Abstract
Adenomyosis is a common disorder of the uterus, and is associated with an enlarged uterus, heavy menstrual bleeding (HMB), pelvic pain, and infertility. It is characterized by endometrial epithelial cells and stromal fibroblasts abnormally found in the myometrium where they elicit hyperplasia and hypertrophy of surrounding smooth muscle cells. While both the mechanistic processes and the pathogenesis of adenomyosis are uncertain, several theories have been put forward addressing how this disease develops. These include intrinsic or induced (1) microtrauma of the endometrial-myometrial interface; (2) enhanced invasion of endometrium into myometrium; (3) metaplasia of stem cells in myometrium; (4) infiltration of endometrial cells in retrograde menstrual effluent into the uterine wall from the serosal side; (5) induction of adenomyotic lesions by aberrant local steroid and pituitary hormones; and (6) abnormal uterine development in response to genetic and epigenetic modifications. Dysmenorrhea, HMB, and infertility are likely results of inflammation, neurogenesis, angiogenesis, and contractile abnormalities in the endometrial and myometrial components. Elucidating mechanisms underlying the pathogenesis of adenomyosis raise possibilities to develop targeted therapies to ameliorate symptoms beyond the current agents that are largely ineffective. Herein, we address these possible etiologies and data that support underlying mechanisms.
Keywords: adenomyosis, mechanisms, pathogenesis, metaplasia, endometrial-myometrial interface
The Myometrial Compartments
Myometrium Anatomy, Embryology, and Structure
The myometrium resides between the endometrium and uterine serosa and is composed of an outer longitudinal layer and an inner circular layer of smooth muscle cells (SMCs), and supporting stromal and vascular tissue1–3 (►Fig. 1a, b). Histologically, the endometrial-myometrial interface (EMI) is a mucosal-muscular interface without an intervening basement membrane, differing markedly from other mucosal tissues (e.g., intestine).2 As the endometrial basalis is in direct contact with the myometrium,4 the question arises about how these compartments maintain their unique structures and functions, whether and how they cross-communicate, and what consequences, if any, ensue with damage or abnormalities in this interface for either or both compartments.
Fig. 1. The structure of normal uterus.
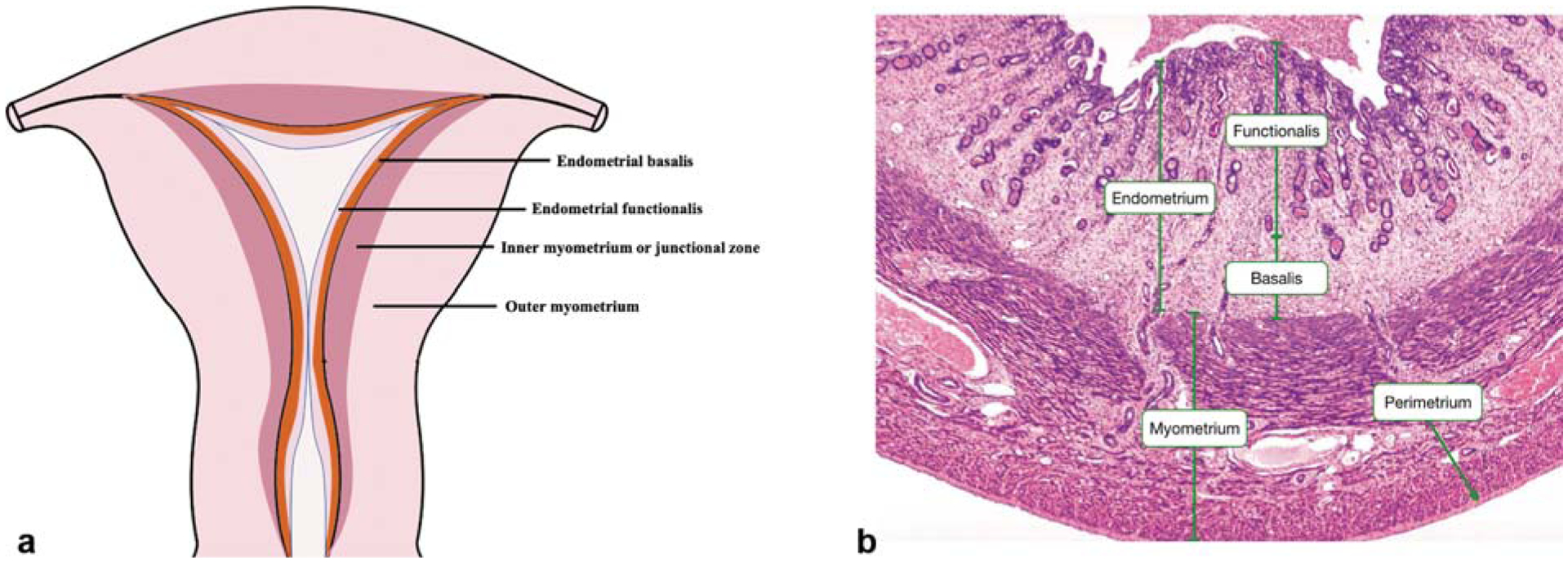
(a) The coronal section of normal uterus. (b) Hematoxylin–eosin staining of the full-thickness of uterus. (With permission from Yale Histology. Available at: http://histology.med.yale.edu/female_reproductive_system/female_reproductive_system_reading.php.)
Relatively recently, a subendometrial layer of the myometrium, called the “inner myometrium” (IM; ►Fig. 1a), has been identified mainly by magnetic resonance imaging (MRI).5 MRI reveals that the uterine wall is composed of high-signal intensity endometrium, medium-signal intensity outer myometrium (OM), and low-signal intensity IM (also called the “junctional zone”) on T2-weighted images.1 Abnormalities in the junctional zone are key in diagnosing adenomyosis by MRI and two- or three-dimensional transvaginal ultrasonography.6
Although there is no histologic distinction on light microscopy between the IM and OM,2 their embryologic origins, structure, and physiological roles differ markedly. During development, the IM arises from the Mullerian ducts, as does the endometrium, whereas the OM is mesenchymal in origin. The OM is absent in egg-laying animals and evolutionarily developed in uteri of viviparous animals, underscoring its role in protecting the fetus throughout gestation and mechanically facilitating expulsion of the conceptus at parturition.2 Lesser appreciated are the potential functions of the IM, which has remained mostly enigmatic in uterine biology (see later).
Myocyte (SMC) density and arrangements differ in IM and OM, with IM exhibiting irregular, predominantly densely packed, circular muscle fibers and more vascularity compared with OM, which exhibits regular and predominantly longitudinal SMC bundles.2,7,8 Ultrastructurally, while IM exhibits increased nuclear:cytoplasm ratio, decreased extracellular matrix (mainly elastin) with lower connective tissue-to-myocyte ratio, and lower water content compared with OM, changes from IM to OM are gradual without distinct zonation.7 At the cellular level, IM is reportedly composed of undifferentiated SMC phenotypes compared with terminally differentiated SMCs in OM.9
Physiology
The IM, which contains fewer contractile elements than the OM, displays cycle-dependent directional (cervix←→fundus) contractions of significantly lower amplitude than in OM.10 The orientation, amplitude, and frequency of IM contractions vary throughout the menstrual cycle. During the follicular phase, retrograde contractions (cervix→fundus) can facilitate sperm transport and promote pregnancy.11 During menses, antegrade-propagated IM contractions (fundus→cervix), as well as increased amplitude of peristalsis, promote desquamation of shed endometrium.12,13 The contractile frequency and amplitude of the IM decreases markedly in the midluteal phase, teleologically not to interfere with embryo nidation.10 The IM is well endowed with estrogen receptors (ERs) and progesterone receptors (PRs) and increases in thickness from the early proliferative to late secretory phases.14,15 Thus, the circular IM plays an important role in female reproduction under the regulation of steroid hormones. In contrast, although ER and PR are present in OM, cyclic changes in sex steroid receptor expression is not as robust as in IM. OM is the major contractile tissue during expulsion of the fetus15,16 under regulation of oxytocin and steroid hormones.17
Theories on the Mechanisms of Adenomyosis
In 1925, Frankl coined the word “adenomyosis”18; and in 1927 Sampson coined the term “peritoneal endometriosis.”19 They noted extraendometrial locations of endometrial glands and stromal cells in the myometrium (adenomyosis) and pelvic cavity (endometriosis), giving rise to the nomenclature of adenomyosis as “endometriosis interna” (i.e., internal to the uterine corpus) and endometriosis as “endometriosis externa” (outside the uterus). Steroid hormone dependence and abnormal steroid hormone responses of these ectopic foci20 are shared in both disorders, and abnormalities in eutopic endometrium and the EMI21 are believed to predispose to both conditions. However, as we learn more about adenomyosis, it has become evident that the pathogenesis of the two disorders is distinct. Endometriosis derives from retrograde shedding of menstrual tissue and cells, invading pelvic structures, and eliciting neuroangiogenic and inflammatory responses, pelvic pain, and scarring, and also may derive from coelomic metaplasia and endometrial stem cells.20 This is in marked contrast to adenomyosis in which different mechanisms establishing disease have been identified, as described later. Remarkably, despite these disorders now considered distinct entities, the 2019 International Statistical Classification of Diseases and Related Health Problems (ICD), a medical classification list by the World Health Organization, cites the ICD10 code for adenomyosis as “endometriosis of the uterus” (www.icd10data.com/ICD10CM/Codes). Herein, we focus on mechanisms underlying adenomyosis pathogenesis, noting that while there are two main forms of adenomyosis (“diffuse” and focal [“adenomyoma”]), most studies do not distinguish between them in describing mechanisms underlying disease formation or pathophysiology (►Fig. 2).
Fig. 2. Theories on the mechanisms of adenomyosis.
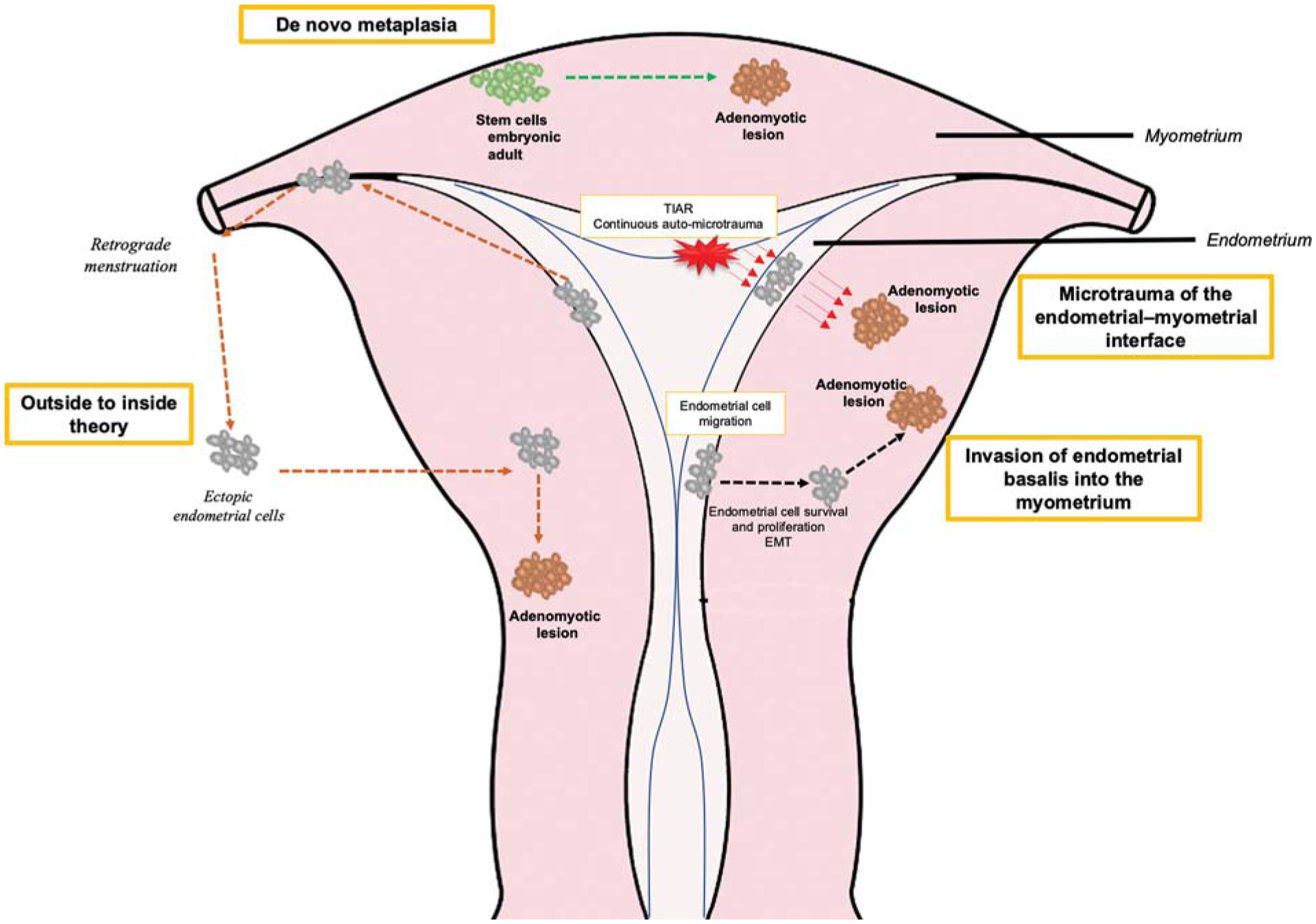
Four theories on the mechanisms of adenomyosis (see text): (1) Invasion of endometrial basalis into the myometrium. (2) Microtrauma of junctional zone induced by TIAR. (3) De novo metaplasia from stem cells. (4) Outside to inside invasion induced by the retrograde menstruation. EMT, epithelial-to-mesenchymal transition; TIAR, tissue injury and repair.
