Abstract
Background:
There is growing evidence that peripheral neuropathy (PN) is common even in the absence of diabetes. However, the clinical sequelae of PN have not been quantified in the general population.
Objective:
We aimed to assess the associations of PN with all-cause and cardiovascular mortality in the general U.S. adult population.
Design:
Prospective cohort study.
Setting:
1999–2004 National Health and Nutrition Examination Survey(NHANES).
Participants:
7,137 participants aged ≥40 years who underwent standardized monofilament testing for PN.
Measurements:
Cox regression to evaluate the associations of PN with all-cause and cardiovascular mortality after adjustment for demographic and cardiovascular risk factors.
Results:
The overall weighted prevalence of PN was 13.5%(SE 0.5%) [27.2%(SE 1.3%) in adults with diabetes and 11.6%(SE 0.5%) in adults without diabetes]. During a median of 13 years of follow-up there were 2140 deaths, including 492 due to cardiovascular causes. In adjusted models, PN was significantly associated with all-cause (HR 1.72, 95% CI 1.28–2.30) and cardiovascular mortality (HR 2.04, 95% CI 1.25–3.32) in participants with diabetes. The association of PN with cardiovascular mortality was particularly evident in adults with long-standing diabetes (HR 3.82, 95% CI 2.12–6.88 for diabetes duration ≥10 years vs <10 years). In participants without diabetes, PN was also significantly associated with both all-cause (HR 1.37, 95% CI 1.21–1.55) and cardiovascular mortality (HR 1.32, 95% CI 1.02–1.72).
Limitations:
PN defined by monofilament testing only, prevalent cardiovascular disease was self-reported.
Conclusion:
PN was common and independently associated with all-cause and cardiovascular mortality in the U.S. population, even in the absence of diabetes. These findings suggest that decreased sensation in the foot may be an under recognized risk factor for mortality in the general population.
Introduction
Peripheral neuropathy results in decreased lower extremity sensation that can lead to substantial morbidity in affected adults (1, 2). Although most commonly associated with diabetes (3, 4), PN affects adults with impaired glucose tolerance and normoglycemia as well (5, 6).
Adults with diabetes and PN have a higher risk of foot ulcers, major amputation, and falls than adults without PN (7–10). Among adults with diabetes who have PN and a foot ulcer, the relative risk of mortality is more than 2-fold higher than adults without PN and a foot ulcer (11). PN has also been described as an independent risk factor for mortality among adults with diabetes (12, 13). However, prior studies have typically focused on clinical populations and the mortality implications of PN have not been examined in the general population.
The clinical sequelae of PN in the absence of diabetes is poorly defined. PN is independently associated with functional impairments, work disability, and lower limb amputations even after adjusting for the presence of diabetes (14). To our knowledge, there are no studies that have examined the association of non-diabetic PN with mortality.
We aimed to assess the associations of prevalent PN with mortality in the general US adult population using the most recent data available from the National Health and Nutrition Examination Survey (NHANES).
Methods
Study population
The National Health and Nutrition Examination Survey (NHANES) are cross-sectional complex survey samples among the U.S. civilian non-institutionalized population conducted by the National Center for Health Statistics (NCHS). Our study included U.S. adults aged 40 years and older who attended the lower extremity disease exam in the NHANES survey cycles 1999–2004 (N = 7902). We excluded participants who were missing covariates of interest (N = 765), for a final sample size of 7137.
The NCHS Institutional Review Board approved the study protocols and informed consent was obtained from all participants.
Measurement of peripheral neuropathy
PN was assessed by trained technicians using a standard monofilament (5.07 Semmes-Weinstein nylon monofilament mounted on a plastic handle, delivering approximately a 10-gram filament force) to apply slight pressure to the bottom of each participant’s each foot on three sites - plantar-first metatarsal head, plantar-fifth metatarsal head and plantar-hallux (15). If the participant’s first response at a site was correct, the test was not repeated at that site. If the participant could not correctly identify where the pressure was applied, the test was repeated for a maximum of three tests per site until a total of two similar responses were obtained. A site was defined as insensate if the participant gave two incorrect or undeterminable responses for that site. PN was defined as having at least one insensate site on either foot.
Other Variable definitions
Age, sex, race-ethnicity, education, smoking status and drinking status were self-reported by participants. Diabetes was defined as self-reported doctor diagnosed diabetes or HbA1c ≥ 6.5%. Normoglycemia was defined as HbA1c < 5.7%. Body mass index (BMI) was calculated as weight (kilograms) divided by squared height (meters2). Hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg, diastolic blood pressure (DBP) ≥ 90 mmHg, or taking blood pressure control medication. Hypercholesterolemia was defined as total cholesterol ≥ 240 mg/dL or taking cholesterol control medication. Participants who self-reported doctor diagnosed coronary heart disease, heart attack or stroke were classified as having cardiovascular disease (CVD). Peripheral artery disease (PAD) was defined as left or right ankle brachial index (ABI) < 0.9. Chronic kidney disease was defined as eGFR < 60 mL/min/1.73m2 or urine albumin to creatinine ratio ≥ 30.
