Abstract
Objective
According to previous studies, anaesthesia type has an important effect on immune response. However, there are limited data determining the effect of low-flow and normal-flow desflurane anaesthesia on inflammatory parameters. This study aimed to investigate the effect of low-flow and normal-flow desflurane anaesthesia on inflammatory parameters in patients undergoing laparoscopic cholecystectomy.
Methods
A total of 92 patients who underwent laparoscopic cholecystectomy were retrospectively included in this study. The patients were divided into the following 2 groups according to the type of anaesthesia they received: low-flow desflurane anaesthesia group (fresh gas flow rate: 0.5 L min−1) and normal-flow desflurane anaesthesia group (fresh gas flow rate: 2 L min−1). Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were obtained before the procedure and 6 hours after the end of the procedure for all patients.
Results
Although pre-procedural NLR and PLR were similar between the normal-flow and low-flow anaesthesia groups, post-procedural NLR (4.38±2.00 vs. 3.51±1.37, p=0.023) and PLR (144.38±71.04 vs. 120.58±35.35, p=0.037) were significantly higher in the normal-flow anaesthesia group. In addition, compared with pre-procedural values, post-procedural NLR (from 2.31±1.02 to 4.38±2.00, p<0.001) and PLR (from 125.60±50.97 to 144.38±71.04, p=0.017) were significantly increased in the normal-flow anaesthesia group, whereas post-procedural NLR (from 2.88±2.51 to 3.51±1.37, p=0.135) and PLR (from 121.86±42.78 to 120.58±35.35, p=0.847) did not change significantly in the low-flow anaesthesia group.
Conclusion
The study results indicated that postoperative inflammatory response was significantly lower with low-flow desflurane anaesthesia than with normal-flow desflurane anaesthesia.
Keywords: Desflurane, inflammatory response, laparoscopic surgery, low-flow anaesthesia
Introduction
It is known that surgical procedures and general anaesthesia affect both the number and distribution of white blood cells (WBCs) because of their effects on the immune system. Previous studies have reported that leucocytosis, neutrophilia, and lymphopenia are typical inflammatory responses during an operation. This inflammatory response is primarily related to the extent of surgical trauma (1, 2). Anaesthesia type may also affect the extent of immune response (3–7). Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) have emerged as simple and cheap markers for inflammatory response (8). Therefore, NLR and PLR measurements may be useful to evaluate the inflammatory response after a surgical operation.
The technical advances in anaesthesia devices and monitors have enabled the development of novel anaesthesia techniques, and low-flow anaesthesia has gained worldwide interest. In contrast to normal-flow anaesthesia, where the rate of gas flow into the breathing system is at 2 L min−1, low-flow anaesthesia, where the rate of gas flow is ≤1 L min−1, aims to administer at least 50% of oxygen to the patient with a sufficient proportion of volatile anaesthetic agent to meet the need of the body after carbon dioxide (CO2) is separated from the gas expired by the patient (9, 10). The most important advantages of this novel anaesthesia technique are reduced cost because of decreased volatile anaesthetic consumption and less air pollution.
Desflurane is one of the most used volatile anaesthetic agents in clinical practice. Because desflurane is less soluble in the blood and tissues, it has a wide range of doses that can be titrated easily and rapidly and can be used as an optimal volatile anaesthetic agent for low-flow anaesthesia (11, 12). Although many previous studies have investigated the effect of anaesthesia type on immune response, there are limited data regarding the effect of low-flow and normal-flow desflurane anaesthesia on inflammatory parameters. In this study, we aimed to compare the effect of low-flow and normal-flow desflurane anaesthesia on inflammatory parameters in patients undergoing laparoscopic cholecystectomy.
Methods
We retrospectively evaluated the archived records of 92 patients undergoing elective laparoscopic cholecystectomy with the American Society of Anesthesiologists (ASA) classification (ASA) I–II. Exclusion criteria were as follows: known renal or hepatic insufficiency; history of coronary artery disease, heart failure, or chronic lung disease; emergent procedures; patients who required open surgery; patients with malignancy or hematologic disorders; ASA III–IV; recent infections; any endocrine or metabolic dysfunction; taking steroids; and chemotherapy or immune system–modulating drugs. The study was performed in accordance with the Declaration of Helsinki, and the Harran University Ethics Committee approved the study design (Date:11.02.2019, Number: HRÜ/19.02.24). Written informed consent was obtained from patients who participated in this study.