Invasion of Endometrial Basalis into the Myometrium
The invagination theory of adenomyosis development has been proposed to result from altered endometrial basalis cells or cell groups invading into the myometrium, crossing an injured or abnormal junctional zone, and subsequently establishing ectopic adenomyotic lesions and inducing hypotrophy and dysfunction of myocytes in the IM and OM.22 In support of this hypothesis are the clinical observations that adenomyosis risk increases with repeated sharp endometrial curettage, cesarean delivery, and prior uterine surgery wherein the EMI is breached23 (see Upson et al this issue). Studies demonstrating enhanced endometrial cell migration, proliferation, epithelial-to-mesenchymal transition (EMT), and resistance to apoptosis and dysregulation of extracellular matrix function in eutopic endometrium of women with adenomyosis compared with controls without disease support mechanistic underpinnings of the invagination theory and are presented later.
Endometrial Cell Migration
Eutopic endometrial stromal fibroblasts (eSFs) from adenomyosis women have a high invasive capacity in vitro compared with controls.24 Moreover, their invasion is augmented by myometrial myocytes from women with versus without disease, suggesting intrinsic abnormalities in the endometrial and myometrial compartments.24 If endometrial mesenchymal cells (e.g., eSF) migrate into the myometrium in vivo, the presence of endometrial epithelial cells (eECs) in this compartment may be due to mesenchymal-epithelial transition or stromal fibroblast-induced cellular transdifferentiation. Collective cell migration is a process of coordinated migration of groups of cells that maintain their cell-cell attachments and is encountered in physiological (wound healing) and pathological (cancer) conditions.25 A previous study suggested that collective cell migration is involved in the invasion process of deep endometriotic lesions in a baboon model,26 and it has been proposed that collective cell migration is a dominant process in invasion events in the late phase of adenomyosis.27 Thus, collective cell migration may also provide a pathway for the presence of eECs in the myometrium (►Fig. 2), although specific mechanistic underpinnings warrant further definition.
Proliferation and Cell Survival
It has been postulated that enhanced proliferation and cell survival of endometrial cells may, along with enhanced migratory properties, enable them to invade into the EMI (►Fig. 2).28 Compared with controls, endometrial tissue in women with adenomyosis displays rare apoptotic bodies, and eSFs are resistant to apoptosis and proliferate more rapidly in women with versus without disease.29 This is supported by bulk tissue transcriptomics revealing marked dysregulation of apoptosis and mammalian target of rapamycin (mTOR) pathways in endometrium of women with versus without adenomyosis.30 Molecular mechanisms underlying decreased apoptosis and increased proliferation likely derive from excessive estradiol (E2) in eutopic endometrium of women with adenomyosis (see later).28,31 Notably, similar proliferation and apoptosis dysfunctionalities are present in adenomyotic lesions.32 Adenomyotic eSFs in vivo display decreased caveolin (CAV1) expression, and CAV1-depleted eSF and eECs demonstrate significantly elevated proliferation, migration, and invasion.33 The phosphoinositide 3-kinases (PI3K)-Akt pathway has been proposed to mediate these processes, via Linc-ROR, a long noncoding RNA that is highly expressed in adenomyosis.34
Dysregulation of Extracellular Cell Matrix Function
A less rigid extracellular cell matrix (ECM) may facilitate endometrial cell migration and invasion into the myometrium and development of adenomyosis. Lysyl oxidase (LOX) is a copper-dependent amine oxidase that plays a critical role in the biogenesis of connective tissue matrices by cross-linking the ECM proteins collagen and elastin. LOX gene expression is highly downregulated in eutopic endometrium of women with versus without adenomyosis,30 consistent with a less rigid ECM. Moreover, matrix metalloproteinases (MMPs) MMP2, MMP3, and MMP9 are upregulated in eutopic endometrium from women with adenomyosis, compared with controls.35,36 Transcriptome sequencing of eutopic endometrium of women with adenomyosis also revealed dysfunction in connective tissues.37 Thus, the dysregulation of ECM function in the eutopic endometrium induced by MMPs, LOX, and junctional proteins may promote invagination of endometrium into myometrium, resulting in adenomyosis.
Epithelial-to-Mesenchymal Transition
While migration of mesenchymal cells (e.g., eSF) into the myometrium is plausible, how endometrial epithelial cells appear in the myometrial compartment is less clear. EMT, a process wherein epithelial cells acquire an invasive and metastatic phenotype, has been proposed for eEC migration into the myometrium38,39 (►Fig. 2). EMT is characterized by loss of E-cadherin and enhanced mesenchymal marker expression and involves β-catenin and other members of the Wnt pathway and the transcription factors such as snail, slug, twist, ZEB1, SIP1, and others. Notably, nuclear β-catenin protein is significantly elevated and E-cadherin expression is decreased, concomitantly with upregulation of N-cadherin and vimentin in eutopic and ectopic endometrium of adenomyosis, compared with normal endometrium.40 In a mouse model of adenomyosis, β-catenin activation induced snail and ZEB1 and inhibited E-cadherin expression, promoting EMT.40 Moreover, EMT of eutopic endometrium can be induced by increased expression and activation of neuropilin 1 and integrin-linked kinase.41,42 Collectively, these data suggest that EMT occurs in endometrial cells contributing to establishment of the disease in the myometrium.
A role for E2 in EMT is supported by the observation that circulating E2 levels correlate positively with vimentin-positive epithelial cells in adenomyosis lesions.38 Moreover, E2 induced morphological changes in Ishikawa cells (an endometrial adenocarcinoma cell line) in vitro from an epithelial to a fibroblast-like phenotype concomitantly with a shift from epithelial to mesenchymal marker expression, upregulation of the EMT regulator slug, and increased migration and invasion—processes that were blocked by the selective ER modulator raloxifene.38 Furthermore, platelet aggregation and activation have been considered as a possible cause of EMT in adenomyosis. In a mouse model of adenomyosis, increased platelet aggregation was observed involving transforming growth factor-β1 (TGF-β1), phospho-smad3, and vimentin.43 Also, ectopic endometrium of women with adenomyosis has significantly higher platelet aggregation and increased EMT markers, compared with eutopic endometrium of women without disease.32 Importantly, anti-platelet treatment is efficacious in suppressing myometrial infiltration by endometrial cells, improving generalized hyperalgesia and reducing uterine hyperactivity in adenomyosis,44 providing a promising nonhormonal treatment for the disease. Thus, platelet activation and high E2 levels may contribute to endometrial EMT in the development of adenomyosis.
Microtrauma of the Endometrial–Myometrial Interface
The disarray of myocyte distribution and irregularities of the nuclear membrane in the IM in the setting of adenomyosis suggest a contributory role of disruption of this compartment to the development of the disease.45 While multiparity is a potential risk factor for adenomyosis,27 nulliparous women without a history of uterine surgeries also develop adenomyosis. These observations together have led to the proposal that physical trauma and physiologic trauma (“microtrauma”) of the EMI contribute to the pathogenesis of adenomyosis.46 “Microtrauma” to the EMI is believed to result from continuous cyclic uterine peristaltic activity throughout a woman’s reproductive lifetime and E2 has been proposed to have dual actions in this process, as described later.46
Tissue Injury and Repair
Tissue injury initiates interleukin-1 (IL-1)-induced activation of cyclooxygenase-2 (COX-2), which results in the production of prostaglandin E2 (PGE2) and in turn activates steroidogenic acute regulatory protein (STAR) and P450 aromatase in the injured tissue.27 This process further increases formation and aromatization of testosterone, resulting in a hyperestrogenic environment that promotes healing via ERβ∙47 Thus, in normal tissue, E2 can be synthesized and promote healing after tissue injury by a process called “tissue injury and repair” (TIAR; ►Fig. 2).
Continuous Auto-microtrauma
It has been suggested that endometrial E2 plays a central role in uterine hyperperistalsis and microtrauma following TIAR.48,49 The peristaltic activity of the subendometrial myometrium (IM) dramatically increases with elevated peripheral E2 levels during controlled ovarian stimulation.49,50 Also, increased local E2 in eutopic endometrium stimulates uterine peristalsis in the EMI through ERα and oxytocin, which impose supraphysiological mechanical strain on myocytes near the fundo-cornual raphe.51 Repeated and sustained overstretching of myocytes and fibroblasts has been proposed to induce micro-traumatization of the EMI. This microtrauma is proposed to activate the TIAR process and promote production of PGE2 (though IL-1-induced COX-2 expression), which then facilitates local production of E2 through STAR and P450 aromatase. While increased E2 promotes healing through ERβ, it also promotes oxytocin-mediated hyperperistalsis through ERα,50,52 which inhibits the healing process and augments further microtrauma in the EMI. Thus, a positive feed forward mechanism is generated by which chronic hyperperistalsis promotes repeated cycles of auto-traumatization and disruption of the EMI, potentially augmenting invagination of the endometrial basalis into the myometrium and eventually leading to establishment of adenomyotic lesions (►Fig. 2). In addition, iatrogenic injuries can promote a similar TIAR, local production of E2, and hyperperistalsis facilitating invasion of the endometrial basalis into the myometrium, inducing adenomyosis lesions. Further functional studies are warranted to validate this theory.
Adenomyosis is also characterized by hyperplasia of myometrial cells surrounding endometrial stroma and glands, with evidence of involvement of growth factors in its pathogenesis. Myostatin, follistatin, and activin A expression are increased in adenomyotic nodules, supporting the increased proliferative activity of myocytes.53 Activin-related proteins are also key regulators of tissue remodelling and repair. Upregulation of these molecules in adenomyotic tissue may be related to the myometrial response to ectopic endometrial cell invasion, supporting the invagination theory.54
De Novo Metaplasia Theory
While several studies favor the endometrial invagination theory of adenomyosis, there is evidence that other mechanisms may be operational. For example, adenomyosis lesions were reported in myometrium of Rokitansky-Kuster-Hauser syndrome patient, who lacked functional endometrium.55,56 Alternative hypotheses propose that ectopic endometrium derives by metaplasia de novo of embryonic epithelial progenitors (remnants) or differentiation of adult endometrial stem cells that transit to the myometrium.
Embryonic Stem Cells (Mullerian Duct)
It has been postulated that during Mullerian duct development and fusion, some remnants of the embryonic tissue may be misplaced in the myometrium, subsequently giving rise in adult women to adenomyosis.27,57 Thus, embryonic Mullerian remnants may undergo metaplastic changes in adult myometrium, leading to establishing de novo ectopic endometrial tissue (►Fig. 2).
