Abstract
Objective
This study aimed to determine the efficacy of prophylactic use of vancomycin powder against surgical site infections in patients with high-risk conditions who underwent posterior spinal instrumentation.
Methods
Data obtained from 209 patients who underwent posterior spinal instrumentation at a single institution from 2014 to 2017 were retrospectively reviewed. Patients were then divided into two groups: control group, including 107 patients (61 females, 46 males; mean age=54 years; age range=16–85 years), and treatment group, including 102 patients (63 females, 39 males; mean age=53 years; age range=14–90 years). All patients received the same standard prophylactic antibiotic regimen. In addition to the prophylactic antibiotic, vancomycin powder was applied locally to the surgical site in the treatment group. All patients were followed up for at least 90 days postoperatively. Infections were categorized as superficial and deep infections. Subgroup analysis of high-risk patients (Syrian refugees) was also performed.
Results
The infection rates were 1.96% (two patients) in the treatment group and 6.54% (seven patients) in the control group. A significant decrease in the infection rates was observed with local vancomycin powder application. Advanced age (>46 years) and prolonged surgical duration (>140 min) were found to be the main risk factors for surgical site infections (p=0.004 and p=0.028, respectively). The infection rates were 3.22% and 8.11% in the treatment and control groups of refugees, respectively. There were three superficial and four deep infections in the control group and one superficial and one deep infection in the treatment group. A dominance of staphylococcus infections was observed in the control group, whereas no significant dominance was observed in the treatment group. Three patients in the control group and one patient in the treatment group received implant removal.
Conclusion
Evidence from this study has revealed that local application of vancomycin powder reduces the rate of surgical site infections after instrumented spinal surgery. The benefit of vancomycin application may be most appreciated in higher risk populations or in clinics with high baseline rates of infection.
Level of Evidence
Level III, Therapeutic Study
Keywords: Surgical site infection, Vancomycin powder, Spine surgery, Posterior instrumentation, Complication
Introduction
Spine surgery is associated with a wide range of complications (1). Surgical site infections are the most devastating complications because they are associated with low postoperative recovery, low patient satisfaction, and high patient morbidity and mortality. In the literature, infection rates of spine surgeries range from 0.7% to 11.9% (2–5). Particularly, instrumented surgeries have higher infection rates than decompression surgeries (5, 6). There are several factors influencing this high range of surgical site infections in spinal surgeries. Socioeconomic status of the patients is one of the factors. The rates of surgical site infections have been found to be much lower in developed countries than those in developing and poor countries (5, 7, 8).
Risk factors associated with surgical site infections include patient- and operation-related factors such as advanced age, diabetes, smoking, obesity, prolonged surgical duration, increased blood loss, and revision surgeries (9–12). To reduce these infections, practices such as preoperative administration of prophylactic antibiotics, appropriate preparation of the skin, and use of sterile technique are recommended.
In the past decade, a new trend has started that involves local application of vancomycin powder to the surgical bed. Although there are some contrasting views, many studies have shown that prophylactic application of vancomycin powder in addition to standard systemic antibiotic prophylaxis leads to a reduction of postoperative surgical site infections after spinal procedures (13–15) However, most of these research studies were performed in developed countries with lower initial infection rates. In this study, we aimed to determine early postoperative infection rates and the advantages and disadvantages of topical vancomycin application in posterior instrumentation spinal surgeries performed in patients with high-risk conditions.
Materials and Methods
Patient selection
The research was conducted according to the principles of the World Medical Association Declaration of Helsinki “Ethical Principles for Medical Research Involving Human Subjects” (amended in October 2013), and Institutional Review Board approval was obtained. This study included 209 patients who underwent posterior instrumentation surgery at a single institution between November 2014 and January 2017. Only posterior approaches and instrumented patients were included in this study. Patients with a history of infection or antibiotic use within the past 1 month or patients who were suffering from infectious or immunodeficiency diseases at the time of enrollment were excluded from the study. All operations were performed by one surgeon (KO). After obtaining a written consent from each patient, patient data, including patient demographics (age, sex, body mass index, tobacco use, comorbidities, American Society of Anesthesiologist [ASA] classification) and surgical parameters (clinical diagnosis, levels of surgery, blood loss, surgical duration, cerebrospinal fluid leak), were retrospectively collected. Systemic diseases, including hypertension, hyperlipidemia, anemia, diabetes mellitus, coronary artery disease, respiratory disease, and chronic kidney disease, were defined as comorbidities of the patients. Clinical diagnoses were classified as degenerative, tumor, and trauma.
