Abstract
Complex visual-motor behaviors dominate human-environment interactions. Letter production, writing individual letters by hand, is an example of a complex visual-motor behavior composed of numerous behavioral components, including the required motor movements and the percepts that those motor movements create. By manipulating and isolating components of letter production, we provide experimental evidence that this complex visual-motor behavior is supported by a widespread neural system that is composed of smaller subsystems related to different sensorimotor components. Adult participants hand-printed letters with and without “ink” on an MR-safe digital writing tablet, perceived static and dynamic representations of their own handwritten letters, and perceived typeface letters during fMRI scanning. Our results can be summarized by three main findings: (1) Frontoparietal systems were associated with the motor component of letter production, whereas temporo-parietal systems were more associated with the visual component. (2) The more anterior regions of the left intraparietal sulcus were more associated with the motor component, whereas the more posterior regions were more associated with the visual component, with an area of visual-motor overlap in the posterior intraparietal sulcus. (3) The left posterior intraparietal sulcus and right fusiform gyrus responded similarly to both visual and motor components, and both regions also responded more during the perception of one’s own handwritten letters compared with perceiving typed letters. These findings suggest that the neural systems recruited during complex visual-motor behaviors are composed of a set of interrelated sensorimotor subsystems that support the full behavior in different ways and, furthermore, that some of these subsystems can be rerecruited during passive perception in the absence of the full visual-motor behavior.
INTRODUCTION
Any human behavior requires the recruitment of a distributed pattern of neural systems that allow for communication among brain regions. How the neural components, or nodes, of the larger system contribute to a given behavior is a question that can help us understand why certain behaviors are supported by particular constellations of nodes or networks. Writing individual letters by hand, for instance, is a behavior that presumably requires numerous motor and visual processes as well as visual-motor integration. This complex visual-motor behavior recruits a widespread frontal, parietal, and ventral-temporal neural system (Longcamp et al., 2014; James & Gauthier, 2006) that displays some similarity to the neural system that supports writing words (Planton, Longcamp, Péran, Démonet, &Jucla, 2017; Planton, Jucla, Roux, & Démonet, 2013). Here, we focus specifically on the neural components that comprise the distributed frontal, parietal, and ventral-temporal letter production system to better understand the contribution of each neural component to the macroscale functioning of the larger neural system.
Letter production is a complex task that involves perceptual and motor behavioral components. Perceptual components include, for instance, visual perception, kinesthesia, and proprioception; motor components include fine motor control, in-hand manipulation, and eye movements (Feder & Majnemer, 2007; James & Gauthier, 2006). As a visually guided action, letter production also requires efficient integration among visual and motor systems. Indeed, tests of visual-motor integration skills repeatedly correlate with the quality of handwritten forms (Klein, Guiltner, Sollereder, & Cui, 2011; Cornhill & Case-Smith, 1996; Tseng & Murray, 1994; Weil & Amundson, 1994; Maeland, 1992). In addition, studies that have investigated the neural systems supporting letter production have repeatedly shown that brain regions associated with motor movements (frontal cortex), visual perceptual processing (ventral-temporal cortex), and perceptual-motor coordination (parietal cortex) are recruited (Longcamp et al., 2014; Yuan & Brown, 2014; but see James & Gauthier, 2006). We propose, therefore, that the frontal, ventral-temporal, and parietal cortices are differentially involved in each behavioral component.
Letter Production as a Perceptual-Motor Behavior
The interrelated behaviors involved with letter production include fine-motor guidance of the fingers, hands, and wrists as they control a writing utensil (among others that will not be focused on here: eye movements, postural control, head movements, arm movements; Trieman & Kessler, 2014; Feder & Majnemer, 2007). In children, for example, the manipulation of the writing utensil is difficult, given their immature fine motor skills. This immaturity results in variations of the standard sequence of hand movements for each letter that they attempt to write. Even in the most proficient adult writers, each time a letterform is produced, the motor behavior changes as a function of desk height, pen weight, paper roughness, lighting, torso positioning, and muscle fatigue. Although the general movements required to produce a given letterform may be fairly standard across productions, the actual force, velocity, and trajectory of each movement are variable from one production to the next (Longstaff & Heath, 1997; Wing & Nimmo-Smith, 1987). If we consider the real-time act of letter production, given that the production of a legible letterform varies from one episode to the next, we can assume that there are unique perceptual-motor interactions involved with each episode (Feder & Majnemer, 2007). It is important to note that, although letter production requires access to motor plans (Longcamp et al., 2014; James & Gauthier, 2006; Longcamp, Anton, Roth, & Velay, 2003), these plans may only serve as a rough guideline for the production of the shape; the in-the-moment production, on the other hand, requires the efficient interplay of online perception and action.
The output of the motor production is the form created on the writing surface that is then visually perceived and used to guide subsequent movements. This perceptual experience involves seeing one’s hand and pencil moving in time with the unfolding letter, the dynamic unfolding of a letterform stroke-by-stroke, and the observation of the final handwritten letter. These experiences not only serve to guide the ongoing motor behaviors in real time but also may be stored to influence subsequent letter perception and/or production—potentially to augment motor plans based on visual feedback. Importantly, the resultant percept is highly variable—not only in its dynamics during production but also in the final percept of the handwritten letter. This perception-action loop culminates in the production of meaningful visual stimuli that are the seemingly simple result of a very complex set of behaviors.
The Candidate Neural Systems Supporting Letter Production
Producing letters by hand is generally supported by a neural system that encompasses perceptual and motor neural subsystems, including regions within frontal, parietal, and ventral-temporal cortices (Longcamp et al., 2008, 2014; Planton et al., 2013; James & Engelhardt, 2012; Longcamp, Hlushchuk, & Hari, 2011; James & Atwood, 2009; James & Gauthier, 2006; Siebner et al., 2001; Seitz et al., 1997). These past studies have provided valuable information concerning how these neural subsystems support the different behavioral components of letter production. Aside from Longcamp et al. (2014), they have not presented visual feedback of the letter being written to the participant as they were writing it, making inferences concerning the visually guided nature of letter production difficult, and few have investigated letter production and perception within the same participants. Below, we review the current hypotheses regarding how these neural subsystems support letter production.
Frontal Motor Regions(Left Precentral Gyrus, Left Superior and Middle Frontal Gyri)
Large bodies of work have documented the recruitment of motor regions in the frontal cortex for action execution (for reviews, see Meier, Aflalo, Kastner, & Graziano, 2008; Graziano, 2006; Schieber, 2001). Not surprisingly then, primary motor and premotor cortices have consistently been shown to be involved in the fine motor movements required during letter and word productions (letters: Haar, Donchin, & Dinstein, 2015; Longcamp et al., 2014; Dufor & Rapp, 2013; James & Gauthier, 2006; words: Planton et al., 2017; for metaanalyses of writing studies, see Planton et al., 2013, and Purcell, Turkeltaub, Eden, & Rapp, 2011) and drawing shapes (Planton et al., 2017; Potgieser & de Jong, 2016; James & Gauthier, 2006; for a meta-analysis of drawing studies, see Yuan & Brown, 2015). The roles of primary and premotor cortices in action execution differ to some degree, however.
Primary motor cortex is a functionally defined region that most often maps onto the precentral gyrus, with the left primary motor cortex being most involved in the execution of movement on the contralateral side of the body (for a review, see Chouinard & Paus, 2006). Even within letter production research, primary motor cortex is closely tied to the actual execution of movements required to produce a written form. For instance, primary motor cortex is recruited when participants write letters (Longcamp et al., 2003, 2014; James & Gauthier, 2006), draw shapes (James & Gauthier, 2006), or draw other forms (Yuan & Brown, 2014). This is not surprising, given the role of primary motor cortex in all forms of action execution.
Premotor cortex, often separated into dorsal premotor cortex, including posterior portions of the superior frontal and middle frontal gyri, and ventral premotor cortex, including posterior portions of the inferior frontal gyri and anterior-ventral portions of the precentral gyri, appears to have a more complex role in form production. Dorsal and ventral premotor cortices participate in movement in different ways. For instance, the left dorsal premotor cortex is recruited during visually guided reach-to-grasp movements with the right hand (Budisavljevic et al., 2017), is associated with finger movement sequencing of the right hand (Budisavljevic et al., 2017; Chouinard & Paus, 2006), and is recruited during letter and shape productions by hand (James & Gauthier, 2006; Longcamp et al., 2003). Ventral premotor cortex, on the other hand, is associated with hand shaping for object manipulation (Budisavljevic et al., 2017) and is recruited more during letter production than during shape drawing (James & Gauthier, 2006).
Dorsal premotor cortex, often referred to as Exner’s area (Planton et al., 2013; Longcamp et al., 2003; Anderson, Damasio, & Damasio, 1990; Exner, 1881), has historically received attention for being one of the more crucial regions for producing written forms by hand. It is routinely recruited during various text production (Purcell et al., 2011; Brownsett & Wise, 2010; Longcamp et al., 2003; Katanoda, Yoshikawa, & Sugishita, 2001) and drawing (Yuan & Brown, 2015) tasks. The exact location of this region remains debatable, however. Dorsal premotor responses during letter production have been reported in the left posterior middle frontal gyrus (Pattamadilok, Ponz, Planton, & Bonnard, 2016), as well as posterior to the left superior frontal gyrus in the superior frontal sulcus (Planton et al., 2017), and are considered to be left-lateralized (Planton et al., 2017; Pattamadilok et al., 2016; Roux et al., 2009).
Some evidence suggests that the left dorsal premotor cortex codes for serial processing of graphemic-motor correspondences (Planton et al., 2017; Pattamadilok et al., 2016; Roux et al., 2009) and has recently been referred to as the graphemic-motor frontal area (Planton et al., 2017). As such, left dorsal premotor responses found during letter production would be attributed to the translation of stored grapheme representations, a stored perceptual representation of the letter “a” for instance, into the series of motor movements required to reproduce those graphemic representations on paper using a writing utensil. The letter production episode is, therefore, characterized by a stepwise reproduction of the letter—one stroke followed by another—until the form is completed. This proposal fits nicely with evidence suggesting that dorsal premotor regions are involved in the sequencing of finger movements required for letter production (James & Gauthier, 2006; Longcamp et al., 2003). Left dorsal premotor recruitment during letter production, however, may not be specific to letter or grapheme production, as it is also recruited during nonletter and nongrapheme production tasks (Planton et al., 2017; Yuan & Brown, 2015; Longcamp et al., 2003, 2014; James & Gauthier, 2006).