All patients were monitored before the procedure in the operating room. Anaesthesia was induced with propofol 2 mg kg−1 and fentanyl 1 μg kg−1. Muscle relaxation was performed with rocuronium 0.5 mg kg−1. Patients were then intubated orotracheally and were connected to the mechanical ventilator with 6–8 mL kg−1 of tidal volume and 35–45 mmHg of end-tidal CO2. Anaesthesia maintenance was continued with desflurane (50% oxygen and 50% air mixture with 6% desflurane). The vaporizer was turned off at approximately 10–15 minutes before the end of the operation. With recovery of spontaneous ventilation, 100% oxygen was administered at 5–6 L min−1 for 3–5 minutes before extubation. Neuromuscular blockade was reversed with atropine sulphate (0.015 mg kg−1) and neostigmine (0.03 mg kg−1), and the patients were extubated. No complications developed during the procedure. After the procedure, tramadol 100 mg was given intravenously to all the patients every 6 hours for pain control. Serious bleeding did not develop in any patient, and no patient required transfusion of blood products.
In our clinic, we received training and gained experience in performing low-flow anaesthesia after 2018. Before this, normal-flow anaesthesia was used in all surgical procedures. We retrospectively investigated the clinical and laboratory characteristics of low-flow and normal-flow anaesthesia groups. Normal-flow anaesthesia was defined as fresh gas flow administered at 4–6 L min−1 for the first 6–8 minutes. After observing minimum alveolar concentration achieved to +1 on the anaesthesia device, fresh gas flow rate was reduced to 2 L min−1 (fresh gas flow rate: 2 L min−1). Low-flow anaesthesia was defined as fresh gas flow administered at 4–6 L min−1 for the first 6–8 minutes. After observing minimum alveolar concentration achieved to +1 on the anaesthesia device, fresh gas flow rate was reduced to 0.5 L min−1 (fresh gas flow rate: 0.5 L min−1).
Complete blood count (CBC) and biochemical analysis of all patients were obtained for the last 24 hours before the surgery. The CBC was also obtained within 6 hours after the end of the procedure. The total counts of WBC and its subtypes were measured using an automated blood cell counter (Coulter LH 780 Haematology Analyser, Beckman Coulter Corp., Hialeah, Florida, USA). Biochemical parameters were measured with the standard laboratory methods. NLR and PLR were calculated for each patient as the ratio of neutrophil-to-lymphocyte counts and platelet-to-lymphocyte counts, respectively. In addition, we calculated the changes in NLR and PLR in our study. These changes were defined as follows: delta NLR=post-procedural NLR–pre-procedural NLR; delta PLR=post-procedural PLR–pre-procedural PLR.
Statistical analysis
The Statistical Package for the Social Sciences 15.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. A post-hoc power analysis was performed and indicated that the power of the study was 94%. One-sample Kolmogorov-Smirnov test was used to test the normality of the data. Continuous data were defined as mean±standard deviation or median (25–75 interquartile range) and compared with the Student’s t test or Mann-Whitney U test according to the normality, whereas categorical data were defined as percentage and compared with the chi-squared test. Paired sample t test or Wilcoxon test were used to compare the pre-procedural and post-procedural data. Pearson and Spearman correlation coefficients were used for correlation analysis. A p value of <0.05 was considered statistically significant.
Results
A total of 92 patients were included in this study. The median age of the study population was 47 (35–56) years, with 65.2% of them being women. A total of 54 (58.7%) patients received normal-flow anaesthesia, whereas 38 (41.3%) received low-flow anaesthesia. Comparison of the baseline characteristics between the normal-flow and low-flow anaesthesia groups is listed in Table 1. There was no significant difference between the 2 groups in terms of baseline characteristics.
Table 1.
Comparison of the baseline characteristics between normal-flow and low-flow anaesthesia groups
| Variables | Normal-flow anaesthesia (n=54) | Low-flow anaesthesia (n=38) | p |
|---|---|---|---|
|
| |||
| Age (year, range) | 47 (36.5–61.0) | 46.5 (33.5–55.3) | 0.614 |
| Sex (female), % | 39 (72.2) | 21 (55.3) | 0.093 |
| Body mass index, kg m−2 | 27.60±4.68 | 28.51±5.74 | 0.405 |
| ASA status, % | |||
| ASA I | 24 (44.4) | 13 (34.2) | |
| ASA II | 30 (55.6) | 25 (65.8) | 0.324 |
| Duration of the procedure (minutes) | 50.37±11.89 | 54.47±11.61 | 0.103 |
ASA: American Society of Anaesthesiologists
Pre-procedural haematological and biochemical variables are presented in Tables 2 and 3. There was no significant difference between the groups in terms of pre-procedural biochemical and haematological variables, including NLR and PLR.