Vancomycin application
All patients received the same standard prophylactic antibiotic, including 1 g cefazolin in the 1-hour period before surgery and another 1 g cefazolin in the postoperative 24-hour period. In 10 patients weighing ≥120 kg, 1.5 g cefazolin was administered in each dose. In the treatment group, vancomycin powder was applied locally to the surgical area in addition to the intravenous antibiotics (Figure 1). In patients undergoing surgery on four spinal levels or less, 1 g vancomycin powder was applied, whereas in those undergoing surgery on five spinal levels or more, 2 g vancomycin powder was applied. Surgical drains (B-vak tissue drainage set, Bicakcilar, İstanbul, Turkey) were used in all the patients enrolled in this study but were removed on the second day after surgery.
Figure 1.
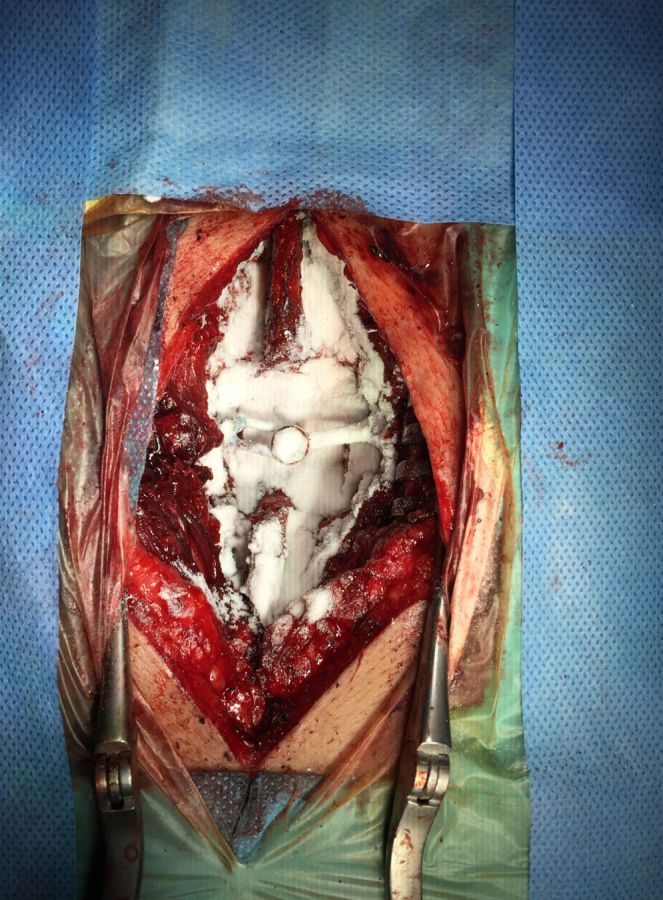
Posterior thoracolumbar instrumentation and posterolateral fusion operation for T12 vertebra fracture. The application of vancomycin powder was demonstrated
Patients follow-up
All patients were followed for at least 90 days postoperatively. Infections were categorized as superficial and deep infections. Infections were classified as superficial if they occurred within 30 days after surgery and involved only the skin and subcutaneous tissue of the incision with at least one of the following characteristics: purulent discharge from incision, positive culture from aseptically collected culture of fluid, clinical signs of tenderness, localized swelling, and redness or warmth. Infections were said to be deep if they occurred within 90 days after surgery, with characteristics of superficial infection and involving the fascial and muscle layers. Superficial infections were treated with wound care, local debridement, and intravenous or oral antibiotics on the basis of the culture results. Deep infections were treated with intravenous antibiotics and deep surgical debridement with or without implant removal.
Statistical analysis
Statistical Package for the Social Sciences version 22.0 (IBM SPSS Corp.; Armonk, NY, USA) and MedCalc 14 (MedCalc Software Ltd., Ostend, Belgium) programs were used to analyze the variables. Data conformance to normal distribution was evaluated by the Shapiro–Wilk test and the variance homogeneity by the Levene test. The independent sample t-test was used in conjunction with the Bootstrap results, and the Mann-Whitney U test was used with the Monte Carlo results to compare the treatment and control groups quantitatively. The Pearson chi-square and Fisher’s exact tests were used together with Monte Carlo and Exact results when comparing the treatment and control groups in terms of the categorical variables. The logistic regression test was used with the backward stepwise (Wald) method to determine the cause-and-effect relation between the explanatory variables and the treatment group variable. The relationship between the classifications made as per the cutoff values and the actual classification based on sensitivity and specificity values were examined and described by receiver operating curve analysis. The quantitative variables were described as mean±standard deviation and the median range (maximum-minimum) and categorical variables as n (%). The variables were examined at 95% confidence level, and p<0.05 was considered significant.
