Abstract
BACKGROUND:
Endocrine disrupting chemicals represent a broad class of compounds, are widespread in the environment and can pose severe health effects.
OBJECTIVES:
The objective of this study was to investigate and compare the overall estrogen and androgen activating potential of PM10 air samples at an urban, rural and industrial location in Flanders, using a human in vitro cell bioassay.
METHODS:
PM10 samples were collected on glass fiber filters every six days between April 2013 and January 2014 using a high-volume sampler. Extraction was executed with a hexane/acetone mixture before analysis using a recombinant estrogen- or androgen responsive human carcinoma cell line. Results were expressed as bioanalytical equivalents (BEQs) per cubic meter of air.
RESULTS:
High fluctuations in estrogenic activity were observed during the entire sampling period, with median BEQs of 32.1, 35.9 and 31.1 fg E2-Eq m−3 in the industrial, urban and rural background area, respectively. Estrogenic activity was measured in 70% of the samples, while no androgenic activity was observed in any of the samples. The estrogenic activity in the industrial area was positively correlated with the airborne concentration of the sum of the non-carcinogenic PAHs pyrene and fluoranthene (rho = 0.48; p < 0.01) and the sum of the carcinogenic PAHs (rho = 0.36 p = 0.05).
CONCLUSIONS:
This study showed that no androgenic activity was present in PM10 and that although the median estrogenic activity was rather low and comparable in the three locations, high fluctuations in estrogenic response exist over time. While atmospheric PAHs contributed to the observed estrogenic response, especially in the industrial area, the chemicals responsible for the majority of estrogenic activity remain to be identified.
Keywords: Estrogen, androgen, PM10, CALUX, EDC, Belgium
1. Introduction
Exogenous substances that act like hormones and disrupt the physiologic function of endogenous hormones in the endocrine system are called endocrine disruptors (EDs) or endocrine disrupting compounds (EDCs). They exert their effects by binding to hormone receptors, disturbing cell-signaling pathways, directly affecting the central neuroendocrine system, inhibiting hormone synthesis and/or by their toxic effects on endocrine responsive organs. Continuous exposure to estrogens can pose severe health risks such as increased risk for breast cancer in adulthood, male and female infertility and precocious puberty (Diamanti-Kandarakis et al., 2009; Krstevska-Konstantinova et al., 2001; Ozen et al., 2012). Due to these health effects, there is growing attention on the presence of EDCs in our environment and our exposure to such chemicals. Unfortunately, since EDCs represent a broad class of molecules such as organochlorine pesticides, industrial chemicals, fuels, plastics and plasticizers and numerous other chemicals, they are essentially ubiquitous. EDCs have been found globally in waste and surface water (Belhaj et al., 2015; Van der Linden et al., 2008), soil and sediment (Houtman et al., 2006), indoor dust and air samples (Kennedy et al., 2010; Vandermarken et al., 2015) and consumer products (Mertl et al., 2014). In the recent years, most studies on EDC pollution have focused on the aquatic environment as part of the Water Framework Directive (WFD 2000/60/EC), which aims at establishing environmental quality standards (EQS) to limit the concentrations of certain chemical substances that pose a significant risk to the environment or to human health, in surface and ground waters. Research on indoor or outdoor air pollution is still more limited and in most cases only a few, well known endocrine disrupting pollutants (like e.g. polycyclic aromatic hydrocarbons or bisphenol A) are quantified instead of a global endocrine activity (Krugly et al., 2014; Rudel et al., 2003; Teil et al., 2016). Few international studies using in vitro bioassays to assess estrogenic or androgenic activity of particulate matter were found. To our knowledge, only one study on androgenic activity in PM is available and while the authors found no androgenic activity, significant anti-androgenic activity was detected (Novák et al., 2013; Novák et al., 2009).
In a Swiss study, estrogenic activity with BEQs between 0.08 and 1.25 pg E2-Eq m−3 were found in PM1 samples, collected in winter during a smog episode (Wenger et al., 2009). In contrast, no estrogenic activity was detected in coarse particulate matter (< 50 μm) and gas phase samples from the Czech Republic (Novák et al., 2009) or in PM10 and gas phase samples from Bosnia and Herzegovina (Novák et al., 2013). However, in both studies, significant anti-estrogenic activity was observed in the PM fractions. A Canadian study from 2001–2002 found low estrogenic activity in PM extracts (Klein et al., 2006), while an Australian study failed to observe any significant estrogenic activity in outdoor air, but high estrogenic activity (in the range of several pg E2-Eq m−3) was measured in indoor office air and suburban homes (Kennedy et al., 2009).
Within this study, which was part of the Joaquin (Joint Air Quality Initiative) project (INTERREG IVB Northwest-Europe program), not only endocrine activity, but also the oxidative potential of PM10, the PM-mediated cellular responses (cytotoxicity, pro-inflammatory changes, DNA damage) of airway epithelial cells and mutagenic potency were assessed. The aims of the study were (1) to verify whether the air quality based on the cellular and oxidative responses to PM10 in the urban and industrial zones is different compared to a rural background site in Flanders, (2) the identification of health-relevant pollutants through analysis of the relationship between the in vitro measurements and PM10 composition.