Parietal Cortex (Left Postcentral Gyrus, Left Superior Parietal Lobe, Bilateral Intraparietal Cortex)
Activation in parietal cortex is routinely found during letter production, very often in the left postcentral gyrus, left superior parietal lobule (SPL), and bilateral intraparietal sulcus (IPS; Longcamp et al., 2014; James & Gauthier, 2006). Activity in the SPL and IPS has traditionally been associated with visual-motor transformations (Ogawa & Inui, 2009; Jackson & Husain, 2006; for a review, see Buneo & Andersen, 2006) and visually guided actions (Milner & Goodale, 2006; Goodale & Milner, 2005), and these regions may serve a similar purpose during letter production. Activity in parietal cortex that included these regions was observed when participants wrote letters (Kadmon Harpaz, Flash, & Dinstein, 2014; Longcamp et al., 2014; Seitz et al., 1997), but also when participants wrote digits (Longcamp et al., 2014), wrote whole words (Planton et al., 2017), copied novel forms (Planton et al., 2017; Yuan & Brown, 2014), and even simply made marks (Haar et al., 2015; Yuan & Brown, 2014). Thus, parietal cortex may not be related to motor plans in the same way that the frontal motor system is but may be more related to in-the-moment visually guided action, communicating with the frontal cortex to potentially augment motor plans (Rizzolatti, Luppino, & Matelli, 1998).
There is some indication of a graded involvement of the IPS in the visual or motor components of letter production. Studies have demonstrated that anterior IPS is more closely associated with motor components of letter production whereas more posterior IPS is more closely associated with the perceptual components of letter production (Haar et al., 2015; Kadmon Harpaz et al., 2014). Activation in the left anterior IPS during letter production has been related to the specification of movement trajectories (Haar et al., 2015; Kadmon Harpaz et al., 2014). For instance, the pattern of activation in the left anterior IPS can be used to predict the letter produced by a participant (Kadmon Harpaz et al., 2014). Posterior IPS, however, is more active if participants are provided visual feedback concerning the location of their cursor (Thaler & Goodale, 2011) or the mark produced by the pen (Yuan & Brown, 2014) than if they are not given visual feedback. The more anterior regions of IPS, then, may be more associated with the motor component of letter production, whereas the posterior regions of IPS may be more important for the visual-motor integration that is required during the act of letter production.
Ventral-Temporal Cortex (Left Fusiform Gyrus)
The ventral-temporal cortex, and, more specifically, the left fusiform gyrus, has traditionally been associated with letter (James, James, Jobard, Wong, & Gauthier, 2005) and word (Dehaene, Le Clec’H, Poline, Le Bihan, & Cohen, 2002; Cohen et al., 2000) processing. The left fusiform gyrus, however, is also more active during letter production than shape production, even without seeing the form produced (James & Gauthier, 2006; Longcamp et al., 2003). This apparent category specificity has led to the interpretation that the left fusiform gyrus is composed of neural traces of prior experiences with letters (i.e., letter representations) that can be accessed in a bottom-up manner through visual perception and in a top-down manner during letter production (Longcamp et al., 2003, 2014; James & Gauthier, 2006). The left fusiform gyrus is therefore generally considered to have some degree of letter specificity and to potentially act as a store for abstract information about letters used for object categorization (e.g., A vs. G vs. D; Rothlein & Rapp, 2014; Dufor & Rapp, 2013; Dehaene et al., 2004; Polk & Farah, 2002; Dehaene et al., 2001).
The right fusiform gyrus, on the other hand, is sensitive to exemplar variation for common objects (Koutstaal et al., 2001), exemplar variation for whole word reading (Barton, Fox, Sekunova, & Iaria, 2010; Barton, Sekunova, et al., 2010; Qiao et al., 2010), and exemplar variation for letters (e.g., A vs. A vs. A) (Barton, Sekunova, et al., 2010; Koutstaal et al., 2001; Gauthier et al., 2000). The right fusiform gyrus is therefore generally considered to be associated with the processing of handwritten letters because handwritten letters are variations from the stereotypical category exemplar of typed letters (Gauthier et al., 2000; although, see Longcamp et al., 2011). Handwritten letter perception may involve more than the perception of category variability, however, because handwritten letters contain information about the motor movements used to produce them that an observer readily perceives (Kandel, Orliaguet, & Viviani, 2000; Orliaguet, Kandel, & Boe, 1997; Babcock & Freyd, 1988; Freyd, 1987).
The Current Study
Here, we sought to better understand the role of brain systems supporting letter production by conducting analyses that focused on the behavioral components of letter production and their neural instantiations relative to the larger neural system recruited during letter production. We used an MR-safe writing tablet with real-time visual feedback provided to the participants to preserve as much as possible the perceptual-motor coupling inherent to the act of handwriting. We decomposed the complex act of letter production by manipulating motor production and visual perception such that we could isolate these components in our analyses.
As suggested by previous work, we predicted that frontal motor regions would be most associated with motor production and that ventral-temporal cortex would be most associated with the visual perception of the forms produced. We also predicted a strong role for the parietal cortex in these productions. We predicted that the posterior parietal cortex would respond to both motor and visual components of the letter production process, because of its role in visually guided action, but would show the graded anterior-posterior response pattern outlined above (Haar et al., 2015; Kadmon Harpaz et al., 2014; Yuan & Brown, 2014; Thaler & Goodale, 2011).
Letter production involves several perceptual components. We therefore endeavored to determine the neural responses associated with each type of percept. During letter production, the observers present themselves with a dynamically unfolding handwritten letter, stroke by stroke, as they are producing the letter. At the completion of letter production, they perceive their own handwritten letter. To better understand the brain systems involved with the perceptual components of letter production, we compared activation found during the perception of one’s own letters dynamically unfolding (i.e., a video recording) with the static presentation of the final product to highlight responses associated with the dynamic unfolding component. We also compared the perception of their final handwritten letter product with the perception of a typed letter to highlight responses associated with the perception of handwritten letters.
We have proposed that the frontal, ventral-temporal, and parietal cortices that comprise the widespread neural system for letter production are, in fact, recruited at varying degrees for each behavioral component. We therefore expected that these neural components of letter production would demonstrate stronger associations with one component of the full behavior than for another, resulting in differing patterns of recruitment within the broader letter production system—different subsystems—that are related to the particular behavioral component. An alternative possibility is that each behavioral component of letter production would recruit only one cluster of activation within the broader neural system, as opposed to a pattern of several clusters, resulting in “modules” of processing (no overlap among clusters during a particular behavior). Another alternative possibility, although unlikely, is that when the behavioral components of letter production are isolated from the full behavior, they will recruit an entirely different neural response that is tangential to the larger letter production system.
METHODS
Participants
Fourteen literate English-speaking adults were recruited by word-of-mouth (8 women, mean age = 20.1 years, SD = 2.5). All participants were right-handed, as indicated by the Edinburgh Handedness Inventory (Oldfield, 1971), screened for neurological trauma, developmental disorders, and MRI contraindications. All participants provided written informed consent according to the guidelines of the Indiana University institutional review board.
Materials
Stimuli
A set of 12 single uppercase letters of the Roman alphabet were selected based on the distinctiveness of their visual forms and their letter names: A, B, C, D, G, H, J, L, Q, R, U, and Y. All letters were presented/produced in white on a black background, one at a time. Typed letters were presented in 120-point Arial font and subtended 4° × 4° of visual angle. All handwritten letters were written within a box that subtended 100° × 10° of visual angle with a pen width of 7 points. The size and form of the letter stimuli within this box differed with and across conditions given the self-produced nature of the written stimuli.
There were two conditions that involved motor production: Write With Ink and Write Without Ink. During the Write With Ink condition, participants wrote a letter of the Roman alphabet on a digitizing tablet that was shown to them in real time in a mirror above the head coil. Writing trajectories and final handwritten letters from the Write With Ink condition were saved so that they could be re-presented in additional conditions (see below). During the Write Without Ink condition, the screen remained blank as they produced their letter, just as if their pen had no ink. Therefore, the motor production in both cases was kept relatively constant, whereas the resultant visual percept of the letter was either present or absent.
There were three conditions that involved passive visual perception: Watch Dynamic, Watch Static, and Watch Typed. During Watch Dynamic, participants saw a playback of their own letter production from the Write With Ink condition (above). The timing of the playback was the same as their own production time. During the Watch Static condition, participants saw their own handwritten letters statically presented. During Watch Typed, participants saw typed letters. The six letters used for the Write With Ink, Watch Static, and Watch Dynamic blocks were necessarily the same set of six letters. For this reason, the same six letters were also displayed in the Watch Typed condition.
For all conditions, the block instructions and letter-name prompts were prerecorded from a female native English speaker and played at the beginning of each block and trial, respectively.
Apparatus
All participants completed fMRI tasks on an MR-safe tablet that records handwriting trajectories and can be used to project participants’ handwritten letters onto a mirror above their head—in real time as they are producing each form (Tam, Churchill, Strother, & Graham, 2011). This handwriting-recording device has been used in several studies (e.g., Kadmon Harpaz et al., 2014; Longcamp et al., 2014).
The tablet was supported by a lap desk that kept it in a fixed position above their lap and within arm’s reach (Figure 1). Participants held an MR-safe stylus throughout the entire session and wore a Wheaton elastic shoulder immobilizer to restrict movement necessary for writing to elbow, wrist, and hand joints.
Figure 1.

Full apparatus setup. The MR-safe writing tablet (Tam et al., 2011), arm pillow, and Wheaton elastic shoulder immobilizer were adjusted for each participant. Participant-specific adjustments ensured that the participants were in a comfortable writing position. Before the experiment began, an experimenter positioned their hand in an appropriate position so that they could feel where their wrist laid on the apparatus. Care was taken to instruct the participants to keep their hand toward the center of the screen.
Auditory instructions and letter-name prompts were presented through MR-safe headphones, and Boom was used to enhance audio clarity. A Mitsubishi XL30 projector displayed all visual presentations onto a mirror in the bore of the scanner attached to the head coil above the head of the participant (Figure 1). An in-house MATLAB program using the Psychophysics Toolbox extensions interfaced with the tablet, headphones, and projector to record and present all stimuli (Brainard, 1997; Pelli, 1997).
Procedure
Before scanning, participants watched a video that demonstrated the tasks they would be asked to perform in the scanner. The video explained that they would be asked to “draw” (i.e., write) and “watch” and that sometimes they would not be able to see what they were writing. After ensuring that the participants understood their task, they proceeded to the imaging environment where they first underwent a high-resolution anatomical scan followed by four fMRI experimental runs. During the fMRI runs, participants wrote letters with and without ink, perceived dynamic and static representations of their own written letters, and perceived typeface letters in a blocked design (Figure 2). This resulted in five experimental conditions: Write With Ink, Write Without Ink, Watch Dynamic, Watch Static, and Watch Typed. The experimental design originally included two additional conditions that were not of primary interest for this particular study.