Results
A total of 209 patients were enrolled in this study with 102 (49%) in the treatment group and 107 (51%) in the control group. Patients’ demographic data, including age, gender, body mass index, comorbidities, ASA scores, and diagnoses, were compared between two groups and shown in Table 1. There were no statistically significant demographic differences between the treatment and control groups (Table 1). The infection rates were 6.54% (seven patients) and 1.96% (two patients) in the control and treatment groups, respectively. There was a statistically significant decrease in the infection rates with local vancomycin powder application.
Table 1.
Demographic data of the patient groups
| Characteristics | Treatment group n=102 (%) |
Control group n=107 (%) |
p |
|---|---|---|---|
| Age (mean) | 53 (14–90) | 54 (16–85) | 0.179 |
| Gender | |||
| Female | 63 (61.76) | 61 (57.01) | 0.424 |
| Male | 39 (38.24) | 46 (42.99) | |
| Nationality | |||
| Refugee | 62 (60.78) | 74 (69.16) | 0.258 |
| Nonrefugee | 40 (39.22) | 33 (30.84) | |
| Body mass index | 27.13±6.74 | 28.58±7.24 | 0.372 |
| Comorbidities | |||
| Hypertension | 41 (40.19) | 39 (36.44) | 0.486 |
| Hyperlipidemia | 38 (37.25) | 34 (31.77) | 0.169 |
| Anemia | 28 (27.45) | 31 (28.97) | 0.241 |
| Diabetes mellitus | 19 (18.62) | 20 (18.69) | 0.824 |
| Coronary artery disease | 14 (13.72) | 13 (12.14) | 0.467 |
| Respiratory disease | 3 (2.94) | 5 (4.67) | 0.542 |
| Chronic kidney disease | 1 (0.98) | 2 (1.86) | 0.174 |
| Smoking | 45 (44.12) | 48 (44.86) | 1 |
| ASA status | |||
| Level I | 53 (51.96) | 59 (55.14) | |
| Level II | 34 (33.33) | 31 (28.97) | |
| Level III | 15 (14.71) | 17 (15.89) | |
| Diagnosis | |||
| Degenerative | 81 (79.41) | 77 (71.96) | 0.462 |
| Trauma | 5 (4.90) | 7 (6.54) | |
| Tumor | 16 (15.69) | 23 (21.50) | |
| Revision | 17 (16.67) | 13 (12.15) | 0.431 |
ASA: American Society of Anesthesiologists
The overall rate of surgical site infections was 4.30% (9 of 209 patients). Some risk factors for surgical site infections were identified. Advanced age, high body mass index, high ASA score, prolonged surgical duration, and increased blood loss during the operation were found to be significant predictors for surgical site infections (Table 2). Particularly, advanced age (>46 years) and prolonged surgical duration (>140 min) were found as the main risk factors for surgical site infections (Table 3; Figures 2 and 3). The patients’ socioeconomic conditions were also reviewed in this study. Of the total study population, 136 patients (65%) were refugees. Six of the seven infected patients in the control group and both infected patients in the treatment group were refugees with poor living conditions. The poor condition of their accommodations was another risk factor for surgical site infections. There was no statistically significant difference in the rates of refugee patients between the treatment and control groups (Table 1). The infection rates were 8.11% and 3.22% in the control and treatment groups of refugees, respectively (Table 4).
Table 2.
Significant predictors for surgical site infections
| Infection | p value | ||
|---|---|---|---|
|
| |||
| Absent | Existent | ||
|
| |||
| Mean±SD | Mean±SD | ||
| Age | 49.11±15.50 | 51.81±13.05 | 0.004 |
| Body mass index | 26.62±4.03 | 30.46±6.25 | 0.005 |
|
| |||
| Median (min/max) | Median (min/max) | ||
|
| |||
| Level | 3 (2/9) | 3 (2/9) | 0.131 |
| ASA score | 1 (1/3) | 2 (1/3) | <0.001 |
| Operation time (minutes) | 140 (80/280) | 180 (100/260) | <0.001 |
| Blood loss (mL) | 450 (250/1500) | 650 (300/1400) | <0.001 |
Independent t-test (Bootstrap): Mann-Whitney U test (Monte Carlo); SD: standard deviation; max: maximum; min: minimum; ASA: American Society of Anesthesiologists
Table 3.