In this paper, the endocrine activity of PM10 air samples and their relationship with the physicochemical composition and meteorological conditions was investigated, while results of the other in vitro tests were described elsewhere (Van Den Heuvel et al., 2016).
2. Materials and methods
2.1. Chemicals and standards
Dimethylsulfoxide (DMSO) (minimum 99.7%), hexane (minimum 96%, suitable for CALUX), acetonitrile (minimum 99.99%) and acetone (minimum 99.95%) were purchased from Biosolve (The Netherlands). The estrogen standard 17β-estradiol (minimum 98%), ultra-pure ethanol, Dulbecco’s Modification of Eagle’s Medium high glucose (DMEM without phenol red) and sodium pyruvate (100 mM, sterile-filtered) were obtained from Sigma-Aldrich (Belgium). Minimum Essential Medium Alpha (α-MEM), penicillin-streptomycin, Fetal Bovine Serum (FBS), FBS charcoal-stripped, L-glutamine (200 mM), trypsine, trypsine without phenol red and Phosphate-Buffered Saline (PBS) were obtained from Life Science Technology (United Kingdom). Glass fiber filters MG 227/1/60 with a diameter of 150 mm were purchased from Sartorius (Belgium). Luciferine reagent and lysis reagent were obtained from Promega (The Netherlands). The recombinant, estrogen-responsive BG1Luc4E2 ovarian carcinoma and the androgen-responsive T47D human breast carcinoma cell lines were previously described (Rogers and Denison, 2000; Rogers and Denison, 2002).
2.2. Sample collection
Between April 2013 and January 2014, the Flanders Environment Agency (VMM) collected particulate matter samples (PM10) at three locations in Flanders (Belgium).
The location Zelzate (51°11’46” N, 3°49’23” E) is representative for an industrial area due to its location near the industrial Canal Zone Ghent-Terneuzen. The urban site Borgerhout (51°12’35” N, 4°25’55” E) is located 700 m from the ring road of the city of Antwerp and is representative for an urban area site with meaningful traffic influence. The site is used as an urban zone for routine PM10 monitoring in the framework of the air quality directive (2008/50/EC), but is located near (30 m) a busy 2×2-lane road (33,500 vehicles day−1 in Feb 2010) (Mishra et al., 2012). The measurement site in Houtem (51°00’59” N, 2°34’56” E) is generally known as a rural background site (VMM, 2013; VMM, 2014a). The measurement site is located in the middle of the polders, 500 m from the French-Belgian border, 2 km from the center of Houtem (700 inhabitants), slightly more than 8 km from the Belgian coast and 15 km from Duinkerke (Figure 1).
Figure 1:

Map of Belgium with the three sampling locations
The PM10 air samples were collected during a 24-h period with high-volume samplers (DHA80-SEQ with 16 filter standard field housing, Digitel) with a theoretical flow of 30 m3 h−1 (720 m3 in a 24-h sampling period). The samplers were equipped with a PM10 size-selective inlet DPM10/30/00 PM10 pre-separator (Digitel) and samples for measurement of EDCs were taken on each location every six days using pre-heated (4 hours at 450 °C) glass fiber filters with a diameter of 150 mm (MG 227/1/60 Sartorius). During the sampling period, also eight field blanks and six procedural (laboratory) blanks were included. All filters were stored at −20°C until further handling.
The VMM provided information on the mass and composition of ambient aerosol throughout the campaign. Data on daily PM concentrations (μg m−3) (PM10 and PM2.5), several PAHs in the total suspended particle (TSP) fraction (fluoranthene, pyrene, benzo(a)anthracene, chrysene, benzo(b)fluoranthene, benzo(k)fluoranthene, benzo(a)pyrene, dibenzo(a,h)anthracene, benzo(g,h,i)- perylene and indeno(1,2,3-cd)pyrene), heavy metal concentrations (ng m−3) in PM10 (Pb, Zn, Cu, Ni, As, Mn, Cd, Cr) and black carbon (BC) (μg m−3) in TSP were available for the whole sampling period. Meteorological conditions, such as air temperature, wind speed, humidity and precipitation were monitored and recorded during the sampling campaigns by VMM and the Royal Meteorological Institute (KMI) from Belgium.
2.3. Extraction and CALUX measurement
Sample pre-treatment and measurement procedures were previously described by Croes et al. (2016). Briefly, sample extraction was carried out with an Accelerated Solvent Extractor using a hexane/acetone (50/50%, v/v) solvent mixture. Samples were then evaporated at 40 °C under a flow of pure air and redissolved in 10 mL hexane for storage until in vitro analysis.
CALUX measurements were executed using recombinant BG1Luc4E2 human ovarian carcinoma and T47D human breast carcinoma cell lines that contain a stably transfected estrogen- or androgen-responsive gene plasmid that responds to estrogenic/androgenic chemicals with the induction of firefly luciferase reporter gene expression (Rogers and Denison, 2000; Rogers and Denison, 2002). Ten standard solutions of 17β-Estradiol (E2) and 4,5-dihydrotestosteron (DHT) were used as a reference standard for calculation of respectively estrogenic and androgenic activity of the sample extracts. For each sample, ten dilution points, ranging between 36 m3 and 0.36 m3 air equivalent were measured in duplicate.