All-cause and cardiovascular mortality
NHANES is linked to death certificate records from the National Death Index. The public-use linked mortality file for 1999–2014 NHANES includes follow-up time and underlying cause of death for NHANES adult participants through December 31, 2015 (16). A detailed description of the linkage methodology and analytic guidelines are available on the NCHS data linkage webpage (17). Cardiovascular mortality was defined as any heart disease-related or cerebrovascular disease-related death.
Statistical analyses
We examined the characteristics of U.S. adults in our study according to both diabetes and PN status. We generated Kaplan-Meier survival curves for all-cause mortality and cardiovascular mortality stratified by diabetes and PN status. We used Cox proportional hazards models to assess the associations of PN with all-cause and cardiovascular mortality in adults with and without diabetes separately. Model 1 was adjusted for age, sex and race-ethnicity. Model 2 was adjusted for all variables in Model 1 as well as other mortality risk factors including education, BMI, smoking status, drinking status, prevalent CVD, hypertension and hypercholesterolemia. We also evaluated the combined association of duration of diabetes and PN status with overall mortality and cardiovascular mortality in participants with diabetes. We then performed a sensitivity analysis assessing the association of PN with all-cause and CVD mortality adjusting for all variables in Model 2 as well as chronic kidney disease (participants with and without diabetes) and retinopathy (participants with diabetes only) separately. We performed an additional sensitivity analysis assessing the association of PN with all-cause and cardiovascular mortality adjusting for all variables in Model 2 among adults with normoglycemia.
We performed all analyses using Stata, version 15.1 (StataCorp) with a P-value less than 0.05 indicating statistical significance. All analyses incorporated sampling weights to obtain unbiased estimates from the complex NHANES design and we used Taylor series (linearization) to estimate standard errors.
Results
The overall prevalence of PN was 13.5% (SE 0.5%) in the general U.S. adult population aged 40 years or older. The prevalence was 27.2% (SE 1.3%) in adults with diabetes and 11.6% (SE 0.5%) in those without diabetes. Adults with PN were older, more frequently male, and had lower levels of education compared to participants without PN (Table 1). Participants with PN also had higher BMI, were more commonly former or current smokers, and had a higher prevalence of hypertension, hypercholesterolemia, and PAD (Table 1). Prevalent CVD was higher among adults with versus without PN in both the diabetes (30.6% vs. 19.0%) and no diabetes (16.6% vs. 8.3%) groups.
Table 1.
Characteristics of U.S. Adults Aged ≥ 40 years (%, SE) According to Diabetes and Peripheral Neuropathy (PN) Status, NHANES, 1999–2004
| No Diabetes | Diabetes | |||
|---|---|---|---|---|
| No PN | PN | No PN | PN | |
| Unweighted N | 5037 | 905 | 838 | 357 |
| % (SE) | % (SE) | % (SE) | % (SE) | |
| Mean age, years | 54.9 (0.2) | 63.3 (0.7) | 60.3 (0.5) | 64.3 (1.0) |
| Age in years | ||||
| 40–49 | 40.7 (1.1) | 19.0 (2.1) | 20.1 (1.9) | 12.2 (2.8) |
| 50–59 | 28.6 (0.9) | 22.9 (1.7) | 29.1 (2.2) | 22.1 (3.0) |
| 60–69 | 16.2 (0.7) | 21.9 (1.6) | 28.2 (1.8) | 28.1 (3.1) |
| 70–79 | 10.6 (0.4) | 22.2 (1.6) | 17.1 (1.5) | 26.9 (3.1) |
| 80+ | 4.0 (0.3) | 13.9 (1.1) | 5.5 (0.6) | 10.7 (1.7) |
| Male | 45.5 (0.6) | 61.1 (1.7) | 47.9 (2.3) | 65.5 (3.6) |
| Race-ethnicity | ||||
| Non-Hispanic White | 80.7 (1.4) | 80.6 (1.8) | 63.8 (3.4) | 69.6 (3.9) |
| Non-Hispanic Black | 7.8 (0.8) | 9.2 (1.2) | 13.8 (1.9) | 13.2 (2.3) |