Main risk factors for surgical site infections
| B | SE | p | Odds ratio | 95% CI for Odds ratio | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Lower | Upper | |||||
| Age (>46 years) | −0.854 | 0.299 | 0.004 | 2.35 | 4.22 | 1.31 |
| Operation Time (>140 min) | −0.668 | 0.305 | 0.028 | 1.95 | 3.54 | 1.07 |
Dependent variable: infection/predicted: Control group = 65.4; Treatment group = 72.5/General: 68.9; p model<0.001; Multiple logistic regression method = backward stepwise (Wald); B: regression coefficient; SE: standard error; CI: confidence interval.
Figure 2.
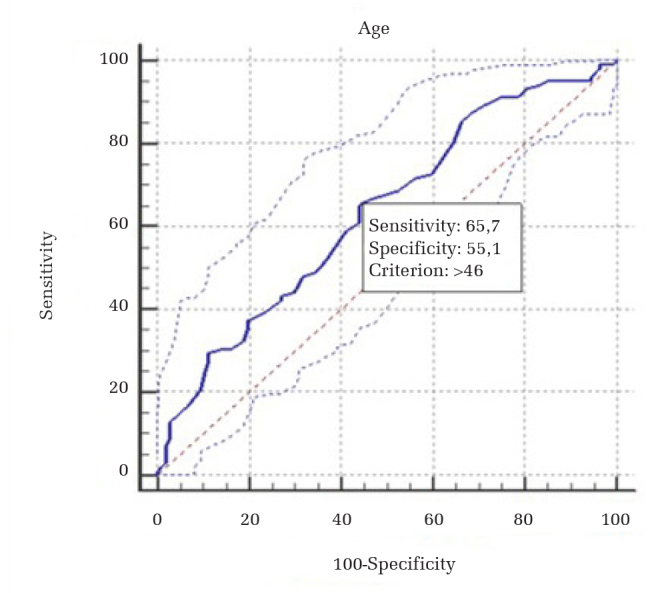
Advanced age ROC curve analysis
ROC: receiver operating curve
Figure 3.
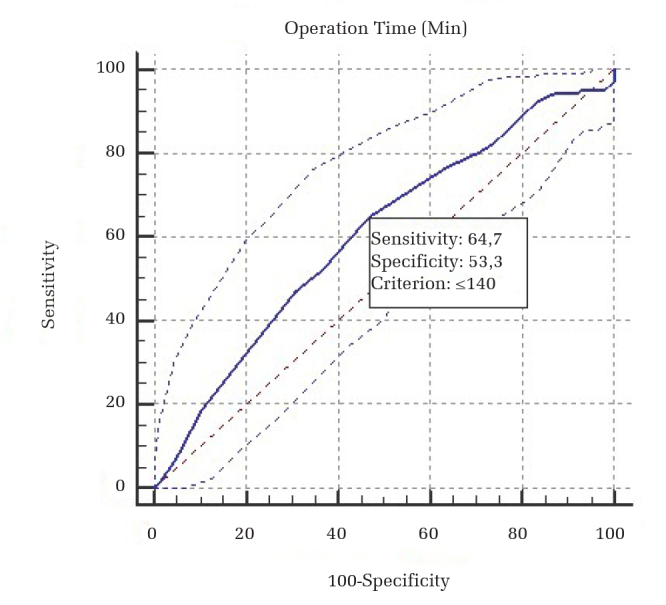
Operation time ROC curve analysis
ROC: receiver operating curve
Table 4.
Distribution of the refugee and nonrefugee patient groups
| Group | Patient n (%) | Infection n (%) |
|---|---|---|
| Control group | ||
| Refugee | 74 (69.16) | 6 (8.11) |
| Nonrefugee | 33 (30.84) | 1 (3.03) |
| Total | 107 (100) | 7 (6.54) |
| Treatment group | ||
| Refugee | 62 (60.78) | 2 (3.22) |
| Nonrefugee | 40 (39.22) | 0 (0) |
| Total | 102 (100) | 2 (1.96) |
There were eight patients who had positive culture results and one patient in the control group where no microorganism was isolated. There were three superficial and four deep infections in the control group and one superficial and one deep infection in the treatment group. Staphylococcus infections dominated in the control group, whereas no statistically significant microorganismal dominance was observed in the treatment group (Table 5). None of the patients had infections of vancomycin-resistant microorganisms in the treatment group. Three patients in the control group and one patient in the treatment group had to undergo implant removal (Table 5).