Table 2.
Comparison of pre-procedural haematological variables between normal-flow and low-flow anaesthesia groups
| Variables | Normal-flow anaesthesia (n=54) | Low-flow anaesthesia (n=38) | p |
|---|---|---|---|
|
| |||
| WBC (×103 μL−1) | 8.59±2.13 | 9.47±3.04 | 0.129 |
| Neutrophil (×103 μL−1) | 5.22±1.78 | 6.05±2.91 | 0.125 |
| Lymphocyte (×103 μL−1) | 2.45±0.73 | 2.50±0.80 | 0.785 |
| Haemoglobin (g dL−1) | 13.29±1.70 | 13.08±1.78 | 0.566 |
| Platelet (×103 μL−1) | 281.57±64.83 | 280.87±72.11 | 0.961 |
| NLR | 2.31±1.02 | 2.88±2.51 | 0.193 |
| PLR | 125.60±50.97 | 121.86±42.78 | 0.713 |
| MCV (fL) | 84.55±8.17 | 84.97±7.54 | 0.801 |
| RDW (%) | 12.25±1.26 | 12.24±1.62 | 0.969 |
WBC: white blood cell; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; MCV: mean corpuscular volume; RDW: red cell distribution width
Table 3.
Comparison of pre-procedural biochemical variables between normal-flow and low-flow anaesthesia groups
| Variables | Normal-flow anaesthesia (n=54) | Low-flow anaesthesia (n=38) | p |
|---|---|---|---|
|
| |||
| Glucose, mg dL−1 | 104 (96–112) | 101 (95–114) | 0.584 |
| Creatinine, mg dL−1 | 0.69±0.18 | 0.73±0.19 | 0.299 |
| AST, U L−1 | 17 (14–22) | 22 (17–32) | 0.075 |
| ALT, U L−1 | 17 (12–23) | 20 (15–31) | 0.210 |
| GGT, U L−1 | 21 (14–35) | 27 (15–45) | 0.820 |
| LDH, U L−1 | 192.71±40.06 | 237.37±135.40 | 0.108 |
| ALP, U L−1 | 82.11±47.57 | 85.65±49.38 | 0.739 |
| Total bilirubin, mg dL−1 | 0.50±0.47 | 0.63±0.86 | 0.362 |
| Direct bilirubin, mg dL−1 | 0.19±0.12 | 0.21±0.27 | 0.634 |
| Amylase, U L−1 | 67.58±19.53 | 62.71±22.72 | 0.294 |
| Lipase, U L−1 | 34.69±18.78 | 33.94±22.06 | 0.869 |
AST: aspartate transaminase; ALT: alanine aminotransferase; GGT: gamma-glutamyl transferase; ALP: alkaline phosphatase; LDH: lactose dehydrogenase
Comparisons of the post-procedural haematological variables between the 2 groups are listed in Table 4. Post-procedural NLR (4.38±2.00 vs. 3.51±1.37, p=0.023) and PLR (144.38±71.04 vs. 120.58±35.35, p=0.037) were significantly higher in the normal-flow anaesthesia group than in the low-flow anaesthesia group. In addition, delta NLR was significantly higher in the normal-flow anaesthesia group than in the low-flow anaesthesia group (1.63 [0.98–2.87] vs. 1.20 [0.02–1.90], p=0.010). However, WBC, neutrophil, and lymphocyte counts were not significantly different between the 2 groups. In addition, post-procedural biochemical variables were similar between the 2 groups (Table 5).
Table 4.
