Abstract
Background:
The safety of operator-directed sedation (ODS) versus anesthesiologist care (AC) during pediatric/congenital catheterizations has been questioned.
Methods:
A multicenter, retrospective cohort study was performed studying procedures habitually performed with ODS or AC at IMPACT® hospitals using ODS for ≥5% of cases. The risks of MAE for ODS and AC cases were compared, adjusted for case-mix. Current recommendations were evaluated by comparing the ratio of observed-to-expected MAE for cases in which ODS was “inappropriate” (inconsistent with those guidelines) to those for similar risk AC cases, as well as those in which ODS or AC was “appropriate.”
Results:
Of the hospitals submitting data to IMPACT®, 28/101 met inclusion criteria. Of the 7,042 cases performed using ODS at these centers, 88% would be “inappropriate”. Use of ODS was associated with lower likelihood of MAE both in observed results (p<0.0001) and after adjusting for case-mix (OR 0.81 p=0.006). Use of AC was also associated with longer adjusted fluoroscopy and procedure times (both p<0.0001). The O/E ratio for ODS cases with high pre-procedural risk (“inappropriate” for ODS) was significantly lower than that for AC cases with comparable pre-procedural risk. Across a range of pre-procedural risk, there was no stratum in which risk of MAE was lower for AC than ODS.
Conclusion:
Across a range of hospitals, ODS was used safely and with improved efficiency. Clinical judgment better identified cases in which ODS could be used than pre-procedural risk score. This should inform future guidelines for the use of ODS/AC in the catheterization laboratory
Keywords: Pediatrics, congenital heart disease, outcomes research, health services research
CONDENSED ABSTRACT:
The safety of operator-directed sedation (ODS) compared to anesthesiologist care (AC) for pediatric/congenital cardiac catheterizations has been questioned. A multicenter retrospective cohort study using data from 28 IMPACT® registry hospitals that habitually used ODS was performed. In adjusted analyses of procedure types performed using both ODS and AC, ODS was associated with a lower risk of major adverse events (MAE) (OR: 0.81 p=0.006), and ODS was at least as safe as AC across a range of pre-procedure risks. The current study demonstrates that at a range of programs ODS was a safe alternative to AC.
Introduction:
Operator-directed sedation without an anesthesiologist (ODS) has been used in the pediatric and congenital catheterization laboratory (PCCL) since the inception of the field. As in other areas of medicine, there has been debate as to whether this practice is appropriate. While PCCL procedures are less noxious than open surgical procedures, they can be technically complex and are also performed in patients with both cardiac and noncardiac conditions that increase the potential for cardiopulmonary instability. In large, multi-center series, the risk of major adverse events (AEs) associated with PCCL procedures is between 10–11%(1, 2). AEs attributable to sedation/anesthesia are rare but some progress to a life-threatening severity(3). There continues to be interest in determining in which patients use of ODS is appropriate versus care from an anesthesiologist (AC).
In 2016, an expert panel with representatives from the Congenital Heart Disease Section of the Society for Cardiac Angiography and Intervention (CHD-SCAI), Society for Pediatric Anesthesia (SPA), and Congenital Cardiac Anesthesia Society (CCAS) published guidelines for the use of ODS and AC during PCCL procedures(4), identifying potential high-risk patient populations, aspects of intra-procedural anesthetic practice, and optimal systems practice. The most prescriptive part of these recommendations was establishing a minimum level of expertise for the person providing sedation/anesthesia based on the Catheterization Risk Score for Pediatrics (CRISP) score(5). Cases with a CRISP score ≥2 would, at a minimum, be staffed by an anesthesiologist with “special expertise in congenital heart disease.”
In a previously published single-center cohort study, 90% of cases in which ODS was used would have been considered “inappropriate” based on these guidelines(6). At the same time, after adjusting for measurable confounding, the use of ODS was associated with lower risk of an AE, as well as shorter case times and lower charges, than in a similar panel of procedures performed with AC(6). This suggested that the care team was identifying cases in which ODS was safe with better discrimination than current recommendations. It was not possible in this study to determine whether these observations were representative of the outcomes at other centers. To overcome this, we used data from a national clinical registry of PCCL procedures to evaluate how widely ODS is used and whether its use was associated with similarly reduced risk of AE in a broad sample of PCCL programs.
Methods:
Data source:
The IMproving Pediatric And Congenital Treatment ® (IMPACT®) registry is a clinical registry funded by the American College of Cardiology and managed by the National Cardiovascular Data Registry with data from 101 North American pediatric and general hospitals performing cardiac catheterizations in children and adults with congenital heart disease at the time of this analysis. Participating centers collect demographics, medical/surgical history, procedural information and AE through hospital discharge on all patients undergoing cardiac catheterization. Data are recorded using standardized data elements and definitions. The database is subject to quality assurance standards(7). The current study used data from IMPACT v1 and v2. The institutional review board of The Children’s Hospital of Philadelphia reviewed the proposed project and determined that it did not represent human subjects research in accordance with the Common Rule (45 CFR 46.102(f)).
Study Design and Population:
We performed a multi-center observational study with two parts. In the first part we sought to describe the use of ODS and AC in all hospitals contributing to the IMPACT® registry. In the second, we evaluated the outcomes of ODS and AC cases at hospitals that habitually utilized ODS. ODS was defined as cases in which an anesthesiologist was not present at the outset of the procedure. AC was defined as cases where an anesthesiologist was present from the start of the case and included those with and without an endotracheal tube (though these data were kept and used for additional sensitivity analyses). This choice was made because current recommendations specify the staffing for cases, but do not provide guidance regarding specific airway management strategies and/or pharmacological regimens(4).
Data were included from hospitals that contributed >25 cases per year for more than four consecutive quarters. For the first part, all centers contributing data to IMPACT® between 1/1/2011 and 6/30/2018 were included. For the second part, we used the same study period but restricted analysis to centers in which ODS was used in ≥5% of cases during the study period. This choice was made to allow us to evaluate the relative results of ODS and AC cases at centers using both strategies. It sacrifices generalizability, excludes data from centers that do not use ODS. However, it prioritizes internal validity addressing the studies central question of whether centers using ODS sort cases for ODS and AC successfully (not increasing the risk of AE in this population). Cases from centers not using ODS are not informative for this question. Elective and urgent cases in subjects from 30 days to 25 years were studied. Cases in patients in whom extracorporeal membrane oxygenation or other mechanical circulatory support was provided were excluded. Cases in which both hemodynamic evaluation and electrophysiology studies were performed were also excluded. Cases with initial ODS that were converted to AC because of an adverse event, hemodynamic instability, or inadequate sedation were included in the ODS cohort as an intention-to-treat analysis.
Next, the subset of PCCL procedures in which the association between ODS or AC and outcome could be fairly compared was identified, specifically procedure types where ODS was used habitually (>10 cases over the study period over the study sample). These procedures were: endomyocardial biopsy after orthotopic heart transplant, biopsy in non-transplant patients, pulmonary vasodilator drug studies, other diagnostic catheterizations, device closure of patent ductus arteriosus, device closure of patent foramen ovale, device closure of atrial septal defect, balloon pulmonary valvuloplasty, balloon aortic valvuloplasty, device or coil occlusion of venous collateral(s) (including baffle leaks and Fontan fenestrations), and occlusion of arterial collaterals (including coronary cameral fistulae) along with balloon angioplasty, stent angioplasty, and stent re-dilation of pulmonary arteries, coarctation, and conduits. The study cohort was then restricted to cases in which one or more of these procedures was performed. Cases with multiple procedures that included procedure types not in this list were excluded.
Study measures:
Demographics, cardiac diagnosis, and pre-procedural risk factors were collected. Procedural information included case times, procedures performed, hemodynamic data, and AEs. Definitions for diagnoses, procedures, and AEs in the database are recorded using standard definitions for the IMPACT® registry. A pair of case times was also collected, specifically total case time (from vascular access until the end of the procedure defined by IMPACT® as the operator breaking scrub) and fluoroscopy time. Together these two times provided complementary measures of procedural length and technical complexity (respectively). SCAI-CHD/SPA/CCAS recommendations for appropriate application of anesthesia in the PCCL are based on the first version CRISP score(4, 5), so it was calculated for each case as described previously(6).
As noted, cases were divided between AC cases, in which an anesthesiologist was present from the start, and ODS cases, in which no anesthesiologist was present at the start of the case. For secondary analyses, cases were divided into three categories: 1) cases where general anesthesia was provided by an anesthesiologist, 2) cases with intravenous or per orum sedation with an anesthesiologist present from the start, and 3) cases with sedation without an anesthesiologist present. Information about individual anesthesiologist’s training and experience, specifically if they have received specific training or degree of experience with pediatric, cardiac, and/or pediatric cardiac anesthesiology is not available in the IMPACT® registry. Likewise, details about the training, staffing, and monitoring programs used for ODS are also not available.