Table 5.
Characteristics of surgical site infections
| Group | Patient | Microorganism isolated | Location |
|---|---|---|---|
| Control group | |||
| 1 | Staphylococcus aureus | Superficial | |
| 2 | Staphylococcus haemolyticus | Deep | |
| 3* | Acinetobacter baumanii | Deep | |
| 4 | Staphylococcus hominis | Superficial | |
| 5* | Pseudomonas aeruginosa | Deep | |
| 6* | Enterococcus faecalis + Staphylococcus aureus | Deep | |
| 7 | – | Superficial | |
| Treatment group | |||
| 1 | Pseudomonas aeruginosa | Superficial | |
| 2* | Escherichia coli + Acinetobacter baumanii | Deep |
Patients who underwent implant removal
Discussion
Because Sweet et al. (16) and Molinari et al. (14) first presented their studies on local vancomycin powder application against spinal surgical site infections in 2011, an increasing number of studies have researched this topic (8–22). Although there are some contrasting views, the general opinion is that local vancomycin application is effective against spinal surgical site infections. In 2014 and 2015, meta-analyses were published on this subject. Chiang et al. (13), Khan et al. (8), and Bakhsheshian et al. (17) have reported that vancomycin application is protective against surgical site infections with an odds ratio of 0.16 (95% CI: 0.09–0.30), 0.34 (95% CI: 0.17–0.66), and 0.43 (95% CI: 0.22–0.82), respectively.
Surgical site infections are one of the most common and devastating complications after spine surgery. The incidence of surgical site infections ranges between 0.7% and 11.9% depending on the type of surgery, indication of the surgery, and use of instrumentation. Specifically, incidence rates were higher in the instrumented posterior approach surgeries (5, 6, 9, 11, 12). Therefore, this study was performed in instrumented patients.
This study was performed in a government hospital in Sanliurfa, which is a Turkish city near the border of Syria. In total, 65% of the patients in this study were Syrians staying in refugee camps or other places with similar living conditions. The higher rates of infection in our study compared with those previously reported in the literature may be due to intensive working conditions of the hospital, a large number of emergent surgeries, lack of patient hygiene, and low rate of patient compliance to the treatment suggestions. Consistent with this hypothesis, infection rates were much higher in the refugee subset of our patient population (Table 4). In this study, the infection rate in the control cohort was 6.54%. After local application of vancomycin powder, the infection rate reduced to 1.96%. The infection rates were 8.11% and 3.22% in the control and treatment groups of refugees, respectively (Table 4). In the meta-analysis by Khan et al. (8), it was suggested that vancomycin application may be of greatest benefit to higher risk populations or in facilities with high baseline rates of infection. The results from our study support this hypothesis.
Risk factors for surgical site infection include comorbidities (particularly diabetes mellitus), old age, smoking, morbid obesity, immunodeficiency, prolonged surgeries, large amount of blood loss, trauma, paralysis, osteoporosis, and postoperative bowel and urinary impairment (6, 15, 22). In this study, advanced age and prolonged surgical duration are the major risk factors for surgical site infections (Table 3). Consistent with the literature, higher body mass index, high ASA score, and large amount of blood loss during surgery were found to be the other risk factors for surgical site infections (Table 2).
Surgical site infections are challenging complications for the patients. Besides the disease itself, the antibiotics used for the treatment can also cause additional complications. Adverse drug effects such as hypotension and renal toxicity or secondary infections owing to antibiotic-resistant microorganisms in the respiratory and genitourinary tracts can be seen in these patients (23). Pharmacokinetic studies have shown that penetration of systemic antibiotics into the spinal region is often poor and may require administration of supratherapeutic doses, leading to more adverse drug effects (11, 24). Therefore, local application of vancomycin powder is advantageous as it allows for maximal levels of antibiotic concentration in the surgical wound with minimal systemic complications (16).
Several studies have performed cost–benefit analysis of local vancomycin powder application (4, 17, 19, 20, 25). A single local application of vancomycin powder costs about $12–$44 and can significantly reduce infection rates and costs of medical care. These studies reported cost savings ranging from $220,000 to $500,000 per 100 patients receiving spine surgery. The majority of these costs are due to re-operations for deep surgical site infections and prolonged usage of antibiotics.