Comparison of post-procedural haematological variables between normal-flow and low-flow anaesthesia groups
| Variables | Normal-flow anaesthesia (n=54) | Low-flow anaesthesia (n=38) | p |
|---|---|---|---|
|
| |||
| WBC (×103 μL−1) | 10.74±2.46 | 10.43±2.71 | 0.582 |
| Neutrophil (×103 μL−1) | 7.91±2.20 | 7.34±2.50 | 0.247 |
| Lymphocyte (×103 μL−1) | 1.99±0.61 | 2.20±0.54 | 0.101 |
| Haemoglobin (g dL−1) | 12.65±1.69 | 12.36±1.59 | 0.413 |
| Platelet (×103 μL−1) | 257.19±61.46 | 254.84±76.15 | 0.871 |
| NLR | 4.38±2.00 | 3.51±1.37 | 0.023 |
| PLR | 144.38±71.04 | 120.58±35.35 | 0.037 |
| MCV (fL) | 85.07±7.84 | 85.60±7.49 | 0.748 |
| RDW (%) | 12.10±1.20 | 11.87±1.12 | 0.341 |
| Delta NLR | 1.63 (0.98–2.87) | 1.20 (0.02–1.90) | 0.010 |
| Delta PLR | 9.59 (−13.49–32.74) | 1.57 (−21.63 2–4.74) | 0.180 |
WBC: white blood cell; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; MCV: mean corpuscular volume; RDW: red cell distribution width
Bold values define statistical significance at the p<0.05 level.
Table 5.
Comparison of post-procedural biochemical variables between normal-flow and low-flow anaesthesia groups
| Variables | Normal-flow anaesthesia (n=54) | Low-flow anaesthesia (n=38) | p |
|---|---|---|---|
|
| |||
| Glucose, mg dL−1 | 114.40±32.36 | 108.00±22.19 | 0.333 |
| Creatinine, mg dL−1 | 7.15±44.54 | 0.85±0.94 | 0.420 |
| AST, U L−1 | 33 (24–40) | 36 (30–47) | 0.889 |
| ALT, U L−1 | 27 (19–40) | 29 (24–42) | 0.746 |
| GGT, U L−1 | 21 (13–39) | 34 (20–51) | 0.061 |
| LDH, U L−1 | 187.03±51.43 | 206.68±63.77 | 0.221 |
| ALP, U L−1 | 78.55±23.65 | 73.25±30.95 | 0.449 |
| Total bilirubin, mg dL−1 | 0.60±0.61 | 0.59±0.44 | 0.908 |
| Direct bilirubin, mgdL−1 | 0.22±0.10 | 0.22±0.14 | 0.929 |
| Amylase, U L−1 | 61.72±35.47 | 53.67±19.64 | 0.261 |
| Lipase, U L−1 | 24.05±11.50 | 23.40±8.82 | 0.796 |
AST: aspartate transaminase; ALT: alanine aminotransferase; GGT: gamma-glutamyl transferase; ALP: alkaline phosphatase; LDH: lactate dehydrogenase
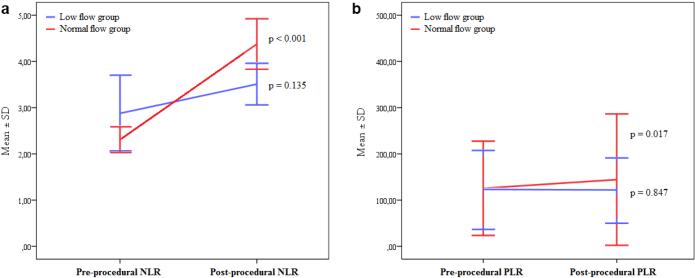
Comparisons of pre-procedural and post-procedural haematological variables according to the normal-flow and low-flow anaesthesia groups are listed in Table 6. When compared with pre-procedural values, neutrophil counts were significantly increased, whereas haemoglobin, lymphocyte, and platelet counts were significantly decreased, in both the normal-flow and low-flow anaesthesia groups. In addition, post-procedural NLR (from 2.31±1.02 to 4.38±2.00, p<0.001) and PLR (from 125.60±50.97 to 144.38±71.04, p=0.017) were significantly increased in the normal-flow anaesthesia group. However, post-procedural NLR (from 2.88±2.51 to 3.51±1.37, p=0.135) and PLR (from 121.86±42.78 to 120.58±35.35, p=0.847) did not change significantly in the low-flow anaesthesia group (Figure 1).
Table 6.