Statistical Analysis:
Descriptive statistics about the use of ODS and AC were calculated. Specifically, trends in the use of ODS as a proportion of total cases over time were measured. Proportions were used because both cases per center and number of centers included in the IMPACT® registry rose over the study period. Similarly, trends in CRISP scores for ODS and AC cases were also evaluated over years. Tests of trends over years were made using the Cochrane-Armitage (linear trend) test.
For the primary analysis, the primary exposure was AC versus ODS. The primary outcome was the occurrence of major adverse events (MAE): death within 30 days, cardiac arrest, new arrhythmia, new heart valve regurgitation, tamponade, air embolus, embolic stroke, device malposition, device embolization, airway events, initiation of dialysis, intubation due to patient instability, initiation of extracorporeal membrane oxygenation, initiation of ventricular assist device, bleeding event, unplanned surgery due to catheterization complication, vascular complication requiring treatment, repeat catheterization due to complication of catheterization, and “other” events. This is the definition of MAE used in the IMPACT® Registry as well as in previous studies(6). Secondary outcomes were total case time and fluoroscopy time. In previous analyses we have evaluated the economic impact of sedation strategy by comparing the charges associated with AC and ODS cases(6), but that information is not available in the IMPACT® registry.
Descriptive statistics for the characteristics of both groups were calculated to evaluate for systematic differences between them. We anticipated that factors influencing the choice between ODS or AC would also influence our outcomes. In previous studies we have used a propensity score to address this potential confounding by indication(8–10). However, in this case the total sample size and event rates made it unlikely that a propensity score would provide superior model validity than conventional regression(11). Therefore, multivariable models were calculated for the association between our primary exposure outcomes adjusted for measurable covariates chosen based on previous studies(3, 6, 12–15). Prematurity was excluded from the model because of overlap with chronic lung disease, and it was felt that the latter was a more specific marker for the sedation/anesthesia associated risk in subjects outside of the neonatal period. Diagnosis category and procedure category were taken from CRISP methodology(5). These were used (rather than the IMPACT® risk adjustment model’s analogous categories) because the CRISP score is the basis for current recommendations for anesthesia care(4). The focus of study was the effect of choice of sedation strategy on outcome and only data that were available prior to the case were included. Elective versus urgent status was not included in the initial model because it is subjective, but as noted previously(6) emergency and salvage cases were excluded. To evaluate the potential for bias, several sensitivity analyses were performed. First, we recalculated models with three strata: AC, sedation with an anesthesiologist, and ODS. This was done to determine if the observed differences between AC and ODS were also seen with sedation cases with an anesthesiologist. Second, because our primary models contained both the CRISP score and many of the individual components of the CRISP score, we also ran models in which the CRISP score was removed, insuring that inclusion of collinear variables had not resulted in errors.
In secondary analysis, analogous models were calculated for case times. Case times are continuous outcomes that are 1) necessarily positive and 2) left skewed. No single strategy is universally accepted for these types of data. Simulation studies have demonstrated that models using a gamma distribution are more robust than other strategies for data with these properties(16). Thus, generalized linear models with a gamma frequency distribution and log link were used. This strategy has been used successfully in previous studies (6, 9, 17–19).
As additional secondary analyses, we sought to measure 1) the degree to which historical practice at the hospitals in our study sample conformed to recent consensus recommendations, and 2) whether practice consistent with these recommendations was associated with improved outcomes. These recommendations state that it is appropriate to perform cases without an anesthesiologist only if the CRISP score is <2 and that cases with CRISP≥2 should be performed with an anesthesiologist (4). These recommendations imply that 1) care by an anesthesiologist reduces the risk of MAE, and 2) risk reduction attributable to anesthesiologist care is proportional to the pre-procedural risk of all AE. We sought to evaluate these two statements by comparing the adjusted risk of MAE in cases across the range of pre-procedure risks. For these comparisons, the ratios of observed to expected (O/E ratio) MAE was used as an outcome expressing risk as a function of the expected risk. The resultant O/E ratios were then compared between ODS and AC cases. For the first analysis we divided these cases into two strata (those with CRISP<2 and those with higher CRISP scores). We hypothesized that case selection by local care teams would have better discrimination than the CRISP-score derived guidelines. If this was correct, 1) the O/E ratio for cases with high CRISP scores performed with ODS would be less than that for cases with lower CRISP scores performed with ODS; and 2) the O/E ratio for AC cases with a low CRISP score would be greater than that for other subgroups, reflecting the notion that this group includes patients at higher risk than predicted by CRISP score. Comparisons of these standardized ratios is qualitative, but 95% confidence intervals were calculated for observed events and O/E ratios to provide a measure of uncertainty. As a secondary analysis, we compared the O/E ratios for ODS and AC cases in each of the CRISP score strata described previously(5). The goal of this was to determine if the difference in outcomes between ODS and AC changed as the estimated pre-procedure risk increased. This would potentially identify if there was a threshold above which AC should be favored over ODS. A post hoc sensitivity analysis was performed restricted to patients under the age of 18 to evaluate to mitigate bias introduced by the inclusion of adults in the sample.
Missing data were limited with two exceptions. As noted previously(13, 20–22), missing data for race and hemodynamic vulnerability are present in >5% of cases. These could not be reasonably imputed from existing data. As in previous studies, a missing variable was created for race to reduce bias. For hemodynamic data, missing data were assumed to be normal. The primary analyses were pre-specified, and other analyses should be considered exploratory. No formal adjustment for multiple comparisons was made. All data analysis was performed using SAS v9.4 (Cary, NC).
Results:
National Utilization of ODS and AC:
During the study period, 110 hospitals contributed information about 165,341 cases. Of the 101 hospitals with sufficient reported case volume, 27% (n=28) utilized ODS for more than 5% of their total case volume (Figure 1). These 28 hospitals have a median volume of 151 cases per year (IQR: 105–373, Range 27–1541) and represent 29% of the total case volume in the IMPACT® registry over the study period (Figure 2).
Figure 1:
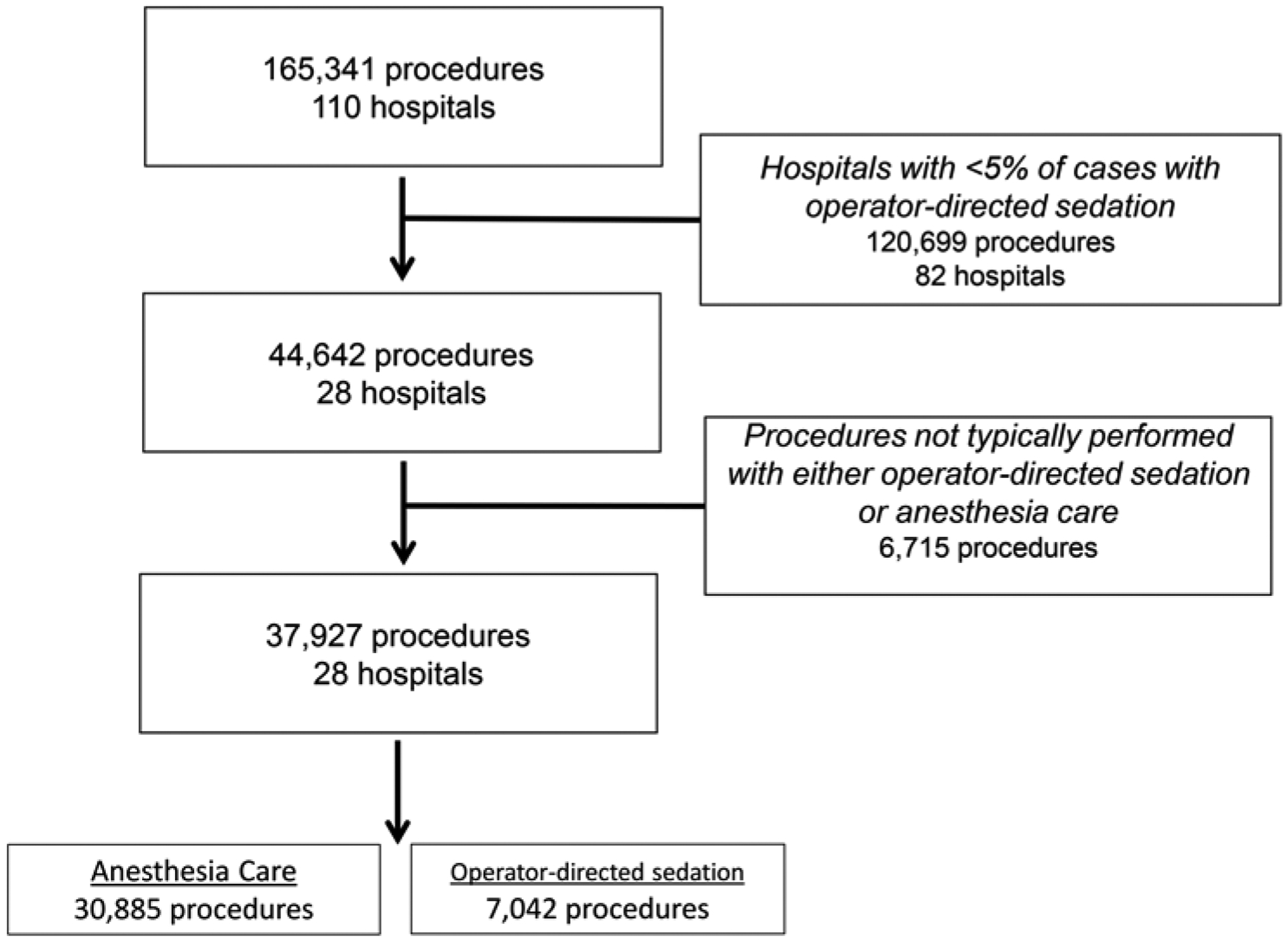
Study population
Figure 2: Hospitals included in the study.