There are some concerns about the complications of vancomycin powder application. In a review by Ghobrial et al., 14 retrospective and 2 prospective studies were identified with a total of 9,721 patients (26). Of these, adverse events were identified in 23 patients. In total, 1 patient had nephropathy, 2 patients had ototoxicity, 1 patient had systemic collapse, and 19 patients had culture negative seroma formation. The overall adverse event rate in patients treated with vancomycin powder was 0.3% (26).
Another concern is the lack of fusion or pseudoarthrosis in the patients treated with vancomycin powder. In vitro studies have shown that high doses of local antibiotic applications have a cytotoxic effect on osteoblasts, leading to reduction in bone healing and fusion (11, 27, 28). Nevertheless, the study by Rathbone et al. reported that vancomycin has less toxic effect on osteoblasts than other commonly used antibiotics (28). In 2016, Mendoza et al. performed an in vivo study to investigate the development of pseudoarthrosis after the local application of vancomycin powder in fusion surgeries (29). Their results indicated that vancomycin powder did not decrease fusion rates at the doses that are routinely used by surgeons. Furthermore, fusion rates were not decreased even after application of a vancomycin powder dose that was 10-fold higher than the usual clinically used dose. Moreover, a change in pseudoarthrosis rate has not been reported in other human clinical studies (15, 16, 21).
There is another feared risk that local application of vancomycin powder may create microorganisms that are resistant to multiple antibiotics (6, 30). In 2017, Chotai et al. performed a study to determine the occurrence of vancomycin-resistant surgical site infections in patients with intrawound application of vancomycin powder (31). They concluded that the local application of vancomycin powder during spine surgeries was beneficial in preventing surgical site infections, and the usage of intrawound vancomycin powder did not seem to create vancomycin-resistant organisms. However, they found a predominance of gram-negative microorganisms and culture negative fluid collection in the vancomycin group. Ghobrial et al. also reported an increase in cultured gram-negative or polymicrobial spine infections when using vancomycin powder for prophylaxis (19).
There are certain limitations of this study. The main limitations are the retrospective nature of the study and the relatively small sample size (209 patients). There is also a lack of pediatric patients in this study group. Nonetheless, this study also has several strengths. For instance, all surgeries were performed by the same surgeon in the same time period. Demographic data of the treatment and control groups are similar, and there is no statistically significant difference between the patient groups.
In conclusion, this study demonstrates that application of local vancomycin powder reduces the rates of surgical site infections in patients undergoing instrumented spinal surgery. The rate of infection was 6.54% in the control group, and it was reduced to 1.96% in the treatment group. Furthermore, no adverse effects were observed related to vancomycin usage. Local application of vancomycin powder has advantages, including ease of usage, relatively low cost, effectiveness against causative microorganisms (particularly staphylococcus infections), and high local antibiotic concentration with minimal systemic circulation. The benefit of vancomycin powder application may be most appreciated in higher risk populations or in clinics with high baseline rates of infection, similar to this study.
HIGHLIGHTS
This study indicated vancomycin powder reduces the rates of surgical site infections.
The benefit of vancomycin powder may be most appreciated in higher risk populations.
Advanced age and prolonged surgical duration were defined as the main risk factors.
Footnotes
Ethics Committee Approval: Ethics committee approval was obtained from the Ethical Committee of the Non-Interventıonal Clinical Researches of Çukurova University School of Medicine (date and protocol number: 09.04.2015, 34/5).
Informed Consent: Written informed consent was obtained from the patients.
