characterized, including seven by single crystal X-ray diffraction. We have investigated their mesogenic properties, and have incorporated them into liposomes.
Results and discussion
Pyridine-2-carboxaldehyde can coordinate to Re(CO)5X and generate a nucleophilic site that can react quickly with primary amines to form Schiff base complexes. A one-pot method is typically used to produce pyridine-2-carboxaldimine (pyca) compounds in a single step. In the past we have used this pyca scaffold to make biologically-based compounds containing amino acids, and anthraquinones.29,34–37 We have also employed this synthetic strategy to make pH sensitive phenol compounds, modular ferrocene containing compounds, Re(CO)3 organometallic polymers, and azobenzene conjugates.30–32,38
The literature of Re(CO)3 pyca complexes containing long chain aliphatic amines is sparse, and primarily discuss synthesis and characterization.39–44 There are a few reports that study the lipophilicity of these pyca conjugates, including work by Alberto and co-workers, which discusses Re(I) Lipiodol mimics that have applications in the imaging of liver cancer.45 A recent 2020 study by Wilson and co-workers examined the cellular uptake and cytotoxic effects these compounds have in HeLa cells.46
A series of Re(CO)3 pyca conjugates with long-chain aliphatic amines (Scheme 1) were synthesized. The reaction of one equivalent of Re(CO)5X (X = Br, Cl) along with single equivalents of both pyridine-2-carboxyaldehyde and various primary alkyl amines (C5-C14) in refluxing toluene produced compounds 1a,b-5a,b as orange solids. We were able to structurally characterize compounds 1a,b, 2a,b, 3a,b, and 5b by single crystal X-ray methods, and the structures of 1b, 3a, and 5b are shown in Fig. 1 (1a, 2a,b, and 3b in Figures S56, S58, S59, and S61). The three carbonyls are bound to the octahedral Re(I) center in a facial coordination geometry. The neutral pyridine-2-aldimine is bound as a bidendate chelate, forming a five membered metalacyclic ring, with the halide in the axial position. Tables S3-S4 lists selected bond lengths and angles for all structurally elucidated compounds. The N-Re-N bite angles are ~75°, and Re-N bond lengths range from ~2.16–2.18 Å, similar to other structures with Re(I) pyridine imines.(ref) Notably, the Re-N bonds are similar in length for both the imine and the pyridine units. The Re-C bond lengths are on the range of ~1.90–1.94 Å, and the carbonyl C-O bond lengths range from ~1.14–1.17 Å, which are typical for Re(I)(CO)3 complexes. With regard to the overall conformation of the complexes, the alkyl chain off of the imine deviates from the plane defined by the Re-pyca unit. We observed similar behavior in a series of palladium and platinum complexes incorporating similar aliphatic chain pyca chelates.47
Fig. 1.
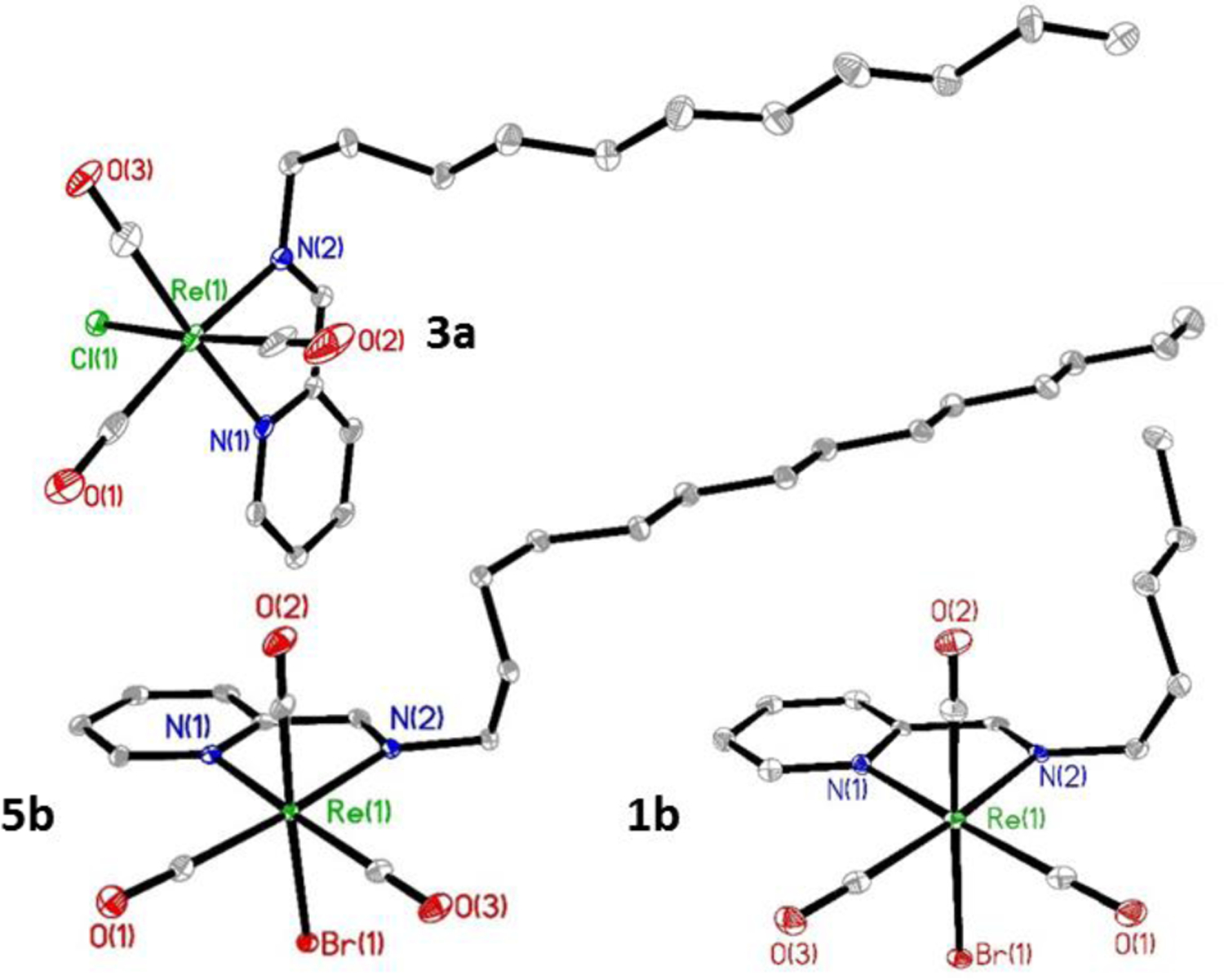
The structures of compounds 1b, 3a, and 5b with 35% thermal ellipsoids. Hydrogen atoms have been omitted for clarity.