2.4. Statistical data treatment
Endocrine activity from the CALUX bioassay was reported as bioanalytical equivalents (BEQs) and expressed in femtograms 17β-estradiol or 4,5-dihydrotestosteron equivalents per cubic meter of air (fg E2-eq/m3 or fg DHT-eq/m3) for respectively the estrogenic and androgenic activity. A Box-Cox transformation was used to characterize the response of the standard solutions and samples. The measured luminescence (expressed in Relative Light Units or RLUs) of an unknown sample was converted into a BEQ by comparison of the slope of the sample dose-response curve with the slope of the standard curve (Elskens et al., 2011; Mihale et al., 2013). This approach was chosen, since the most concentrated sample extracts were often toxic to the cells and thus no full concentration-response curves (with upper plateau up to 100% RLU induction) could be obtained. Accordingly, the Box-Cox transformation with calculation of the slopes could provide a reliable alternative for the Hill function. For samples with a BEQ-response below the value of the blank filter, half of the median of all procedural and field blanks was used. Kruskal-Wallis and Analysis of variance (two-factor ANOVA) tests were used to determine possible differences between sampling dates and locations. Statistical significance was defined at 5%. To define correlations between different biological responses and PM characteristics and meteorological conditions, a Spearman rank correlation test was used. In addition, principal component analysis (PCA) was conducted to study the correlations between variables (estrogenic activity - ER, sum carcinogenic PAHs - ΣC, sum non-carcinogenic PAHs - ΣNC, particulate matter in air samples – PM10, black carbon - BC, temperature – T and wind speed – W) and to visualize observations in a 2-dimensional space in order to identify uniform or atypical groups of observations (Borgerhout, Zelzate and Houtem). Variables with a non-Gaussian distribution were logarithmically transformed. PCA and related statistics were performed using the software XLSTAT, version 2015.6.01.23990 of the Addinsoft company (USA). Since the PAHs were strongly correlating, they were categorized as non-carcinogenic (sum of pyrene and fluoranthene) and carcinogenic (sum of benzo(a)anthracene, chrysene, benzo(b)fluoranthene, benzo(k)fluoranthene, benzo(a)pyrene, dibenzo(a,h)anthracene, benzo(g,h,i)-perylene and indeno(1,2,3-cd)pyrene) PAHs before inclusion in the model (Brits et al., 2004; Sevastyanova et al., 2008; Taioli et al., 2007). Although PAHs were measured in the TSP fraction, correlation with estrogenic activity can be defined since PAHs are most present in the fine fraction (Schönbuchner et al., 2001; Thongsanit et al., 2003; Xu et al., 2015). Metal concentrations were not included in the model, since previous research showed that metals were not present in the CALUX sample extracts (Croes et al., 2016).
3. Results and discussion
3.1. Estrogenic and androgenic activity
Figures 2 and table S1 present an overview of the estrogenic activity (BEQ expressed as fg E2-Eq m−3) of all samples. The dashed line in figure 3 represents half of the median value of all procedural/field blanks (8.29 ± 2.72 fg E2-Eq m−3), while cytotoxic samples were put at zero on this graph. Cytotoxic samples were not taken into account for further calculations, while all samples below the blank response (n=26) were set at half of the median value for further calculations.