Comparison of pre-procedural and post-procedural haematological variables in normal-flow and low-flow anaesthesia groups
| Normal-flow anaesthesia (n=54) | Low-flow anaesthesia (n=38) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Pre-procedural | Post-procedural | p | Pre-procedural | Post-procedural | p | |
|
| ||||||
| WBC (×103 μL−1) | 8.59±2.13 | 10.74±2.46 | <0.001 | 9.47±3.04 | 10.43±2.71 | 0.067 |
| Neutrophil (×103 μL−1) | 5.22±1.78 | 7.91±2.20 | <0.001 | 6.05±2.91 | 7.34±2.50 | 0.019 |
| Lymphocyte (×103 μL−1) | 2.45±0.74 | 1.99±0.61 | <0.001 | 2.50±0.80 | 2.20±0.54 | 0.013 |
| Haemoglobin (g dL−1) | 13.29±1.70 | 12.65±1.69 | <0.001 | 13.08±1.78 | 12.36±1.59 | <0.001 |
| Platelet (×103 μL−1) | 281.57±64.83 | 257.19±61.46 | <0.001 | 280.87±72.11 | 254.84±76.15 | 0.001 |
| NLR | 2.31±1.02 | 4.38±2.00 | <0.001 | 2.88±2.51 | 3.51±1.37 | 0.135 |
| PLR | 125.60±50.97 | 144.38±71.04 | 0.017 | 121.86±42.78 | 120.58±35.35 | 0.847 |
| MCV (fL) | 84.55±8.17 | 85.07±7.84 | 0.055 | 84.97±7.54 | 85.60±7.49 | 0.199 |
| RDW (%) | 12.25±1.26 | 12.10±1.20 | 0.071 | 12.24±1.62 | 11.87±1.12 | 0.063 |
WBC: white blood cell; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; MCV: mean corpuscular volume; RDW: red cell distribution width
Bold values define statistical significance at the p<0.05 level
Figure 1.
a, b. Comparison of pre-procedural and post-procedural neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in normal-flow and low-flow anaesthesia groups
In correlation analysis, pre-procedural NLR positively correlated with pre-procedural PLR (r=0.396, p<0.001), whereas post-procedural NLR positively correlated with post-procedural PLR (r=0.719, p<0.001). Linear regression analysis was performed to determine the independent relationship between the anaesthesia technique and inflammatory response. Anaesthesia technique was independently associated with both delta NLR and delta PLR (Table 7).
Table 7.
Multivariate linear regression analysis showing independent predictors of delta NLR and PLR
| Unstandardized coefficients | Standardized coefficients | p | |||
|---|---|---|---|---|---|
|
|
|
||||
| B | SE | β | t | ||
|
| |||||
| Delta NLR | |||||
| Anaesthesia technique | −1.640 | 0.552 | −0.337 | −2.973 | 0.004 |
| Age | −0.006 | 0.026 | −0.035 | −0.244 | 0.808 |
| Sex | −0.440 | 0.538 | −0.087 | −0.818 | 0.416 |
| ASA status | 0.367 | 0.750 | 0.075 | 0.490 | 0.626 |
| BMI | 0.0035 | 0.052 | 0.012 | 0.105 | 0.916 |
| Delta PLR | |||||
| Anaesthesia technique | −27.097 | 11.830 | −0.264 | −2.290 | 0.024 |
| Age | −0.144 | 0.556 | −0.038 | −0.625 | 0.796 |
| Sex | −15.138 | 11.530 | −0.143 | −1.313 | 0.193 |
| ASA status | 6.505 | 16.096 | 0.063 | 0.404 | 0.687 |
| BMI | 1.004 | 1.116 | 0.101 | 0.900 | 0.371 |
B: unstandardized regression coefficient; SE: standard error; β: standardized β coefficient; BMI: body mass index; ASA: American Society of Anaesthesiologists; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio
Bold values define statistical significance at the p<0.05 level
Discussion
The main finding of our study was that normal-flow desflurane anaesthesia significantly increased NLR and PLR values, whereas low-flow desflurane anaesthesia did not significantly change those values. In addition, post-procedural NLR and PLR were significantly higher in the normal-flow desflurane anaesthesia group than in the low-flow desflurane anaesthesia group. Our results suggest that the immune system is less affected by low-flow anaesthesia than normal-flow anaesthesia.