The IR spectra show CO stretching frequencies that range from ~2013–2018 cm−1 and ~1876–1891 cm−1. These values are typical of a pseudo-C3v symmetry resulting from a facial arrangement of carbonyls on the Re(CO)3 unit. All compounds have been characterized by NMR spectroscopy (Figures S1-S10). The diagnostic imine protons in the 1H NMR spectra can be readily assigned at 8.73 ppm (X = Cl), and 8.70 ppm (X = Br).
All Re(I) compounds have UV-Visible transitions in the visible range as well as strong absorbing π-π* transitions in the UV range. The spectra of 5a and 5b are shown in Fig. 2 in dichloromethane solvent. The spectra of 1a,b-4a,b are analogous and can be found in Figures S31-S35. A low energy absorption at ~415 nm can be seen for the chlorides and is shifted to the red by about 6 nm for the bromides at ~421 nm. These transitions arise from a MLCT with extinction coefficients on the range of ~3,200–3,300 M−1cm-1. These values are typical for other Re(I)pyca conjugates found in the literature.30,34,37 To confirm the presence of a MLCT transition, solvatochromic studies were carried out. Seven solvents systems were investigated, ranging from non-polar toluene to highly polar DMSO. The solvent polarity studies are displayed in Figures S36-S45. A MLCT was confirmed by a hypsochromic shift ~40 nm with increasing solvent polarity.
Fig. 2.
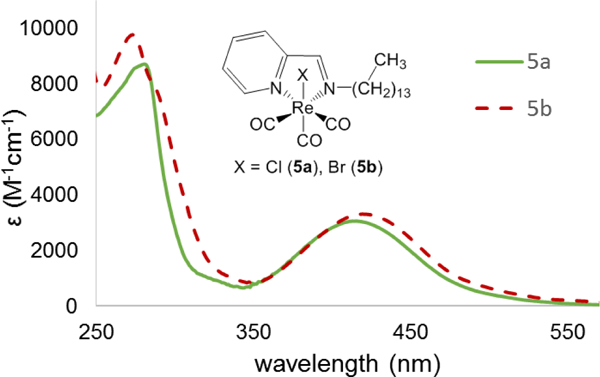
UV-visible spectra for compounds 5a and 5b in DCM.
The metal compounds are microcrystalline powders at room temperature, and the melting points of the compounds with alkyl chains n ≥ 11 carbons had lower melting points than compounds with n = 5, and 7 carbons. To determine the presence of liquid crystalline phases in the compounds, differential scanning calorimetry thermograms were collected (Figures S46-S55). Metallomesogenic compounds combine a metal scaffold with a ligand system to generate liquid crystalline materials. Examples of ligands include imines like salicylaldimines,47–52 pyridyl ring systems,53–56 and peripherally substituted porphyrins and phthalocyanines.57–59 Re(I) complexes of bipyridine, aldimines, and phenanthrolines have been studied extensively in the early 90s by Bruce and co-workers.60–65
The chloride derivatives were observed to have lower melting points than their bromide counterparts. As the length of the alkyl chain increases, the melting points of the compounds were observed to decrease in temperature. This phenomenon has been observed previously with the introduction of liquid crystal mesophases.47,66–68 An initial heating cycle revealed single melting points for 2a, and 3a. All other compounds have multiple transitions in their DSC thermograms. Results from TGA indicate the compounds are stable up to 300 °C and don’t begin to lose mass until 250 °C. The DSC thermograms of the metal compounds are complex and the transitions could arise from chemical reactions due to replacement of the halide at the metal center or liquid crystalline phases.
We surmised the lipophilic Re(I) compounds could be incorporated into a lipid bilayer membrane of cells, or vesicles like liposomes. It is well known that molecules with long chain hydrocarbons, and polyethers can be integrated into membranes for drug delivery, gene therapy, and antimicrobial activity.69–73 To determine if we could potentially use these compounds as metal carbonyl IR dyes in biological samples we chose compound 5a for our studies. All compounds were found to have similar solubilities. Given that no major spectroscopic variations were observed between the bromides and chlorides, only one Re(I) complex (5a) was investigated for this report. Compound 5a was studied for its ability to form vesicles with 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC). DOPC is commonly used in model lipid membranes and drug delivery systems.74,75 We prepared two samples of vesicles composed of 100 mol % DOPC, and 95 mol % DOPC, with 5 mol % by Re(I) content of compound 5a. The lipid contents were combined in chloroform and reduced in volume by rotary evaporation. The lipid films were re-suspended in water and used immediately for IR imaging experiments.
Fig. 3 presents images collected by the FTIR microscope. Fig. 3a contains vesicles of the control lipid sample with a single lipid component of 100 % mol DOPC. Vesicles of varying size and morphology are observed for the control. The sizes range from ~1–10 μm in diameter, with the average size being around 5 μm in diameter and are best described as giant unilamellar vesicles (GUVs) (1–100 μm). The addition of 5 mol % Re(I) does not inhibit the ability for vesicle formation (Fig. 3b). The introduction of the metal compound does alter the liposome size and morphology with the average size being about half of the control vesicles. Smaller spherical vesicles with a diameter of ~2.5 μm are observed for the Re(I) containing sample.
Fig. 3.