Figure 2:
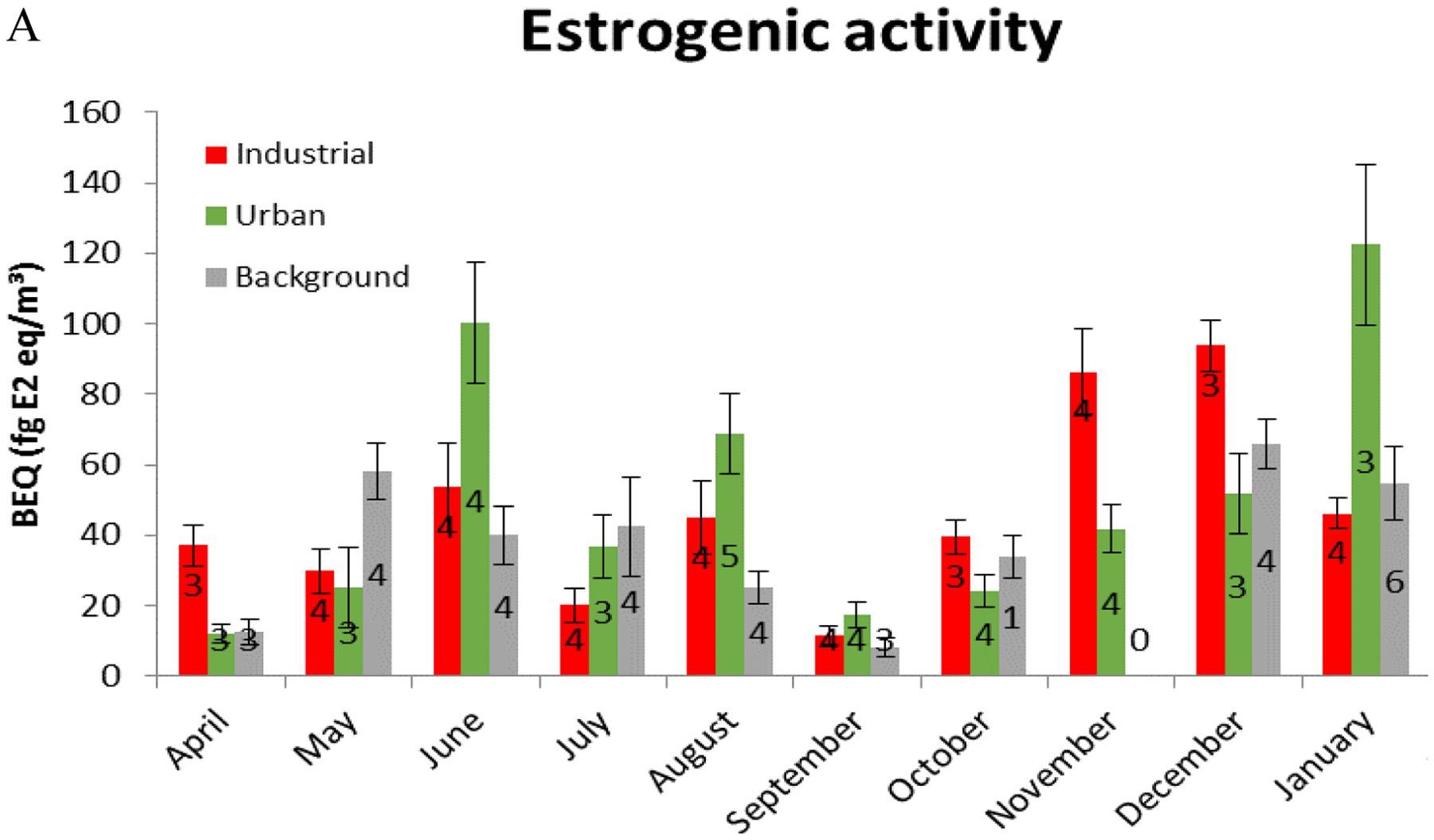

A. Mean monthly and B. total estrogenic activity between April 2013 and January 2014. A. the numbers indicate the monthly number of samples analyzed (with exclusion of ten cytotoxic sample extracts). Error bars define the variability of the measured BEQ within the respective month. B. box plots for the total estrogenic activity (from the whole campaign), with median, quartiles and outliers/extremes indicated for each sampling area.
Figure 3:

Estrogenic activity of individual PM10 air samples between April 2013 and January 2014. In the rural background location, no samples were available between 13 October and 6 December 2013 (dashed grey line). The dashed line at 8.29 fg E2-Eq/m3 shows half of the median field blank activity.
From these figures, it is clear that high fluctuations in estrogenic activity were observed in PM10 air samples, with significant differences between the monthly activities (months p=0.03, location p=0.45, two-factor ANOVA) and borderline significant differences between the individual measurement days (sampling day p=0.058, location p=0.25, two-factor ANOVA). No significant differences were found in average results between the three sampling locations with median BEQs of 32.1, 35.9 and 31.1 fg E2-Eq m−3 for the industrial area, urban area and rural background location, respectively. In 26 out of 116 samples, no estrogenic activity was detected, while for ten samples the BEQ could not be calculated due to high cytotoxicity of the extract. For most samples, induction levels ranged from 20 to 50% of the maximum E2 induction, which corresponds to BEQs of 20 to 60 fg E2-Eq m−3. In the urban area of Borgerhout, several samples collected during June and August 2013 and January 2014 showed very high estrogenic activity (with peaks above 120 fg E2-Eq m−3), while in the industrial area of Zelzate the highest peak concentrations were found during the winter period (November-December, also with peaks above 120 fg E2-Eq m−3) (Figure 3). Thus, it seems that in the industrial area there was a tendency towards higher concentrations in winter, while at the urban area and, to a lesser extent, at the rural background location these high peak concentrations were not concentrated in the winter period and it seems thus that episodic events have influenced the observed mean monthly values.
In this study, androgenic activity in PM10 was also determined for the first time in Flanders. For each location, ten samples were randomly selected and ten dilution points of each extract were measured using the androgenic cell line. No androgenic activity was observed in any of the samples or blanks. The lack of androgenic activity was not surprising, given that most pollutants typically present in the atmosphere (e.g. PAHs and their metabolites, pesticides and brominated flame retardants) are not androgenic or anti-androgenic (Liscio et al., 2014; Sonneveld et al., 2005; Weiss et al., 2009). In contrast, water and sediment samples, often contain androgenic activity (Van der Linden et al., 2008; Weiss et al., 2009). This activity is generally associated with known androgenic compounds, including natural and synthetic steroid hormones (e.g. testosterone, nandrolone and levonorgestrel), UV-filters and perfluorinated compounds, all of which have been previously found in these environments (Bjerregaard-Olesen et al., 2014; Freyberger et al., 2010; Sonneveld et al., 2005).