Figure 2.
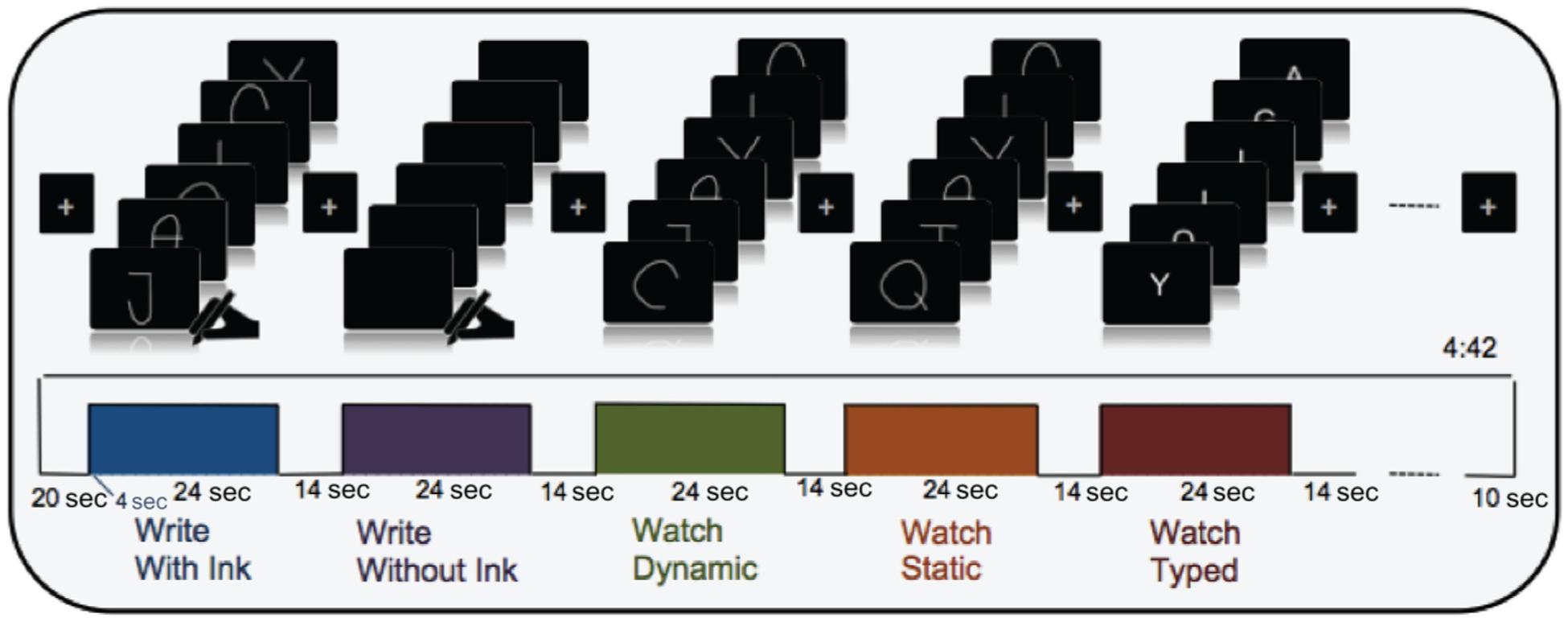
Stimulation protocol. The figure presents a depiction of the blocks within each run and the trials within each block. Block orders were pseudorandomized and counterbalanced across runs. The six letters used for the Write With Ink, Watch Static, and Watch Dynamic blocks were necessarily the same set of six letters, and the same set of six letters was also displayed in the Watch Typed condition. Letter orders within a block were randomized. Block instructions and letter names were prerecorded. Block instructions were played at the beginning of each block to alert participants to the task. Letter names were played at the beginning of each trial to alert the participants to the letter that they should write or to the letter that would be displayed.
Each run contained one block of each condition. At the beginning of each run, six letters were selected randomly without replacement from the full stimulus set of 12 letters with the additional restriction that a particular selection may not contain highly confusable letter names, such as “b” and “g” (Hull, 1973; Conrad, 1964). Block orders were pseudorandomized, as opposed to fully randomized, to ensure that the Write With Ink condition occurred before Watch Static and Watch Dynamic conditions in each run. Given that each condition presented the same six letters, but in a different format, it was imperative to ensure that block order was randomized to prevent possible repetition suppression effects affecting one condition more than another. Because we averaged activation across all runs, we are confident that, if repetition suppression did occur, it would not be more pronounced in one condition over another.
Each block consisted of six stimuli, one presented in each of the six trials within a block. The order of the six letters within each block was randomized. Each trial lasted for 4 sec to ensure that the participants had enough time to write with and without ink. There was no gap between trials, resulting in 24-sec-long blocks. Each block was separated by a 14-sec interblock interval, the last 2 sec of which included auditory instructions for the next block. Auditory instructions were kept to a set of two simple one-word imperatives: “draw” and “watch.” During the interblock interval, only the fixation cross was visible in the mirror. The same fixation cross was presented for 20 sec before the first block of each run and for 10 sec after the last block of each run. Each run, therefore, totaled 4 min 42 sec.
Scanning Parameters
Neuroimaging was performed using a Siemens Magnetom Tim Trio 3-T whole-body MRI system housed in the Indiana University Imaging Research Facility. High-resolution T1-weighted anatomical volumes were acquired using a Turbo-flash 3-D sequence: inversion time = 900 msec, echo time = 2.67 msec, repetition time = 2000 msec, flip angle = 9°, with 120 sagittal slices of 1.5-mm thickness, a field of view of 192 × 192 mm, and an isometric voxel size of 1.5 mm3. For functional images, the field of view was 192 × 192 mm, with an in-plane resolution of 64 × 64 pixels and 33 axial slices of 3.8-mm thickness per volume with 0% slice gap, producing 3.0 × 3.0 × 3.8 mm voxels. Functional images were acquired using a gradient-echo EPI sequence with interleaved slice order: echo time = 30 msec, repetition time = 2000 msec, and flip angle = 70° for BOLD imaging.
Analyses
The main analyses consisted of a standard preprocessing pipeline for fMRI data and additional motion correction steps, followed by a series of planned whole-brain contrasts and a conjunction analysis. All but one analysis were performed in BrainVoyager QX 2.8.2. For this one analysis, an in-house MATLAB routine was used to extract the phase time course from the complex MR signal and insert it as a predictor in the BrainVoyager design matrix file (see Preprocessing and Motion Correction section).
Preprocessing and Motion Correction
Individual anatomical volumes were normalized to Talairach space (Talairach & Tournoux, 1988). Preprocessing of functional data included slice scan time correction, 3-D motion correction using trilinear/sinc interpolation, and 3-D Gaussian spatial blurring with an FWHM of 6 mm. Temporal high-pass filtering was performed using a voxel-wise general linear model (GLM) with predictors that included a Fourier basis set with a cutoff value of 2 sine/cosine pairs and a linear trend predictor. During normalization, functional data were resampled to 3-mm3 isometric voxels. Coregistration of functional volumes to anatomical volumes was performed using a rigid body transformation.
To account for head motion, rigid body transformation parameters were included in the design matrix as predictors of no interest (Bullmore et al., 1999) along with spike regressors for each time point at which the relative root mean squared time course exceeded 0.5 mm (Satterthwaite et al., 2013). To account for possible perturbations in the static magnetic field due to movements outside the field of view (e.g., arm movements), demeaned “phase regressors” were included in the design matrix as predictors of no interest (Cheng & Puce, 2014; Barry et al., 2010; Cheng & Li, 2010).
Data Analyses
The statistical analyses began with a voxel-wise GLM with one predictor of interest for each condition and seven predictors of no interest that were included for motion correction purposes only (see Preprocessing and Motion Correction section). Each predictor of interest was convolved with a double-gamma hemodynamic response function (Boynton, Engel, Glover, & Heeger, 1996). The resulting design matrix was subjected to a random effects GLM analysis for planned contrasts and a conjunction analysis. Resulting t maps were subjected to a standard voxel-wise threshold of pvoxel < .001 and a cluster threshold of 40 contiguous 3-mm isometric voxels. Correspondence between anatomical locations and significant activation clusters was determined by, first, referencing the Talairach Daemon and, second, referencing the Duvernoy (1999) human brain atlas to verify. In cases where the Talairach Daemon and Duvernoy label disagreed, the Duvernoy label was selected.
Our first contrast was designed to identify the entire letter production system, as has been done in prior work (Longcamp et al., 2014). To this end, we compared letter production with fixation (Write With Ink > Fixation) to identify regions broadly associated with producing letters by hand. We followed this preliminary contrast with several whole-brain contrasts designed to determine the neural subsystems of the broader letter production system that may be more or less involved in certain behavioral components of letter production.
Two contrasts and a conjunction analysis were used to identify regions that were related to the motor, visual, and/or visual-motor components of letter production. The first contrast identified the motor component of production by comparing activation during Write With Ink with activation during Watch Dynamic. These two conditions were identical (i.e., yoked) in terms of the visual information shown to the participant and served to identify the neural subsystem of letter production associated with the motor behavioral component. The second contrast identified the visual component of letter production by comparing Write With Ink with Write Without Ink. These two conditions were identical (i.e., yoked) in terms of the motor action required from the participant and served to identify the neural subsystem of letter production associated with the visual behavioral component.
A conjunction between the motor component contrast and the visual component contrast was performed to identify regions that were involved in both motor and visual components to an equal extent. Regions revealed by this conjunction analysis would demonstrate a significant difference between activation during Write With Ink and Watch Dynamic (i.e., the motor component) that is not significantly different than the difference between activation during Write With Ink and Write Without Ink (i.e., the visual component). The resulting clusters revealed by this conjunction would therefore be better characterized as regions associated with the visual-motor component of letter production as opposed to only the motor or visual component.
Two additional contrasts were designed to identify brain regions that were responsive to particular visual percepts produced by letter production. The first contrast identified areas associated with the unfolding of the form produced over time (Watch Dynamic > Watch Static) to assess the effects of the perception of motion information during letter production. In both of these conditions, participants were presented with their own handwritten letter—both from the same letter production episode—with the only difference being that the presentation of the letterform unfolded overtime in Watch Dynamic and was presented statically in Watch Static. The second contrast compared the effects of viewing one’s own handwritten form with a typed category exemplar (Watch Static > Watch Typed) to identify regions that might be more sensitive to the perception of handwritten forms compared with typed forms.