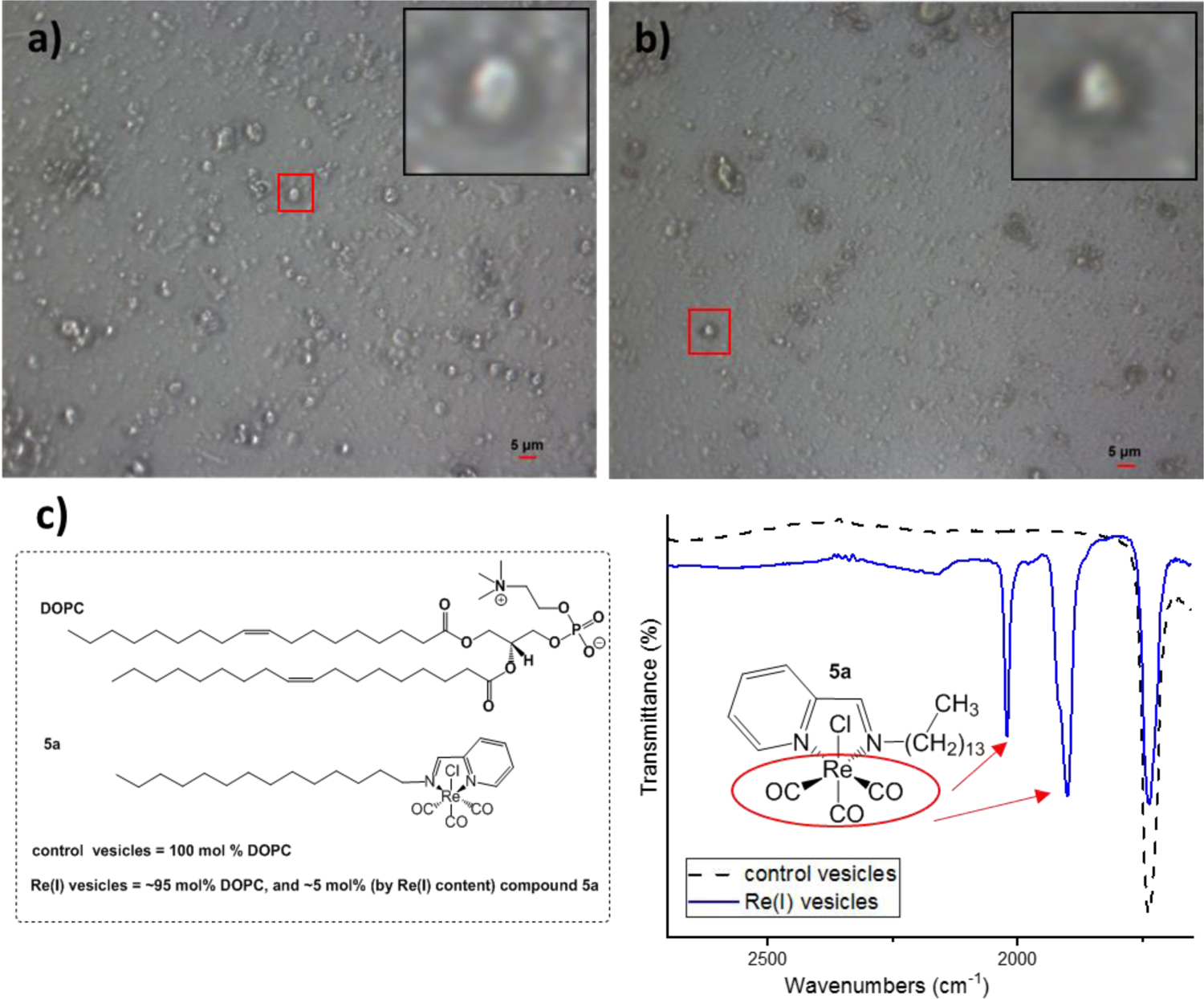
a) IR microscope image of control vesicles containing 100 mol % DOPC. Zoomed in region shows the single vesicle the IR spectrum was taken of. b) IR microscope image of Re(I) containing vesicles with 95 mol % DOPC, and 5 mol % (by Re(I) content) of compound 5a. Zoomed in region shows the single vesicle the IR spectrum was taken of c) FTIR spectra in transmittance mode of the single control vesicle and a vesicle with Re(I). Zoomed in region shows M-CO stretches.
The FTIR spectra of the control and Re(I) containing vesicle samples are seen in Fig. 3c. A spectrum was collected through an aperture size of 5×5 microns, about the size of a single vesicle. The spectral region of interest is between the range of 1,650–2,700 cm−1 (full spectra in Figures S63-S64). A strong carbonyl stretch at 1734 cm−1 is observed in both samples due to the ester carbonyl from the DOPC, and is confirmed by literature values.76,77 The Re(I) containing vesicles shows additional peaks in this region from the metal carbonyl moiety. The FTIR spectrum clearly show the A1 and E-bands of the facial Re(CO)3 unit at 2021 and 1900 cm−1 and these bands do not appear in the control liposomes. Additionally, the IR spectrum of background without any liposomes shows no such signal. This shows that compound 5a has been successfully incorporated into the liposome membrane. While these compounds are highly lipophilic and lack appreciable water solubility, application to cells can be readily accomplished as these compounds are readily soluble in DMSO as the solvent. With regard to biological activity, compounds 1a, and 4a were recently reported by Wilson and coworkers and cellular uptake was carried out with DMSO solutions in media. Complexes with longer alkyl chains were observed to have rapid cellular uptake, and HeLa cells treated at 50 μM displayed time dependent cytotoxicity.46 To increase the water solubility of these compounds, the corresponding cationic species could potentially be made. It is well known that the halide can be removed via a silver salt metathesis and replaced with a nitrogenous base like pyridine.37,78–81 This would afford a lipophilic complex with a cationic rhenium head. These charged variants should exhibit improved solubility, but it is unclear how the cationic metal centre would affect lipid incorporation.