Compared to other international studies, our results suggests that although estrogenic activity was measured in 70% of the samples in the present study, the BEQs were generally low compared to those from other studies reporting on indoor environments and outdoor air samples from smog episodes (Wenger et al., 2009; Novák et al., 2009; Novák et al., 2013; Klein et al., 2006; Kennedy et al., 2009). A possible explanation for these lower estrogenic BEQs and the absence of androgenic activity could be differences in sample pre-treatment and bioassay analysis and/or the presence of antagonistic compounds that reduce the overall estrogenic and/or androgenic responses. Further molecule specific analysis (GC- and/or LC-MS) will be necessary to identify the pollutants present in the extract that contribute to the observed BEQ, since the CALUX test only gives an overall activity of all compounds present in the sample. This overall activity gives important information, since it includes mixture effects, but for policy makers identification of pollutants remains important for implementation of actions to reduce the exposure to endocrine pollutants and their possible adverse health effects.
3.2. Correlations with PM characteristics and meteorological conditions
In order to identify which physical and chemical determinants may provide a possible explanation for the observed endocrine activity, Spearman rank correlation tests, followed by Principal Component Analysis, were executed on the whole dataset from the three locations and on the data from the individual locations. The data were first log-transformed to stabilize the variance and generate nearly symmetrical distributions (−0.4 ≤ skew ≤ 0.7).
In the PCA analysis, three principal components were selected for interpreting the data, following Kaiser’s rule (Jolliffe, 1986). These new components explain about 84% of the total variance of the system. The PCA loading plots (Figure 4) display the relationships between all seven variables at the same time. The first component axis explains 43.7% of the variation, the second one 24.9% and the third one 15.1%. The coordinates of the original variables in the plane represent their Spearman correlations with the new components. In other words, the further away a variable lays from the origin, the stronger the impact of that variable on the model. In our study, PC1 was mainly explained by PM10, BC, ΣC and ΣNC, while PC2 was mainly explained by the physical characteristics T and W. Chemical composition and meteorological variables showed little contribution to PC3, which was strongly represented by ER. When two variables are both close to each other and close to the correlation circle, their correlation is high and significant (e.g. PM10 vs BC and ΣC vs ΣNC, Spearman rank coefficient rho > 0.8; p <0.001). If they are diametrically opposed, they are anti-correlated (e.g. variables T vs W, rho = −0.4; p = 0.001). If they are orthogonal, they are not correlated (e.g. ΣC and ΣNC vs W; PM10 and BC vs T). It is noted that ER is not at all dependent on the other variables, while it is almost fully determined by PC3 (> 93%). With these results of the PCA loading plot in mind, interpretation of the score plot (Figure 5), which provides a map of how the sample sites relate to each other, is greatly facilitated. Samples close to each other have similar properties, whereas those far from each other are dissimilar with respect to the physicochemical characteristics shared by the new components. There is clearly no segregation according to the vertical plane along PC2, i.e. no significant impacts of the meteorological gradients (wind speed and temperature) on the measurement sites (Borgerhout, Zelzate and Houtem), but there is a slight segregation along PC1 between Borgerhout (urban) and Zelzate (industrial) on the one hand and Houtem (rural background) on the other. Overall, the concentrations of BC, PM10, ΣC and ΣNC were significantly lower in Houtem (Pairwise Multiple Comparison Holm-Sidak p < 0.05) than at the other sites. No segregation occurs along the PC3 axis, indicating that the estrogenic activities are similar at the three measurement sites. Extreme observations or outliers were not detected in the data set, with most of the observations falling inside the ellipse corresponding to the 95% confidence region (Figure 5), specified by the Hotelling’s T2 test (Prokhorov, 2001).
Figure 4:

PCA loading plot for the entire dataset. The data were log-transformed prior to analysis. The factor loadings (vectors) represent the Spearman rank correlations between the original variables and the principal components (PC). Estrogenic activity = ER; sum carcinogenic PAHs - ΣC; sum non-carcinogenic PAHs = ΣNC; particulate matter in air samples = PM10; black carbon concentration = BC; temperature °C = T and wind speed= W. The continuous line represents the correlation circle
Figure 5:

PCA score plot for the entire dataset. The data were log transformed prior to analysis. The continuous line represents the ellipse corresponding to the 95% confidence region, as specified by the Hotelling’s T2 statistic. Borgerhout = urban; Zelzate= industrial; Houtem= rural background area.
When investigating the different locations independently, significant correlations were only found between estrogenic activities and PAH concentrations in the industrial area of Zelzate (Table 1). Both the sum of all PAHs (rho = 0.42; p = 0.02), the sum of the non-carcinogenic (rho = 0.48; p < 0.01) and carcinogenic PAHs (rho = 0.36 p = 0.05) were positively correlated with the estrogenic response, while for the mean daily temperature, a significant negative correlation was found (rho = −0.41; p = 0.04). These results are not surprising, since this industrial area is known for high airborne PAH concentrations (VMM, 2014b). Furthermore, PAHs are known to exert estrogenic effects and as previously mentioned, a Swiss study reported a positive relationship between estrogenic activities in the PM1 fraction and concentrations of hydroxyl-PAHs at an urban and a rural site (Wenger et al., 2009).