Author Contributions: Concept - K.M.Ö.; Design - K.O.; Supervision - T.E., A.G.; Data Collection and/or Processing - K.O.; Analysis and/or Interpretation - K.M.Ö., N.E.Ç.; Literature Review - N.E.Ç.; Writing - K.O.; Critical Review - T.E., A.G.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Beier AD, Soo TM, Claybrooks R. Subdural hematoma after microdiscectomy: a case report and review of the literature. Spine J. 2009;9:E9–E12. doi: 10.1016/j.spinee.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Glassman SD, Dimar JR, Puno RM, Johnson JR. Salvage of instrumental lumbar fusions complicated by surgical wound infection. Spine (Phila Pa 1976) 1996;21:2163–9. doi: 10.1097/00007632-199609150-00021. [DOI] [PubMed] [Google Scholar]
- 3.Hill BW, Emohare O, Song B, Davis R, Kang MM. The use of vancomycin powder reduces surgical reoperation in posterior instrumented and noninstrumented spinal surgery. Acta Neurochir (Wien) 2014;156:749–54. doi: 10.1007/s00701-014-2022-z. [DOI] [PubMed] [Google Scholar]
- 4.Theologis AA, Demirkıran G, Callahan M, Pekmezci M, Ames C, Deviren V. Local intrawound vancomycin powder decreases the risk of surgical site infections in complex adult deformity reconstruction: A cost analysis. Spine (Phila Pa 1976) 2014;39:1875–80. doi: 10.1097/BRS.0000000000000533. [DOI] [PubMed] [Google Scholar]
- 5.Weinstein MA, McCabe JP, Cammisa FP., Jr Postoperative spinal wound infection: a review of 2,391 consecutive index procedures. J Spinal Disord. 2000;13:422–6. doi: 10.1097/00002517-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Schroeder JE, Girardi FP, Sandhu H, Weinstein J, Cammisa FP, Sama A. The use of local vancomycin powder in degenerative spine surgery. Eur Spine J. 2016;25:1029–33. doi: 10.1007/s00586-015-4119-3. [DOI] [PubMed] [Google Scholar]
- 7.Farshad M, Bauer DE, Wechsler C, Gerber C, Aichmair A. Risk factors for perioperative morbidity in spine surgeries of different complexities: A multivariate analysis of 1,009 consecutive patients. Spine J. 2018;18:1625–31. doi: 10.1016/j.spinee.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Khan NR, Thompson CJ, DeCuypere M, et al. A meta-analysis of spinal surgical site infection and vancomycin powder. J Neurosurg Spine. 2014;21:974–83. doi: 10.3171/2014.8.SPINE1445. [DOI] [PubMed] [Google Scholar]
- 9.Calderone RR, Garland DE, Capen DA, Oster H. Cost of medical care for postoperative spinal infections. Orthop Clin North Am. 1996;27:171–82. [PubMed] [Google Scholar]
- 10.Eren B, Karagöz Güzey F, Kitiş S, Özkan N, Korkut C. The effectiveness of pedicle screw immersion in vancomycin and ceftriaxone solution for the prevention of postoperative spinal infection: A prospective comparative study. Acta Orthop Traumatol Turc. 2018;52:289–93. doi: 10.1016/j.aott.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hey HW, Thiam DW, Koh ZS, et al. Is intraoperative local vancomycin powder the answer to surgical site infections in spine surgery? Spine (Phila Pa 1976) 2017;42:267–74. doi: 10.1097/BRS.0000000000001710. [DOI] [PubMed] [Google Scholar]
- 12.Whitmore RG, Stephen J, Stein SC, et al. Patient comorbidities and complications after spinal surgery: A societal-based cost analysis. Spine (Phila Pa 1976) 2012;37:1065–71. doi: 10.1097/BRS.0b013e31823da22d. [DOI] [PubMed] [Google Scholar]
- 13.Chiang HY, Herwaldt LA, Blevins AE, Cho E, Schweizer ML. Effectiveness of local vancomycin powder to decrease surgical site infections: A meta-analysis. Spine J. 2014;14:397–407. doi: 10.1016/j.spinee.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Molinari RW, Khera OA, Molinari WJ. Prophylactic intraoperative powdered vancomycin and postoperative deep spinal wound infection: 1,512 consecutive surgical cases over a 6-year period. Eur Spine J. 2011;21(Suppl 4):S476–S82. doi: 10.1007/s00586-011-2104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strom RG, Pacione D, Kalhorn SP, Frempong-Boadu AK. Lumbar laminectomy and fusion with routine local application of vancomycin powder: Decreased infection rate in instrumented and non-instrumented cases. Clin Neurol Neurosurg. 2013;115:1766–9. doi: 10.1016/j.clineuro.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Sweet FA, Roh M, Sliva C. Intrawound application of vancomycin for prophylaxis in instrumented thoracolumbar fusions. Spine (Phila Pa 1976) 2011;36:2084–8. doi: 10.1097/BRS.0b013e3181ff2cb1. [DOI] [PubMed] [Google Scholar]