Conclusions
Ten Re(I)(CO)3 compounds have been made via a one-pot reaction with pyridine-2-carboxaldehyde, Re(CO)5X [X = Cl, Br], and various aliphatic amines (C5-C14). All compounds were fully characterized, and the crystal structures of seven compounds were structurally elucidated. We have successfully demonstrated that liposomes can be made to incorporate an organometallic complex. The metal carbonyl stretches were detected in the FTIR spectrum between 1,650–2,700 cm−1 when imaging a single GUV. The spectral region does not have interference from the DOPC lipid, or other small molecules like water making these compounds potentially ideal for future IR microscopy experiments. We are continuing our investigations into these compounds including their ability to target and incorporate into cellular membranes for biological imaging.
General Information
Experimental
All reagents and starting materials were purchased from commercial vendors and used without further purification. Deuterated solvents were purchased from Cambridge Isotope Laboratories and used as received.
NMR spectra were recorded on 300 MHz and 500 MHz spectrometers and chemical shifts were given in ppm relative to residual solvent resonances (1H NMR and 13C NMR spectra). High-resolution mass spectrometry experiments were performed on a Bruker MicroTOF-III and MicroTOF-qIII instruments. Infrared spectra were collected on Thermo Scientific Nicolet iS5 that was equipped with an iD5 ATR. UV-visible spectra were recorded on a Cary 100 Bio UV-visible spectrometer. Differential scanning calorimetry (DSC) was conducted at 10 °C min−1 using a TA Instruments Q200 Differential Scanning Calorimeter. Data were processed with the TA Universal Analysis software program.
X-ray intensity data were measured on a Bruker CCD-based and PHOTON II CPAD-based diffractometer with dual Cu/Mo ImuS microfocus optics (Cu Kα radiation, λ = 1.54178 Å, Mo Kα radiation, λ =0.71073 Å). Crystals were mounted on a cryoloop using Paratone oil and placed under a steam of nitrogen at 100 K (Oxford Cryosystems). The detector was placed at a distance of 5.00 cm from the crystal. The data were corrected for absorption with the SADABS program. The structures were refined using the Bruker SHELXTL Software Package (Version 6.1),82 and were solved using direct methods until the final anisotropic full-matrix, least squares refinement of F2 converged. CCDC 2040147–2040153 contains the supplementary crystallographic data for this paper. The data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/structures.
Preparation of Phospholipid Vesicles
DOPC lipids for IR microscopy measurements were prepared as follows. DOPC was purchased from Cayman Chemical and used as is. A mixture of DOPC, and compound 5a (0 mol %, and 5 mol %) lipids were dissolved in chloroform. The solutions were dried under vacuum, and resuspended in water (~5 mg/mL). The lipids were not extruded through a membrane and used immediately.
FT-IR Imaging Experiments
The infrared spectra were recorded on a Nicolet iS50 FTIR with a continuum FTIR microscopy accessory in transmittance mode. For the solution samples a drop of the lipid vesicle suspension was placed on the diamond compression cell and imaged with a CCD color digital camera attachment. The imaged vesicle was dried and a spectrum of the film was collected through an aperture size set to 5×5 microns.
Syntheses
Synthesis of 1a,b-5a,b. The procedure for generating 1a is representative for these syntheses except heptylamine (0.024 g, 0.21 mmol, 31 µL) was used in 2a,b, undecylamine (0.036 g, 0.21 mmol, 45 µL) was used in 3a,b, dodecylamine (0.038 g, 0.21 mmol) was used in 4a,b, and tetradecylamine (0.044 g, 0.21 mmol) was used in 5a,b. Additionally, the reactions to make 1b-5b employ Re(CO)5Br (0.084 g, 0.21 mmol). Re(CO)5Cl (0.075 g, 0.21 mmol), pyridine-2-carboxaldehyde (0.022 g, 0.21 mmol, 20 µL), pentylamine (0.018 g, 0.21 mmol, 24 µL), and toluene (2.00 mL) was heated to reflux for 6 hours. The solution was cooled to room temperature, and hexane was added to induce precipitation. The resultant orange solids were filtered and washed with hexane. Crystals suitable for X-ray diffraction were grown by slow evaporation of toluene.
1a: Yield: 0.086 g (86%). MP: 143–145 °C. IR: 2013, 1908, 1876 cm−1 (νCO). 1H NMR (300 MHz, CDCl3): δ = 9.03 (d, J = 5.3 Hz, 1H), 8.73 (s, 1H), 8.07 (t, J = 7.6 Hz, 1H), 7.88 (d, J = 7.6 Hz, 1H), 7.58 (t, J = 7.6 Hz, 1H), 4.24 (m, 1H), 4.06 (m, 1H), 2.08 (m, 2H), 1.41 (m, 4H), 0.95 (t, J = 7.0 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ = 196.2 (CO), 196.1, 165.6, 155.1, 153.1, 139.1, 128.3, 127.7, 66.0, 29.4, 28.9, 22.2, 13.9. HRMS (ESI-TOF, positive mode) m/z: calcd for C14H16ClN2NaO3Re 505.0299, found 505.0309 [M+Na]+.
1b: Yield: 0.088 g (80%). MP: 145–151 °C. IR: 2013, 1912, 1880 cm−1 (νCO). 1H NMR (300 MHz, CDCl3): δ = 9.05 (d, J = 5.3 Hz, 1H), 8.70 (s, 1H), 8.06 (t, J = 7.9 Hz, 1H), 7.92 (d, J = 7.6 Hz, 1H), 7.57 (t, J = 6.4 Hz, 1H), 4.27 (m, 1H), 4.04 (m, 1H), 2.08 (m, 2H), 1.41 (m, 4H), 0.95 (t, J = 7.0 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ = 196.6, 195.8, 165.4, 155.1, 153.3, 138.9, 128.2, 127.8, 66.2, 29.6, 28.9, 22.2, 13.9. HRMS (ESI-TOF, positive mode) m/z: calcd for C14H16BrN2NaO3Re 548.9794, found 548.9753 [M+Na]+.