Table 1:
Spearman rank correlation coefficients for estrogenic activity in the industrial area of Zelzate. Significant correlations are indicated in bold. n= number of data points.
| Estrogenic activity | n | Spearman rho | p-value |
|---|---|---|---|
| Sum all PAHs | 29 | 0.42 | 0.022 |
| Sum carc. PAHs | 29 | 0.36 | 0.052 |
| Sum non-carc. PAHs | 29 | 0.48 | 0.008 |
| Wind direction | 38 | −0.02 | 0.889 |
| Wind speed | 38 | 0.16 | 0.338 |
| Temperature | 38 | −0.33 | 0.044 |
| Precipitation | 38 | −0.16 | 0.338 |
| Black carbon | 38 | 0.26 | 0.111 |
| PM10 | 28 | 0.35 | 0.064 |
A negative correlation with temperature was also not unexpected, since higher temperatures cause volatilization of certain (especially lower molecular weight) pollutants, leading to higher concentrations in the gas phase and lower levels bound to the PM fraction (Lammel et al., 2011; Ravindra et al., 2006; Vecchi et al., 2004). This hypothesis was confirmed in our study by the negative Spearman rank correlations between temperature and all individual PAHs (both carcinogenic and non-carcinogenic) that were measured in the TSP fractions from the three locations (results not shown). However, one also need to take into account that the situation is more complex, since in winter time there is usually more biomass burning (domestic heating) and this also contributes to the higher atmospheric concentrations of PAHs. In summary, our results suggests that the non-carcinogenic PAHs measured in the industrial area of Zelzate account for ~25% of the variability in the observed estrogenic activity, while for all data points from the three locations less than 5% of the variance could be attributed to the atmospheric PAH concentrations. This means that the observed correlations can not completely explain the measured estrogenic activities, indicating that other pollutants, which were not measured in this study, have a significant impact on the in vitro cell response.
4. Conclusions
A case study at three different locations in Flanders showed no androgenic, but significant estrogenic activity in PM10 of most samples, but generally low estrogenic values were found if compared to indoor air or samples from smog episodes. No clear seasonal (summer/winter) trends were observed in the urban and rural background area, while a tendency towards higher winter concentrations of estrogenic activity was found in the industrial area of Zelzate. Statistical analysis showed significant positive correlations between estrogenic activity and the sum of the non-carcinogenic PAHs (pyrene and fluoranthene) when taking all data points together, but this could only account for less than 5% of the observed variance in estrogenic activity. However, when only taking into account the data from the industrial area (a region with higher airborne PAH concentrations compared to the other measurement sites) highly significant positive correlations with PAH concentrations were found, explaining around 25% of the observed variance in estrogenic activity. Thus, it can be concluded that although the median estrogenic activity was rather low and comparable in the three locations, high fluctuations in the estrogenic response were measured and other unidentified pollutants seem to be contributing to the observed response in these regions. The identity of these atmospheric estrogenic chemicals and their potential to produce endocrine disrupting effects remain to be elucidated. Furthermore, although no differences in mean endocrine activity were found between the three areas, significant differences were found for other endpoints, like oxidative potential, inflammatory potential of particles and mutagenic activity (Van Den Heuvel et al., 2016). This shows that combining several assays with different endpoints is beneficial for interpreting the overall air quality.
Supplementary Material
Acknowledgements
This study was partly commissioned, financed and steered by the Ministry of the Flemish Community (Department of Economics, Science and Innovation; Flemish Agency for Care and Health; and Department of Environment, Nature and Energy). This study was supported by Joaquin (Joint Air Quality Initiative), an EU cooperation project funded by the INTERREG IVB Northwest-Europe programme.
The BG1LUC4E2 and the T47D cell lines were developed by the University of California-Davis (USA) with funding from a Superfund Research Program grant (ES04699) from the National Institute of Environmental Health Sciences.
The authors acknowledge the technicians from the Flanders Environment Agency for collection of the PM10 air samples.
References:
- Belhaj D, Baccar R, Jaabiri I, Bouzid J, Kallel M, Ayadi H, et al. Fate of selected estrogenic hormones in an urban sewage treatment plant in Tunisia (North Africa). Science of the total Environment 2015; 505: 154–160. [DOI] [PubMed] [Google Scholar]
- Bjerregaard-Olesen C, Bossi R, Bech BH, Bonefeld-Jørgensen EC. Extraction of perfluorinated alkyl acids from human serum for determination of the combined xenoestrogenic transactivity: A method development. Chemosphere 2014. [DOI] [PubMed] [Google Scholar]