- 17.Bakhsheshian J, Dahdaleh NS, Lam SK, Savage JW, Smith ZA. The use of vancomycin powder in modern spine surgery: systematic review and meta-analysis of the clinical evidence. World Neurosurg. 2015;83:816–23. doi: 10.1016/j.wneu.2014.12.033. [DOI] [PubMed] [Google Scholar]
- 18.Caroom C, Tullar JM, Benton EG, Jr, Jones JR, Chaput CD. Intrawound vancomycin powder reduces surgical site infections in posterior cervical fusion. Spine (Phila Pa 1976) 2013;38:1183–7. doi: 10.1097/BRS.0b013e31828fcfb5. [DOI] [PubMed] [Google Scholar]
- 19.Ghobrial GM, Thakkar V, Andrews E, et al. Intraoperative vancomycin use in spinal surgery: single institution experience and microbial trends. Spine (Phila Pa 1976) 2014;39:550–5. doi: 10.1097/BRS.0000000000000241. [DOI] [PubMed] [Google Scholar]
- 20.Godil SS, Parker SL, O’Neill KR, Devin CJ, McGirt MJ. Comparative effectiveness and cost-benefit analysis of local application of vancomycin powder in posterior spinal fusion for spine trauma: Clinical article. J Neurosurg Spine. 2013;19:331–5. doi: 10.3171/2013.6.SPINE121105. [DOI] [PubMed] [Google Scholar]
- 21.Martin JR, Adogwa O, Brown CR, et al. Experience with intrawound vancomycin powder for spinal deformity surgery. Spine (Phila Pa 1976) 2014;39:177–84. doi: 10.1097/BRS.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe M, Sakai D, Matsuyama D, Yamamoto Y, Sato M, Mochida J. Risk factors for surgical site infection following spine surgery: Efficacy of intraoperative saline irrigation. J Neurosurg Spine. 2010;12:540–6. doi: 10.3171/2009.11.SPINE09308. [DOI] [PubMed] [Google Scholar]
- 23.Moise PA, Smyth DS, El-fawal N, et al. Microbiological effects of prior vancomycin use in patients with methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother. 2008;61:85–90. doi: 10.1093/jac/dkm445. [DOI] [PubMed] [Google Scholar]
- 24.Gibson MJ, Karpinski MR, Slack RC, Cowlishaw WA, Webb JK. The penetration of antibiotics into the normal intervertebral disc. J Bone Joint Surg Br. 1987;69:784–6. doi: 10.1302/0301-620X.69B5.3680343. [DOI] [PubMed] [Google Scholar]
- 25.Emohare O, Ledonio CG, Hill BW, Davis RA, Polly DW, Jr, Kang MM. Cost savings analysis of intrawound vancomycin powder in posterior spinal surgery. Spine J. 2014;14:2710–5. doi: 10.1016/j.spinee.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Ghobrial GM, Cadotte DW, Williams K, Jr, Fehlings MG, Harrop JS. Complications from the use of intrawound vancomycin in lumbar spinal surgery: a systematic review. Neurosurg Focus. 2015;39:E11. doi: 10.3171/2015.7.FOCUS15258. [DOI] [PubMed] [Google Scholar]
- 27.Edin ML, Miclau T, Lester GE, Lindsey RW, Dahners LE. Effect of cefazolin and vancomycin on osteoblasts in vitro. Clin Orthop Relat Res. 1996;333:245–51. doi: 10.1097/00003086-199612000-00027. [DOI] [PubMed] [Google Scholar]
- 28.Rathbone CR, Cross JD, Brown KV, Murray CK, Wenke JC. Effect of various concentrations of antibiotics on osteogenic cell viability and activity. J Orthop Res. 2011;29:1070–4. doi: 10.1002/jor.21343. [DOI] [PubMed] [Google Scholar]
- 29.Mendoza MC, Sonn KA, Kannan AS, et al. The effect of vancomycin powder on bone healing in a rat spinal rhBMP-2 model. J Neurosurg Spine. 2016;25:147–53. doi: 10.3171/2015.11.SPINE15536. [DOI] [PubMed] [Google Scholar]
- 30.Nordmann P, Naas T, Fortineau N, Poirel L. Superbugs in the coming new decade; multidrug resistance and prospects for treatment of Staphylococcus aureus, Enterococcus spp. and Pseudomonas aeruginosa in 2010. Curr Opin Microbiol. 2007;10:436–40. doi: 10.1016/j.mib.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Chotai S, Wright PW, Hale AT, et al. Does intrawound vancomycin application during spine surgery create vancomycin-resistant organism? Neurosurgery. 2017;80:746–53. doi: 10.1093/neuros/nyw097. [DOI] [PubMed] [Google Scholar]