RESULTS
Letter Production
The comparison of producing a letter with ink to visual fixation produced a distributed neural system that included the left precentral and postcentral gyri, right precentral gyrus, bilateral posterior superior frontal gyri, bilateral parietal cortices, bilateral occipital and ventral-temporal regions, and cerebellum (Figure 3A and Table 1). The expanse of this contrast was expected as it served to provide a general display of brain regions involved in the letter production task and is consistent with the general response during letter production reported in Longcamp et al. (2014) using a similar apparatus and procedure. No areas were more responsive during fixation than during letter production. The absence of auditory cortex recruitment in this contrast can be attributed to the auditory instructions received during the last 2 sec of the fixation period, resulting in a cancellation of the auditory activation that would have been expected because of the auditory prompts during Write With Ink when compared with fixation.
Figure 3.
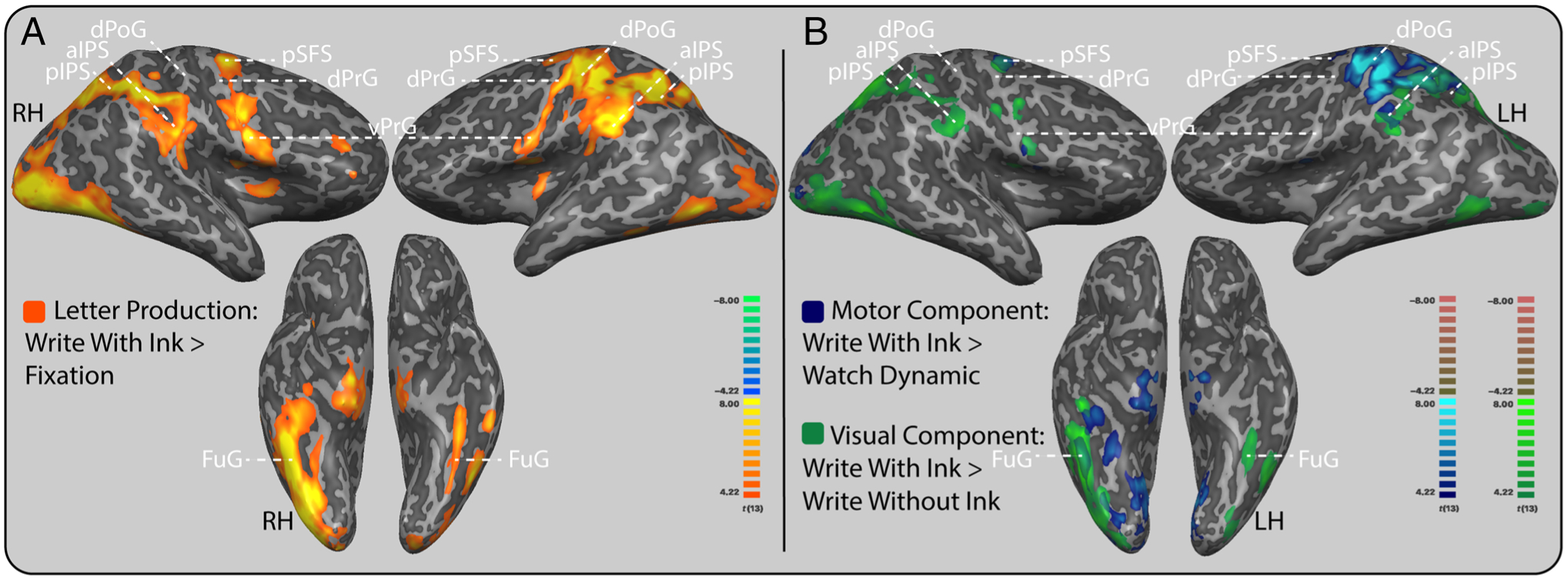
Motor and visual components of letter production from whole-brain contrasts. Letter production recruited a broad frontal motor, parietal, and ventral-temporal response, with frontal motor and anterior parietal cortices being associated with the motor component and posterior parietal and ventral-temporal cortices being associated with the visual component. (A) Letter production system. Areas that are orange were more active during letter production than at rest. (B) Motor and visual neural components of letter production. Areas that are blue were more active during letter production than watching the letter unfold (motor component), and areas that are green are more active during letter production than during letter production without ink (visual component). Group level results are displayed at a standard voxel-wise threshold of pvoxel < .001, and a cluster threshold of 40 contiguous 3-mm isometric voxels overlaid on an inflated anatomical image from a single participant. Anatomical label abbreviations: pSFS = posterior superior frontal sulcus; dPrG = dorsal precentral gyrus; vPrG = ventral precentral gyrus; dPoG = dorsal postcentral gyrus; aIPS = anterior IPS; pIPS = posterior IPS; FuG = fusiform gyrus; LH = left hemisphere; RH = right hemisphere.
Table 1.
Whole-Brain Contrasts
| Contrast | Number of Clusters | Cluster Size (Voxels) | Talairach Coordinates | Peak T | Anatomical Location | ||
|---|---|---|---|---|---|---|---|
| Peak x | Peak y | Peak z | |||||
| Write With Ink > fixation | 4 | 118,590 | 18 | −46 | −26 | 13.9 | Right cerebellum |
| −24 | −58 | −26 | 12.7 | Left cerebellum | |||
| 35 | −46 | −14 | 7.5 | Right posterior fusiform gyrus | |||
| 29 | −90 | 0 | 7.5 | Right occipital lobe | |||
| −44 | −42 | −23 | 7.0 | Left posterior fusiform gyrus | |||
| 49,347 | −33 | −43 | 43 | 21.3 | Left IPS | ||
| −39 | −22 | 52 | 11.7 | Left dorsal postcentral gyrus | |||
| −33 | −19 | 52 | 10.8 | Left dorsal precentral gyrus | |||
| −36 | −4 | 13 | 10.7 | Left insula | |||
| −24 | −13 | 49 | 9.1 | Left posterior superior frontal sulcus | |||
| −6 | −7 | 55 | 8.6 | Left ACC | |||
| −51 | −4 | 37 | 7.1 | Left ventral precentral gyrus | |||
| 31,838 | 27 | −40 | 40 | 11.8 | Right IPS | ||
| 18,495 | 48 | 2 | 31 | 9.5 | Right ventral precentral gyrus | ||
| 24 | −13 | 49 | 9.3 | Right posterior superior frontal sulcus | |||
| Motor component: Write With Ink > Watch Dynamic | 3 | 118,665 | 24 | −40 | −26 | 10.3 | Right cerebellum |
| −24 | −55 | −29 | 8.8 | Left cerebellum | |||
| 36 | −55 | −11 | 7.2 | Right posterior fusiform gyrus | |||
| 30,254 | −30 | −40 | 46 | 10.3 | Left IPS | ||
| −33 | −19 | 49 | 10.2 | Left dorsal postcentral gyrus | |||
| −1 | 5 | 46 | 6.3 | Left ACC | |||
| 2,152 | 21 | −10 | 55 | 6.9 | Right posterior superior frontal sulcus | ||
| Visual component: Write With Ink > Write Without Ink | 6 | 24,288 | 42 | −40 | −17 | 11.4 | Right posterior fusiform gyrus |
| 33 | −79 | 4 | 7.6 | Right middle occipital gyrus | |||
| 17,273 | 24 | −61 | 52 | 8.8 | Right IPS | ||
| 13,946 | −18 | −67 | 46 | 7.6 | Left IPS | ||
| 12,885 | −42 | −67 | −11 | 6.9 | Left posterior fusiform gyrus | ||
| 3,034 | 42 | −4 | 40 | 1.1 | Right ventral precentral gyrus | ||
| 1,791 | 30 | −10 | 55 | 7.1 | Right posterior superior frontal sulcus | ||
| Visual-motor component: Write With Ink > Watch Dynamic and Write With Ink > Write Without Ink | 3 | 10,484 | 36 | −58 | −11 | 7.1 | Right posterior fusiform gyrus |
| 5,452 | −30 | −46 | 46 | 6.3 | Left posterior IPS | ||
| 1,114 | 27 | −13 | 52 | 6.6 | Right posterior superior frontal sulcus | ||
| Unfolding: Watch Dynamic > Watch Static | 0 | – | – | – | – | – | – |
| Handwritten forms: Watch Static > Watch Typed | 5 | 2,002 | −24 | −64 | 52 | 8.4 | Left posterior IPS |
| 1,916 | 45 | −61 | −8 | 12.6 | Right posterior fusiform gyrus | ||
| 1,219 | 18 | −61 | 49 | 6.9 | Right posterior IPS | ||
| 1,025 | 42 | 44 | 22 | 6.4 | Right pFC | ||
| 711 | 27 | −88 | 9 | 7.0 | Right middle occipital gyrus | ||
Local peaks with a T statistic greater than 6.0 are reported for large clusters that spanned several anatomical locations.
Motor Component
The left dorsal postcentral gyrus, left IPS, right ventral temporal cortex, right posterior superior frontal gyrus, and bilateral cerebellar responses were associated with the motor component of the letter production task (Figure 3B). Included in the frontoparietal regions were dorsal and lateral aspects of the left precentral and left postcentral gyri, the right posterior middle frontal gyrus, the left superior parietal cortex, and the left anterior to middle IPS. The right ventral temporal activations included a posterior portion of the right fusiform gyrus and a lateral portion of the right posterior inferior temporal gyrus. No areas responded more for the reverse contrast, the perception of dynamic unfolding compared to producing letters with ink.
Visual Component
Bilateral parietal and ventral-temporal cortices were associated with the visual component of the letter production task as well as the right posterior superior frontal gyrus. The bilateral parietal regions were middle to posterior IPS (Figure 3B). The left ventral-temporal activation included a posterior portion of the inferior temporal gyri and the posterior fusiform gyrus. The right ventral-temporal response was notably broader than the left (Figure 3B). No cerebellar activation was found for this contrast. No regions responded more for the reverse contrast, producing letters without ink compared to producing letters with ink.
Visual-Motor Component
Areas of overlap among the visual and motor components of letter production were observed in the right ventral-temporal cortex, including the posterior inferior temporal gyrus and posterior fusiform gyrus; in the left posterior IPS; and in the right posterior middle frontal gyrus (Figure 4A).
Figure 4.