Adult Stem Cells
Endometrial epithelial progenitor and mesenchymal stem cells (eMSCs) have been identified in the endometrium and can differentiate into the eECs and eSF, respectively.58–60 Notably, eSFs cultured from ectopic endometrium of adenomyosis patients differentiate down mesenchymal lineages and express MSCs surface markers.61 In contrast, most epithelial cells and a proportion of stromal cells in adenomyotic lesions express Musashi-1 + , an adult stem cell marker.62 Thus, adenomyotic lesions contain several adult stem cells that have the potential to differentiate into functional endometrium. Importantly, recent somatic mutation analyses demonstrate that epithelial cells in adenomyotic lesions and in their associated eutopic endometrium derive from the same endometrial epithelial progenitor cell.63 Moreover, Gargett demonstrated that abnormal differentiation of eMSCs can promote myometrial smooth muscle hyperplasia.64 Thus, adult stem cells residing in the endometrium basalis, adjacent to the myometrium, may cross the EMI and proliferate and differentiate into adenomyosis lesions and promote myometrial SMC hyperplasia and hypertrophy that are pathognomonic of the disease.65–67 An alternative hypothesis involves stem-like “pale cells” in basalis endometrium that actively migrate and possibly transit through the EMI into the myometrium, resulting in adenomyotic foci.45
Several mechanisms that may induce dislocation of endometrial adult stem cells into the myometrium have been proposed. For example, tissue injury typically activates adult stem cells,68 and thus, hyperestrogenism, peristalsis, and TIAR-induced microtrauma in the EMI could disrupt endometrial stem/progenitor cell niches and promote their invasion into the myometrium. Moreover, this migration process may be enhanced by elevated expression of MMP-2 and −9 in eSF, disrupting the normal ECM and facilitating migration of stem cells into the myometrial compartment.36,69 Understanding triggers and mechanisms underlying differentiation of stem and progenitor cells purportedly participating in adenomyosis formation is greatly needed.
Outside to Inside Invasion
In addition to invasion of stem cells from endometrial basalis directly into the myometrium, retrograde menstruation may also result in deposition of adult stem cells into the myometrium. Perivascular eMSCs reside in both the endometrial basalis and functionalis layers,70–72 and they are components of menstrual blood.70,73 Chapron et al proposed the “from outside to inside invasion” theory in which adult endometrial cells (or stem cells) in retrograde menstrual effluent have the potential to infiltrate the uterine serosa and penetrate into the OM and develop into intramyometrial endometrial implants (i.e., adenomyotic foci27,74,75; ►Fig. 2). This theory is supported by the high concurrence of posterior focal adenomyosis of the OM and deep infiltrating endometriosis nodules in the posterior compartment in endometriosis/adenomyosis patients.75
Pathogenesis of Adenomyosis
Genetics
Genetic variants involving key processes in adenomyosis development include steroid hormone function, ECM dysregulation, angiogenesis, TIAR, and inflammation (►Fig. 3).
Fig. 3. Pathogenesis of adenomyosis.
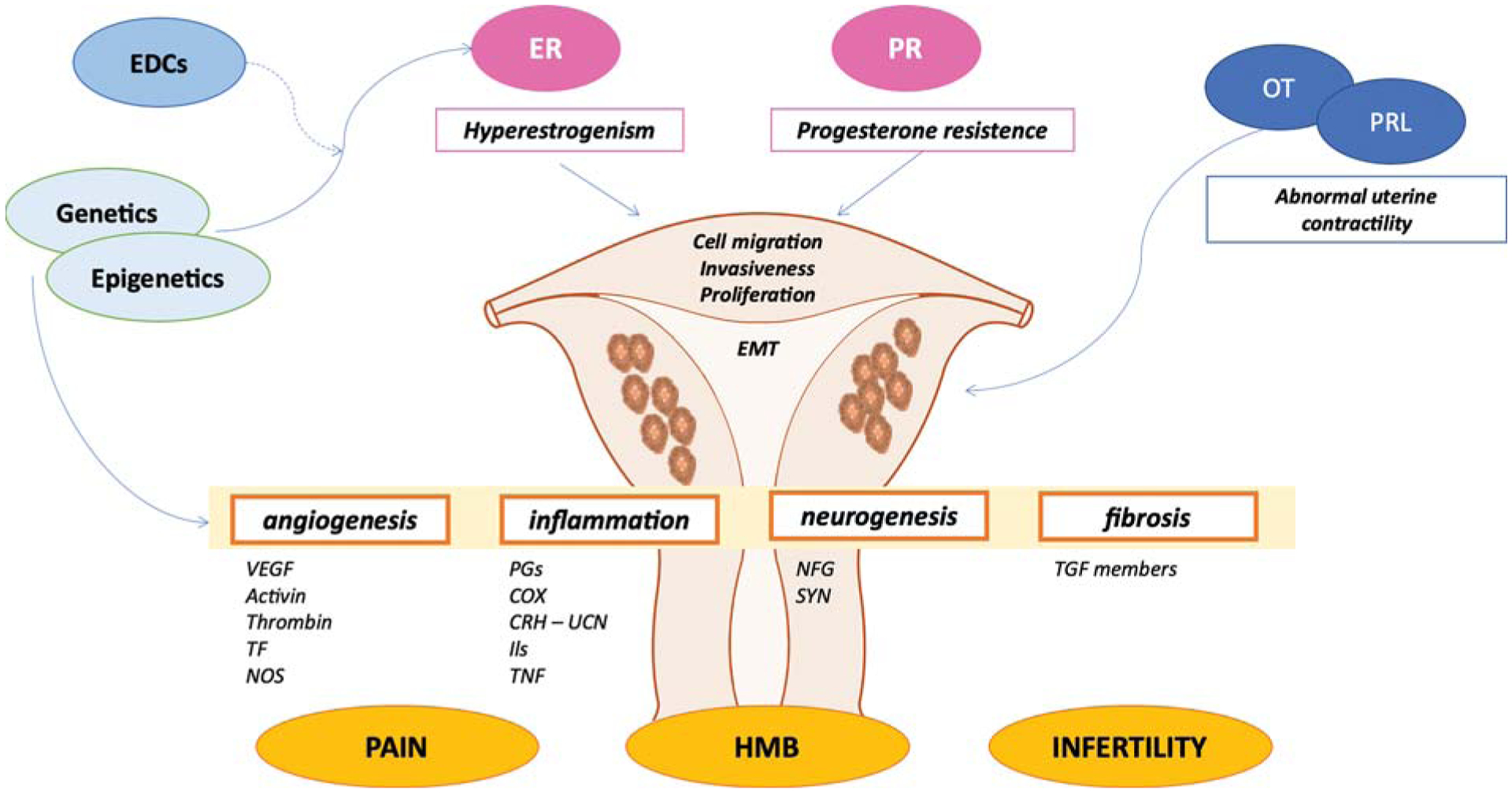
Abnormal genetic and epigenetic factors result in the hyperestrogenism and progesterone resistance, promoting cell proliferation, migration, EMT, and invasiveness of endometrial cellular components into the myometrial compartment. Moreover, genetics and epigenetics modifications also contribute to the development of pelvic pain, infertility, and HMB experienced by women with adenomyosis through different mediators. In addition, pituitary hormones such as PRL and OT also play a role in the pathogenesis of adenomyosis through abnormal uterine contractility. EDCs, endocrine disrupting chemicals; EMT, epithelial-to-mesenchymal transition; ER, estrogen receptor; HMB, heavy menstrual bleeding; OT, oxytocin; PR, progesterone receptor; PRL, prolactin.
CYP1A1 and 1A2 catalyze the formation of 2-hydroxyestrone—a metabolite that has weak estrogenic activity and does not promote proliferative effects, while CYP19 aromatase converts androstenedione and testosterone into estrone (E1) and E2, respectively, through aromatization.76,77 Moreover, catechol-O-methyltransferase (COMT) is also involved in estrogen metabolism. Many studies have found that CYP genes and COMT gene variants could influence the enzyme activity and increase the risk of estrogen-dependent diseases, including adenomyosis.78 Notably, patients with adenomyosis have increased frequency of the C allele in the T/C and C/C genotypes of the CYP1A1 gene, A allele in the C/A and A/A genotypes of the CYP1A2 gene, and the T allele and C/T and C/C genotypes of the CYP19 gene compared with women without adenomyosis.79 Additionally, COMT 158 G/A gene polymorphisms contribute to the high risk of developing adenomyosis, particularly in Asian populations.80 These results further demonstrate the role of polymorphisms of estrogen metabolism genes in adenomyosis. Additionally, ER and PR gene polymorphisms were also detected in adenomyosis patients. The transmembrane G protein-coupled receptor (GPR30) has been reported to bind E2 with high affinity, 10 times higher than ERα,81 and the C allele of the SNP rs4266553 of GPR30 displays higher frequency in women with adenomyosis than in women without adenomyosis.82 Several studies have also reported that PVUII polymorphism of the ERα gene and PR gene polymorphic allele +331A are associated with a reduced risk of deep infiltrating endometriosis and adenomyosis.83,84 Hence, the metabolism and functions of steroid hormones and their receptors regulated by genetic polymorphisms appear to be involved in the pathogenesis of adenomyosis.
Meta-analyses have also suggested association of MMP-1-1607 1G/2Gs polymorphism and MMP-2 21306C/T polymorphism with high risk of adenomyosis, supporting a role for ECM dysfunction in adenomyosis development.85,86 Fibroblast growth factors (FGF) 1 and 2 play vital roles in angiogenesis, and FGF2 754C/G polymorphism correlates with a high risk of developing adenomyosis in northern Chinese women.87 Furthermore, vascular endothelial growth factor (VEGF) gene polymorphism is also associated with susceptibility to adenomyosis.88,89 As discussed earlier, COX-2 is key in TIAR and the microtrauma positive feedforward loop in the pathogenesis of adenomyosis. Importantly, genetic variation of G to A at −1195 locus in the promoter region of COX-2 gene has been shown to increase the risk of adenomyosis.90 Thus, current evidence supports genetics as a driver in the pathogenesis of adenomyosis through alterations in gene functions governing steroid hormone function, ECM dysregulation, angiogenesis, TIAR, and inflammatory mediators.
Epigenetics
Epigenetic alterations have been detected in adenomyosis. Deoxyribonucleic acid methyltransferases (DNMTs) comprise a family of enzymes that catalyze transfer of a methyl group to DNA. DNMT1 and DNMT3B are significantly increased in ectopic endometrium, while DNMT3A levels are reduced in eutopic and ectopic endometrium of women with adenomyosis.91 Furthermore, promoter hypermethylation of PRB, and silencing of PRB expression, was detected in eutopic endometrium of women with adenomyosis,92 resulting in progesterone resistance. DNA hypomethylation and increased expression of CCAAT/enhancer-binding protein β (CEBPB), a transcription factor regulating gene expression to control cellular proliferation, differentiation, and metabolism, were also associated with the incidence of adenomyosis.37
Besides DNA methylation, aberrant expression and localization of class I histone deacetylases (HDACs) in endometrium of women with adenomyosis have also been demonstrated. Compared with normal endometrium, expression of HDAC1 and HDAC3 was higher in both eutopic and ectopic endometrium of women with adenomyosis, while the use of valproic acid, a histone deacetylase inhibitor reportedly, is effective in treating refractory disease.93 These findings suggest potential involvement of histone acetylation in the pathogenesis of adenomyosis and possible targets for therapies.92,94
RNA methylation, especially N6-methyladenosine (m6A) and its regulators, plays important roles in regulating some physiological processes and invasive disorders. Our recent data-mining and laboratory-based study of gene expression and the interactome of m6A RNA methylation regulators (methyltransferase‐like 3 [METTL3], a writer promoting methylation of RNA95) and total m6A levels demonstrated that METTL3 and total m6A levels in eutopic endometrium of adenomyosis patients were decreased compared with controls. Moreover, in myometrium, m6A RNA methylation-clustered regulators involved in cell adhesion, muscle contraction, and immune response also differed in women with and without disease. Thus, m6A RNA methylation regulators may be involved in the pathogenesis of adenomyosis through aberrant expression and actions in both the uterine endometrium and myometrium.96
Ovarian and Local Steroid Hormones Aberration
Hyperestrogenism and Progesterone Resistance
That adenomyosis as an estrogen-dependent disease is based on multiple observations.27 For example, in a mouse model, prolonged treatment with estrogen results in inducing adenomyosis.97 In women, elevated E2 levels in menstrual blood of those with adenomyosis without concomitant elevations in peripheral blood, compared with controls, suggest that local rather than systemic hyperestrogenism contributes to the development of the disease.98 Moreover, aromatase cytochrome P450, a key enzyme in synthesis of E2, is expressed in eutopic endometrium of women with adenomyosis.99 And lower expression of 17-β hydroxysteroid dehydrogenase type 2 (17-β HSD2), which converts E2 to the less potent estrogen, E1, has been reported in the eutopic and ectopic endometrium of adenomyosis patients, compared with controls.100 Thus, increased biosynthesis and decreased conversion of E2 contribute to a local hyperestrogenic milieu, suggesting a key role for E2 in the development of disease.101
Uterine ER and PR expression of adenomyosis patients differ from normal controls. Specifically, ERα is decreased in eutopic endometrium of adenomyosis patients during the middle secretary phase compared with controls, but is equivalently expressed in the inner and outer myometrial layers. ERβ expression is significantly elevated in adenomyotic lesions during the proliferative phase and throughout the myometrium across the menstrual cycle. Because of reduced expression of PR (PRA and PRB) in the basalis stroma and IM and OM in adenomyosis, resistance to progesterone has been suggested in adenomyosis, as in endometriosis,102 which may have therapeutic implications.