Previous studies have reported that NLR and PLR are stronger markers of systemic inflammation than other WBC subtypes (13–16). Both of them are inexpensive and easily obtainable parameters from CBC. Leucocytosis, neutrophilia, and lymphopenia are typical inflammatory responses after general anaesthesia and surgery (1). Although it is believed that surgical procedures affect the immune system more than anaesthesia, some studies have reported that the immune system may also be affected by anaesthesia types and anaesthetic agents. Therefore, researchers have recently focused on the effects of anaesthesia types and anaesthetic agents on the immune system. It has been reported that spinal anaesthesia was associated with less increase in the NLR than general anaesthesia (6). In addition, it has been reported that volatile anaesthetic agents trigger a higher immune response than propofol (7). Furthermore, it was found that total intravenous anaesthesia had significantly lower serum levels of immune mediators than inhalational anaesthesia (3, 4). These studies suggest that volatile anaesthetics may trigger a more widespread inflammatory response. Effect of general anaesthesia on the immune system may be explained by the fact that it could trigger an inflammatory response by disturbing the functions of the immune system cells or by modulation of the stress response.
Low-flow anaesthesia is a novel anaesthesia technique, which reduces anaesthetic gas consumption, decreases atmospheric pollution, and reduces costs owing to decreased gas consumption (9, 10, 17). Although all volatile anaesthetic agents, including sevoflurane, desflurane, and isoflurane, are effective, desflurane is considered as the optimal volatile anaesthetic agent for low-flow anaesthesia because of its low solubility and short wash-in period properties (11, 12). In a previous study, Bilgi et al. (18) compared the effects of low-flow and normal-flow desflurane anaesthesia on mucociliary clearance and pulmonary function. They found that respiratory function and mucociliary clearance were preserved better during low-flow desflurane anaesthesia than during normal-flow desflurane anaesthesia. However, the effects of low-flow and normal-flow desflurane anaesthesia techniques on immune response have not been exactly investigated yet.
Low-flow and normal-flow administration of volatile anaesthetic agents could lead to different immune responses independent of surgery type. Pirbudak Cocelli et al. (19) have found that low-flow sevoflurane anaesthesia exerted minimal effects on neutrophil and T-cell populations compared with low-flow desflurane anaesthesia. In this study, we compared the effects of normal-flow and low-flow desflurane anaesthesia on the immune system of patients undergoing laparoscopic cholecystectomy and observed that normal-flow desflurane anaesthesia significantly increased the NLR and PLR values, whereas low-flow desflurane anaesthesia did not significantly change these values. These results suggest that the low-flow anaesthesia technique may trigger a lower inflammatory response than the normal-flow anaesthesia technique. Because higher inflammatory response was found to be associated with increased perioperative and postoperative complications, it can be concluded that low-flow desflurane anaesthesia has better outcomes in clinical practice. We believe that further studies with more participants are required to better elucidate the effects of low-flow desflurane anaesthesia on inflammatory response and post-procedural outcomes.
Laparoscopic surgery is a commonly preferred surgical technique than open surgery owing to shorter hospital stay, less tissue damage at the surgery sites, and lower morbidity (20, 21). Previous studies have reported that inflammatory response was significantly lower during a laparoscopic procedure than during an open surgery (22, 23). Because there is less tissue damage, it is possible that laparoscopic surgery has a minor effect on the immune system compared with that of open surgery (4). In our study, laparoscopic technique was used in all patients and anaesthesia technique was found to be independently associated with postoperative inflammatory response in multivariate analysis. Therefore, we believe that the increase in the postoperative NLR and PLR could be attributed to the different rates of fresh gas flow than the surgery type.
Our study had several limitations. The main limitation of our study was the small sample size and its retrospective design. However, when we performed a post-hoc power analysis, we calculated the power of the study as 94% (effect size: 0.68, α=0.05). We also did not evaluate other inflammatory markers, such as C-reactive protein, because it was not as cheap as CBC and not routinely measured. Another limitation could be that CBC was obtained only within 6 hours after the end of the procedure and serial CBC measurements after surgery were not performed. It might be beneficial to perform serial CBC measurements. Furthermore, we could not investigate the association between in-hospital complications and the NLR and PLR values. Finally, although the anaesthesia technique was independently associated with postoperative NLR and PLR, the possibility of residual confounding factors from unmeasured covariates could not be excluded.
Conclusion
NLR and PLR are easily obtainable parameters from the CBC. In this study, we reported that fresh gas flow rates may variably affect the postoperative inflammatory response. It was observed that postoperative NLR and PLR values were significantly increased with normal-flow desflurane anaesthesia, whereas they did not significantly change with low-flow anaesthesia. Therefore, we suggest that low-flow anaesthesia may have a beneficial effect on inflammatory response than normal-flow desflurane anaesthesia. Larger prospective studies are required to confirm our findings.