- Brits E, Schoeters G, Verschaeve L. Genotoxicity of PM 10 and extracted organics collected in an industrial, urban and rural area in Flanders, Belgium. Environmental research 2004; 96: 109–118. [DOI] [PubMed] [Google Scholar]
- Croes K, Debaillie P, Van den Bril B, Staelens J, Vandermarken T, Van Langenhove K, et al. Assessment of estrogenic activity in PM10 air samples with the ERE-CALUX bioassay: Method optimization and implementation at an urban location in Flanders (Belgium). Chemosphere 2016; 144: 392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon J-P, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocrine reviews 2009; 30: 293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elskens M, Baston D, Stumpf C, Haedrich J, Keupers I, Croes K, et al. CALUX measurements: Statistical inferences for the dose-response curve. Talanta 2011; 85: 1966–1973. [DOI] [PubMed] [Google Scholar]
- Freyberger A, Witters H, Weimer M, Lofink W, Berckmans P, Ahr HJ. Screening for (anti)androgenic properties using a standard operation protocol based on the human stably transfected androgen sensitive PALM cell line. First steps towards validation. Reproductive Toxicology 2010; 30: 9–17. [DOI] [PubMed] [Google Scholar]
- Houtman CJ, Booij P, Jover E, Del Rio DP, Swart K, Van Velzen M, et al. Estrogenic and dioxin-like compounds in sediment from Zierikzee harbour identified with CALUX assay-directed fractionation combined with one and two dimensional gas chromatography analyses. Chemosphere 2006; 65: 2244–2252. [DOI] [PubMed] [Google Scholar]
- Jolliffe I Principal Component Analysis: Springer, 1986. [Google Scholar]
- Kennedy K, Macova M, Bartkow ME, Hawker DW, Zhao B, Denison MS, et al. Effect based monitoring of seasonal ambient air exposures in Australia sampled by PUF passive air samplers. Atmos Pollut Res. 2010; 1: 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy K, Macova M, Leusch F, Bartkow ME, Hawker DW, Zhao B, et al. Assessing indoor air exposures using passive sampling with bioanalytical methods for estrogenicity and aryl hydrocarbon receptor activity. Analytical and Bioanalytical Chemistry 2009; 394: 1413–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein GP, Hodge EM, Diamond ML, Yip A, Dann T, Stern G, et al. Gas-phase ambient air contaminants exhibit significant dioxin-like and estrogen-like activity in vitro. Environmental health perspectives 2006; 114: 697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krstevska-Konstantinova M, Charlier C, Craen M, Du Caju M, Heinrichs C, de Beaufort C, et al. Sexual precocity after immigration from developing countries to Belgium: evidence of previous exposure to organochlorine pesticides. Human reproduction 2001; 16: 1020–1026. [DOI] [PubMed] [Google Scholar]
- Krugly E, Martuzevicius D, Sidaraviciute R, Ciuzas D, Prasauskas T, Kauneliene V, et al. Characterization of particulate and vapor phase polycyclic aromatic hydrocarbons in indoor and outdoor air of primary schools. Atmospheric Environment 2014; 82: 298–306. [Google Scholar]
- Lammel G, Novák J, Landlová L, Dvorská A, Klánová J, Čupr P, et al. Sources and distributions of polycyclic aromatic hydrocarbons and toxicity of polluted atmosphere aerosols. Urban Airborne Particulate Matter 2011: 39–62. [Google Scholar]
- Liscio C, Abdul-Sada A, Al-Salhi R, Ramsey MH, Hill EM. Methodology for profiling anti-androgen mixtures in river water using multiple passive samplers and bioassay-directed analyses. Water Research 2014; 57: 258–269. [DOI] [PubMed] [Google Scholar]
- Mertl J, Kirchnawy C, Osorio V, Grininger A, Richter A, Bergmair J, et al. Characterization of Estrogen and Androgen Activity of Food Contact Materials by Different In Vitro Bioassays (YES, YAS, ERα and AR CALUX) and Chromatographic Analysis (GC-MS, HPLC-MS). PloS one 2014; 9: e100952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihale M, Croes K, Tungaraza C, Baeyens W, Van Langenhove K. PCDD/F and Dioxin-Like PCB Determinations in Mtoni Estuarine Sediments (Tanzania) Using the Chemically Activated Luciferase Gene Expression (CALUX) Bioassay. Environment and Pollution 2013; 2: 1–19. [Google Scholar]
- Mishra VK, Kumar P, Van Poppel M, Bleux N, Frijns E, Reggente M, et al. Wintertime spatio-temporal variation of ultrafine particles in a Belgian city. Science of the total Environment 2012; 431: 307–313. [DOI] [PubMed] [Google Scholar]
- Novák J, Giesy JP, Klánová J, Hilscherová K. In vitro effects of pollutants from particulate and volatile fractions of air samples—day and night variability. Environmental Science and Pollution Research 2013; 20: 6620–6627. [DOI] [PubMed] [Google Scholar]
- Novák J, Jálová V, Giesy JP, Hilscherová K. Pollutants in particulate and gaseous fractions of ambient air interfere with multiple signaling pathways in vitro. Environment international 2009; 35: 43–49. [DOI] [PubMed] [Google Scholar]
- Ozen S, Darcan S, Bayindir P, Karasulu E, Simsek D, Gurler T. Effects of pesticides used in agriculture on the development of precocious puberty. Environmental monitoring and assessment 2012; 184: 4223–4232. [DOI] [PubMed] [Google Scholar]
- Prokhorov A Hotelling T2-distribution. Encyclopedia of Mathematics: Springer; 2001. [Google Scholar]