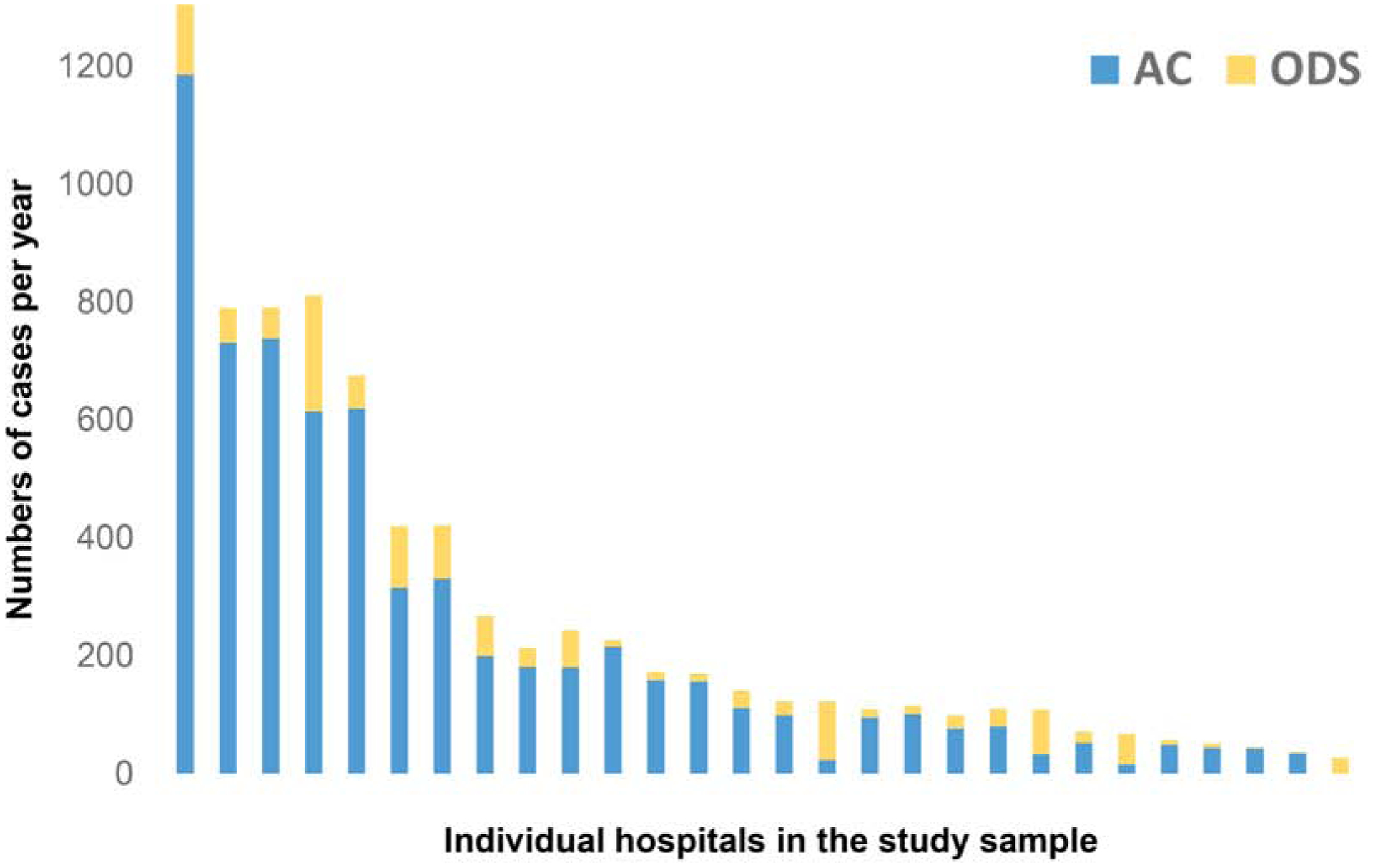
This stacked bar graph depicts the total number of cases using operator-directed sedation (ODS gold) and anesthesia care (AC blue) at each hospital included in the study sorted by decreasing total annual case volume (y-axis). These 28 hospitals reflect 29% of the total cases in the IMPACT® registry.
Over the study period, the overall percentage of cases using ODS has decreased significantly from 19.0% in 2011 to 3.9% in 2018 (p<0.001) (Figure 3). To confirm that the observed trend was not due to addition of hospitals with low utilization of ODS, the trend in ODS utilization amongst hospitals contributing data continuously from 2011 to 2018 was performed, demonstrating an identical pattern (19.1% in 2011 to 3.3% in 2018, p<0.001). Over the same time, the pre-procedural risk of procedures (measured by mean/median CRISP score) of ODS cases decreased (p<0.001) from a mean of 3.2±1.9 in 2011 to 2.9±1.6 in 2018. Similarly, there was also a statistically significant but relatively small decrease in the CRISP score of AC cases, from 4.7± 2.6 in 2011 to 4.6±2.6 in in 2018 (p<0.001) (Supplementary Table 1).
Figure 3: Trends in ODS utilization over the study period.
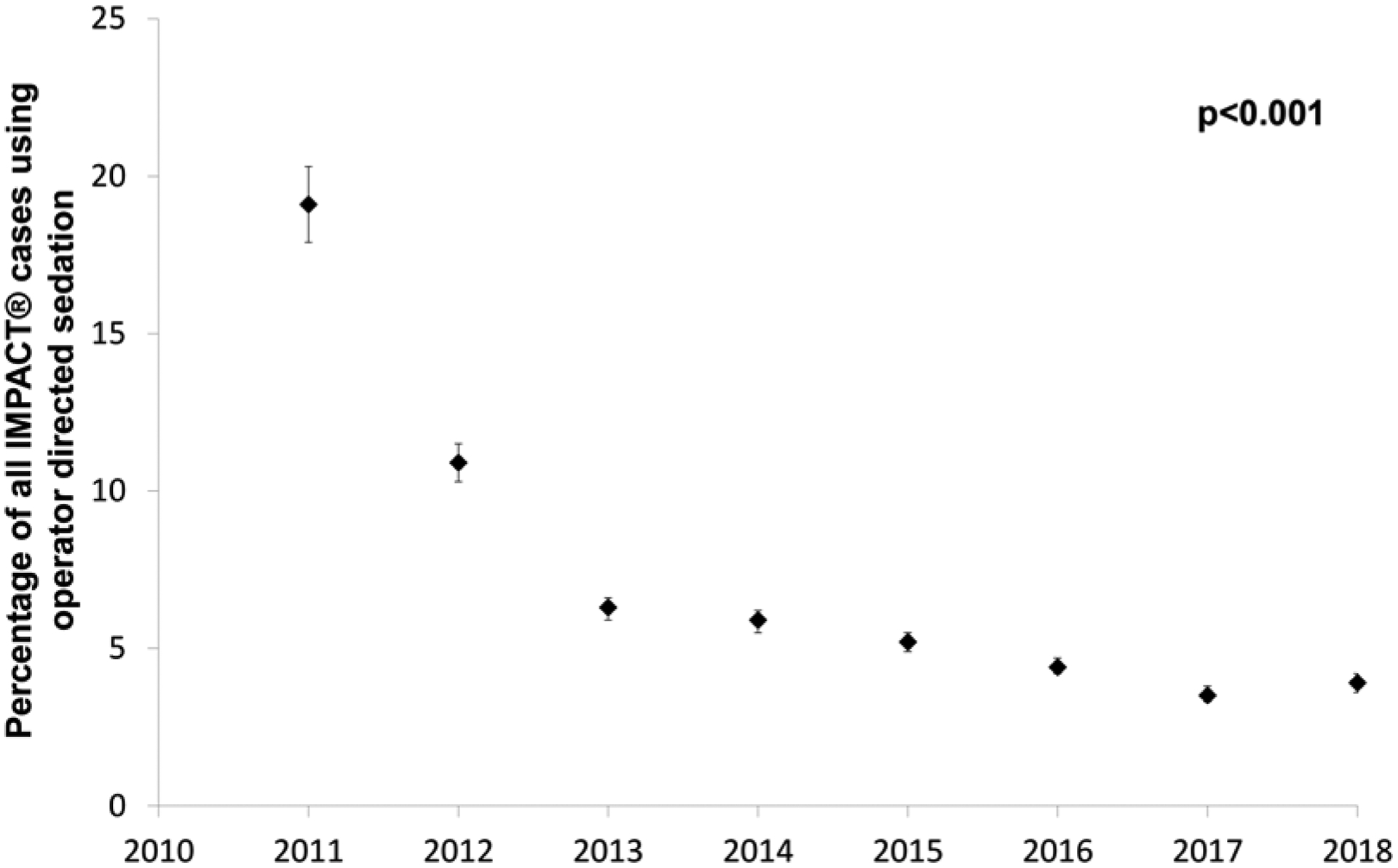
The proportion of cases performed using ODS (black diamonds boxes, brackets reflect 95% confidence intervals) at IMPACT® hospitals over the study period. That percentage decreased significantly over the study period (p<0.001).