2a: Yield: 0.086 g (78%). MP: 115–119 °C. IR: 2018, 1917, 1878 cm−1 (νCO). 1H NMR (300 MHz, CDCl3): δ = 9.03 (d, J = 5.6 Hz, 1H), 8.73 (s, 1H), 8.07 (t, J = 7.9 Hz, 1H), 7.88 (d, J = 7.6 Hz, 1H), 7.58 (t, J = 6.4 Hz, 1H), 4.24 (m, 1H), 4.06 (m, 1H), 2.07 (m, 2H), 1.40 (m, 4H), 1.31 (m, 4H), 0.90 (t, J = 6.4 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ = 197.0, 196.2, 186.7, 165.6, 155.1, 153.1, 139.1, 128.1, 127.7, 66.0, 31.6, 29.8, 28.7, 26.8, 22.8, 14.0. HRMS (ESI-TOF, positive mode) m/z: calcd for C16H20ClN2NaO3Re 533.0612, found 533.0614 [M+Na]+.
2b: Yield: 0.090 g (78%). MP: 130–132 °C. IR: 2015, 1885 cm−1 (νCO). 1H NMR (300 MHz, CDCl3): δ = 9.05 (d, J = 5.6 Hz, 1H), 8.70 (s, 1H), 8.06 (t, J = 7.6 Hz, 1H), 7.89 (d, J = 7.6 Hz, 1H), 7.57 (t, J = 6.7 Hz, 1H), 4.26 (m, 1H), 4.07 (m, 1H), 2.07 (m, 2H), 1.40 (m, 4H), 1.31 (m, 4H), 0.90 (t, J = 6.1 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ = 196.6, 195.8, 186.1, 165.4, 155.1, 153.3, 139.0, 128.2, 127.8, 66.2, 31.6, 29.9, 28.7, 26.8, 22.5, 14.0. HRMS (ESI-TOF, positive mode) m/z: calcd for C16H20BrN2NaO3Re 577.0107, found 577.0077 [M+Na]+.
3a: Yield: 0.087 g (74%). MP: 101–106 °C. IR: 2015, 1884 cm−1 (νCO). 1H NMR (300 MHz, CDCl3): δ = 9.03 (d, J = 5.3 Hz, 1H), 8.73 (s, 1H), 8.07 (t, J = 7.6 Hz, 1H), 7.88 (d, J = 7.9 Hz, 1H), 7.58 (t, J = 6.5 Hz, 1H), 4.24 (m, 1H), 4.02 (m, 1H), 2.05 (m, 2H), 1.40 (m, 4H), 1.27 (m, 12H), 0.89 (t, J = 6.1 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ = 196.1, 165.6, 155.2, 153.2, 139.0, 128.3, 127.6, 66.0, 31.9, 29.8, 29.5, 29.4, 29.3, 29.1, 26.8, 22.6, 14.1. HRMS (ESI-TOF, positive mode) m/z: calcd for C20H28ClN2NaO3Re 589.1238, found 589.1262 [M+Na]+.
3b: Yield: 0.095 g (75%). MP: 116–119 °C. IR: 2016, 1887 cm−1 (νCO). 1H NMR (300 MHz, CDCl3): δ = 9.05 (d, J = 5.6 Hz, 1H), 8.70 (s, 1H), 8.06 (t, J = 7.0 Hz, 1H), 7.89 (d, J = 7.9 Hz, 1H), 7.57 (t, J = 5.9 Hz, 1H), 4.29 (m, 1H), 4.04 (m, 1H), 2.06 (m, 2H), 1.40 (m, 4H), 1.27 (m, 12H), 0.89 (t, J = 6.1 Hz, 3H). ). 13C{1H} NMR (125 MHz, CDCl3): δ = 196.6, 195.8, 186.1, 165.4, 155.1, 153.3, 138.9, 128.2, 127.8, 66.2, 31.9, 29.9, 29.5, 29.4, 29.3, 29.1, 26.8, 22.6, 14.1. HRMS (ESI-TOF, positive mode) m/z: calcd for C20H28BrN2NaO3Re 633.0733, found 633.0750 [M+Na]+.
4a: Yield: 0.093 g (77%). MP: 103–109 °C. IR: 2015, 1886 cm−1 (νCO). 1H NMR (300 MHz, CDCl3): δ = 9.02 (d, J = 5.6 Hz, 1H), 8.73 (s, 1H), 8.07 (t, J = 7.6 Hz, 1H), 7.88 (d, J = 7.6 Hz, 1H), 7.58 (t, J = 6.7 Hz, 1H), 4.23 (m, 1H), 4.03 (m, 1H), 2.06 (m, 2H), 1.40 (m, 4H), 1.26 (m, 14H), 0.89 (t, J = 5.8 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ = 197.0, 196.2, 186.7, 165.6, 155.1, 153.1, 139.1, 128.3, 127.7, 66.0, 31.9, 29.8, 29.6, 29.5, 29.4, 29.3, 29.1, 26.8, 22.6, 14.1. HRMS (ESI-TOF, positive mode) m/z: calcd for C21H30ClN2NaO3Re 603.1395, found 603.1340 [M+Na]+.
4b: Yield: 0.11 g (83%). MP: 118–121 °C. IR: 2016, 1889 cm−1 (νCO). 1H NMR (300 MHz, CDCl3): δ = 9.05 (d, J = 5.3 Hz, 1H), 8.70 (s, 1H), 8.06 (t, J = 7.6 Hz, 1H), 7.89 (d, J = 7.9 Hz, 1H), 7.57 (t, J = 5.9 Hz, 1H), 4.26 (m, 1H), 4.03 (m, 1H), 2.06 (m, 2H), 1.40 (m, 4H), 1.27 (m, 14H), 0.89 (t, J = 6.7 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ = 196.6, 195.7, 186.1, 165.3, 155.1, 153.3, 138.9, 128.2, 127.7, 66.2, 31.9, 29.9, 29.6, 29.5, 29.4, 29.3, 29.1, 26.8, 22.6, 14.1. HRMS (ESI-TOF, positive mode) m/z: calcd for C21H30BrN2NaO3Re 647.0890, found 647.0854 [M+Na]+.