- Ravindra K, Bencs L, Wauters E, De Hoog J, Deutsch F, Roekens E, et al. Seasonal and site-specific variation in vapour and aerosol phase PAHs over Flanders (Belgium) and their relation with anthropogenic activities. Atmospheric Environment 2006; 40: 771–785. [Google Scholar]
- Rogers J, Denison M. Recombinant cell bioassays for endocrine disruptors: development of a stably transfected human ovarian cell line for the detection of estrogenic and anti-estrogenic chemicals. In vitro & molecular toxicology 2000; 13: 67–82. [PubMed] [Google Scholar]
- Rogers JM, Denison MS. Analysis of the Antiestrogenic Activity of 2,3,7,8-Tetrachlorodibenzo-p-dioxin in Human Ovarian Carcinoma BG-1 Cells. Molecular pharmacology 2002; 61: 1393–1403. [DOI] [PubMed] [Google Scholar]
- Rudel RA, Camann DE, Spengler JD, Korn LR, Brody JG. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environmental science & technology 2003; 37: 4543–4553. [DOI] [PubMed] [Google Scholar]
- Schönbuchner H, Guggenberger G, Peters K, Bergmann H, Zech W. Particle-size distribution of PAH in the air of a remote Norway spruce forest in northern Bavaria. Water, air, and soil pollution 2001; 128: 355–367. [Google Scholar]
- Sevastyanova O, Novakova Z, Hanzalova K, Binkova B, Sram R, Topinka J. Temporal variation in the genotoxic potential of urban air particulate matter. Mutation Research/Genetic Toxicology and Environmental Mutagenesis 2008; 649: 179–186. [DOI] [PubMed] [Google Scholar]
- Sonneveld E, Jansen HJ, Riteco JA, Brouwer A, van der Burg B. Development of androgen-and estrogen-responsive bioassays, members of a panel of human cell line-based highly selective steroid-responsive bioassays. Toxicological Sciences 2005; 83: 136–148. [DOI] [PubMed] [Google Scholar]
- Taioli E, Sram RJ, Garte S, Kalina I, Popov TA, Farmer PB. Effects of polycyclic aromatic hydrocarbons (PAHs) in environmental pollution on exogenous and oxidative DNA damage (EXPAH project): description of the population under study. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 2007; 620: 1–6. [DOI] [PubMed] [Google Scholar]
- Teil M-J, Moreau-Guigon E, Blanchard M, Alliot F, Gasperi J, Cladière M, et al. Endocrine disrupting compounds in gaseous and particulate outdoor air phases according to environmental factors. Chemosphere 2016; 146: 94–104. [DOI] [PubMed] [Google Scholar]
- Thongsanit P, Jinsart W, Hooper B, Hooper M, Limpaseni W. Atmospheric particulate matter and polycyclic aromatic hydrocarbons for PM10 and size-segregated samples in Bangkok. Journal of the Air & Waste Management Association 2003; 53: 1490–1498. [DOI] [PubMed] [Google Scholar]
- Van Den Heuvel R, Den Hond E, Govarts E, Colles A, Koppen G, Staelens J, et al. Toxicity of ambient PM10 collected at three sites in Flanders is related to physicochemical characteristics of PM10. Environmental Research 2016; in press. [Google Scholar]
- Van der Linden SC, Heringa MB, Man H-Y, Sonneveld E, Puijker LM, Brouwer A, et al. Detection of multiple hormonal activities in wastewater effluents and surface water, using a panel of steroid receptor CALUX bioassays. Environmental science & technology 2008; 42: 5814–5820. [DOI] [PubMed] [Google Scholar]
- Vandermarken T, De Galan S, Croes K, Van Langenhove K, Vercammen J, Sanctorum H, et al. Characterisation and implementation of the ERE-CALUX bioassay on indoor dust samples of kindergartens to assess estrogenic potencies. The Journal of steroid biochemistry and molecular biology 2015. [DOI] [PubMed] [Google Scholar]
- Vecchi R, Marcazzan G, Valli G, Ceriani M, Antoniazzi C. The role of atmospheric dispersion in the seasonal variation of PM1 and PM2. 5 concentration and composition in the urban area of Milan (Italy). Atmospheric Environment 2004; 38: 4437–4446. [Google Scholar]
- VMM. Luchtkwaliteit in het Vlaamse Gewest. Jaarverslag immissiemeetnetten. Kalenderjaar 2012. Jaarverslag immissiemeetnetten VMM, 2013. [Google Scholar]
- VMM. Luchtkwaliteit in het Vlaamse Gewest. Jaarverslag immissiemeetnetten. Kalenderjaar 2013. Jaarverslag immissiemeetnetten VMM, 2014a, pp. 112. [Google Scholar]
- VMM. Luchtkwaliteit in Vlaanderen: Polycyclische aromatische koolwaterstoffen – jaarrapport 2013. jaarrapport, 2014b. [Google Scholar]
- Weiss JM, Hamers T, Thomas KV, van der Linden S, Leonards PE, Lamoree MH. Masking effect of anti-androgens on androgenic activity in European river sediment unveiled by effect-directed analysis. Analytical and Bioanalytical Chemistry 2009; 394: 1385–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger D, Gerecke AC, Heeb NV, Schmid P, Hueglin C, Naegeli H, et al. In vitro estrogenicity of ambient particulate matter: contribution of hydroxylated polycyclic aromatic hydrocarbons. Journal of Applied Toxicology 2009; 29: 223–232. [DOI] [PubMed] [Google Scholar]
- Xu H, Guinot B, Niu X, Cao J, Ho KF, Zhao Z, et al. Concentrations, particle-size distributions, and indoor/outdoor differences of polycyclic aromatic hydrocarbons (PAHs) in a middle school classroom in Xi’an, China. Environmental geochemistry and health 2015; 37: 861–873. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