| Hispanic | 3.9 (0.8) | 5.2 (1.3) | 7.5 (2.5) | 2.8 (1.3) |
| Other | 7.7 (0.8) | 5.1 (0.9) | 14.9 (1.9) | 14.4 (3.1) |
| Education | ||||
| Less than high school | 17.0 (0.8) | 27.2 (2.3) | 30.7 (1.9) | 34.9 (3.7) |
| High school or equivalent | 26.3 (0.9) | 26.4 (2.0) | 24.4 (1.9) | 31.0 (3.3) |
| College and above | 56.8 (1.3) | 46.3 (2.6) | 44.9 (2.2) | 34.2 (3.7) |
| BMI (kg/m2) | ||||
| <25.0 | 31.7 (1.2) | 25.9 (1.7) | 16.4 (1.9) | 13.5 (2.5) |
| 25.0–29.9 | 38.5 (0.9) | 39.4 (2.5) | 32.9 (2.1) | 32.5 (3.6) |
| ≥30.0 | 29.7 (1.0) | 34.7 (2.8) | 50.6 (2.8) | 54.0 (3.6) |
| Smoking status | ||||
| Never | 47.1 (1.0) | 43.1 (2.4) | 46.7 (2.9) | 44.6 (3.6) |
| Former | 32.1 (1.0) | 38.8 (2.2) | 34.9 (2.5) | 38.1 (4.0) |
| Current | 20.7 (0.7) | 18.2 (1.8) | 18.4 (1.6) | 17.4 (3.1) |
| Drinking status | ||||
| Never | 11.2 (1.1) | 15.5 (1.7) | 18.3 (1.8) | 20.9 (2.6) |
| Former | 19.4 (1.2) | 24.6 (2.0) | 34.3 (2.2) | 36.9 (4.3) |
| Current | 69.4 (1.8) | 59.9 (2.4) | 47.4 (2.8) | 42.2 (4.6) |
| Cardiovascular disease | 8.3 (0.5) | 16.6 (1.5) | 19.0 (1.6) | 30.6 (3.8) |
| Hypertension | 39.0 (1.1) | 52.8 (2.3) | 63.4 (2.1) | 71.1 (3.2) |
| Hypercholesterolemia | 33.2 (0.9) | 34.6 (2.0) | 49.2 (2.5) | 46.3 (4.1) |
| Peripheral artery diseasea | 4.2 (0.3) | 8.3 (1.0) | 7.6 (1.5) | 17.7 (3.3) |
| Cancer | 10.6 (0.4) | 17.0 (1.5) | 14.6 (1.6) | 19.5 (2.8) |
| Hemoglobin A1c, % (SE) | 5.4 (0.01) | 5.5 (0.01) | 7.4 (0.07) | 7.6 (0.13) |
In the sub-population with PAD data (N=6101)
Over a median follow-up of 13 years, there were 2140 total deaths and 492 deaths due to cardiovascular causes. The incidence rate (per 1000 person-years) of all-cause mortality through 2015 (maximum follow-up, 17 years) was 58.0 (95% CI 48.8–69.0) in adults with diabetes and PN, 27.4 (95% CI 23.7–31.7) in adults with diabetes and no PN, 34.3 (95% CI 30.3–38.8) in adults with no diabetes and PN, and 13.0 (95% CI 12.2–14.0) in adults with no diabetes and no PN (Table 2). The leading cause of death among participants with diabetes was cardiovascular disease (31% of total deaths), whereas the leading cause of death among participants without diabetes was malignant neoplasms (27% of total deaths) (Appendix Table 1). In crude Kaplan-Meier analyses, the risk of all-cause and cardiovascular mortality was highest among adults with diabetes and PN, but PN was also associated with mortality in the absence of diabetes (Figure 1). The risk of all-cause mortality among adults with PN in the absence of diabetes was nearly equivalent to that for adults with diabetes but no PN (Figure 1A).
Table 2.
Hazard ratios (95% CIs) of Peripheral Neuropathy with All-cause and Cardiovascular Mortality in U.S. Adults Aged ≥ 40 years, NHANES, 1999–2004
| No Diabetes | With Diabetes | |||||||
|---|---|---|---|---|---|---|---|---|
| Events/N | Incidence Rate per 1000 (95% CI) | Model 1 HR (95% CI) | Model 2 HR (95% CI) | Events/N | Incidence Rate per 1000 (95% CI) | Model 1 HR (95% CI) | Model 2 HR (95% CI) | |
| All-cause mortality | Model 1 | Model 2 | ||||||
| No PN | 1186/5037 | 13.0 (12.2–14.0) | 1 (Ref) | 1 (Ref) | 314/838 | 27.4 (23.7–31.7) | 1 (Ref) | 1 (Ref) |
| With PN | 430/905 | 34.3 (30.3–38.8) | 1.40 (1.26–1.56) | 1.37 (1.21–1.55) | 210/357 | 58.0 (48.8–69.0) | 1.79 (1.36–2.36) | 1.72 (1.28–2.30) |
| Cardiovascular mortality | ||||||||
| No PN | 241/5037 | 2.4 (2.0–2.8) | 1 (Ref) | 1 (Ref) | 85/838 | 7.7 (5.9–10.2) | 1 (Ref) | 1 (Ref) |
| With PN | 104/905 | 7.3 (5.8–9.3) | 1.40 (1.09–1.79) | 1.32 (1.02–1.72) | 62/357 | 19.8 (14.5–27.7) | 2.11 (1.35–3.29) | 2.04 (1.25–3.32) |
Model 1: Adjusted for age, sex, and race-ethnicity
Model 2: Adjusted for variables in Model 1 plus education, BMI, smoking status, drinking status, cardiovascular disease, hypertension and hypercholesterolemia
Figure 1.