5a: Yield: 0.11 g (86%). MP: 110–114 °C. IR: 2016, 1886 cm−1 (νCO). 1H NMR (300 MHz, CDCl3): δ = 9.02 (d, J = 5.3 Hz, 1H), 8.73 (s, 1H), 8.07 (t, J = 6.4 Hz, 1H), 7.88 (d, J = 7.9 Hz, 1H), 7.58 (t, J = 7.6 Hz, 1H), 4.23 (m, 1H), 4.04 (m, 1H), 2.04 (m, 2H), 1.40 (m, 4H), 1.26 (m, 18H), 0.89 (t, J = 6.4 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ = 197.0, 196.2, 186.7, 165.6, 155.1, 153.1, 139.1, 128.3, 127.7, 66.0, 31.9, 29.8, 29.7, 29.63, 29.61, 29.6, 29.5, 29.4, 29.3, 29.1, 26.8, 22.7, 14.1. HRMS (ESI-TOF, positive mode) m/z: calcd for C23H34ClN2NaO3Re 631.1708, found 631.1647 [M+Na]+.
5b: Yield: 0.012 g (88%). MP: 120–124 °C. IR: 2016, 1891 cm−1 (νCO). 9.05 (d, J = 6.2 Hz, 1H), 8.70 (s, 1H), 8.06 (t, J = 7.6 Hz, 1H), 7.89 (d, J = 7.3 Hz, 1H), 7.56 (t, J = 7.6 Hz, 1H), 4.26 (m, 1H), 4.04 (m, 1H), 2.06 (m, 2H), 1.39 (m, 4H), 1.26 (m, 18H), 0.89 (t, J = 6.2 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ = 196.6, 195.8, 186.1, 165.4, 155.1, 153.3, 139.9, 128.2, 127.8, 66.1, 31.9, 29.9, 29.7, 29.63, 29.61, 29.6, 29.5, 29.4, 29.3, 29.1, 26.8, 22.7, 14.1. HRMS (ESI-TOF, positive mode) m/z: calcd for C23H34BrN2NaO3Re 675.1203, found 675.1176 [M+Na]+.
Supplementary Material
Acknowledgements
CJZ acknowledges the National Institutes of Health (1R15GM119030) for support of this research.
Footnotes
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/d0dt03743e
Conflicts of interest
There are no conflicts to declare.
Notes and references
- 1.Kikuchi K, Adv. Biochem. Eng. Biotechnol, 2010, 119, 63–78. [DOI] [PubMed] [Google Scholar]
- 2.Chen M, He X, Wang K, He D, Yang X and Shi H, TrAC, Trends Anal. Chem, 2014, 58, 120–129. [Google Scholar]
- 3.Fernandez A and Vendrell M, Chem. Soc. Rev, 2016, 45, 1182–1196. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Z, Chen W, Zhao Y and Yang Y, Neurosci. Bull, 2018, 34, 875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma Y, Yin J, Li G, Gao W and Lin W, Coord. Chem. Rev, 2020, 406, 213144. [Google Scholar]
- 6.McCullough BS and Barrios AM, Curr. Opin. Chem. Biol, 2020, 57, 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin W, Colombani-Garay D, Huang L, Duan C and Han G, Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology, 2020, 12, e1627. [DOI] [PubMed] [Google Scholar]
- 8.Shi L, Liu X, Shi L, Stinson HT, Rowlette J, Kahl LJ, Evans CR, Zheng C, Dietrich LEP and Min W, Nat. Methods, 2020, 17, 844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo S, Zhang E, Su Y, Cheng T and Shi C, Biomaterials, 2011, 32, 7127–7138. [DOI] [PubMed] [Google Scholar]
- 10.Zhu S, Tian R, Antaris AL, Chen X and Dai H, Adv. Mater, 2019, 31, 1900321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L, Dong X, Li J and Wei J, Dye. Pigment, 2020, 183, 108756. [Google Scholar]
- 12.Bellisola G and Sorio C, Am. J. Cancer Res, 2012, 2, 1–21. [PMC free article] [PubMed] [Google Scholar]
- 13.Baker MJ, Trevisan J, Bassan P, Bhargava R, Butler HJ, Dorling KM, Fielden PR, Fogarty SW, Fullwood NJ, Heys KA, Hughes C, Lasch P, Martin-Hirsch PL, Obinaju B, Sockalingum GD, Sule-Suso J, Strong RJ, Walsh MJ, Wood BR, Gardner P and Martin FL, Nat. Protoc, 2014, 9, 1771–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang D, Li C, Zhang C, Slipchenko MN, Eakins G and Cheng J-X, Sci. Adv, 2016, 2, e1600521/1–e1600521/7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quaroni L and Zobi F, Inorg. Chem. Biol, 2014, 149–182. [Google Scholar]
- 16.Zobi F, Quaroni L, Santoro G, Zlateva T, Blacque O, Sarafimov B, Schaub MC and Bogdanova AY, J. Med. Chem, 2013, 56, 6719–6731. [DOI] [PubMed] [Google Scholar]
- 17.Quaroni L, Obst M, Nowak M and Zobi F, Angew. Chemie Int. Ed, 2015, 54, 318–322. [DOI] [PubMed] [Google Scholar]
- 18.Kong KV, Chew W, Lim LHK, Fan WY and Leong WK, Bioconjug. Chem, 2007, 18, 1370–1374. [DOI] [PubMed] [Google Scholar]
- 19.Clede S, Lambert F, Sandt C, Gueroui Z, Delsuc N, Dumas P, Vessieres A and Policar C, Biotechnol. Adv, 2013, 31, 393–395. [DOI] [PubMed] [Google Scholar]
- 20.Clède S, Lambert F, Sandt C, Gueroui Z, Réfrégiers M, Plamont M-A, Dumas P, Vessières A and Policar C, Chem. Commun, 2012, 48, 7729–7731. [DOI] [PubMed] [Google Scholar]