Cohort of ODS and comparable AC cases:
After applying inclusion and exclusion criteria, 37,927 cases from 28 hospitals were included in our analysis of which 23% (n=7042) were performed with ODS. The ODS cohort differed from the AC cohort in several ways (Table 1). The proportion of infants (age 30 days to 1 year) was lower (8% vs. 21%) in the ODS cohort and the proportion of adult patients (≥18 years) was higher (58% vs. 13%). The proportion of cases in which subjects had a genetic syndrome, chronic lung disease, single ventricle, renal insufficiency, and pre-procedure inotrope were all higher in AC vs. ODS cohorts. These differences were reflected in higher median CRISP score in AC cohort (4 IQR: 3–6) than ODS (3 IQR: 2–3) cohort.
Table 1:
Study population
| Anesthesia care N=30,885 |
Operator-directed sedation N=7,042 |
p | |
|---|---|---|---|
| Male sex | 16068 (52%) | 3621 (51%) | 0.36 |
| Age | |||
| 30 days – 1 year | 6340 (21%) | 539 (8%) | <0.001 |
| 1–8 years | 12713 (41%) | 989 (14%) | |
| 8–18 years | 7786 (25%) | 1396 (19%) | |
| 18–25 years | 4046 (13%) | 4118 (58%) | |
| Race | |||
| White | 1819 (56%) | 728 (61%) | <0.001 |
| Black | 633 (20%) | 256 (22%) | |
| Asian | 123 (4%) | 50 (4%) | |
| Other/Missing | 661 (20%) | 154 (13%) | |
| Premature infant | 500 (16%) | 73 (6%) | <0.001 |
| Genetic syndrome | 2457 (8%) | 147 (1%) | <0.001 |
| 22q11.2 microdeletion syndrome | 822 (2.7%) | 66 (0.9%) | <0.001 |
| Alagille syndrome | 185 (0.6%) | 5 (0.1%) | <0.001 |
| Trisomy 21 | 1078 (3.5%) | 59 (0.8%) | <0.001 |
| Trisomy 13 | 21 (0.1%) | 2 (0.03%) | 0.22 |
| Trisomy 18 | 26 (0.1%) | 2 (0.03%) | 0.12 |
| Turner syndrome | 101 (0.3%) | 11 (0.2%) | 0.02 |
| Williams-Beuren syndrome | 233 (0.8%) | 4 (0.1%) | <0.001 |
| Coagulation disorder | |||
| Hypocoagulation | 152 (0.5%) | 42 (0.6%) | 0.27 |
| Hypercoagulation | 425 (1%) | 160 (2%) | <0.001 |
| Single ventricle | 6019 (20%) | 727 (10%) | <0.001 |
| Chronic lung disease | 2523 (8%) | 364 (5%) | <0.001 |
| Renal insufficiency | 1096 (4%) | 412 (6%) | <0.001 |
| Hepatic disease | 444 (1%) | 187 (3%) | <0.001 |
| Pre-procedural inotrope | 2521 (8%) | 140 (2%) | <0.001 |
| Status | <0.001 | ||
| Elective | 27971 (91%) | 6546 (93%) | |
| Urgent | 3330 (9%) | 490 (7%) | |
| CRISP Score | 4 (IQR: 3–6) | 3 (IQR: 2–3) | <0.001 |
| CRISP Score >2 | 29555 (96%) | 6198 (88%) | <0.001 |
| Procedure type | |||
| Diagnostic catheterization | 9926 (32%) | 2767 (39%) | <0.001 |
| Endomyocardial biopsy after heart transplant | 6079 (20%) | 1190 (17%) | <0.001 |
| Other endomyocardial biopsy | 194 (0.6%) | 38 (0.5%) | <0.001 |
| Pulmonary vasodilator drug study | 1759 (6%) | 229 (3%) | <0.001 |
| Device closure of patent ductus arteriosus | 658 (2%) | 178 (3%) | 0.17 |
| Device closure of PFO | 2091 (7%) | 509 (7%) | <0.001 |
| Device closure of ASD | 2135 (7%) | 921 (13%) | <0.001 |
| Balloon aortic valvuloplasty | 398 (1%) | 57 (1%) | <0.001 |
| Balloon pulmonary valvuloplasty | 658 (2%) | 178 (3%) | 0.04 |
| Pulmonary artery balloon angioplasty | 2437 (8%) | 124 (2%) | <0.001 |
| Pulmonary artery stent angioplasty | 1467 (5%) | 241 (3%) | <0.001 |
| Pulmonary artery/conduit stent redilation | 876 (3%) | 32 (0.5%) | <0.001 |
| Coarctation balloon angioplasty | 116 (4%) | 14 (1%) | <0.001 |
| Coarctation stent angioplasty | 557 (2%) | 46 (1%) | <0.001 |
| Conduit balloon angioplasty | 614 (2%) | 42 (0.6%) | <0.001 |
| Conduit stent angioplasty | 561 (2%) | 29 (0.4%) | <0.001 |
| Device or coil occlusion of veno-venous collaterals and/or Fontan fenestration | 1080 (4%) | 153 (2%) | <0.001 |
| Device or coil occlusion of arterial collaterals | 1662 (5%) | 57 (0.8%) | <0.001 |
| Multiple of the above interventions | 3775 (12%) | 509 (7%) | <0.001 |
Abbreviations: IQR interquartile range
Risk of major adverse events:
The risk of all MAE in the population was 7% (2496/37927) and was higher (7%) in AC than ODS (4%) cohorts (p<0.001, Table 2). In-hospital deaths within 30 days were more frequent in AC (1.9%) than ODS (0.6%) cohorts (p<0.0001). Receipt of AC was associated with higher risk for cardiac arrest (p=0.01), new arrhythmia (p=0.02), device malposition (p=0.002), vascular complications (p=0.01), and miscellaneous adverse events (p<0.001). ODS was associated with higher risk of initiation of dialysis (p=0.009) and tamponade (p=0.01). Airway events were also more common in ODS cases (p=0.03).