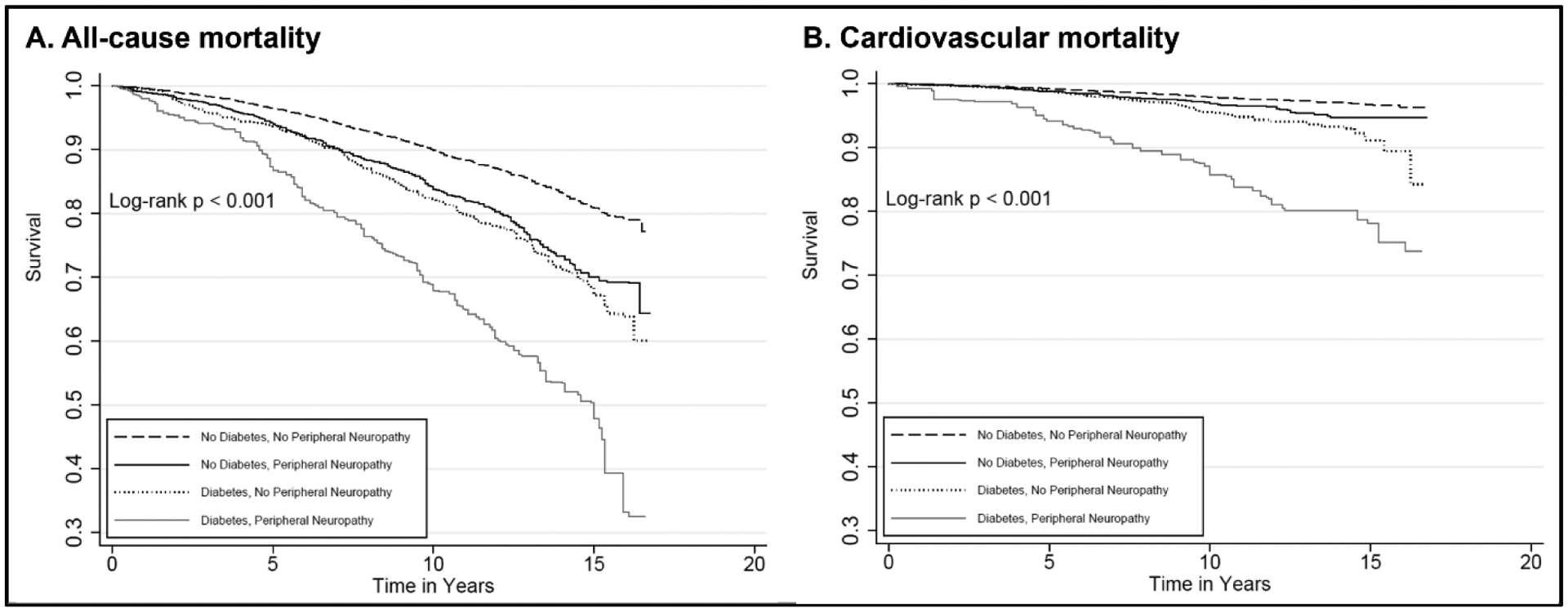
Kaplan-Meier (crude) survival curves according to peripheral neuropathy and diabetes status for all-cause mortality (Panel A) and cardiovascular mortality (Panel B), U.S. Adults Aged ≥ 40 years, NHANES, 1999–2004
After adjusting for age, sex, and race-ethnicity, PN was significantly associated with all-cause and CVD mortality in participants with and without diabetes (Table 2, Model 1). After adjusting for additional risk factors including prevalent CVD, PN was significantly associated with all-cause (HR 1.72, 95% CI 1.28–2.30) and cardiovascular mortality (HR 2.04, 95% CI 1.25–3.32) in participants with diabetes (Model 2). The association of PN with all-cause and cardiovascular mortality among participants with diabetes was strongest for those participants with long-standing diabetes (i.e. ≥10 years) (Table 3). In participants without diabetes, PN was significantly associated with both all-cause (HR 1.37, 95% CI 1.21–1.55) and cardiovascular mortality (HR 1.32, 95% CI 1.02–1.72) in Model 2. Our results did not substantially change after additionally adjusting for chronic kidney disease or retinopathy (Appendix Table 2).
Table 3.