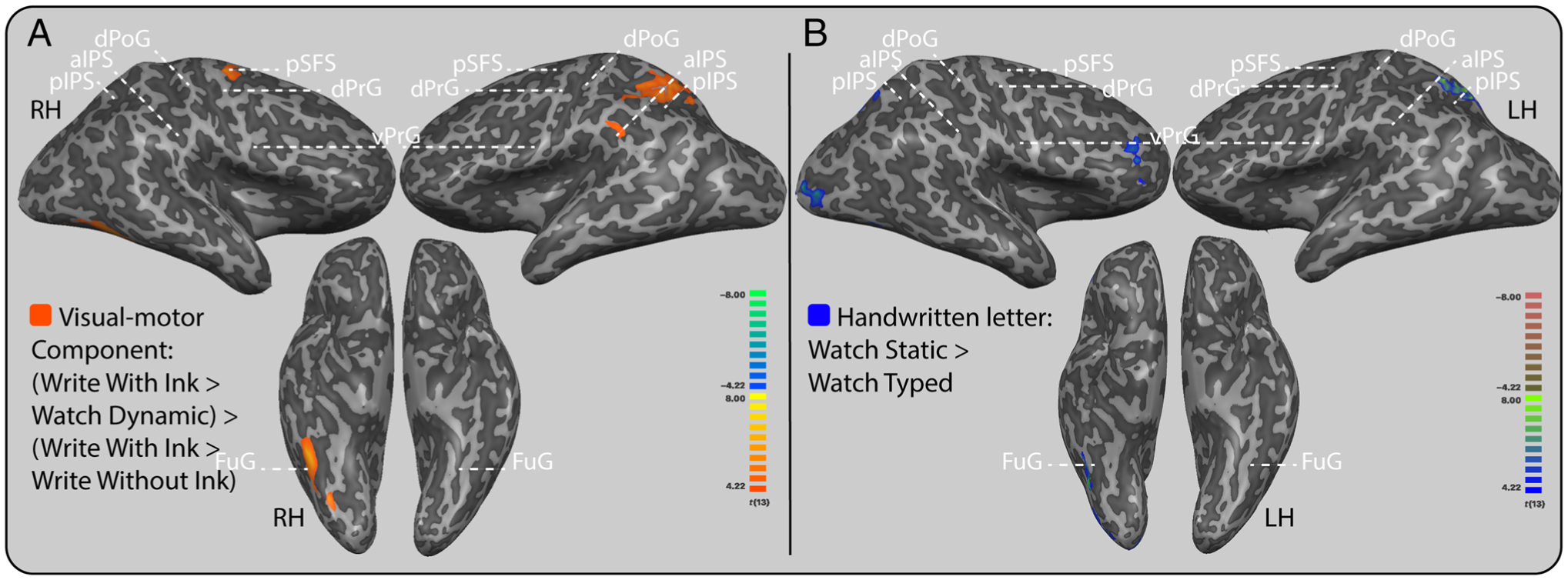
Conjunction of motor and visual components of letter production and handwritten form perception. A similar neural response occurred for areas involved in the visual-motor component of letter production and the perception of handwritten forms. (A) Overlap between motor and visual components of letter production. Areas in orange are areas that responded equally for the motor and visual components of letter production. The results of the conjunction analysis statistically confirm an overlap between motor and visual components in the bilateral posterior IPS and in the right inferior temporal gyrus. (B) Perception of handwritten forms. Areas that are in magenta were more active while looking at a static handwritten letter than while looking at a static typed letter; these include the left posterior IPS, right ventral-temporal cortex, and right posterior middle frontal gyrus. Group level results are displayed at a standard voxel-wise threshold of pvoxel < .001, and a cluster threshold of 40 contiguous 3-mm isometric voxels overlaid on an inflated anatomical image from a single participant. Anatomical label abbreviations: pSFS = posterior superior frontal sulcus; dPrG = dorsal precentral gyrus; vPrG = ventral precentral gyrus; dPoG = dorsal postcentral gyrus; aIPS = anterior IPS; pIPS = posterior IPS; FuG = fusiform gyrus; LH = left hemisphere; RH = right hemisphere.
Motor and Visual Processing in the Left Dorsal Premotor Cortex
We found no activation in the left middle frontal gyrus, commonly referred to as Exner’s area (Planton et al., 2013; Longcamp et al., 2003; Anderson et al., 1990; Exner, 1881), for any of our contrasts and only found a response in the left superior middle frontal sulci when we compared letter production with a fixation baseline. We were interested in further exploring this result because we would have expected to see a response in the left dorsal premotor cortex for the motor component of letter production. Recent work has suggested that the left dorsal premotor cortex may function as a motoric buffer that supports the serial, stroke-by-stroke creation of written forms from stored graphemic representations (Planton et al., 2017; Pattamadilok et al., 2016; Roux et al., 2009) while also showing sensitivity to the visual perception of the unfolding stroke orders (Nakamura et al., 2012). We thought it possible that the left dorsal premotor cortex might be involved in serial visual and motor processing, resulting in no significant differences between any of our conditions except between letter production and fixation.
To provide some evidence on the use of left dorsal premotor cortex for either serial motoric and/or visual processing, we conducted two additional contrasts. In the first contrast, we compared Write Without Ink with Watch Typed with the thought that writing without ink would correspond to serial motoric processing whereas watching a static typed letter would not engage serial processing but would control for “letters.” In the second contrast, we compared Watch Dynamic with Watch Typed. In this contrast, Watch Dynamic would engage serial visual processing, whereas Watch Typed would not. We did not use Watch Static as our control condition because there is evidence that perceiving static handwritten letters evokes similar neural mechanisms as producing letters by hand (Orliaguet et al., 1997; Babcock & Freyd, 1988; Freyd, 1987).
These contrasts revealed activity in the left posterior superior frontal sulcus in the left dorsal premotor cortex for both serial visual and serial motoric processing. We found responses in the bilateral posterior superior frontal sulci, bilateral posterior parietal cortices, and bilateral ventral temporal cortex for serial visual processing, that is, the comparison between Watch Dynamic and Watch Typed (Figure 5). We found responses in the left posterior superior frontal sulci, left dorsal precentral gyrus, left anterior IPS/SPL, bilateral occipitotemporal cortices, and bilateral cerebellum for serial motoric processing, that is, the comparison between Write Without Ink and Watch Typed (Figure 5).
Figure 5.
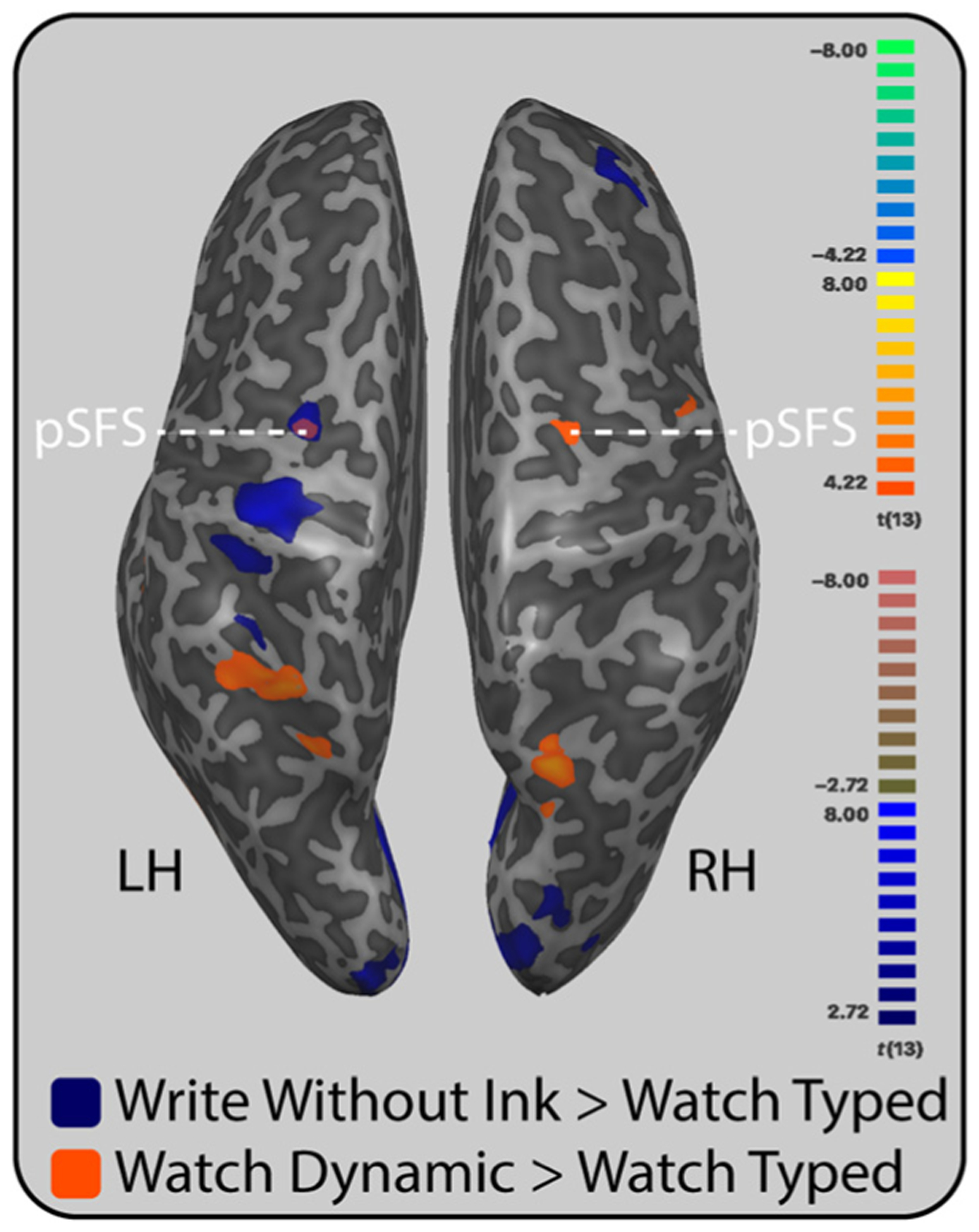
Motor and visual processing in the left dorsal premotor cortex. The left superior frontal sulcus responded for serial processing in both motor and visual domains. Blue indicates regions that were more active while participants wrote letters without ink when compared with a statically presented typed letter. Orange indicates regions that were more active while participants watched a handwritten letter dynamically unfold when compared with a statically presented typed letter. Anatomical label abbreviations: pSFS = posterior superior frontal sulcus; LH = left hemisphere; RH = right hemisphere.
Visual Percepts Produced by Letter Production
Unfolding
We contrasted activation levels during Watch Dynamic to Watch Static to determine areas that were related to viewing a dynamically unfolding percept. This contrast revealed no significant differences, lending support to the idea that the static handwritten percept evokes dynamic production information.