Thus, elevated local E2 concentration and ER receptor expression and decreased PR expression are central to adenomyosis pathogenesis. This can be manifested in high local E2-induced hyperperistalsis and microtrauma of the junctional zone (first step injury), initiating TIAR and promoting a positive feed-forward (second step injury) cycle that facilitates the microtrauma of the junctional zone and invasion of the endometrial basalis into the myometrium, as discussed earlier. Moreover, hyperestrogenism and progesterone resistance can result in increased proliferation of eutopic endometrial cells, and E2 induces hyperproliferation of SMCs in the junctional zone and myometrium—common features of adenomyosis.103 E2 induces a shift of epithelial to mesenchymal markers and increases endometrial cell migration and invasion, thereby facilitating implantation of ectopic endometrium into the myometrium, as described earlier.38 Elevated expression of annexinA2 (ANXA2) has been found in adenomyosis versus normal endometrium and is E2-regulated. It promotes phenotypic mesenchymal-like cellular changes, mediated by β-catenin/T cell factor signaling and angiogenesis.54
In conclusion, hyperestrogenism and progesterone resistance of local endometrium likely contribute to the EMT process, enhanced endometrial and myometrial smooth muscle proliferation, endometrium angiogenesis, and micro-trauma of the junctional zone—playing key roles in the pathogenesis of adenomyosis (►Fig. 3).
The Role of Pituitary Hormones
Prolactin
A remarkable experiment in mice nearly 40 years ago revealed that ectopic pituitary transplantation induced uterine adenomyosis.104 Subsequent studies showed that infusion of prolactin (PRL) or administration of dopamine antagonists causing hyperprolactinemia triggered adenomyosis in a mouse model.105 Moreover, PRL receptor (PRLR) mRNA is overexpressed in uteri of mice with versus without adenomyosis,106 and spontaneous adenomyosis with advancing age in female mice was prevented by the dopamine agonist bromocriptine.107 Similar results were found in a bovine model.108 In humans, higher levels of PRL have been reported in women with than without adenomyosis,109 and vaginal administration of bromocriptine significantly decreased menstrual bleeding and pain and improved quality of life in women with disease.110
Mechanisms underlying elevated PRL and PRLR in the pathogenesis of adenomyosis are still unclear. In the normal bovine uterus, endometrial cells are the main source of PRL, whereas in the setting of adenomyosis, it is intensely synthesized by myometrial cells.108 Moreover, PRL is a smooth muscle mitogen in vitro,111 suggesting that uterine myometrium may be the main uterine tissue compartment that is affected by PRL during initiation and progression of adenomyosis. Mori et al proposed that PRL directly affects the myometrium and leads to the degeneration of SMCs and induces adenomyosis.112 In women, whether endometrial prolactin secreted during stromal fibroblast decidualization contributes to adenomyosis pathogenesis seems unlikely, as progesterone resistance may limit decidualization in eutopic endometrium of women with disease. Overall, local more than systemic PRL may participate in the pathogenesis and pathophysiology of adenomyosis with specific mechanisms awaiting further delineation.
Oxytocin
As described earlier, oxytocin (OT) plays a major role in microtrauma of the EMI theory through E2-mediated IM contractions and hyperperistalsis. Oxytocin receptor (OTR) is expressed in normal endometrial and myometrial cells in both IM and OM of the human uterus and varies by cycle phase, suggesting it may be involved in endometrial functions under steroid hormone regulation.113 Myometrial OTR is essential for myometrial contraction and microtrauma of the EMI. Expression of myometrial OTR positively correlates with severity of adenomyosis and is significantly higher in the secretory phase in the setting of adenomyosis.114 In the mouse model of disease, tamoxifen induced OTR mRNA expression.115 In the fundal region, significantly higher expression of OTR in EMI of adenomyosis uteri was detected, compared with controls in the proliferative and secretory phases, and a prior study also showed that OTR expression correlated positively with contractile amplitude in myometrial SMCs.114 Thus, overexpression of fundal myometrial OTR in adenomyosis uteri can account for the hyperperistalsis and dysperistalsis of EMI contractions, further inducing microtrauma and development of adenomyosis. Moreover, in contrast to controls, expression of OTR in the isthmic region was significantly lower than in the fundal region in the proliferative phase of women with adenomyosis. The opposite expression pattern of OTR in isthmic and fundal regions may disturb the direction of the EMI contractions, potentially interfering with sperm transport and fertility. Thus, the myometrial OTR system, regulated by E2, and PRL appear to play roles in the pathogenesis of adenomyosis (►Fig. 3). Targeting local PRL or OTRs may have promise for disease management.
Endocrine Disrupting Chemicals
Animal models have made major contributions to understanding the roles of estrogen-mimetic endocrine disrupting chemicals (EDCs) in promoting the development of adenomyosis. For example, mice exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) developed deep adenomyosis, whereas control mice did not develop the disease. Moreover, TCDD-exposed mice had significantly reduced ERα:ERβ ratio, compared with controls. The authors proposed that epigenetic alterations affecting steroid-sensitive immune-endocrine crosstalk within the uterus is responsible for the development of adenomyosis after TCDD exposure,116,117 although precise mechanisms remain to be determined. Moreover, exposure to the estrogen mimetics bisphenol A and diethylstilbestrol during fetal development and in the neonatal period resulted in increased incidence of adenomyosis in mice.118,119 Effects of EDCs in the context of genetics have also been investigated. Phthalates are EDCs that have antiandrogenic effects, and glutathione S-transferase M1 (GSTM1) is a major phthalate detoxification enzyme. Notably, GSTM1 null genotype and phthalate exposure are related to the risk of adenomyosis,120 suggesting gene-environment interactions of these EDCs and genotype polymorphism, giving rise to the development of adenomyosis (►Fig. 3). Additional studies are warranted as preventive strategies could be envisioned.
Immune Disorder and Inflammatory Mediators
Immune Response
In adenomyosis, a series of immune responses is activated, including changes in both cellular and humoral immunity, particularly in endometrium and involving expression of human leukocyte (cell surface) antigens (HLAs) and increased numbers of macrophages and other immune cells in adenomyosis.121 HLAs are key regulators of the immune system, and elevated expression of HLA class II antigens in eutopic and ectopic endometrium of adenomyosis patients has been reported.122 Also, HLA-G is uniquely expressed in both eutopic and ectopic endometrium of adenomyosis women, without expression in control endometrium.123 Thus, it has been proposed that expression of these specific HLA classes in the endometrium of adenomyosis women may activate the autoimmune system, resulting in a dysregulated immune response.121
Increased numbers of macrophages and gamma-delta T cells (γδT cells) in eutopic and ectopic endometrium in women with adenomyosis have been reported in one121 but not another study. Moreover, no differences in eutopic endometrial T cells, interferon (IFN)-γ, and HLA-DR-positive cells were found, compared with unaffected controls.124 Interestingly, circulating B-cell-derived autoantibodies, mainly against phospholipids, have been detected in adenomyosis patients,125 which decrease markedly in women after hysterectomy for adenomyosis versus controls, suggesting an antigen-antibody response involving uterine antigens in adenomyosis.121 Further studies are needed to understand these observations and their possible physiologic relevance.
In summary, aberrant expression of HLA molecules, altered activation of T cells, B cells, and macrophages in eutopic and ectopic endometrium have been proposed to be involved in immunological responses contributing to the pathogenesis and perhaps the pathophysiology of adenomyosis.
Inflammatory Mediators
In 1925, Frankl used the term “adenomyosis uteri” because “it does not suggest any inflammatory origin” to describe adenomyosis in the myometrium.18 Much has changed over the past nearly 100 years, and now there is considerable evidence that adenomyosis is indeed associated with expression of uterine inflammatory mediators and cytokines. Increased expression of IL-1, IL-18, and tumor necrosis factor-α,β (TNF-α,β) and altered expression of COX-2 in the endometrium of adenomyosis uteri demonstrate involvement of inflammatory pathways in the pathogenesis and pathophysiology of adenomyosis35,126,127 and are summarized later and in ►Fig. 3.
Both eutopic endometrium and adenomyotic nodules highly express IL-1β and corticotropin-releasing hormone (CRH),128 key inflammatory mediators in the development of endometriosis.129–132 Elevated eutopic endometrial IL-18R, the receptor of IL-18, in women with adenomyosis versus controls further implicates a role of chronic inflammation in developing adenomyotic lesions.133,134 Increased expression of nuclear factor kappa light-chain enhancer of activated B cells (NF-κB) and p65 subunit have been reported in eutopic endometrium and adenomyotic nodules of adenomyosis patients.127 Moreover, andrographolide, a novel NF-κB inhibitor, suppressed myometrial infiltration, suppressed uterine contractions, and improved hyperalgesia in a mouse model of adenomyosis,135 supporting the hypothesis that altered expression of NF-κB endometrium plays a role in the pathogenesis of adenomyosis. Also, lipopolysaccharide (LPS)-induced toll-like receptor (TLR4) signaling activation facilitated proliferation and invasion of stromal cells and amplified a local inflammatory response through various growth factors,136 leading to the development of adenomyosis.31 Additionally, partially effectiveness of nonsteroidal anti-inflammatory drugs, nonhormonal compounds commonly used to treat symptomatic adenomyosis, further underscores a role for inflammatory mediators in the disorder.137
Thus, numerous inflammatory mediators are aberrantly expressed in the endometrium of adenomyosis patients, setting up a network to promote inflammation in which increased TNF-α promotes secretion of IL-1β,138 activation of NF-κB pathway,139 and engagement of IL-18/IL-18R complex.140 High expression of CRH in adenomyosis correlates with increased COX-2 and PGE2 synthesis,141 which can facilitate the development of the disease.
Neuroangiogenesis and Fibrosis
Neuroangiogenesis plays a major role in the pathogenesis and pathophysiology of adenomyosis, as a relevant contributing factor to pain and HMB (►Fig. 3). Anatomically, a subserosal plexus innervates the myometrium and the endometrial-myometrial junction.142 In adenomyotic lesions, higher expression of neurogenic factors, such as nerve growth factors (NGFs), synaptophysin (SYN), and microtubule-associated protein 2 (MAP2), has been observed compared with controls. Besides, NGF expression is induced by urocortin in cultured human endometrial stromal cells, providing evidence to support a link between inflammatory and neurogenic pathways.143 Sensory unmyelinated C nerve fibers in the functional layer of the endometrium may be sensitized by inflammatory mediators, such as PGE2 and prostacyclin, causing neurogenic inflammation and thus pain.144 In symptomatic adenomyosis, the presence of PGP9.5-positive nerve fibers in the functional layer of the endometrium was reported.145,146 Additionally, neurofilament (NF) protein-positive cells were detected in the endometrium and myometrium of women with symptomatic adenomyosis.147 In a mouse model, NGF-β and its receptor levels increase as the disease worsens in terms of pain symptoms.148 In addition, mice with induced adenomyosis have a decreased number of glutamate decarboxylase (GAD)-65-expressing neurons with resulting loss of GABAergic inhibition and hyperalgesia.149
The glycoprotein neural cell adhesion molecule, also known as CD56, is highly expressed in endometrial glandular epithelium in adenomyosis uteri and stimulates nerve growth in the stroma, supporting the neurogenic origin of adenomyosis pain.150 CD56, and the slit-robo system,151 and other neurotrophic factors found in adenomyosis lesions increase neurogenic inflammation and sensitivity of nociceptors in myometrium, triggering pelvic pain.