Hazard Ratios (95% CIs) of All-cause and Cardiovascular Mortality with Duration of Diabetes and Peripheral Neuropathy in U.S. Adults Aged ≥ 40 years with Diabetes, NHANES, 1999–2004
| PN | Events/N | Incidence Rate per 1000 (95% CI) | Model 1 HR (95% CI) | Model 2 HR (95% CI) | |
|---|---|---|---|---|---|
| All-cause mortality | |||||
| Diabetes duration <10 years | No | 178/555 | 22.0 (18.2–26.8) | 1 (Ref) | 1 (Ref) |
| Diabetes duration <10 years | Yes | 87/172 | 49.3 (38.4–63.6) | 1.80 (1.25–2.57) | 1.62 (1.10–2.39) |
| Diabetes duration ≥10 years | No | 134/281 | 39.4 (31.8–49.1) | 1.50 (1.14–1.99) | 1.37 (1.02–1.84) |
| Diabetes duration ≥10 years | Yes | 121/180 | 68.3 (53.3–87.4) | 2.53 (1.84–3.49) | 2.42 (1.75–3.33) |
| Cardiovascular mortality | |||||
| Diabetes duration <10 years | No | 45/555 | 5.5 (3.8–8.3) | 1 (Ref) | 1 (Ref) |
| Diabetes duration <10 years | Yes | 21/172 | 11.3 (6.5–21.4) | 1.57 (0.67–3.69) | 1.40 (0.58–3.33) |
| Diabetes duration ≥10 years | No | 39/281 | 12.3 (8.5–18.4) | 1.87 (1.02–3.44) | 1.56 (0.85–2.86) |
| Diabetes duration ≥10 years | Yes | 46/180 | 29.5 (19.9–44.8) | 4.13 (2.26–7.56) | 3.82 (2.12–6.88) |
Model 1: Adjusted for age, sex and race-ethnicity
Model 2: Adjusted for variables in Model 1 plus education, BMI, smoking status, drinking status, cardiovascular disease, hypertension and hypercholesterolemia
In a sensitivity analysis limited to adults with normoglycemia (AppendixTable 3), the risk-adjusted association of PN with all-cause mortality persisted (HR 1.37, 95% CI 1.18–1.60). The association of PN with cardiovascular mortality was significant after adjusting for age, sex, and race-ethnicity (HR 1.39, 95% CI 1.07–1.83), but was attenuated after further adjusting for additional risk factors (HR 1.28, 95% CI 0.94–1.74).
Discussion
We found that the prevalence of PN in the U.S. population was substantial, even among adults without diabetes. PN was associated with both all-cause and cardiovascular mortality in adults with and without diabetes, and these associations persisted even after adjustment for prevalent cardiovascular disease and other mortality risk factors. Among adults with diabetes, the association of PN with mortality was strongest for those with long-standing diabetes ≥10 years. Overall, our data suggest that PN is a marker of increased mortality risk among U.S adults.
We found that the prevalence of PN was 27.2% in U.S. adults aged ≥40 years with diabetes and 11.6% in U.S. adults aged ≥40 years without diabetes, equivalent to 35 million adults based on the 2020 U.S. Census. These estimates are consistent with previously published reports (3, 4, 18). While diabetes is a well-established risk factor for PN (19–21), the high prevalence of PN among adults without diabetes is notable.
The etiology of PN in the absence of diabetes is unclear. There is some evidence to suggest that adults with PN who do not have diabetes may have impaired glucose tolerance or prediabetes (5, 6, 22). However, we found a persistent association of PN with all-cause mortality among adults with normoglycemia. Prior studies have reported an association of normoglycemic PN with features of metabolic syndrome (other than hyperglycemia) (23, 24), suggesting that chronic metabolic derangements may play a role in the pathogenesis of PN (25). We recently reported a significant association of body mass index and hypertension with PN among adults both with and without diabetes (Hicks et al., Under review). While diabetes is a well-established risk factor for PN, our data suggest that the prevalence of PN among adults without diabetes is substantial and may be an under-recognized risk factor for mortality.
The risks of all-cause and cardiovascular mortality were significantly higher for adults with diabetes and PN compared to those without PN in our study. Adults with diabetes are known to have a 2–4 times higher cardiovascular risk compared to adults without diabetes (26). However, the specific mortality associated with PN has not previously been described. Previous studies have reported that adults with diabetes and neuropathic sequelae, such as foot ulcers, have a higher risk of death than adults with diabetes who do not have neuropathic sequelae (27–30). In the past, these elevated mortality rates have largely been attributed to subsequent major amputation and/or a higher prevalence of macrovascular atherosclerotic disease in affected patients (29, 31). As a result, it is possible that elevated mortality among adults with PN is partially attributable to infected foot ulcers and/or subsequent major amputation. However, we also found that the risk of cardiovascular mortality remained higher among adults with diabetes and PN even after adjusting for cardiovascular risk factors, prevalent cardiovascular disease, and PAD. The presence of PN may portend a higher risk of concomitant cardiac autonomic neuropathy or other unmeasured conditions. PN confers significantly higher risk of cardiac autonomic neuropathy among adults with diabetes (32). We have previously reported elevated high-sensitivity troponin T, brain natriuretic peptide levels, and kidney biomarkers among adults with PN (Hicks et al., In press), suggesting that the presence of PN may reflect systemic subclinical microvascular disease (33, 34). Our study contributes to the growing body of literature supporting the concept that PN is associated with substantial clinical sequelae.