Handwritten Forms
Areas that had a larger response to one’s own handwritten letter than a static typed letter included bilateral posterior IPS, right occipital cortex, right posterior inferior temporal gyrus, ventral-temporal cortex, and right pFC (Figure 4B). No areas responded more for typed letter perception than for the perception of one’s own handwritten letter.
DISCUSSION
Understanding the function of a widespread neural system requires that we understand how its components contribute to its function. In this research, we determined that the letter production system, one aspect of handwriting, involved frontal motor regions, cerebellum, ventral temporal cortex, and parietal cortex, replicating previous work (e.g., Longcamp et al., 2014) and further determined the relative involvement of these regions in different behavioral components of letter production (e.g., motor, visual perceptual, visual-motor). Our results suggest that the widespread neural systems that support complex sensorimotor behaviors arise from the coordination of relatively distinct neural subsystems that can be related back to the sensorimotor components of the full complex behavior.
In what follows, we discuss these results in light of previous work and discuss the role of each neural component of the larger sensorimotor system.
Precentral Gyri
The precentral gyri were recruited bilaterally for the broad contrast of letter production compared with fixation. When we isolated the motor component of letter production by contrasting letter production and watching the dynamic visual unfolding of the letter, we found only the left precentral gyrus, not the right. This primary motor region was only active during actual letter production and did not respond to the visual information that resulted from letter production. Longcamp et al. (2014) found that the left dorsal precentral gyrus responded more during letter production. As Longcamp et al. (2014) provided real-time visual feedback of the letters as they were being produced, it was not clear whether the left dorsal precentral gyrus was related to the motor movements, the visual presentation of letters that occurred during production, or the use of vision to guide the motor movements. We extend this work by showing that the activation of the left precentral gyrus is specific to motor production. These results are in line with the results of James and Gauthier (2006) that demonstrated that the left precentral gyrus was more active while participants wrote a letter without visual feedback compared with when they imagined a letter. They are also in line with work indicating that the left precentral gyrus is involved with the execution of movements, in general (for reviews, see Meier et al., 2008; Chouinard & Paus, 2006; Graziano, 2006; Schieber, 2001).
Superior and Middle Frontal Gyri
The only comparison that revealed a response in any dorsal premotor region was the comparison between letter production and fixation. Neither the motor component contrast nor the visual component contrast revealed activation in the dorsal premotor cortex. We interpret these findings to indicate that the dorsal premotor response in the posterior superior frontal sulcus was both motor and visual in nature. We suggest that the motor processes that might occur in this region are mediated by visual feedback during letter production, perhaps through parietal connections.
We interpret the response in the left superior frontal sulcus during letter production to be analogous to the graphemic-motor frontal area (Planton et al., 2017; Pattamadilok et al., 2016; Roux et al., 2009). As such, we would expect this response to be related to serial motoric processing, holding a perceptual representation in a buffer for the purpose of manually producing it stroke by stroke, as it occurs during letter production (Planton et al., 2017; Pattamadilok et al., 2016; Roux et al., 2009). We found some support for this idea. We found that the left superior frontal sulcus was more active when participants wrote letters without ink (serial motoric processing) than when they watched a statically presented typed letter. We also found, however, that the left superior frontal sulcus was more active when participants watched a letter dynamically unfolding (serial visual processing) than while watching a statically presented typed letter. These results suggest that the left posterior superior frontal sulcus is related to serial motoric processing but remains sensitive to serial visual processing. This suggestion is consistent with the absence of activity in this region for all of our planned contrasts besides the comparison between letter production and fixation and fits nicely with prior work demonstrating the involvement of the dorsal premotor cortex for serial motoric (Planton et al., 2017; Pattamadilok et al., 2016; Roux et al., 2009) and visual (Nakamura et al., 2012) processing during letter production.
Although research on letter production typically focuses on motor sequencing of hand movements, eye movement sequencing must also occur. The sensitivity to serial visual processing that we found in the left dorsal premotor cortex may be related to the visual sampling of a handwritten letter for the purposes of updating motor movement parameters whether or not an overt motor movement is produced and can, conversely, be used for the motor sampling of a handwritten form for the purposes of updating eye gaze parameters. This interpretation of the left dorsal premotor cortex’s role in letter production is in line with work suggesting that dorsal premotor responses that have typically been attributed to Exner area functions may be strongly related to eye movements that occur during letter production. Yuan and Brown (2015) performed a meta-analysis of studies of handwriting and drawing and found that peak activations in coordinates found for handwriting and drawing were similar to those found in a meta-analysis of saccadic eye movements (Grosbras, Laird, & Paus, 2005). They therefore suggested that premotor responses during handwriting might be tightly linked, and perhaps synonymous, with the FEFs (Yuan & Brown, 2015; but see Matsuo et al., 2003).
Eye movement sequencing naturally co-occurs with hand and finger movement sequencing during letter production, and indeed, both are serial processes that likely support each other. Prior work has shown that activation in the left posterior superior frontal sulcus is associated with the serial conversion of a grapheme, a stored perceptual representation of a letterform, into the step-by-step motor commands necessary to recreate the letter using pen and paper (Planton et al., 2017; Pattamadilok et al., 2016; Roux et al., 2009). We extend this work by demonstrating that this serial processing may have some relationship to the serial visual sampling that naturally co-occurs with hand movements during letter production. Further work is needed to disentangle the coordination of eye and finger movement sequencing.
Bilateral Cerebellum
Cerebellar involvement, particularly the cerebellar lobe ipsilateral to the hand used to write, is very consistently reported in studies of handwriting as being related to the motor component of letter production (for a meta-analysis, see Planton et al., 2013), although the cerebellum has also been attributed cognitive and linguistic functions (Stoodley & Schmahmann, 2009). We found a bilateral cerebellar response that was weighted more heavily to the right hemisphere for only the motor contrast. We interpret this cerebellar response to be related to hand movements performed during the Write With Ink task that were not necessary during the Watch Dynamic task. This interpretation is consistent with a vast literature relating ipsilateral cerebellar involvement (relative to the effector) to sensorimotor execution processes (Manni & Petrosini, 2004), even for the fine motor movements required for written form production (Planton et al., 2013; Baillieux et al., 2010; Mariën et al., 2009; Haggard, Jenner, & Wing, 1994). More work is necessary to determine the exact role of the cerebellum in this sensorimotor execution process and the possibility that the cerebellum may also be performing additional cognitive and linguistic processes (Manto et al., 2012; Stoodley & Schmahmann, 2009).
Parietal Cortex (IPS)
The IPS has long been associated with visually guided action (e.g., Milner & Goodale, 2006; Goodale & Milner, 2005). As such, it is thought to be a region that combines and integrates visual information in real time for the purpose of supporting motor actions. Prior work suggests that the involvement of parietal cortex in the visual and motor components of writing and drawing is graded, such that more anterior portions of the IPS are related to the motor component and more posterior portions are related to the visual component (Haar et al., 2015; Kadmon Harpaz et al., 2014; Yuan & Brown, 2014; Thaler & Goodale, 2011). We found a similar pattern with the visual and motor components of letter production in the current study and found, further, an area of overlap between motor and visual components in the left posterior IPS. The left anterior IPS was only associated with the motor component of letter production, whereas the conjunction analysis revealed that the left posterior IPS was associated with both the visual and motor components of letter production. Indeed, the left SPL and left anterior IPS did not demonstrate more activation for any of our other contrasts that were designed to look at responses associated with the different visual percepts produced as a result of letter production.
The role of the left posterior IPS in processing the visual percepts that result from—or that guide—the motor production of forms is further demonstrated by its response during purely visual tasks that involve motor-ically produced percepts. That is, when we compared the perception of handwritten forms with typed forms, we found recruitment of the left posterior IPS. This same contrast in previous work only showed recruitment of the frontal cortex (which we do not replicate; Longcamp et al., 2011). We believe that these two seemingly contradictory results provide an interesting insight concerning the nature of perceiving letters and the motor responses that this perception invokes. For instance, Longcamp et al. (2011) used fMRI to compare the perception of lowercase cursive letters that were not written by the participants themselves with typed letters and found greater frontal motor cortex activation to the cursive letters than the typed letters. This finding was interpreted as demonstrating that cursive letters invoke a generalized motor plan during visual perception. Behavioral work supports this interpretation. Individuals can infer motor production steps from simply viewing a letter or word produced in a cursive font (Orliaguet et al., 1997; Babcock & Freyd, 1988; Freyd, 1987). However, when we compared one’s own handwritten (printed, not cursive) letters with typed letters, we did not find motor cortex activation but rather recruitment of the left IPS and right ventral temporal cortex. We hypothesize that this seem-ingly discrepant result actually fits well with previous interpretations in that perception of one’s own produced form may not activate generalized motor plans but rather may reactivate individual visually guided action events based on exemplar perception.
Support for this interpretation comes from a long history of research on the differences among frontal motor systems and parietal motor systems (for a review, see Rizzolatti et al., 1998). The motor plans in the frontal cortex are created from the experiences of acting, and perceiving actions, and are thought to be generalized from numerous instances throughout a lifetime. As such, they are flexible and can be used in many different situations to facilitate motor interaction. In contrast, the motor information in the parietal cortex, and more specifically in the IPS, is more specific to individual visually guided action events and pairs the motor information with a specific visual percept and, perhaps, prior kinesthetic percepts. This perceptual-motor information can be reactivated through associative mechanisms (as in viewing one’s own handwritten letter) but may not be stored for use in subsequent generalized behaviors because the association is too specific to a particular visually guided event (Milner & Goodale, 2006; Goodale & Milner, 2005). Support for this suggestion comes from prior work that suggested a frontoparietal system associated with the motor production of written forms (for a review, see Nakamura & Kouider, 2003) as well as other contrasts in this study.
Furthermore, we found that there was no difference between the condition where participants perceived their own handwritten letter unfold over time compared with perceiving a static, handwritten version of that same letter. We infer from this that both types of percepts are associated with the prior visually guided production of the letter and therefore will not show differential activation in the IPS. Indeed, when we compared the perception of their own handwritten letter unfolding over time with a static, typed version of the same letter, we saw bilateral frontal (superior frontal sulci) and parietal (IPS) responses. More direct comparisons among self-produced handwriting and various other versions produced by oneself and others would be necessary to make strong conclusions based on these results.
