Abstract
An emergency operation was planned for a patient who developed pneumothorax, subcutaneous emphysema and pneumomediastinum, which was thought to develop secondary to acute diverticulitis. Polymerase chain reaction (PCR) test for coronavirus disease 2019 (COVID-19) diagnosis could not be performed before the operation. In COVID-19 infection, it has been reported that pneumonia, pneumomediastinum and subcutaneous emphysema could be seen in thoracic computed tomography (CT) scan in addition to classic ground-glass opacities. In this study, a modified closed chest drainage system (CCDS) is presented to prevent COVID-19 aerosolisation in a patient undergoing intraoperative tube thoracostomy.
Keywords: Chest drainage system, COVID-19, diverticulitis, subcutaneousemphysema
Introduction
Colon perforation is among the complications of acute diverticulitis, which leads to pneumomediastinum, pneumothorax and subcutaneous emphysema and may require surgery as a treatment protocol (1). During the period of coronavirus disease 2019 (COVID-19) pandemic, it should be noted that patients who need to be operated urgently may be asymptomatic, and the risk of healthcare workers’ exposure to the disease should be avoided. It has been reported that thoracic computed tomography (CT) scan may show images of pneumothorax, pleural effusion, pneumomediastinum and subcutaneous emphysema apart from the classic ground-glass appearance in COVID-19 infection (2, 3).
In this study, we explained our approach as an anaesthesiology team to a patient undergoing an emergency surgery, and also explained our modified closed chest drainage system.
Case Presentation
An 81-year-old male patient who had no systemic disease other than hypertension was admitted to the emergency room with a complaint of right lower quadrant pain in his abdomen, constipation and shortness of breath that had been going on for a week. The patient’s general condition was normal; he was conscious, cooperative and oriented. His blood pressure (BP) was 160/90 mmHg, heart rate (HR) was 86 beats min−1 and body temperature was 36.8°C. There were bilateral crepitant crackles in the lungs, abdominal distension and rebound tenderness and extensive subcutaneous emphysema in the neck. In laboratory tests, leucocytosis (25.5 × 103μL−1) and lymphopenia (0.71×103μL−1) were detected; C-reactive protein (CRP) (349 m gL−1), D-dimer (12.4 μg mL−1), ferritin (279 ng dL−1), lactate dehydrogenase (LDH) (604 UL−1) and prothrombin time (PT) (17.2 s), international normalized ratio (INR) (1.28) levels were found to be increased. The liver and kidney functions were impaired. In arterial blood gas (ABG) with oxygen supported via face mask, pH was 7.30, partial pressure of carbon dioxide (PaCO2) was 30 mmHg, partial pressure of oxygen (PaO2)was 47.8 mmHg, arterial oxygen saturation (SaO2) was 76.9% mmHg, lactate was 43 mg dL−1, and BE was −10.7. Electrocardiogram (ECG) showed a normal sinus rhythm. In the CT scan, subcutaneous emphysema in the neck and thoracic wall, consolidation in the bilateral lower lobe of the lungs, pneumomediastinum, bilateral pneumothorax and air patches in the pericaecal area and a necrotic collection at the right lower quadrant were observed (Figure 1). It was stated that these findings may have developed secondary to perforated caecal diverticulitis. An emergency laparotomy was planned. Because pneumothorax was minimal, thoracic surgery was not considered as a preoperatvie intervention.
Figure 1.