The association of PN with mortality was highest among adults with long-standing diabetes. Adults with diabetes ≥10 years in duration and PN had a 2.4-fold higher risk of all-cause mortality and a 3.8-fold higher risk of cardiovascular mortality than adults with diabetes <10 years duration and no PN. Diabetes duration is a known risk factor for PN (35, 36) and cardiovascular mortality (37). Consistent with this notion, we found that the risk of cardiovascular mortality was 1.6-times higher among adults with diabetes ≥10 years duration and no PN, but that this risk more than doubled (HR 3.8) among adults with long-standing diabetes and PN.
The risk of all-cause and cardiovascular mortality was also significantly higher for adults with versus without PN in the absence of diabetes. Even after adjusting for prevalent cardiovascular disease and other mortality risk factors, the risk of death associated with PN was more than 30% higher in the non-diabetic population. There is a known association of PN with cancer related to the neurotoxic effects of some chemotherapies (38), although a history of cancer was rare in this population-based study. We also found an association of PN with cardiovascular mortality. Indeed, we found that PN in the absence of diabetes conferred approximately the same overall and cardiovascular mortality risk as compared to a diagnosis of diabetes but no PN. We also found an association of PN with mortality among adults with normoglycemia, suggesting that hyperglycemia is not the only etiology for the disease. PN assessed by monofilament testing appears to be an unrecognized marker for mortality risk.
The limitations of our study include self-report of prevalent cardiovascular disease. We also do not have data on non-diabetic etiologies of PN (e.g. toxin exposures, autoimmune disease, vitamin deficiencies, etc.) or the duration of disease. PN was assessed using a cross-sectional evaluation of NHANES participants, so we could not evaluate long-standing vs. shorter term PN. Finally, we did not have eletromyography and nerve conduction tests to diagnose PN. NHANES did not collect data on participant symptoms using symptom-based screening instruments (39, 40), and did not evaluate participants using a tuning fork or vibration perception test, which may be more sensitive than monofilament testing (41). However, PN was assessed using a 10-g standard monofilament test, which reflects clinical practice for PN screening and is the recommended test to diagnose PN in its more severe form, loss of protective sensation (42). As such, it is important to distinguish that we found an association of PN in the form of impaired sensation with all-cause and cardiovascular mortality.
Strengths of our study include the large sample size and collection of major risk factors in a standardized fashion by trained personnel. NHANES data are representative of the U.S. population, meaning that our findings are generalizable to U.S. adults ≥ 40 years of age. BMI, hypertension, PAD, and chronic kidney disease were all based on standard measurements performed in NHANES, and diabetes was defined based on hemoglobin A1c in participants without an established diagnosis of diabetes. Finally, mortality was identified using the National Death Index, which allowed for accurate mortality risk estimates.
In conclusion, the prevalence of PN as assessed by monofilament testing is substantial in the U.S. population, even among adults without diabetes. PN was independently associated with all-cause and cardiovascular mortality regardless of diabetes status and other cardiovascular risk factors. These findings suggest that decreased sensation in the foot may be an under recognized risk factor for mortality in the general population.
Supplementary Material
Funding:
Dr. Selvin was supported by NIH/NIDDK grants K24DK106414 and R01DK089174. Dr. Matsushita was supported by NHLBI grant R21HL133694. The study sponsor/funder was not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; and did not impose any restrictions regarding the publication of the report.