Table 2:
Adverse events
| Anesthesia Care N=30885 |
Operator Directed Sedation N=7042 |
p | |
|---|---|---|---|
| Total | 2248 (7%) | 248 (4%) | <0.001 |
| 30 day in-hospital mortality | 599 (1.9%) | 41 (0.6%) | <0.001 |
| Cardiac arrest | 164 (0.5%) | 21 (0.3%) | 0.01 |
| New arrhythmia | 544 (1.8%) | 96 (1.4%) | 0.02 |
| New heart valve regurgitation | 6 (<0.1%) | 1 (<0.1%) | 0.77 |
| Tamponade | 7 (<0.1%) | 6 (0.1%) | 0.01 |
| Air embolus | 8 (<0.1%) | 1 (<0.1%) | 0.91 |
| Embolic stroke | 19 (0.1%) | 2 (<0.1%) | 0.29 |
| Device malposition | 49 (0.2%) | 1 (<0.1%) | 0.002 |
| Device embolization | 116 (0.4%) | 16 (0.2%) | 0.06 |
| Airway event | 154 (0.5%) | 50 (0.7%) | 0.03 |
| Initiation of dialysis | 9 (<0.1%) | 7 (0.1%) | 0.009 |
| Initiation of extracorporeal membrane oxygenation | 28 (0.1%) | 9 (0.1%) | 0.37 |
| Initiation of ventricular assist device | 6 (<0.1%) | 0 (0%) | 0.24 |
| Bleeding event | 294 (1.0%) | 62 (0.9%) | 0.58 |
| Unplanned cardiac/vascular/other surgery | 7 (0.2%) | 0 (0%) | 0.24 |
| Vascular complication | 171 (0.6%) | 18 (0.3%) | 0.01 |
| Repeat catheterization | 59 (0.2%) | 8 (0.1%) | 0.16 |
| Other | 894 (3%) | 49 (0.7%) | <0.001 |
In models adjusted for pre-procedural risk (Central Figure Panel A), AC was associated with increased odds of MAE relative to ODS (OR: 1.2, 95% CI: 1.03–1.40 p=0.02). As a secondary analysis, risk of MAE was compared between ODS, sedation with an anesthesiologist, and general endotracheal anesthesia (Supplementary Table 2). In this analysis, the odds of MAE for cases with sedation provided by an anesthesiologist and those with ODS were not statistically different (p=0.55). Cases with general endotracheal intubation continued to be associated with greater odds of MAE (OR: 1.82, 95% CI: 1.54–2.17, p<0.0001). Sensitivity analyses in which the CRISP score was taken out of the model had no effect on the observed associations (Supplementary Table 3). In a sensitivity analysis restricted to patients <18 years of age, the association between sedation strategy and risk was unchanged in its direction (OR: 1.04) but not statistically significant (p=0.66, rest of data not shown).
Central Figure: Multivariable model for risk of major adverse events.
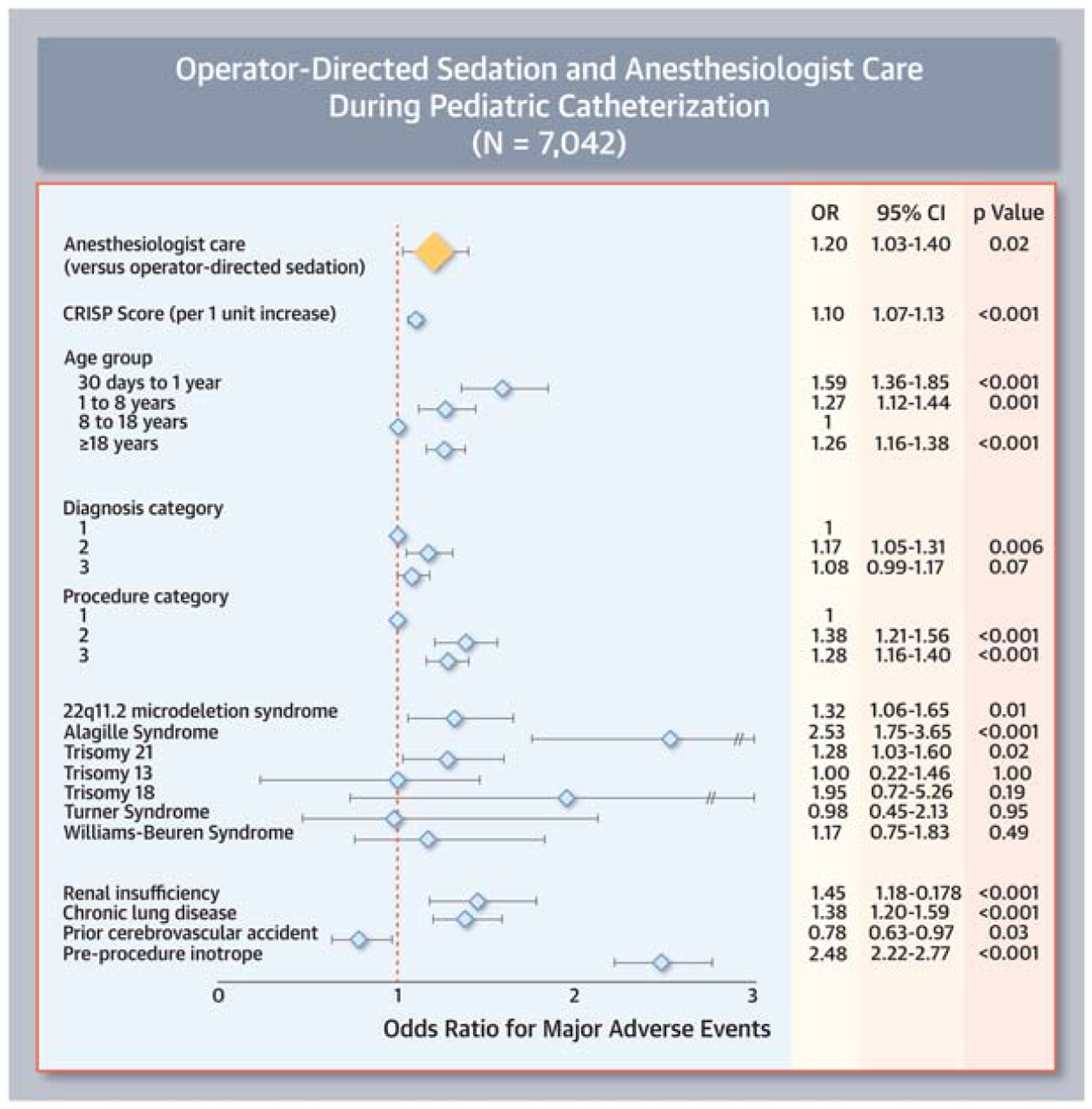
This Forest plot demonstrates that after adjusting for measurable confounders (light blue diamonds) use of anesthesiologist care was associated with increased odds of major adverse events (yellow diamond). 95% Confidence intervals are depicted as brackets.
In a pre-planned secondary analysis, we evaluated the degree to which practice at the institutions in our study sample conformed to the recent SCAI-CHD/SPA/CCAS consensus document. Of ODS cases, the overwhelming majority fell outside of current recommendations; only 12% (844/7042) had CRISP scores (CRISP <2) that would have been “appropriate” to be performed without an anesthesiologist. According to these recommendations, 4% (1330/30885) of cases performed with AC could have appropriately been performed with ODS (CRISP score <2).
Given this discrepancy, we sought to evaluate whether the case-mix adjusted risk of adverse event (represented by O/E ratio for MAE) was different between cases where there was a deviation from guidelines (Figure 4). The point estimate for O/E ratio for ODS cases with higher CRISP scores (1.2, 95% CI: 1.0–1.4) was significantly lower than that of high CRISP score cases performed with AC (1.5, 95% CI: 1.5–1.6) as well as AC cases in subjects with CRISP<2 whose O/E ratio (3.3, 95% CI: 2.3–4.2) was not significantly different from O/E ratio for ODS cases with CRISP<2 (3.9, 95% CI: 2.6–5.2).
Figure 4: Ratios of observed to expected major adverse events.