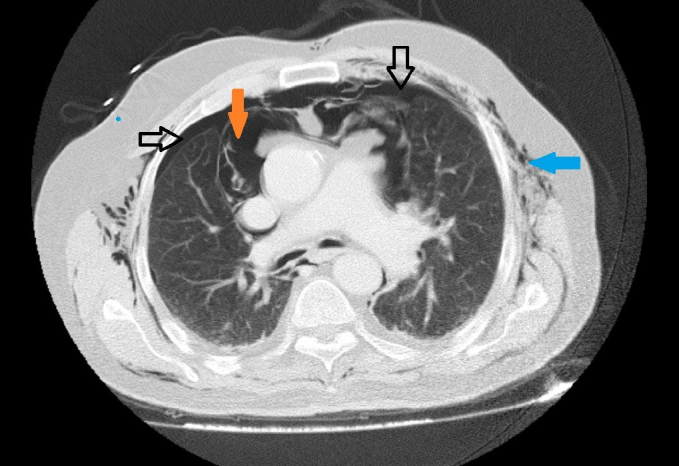
Subcutaneous emphysema in the thoracic wall, pneumomediastinum and bilateral millimetre-sized pneumothorax was observed in the thoracic computed tomography scan of the patient before the operation
Black arrows: pneumothorax
Orange arrow: pneumomediastinum
Blue arrow: subcutaneous emphysema
All the healthcare providers used personal protective equipment (PPE). The patient was taken to the operation room and the following values were monitored: BP: 135/86 mmHg, HR: 92 beats min−1 and SpO2: 85%. After intravenous anaesthesia induction with propofol and fentanyl, a neuromuscular agent was given, and the patient was intubated with rapid sequence intubation with video laryngoscope. In the mechanical ventilator, the tidal volume was set at 6 mL kg−1 and the frequency was 10 min−1 in volume control mode. In the 10th minute of anaesthesia, peripheral oxygen saturation (SpO2) of the patient began to decrease gradually. Peak and plateau airway pressures were found to be increased. With auscultation, respiratory sounds could not be obtained in the left hemithorax and pneumothorax was thought to be increased, and hence, a thoracic surgery consultation was requested. In ABG analysis with 100% oxygen, pH was 7.22, PaCO2 was 40 mmHg, PaO2 was 57 mmHg and SaO2 was 83%. Thoracentesis was performed on the left hemithorax; air was aspirated. After tube thoracostomy was performed, it was connected to a closed chest drainage system (CCDS). Owing to COVID-19 infection suspicion, CCDS, which has a high risk of aerosolisation, was modified as suggested by Bilkhu et al. (4). The 9:0 endotracheal tube was cut approximately 5 cm at its proximal end portion; a bacterial/viral respiratory filter (Disposet, Ankara, Turkey) was inserted on its tip, and it was placed into the open end of the CCDS (Figure 2). It was ensured that oscillations continued. Until the end of the surgery, fraction of inspired oxygen (FiO2) could be reduced by up to 50%. pH was 7.30, PaCO2 was 38.00 mmHg, PaO2 was 74.6 mmHg, lactate was 40 mg dL−1, BE was −10 and SaO2 was 89.6% in ABG. Haemodynamics remained stable during the intraoperative period. The patient received vacuum-assisted surgical drainage treatment after drainage and colostomy. Laparotomy was preferred over laparoscopy, and the use of electrocautery was minimised to prevent viral aerosolisation. After the surgery, the patient was shifted to an isolated room in the intensive care unit, as he was intubated. Polymerase chain reaction (PCR) test COVID-19 detection was requested, and the patient was treated as a COVID-19 suspect patient until the test results were obtained. No expansion defect in bilateral hemithorax was observed on the anteroposterior chest X-ray image taken after tube thoracostomy (Figure 3). When the patient’s PCR test result was negative after 5 hours, the part mounted to the CCDS was removed.
Figure 2.

Closed drainage system and the mounted part
Black arrow: closed chest drainage system
Yellow arrow: intubation tube part
Blue arrow: bacterial/viral respiratory filter
Figure 3.
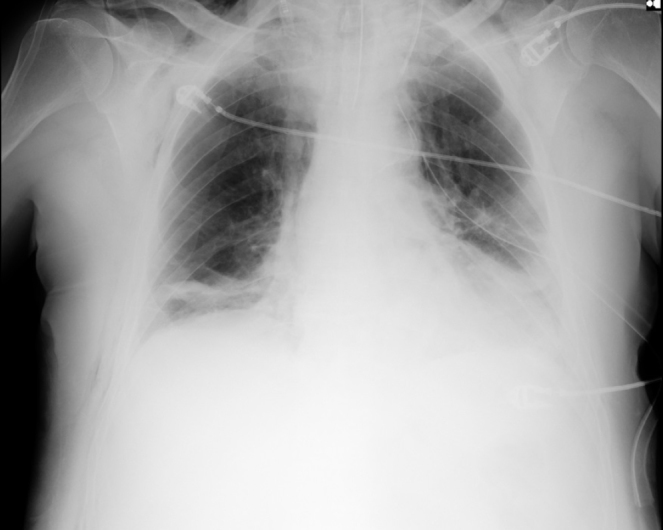
Anteroposterior chest radiography after tube thoracostomy
The relatives of the patient were informed about the patient’s condition and consent was obtained from a first-degree relative to use their data for scientific studies.