- 21.Policar C, Waern JB, Plamont M-A, Clède S, Mayet C, Prazeres R, Ortega J-M, Vessières A and Dazzi A, Angew. Chemie Int. Ed, 2011, 50, 860–864. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez CM, García-Rodríguez R and Miguel D, Dalt. Trans, 2007, 3, 3546. [DOI] [PubMed] [Google Scholar]
- 23.Kianfar E, Kaiser M and Knör G, J. Organomet. Chem, 2015, 799–800, 13–18. [Google Scholar]
- 24.Bourkoula A, Paravatou-Petsotas M, Papadopoulos A, Santos I, Pietzsch H-J, Livaniou E, Pelecanou M, Papadopoulos M and Pirmettis I, Eur. J. Med. Chem, 2009, 44, 4021–4027. [DOI] [PubMed] [Google Scholar]
- 25.Kottelat E, Lucarini F, Crochet A, Ruggi A and Zobi F, Eur. J. Inorg. Chem, 2019, 2019, 3758–3768. [Google Scholar]
- 26.Konkankit CC, Vaughn BA, MacMillan SN, Boros E and Wilson JJ, Inorg. Chem, 2019, 58, 3895–3909. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Chu BW-K, Zhu N and Yam VW-W, Organometallics, 2007, 26, 5423–5429. [Google Scholar]
- 28.Schrage BR, Herrick RS and Ziegler CJ, J. Organomet. Chem, 2019, 880, 170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chanawanno K, Kondeti V, Caporoso J, Paruchuri S, Leeper TC, Herrick RS and Ziegler CJ, Dalt. Trans, 2016, 45, 4729–4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasheminasab A, Engle JT, Bass J, Herrick RS and Ziegler CJ, Eur. J. Inorg. Chem, 2014, 2014, 2643–2652. [Google Scholar]
- 31.Chanawanno K, Rhoda HM, Hasheminasab A, Crandall LA, King AJ, Herrick RS, Nemykin VN and Ziegler CJ, J. Organomet. Chem, 2016, 818, 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chanawanno K, Engle JT, Le KX, Herrick RS and Ziegler CJ, Dalt. Trans, 2013, 42, 13679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ball PJ, Shtoyko TR, Krause Bauer JA, Oldham WJ and Connick WB, Inorg. Chem, 2004, 43, 622–632. [DOI] [PubMed] [Google Scholar]
- 34.Costa R, Chanawanno K, Engle JT, Baroody B, Herrick RS and Ziegler CJ, J. Organomet. Chem, 2013, 734, 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qayyum H, Herrick RS and Ziegler CJ, Dalt. Trans, 2011, 40, 7442. [DOI] [PubMed] [Google Scholar]
- 36.Herrick RS, Wrona I, McMicken N, Jones G, Ziegler CJ and Shaw J, J. Organomet. Chem, 2004, 689, 4848–4855. [Google Scholar]
- 37.Schrage BR, Herrick RS and Ziegler CJ, J. Organomet. Chem, , DOI: 10.1016/j.jorganchem.2018.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasheminasab A, Wang L, Dawadi MB, Bass J, Herrick RS, Rack JJ and Ziegler CJ, Dalt. Trans, 2015, 44, 15400–15403. [DOI] [PubMed] [Google Scholar]
- 39.Wang C, Lam H-C, Zhu N and Wong KM-C, Dalt. Trans, 2015, 44, 15250–15263. [DOI] [PubMed] [Google Scholar]
- 40.Santos IG, Abram U, Alberto R, Lopez EV and Sanchez A, Inorg. Chem, 2004, 43, 1834–1836. [DOI] [PubMed] [Google Scholar]
- 41.Wang W, Spingler B and Alberto R, Inorganica Chim. Acta, 2003, 355, 386–393. [Google Scholar]
- 42.Servaas PC, Stor GJ, Stufkens DJ and Oskam A, Inorganica Chim. Acta, 1990, 178, 185–194. [Google Scholar]
- 43.Boros E, Hafeli UO, Patrick BO, Adam MJ and Orvig C, Bioconjug. Chem, 2009, 20, 1002–1009. [DOI] [PubMed] [Google Scholar]
- 44.Bowen ML, Chen Z-F, Roos AM, Misri R, Häfeli U, Adam MJ and Orvig C, Dalt. Trans, 2009, 9228–9236. [DOI] [PubMed] [Google Scholar]
- 45.Saw MM, Kurz P, Agorastos N, Hor TSA, Sundram FX, Yan YK and Alberto R, Inorganica Chim. Acta, 2006, 359, 4087–4094. [Google Scholar]
- 46.Konkankit CC, Vaughn BA, Huang Z, Boros E and Wilson JJ, Dalt. Trans, 2020, Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herrick RS, Ziegler CJ, Ding T, Shaw J, Wrona I, Beaver M, Giguere J, Maus C and Müller P, J. Coord. Chem, 2017, 70, 3488–3500. [Google Scholar]
- 48.Wang Y, Liu Y, Luo J, Qi H, Li X, Nin M, Liu M, Shi D, Zhu W and Cao Y, Dalt. Trans, 2011, 40, 5046–5051. [DOI] [PubMed] [Google Scholar]
- 49.Binnemans K, Lodewyckx K, Donnio B and Guillon D, Chem. – A Eur. J, 2002, 8, 1101–1105. [DOI] [PubMed] [Google Scholar]
- 50.Lai CK, Chang C and Tsai C, J. Mater. Chem, 1998, 8, 599–602. [Google Scholar]
- 51.Wang Y-J, Song J-H, Lin Y-S, Lin C, Sheu H-S, Lee G-H and Lai CK, Chem. Commun, 2006, 4912–4914. [DOI] [PubMed] [Google Scholar]
- 52.Yeap G-Y and Heng B-T, J. Chem. Sci, 2014, 126, 247–254. [Google Scholar]