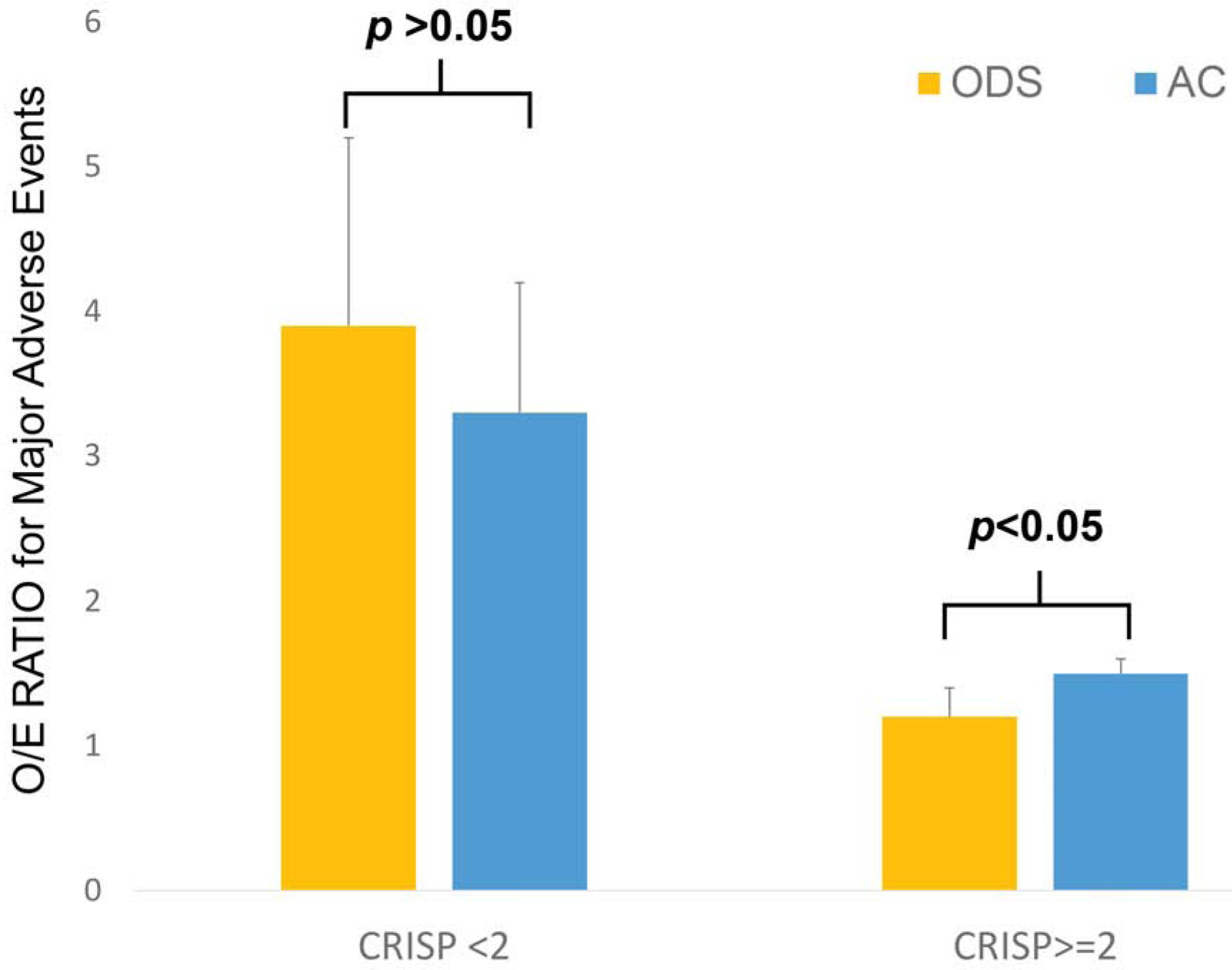
Ratio of observed to expected outcomes (O/E ratio) for operator directed sedation (gold) and general anesthesia (blue) cases are depicted along with the top bound of 95% confidence intervals. Cases divided according to categories of CRISP score as per recent CHD-SCAI/SPA/CCAS recommendations.
To evaluate whether there was a threshold based on CRISP scores where cases in current practice would have a higher O/E ratio for ODS cases than AC counterparts, O/E ratios were calculated for the full range of CRISP scores (Table 3). There is no threshold level for CRISP score at which the O/E ratio was significantly higher for the ODS cases than that for AC cases, and the O/E ratio is significantly lower for ODS cases for CRISP score between 0–2 and 3–5.
Table 3:
Ratios of observed to expected major adverse events across range of CRISP Scores
| CRISP SCORE | Operator directed sedation | Anesthesiologist care |
|---|---|---|
| 0–2 | 2.9 (2.2–3.7) | 3.8 (3.2–4.3) |
| 3–5 | 1.0 (0.8–1.2) | 1.7 (1.5–1.8) |
| 6–9 | 1.4 (1.1–1.8) | 1.5 (1.4–1.6) |
| 10–14 | 1.2 (0.7–1.8) | 1.2 (1.2–1.3) |
| 15+ | n/a | 0.6 (0.1–1.0) |
Comparison of case times:
Case-mix adjusted models were calculated to compare case times between ODS and AC cases. AC was associated with higher fluoroscopy time (ratio: 1.08, 95% CI 1.06–1.11, p<0.0001, Table 4) and total case time (ratio: 1.13, 95% CI: 1.11 to 1.15, p<0.0001, Table 5) relative to ODS.
Table 4:
Multivariable model for fluoroscopy time
| Ratio | 95% CI | p | |
|---|---|---|---|
| Anesthesiologist care (versus Operator directed sedation) | 1.08 | 1.06–1.11 | <0.0001 |
| CRISP (per 1 unit increase) | 1.06 | 1.06–1.07 | <0.0001 |
| Age group category | |||
| 30 days to 1 year | 1.09 | 1.06–1.13 | <0.0001 |
| 1 year to 8 years | 1.23 | 1.21–1.26 | <0.0001 |
| 8 years to 18 years | 1 | n/a | n/a |
| ≥18 years | 1.29 | 1.26–1.32 | <0.0001 |
| Diagnosis category | |||
| 1 | 1 | n/a | n/a |
| 2 | 1.43 | 1.40–1.46 | <0.0001 |
| 3 | 1.04 | 1.00–1.46 | 0.03 |
| Procedure category | |||
| 1 | 1 | n/a | n/a |
| 2 | 1.49 | 1.45–1.54 | <0.0001 |
| 3 | 1.94 | 1.86–2.03 | <0.0001 |
| 22q11.2 microdeletion syndrome | 1.26 | 1.20–1.33 | <0.0001 |
| Alagille syndrome | 1.26 | 1.12–1.41 | 0.0001 |
| Trisomy 21 | 1.05 | 1.00–1.10 | 0.04 |
| Trisomy 13 | 1.20 | 0.88–1.63 | 0.25 |
| Trisomy 18 | 1.30 | 0.99–1.69 | 0.06 |
| Turner syndrome | 0.81 | 0.70–0.94 | 0.006 |
| Williams-Beuren syndrome | 1.09 | 0.99–1.21 | 0.09 |
| Renal insufficiency | 0.86 | 0.82–0.89 | <0.0001 |
| Chronic lung disease | 1.07 | 1.04–1.11 | <0.0001 |
| Prior cerebrovascular accident | 0.89 | 0.86–0.91 | <0.0001 |
| Pre-procedure inotrope | 1.28 | 1.25–1.32 | <0.0001 |
Table 5:
Multivariable model for total case time
| Ratio | 95% CI | p | |
|---|---|---|---|
| Anesthesiologist care (versus Operator directed sedation) | 1.13 | 1.11–1.15 | <0.0001 |
| CRISP (per 1 unit increase) | 1.05 | 1.04–1.05 | <0.0001 |
| Age group category | |||
| 30 days to 1 year | 0.99 | 0.96–1.01 | 0.19 |
| 1 year to 8 years | 1.10 | 1.08–1.12 | <0.0001 |
| 8 years to 18 years | 1 | n/a | n/a |
| ≥18 years | 1.28 | 1.26–1.30 | <0.0001 |
| Diagnosis category | |||
| 1 | 1 | n/a | n/a |
| 2 | 1.28 | 1.26–1.30 | <0.0001 |
| 3 | 1.14 | 1.11–1.16 | <0.0001 |
| Procedure category | |||
| 1 | 1 | n/a | n/a |
| 2 | 1.35 | 1.33–1.38 | <0.0001 |
| 3 | 1.43 | 1.38–1.47 | <0.0001 |
| 22q11.2 microdeletion syndrome | 1.11 | 1.07–1.16 | <0.0001 |
| Alagille syndrome | 1.06 | 0.98–1.15 | 0.16 |
| Trisomy 21 | 1.12 | 1.09–1.16 | <0.0001 |
| Trisomy 13 | 1.33 | 1.05–1.67 | 0.02 |
| Trisomy 18 | 1.11 | 0.91–1.6 | 0.30 |
| Turner syndrome | 0.94 | 0.84–1.04 | 0.23 |
| Williams-Beuren syndrome | 0.97 | 0.90–1.05 | 0.49 |
| Renal insufficiency | 0.81 | 0.79–0.84 | <0.0001 |
| Chronic lung disease | 1.08 | 1.06–1.10 | <0.0001 |
| Prior cerebrovascular accident | 0.89 | 0.87–0.91 | <0.0001 |
| Pre-procedure inotrope | 1.11 | 1.09–1.14 | <0.0001 |
Discussion:
This multicenter retrospective cohort study evaluated the experience of North American PCCL programs’ use of ODS for a subset of catheterization procedures between 2011 and 2018. For a subset of procedures performed habitually with ODS, use of ODS was associated with a lower risk of MAE compared to similar cases performed using AC. This was true not only in comparisons of observed rates of MAE but also in analyses adjusted for case mix. As a secondary analysis, the ramifications of recent published guidelines were evaluated. Nearly 90% of current ODS cases would be considered “inappropriate” by these guidelines. However, the case-mix adjusted risk of MAE was not higher in these “inappropriate” cases. In fact, the O/E ratio was lower than the O/E ratio in similar cases with “appropriate” use of AC. Looking at the range of procedures in which ODS is used, in the lower risk strata the case-mix adjusted risk of MAE was lower in ODS. Above a CRISP score of 5, there was no significant difference in case-mix adjusted risk of MAE. In addition, our analysis of case times found that both fluoroscopy time and total case times were lower in ODS cases, suggesting that the expected benefits of AC (greater control over patient hemodynamic stability and movement) were not manifest as reduced procedural case times. The duration of the periods before vascular access and after the interventional cardiologist breaks scrub, i.e. the times where anesthesia care might be expected to be associated with largest marginal increases in case time for induction, intubation, and extubation, are not recorded in the IMPACT® registry. It would be reasonable to expect that the increases in time associated with AC would be even greater for these periods and (in combination with observed increases in time) would result in even greater increases in the total time spent in the catheterization laboratory for each case. This was previously demonstrated in our previous single center study, where use of general anesthesia was associated with an increase in both case-mix adjusted total room time (120% p<0.001) and exit time (167% p<0.001)(6). These findings reinforce that, for a subset of PCCL procedures, the use of ODS is associated with high quality efficient care, and that there is no evidence for this subset of procedures that the alternative use of AC would improve outcomes. The observation that ODS was associated with shorter fluoroscopy times was not expected. This is likely due to unmeasured confounding, that the ODS cases were more technically simple (thereby requiring less fluoroscopy time). Though it is speculation, these differences may be additional evidence that the care team is successfully selecting cases for ODS. At the very least, it demonstrates that for the selected cases additional fluoroscopy time is not incurred because of patient instability or movement with ODS.