Discussion
In this case, we considered the patient as a COVID-19 suspect patient. The reason for this is that laboratory test results supported COVID-19 infection (lymphopenia; elevated CRP, LDH, ferritin and D-dimer levels; crepitant crackles; respiratory distress with hypoxia and basal consolidation findings in thoracic CT). PCR test for COVID-19 was not performed as it was an emergency case. Moreover, in addition to ground-glass appearance in the sub-pleural areas of the lower lobes, there are case reports in the literature reporting that patients with COVID-19 infection may present with mediastinal emphysema, subcutaneous emphysema, pneumothorax and pleural effusion in the thoracic CT findings (2, 3, 5). Bilkhu et al. (4) have described CCDS as silent ‘super spreaders’ in the treatment of pneumothorax by tube thoracostomy in patients with COVID-19 infection. In their study, they used a Filta-GuardTM (Intersurgical Ltd, Wokingham, England) ventilator filter and reported that such filters would be effective for 60–140 nm size of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) isolation (4). The authors have stated that this system may create resistance to the air flow (4). The oscillation and air leak detection showed that the part we mounted on the CDSS did not cause air resistance. In addition, no expansion defect was observed on the chest X-ray before removing the mounted part (Figure 3). However, our patient was intubated, and the effect on patient’s breathing spontaneously might have been different.
Although it is recommended that consultations should not be requested for a patient with suspected COVID-19 infection whenever possible during the intraoperative process, it may be necessary to go beyond the suggestions in life-threatening situations.
Conclusion
If there are any findings, laboratory test results or imaging findings that support COVID-19 infection, especially in patients undergoing emergency surgery without performing a test during the COVID-19 pandemic, patients should be considered as COVID-19 suspect patients until otherwise is proven. In this process, the anaesthesia team bears great responsibilities both in ensuring patient safety and in protecting the operating room environment and other operating staff. Since CCDS increases aerosolisation in COVID-19 infection, adding a bacterial/viral respiratory filter to this system may be beneficial for protection of the operating room environment and other operating staff from patients without a COVID-19 test.
Main Points:
Patients who need emergency surgery may also have symptoms suggestive of coronavirus disease 2019 (COVID-19) infection during the COVID-19 pandemic period.
In order to ensure safety of the operating room environment, operating room staff and other patients, these patients should be considered as COVID-19 suspects until proven otherwise, and necessary precautions should be taken.
Anaesthesiologists have important duties in this regard.
Chest drainage tubes are an important risk source for the spread of COVID-19 infection. Aerosolisation of the virus can be prevented by the endotracheal tube and bacterial/viral respiratory filter added to the outlet part of the drainage system.
Footnotes
Informed Consent: The relatives of the patient were informed about the patient’s condition and consent was obtained from a first-degree relative to use their data for scientific studies.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – T.Ç.; Design – T.Ç.; Supervision – T.Ç.; Resources – A.E., H.F.S.; Materials – T.Ç., E.Ö.Ç., A.E., H.F.S.; Data Collection and/or Processing – E.Ö.Ç., A.E., H.F.S.; Analysis and/or Interpretation – T.Ç.; Literature Search – T.Ç., E.Ö.Ç.; Writing Manuscript – T.Ç., E.Ö.Ç.; Critical Review – T.Ç.; Other – T.Ç., E.Ö.Ç.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Ritz JP, Lehmann KS, Frericks B, Stroux A, Buhr HJ, Holmer C. Outcome of patients with acute sigmoid diverticulitis: multivariate analysis of risk factors for free perforation. Surgery. 2011;149:606–13. doi: 10.1016/j.surg.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Wang W, Gao R, Zheng Y, Jiang L. COVID-19 with spontaneous pneumothorax, pneumomediastinum and subcutaneous emphysema. J Travel Med. 2020;27:taaa062. doi: 10.1093/jtm/taaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun R, Liu H, Wang X. Mediastinal Emphysema, Giant Bulla, and Pneumothorax Developed during the Course of COVID-19 Pneumonia. Korean J Radiol. 2020;21:541–4. doi: 10.3348/kjr.2020.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilkhu R, Viviano A, Saftic I, Bille A. COVID-19: Chest Drains With Air Leak - The Silent ‘Super Spreader’? 2020 Apr; doi: 10.25373/ctsnet.12089130. [DOI] [Google Scholar]
- 5.Lin X, Gong Z, Xiao Z, Xiong J, Fan B, Liu J. Novel coronavirus pneumonia outbreak in 2019: computed tomographic findings in two cases. Korean J Radiol. 2020;21:365–8. doi: 10.3348/kjr.2020.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]