- 53.Plasseraud L, Cuervo LG, Guillon D, Süss-Fink G, Deschenaux R, Bruce DW and Donnio B, J. Mater. Chem, 2002, 12, 2653–2658. [Google Scholar]
- 54.Wu X, Zhu M, Bruce DW, Zhu W and Wang Y, J. Mater. Chem. C, 2018, 6, 9848–9860. [Google Scholar]
- 55.Cuerva C, Ovejero P, Campo JA and Cano M, New J. Chem, 2014, 38, 511–517. [Google Scholar]
- 56.Santoro A, Prokhorov AM, Kozhevnikov VN, Whitwood AC, Donnio B, Williams JAG and Bruce DW, J. Am. Chem. Soc, 2011, 133, 5248–5251. [DOI] [PubMed] [Google Scholar]
- 57.Park M, Kang D-G, Ko H, Rim M, Tran DT, Park S, Kang M, Kim T-W, Kim N and Jeong K-U, Mater. Horiz, 2020, 7, 2635–2642. [Google Scholar]
- 58.Concellón A, Termine R, Golemme A, Romero P, Marcos M and Serrano JL, Org. Chem. Front, 2020, 7, 2008–2015. [Google Scholar]
- 59.Gürek AG, Durmuş M and Ahsen V, New J. Chem, 2004, 28, 693–699. [Google Scholar]
- 60.Morrone S, Guillon D and Bruce DW, Inorg. Chem, 1996, 35, 7041–7048. [DOI] [PubMed] [Google Scholar]
- 61.Rowe KE and Bruce DW, J. Chem. Soc. Dalt. Trans, 1996, 3913–3915. [Google Scholar]
- 62.Rowe KE and Bruce DW, Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A. Mol. Cryst. Liq. Cryst, 1999, 326, 15–40. [Google Scholar]
- 63.Cardinaels T, Ramaekers J, Nockemann P, Driesen K, Van Hecke K, Van Meervelt L, Lei S, De Feyter S, Guillon D, Donnio B and Binnemans K, Chem. Mater, 2008, 20, 1278–1291. [Google Scholar]
- 64.Guillevic M-A, Light ME, Coles SJ, Gelbrich T, Hursthouse MB and Bruce DW, J. Chem. Soc. Dalt. Trans, 2000, 1437–1445. [Google Scholar]
- 65.Cardinaels T, Driesen K, Parac-Vogt TN, Heinrich B, Bourgogne C, Guillon D, Donnio B and Binnemans K, Chem. Mater, 2005, 17, 6589–6598. [Google Scholar]
- 66.Hu T, Wang Y, Dong M, Wu J, Miao X, Hu Y and Deng W, J. Phys. Chem. C, 2020, 124, 1646–1654. [Google Scholar]
- 67.Dey KC, Mandal PK and Kula P, J. Mol. Liq, 2020, 298, 112056. [Google Scholar]
- 68.López-Martin I, Burello E, Davey PN, Seddon KR and Rothenberg G, ChemPhysChem, 2007, 8, 690–695. [DOI] [PubMed] [Google Scholar]
- 69.Bornemann S, Herzog M, Roling L, Paulisch TO, Brandis D, Kriegler S, Galla H-J, Glorius F and Winter R, Phys. Chem. Chem. Phys, 2020, 22, 9775–9788. [DOI] [PubMed] [Google Scholar]
- 70.Sasaki R, Sato K and Kinbara K, ChemistryOpen, 2020, 9, 301–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang D, Richter C, Rühling A, Drücker P, Siegmund D, Metzler-Nolte N, Glorius F and Galla H-J, Chem. – A Eur. J, 2015, 21, 15123–15126. [DOI] [PubMed] [Google Scholar]
- 72.Mahmoud NN, Alhusban AA, Ali JI, Al-Bakri AG, Hamed R and Khalil EA, Sci. Rep, 2019, 9, 5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Witzigmann D, Kulkarni JA, Leung J, Chen S, Cullis PR and van der Meel R, Adv. Drug Deliv. Rev, , DOI: 10.1016/j.addr.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li J, Wang X, Zhang T, Wang C, Huang Z, Luo X and Deng Y, Asian J. Pharm. Sci, 2015, 10, 81–98. [Google Scholar]
- 75.Beltran-Gracia E, Lopez-Camacho A, Higuera-Ciapara I, Velazquez-Fernandez JB and Vallejo-Cardona AA, Cancer Nanotechnol, 2019, 10, 11. [Google Scholar]
- 76.Blume A, Curr. Opin. Colloid Interface Sci, 1996, 1, 64–77. [Google Scholar]
- 77.Generosi J, Margaritondo G, Sanghera JS, Aggarwal ID, Tolk NH, Piston DW, Castellano AC and Cricenti A, Appl. Phys. Lett, 2006, 89, 233906. [DOI] [PubMed] [Google Scholar]
- 78.Jiménez-Pulido SB, Illán-Cabeza NA, Hueso-Ureña F, Maldonado CR, Sánchez-Sánchez P, Fernández-Liencres MP, Fernández-Gómez M and Moreno-Carretero MN, Dalt. Trans, 2016, 45, 15142–15154. [DOI] [PubMed] [Google Scholar]
- 79.Espinal Viguri M, Huertos MA, Perez J, Riera L and Ara I, J. Am. Chem. Soc, 2012, 134, 20326–20329. [DOI] [PubMed] [Google Scholar]
- 80.Wright PJ, Muzzioli S, V Werrett M, Raiteri P, Skelton BW, Silvester DS, Stagni S and Massi M, Organometallics, 2012, 31, 7566–7578. [Google Scholar]
- 81.Yang J, Zhao J-X, Cao Q, Hao L, Zhou D, Gan Z, Ji L-N and Mao Z-W, ACS Appl. Mater. Interfaces, 2017, 9, 13900–13912. [DOI] [PubMed] [Google Scholar]
- 82.Sheldrick GM, Acta Crystallogr. Sect. A Found. Crystallogr, 2008, 64, 112–122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