These findings are consistent with previous work reviewing the experience using ODS over a similar time frame at one large-volume PCCL program, which demonstrated that ODS was associated with lower odds of MAE and shorter procedural times than AC(6). That study also demonstrated that these cases were also associated with reduced charges relative to AC cases. Care was taken to address confounding by indication using propensity score adjustment, but a major limitation of this study was its applicability. Specifically, it was not clear whether the observed benefits were generalizable to other PCCL programs. The current study addresses this concern directly, demonstrating that, in a sample of nearly thirty programs of varying sizes, similar benefits for ODS cases were observed. It is important to note that these findings do not imply that the involvement of an anesthesiologist increases the risk of MAE. Rather, these findings suggest that local care teams are able to identify patients and procedures in whom the use of ODS is safe and appropriate, presumably based on factors that are not incorporated in the CRISP score. Further, these findings suggest that reliance on practice guidelines based on a pre-procedural risk score that was not derived with the goal of informing sedation strategy may be misguided, as it appears that the CRISP score does not fully capture the risk that is mitigated by the presence of an anesthesiologist.
Across disciplines, the conventional wisdom states that formalizing sedation practice is necessary because patients are being placed at excessive risk by current practices. We agree that identifying the boundaries of good practice is an important aspect of providing high quality care. To try to achieve this, the current CHD-SCAI/SPA/CCAS guidelines make a series of well-intentioned assumptions about how the use of anesthesia might improve safety. Their recommendations assume that an important fraction of MAE could be mitigated or eliminated by the presence of an anesthesiologist. It also assumes that the risk of pooled adverse events is an accurate way to determine which cases would be best served by the presence of an anesthesiologist. The current study demonstrates that neither of these intuitions are true for the subset of procedures historically performed with ODS. This is likely due to the fact that the benefit of having an anesthesiologist present is not the same for all adverse events, and that the potential benefit is not proportional to the risk of adverse events. In addition, the guidelines call for an anesthesiologist with “special expertise in congenital heart disease”, the extent of whose experience varies widely between centers. There is a natural desire to try to formulate a simple metric to guide clinical decision-making, but this example underscores the importance of using metrics that specifically address the risk that is mitigated by the intervention in question. It is also possible that different procedures may be more or less facilitated by the presence of an anesthesiologist (e.g. procedures that are particularly painful, where immobility is more important, or where there is greater potential for hemodynamic instability). However, it is clear that across centers, a subset of case types (diagnostic procedures, valvuloplasty, angioplasty, and some relatively straightforward closure/occlusion procedures) has been identified that can be performed safely without anesthesia (and that these procedures defy current recommendations).
It is not possible in the current study to determine how much of an effect the CHD-SCAI/SPA/CCAS recommendations have had on practice. In fact, it appears that ODS use was decreasing in frequency even before the 2016 publication of the guidelines. What is clear is that these recommendations are part of a larger trend to regulate and limit the use of procedural sedation, and that overall ODS is being used far less frequently now than in the previous decade and that the population receiving ODS is in small but real ways a lower risk strata. Because ODS (like any clinical practice) requires a minimum volume to maintain quality practice, this trend may result in the use of ODS becoming impractical at individual centers and more broadly. This would be regrettable because as the current study and previous research(6) have shown, ODS represents an example of high value care (both safe and efficient in terms of time and economic impact). Eliminating it because of well-intentioned but misconceived recommendations represents a missed opportunity to provide the best care to patients undergoing catheterization procedures.
Moving forward, it is incumbent to consider whether there are generalizable lessons about producing guidelines that can be drawn from this example. Across congenital cardiology, there are limitations in the quality of data available upon which to base recommendations. Almost all practice guidelines in congenital cardiac catheterization are based in consensus and extrapolation from limited observational studies instead of clinical trials(23). The availability of registries, such as IMPACT®, provide an opportunity to base recommendations on real-world data. The current study shows how recommendations can be evaluated retrospectively, but the same techniques could be used prior to publication of guidelines (or even in preparation of them) to evaluate whether 1) the guideline would change practice and 2) whether the change would be associated with improved outcomes. A data-driven approach might remedy the current situation in which there is demonstrable evidence that adherence to guidelines is poor(24, 25).
Limitations:
We acknowledge that this, as with all studies, has limitations. Identifying the proportion of major adverse events attributable to sedation practices in the ODS and AC subgroups would be useful. Adjudicating culpability for individual events is not possible in this observational study, so all MAE were studied without restriction. Counting how often adverse events were averted or rescued in both case types would be informative, but this was also not possible. Finally, we acknowledge that the definitions of AE in the CRISP methodology and in IMPACT® (the method used in our catheterization laboratory database) differ. The IMPACT® definitions include more minor events that would not be included in the CRISP model. The presented AE rates and O/E ratios are therefore inflated.
There are several other limitations to this study. The study population was limited to the practice at the institutions using ODS regularly. The results of this study are not, strictly speaking, generalizable beyond the studied procedures. However, the fact that some procedures were not done habitually with ODS does not imply that it is not possible/appropriate to do so. Though care was chosen in data collected and analysis, we also acknowledge the possibility of unmeasured confounding. We acknowledge that the anesthesia expertise in pediatric cardiac anesthesia is not recorded in the IMPACT® Registry, and that the potential bias introduced by this is not ascertainable. It is also not possible to determine the training path of the interventional cardiologist (internal medicine vs. pediatrics), nor is it possible to determine whether cases are performed at free-standing children’s or general hospitals. The age at which AC is preferable to ODS is not evaluable in this study. The majority of subjects were adults, but analyses were performed adjusting for age mitigating bias. In a post hoc sensitivity analysis restricted to subjects <18 years, the association between AC and increased risk of AE was no longer statistically significant. However, this analysis was limited by the inevitable reduction in statistical power and therefore susceptible to type II error. Finally, even in this relatively large sample, the number of cases for individual procedures was not sufficient to evaluate the relative risk for ODS and AC for each procedure.
Conclusion:
Acknowledging these limitations, we conclude that the current study demonstrates that ODS can be used across a range of centers with safety. Current recommendations using pre-procedural risk of MAE to determine whether ODS is appropriate are ineffective and perform less well than the clinical judgment of teams that habitually use ODS. These findings should inform future guidelines governing the use of procedural sedation in PCCL.
Supplementary Material
CLINICAL PERSPECTIVE:
What’s known?
Operator-directed sedation (ODS) has been used broadly in pediatric/congenital cardiac catheterization. However, the safety of ODS relative to care by an anesthesiologist has been questioned, and its “appropriate” use is restricted to the lowest risk strata of patients in current practice guidelines. A previous single-center study demonstrated that ODS was associated with a reduced risk of major adverse events, lower cost, and shorter procedure time, but the generalizability of these findings have not been studied.
What’s new?