In adenomyosis, along with neurogenesis, increased angiogenesis is observed.152,153 Overexpression of VEGF, one of the most potent angiogenic factors known, which enhances vascular sprouting and augments vascular permeability,154 has been shown in ectopic and eutopic endometrium of adenomyotic uteri.32,155 Also, follistatin and activin A, members of TGF-β family, act as a proangiogenic factors in adenomyosis.53 In adenomyosis, a higher microvessel density (MVD) is observed in eutopic endometrium,156,157 with an increased mean and total surface area of capillaries.158
Adenomyosis is also characterized by a certain degree of fibrosis, as a result of repeated cycles of TIAR. TGF-β signaling plays a major role in EMT and collagen production, leading to smooth muscle metaplasia (SMM) and ultimately to fibrosis.32
Mechanisms Underlying Pain, HMB, and Infertility
The main symptoms of women with adenomyosis are pain, HMB, infertility, and miscarriage68 (►Fig. 3). Dysmenorrhea and dyspareunia can be explained by myometrial hypercontractility, suggested by higher expression of OTRs and increased contractile amplitude of uterine smooth muscle cells (uSMCs) in adenomyotic uteri.114,159 Altered membrane depolarization of uSMCs, due to dysfunction of potassium channels, cooperates to determine abnormal uterine contractility inadenomyosis.160 Overexpression of OTR in adenomyosis-surrounding myometrium coupled with vasopressin receptor (VP1αR) expression in blood vessels and myometrium161 contributes to altered microcirculation.162 Inflammatory factors described earlier also play a role in pain symptoms. The high expression of IL-1β, CRH, and UCN found in adenomyotic lesions143 may mediate prostaglandins synthesis. Adenomyosis-induced pain has also a relevant neuropathic nature, and all the data presented in the pathogenesis support this hypothesis. Increased expression of NGF, SYN, and NF protein may explain the high uterine pain perception.143,147,163 Furthermore, in human clinical studies, women with painful symptoms have a higher density of PGP9.5- and NF-positive nerve fibers than those with asymptomatic adenomyosis.164 The severity of dysmenorrhea also correlates with microvessel density (MVD) and slit-robo system immunoreactivity, a neuronal factor, in the eutopic endometrium.151
Mechanisms underlying HMB in adenomyosis include neoangiogenesis, abnormal uterine contractility, and high MVD. Events leading to increased proangiogenic factors expression, such as VEGF, are triggered by TIAR, hypoxia, and hormonal dysfunction.153 Tissue factor is a key glycoprotein of the clotting cascade, and it is increased in adenomyosis, along with VEGF, and correlating with the amount of menstrual bleeding.165 Furthermore, in women with adenomyosis-related HMB, the endometrium and myometrium present higher expression of endothelial nitric oxide synthase, supporting the role of NO in regulating the amount of menstrual bleeding.40
Infertility and poor implantation outcomes are common findings is women with adenomyosis, purportedly due to an altered uterine environment, abnormal uterine contractility, increased inflammation, and abnormal eutopic endometrial function.21,166 Poor uterine receptivity is supported by a dysregulation of immune factors, apoptosis, and cell proliferation, and an increase in inflammatory mediators and oxidative stress. Several implantation-associated factors, such as HOXA, leukemia inhibitory factor, and MMP2, are dysregulated, impairing decidualization.167,168 Endometrial inflammation is mediated by CRH and IL-1β,143 and reactive oxygen species released by immune cells, together with altered expression of prooxidant and antioxidant enzymes.121,169 Furthermore, decreased expression of integrin family proteins, cell adhesion receptors, and ECM enzymes, such as osteopontin or integrin β3,167 reduces uterine receptivity to embryo implantation. Since adenomyosis likely originates from an invasion of endometrial cells into the myometrium, a consequent activation of a cascade of events, including local effects of sex steroid and pituitary hormones, the role of immune and inflammatory factors, and neuro- and angiogenic mediators, determine the clinical presentation of pelvic pain, HMB, and infertility (►Fig. 3).
Summary and Conclusions
The bulk of evidence supports that adenomyosis is an estrogen-dependent gynecological disorder resulting from one of several mechanisms: invasion of the endometrial basalis into the myometrium induced by enhanced cell survival, EMT, and cell migration; continuous auto-microtrauma of the junctional zone; de novo metaplasia from adult stem cells and embryonic Mullerian remnants; and “from outside to inside invasion.” There are also genetic, epigenomic, environmental, and pituitary triggers of the disease. In addition to a central role for local hyperestrogenism, progesterone resistance, and inflammation in the pathogenesis of adenomyosis, these factors along with abnormal uterine contractility, neurogenesis, and neoangiogenesis are key pathogenic mediators of the pain, HMB, and infertility experienced by women with adenomyosis. Two other common disorders of the uterus, endometriosis, and uterine fibroids are also estrogen dependent and, like adenomyosis, have accompanying symptoms of dysmenorrhea, pelvic pain, abnormal uterine bleeding, and infertility, and they often coexist (18% of women with adenomyosis had concurrent endometriosis and 47% had uterine fibroids, based on hospital codes170). Despite these similarities, the underlying pathogenesis, pathophysiology, risk factors, and therapies for these conditions mostly differ and warrant further comparative study. Prioritization of further molecular, genetic, clinical, and epidemiologic investigation on adenomyosis is warranted, as the impact on quality of life is enormous in those affected. Integrating current knowledge and advancing in new directions with novel models, novel approaches, new technologies, and big data are essential to understand the pathogenesis, pathophysiology, and determinants of disease risk of this disorder. A major paradigm shift needs to occur to develop targeted therapies to mitigate symptoms that are poorly regulated by current treatments and also for preventive strategies and the possibility of cure.
Funding
This review was supported by the NIH Eunice Kennedy Shriver National Institute for Child Health and Human Development (P50 HD055764-12 [LCG]).
Footnotes
Conflict of Interest
None declared.
References
- 1.Brosens JJ, de Souza NM, Barker FG. Uterine junctional zone: function and disease. Lancet 1995;346(8974):558–560 [DOI] [PubMed] [Google Scholar]
- 2.Naftalin J, Jurkovic D. The endometrial-myometrial junction: a fresh look at a busy crossing. Ultrasound Obstet Gynecol 2009;34(01):1–11 [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Zhou L, Li TC, Duan H, Yu P, Wang HY. Ultrastructural features of endometrial-myometrial interface and its alteration in adenomyosis. Int J Clin Exp Pathol 2014;7(04):1469–1477 [PMC free article] [PubMed] [Google Scholar]
- 4.Uduwela AS, Perera MA, Aiqing L, Fraser IS. Endometrial-myometrial interface: relationship to adenomyosis and changes in pregnancy. Obstet Gynecol Surv 2000;55(06):390–400 [DOI] [PubMed] [Google Scholar]
- 5.Hricak H, Alpers C, Crooks LE, Sheldon PE. Magnetic resonance imaging of the female pelvis: initial experience. AJR Am J Roentgenol 1983;141(06):1119–1128 [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen CK, Hansen ES, Dueholm M. Two- and three-dimensional ultrasonographic features related to histopathology of the uterine endometrial-myometrial junctional zone. Acta Obstet Gynecol Scand 2019;98(02):205–214 [DOI] [PubMed] [Google Scholar]
- 7.Mehasseb MK, Bell SC, Brown L, Pringle JH, Habiba M. Phenotypic characterisation of the inner and outer myometrium in normal and adenomyotic uteri. Gynecol Obstet Invest 2011;71(04): 217–224 [DOI] [PubMed] [Google Scholar]
- 8.Tetlow RL, Richmond I, Manton DJ, Greenman J, Turnbull LW, Killick SR. Histological analysis of the uterine junctional zone as seen by transvaginal ultrasound. Ultrasound Obstet Gynecol 1999;14(03):188–193 [DOI] [PubMed] [Google Scholar]
- 9.Kishi Y, Shimada K, Fujii T, et al. Phenotypic characterization of adenomyosis occurring at the inner and outer myometrium. PLoS One 2017;12(12):e0189522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ijland MM, Evers JL, Dunselman GA, van Katwijk C, Lo CR, Hoogland HJ. Endometrial wavelike movements during the menstrual cycle. Fertil Steril 1996;65(04):746–749 [DOI] [PubMed] [Google Scholar]
- 11.Kunz G, Beil D, Deininger H, Wildt L, Leyendecker G. The dynamics of rapid sperm transport through the female genital tract: evidence from vaginal sonography of uterine peristalsis and hysterosalpingoscintigraphy. Hum Reprod 1996;11(03): 627–632 [DOI] [PubMed] [Google Scholar]
- 12.de Vries K, Lyons EA, Ballard G, Levi CS, Lindsay DJ. Contractions of the inner third of the myometrium. Am J Obstet Gynecol 1990; 162(03):679–682 [DOI] [PubMed] [Google Scholar]
- 13.Lyons EA, Taylor PJ, Zheng XH, Ballard G, Levi CS, Kredentser JV. Characterization of subendometrial myometrial contractions throughout the menstrual cycle in normal fertile women. Fertil Steril 1991;55(04):771–774 [DOI] [PubMed] [Google Scholar]
- 14.Hoad CL, Raine-Fenning NJ, Fulford J, Campbell BK, Johnson IR, Gowland PA. Uterine tissue development in healthy women during the normal menstrual cycle and investigations with magnetic resonance imaging. Am J Obstet Gynecol 2005;192(02):648–654 [DOI] [PubMed] [Google Scholar]
- 15.Noe M, Kunz G, Herbertz M, Mall G, Leyendecker G. The cyclic pattern of the immunocytochemical expression of oestrogen and progesterone receptors in human myometrial and endometrial layers: characterization of the endometrial-subendometrial unit. Hum Reprod 1999;14(01):190–197 [DOI] [PubMed] [Google Scholar]
- 16.Aguilar HN, Mitchell BF. Physiological pathways and molecular mechanisms regulating uterine contractility. Hum Reprod Update 2010;16(06):725–744 [DOI] [PubMed] [Google Scholar]
- 17.Kurowicka B, Franczak A, Oponowicz A, Kotwica G. In vitro contractile activity of porcine myometrium during luteolysis and early pregnancy: effect of oxytocin and progesterone. Reprod Biol 2005;5(02):151–169 [PubMed] [Google Scholar]
- 18.Frankl O Adenomyosis uteri. Am J Obstet Gynecol 1925; 10:680–684 [Google Scholar]
- 19.Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol 1927;14:422–469 [Google Scholar]
- 20.Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril 2012;98(03):511–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benagiano G, Brosens I, Habiba M. Structural and molecular features of the endomyometrium in endometriosis and adenomyosis. Hum Reprod Update 2014;20(03):386–402 [DOI] [PubMed] [Google Scholar]