References
- 1.Dyck PJ, Litchy WJ, Lehman KA, Hokanson JL, Low PA, O’Brien PC. Variables influencing neuropathic endpoints: the Rochester Diabetic Neuropathy Study of Healthy Subjects. Neurology. 1995;45(6):1115–21. [DOI] [PubMed] [Google Scholar]
- 2.Koski K, Luukinen H, Laippala P, Kivela SL. Risk factors for major injurious falls among the home-dwelling elderly by functional abilities. A prospective population-based study. Gerontology. 1998;44(4):232–8. [DOI] [PubMed] [Google Scholar]
- 3.Gregg EW, Gu Q, Williams D, de Rekeneire N, Cheng YJ, Geiss L, et al. Prevalence of lower extremity diseases associated with normal glucose levels, impaired fasting glucose, and diabetes among U.S. adults aged 40 or older. Diabetes Res Clin Pract. 2007;77(3):485–8. [DOI] [PubMed] [Google Scholar]
- 4.Katon JG, Reiber GE, Nelson KM. Peripheral neuropathy defined by monofilament insensitivity and diabetes status: NHANES 1999–2004. Diabetes Care. 2013;36(6):1604–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franklin GM, Kahn LB, Baxter J, Marshall JA, Hamman RF. Sensory neuropathy in non-insulin-dependent diabetes mellitus. The San Luis Valley Diabetes Study. Am J Epidemiol. 1990;131(4):633–43. [DOI] [PubMed] [Google Scholar]
- 6.Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A, Group KS. Neuropathic pain in diabetes, prediabetes and normal glucose tolerance: the MONICA/KORA Augsburg Surveys S2 and S3. Pain Med. 2009;10(2):393–400. [DOI] [PubMed] [Google Scholar]
- 7.Boulton AJ. Diabetic neuropathy and foot complications. Handb Clin Neurol. 2014;126:97–107. [DOI] [PubMed] [Google Scholar]
- 8.Boulton AJ. The pathway to foot ulceration in diabetes. Med Clin North Am. 2013;97(5):775–90. [DOI] [PubMed] [Google Scholar]
- 9.Kumar S, Ashe HA, Parnell LN, Fernando DJ, Tsigos C, Young RJ, et al. The prevalence of foot ulceration and its correlates in type 2 diabetic patients: a population-based study. Diabet Med. 1994;11(5):480–4. [DOI] [PubMed] [Google Scholar]
- 10.Margolis DJ, Jeffcoate W. Epidemiology of foot ulceration and amputation: can global variation be explained? Med Clin North Am. 2013;97(5):791–805. [DOI] [PubMed] [Google Scholar]
- 11.Boyko EJ, Ahroni JH, Smith DG, Davignon D. Increased mortality associated with diabetic foot ulcer. Diabet Med. 1996;13(11):967–72. [DOI] [PubMed] [Google Scholar]
- 12.Forsblom CM, Sane T, Groop PH, Totterman KJ, Kallio M, Saloranta C, et al. Risk factors for mortality in Type II (non-insulin-dependent) diabetes: evidence of a role for neuropathy and a protective effect of HLA-DR4. Diabetologia. 1998;41(11):1253–62. [DOI] [PubMed] [Google Scholar]
- 13.Coppini DV, Bowtell PA, Weng C, Young PJ, Sonksen PH. Showing neuropathy is related to increased mortality in diabetic patients - a survival analysis using an accelerated failure time model. J Clin Epidemiol. 2000;53(5):519–23. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman EM, Staff NP, Robb JM, St Sauver JL, Dyck PJ, Klein CJ. Impairments and comorbidities of polyneuropathy revealed by population-based analyses. Neurology. 2015;84(16):1644–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey 1999–2000 Data Documentation, Codebook and Frequencies: Lower Extremity Disease - Peripheral Neuropathy. Available at: https://wwwn.cdc.gov/Nchs/Nhanes/1999-2000/LEXPN.htm. Updated 06/2002. Accessed 08/14 2019.
- 16.Centers for Disease Control and Prevention. Codebook for the 2015 Public-Use Lnked Mortality File (LMF). Available at: https://www.cdc.gov/nchs/data/datalinkage/publicuse-2015-linked-mortality-files-data-dictionary.pdf. Updated 07/12/2019. Accessed 03/30 2020.
- 17.Office of Analysis and Epidemiology, Centers for Disease Control and Prevention. The Linkage of National Center for Health Statistics Survey Data to the National Death Index - 2015 Linked Mortality File: Methodology Overview and Analytic Considerations. Available at: https://www.cdc.gov/nchs/data/datalinkage/LMF2015_Methodology_Analytic_Considerations.pdf. Updated 04/11/2019. Accessed 02/12/2020 2020.