Using data from the IMPACT® registry, the current study demonstrated that ODS for diagnostic and interventional procedures in the pediatric/congenital cardiac catheterization laboratory was safe with at least comparable safety to the anesthesia care for comparable procedures. The non-inferiority of ODS was demonstrated across the entire range of pre-procedural risks. Expected benefits in terms of intra-procedural time were not seen. This suggests that the judicious use of operator directed sedation provides safe, effective, and efficient care for young patients with cardiac disease.
What’s next?:
Previous guidelines for the use of anesthesia care during pediatric/congenital cardiac catheterization cases were based on a score estimating the pre-procedural risk of composite adverse events. It is important to direct future guidelines to risk adjustment systems that address the attributable risk of the practice. Additionally, without randomized clinical trials to guide care, the current study demonstrates how, in the future, clinical registries may provide a means to evaluate future guidelines prior to publication.
Funding:
Dr. O’Byrne receives research support from the National Institute of Health/National Heart Lung and Blood Institute (K23 HL130420-01). Dr. Hill receives research support from the National Centers for Advancing Translational Sciences (U01TR-001803-01). Dr. Chamberlain is supported by the National Institute of General Medical Sciences and the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (T32GM086330). The specific study was supported by the American College of Cardiology Foundation. It was reviewed by the IMPACT® Registry research and publications committee during its planning as was the resulting manuscript. However, the funding agencies had no role in the planning or execution of the study, nor did they edit the manuscript as presented. The manuscript represents the opinions of the authors alone.
ABBREVIATIONS:
- AC
anesthesiologist
- AE
adverse event
- CHD-SCAI/SPA/CCAS
Congenital Heart Disease section of the Society for Cardiac Angiography and Intervention, Society for Pediatric Anesthesia, and Congenital Cardiac Anesthesia Society
- CRISP
Catheterization Risk Score for Pediatrics
- IMPACT®
IMProving Adult and Congenital Treatment registry
- IQR
interquartile range
- MAE
major adverse event
- ODS
operator directed sedation
- PCCL
pediatric congenital cardiac catheterization laboratory
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: No pertinent financial conflicts of interest to disclose.
References:
- 1.Vincent RN, Moore J, Beekman RH, et al. Procedural characteristics and adverse events in diagnostic and interventional catheterisations in paediatric and adult CHD: initial report from the IMPACT Registry. Cardiology in the Young 2016;26:70–78. [DOI] [PubMed] [Google Scholar]
- 2.Moore JW, Vincent RN, Beekman RH, et al. Procedural results and safety of common interventional procedures in congenital heart disease: initial report from the National Cardiovascular Data Registry. J Am Coll Cardiol 2014;64:2439–2451. [DOI] [PubMed] [Google Scholar]
- 3.Lin CH, Desai S, Nicolas R, et al. Sedation and Anesthesia in Pediatric and Congenital Cardiac Catheterization: A Prospective Multicenter Experience. Pediatr Cardiol 2015;36:1363–1375. [DOI] [PubMed] [Google Scholar]
- 4.Odegard KC, Vincent R, Baijal R, et al. SCAI/CCAS/SPA expert consensus statement for anesthesia and sedation practice: Recommendations for patients undergoing diagnostic and therapeutic procedures in the pediatric and congenital cardiac catheterization laboratory. 2016;88:912–922. [DOI] [PubMed] [Google Scholar]
- 5.Nykanen DG, Forbes TJ, Du W, et al. CRISP: Catheterization RISk score for pediatrics: A Report from the Congenital Cardiac Interventional Study Consortium (CCISC). Cathet. Cardiovasc. Intervent 2015. [DOI] [PubMed] [Google Scholar]
- 6.O’Byrne ML, Millenson ME, Steven JM, et al. Operator-Directed Procedural Sedation in the Congenital Cardiac Catheterization Laboratory. JACC Cardiovasc Interv 2019;12:835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Messenger JC, Ho KKL, Young CH, et al. The National Cardiovascular Data Registry (NCDR) Data Quality Brief: the NCDR Data Quality Program in 2012. J Am Coll Cardiol 2012;60:1484–1488. [DOI] [PubMed] [Google Scholar]
- 8.O’Byrne ML, Gillespie MJ, Shinohara RT, Dori Y, Rome JJ, Glatz AC. Cost comparison of transcatheter and operative closures of ostium secundum atrial septal defects. Am Heart J 2015;169:727–735.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Byrne ML, Gillespie MJ, Shinohara RT, Dori Y, Rome JJ, Glatz AC. Cost comparison of Transcatheter and Operative Pulmonary Valve Replacement (from the Pediatric Health Information Systems Database). Am J Cardiol 2016;117:121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuller S, Ramachandran A, Awh K, et al. Comparison of outcomes of pulmonary valve replacement in adult versus paediatric hospitals: institutional influence†. Eur J Cardiothorac Surg 2019;56:891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cepeda MS, Boston R, Farrar JT, Strom BL. Comparison of logistic regression versus propensity score when the number of events is low and there are multiple confounders. AmJEpidemiol 2003;158:280–287. [DOI] [PubMed] [Google Scholar]
- 12.Jayaram N, Spertus JA, Kennedy KF, et al. Modeling Major Adverse Outcomes of Pediatric and Adult Patients With Congenital Heart Disease Undergoing Cardiac Catheterization: Observations From the NCDR IMPACT Registry (National Cardiovascular Data Registry Improving Pediatric and Adult Congenital Treatment). Circulation 2017;136:2009–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jayaram N, Spertus JA, O’Byrne ML, et al. Relationship between hospital procedure volume and complications following congenital cardiac catheterization: A report from the IMproving Pediatric and Adult Congenital Treatment (IMPACT) registry. Am Heart J 2017;183:118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Byrne ML, Glatz AC, Shinohara RT, et al. Effect of center catheterization volume on risk of catastrophic adverse event after cardiac catheterization in children. Am Heart J 2015;169:823–832.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergersen L, Gauvreau K, Foerster SR, et al. Catheterization for Congenital Heart Disease Adjustment for Risk Method (CHARM). JACC Cardiovasc Interv 2011;4:1037–1046. [DOI] [PubMed] [Google Scholar]
- 16.Manning WG, Basu A, Mullahy J. Generalized modeling approaches to risk adjustment of skewed outcomes data. J Health Econ 2005;24:465–488. [DOI] [PubMed] [Google Scholar]
- 17.O’Byrne ML, Shinohara RT, Grant EK, et al. Increasing propensity to pursue operative closure of atrial septal defects following changes in the instructions for use of the Amplatzer Septal Occluder device: An observational study using data from the Pediatric Health Information Systems database. Am Heart J 2017;192:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Byrne ML, Glatz AC, Mercer-Rosa L, et al. Trends in pulmonary valve replacement in children and adults with tetralogy of fallot. Am J Cardiol 2015;115:118–124. Available at: http://linkinghub.elsevier.com/retrieve/pii/S0002914914019389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Byrne ML, Glatz AC, Faerber JA, et al. Interhospital Variation in the Costs of Pediatric/Congenital Cardiac Catheterization Laboratory Procedures: Analysis of Data From the Pediatric Health Information Systems Database. J Amer Heart Assoc 2019;8:e011543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jayaram N, Beekman RH, Benson L, et al. Adjusting for Risk Associated With Pediatric and Congenital Cardiac Catheterization: A Report From the NCDR IMPACT Registry. Circulation 2015;132:1863–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Byrne ML, Kennedy KF, Jayaram N, et al. Failure to Rescue as an Outcome Metric for Pediatric and Congenital Cardiac Catheterization Laboratory Programs: Analysis of Data From the IMPACT Registry. J Amer Heart Assoc 2019;8:e013151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Byrne ML, Kennedy KF, Kanter JP, Berger JT, Glatz AC. Risk Factors for Major Early Adverse Events Related to Cardiac Catheterization in Children and Young Adults With Pulmonary Hypertension: An Analysis of Data From the IMPACT (Improving Adult and Congenital Treatment) Registry. J Amer Heart Assoc 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feltes TF, Bacha E, Beekman RH, et al. Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from the American Heart Association. Circulation 2011;123:2607–2652. [DOI] [PubMed] [Google Scholar]
- 24.Glatz AC, Kennedy KF, Rome JJ, O’Byrne ML. Variations in Practice Patterns and Consistency With Published Guidelines for Balloon Aortic and Pulmonary Valvuloplasty. JACC Cardiovasc Interv 2018;11:529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Byrne ML, Kennedy KF, Rome JJ, Glatz AC. Variation in practice patterns in device closure of atrial septal defects and patent ductus arteriosus: An analysis of data from the IMproving Pediatric and Adult Congenital Treatment (IMPACT) registry. Am Heart J 2018;196:119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