- 22.Bergeron C, Amant F, Ferenczy A. Pathology and physiopathology of adenomyosis. Best Pract Res Clin Obstet Gynaecol 2006;20(04):511–521 [DOI] [PubMed] [Google Scholar]
- 23.Curtis KM, Hillis SD, Marchbanks PA, Peterson HB. Disruption of the endometrial-myometrial border during pregnancy as a risk factor for adenomyosis. Am J Obstet Gynecol 2002;187(03): 543–544 [DOI] [PubMed] [Google Scholar]
- 24.Mehasseb MK, Taylor AH, Pringle JH, Bell SC, Habiba M. Enhanced invasion of stromal cells from adenomyosis in a three-dimensional coculture model is augmented by the presence of myocytes from affected uteri. Fertil Steril 2010;94(07):2547–2551 [DOI] [PubMed] [Google Scholar]
- 25.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol 2009;10(07): 445–457 [DOI] [PubMed] [Google Scholar]
- 26.Donnez O, Orellana R, Van Kerk O, Dehoux JP, Donnez J, Dolmans MM. Invasion process of induced deep nodular endometriosis in an experimental baboon model: similarities with collective cell migration? Fertil Steril 2015;104(02):491–7.e2 [DOI] [PubMed] [Google Scholar]
- 27.García-Solares J, Donnez J, Donnez O, Dolmans MM. Pathogenesis of uterine adenomyosis: invagination or metaplasia? Fertil Steril 2018;109(03):371–379 [DOI] [PubMed] [Google Scholar]
- 28.Li J, Yanyan M, Mu L, Chen X, Zheng W. The expression of Bcl-2 in adenomyosis and its effect on proliferation, migration, and apoptosis of endometrial stromal cells. Pathol Res Pract 2019; 215(08):152477. [DOI] [PubMed] [Google Scholar]
- 29.Jones RK, Searle RF, Bulmer JN. Apoptosis and bcl-2 expression in normal human endometrium, endometriosis and adenomyosis. Hum Reprod 1998;13(12):3496–3502 [DOI] [PubMed] [Google Scholar]
- 30.Herndon CN, Aghajanova L, Balayan S, et al. Global transcriptome abnormalities of the eutopic endometrium from women with adenomyosis. Reprod Sci 2016;23(10):1289–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo J, Chen L, Luo N, et al. LPS/TLR4-mediated stromal cells acquire an invasive phenotype and are implicated in the pathogenesis of adenomyosis. Sci Rep 2016;6:21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X, Shen M, Qi Q, Zhang H, Guo SW. Corroborating evidence for platelet-induced epithelial-mesenchymal transition and fibro-blast-to-myofibroblast transdifferentiation in the development of adenomyosis. Hum Reprod 2016;31(04):734–749 [DOI] [PubMed] [Google Scholar]
- 33.Zhao L, Zhou S, Zou L, Zhao X. The expression and functionality of stromal caveolin 1 in human adenomyosis. Hum Reprod 2013;28(05):1324–1338 [DOI] [PubMed] [Google Scholar]
- 34.Xu XY, Zhang J, Qi YH, Kong M, Liu SA, Hu JJ. Linc-ROR promotes endometrial cell proliferation by activating the PI3K-Akt pathway. Eur Rev Med Pharmacol Sci 2018;22(08):2218–2225 [DOI] [PubMed] [Google Scholar]
- 35.Tokyol C, Aktepe F, Dilek FH, Sahin O, Arioz DT. Expression of cyclooxygenase-2 and matrix metalloproteinase-2 in adenomyosis and endometrial polyps and its correlation with angiogenesis. Int J Gynecol Pathol 2009;28(02):148–156 [DOI] [PubMed] [Google Scholar]
- 36.Li T, Li YG, Pu DM. Matrix metalloproteinase-2 and −9 expression correlated with angiogenesis in human adenomyosis. Gynecol Obstet Invest 2006;62(04):229–235 [DOI] [PubMed] [Google Scholar]
- 37.Xiang Y, Sun Y, Yang B, et al. Transcriptome sequencing of adenomyosis eutopic endometrium: a new insight into its pathophysiology. J Cell Mol Med 2019;23(12):8381–8391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen YJ, Li HY, Huang CH, et al. Oestrogen-induced epithelial-mesenchymal transition of endometrial epithelial cells contributes to the development of adenomyosis. J Pathol 2010;222(03): 261–270 [DOI] [PubMed] [Google Scholar]
- 39.Khan KN, Kitajima M, Hiraki K, Fujishita A, Nakashima M, Masuzaki H. Involvement of hepatocyte growth factor-induced epithelial-mesenchymal transition in human adenomyosis. Biol Reprod 2015;92(02):35. [DOI] [PubMed] [Google Scholar]
- 40.Oh SJ, Shin JH, Kim TH, et al. β-Catenin activation contributes to the pathogenesis of adenomyosis through epithelial-mesenchymal transition. J Pathol 2013;231(02):210–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou W, Peng Z, Zhang C, Liu S, Zhang Y. ILK-induced epithelial-mesenchymal transition promotes the invasive phenotype in adenomyosis. Biochem Biophys Res Commun 2018;497(04): 950–956 [DOI] [PubMed] [Google Scholar]
- 42.Hu R, Peng GQ, Ban DY, Zhang C, Zhang XQ, Li YP. High-expression of neuropilin 1 correlates to estrogen-induced epithelial-mesenchymal transition of endometrial cells in adenomyosis. Reprod Sci 2020;27(01):395–403 [DOI] [PubMed] [Google Scholar]
- 43.Shen M, Liu X, Zhang H, Guo SW. Transforming growth factor β1 signaling coincides with epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis in mice. Hum Reprod 2016;31(02): 355–369 [DOI] [PubMed] [Google Scholar]
- 44.Zhu B, Chen Y, Shen X, Liu X, Guo SW. Anti-platelet therapy holds promises in treating adenomyosis: experimental evidence. Reprod Biol Endocrinol 2016;14(01):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ibrahim MG, Chiantera V, Frangini S, et al. Ultramicro-trauma in the endometrial-myometrial junctional zone and pale cell migration in adenomyosis. Fertil Steril 2015;104(06):1475–83.e1, 3 [DOI] [PubMed] [Google Scholar]
- 46.Kunz G, Beil D, Huppert P, Noe M, Kissler S, Leyendecker G. Adenomyosis in endometriosis–prevalence and impact on fertility. Evidence from magnetic resonance imaging. Hum Reprod 2005;20(08):2309–2316 [DOI] [PubMed] [Google Scholar]
- 47.Yang G, Im HJ, Wang JH. Repetitive mechanical stretching modulates IL-1beta induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene 2005;363:166–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mowa CN, Hoch R, Montavon CL, Jesmin S, Hindman G, Hou G. Estrogen enhances wound healing in the penis of rats. Biomed Res 2008;29(05):267–270 [DOI] [PubMed] [Google Scholar]
- 49.Leyendecker G, Kunz G, Wildt L, Beil D, Deininger H. Uterine hyperperistalsis and dysperistalsis as dysfunctions of the mechanism of rapid sperm transport in patients with endometriosis and infertility. Hum Reprod 1996;11(07):1542–1551 [DOI] [PubMed] [Google Scholar]
- 50.Kunz G, Noe M, Herbertz M, Leyendecker G. Uterine peristalsis during the follicular phase of the menstrual cycle: effects of oestrogen, antioestrogen and oxytocin. Hum Reprod Update 1998;4(05):647–654 [DOI] [PubMed] [Google Scholar]
- 51.Leyendecker G, Wildt L, Mall G. The pathophysiology of endometriosis and adenomyosis: tissue injury and repair. Arch Gynecol Obstet 2009;280(04):529–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leyendecker G, Bilgicyildirim A, Inacker M, et al. Adenomyosis and endometriosis. Re-visiting their association and further insights into the mechanisms of auto-traumatisation. An MRI study. Arch Gynecol Obstet 2015;291(04):917–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carrarelli P, Yen CF, Arcuri F, et al. Myostatin, follistatin and activin type II receptors are highly expressed in adenomyosis. Fertil Steril 2015;104(03):744–52.e1 [DOI] [PubMed] [Google Scholar]
- 54.Zhou S, Yi T, Liu R, et al. Proteomics identification of annexin A2 as a key mediator in the metastasis and proangiogenesis of endometrial cells in human adenomyosis. Mol Cell Proteomics 2012;11(07):017988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chun S, Kim YM, Ji YI. Uterine adenomyosis which developed from hypoplastic uterus in postmenopausal woman with Mayer-Rokitansky-Kuster-Hauser syndrome: a case report. J Menopausal Med 2013;19(03):135–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoo PS, Norhaslinda AR, Reza JN. Rare case of leiomyoma and adenomyosis in Mayer-Rokitansky-Kuster-Hauser syndrome. Case Rep Obstet Gynecol 2016;2016:3725043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferenczy A Pathophysiology of adenomyosis. Hum Reprod Update 1998;4(04):312–322 [DOI] [PubMed] [Google Scholar]
- 58.Schwab KE, Gargett CE. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod 2007;22(11):2903–2911 [DOI] [PubMed] [Google Scholar]
- 59.Masuda H, Anwar SS, Bühring HJ, Rao JR, Gargett CE. A novel marker of human endometrial mesenchymal stem-like cells. Cell Transplant 2012;21(10):2201–2214 [DOI] [PubMed] [Google Scholar]
- 60.Gargett CE, Schwab KE, Zillwood RM, Nguyen HP, Wu D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod 2009;80(06): 1136–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen YJ, Li HY, Chang YL, et al. Suppression of migratory/invasive ability and induction of apoptosis in adenomyosis-derived mesenchymal stem cells by cyclooxygenase-2 inhibitors. Fertil Steril 2010;94(06):1972–1979, 1979.e1–1979.e4 [DOI] [PubMed] [Google Scholar]
- 62.Götte M, Wolf M, Staebler A, et al. Increased expression of the adult stem cell marker Musashi-1 in endometriosis and endometrial carcinoma. J Pathol 2008;215(03):317–329 [DOI] [PubMed] [Google Scholar]
- 63.Inoue S, Hirota Y, Ueno T, et al. Uterine adenomyosis is an oligoclonal disorder associated with KRAS mutations. Nat Commun 2019;10(01):5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gargett CE. Uterine stem cells: What is the evidence? Hum Reprod Update 2007;13(01):87–101 [DOI] [PubMed] [Google Scholar]
- 65.Alawadhi F, Du H, Cakmak H, Taylor HS. Bone marrow-derived stem cell (BMDSC) transplantation improves fertility in a murine model of Asherman’s syndrome. PLoS One 2014;9(05):e96662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garcia L, Isaacson K. Adenomyosis: review of the literature. J Minim Invasive Gynecol 2011;18(04):428–437 [DOI] [PubMed] [Google Scholar]
- 67.Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod 2004;70(06):1738–1750 [DOI] [PubMed] [Google Scholar]
- 68.Vannuccini S, Tosti C, Carmona F, et al. Pathogenesis of adenomyosis: an update on molecular mechanisms. Reprod Biomed Online 2017;35(05):592–601 [DOI] [PubMed] [Google Scholar]
- 69.Yi KW, Kim SH, Ihm HJ, et al. Increased expression of p21-activated kinase 4 in adenomyosis and its regulation of matrix metalloproteinase-2 and −9 in endometrial cells. Fertil Steril 2015;103(04):1089–1097.e2 [DOI] [PubMed] [Google Scholar]
- 70.Gargett CE, Masuda H. Adult stem cells in the endometrium. Mol Hum Reprod 2010;16(11):818–834 [DOI] [PubMed] [Google Scholar]
- 71.Schüring AN, Schulte N, Kelsch R, Röpke A, Kiesel L, Götte M. Characterization of endometrial mesenchymal stem-like cells obtained by endometrial biopsy during routine diagnostics. Fertil Steril 2011;95(01):423–426 [DOI] [PubMed] [Google Scholar]