- 18.Martyn CN, Hughes RA. Epidemiology of peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1997;62(4):310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cabezas-Cerrato J The prevalence of clinical diabetic polyneuropathy in Spain: a study in primary care and hospital clinic groups. Neuropathy Spanish Study Group of the Spanish Diabetes Society (SDS). Diabetologia. 1998;41(11):1263–9. [DOI] [PubMed] [Google Scholar]
- 20.Tovi J, Svanborg E, Nilsson BY, Engfeldt P. Diabetic neuropathy in elderly Type 2 diabetic patients: effects of insulin treatment. Acta Neurol Scand. 1998;98(5):346–53. [DOI] [PubMed] [Google Scholar]
- 21.Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993;36(2):150–4. [DOI] [PubMed] [Google Scholar]
- 22.Novella SP, Inzucchi SE, Goldstein JM. The frequency of undiagnosed diabetes and impaired glucose tolerance in patients with idiopathic sensory neuropathy. Muscle Nerve. 2001;24(9):1229–31. [DOI] [PubMed] [Google Scholar]
- 23.Visser NA, Vrancken AF, van der Schouw YT, van den Berg LH, Notermans NC. Chronic idiopathic axonal polyneuropathy is associated with the metabolic syndrome. Diabetes Care. 2013;36(4):817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith AG, Rose K, Singleton JR. Idiopathic neuropathy patients are at high risk for metabolic syndrome. J Neurol Sci. 2008;273(1–2):25–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Callaghan B, Feldman E. The metabolic syndrome and neuropathy: therapeutic challenges and opportunities. Ann Neurol. 2013;74(3):397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dal Canto E, Ceriello A, Ryden L, Ferrini M, Hansen TB, Schnell O, et al. Diabetes as a cardiovascular risk factor: An overview of global trends of macro and micro vascular complications. Eur J Prev Cardiol. 2019;26(2_suppl):25–32. [DOI] [PubMed] [Google Scholar]
- 27.Tesic DSP N; Mitrovic M; Medic-Stojanoska M; Bajkin I; Benc D; Icin T; Novakovic-Paro J; Pejin R; Popovic D; tesic D; Tomic D; Vukovic B 2016. Severity of foot pathology (IWGDF categories 2 and 3) shows the strongest association with mortality in diabetes. Eauropean Association for the Study of Diabetes Virtual Meeting 2016. Available at: https://www.easd.org/virtualmeeting/home.html#!resources/severity-of-foot-pathologyiwgdf-categories-2-and-3-shows-the-strongest-association-with-mortality-in-diabetes. Accessed 02/12/2020 2020. [Google Scholar]
- 28.Brownrigg JR, Griffin M, Hughes CO, Jones KG, Patel N, Thompson MM, et al. Influence of foot ulceration on cause-specific mortality in patients with diabetes mellitus. J Vasc Surg. 2014;60(4):982–6 e3. [DOI] [PubMed] [Google Scholar]
- 29.Brownrigg JR, Davey J, Holt PJ, Davis WA, Thompson MM, Ray KK, et al. The association of ulceration of the foot with cardiovascular and all-cause mortality in patients with diabetes: a meta-analysis. Diabetologia. 2012;55(11):2906–12. [DOI] [PubMed] [Google Scholar]
- 30.Amadou C, Carlier A, Amouyal C, Bourron O, Aubert C, Couture T, et al. Five-year mortality in patients with diabetic foot ulcer during 2009–2010 was lower than expected. Diabetes Metab. 2019. [DOI] [PubMed] [Google Scholar]
- 31.Moulik PK, Mtonga R, Gill GV. Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care. 2003;26(2):491–4. [DOI] [PubMed] [Google Scholar]
- 32.Motataianu A, Maier S, Bajko Z, Voidazan S, Balasa R, Stoian A. Cardiac autonomic neuropathy in type 1 and type 2 diabetes patients. BMC Neurol. 2018;18(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinto A, Tuttolomondo A, Di Raimondo D, Fernandez P, La Placa S, Di Gati M, et al. Cardiovascular risk profile and morbidity in subjects affected by type 2 diabetes mellitus with and without diabetic foot. Metabolism. 2008;57(5):676–82. [DOI] [PubMed] [Google Scholar]
- 34.Ang L, Jaiswal M, Martin C, Pop-Busui R. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep. 2014;14(9):528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oguejiofor OC, Odenigbo CU, Oguejiofor CB. Evaluation of the effect of duration of diabetes mellitus on peripheral neuropathy using the United Kingdom screening test scoring system, bio-thesiometry and aesthesiometry. Niger J Clin Pract. 2010;13(3):240–7. [PubMed] [Google Scholar]
- 36.Nisar MU, Asad A, Waqas A, Ali N, Nisar A, Qayyum MA, et al. Association of Diabetic Neuropathy with Duration of Type 2 Diabetes and Glycemic Control. Cureus. 2015;7(8):e302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox CS, Sullivan L, D’Agostino RB Sr., Wilson PW, Framingham Heart S. The significant effect of diabetes duration on coronary heart disease mortality: the Framingham Heart Study. Diabetes Care. 2004;27(3):704–8. [DOI] [PubMed] [Google Scholar]
- 38.Gordon-Williams R, Farquhar-Smith P. Recent advances in understanding chemotherapy-induced peripheral neuropathy. F1000Res. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herman WH, Pop-Busui R, Braffett BH, Martin CL, Cleary PA, Albers JW, et al. Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med. 2012;29(7):937–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17(11):1281–9. [DOI] [PubMed] [Google Scholar]
- 41.Gin H, Rigalleau V, Baillet L, Rabemanantsoa C. Comparison between monofilament, tuning fork and vibration perception tests for screening patients at risk of foot complication. Diabetes Metab. 2002;28(6 Pt 1):457–61. [PubMed] [Google Scholar]
- 42.American Diabetes A 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S124–S38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