Finding parietal activation for visual perception of letters is, nonetheless, a novel finding that suggests that the parietal cortex might have some “memory” for objects—at least objects with which we have a visual-motor history. Traditional accounts of the dorsal visual stream suggest that the visually guided actions mediated by the parietal cortex are completely online and do not use stored information (Milner & Goodale, 2006), although more recent accounts suggest that the dorsal stream can be further divided into a dorsal-dorsal stream and a ventral-dorsal stream (Rizzolatti & Matelli, 2003). Of these two, the ventral-dorsal stream has potential for “memory” as it shares many characteristics with the ventral visual stream that is thought to have long-term storage capabilities for such things as object identity (Milner & Goodale, 2006). Our study differed from prior work in that participants’ handwritten letters were presented back to them within the same experimental run that they had written them. It may be that the “memories” in the parietal cortex for these specific visual cues are relatively short in comparison with, for example, the memory for object identities in the ventral-temporal cortex (Rothlein & Rapp, 2014; Dufor & Rapp, 2013) and may, in having any memory at all, rely upon ventral-dorsal stream mechanisms.
Ventral Temporal Cortex
The visual component of letter production (Write With Ink > Write Without Ink) recruited the ventral temporal cortices bilaterally. This is not surprising given the substantial amount of research on the involvement of ventral temporal cortex in letter perception (Kersey & James, 2013; James & Engelhardt, 2012; James, 2010; James & Atwood, 2009; James & Gauthier, 2006; James et al., 2005; Longcamp et al., 2003) and letter production (Planton et al., 2017; Longcamp et al., 2014; Dufor & Rapp, 2013; James & Gauthier, 2006). The present finding specifies the role of the left ventral temporal cortex during letter production by demonstrating that it is only recruited during the visual components of letter production. Moreover, we found a greater involvement of the right ventral temporal cortex not only in the visual component of letter production but also in several of our other contrasts.
Prior work has not typically reported right ventral temporal involvement during letter production. We hypothesize that this may be due to a difference in methodologies between prior work and this study. In this study, participants were able to see what they were writing as they were writing it, whereas prior work has not typically provided visual feedback during letter production. Only one other study, Longcamp et al. (2014), has studied letter production with visual feedback of the form being created. They found, as did we, a response in the right ventral temporal cortex for letter production. They also found this response for digit production, however, and on the basis of additional analyses, concluded that this response was likely involved in lower level effects that would vary between writing instances. One such variation could be the use of visual cues to guide motor movements.
We suggest that the right ventral-temporal response for the motor component may be related to the use of subtle variations in the letterform being produced that may be used during letter production. It is the nature of the fine motor system that each production of a given letter will be slightly different, even in proficient writers, so that each time a letter is written, it is essentially in a new “font.” The right ventral-temporal response may, accordingly, reflect the detection of instance variability and is in line with findings that link the right fusiform gyrus to the perception of font variations (Rothlein & Rapp, 2014; Barton, Fox, et al., 2010; Barton, Sekunova, et al., 2010; Qiao et al., 2010; Gauthier et al., 2000). This interpretation is supported by our other findings: that the right fusiform gyrus responded more during the perception of one’s own handwritten letters than for typed letters, that it did not respond stronger for dynamically unfolding handwritten letters compared with static handwritten letters, and that it responded similarly for both the visual and motor components of letter production. The role of the right fusiform gyrus in letter production may therefore be related to the detection and/or use of subtle visual cues that are suggestive of motor movements, whether this is occurring during the production or not.
Conclusion
Producing symbols by hand is a complex task, requiring the coordination of several neural systems. Here, we have decomposed letter production into several behavioral components and related these behavioral components to relatively distinct neural subsystems within the widespread neural system that supports letter production (Longcamp et al., 2014; James & Gauthier, 2006). By using a novel design during the measurement of BOLD activation, we have documented the neural subsystems involved in a foundational aspect of handwriting and the relationship of these neural subsystems to the various behavioral components of letter production. An interesting line of future work would be to investigate how similarly each letter is processed and/or represented across tasks using multivariate techniques. An analysis such as this would shed light on the differences and similarities in the representations of letters across tasks and may provide further insight into the differences and similarities in letter representations across modalities.
Acknowledgments
We thank members of the Imaging Research Facility at Indiana University for conversations and suggestions concerning study procedures. S. V. B. was supported by the National Institute of Health 2T32 grant #HD007475-21. The research outlined here was partially supported by the Indiana University Office of the Vice President for Research Emerging Area of Research Initiative, Learning: Brains, Machines, and Children. The IUB Imaging Research Facility Brain Scan Credit Program provided additional imaging funds.
REFERENCES
- Anderson SW, Damasio AR, & Damasio H (1990). Troubled letters but not numbers: Domain specific cognitive impairments following focal damage in frontal cortex. Brain, 113, 749–766. [DOI] [PubMed] [Google Scholar]
- Babcock MK, & Freyd JJ (1988). Perception of dynamic information in static handwritten forms. American Journal of Psychology, 101, 111–130. [PubMed] [Google Scholar]
- Baillieux H, De Smet HJ, Dobbeleir A, Paquier PF, De Deyn PP, & Mariën P (2010). Cognitive and affective disturbances following focal cerebellar damage in adults: A neuropsychological and SPECT study. Cortex, 46, 869–879. [DOI] [PubMed] [Google Scholar]
- Barry RL, Williams JM, Klassen LM, Gallivan JP, Culham JC, & Menon RS (2010). Evaluation of preprocessing steps to compensate for magnetic field distortions due to body movements in BOLD fMRI. Magnetic Resonance Imaging, 28, 235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton JJ, Fox CJ, Sekunova A, & Iaria G (2010). Encoding in the visual word form area: An fMRI adaptation study of words versus handwriting. Journal of Cognitive Neuroscience, 22, 1649–1661. [DOI] [PubMed] [Google Scholar]
- Barton JJ, Sekunova A, Sheldon C, Johnston S, Iaria G, & Scheel M (2010). Reading words, seeing style: The neuropsychology of word, font and handwriting perception. Neuropsychologia, 48, 3868–3877. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, & Heeger DJ (1996). Linear systems analysis of functional magnetic resonance imaging in human V1. Journal of Neuroscience, 16, 4207–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH (1997). The psychophysics toolbox. Spatial Vision, 10, 433–436. [PubMed] [Google Scholar]
- Brownsett SLE, & Wise RJS (2010). The contribution of the parietal lobes to speaking and writing. Cerebral Cortex, 20, 517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budisavljevic S, Dell’Acqua F, Djordjilovic V, Miotto D, Motta R, & Castiello U (2017). The role of the frontal aslant tract and premotor connections in visually guided hand movements. Neuroimage, 146, 419–428. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Brammer MJ, Rabe-Hesketh S, Curtis VA, Morris RG, Williams SCR, et al. (1999). Methods for diagnosis and treatment of stimulus-correlated motion in generic brain activation studies using fMRI. Human Brain Mapping, 7, 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buneo CA, & Andersen RA (2006). The posterior parietal cortex: Sensorimotor interface for the planning and online control of visually guided movements. Neuropsychologia, 44, 2594–2606. [DOI] [PubMed] [Google Scholar]
- Cheng H, & Li Y (2010). Respiratory noise correction using phase information. Magnetic Resonance Imaging, 28, 574–582. [DOI] [PubMed] [Google Scholar]
- Cheng H, & Puce A (2014). Reducing respiratory effect in motion correction for EPI images with sequential slice acquisition order. Journal of Neuroscience Methods, 227, 83–89. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, & Paus T (2006). The primary motor and premotor areas of the human cerebral cortex. Neuroscientist, 12, 143–152. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehéricy S, Dehaene-Lambertz G, Henaff MA, et al. (2000). The visual word form area: Spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain, 123, 291–307. [DOI] [PubMed] [Google Scholar]
- Conrad R (1964). Acoustic confusions in immediate memory. British Journal of Psychology, 55, 75–84. [Google Scholar]
- Cornhill H, & Case-Smith J (1996). Factors that relate to good and poor handwriting. American Journal of Occupational Therapy, 50, 732–739. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Jobert A, Naccache L, Ciuciu P, Poline JB, Le Bihan D, et al. (2004). Letter binding and invariant recognition of masked words: Behavioral and neuroimaging evidence. Psychological Science, 15, 307–313. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Le Clec’H G, Poline JB, Le Bihan D, & Cohen L (2002). The visual word form area: A prelexical representation of visual words in the fusiform gyrus. NeuroReport, 13, 321–325. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Naccache L, Cohen L, Bihan DL, Mangin JF, Poline JB, et al. (2001). Cerebral mechanisms of word masking and unconscious repetition priming. Nature Neuroscience, 4, 752–758. [DOI] [PubMed] [Google Scholar]
- Dufor O, & Rapp B (2013). Letter representations in writing: An fMRI adaptation approach. Frontiers in Psychology, 4, 781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM (1999). Human brain atlas (2nd ed.). New York: Springer. [Google Scholar]
- Exner S (1881). Untersuchungen über die localisation der functionen in der grosshirnrinde des menschen. Wien, Austria: Wilhelm Braumüller. [Google Scholar]
- Feder KP, & Majnemer A (2007). Handwriting development, competency, and intervention. Developmental Medicine & Child Neurology, 49, 312–317. [DOI] [PubMed] [Google Scholar]
- Freyd JJ (1987). Dynamic mental representations. Psychological Review, 94, 427–438. [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Moylan J, Skudlarski P, Gore JC, & Anderson AW (2000). The fusiform “face area” is part of a network that processes faces at the individual level. Journal of Cognitive Neuroscience, 12, 495–504. [DOI] [PubMed] [Google Scholar]
- Goodale MA, & Milner AD (2005). Sight unseen. Oxford: Oxford University Press. [Google Scholar]
- Graziano M (2006). The organization of behavioral repertoire in motor cortex. Annual Review of Neuroscience, 29, 105–134. [DOI] [PubMed] [Google Scholar]