- 72.Spitzer TL, Rojas A, Zelenko Z, et al. Perivascular human endometrial mesenchymal stem cells express pathways relevant to self-renewal, lineage specification, and functional phenotype. Biol Reprod 2012;86(02):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Musina RA, Belyavski AV, Tarusova OV, Solovyova EV, Sukhikh GT. Endometrial mesenchymal stem cells isolated from the menstrual blood. Bull Exp Biol Med 2008;145(04):539–543 [DOI] [PubMed] [Google Scholar]
- 74.Vannuccini S, Petraglia F. Recent advances in understanding and managing adenomyosis. F1000 Res 2019;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chapron C, Tosti C, Marcellin L, et al. Relationship between the magnetic resonance imaging appearance of adenomyosis and endometriosis phenotypes. Hum Reprod 2017;32(07): 1393–1401 [DOI] [PubMed] [Google Scholar]
- 76.El-Shennawy GA, Elbialy AA, Isamil AE, El Behery MM. Is genetic polymorphism of ER-α, CYP1A1, and CYP1B1 a risk factor for uterine leiomyoma? Arch Gynecol Obstet 2011;283(06): 1313–1318 [DOI] [PubMed] [Google Scholar]
- 77.Bulun SE, Chen D, Moy I, Brooks DC, Zhao H. Aromatase, breast cancer and obesity: a complex interaction. Trends Endocrinol Metab 2012;23(02):83–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsuchiya M, Tsukino H, Iwasaki M, et al. Interaction between cytochrome P450 gene polymorphisms and serum organochlorine TEQ levels in the risk of endometriosis. Mol Hum Reprod 2007;13(06):399–404 [DOI] [PubMed] [Google Scholar]
- 79.Artymuk N, Zotova O, Gulyaeva L. Adenomyosis: genetics of estrogen metabolism. Horm Mol Biol Clin Investig 2019;37(02): 37. [DOI] [PubMed] [Google Scholar]
- 80.Tong X, Li Z, Wu Y, Fu X, Zhang Y, Fan H. COMT 158G/A and CYP1B1 432C/G polymorphisms increase the risk of endometriosis and adenomyosis: a meta-analysis. Eur J Obstet Gynecol Reprod Biol 2014;179:17–21 [DOI] [PubMed] [Google Scholar]
- 81.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 2005;146(02):624–632 [DOI] [PubMed] [Google Scholar]
- 82.Hong DG, Park JY, Chong GO, et al. Transmembrane G protein-coupled receptor 30 gene polymorphisms and uterine adenomyosis in Korean women. Gynecol Endocrinol 2019;35(06): 498–501 [DOI] [PubMed] [Google Scholar]
- 83.van Kaam KJ, Romano A, Schouten JP, Dunselman GA, Groothuis PG. Progesterone receptor polymorphism þ331G/A is associated with a decreased risk of deep infiltrating endometriosis. Hum Reprod 2007;22(01):129–135 [DOI] [PubMed] [Google Scholar]
- 84.Kitawaki J, Obayashi H, Ishihara H, et al. Oestrogen receptor-alpha gene polymorphism is associated with endometriosis, adenomyosis and leiomyomata. Hum Reprod 2001;16(01): 51–55 [DOI] [PubMed] [Google Scholar]
- 85.Ye H, He Y, Wang J, et al. Effect of matrix metalloproteinase promoter polymorphisms on endometriosis and adenomyosis risk: evidence from a meta-analysis. J Genet 2016;95(03):611–619 [DOI] [PubMed] [Google Scholar]
- 86.Kang S, Zhao X, Xing H, et al. Polymorphisms in the matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 and the risk of human adenomyosis. Environ Mol Mutagen 2008; 49(03):226–231 [DOI] [PubMed] [Google Scholar]
- 87.Kang S, Li SZ, Wang N, et al. Association between genetic polymorphisms in fibroblast growth factor (FGF)1 and FGF2 and risk of endometriosis and adenomyosis in Chinese women. Hum Reprod 2010;25(07):1806–1811 [DOI] [PubMed] [Google Scholar]
- 88.Kang S, Zhao J, Liu Q, Zhou R, Wang N, Li Y. Vascular endothelial growth factor gene polymorphisms are associated with the risk of developing adenomyosis. Environ Mol Mutagen 2009;50(05): 361–366 [DOI] [PubMed] [Google Scholar]
- 89.Liu Q, Li Y, Zhao J, et al. [Association of single nucleotide polymorphisms in VEGF gene with the risk of endometriosis and adenomyosis]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2009;26(02):165–169 [DOI] [PubMed] [Google Scholar]
- 90.Wang Y, Qu Y, Song W. Genetic variation in COX-2 −1195 and the risk of endometriosis and adenomyosis. Clin Exp Obstet Gynecol 2015;42(02):168–172 [PubMed] [Google Scholar]
- 91.Liu X, Guo SW. Aberrant immunoreactivity of deoxyribonucleic acid methyltransferases in adenomyosis. Gynecol Obstet Invest 2012;74(02):100–108 [DOI] [PubMed] [Google Scholar]
- 92.Nie J, Liu X, Guo SW. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in adenomyosis and its rectification by a histone deacetylase inhibitor and a demethylation agent. Reprod Sci 2010;17(11):995–1005 [DOI] [PubMed] [Google Scholar]
- 93.Liu X, Guo SW. Valproic acid alleviates generalized hyperalgesia in mice with induced adenomyosis. J Obstet Gynaecol Res 2011; 37(07):696–708 [DOI] [PubMed] [Google Scholar]
- 94.Liu X, Guo SW. A pilot study on the off-label use of valproic acid to treat adenomyosis. Fertil Steril 2008;89(01):246–250 [DOI] [PubMed] [Google Scholar]
- 95.Wen J, Lv R, Ma H, et al. Zc3h13 regulates nuclear RNA m6A methylation and mouse embryonic stem cell self-renewal. Mol Cell 2018;69(06):1028–1038.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhai J, Li S, Sen S, et al. m6A RNA methylation regulators contribute. to eutopic endometrium and myometrium dysfunction in adenomyosis. Front Genet 2020;11:716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pan SC, Gardner WU. Carcinomas of the uterine cervix and vagina in estrogen- and androgen-treated hybrid mice. Cancer Res 1948;8(07):337–345 [PubMed] [Google Scholar]
- 98.Takahashi K, Nagata H, Kitao M. Clinical usefulness of determination of estradiol level in the menstrual blood for patients with endometriosis. Nippon Sanka Fujinka Gakkai Zasshi 1989;41(11):1849–1850 [PubMed] [Google Scholar]
- 99.Kitawaki J, Noguchi T, Amatsu T, et al. Expression of aromatase cytochrome P450 protein and messenger ribonucleic acid in human endometriotic and adenomyotic tissues but not in normal endometrium. Biol Reprod 1997;57(03):514–519 [DOI] [PubMed] [Google Scholar]
- 100.Kitawaki J, Koshiba H, Ishihara H, Kusuki I, Tsukamoto K, Honjo H. Progesterone induction of 17beta-hydroxysteroid dehydrogenase type 2 during the secretory phase occurs in the endometrium of estrogen-dependent benign diseases but not in normal endometrium. J Clin Endocrinol Metab 2000;85(09):3292–3296 [DOI] [PubMed] [Google Scholar]
- 101.Ezaki K, Motoyama H, Sasaki H. Immunohistologic localization of estrone sulfatase in uterine endometrium and adenomyosis. Obstet Gynecol 2001;98(5, Pt 1):815–819 [DOI] [PubMed] [Google Scholar]
- 102.Mehasseb MK, Panchal R, Taylor AH, Brown L, Bell SC, Habiba M. Estrogen and progesterone receptor isoform distribution through the menstrual cycle in uteri with and without adenomyosis. Fertil Steril 2011;95(07):2228–2235, 2235.e1 [DOI] [PubMed] [Google Scholar]
- 103.Wang S, Duan H, Zhang Y, Wang L, Zhang H, Li G. [Mechanism of 17β-estrogen on intracellular free calcium regulation in smooth muscle cells at the endometrial-myometrial interface in uteri with adenomyosis]. Zhonghua Fu Chan Ke Za Zhi 2015;50(07):510–515 [PubMed] [Google Scholar]
- 104.Mori T, Nagasawa H, Takahashi S. The induction of adenomyosis in mice by intrauterine pituitary isografts. Life Sci 1981;29(12): 1277–1282 [DOI] [PubMed] [Google Scholar]
- 105.Singtripop T, Mori T, Park MK, Sakamoto S, Kawashima S. Development of uterine adenomyosis after treatment with dopamine antagonists in mice. Life Sci 1991;49(03):201–206 [DOI] [PubMed] [Google Scholar]
- 106.Yamashita M, Matsuda M, Mori T. Increased expression of prolactin receptor mRNA in adenomyotic uterus in mice. Life Sci 1997;60(17):1437–1446 [DOI] [PubMed] [Google Scholar]
- 107.Nagasawa H, Mori T. Stimulation of mammary tumorigenesis and suppression of uterine adenomyosis by temporary inhibition of pituitary prolactin secretion during youth in mice (41492). Proc Soc Exp Biol Med 1982;171(02):164–167 [DOI] [PubMed] [Google Scholar]
- 108.Łupicka M, Socha BM, Szczepańska AA, Korzekwa AJ. Prolactin role in the bovine uterus during adenomyosis. Domest Anim Endocrinol 2017;58:1–13 [DOI] [PubMed] [Google Scholar]
- 109.Sengupta P, Sharma A, Mazumdar G, et al. The possible role of fluoxetine in adenomyosis: an animal experiment with clinical correlations. J Clin Diagn Res 2013;7(07):1530–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Andersson JK, Khan Z, Weaver AL, Vaughan LE, Gemzell-Danielsson K, Stewart EA. Vaginal bromocriptine improves pain, menstrual bleeding and quality of life in women with adenomyosis: a pilot study. Acta Obstet Gynecol Scand 2019;98(10): 1341–1350 [DOI] [PubMed] [Google Scholar]
- 111.Nowak RA, Mora S, Diehl T, Rhoades AR, Stewart EA. Prolactin is an autocrine or paracrine growth factor for human myometrial and leiomyoma cells. Gynecol Obstet Invest 1999;48(02): 127–132 [DOI] [PubMed] [Google Scholar]
- 112.Mori T, Singtripop T, Kawashima S. Animal model of uterine adenomyosis: is prolactin a potent inducer of adenomyosis in mice? Am J Obstet Gynecol 1991;165(01):232–234 [DOI] [PubMed] [Google Scholar]
- 113.Takemura M, Nomura S, Kimura T, et al. Expression and localization of oxytocin receptor gene in human uterine endometrium in relation to the menstrual cycle. Endocrinology 1993;132(04): 1830–1835 [DOI] [PubMed] [Google Scholar]
- 114.Guo SW, Mao X, Ma Q, Liu X. Dysmenorrhea and its severity are associated with increased uterine contractility and overexpression of oxytocin receptor (OTR) in women with symptomatic adenomyosis. Fertil Steril 2013;99(01):231–240 [DOI] [PubMed] [Google Scholar]
- 115.Nie J, Liu X. Leonurine attenuates hyperalgesia in mice with induced adenomyosis. Med Sci Monit 2017;23:1701–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bruner-Tran KL, Gnecco J, Ding T, Glore DR, Pensabene V, Osteen KG. Exposure to the environmental endocrine disruptor TCDD and human reproductive dysfunction: Translating lessons from murine models. Reprod Toxicol 2017;68:59–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bruner-Tran KL, Duleba AJ, Taylor HS, Osteen KG. Developmental toxicant exposure is associated with transgenerational adenomyosis in a murine model. Biol Reprod 2016;95(04):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Newbold RR, Jefferson WN, Padilla-Banks E. Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract. Reprod Toxicol 2007;24(02):253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bennett LM, McAllister KA, Malphurs J, et al. Mice heterozygous for a Brca1 or Brca2 mutation display distinct mammary gland and ovarian phenotypes in response to diethylstilbestrol. Cancer Res 2000;60(13):3461–3469 [PubMed] [Google Scholar]
- 120.Huang PC, Tsai EM, Li WF, et al. Association between phthalate exposure and glutathione S-transferase M1 polymorphism in adenomyosis, leiomyoma and endometriosis. Hum Reprod 2010;25(04):986–994 [DOI] [PubMed] [Google Scholar]
- 121.Ota H, Igarashi S, Hatazawa J, Tanaka T. Is adenomyosis an immune disease? Hum Reprod Update 1998;4(04):360–367 [DOI] [PubMed] [Google Scholar]