Main Points:
It is known that anaesthesia type may affect the extent of inflammatory response.
Low-flow anaesthesia is a novel anaesthesia technique, which reduces the cost owing to decreased anaesthetic gas consumption and decreases air pollution.
Low-flow anaesthesia could also lead to different immune responses independent of surgery type.
We found that postoperative inflammatory response was remarkably lower with low-flow anaesthesia compared to normal-flow anaesthesia.
Footnotes
This study was presented as oral presentation in the 53rd National Congress of Turkish Anesthesiology and Reanimation Congress, 7–10 November 2019, Antalya, Turkey.
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Harran University (Date:11.02.2019, Number: HRÜ/19.02.24).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – T.B.T., A.G.H., M.T., A.K., G.P.; Design – T.B.T., A.G.H., M.T., A.K., G.P.; Supervision – T.B.T., A.G.H., G.P.; Resources – T.B.T., A.G.H., M.T., A.K., G.P.; Data Collection and/or Processing – T.B.T., A.G.H., M.T.; Analysis and/or Interpretation – T.B.T., A.K., G.P.; Literature Search – T.B.T., M.T.; Writing Manuscript – T.B.T., A.G.H., M.T.; Critical Review – T.B.T., M.T., A.G.H., A.K., G.P.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Salo M. Effects of anesthesia and surgery on the immune response. Acta Anaesthesiol Scand. 1992;36:201–20. doi: 10.1111/j.1399-6576.1992.tb03452.x. [DOI] [PubMed] [Google Scholar]
- 2.Helmy SA, Wahby MA, El-Nawaway M. The effect of anaesthesia and surgery on plasma cytokine production. Anaesthesia. 1999;54:733–8. doi: 10.1046/j.1365-2044.1999.00947.x. [DOI] [PubMed] [Google Scholar]
- 3.Ke JJ, Zhan J, Feng XB, Wu Y, Rao Y, Wang YL. A comparison of the effect of total intravenous anaesthesia with propofol and remifentanil and inhalational anaesthesia with isoflurane on the release of pro- and anti-inflammatory cytokines in patients undergoing open cholecystectomy. Anaesth Intensive Care. 2008;36:74–8. doi: 10.1177/0310057X0803600113. [DOI] [PubMed] [Google Scholar]
- 4.Zheng L, Hagan KB, Villarreal J, Keerty V, Chen J, Cata JP. Scalp block for glioblastoma surgery is associated with lower inflammatory scores and improved survival. Minerva Anestesiol. 2017;83:1137–45. doi: 10.23736/S0375-9393.17.11881-X. [DOI] [PubMed] [Google Scholar]
- 5.Inada T, Yamanouchi Y, Jomura S, Sakamoto S, Takahashi M, Kambara T, et al. Effect of propofol and isoflurane anaesthesia on the immune response to surgery. Anaesthesia. 2004;59:954–9. doi: 10.1111/j.1365-2044.2004.03837.x. [DOI] [PubMed] [Google Scholar]
- 6.Erbaş M, Toman H, Gencer M, Şahin H, Kiraz HA, Şimşek T, et al. The effect of general and spinal anesthesia on neutrophil to lymphocyte ratio in patients undergoing cesarian section. Anaesthesia, Pain & Intensive Care. 2015;19:485–8. [Google Scholar]
- 7.Kalimeris K, Christodoulaki K, Karakitsos P, Batistatou A, Lekka M, Bai M, et al. Influence of propofol and volatile anaesthetics on the inflammatory response in the ventilated lung. Acta Anaesthesiol Scand. 2011;55:740–8. doi: 10.1111/j.1399-6576.2011.02461.x. [DOI] [PubMed] [Google Scholar]
- 8.Zheng J, Cai J, Li H, Zeng K, He L, Fu H, et al. Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio as Prognostic Predictors for Hepatocellular Carcinoma Patients with Various Treatments: a Meta-Analysis and systematic Review. Cell Physiol Biochem. 2017;44:967–81. doi: 10.1159/000485396. [DOI] [PubMed] [Google Scholar]
- 9.Baum JA, Aitkenhead AR. Low-flow anaesthesia. Anaesthesia. 1995;50:37–44. doi: 10.1111/j.1365-2044.1995.tb06189.x. [DOI] [PubMed] [Google Scholar]