- Grosbras MH, Laird AR, & Paus T (2005). Cortical regions involved in eye movements, shifts of attention, and gaze perception. Human Brain Mapping, 25, 140–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haar S, Donchin O, & Dinstein I (2015). Dissociating visual and motor directional selectivity using visuomotor adaptation. Journal of Neuroscience, 35, 6813–6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggard P, Jenner J, & Wing A (1994). Coordination of aimed movements in a case of unilateral cerebellar damage. Neuropsychologia, 32, 827–846. [DOI] [PubMed] [Google Scholar]
- Hull AJ (1973). A letter-digit matrix of auditory confusions. British Journal of Psychology, 64, 579–585. [DOI] [PubMed] [Google Scholar]
- Jackson SR, & Husain M (2006). Visuomotor functions of the posterior parietal cortex. Neuropsychologia, 44, 2589–2593. [DOI] [PubMed] [Google Scholar]
- James KH (2010). Sensorimotor experience leads to changes in visual processing in the developing brain. Developmental Science, 13, 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James KH, & Atwood TP (2009). The role of sensorimotor learning in the perception of letter-like forms: Tracking the causes of neural specialization for letters. Cognitive Neuropsychology, 26, 91–110. [DOI] [PubMed] [Google Scholar]
- James KH, & Engelhardt L (2012). The effects of handwriting experience on functional brain development in pre-literate children. Trends in Neuroscience and Education, 1, 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James KH, & Gauthier I (2006). Letter processing automatically recruits a sensory-motor brain network. Neuropsychologia, 44, 2937–2949. [DOI] [PubMed] [Google Scholar]
- James KH, James TW, Jobard G, Wong ACN, & Gauthier I (2005). Letter processing in the visual system: Different activation patterns for single letters and strings. Cognitive, Affective & Behavioral Neuroscience, 5, 452–466. [DOI] [PubMed] [Google Scholar]
- Kadmon Harpaz N, Flash T, & Dinstein I (2014). Scale-invariant movement encoding in the human motor system. Neuron, 81, 452–462. [DOI] [PubMed] [Google Scholar]
- Kandel S, Orliaguet JP, & Viviani P (2000). Perceptual anticipation in handwriting: The role of implicit motor competence. Attention, Perception, & Psychophysics, 62, 706–716. [DOI] [PubMed] [Google Scholar]
- Katanoda K, Yoshikawa K, & Sugishita M (2001). A functional MRI study on the neural substrates for writing. Human Brain Mapping, 13, 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersey AJ, & James KH (2013). Brain activation patterns resulting from learning letter forms through active self-production and passive observation in young children. Frontiers in Psychology, 4, 567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S, Guiltner V, Sollereder P, & Cui Y (2011). Relationships between fine-motor, visual-motor, and visual perception scores and handwriting legibility and speed. Physical & Occupational Therapy in Pediatrics, 31, 103–114. [DOI] [PubMed] [Google Scholar]
- Koutstaal W, Wagner AD, Rotte M, Maril A, Buckner RL, & Schacter DL (2001). Perceptual specificity in visual object priming: Functional magnetic resonance imaging evidence for a laterality difference in fusiform cortex. Neuropsychologia, 39, 184–199. [DOI] [PubMed] [Google Scholar]
- Longcamp M, Anton J-L, Roth M, & Velay J-L (2003). Visual presentation of single letters activates a premotor area involved in writing. Neuroimage, 19, 1492–1500. [DOI] [PubMed] [Google Scholar]
- Longcamp M, Boucard C, Gilhodes J-C, Anton J-L, Roth M, Nazarian B, et al. (2008). Learning through hand- or typewriting influences visual recognition of new graphic shapes: Behavioral and functional imaging evidence. Journal of Cognitive Neuroscience, 20, 802–815. [DOI] [PubMed] [Google Scholar]
- Longcamp M, Hlushchuk Y, & Hari R (2011). What differs in visual recognition of handwritten vs. printed letters? An fMRI study. Human Brain Mapping, 32, 1250–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longcamp M, Lagarrigue A, Nazarian B, Roth M, Anton J-L, Alario F-X, et al. (2014). Functional specificity in the motor system: Evidence from coupled fMRI and kinematic recordings during letter and digit writing. Human Brain Mapping, 35, 6077–6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longstaff MG, & Heath RA (1997). Space-time invariance in adult handwriting. Acta Psychologica, 97, 201–214. [Google Scholar]
- Maeland AF (1992). Handwriting and perceptual-motor skills in clumsy, dysgraphic, and “normal” children. Perceptual and Motor Skills, 75(3 Suppl), 1207–1217. [DOI] [PubMed] [Google Scholar]
- Manni E, & Petrosini L (2004). A century of cerebellar somatotopy: A debated representation. Nature Reviews Neuroscience, 5, 241–249. [DOI] [PubMed] [Google Scholar]
- Manto M, Bower JM, Conforto AB, Delgado-Garcia JM, da Guarda SNF, Gerwig M, et al. (2012). Consensus paper: Roles of the cerebellum in motor control—The diversity of ideas on cerebellar involvement in movement. Cerebellum, 11, 457–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariën P, Baillieux H, De Smet HJ, Engelborghs S, Wilssens I, Paquier P, et al. (2009). Cognitive, linguistic and affective disturbances following a right superior cerebellar artery infarction: A case study. Cortex, 45, 527–536. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Kato C, Sumiyoshi C, Toma K, Duy Thuy DH, Moriya T, et al. (2003). Discrimination of Exner’s area and the frontal eye field in humans—Functional magnetic resonance imaging during language and saccade tasks. Neuroscience Letters, 340, 13–16. [DOI] [PubMed] [Google Scholar]
- Meier JD, Aflalo TN, Kastner S, & Graziano MS (2008). Complex organization of human primary motor cortex: A high-resolution fMRI study. Journal of Neurophysiology, 100, 1800–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner AD, & Goodale MA (2006). The visual brain in action (2nd ed.). Oxford: Oxford University Press. [Google Scholar]
- Nakamura K, & Kouider S (2003). Functional neuroanatomy of Japanese writing systems. Aphasiology, 17, 667–683. [Google Scholar]
- Nakamura K, Kuo W-J, Pegado F, Cohen L, Tzeng OJL, & Dehaene S (2012). Universal brain systems for recognizing word shapes and handwriting gestures during reading. Proceedings of the National Academy of Sciences, U.S.A, 109, 20762–20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K, & Inui T (2009). The role of the posterior parietal cortex in drawing by copying. Neuropsychologia, 47, 1013–1022. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Orliaguet JP, Kandel S, & Boë LJ (1997). Visual perception of motor anticipation in cursive handwriting: Influence of spatial and movement information on the prediction of forthcoming letters. Perception, 26, 905–912. [DOI] [PubMed] [Google Scholar]
- Pattamadilok C, Ponz A, Planton S, & Bonnard M (2016). Contribution of writing to reading: Dissociation between cognitive and motor process in the left dorsal premotor cortex. Human Brain Mapping, 37, 1531–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG (1997). The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision, 10, 437–442. [PubMed] [Google Scholar]
- Planton S, Jucla M, Roux F-E, & Démonet J-F (2013). The “handwriting brain”: A meta-analysis of neuroimaging studies of motor versus orthographic processes. Cortex, 49, 2772–2787. [DOI] [PubMed] [Google Scholar]
- Planton S, Longcamp M, Péran P, Démonet J-F, &Jucla M (2017). How specialized are writing-specific brain regions? An fMRI study of writing, drawing and oral spelling. Cortex, 88, 66–80. [DOI] [PubMed] [Google Scholar]
- Polk TA, & Farah MJ (2002). Functional MRI evidence for an abstract, not perceptual, word form area. Journal of Experimental Psychology: General, 131, 65. [DOI] [PubMed] [Google Scholar]
- Potgieser ARE, & de Jong BM (2016). Visuomotor dissociation in cerebral scaling of size. PloS One, 11, e0151484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell JJ, Turkeltaub PE, Eden GF, & Rapp B (2011). Examining the central and peripheral processes of written word production through meta-analysis. Frontiers in Psychology, 2, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao E, Vinckier F, Szwed M, Naccache L, Valabregue R, Dehaene S, et al. (2010). Unconsciously deciphering handwriting: Subliminal invariance for handwritten words in the visual word form area. Neuroimage, 49, 1786–1799. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G, & Matelli M (1998). The organization of the cortical motor system: New concepts. Clinical Neurophysiology, 106, 283–296. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, & Matelli M (2003). Two different streams form the dorsal visual system: Anatomy and functions. Experimental Brain Research, 153, 146–157. [DOI] [PubMed] [Google Scholar]
- Rothlein D, & Rapp B (2014). The similarity structure of distributed neural responses reveals the multiple representations of letters. Neuroimage, 89, 331–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux F-E, Dufor O, Giussani C, Wamain Y, Draper L, Longcamp M, et al. (2009). The graphemic/motor frontal area Exner’s area revisited. Annals of Neurology, 66, 537–545. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, et al. (2013). An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage, 64, 240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber MH (2001). Constraints on somatotopic organization in the primary motor cortex. Journal of Neurophysiology, 86, 2125–2143. [DOI] [PubMed] [Google Scholar]
- Seitz RJ, Canavan AGM, Yágüez L, Herzog H, Tellmann L, Knorr U, et al. (1997). Representations of graphomotor trajectories in the human parietal cortex: Evidence for controlled processing and automatic performance. European Journal of Neuroscience, 9, 378–389. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Limmer C, Peinemann A, Bartenstein P, Drzezga A, & Conrad B (2001). Brain correlates of fast and slow handwriting in humans: A PET-performance correlation analysis. European Journal of Neuroscience, 14, 726–736. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, & Schmahmann JD (2009). Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. Neuroimage, 44, 489–501. [DOI] [PubMed] [Google Scholar]
- Talairach J, & Tournoux P (1988). Co-planar stereotaxic atlas of the human brain. 3-Dimensional proportional system: An approach to cerebral imaging. New York: Thieme. [Google Scholar]
- Tam F, Churchill NW, Strother SC, & Graham SJ (2011). A new tablet for writing and drawing during functional MRI. Human Brain Mapping, 32, 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler L, & Goodale MA (2011). Neural substrates of visual spatial coding and visual feedback control for hand movements in allocentric and target-directed tasks. Frontiers in Human Neuroscience, 5, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieman R, & Kessler B (2014). How children learn to write words. New York: Oxford University Press. [Google Scholar]
- Tseng MH, & Murray EA (1994). Differences in perceptual-motor measures in children with good and poor handwriting. The Occupational Therapy Journal of Research, 14, 19–36. [Google Scholar]
- Weil MJ, & Amundson SJC (1994). Relationship between visuomotor and handwriting skills of children in kindergarten. American Journal of Occupational Therapy, 48, 982–988. [DOI] [PubMed] [Google Scholar]
- Wing AM, & Nimmo-Smith I (1987). The variability of cursive handwriting measure defined along a continuum: Letter specificity. Journal of the Forensic Science Society, 27, 297–306. [Google Scholar]
- Yuan Y, & Brown S (2014). The neural basis of mark making: A functional MRI study of drawing. PLoS One, 9, e108628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, & Brown S (2015). Drawing and writing: An ALE meta-analysis of sensorimotor activations. Brain and Cognition, 98, 15–26. [DOI] [PubMed] [Google Scholar]