- 122.Ota H, Igarashi S. Expression of major histocompatibility complex class II antigen in endometriotic tissue in patients with endometriosis and adenomyosis. Fertil Steril 1993;60(05): 834–838 [DOI] [PubMed] [Google Scholar]
- 123.Wang F, Wen Z, Li H, Yang Z, Zhao X, Yao X. Human leukocyte antigen-G is expressed by the eutopic and ectopic endometrium of adenomyosis. Fertil Steril 2008;90(05):1599–1604 [DOI] [PubMed] [Google Scholar]
- 124.Chiang CM, Hill JA. Localization of T cells, interferon-gamma and HLA-DR in eutopic and ectopic human endometrium. Gynecol Obstet Invest 1997;43(04):245–250 [DOI] [PubMed] [Google Scholar]
- 125.Ota H, Maki M, Shidara Y, et al. Effects of danazol at the immunologic level in patients with adenomyosis, with special reference to autoantibodies: a multi-center cooperative study. Am J Obstet Gynecol 1992;167(02):481–486 [DOI] [PubMed] [Google Scholar]
- 126.Wang F, Li H, Yang Z, Du X, Cui M, Wen Z. Expression of interleukin-10 in patients with adenomyosis. Fertil Steril 2009;91(05):1681–1685 [DOI] [PubMed] [Google Scholar]
- 127.Li B, Chen M, Liu X, Guo SW. Constitutive and tumor necrosis factor-α-induced activation of nuclear factor-κB in adenomyosis and its inhibition by andrographolide. Fertil Steril 2013;100(02):568–577 [DOI] [PubMed] [Google Scholar]
- 128.Carrarelli P, Yen CF, Funghi L, et al. Expression of inflammatory and neurogenic mediators in adenomyosis. Reprod Sci 2017;24(03):369–375 [DOI] [PubMed] [Google Scholar]
- 129.Baigent SM. Peripheral corticotropin-releasing hormone and urocortin in the control of the immune response. Peptides 2001;22(05):809–820 [DOI] [PubMed] [Google Scholar]
- 130.Tsatsanis C, Androulidaki A, Dermitzaki E, Gravanis A, Margioris AN. Corticotropin releasing factor receptor 1 (CRF1) and CRF2 agonists exert an anti-inflammatory effect during the early phase of inflammation suppressing LPS-induced TNF-alpha release from macrophages via induction of COX-2 and PGE2. J Cell Physiol 2007;210(03):774–783 [DOI] [PubMed] [Google Scholar]
- 131.Vergetaki A, Jeschke U, Vrekoussis T, et al. Differential expression of CRH, UCN, CRHR1 and CRHR2 in eutopic and ectopic endometrium of women with endometriosis. PLoS One 2013;8(04): e62313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Brawn J, Morotti M, Zondervan KT, Becker CM, Vincent K. Central changes associated with chronic pelvic pain and endometriosis. Hum Reprod Update 2014;20(05):737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huang Y, Zheng W, Mu L, Ren Y, Chen X, Liu F. Expression of tyrosine kinase receptor B in eutopic endometrium of women with adenomyosis. Arch Gynecol Obstet 2011;283(04):775–780 [DOI] [PubMed] [Google Scholar]
- 134.Huang HY, Yu HT, Chan SH, Lee CL, Wang HS, Soong YK. Eutopic endometrial interleukin-18 system mRNA and protein expression at the level of endometrial-myometrial interface in adenomyosis patients. Fertil Steril 2010;94(01):33–39 [DOI] [PubMed] [Google Scholar]
- 135.Mao X, Wang Y, Carter AV, Zhen X, Guo SW. The retardation of myometrial infiltration, reduction of uterine contractility, and alleviation of generalized hyperalgesia in mice with induced adenomyosis by levo-tetrahydropalmatine (l-THP) and andrographolide. Reprod Sci 2011;18(10):1025–1037 [DOI] [PubMed] [Google Scholar]
- 136.Armstrong L, Medford AR, Uppington KM, et al. Expression of functional toll-like receptor-2 and −4 on alveolar epithelial cells. Am J Respir Cell Mol Biol 2004;31(02):241–245 [DOI] [PubMed] [Google Scholar]
- 137.Vannuccini S, Luisi S, Tosti C, Sorbi F, Petraglia F. Role of medical therapy in the management of uterine adenomyosis. Fertil Steril 2018;109(03):398–405 [DOI] [PubMed] [Google Scholar]
- 138.Yang Y, Bin W, Aksoy MO, Kelsen SG. Regulation of interleukin-1beta and interleukin-1beta inhibitor release by human airway epithelial cells. Eur Respir J 2004;24(03):360–366 [DOI] [PubMed] [Google Scholar]
- 139.Hayden MS, Ghosh S. Regulation of NF-κB by TNF family cytokines. Semin Immunol 2014;26(03):253–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kojima H, Aizawa Y, Yanai Y, et al. An essential role for NF-kappa B in IL-18-induced IFN-gamma expression in KG-1 cells. J Immunol 1999;162(09):5063–5069 [PubMed] [Google Scholar]
- 141.McEvoy AN, Bresnihan B, FitzGerald O, Murphy EP. Cyclooxygenase 2-derived prostaglandin E2 production by corticotropin-releasing hormone contributes to the activated cAMP response element binding protein content in rheumatoid arthritis synovial tissue. Arthritis Rheum 2004;50(04):1132–1145 [DOI] [PubMed] [Google Scholar]
- 142.Krantz KE. Innervation of the human uterus. Ann N Y Acad Sci 1959;75:770–784 [DOI] [PubMed] [Google Scholar]
- 143.Carrarelli P, Luddi A, Funghi L, et al. Urocortin and corticotrophin-releasing hormone receptor type 2 mRNA are highly expressed in deep infiltrating endometriotic lesions. Reprod Biomed Online 2016;33(04):476–483 [DOI] [PubMed] [Google Scholar]
- 144.Apfel SC. Neurotrophic factors and pain. Clin J Pain 2000;16(02):S7–S11 [DOI] [PubMed] [Google Scholar]
- 145.Zhang X, Lu B, Huang X, Xu H, Zhou C, Lin J. Endometrial nerve fibers in women with endometriosis, adenomyosis, and uterine fibroids. Fertil Steril 2009;92(05):1799–1801 [DOI] [PubMed] [Google Scholar]
- 146.Zhang X, Lu B, Huang X, Xu H, Zhou C, Lin J. Innervation of endometrium and myometrium in women with painful adenomyosis and uterine fibroids. Fertil Steril 2010;94(02):730–737 [DOI] [PubMed] [Google Scholar]
- 147.Choi YJ, Chang JA, Kim YA, Chang SH, Chun KC, Koh JW. Innervation in women with uterine myoma and adenomyosis. Obstet Gynecol Sci 2015;58(02):150–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li Y, Zhang SF, Zou SE, Xia X, Bao L. Accumulation of nerve growth factor and its receptors in the uterus and dorsal root ganglia in a mouse model of adenomyosis. Reprod Biol Endocrinol 2011;9:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen Y, Zhu B, Zhang H, Liu X, Guo SW. Epigallocatechin-3-gallate reduces myometrial infiltration, uterine hyperactivity, and stress levels and alleviates generalized hyperalgesia in mice induced with adenomyosis. Reprod Sci 2013;20(12):1478–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang F, Shi X, Qin X, Wen Z, Zhao X, Li C. Expression of CD56 in patients with adenomyosis and its correlation with dysmenorrhea. Eur J Obstet Gynecol Reprod Biol 2015;194:101–105 [DOI] [PubMed] [Google Scholar]
- 151.Nie J, Liu X, Zheng Y, Geng JG, Guo SW. Increased immunoreactivity to SLIT/ROBO1 and its correlation with severity of dysmenorrhea in adenomyosis. Fertil Steril 2011;95(03):1164–1167 [DOI] [PubMed] [Google Scholar]
- 152.Wang J, Deng X, Yang Y, Yang X, Kong B, Chao L. Expression of GRIM-19 in adenomyosis and its possible role in pathogenesis. Fertil Steril 2016;105(04):1093–1101 [DOI] [PubMed] [Google Scholar]
- 153.Harmsen MJ, Wong CFC, Mijatovic V, et al. Role of angiogenesis in adenomyosis-associated abnormal uterine bleeding and subfertility: a systematic review. Hum Reprod Update 2019;25(05): 647–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hillen F, Griffioen AW. Tumour vascularization: sprouting angiogenesis and beyond. Cancer Metastasis Rev 2007;26(3–4):489–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Huang TS, Chen YJ, Chou TY, et al. Oestrogen-induced angiogenesis promotes adenomyosis by activating the Slug-VEGF axis in endometrial epithelial cells. J Cell Mol Med 2014;18(07): 1358–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schindl M, Birner P, Obermair A, Kiesel L, Wenzl R. Increased microvessel density in adenomyosis uteri. Fertil Steril 2001;75(01):131–135 [DOI] [PubMed] [Google Scholar]
- 157.Goteri G, Lucarini G, Montik N, et al. Expression of vascular endothelial growth factor (VEGF), hypoxia inducible factor-1alpha (HIF-1alpha), and microvessel density in endometrial tissue in women with adenomyosis. Int J Gynecol Pathol 2009;28(02): 157–163 [DOI] [PubMed] [Google Scholar]
- 158.Ota H, Igarashi S, Tanaka T. Morphometric evaluation of stromal vascularization in the endometrium in adenomyosis. Hum Reprod 1998;13(03):715–719 [DOI] [PubMed] [Google Scholar]
- 159.Nie J, Liu X, Guo SW. Immunoreactivity of oxytocin receptor and transient receptor potential vanilloid type 1 and its correlation with dysmenorrhea in adenomyosis. Am J Obstet Gynecol 2010; 202(04):346.e1–346.e8 [DOI] [PubMed] [Google Scholar]
- 160.Brainard AM, Korovkina VP, England SK. Potassium channels and uterine function. Semin Cell Dev Biol 2007;18(03):332–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mechsner S, Grum B, Gericke C, Loddenkemper C, Dudenhausen JW, Ebert AD. Possible roles of oxytocin receptor and vasopressin-1α receptor in the pathomechanism of dysperistalsis and dysmenorrhea in patients with adenomyosis uteri. Fertil Steril 2010;94(07):2541–2546 [DOI] [PubMed] [Google Scholar]
- 162.Shi H, Li R, Qiang J, Li Y, Wang L, Sun R. Computed tomography perfusion imaging detection of microcirculatory dysfunction in small intestinal ischemia-reperfusion injury in a porcine model. PLoS One 2016;11(07):e0160102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen Y, Li D, Zhang Z, Takushige N, Kong BH, Wang GY. Effect of siRNA against β-NGF on nerve fibers of a rat model with endometriosis. Reprod Sci 2014;21(03):329–339 [DOI] [PubMed] [Google Scholar]
- 164.Lertvikool S, Sukprasert M, Pansrikaew P, Rattanasiri S, Weerakiet S. Comparative study of nerve fiber density between adenomyosis patients with moderate to severe pain and mild pain. J Med Assoc Thai 2014;97(08):791–797 [PubMed] [Google Scholar]
- 165.Liu X, Nie J, Guo SW. Elevated immunoreactivity to tissue factor and its association with dysmenorrhea severity and the amount of menses in adenomyosis. Hum Reprod 2011;26(02):337–345 [DOI] [PubMed] [Google Scholar]
- 166.Campo S, Campo V, Benagiano G. Adenomyosis and infertility. Reprod Biomed Online 2012;24(01):35–46 [DOI] [PubMed] [Google Scholar]
- 167.Xiao Y, Li T, Xia E, Yang X, Sun X, Zhou Y. Expression of integrin β3 and osteopontin in the eutopic endometrium of adenomyosis during the implantation window. Eur J Obstet Gynecol Reprod Biol 2013;170(02):419–422 [DOI] [PubMed] [Google Scholar]
- 168.Fischer CP, Kayisili U, Taylor HS. HOXA10 expression is decreased in endometrium of women with adenomyosis. Fertil Steril 2011; 95(03):1133–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Van Langendonckt A, Casanas-Roux F, Donnez J. Oxidative stress and peritoneal endometriosis. Fertil Steril 2002;77(05): 861–870 [DOI] [PubMed] [Google Scholar]
- 170.Yu O, Schulze-Rath R, Grafton J, Hansen K, Scholes D, Reed SD. Adenomyosis incidence, prevalence and treatment: United States population-based study 2006–2015. Am J Obstet Gynecol 2020;223(01):94.e1–94.e10 [DOI] [PubMed] [Google Scholar]