- 10.Duymaz G, Yağar S, Özgök A. Comparison of Effects of Low-Flow Sevoflurane and Low-Flow Desflurane Anaesthesia on Renal Functions Using Cystatin C. Turk J Anaesthesiol Reanim. 2017;45:93–7. doi: 10.5152/TJAR.2017.72325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hargasser S, Hipp R, Breinbauer B, Mielke L, Entholzner E, Rust M. A lower solubility recommends the use of desflurane more than isoflurane, halothane, and enflurane under low-flow conditions. J Clin Anesth. 1995;7:49–53. doi: 10.1016/0952-8180(94)00003-M. [DOI] [PubMed] [Google Scholar]
- 12.Kazancıoğlu L, Batçık Ş, Erdivanlı B, Şen A, Dursun E. Comparison of the Effects of Minimal and High-Flow Anaesthesia on Cerebral Perfusion During Septorhinoplasty. Turk J Anaesthesiol Reanim. 2021;47:12–6. doi: 10.5152/TJAR.2018.36786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176–81. doi: 10.1016/j.lungcan.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 14.Mochizuki T, Kawahara T, Takamoto D, Makiyama K, Hattori Y, Teranishi JI, et al. The neutrophil-to-lymphocyte ratio (NLR) predicts adrenocortical carcinoma and is correlated with the prognosis. BMC Urol. 2017;17:49. doi: 10.1186/s12894-017-0240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanriverdi Z, Colluoglu T, Dursun H, Kaya D. The Relationship between neutrophil-to-lymphocyte ratio and fragmented QRS in acute STEMI patients treated with primary PCI. J Electrocardiol. 2017;50:876–83. doi: 10.1016/j.jelectrocard.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Li MX, Liu XM, Zhang XF, Zhang JF, Wang WL, Zhu Y, et al. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Int J Cancer. 2014;134:2403–13. doi: 10.1002/ijc.28536. [DOI] [PubMed] [Google Scholar]
- 17.Baum JA. Low-flow anesthesia: theory, practice, technical preconditions, advantages, and foreign gas accumulation. J Anesth. 1999;13:166–74. doi: 10.1007/s005400050050. [DOI] [PubMed] [Google Scholar]
- 18.Bilgi M, Goksu S, Mizrak A, Cevik C, Gul R, Koruk S, et al. Comparison of the effects of low-flow and normal flow inhalational anaesthesia with nitrous oxide and desflurane on mucociliary activity and pulmonary function tests. Eur J Anaesthesiol. 2011;28:279–83. doi: 10.1097/EJA.0b013e3283414cb7. [DOI] [PubMed] [Google Scholar]
- 19.Pirbudak Cocelli L, Ugur MG, Karadasli H. Comparison of effects of low-flow sevoflurane and desflurane anesthesia on neutrophil and T-cell populations. Curr Ther Res Clin Exp. 2012;73:41–51. doi: 10.1016/j.curtheres.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valenza F, Chevallard G, Fossali T, Salice V, Pizzocri M, Gattinoni L. Management of mechanical ventilation during laparoscopic surgery. Best Pract Res Clin Anaesthesiol. 2010;24:227–41. doi: 10.1016/j.bpa.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Lo HC, Wang YC, Su LT, Hsieh CH. Can early laparoscopic cholecystectomy be the optimal management of cholecystitis with gallbladder perforation? A single institute experience of 74 cases. Surg Endosc. 2012;26:3301–6. doi: 10.1007/s00464-012-2344-y. [DOI] [PubMed] [Google Scholar]
- 22.Veenhof AA, Vlug MS, van der Pas MH, Sietses C, van der Peet DL, de Lange-de Klerk ES, et al. Surgical stress response and postoperative immune function after laparoscopy or open surgery with fast track or standard perioperative care: a randomized trial. Ann Surg. 2012;255:216–21. doi: 10.1097/SLA.0b013e31824336e2. [DOI] [PubMed] [Google Scholar]
- 23.Schietroma M, Carlei F, Franchi L, Mazzotta C, Sozio A, Lygidakis NJ, et al. A comparison of serum interleukin-6 concentrations in patients treated by cholecystectomy via laparotomy or laparoscopy. Hepatogastroenterology. 2004;51:1595–9. [PubMed] [Google Scholar]