Abstract
Objective
The aim of this study was to immunohistochemically identify and characterize the presence of sensory nerve endings (SNEs) in pulvinar, ligamentum teres (LT), and hip joint capsule (HJC) of children with developmental dysplasia of the hip (DDH).
Methods
Pulvinar, LT, and HJC specimens were obtained from 38 hips of 36 children (31 girls, five boys; mean age=49 months; age range=18–132 months) during open reduction surgery for DDH. All specimens underwent subsequent routine tissue processing (formalin fixation and paraffin embedding). To determine tissue morphology, haematoxylin and eosin staining was used. SNEs were analyzed immunohistochemically using a mouse monoclonal antibody against S-100 Beta Protein based on the classification of Freeman and Wyke including four types of SNEs including mechanoreceptors: type I Ruffini corpuscles, type II Pacini corpuscles, type III Golgi organs, and type IVa unmyelinated free nerve endings (FNEs). Additionally, children were sorted into three groups based on their age at the time of surgery: Group 1 (age <3 years; 19 hips of 18), Group 2 (age: 3–5 years; 10 hips of 10 children), and Group 3 (age >5 years; 9 hips of 8 children).
Results
Although no Type I, II, or III SNEs were identified in any specimen, type IVa mechanoreceptor (FNEs) was immunohistochemically characterized in 13 (34%) pulvinar, 19 (50%) LT, and 16 (42%) HJC specimens. The total density of FNEs was 3.31±5.70)/50 mm2 (range 0–21) in pulvinar specimens, 3.18 ± 5.92)/50 mm2 (range 0–24) in HJC specimens, and 4.51±6.61/50 mm2 (range 0–22) in LT specimens. Furthermore, the operated side, gender, and the number of FNEs in specimens did not differ significantly among the age groups (p>0.05 for all), and the number of FNEs was not significantly correlated with age, gender, or the operated side (p>0.05 for all).
Conclusion
Evidence from this study revealed that pulvinar, LT, and HJC include only FNEs, which play a role in pain sensation, among mechanoreceptors. Surgical excision of these tissues may not cause a significant loss of sensory function in the hip joint of children with DDH.
Level of Evidence
Level II, Therapeutic Study
Keywords: Developmental dysplasia of the hip, Ligamentum teres, Pulvinar, Sensory nerve endings, Hip joint capsule
Introduction
To achieve joint stability, both static and dynamic elements of the joint structure are important (1). Although static joint stability is established by the anatomical congruity of the joint surfaces, dynamic joint stability is associated with sensorial control of compressive and directional muscular forces acting on a joint (1, 2).
Sensory nerve endings (SNEs) are afferent neural elements that include mechanoreceptors. These specialized neurons, which transmit mechanical distortion information through electrical signals, play a role in pain sensation, tactile sense, and proprioception. Type I (Ruffini) mechanoreceptors are receptors for deep sensation and temperature sense and type II (Pacini) and type III (Golgi) corpuscles are pressure receptors. Type IV receptors (free nerve endings [FNEs]) are nociceptors, reacting to inflammatory or noxious stimuli (3, 4). Stimulation of these receptors results in muscle contraction and coordinates movements in the joints. Thus, mechanoreceptors are responsible for dynamic joint stability (5). Therefore, analysis of SNEs in periarticular tissue is important to increase our understanding of dynamic joint stability (6).
SNEs have been investigated in various joints (7), and studies are on-going to characterize SNEs in the hip joint. Although the mechanoreceptors of the hip joint have been studied in healthy animals (8, 9), it is not possible to perform these studies in healthy people due to ethical concerns. Therefore, studies in humans are usually performed in tissues excised during hip surgery or in cadavers (7, 10–14). The majority of samples for human hip joint assessment have been obtained from specimens excised during surgical treatment of coxarthrosis or patients with developmental dysplasia of the hip (DDH) (7, 10–13).
DDH is important in pediatrics. The static stability of the hip joint depends on the shape of the articular surfaces and on the soft tissue of the capsule (1, 15–17). Thus, an elongated hip joint capsule (HJC), hypertrophic ligamentum teres (LT), and excessive pulvinar have primarily been considered obstructions to hip reduction in DDH (17, 18). Therefore, several authors have described the excision and plication of an elongated HJC and resection of the hypertrophic LT and excessive pulvinar as part of open hip reduction in DDH (15, 17, 18).
The presence of SNEs in the LT and the HJC in patients with DDH has been previously studied (11, 12). However, to the best of our knowledge, no study in the English-language literature has explored SNEs in the pulvinar of patients with DDH. Therefore, we investigated the prevalence of SNEs in the pulvinar by performing haematoxylin and eosin (H&E) staining and S-100 immunohistochemical staining. We also investigated studies of the HJC and LT for comparison with previous reports.
Materials and Methods
Study population
This study was performed initially at the author’s previous institute (Department of Orthopaedic Surgery, Harran University, School of Medicine, Şanlıurfa, Turkey). Data analysis and manuscript writing were completed at the author’s current institute (Department of Orthopaedic Surgery, İstanbul Kanuni Sultan Süleyman Training and Research Hospital, İstanbul, Turkey). The data belong to Harran University, and ethical approval was obtained from the local ethics committee there (approval ID number: B.30.2.HRU.0.0.20.05.00.101.5/.031). Informed consent from all patients was obtained before this study. Biopsy specimens were obtained from 38 hips of 36 patients who were surgically treated for DDH at Harran University Hospital between January 2010 and July 2012.
Clinical and radiological assessments and surgical technique
All patients were diagnosed with DDH; none had been previously neglected or untreated. A total of 31 females (33 hips) and 5 males (5 hips) were included in the study. Two patients presented with bilateral dislocations. The cases included 15 right hips and 23 left hips, and the average age at the time of surgery was 48.9±30.6 months (range: 18–132 months). As the patients ages varied, we divided them into groups to analyse the relationship of age with the presence of SNEs. Although there are no definite criteria for separating the age groups, previous studies using similar surgical procedures were referenced (19). Thus, our patients were distributed into three groups according to the age at which they were operated: Group 1 included 18 patients (19 hips) aged <3 years, Group 2 comprised 10 patients (10 hips) aged 3–5 years, and Group 3 included 8 patients (9 hips) aged >5 years (Table 1). The age groups did not differ significantly in gender or operated side (p>0.05).
Table 1.
Demographic data for FNEs assessment
| Case | Age (months) | Group | Side | Gender | FNEs/pulvinar | FNEs/HJC | FNEs/LT |
|---|---|---|---|---|---|---|---|
| 1 | 31 | 1 | 1 | 2 | 0 | 0 | 4 |
| 2 | 20 | 1 | 2 | 2 | 12 | 3 | 5 |
| 3 | 18 | 1 | 1 | 2 | 0 | 4 | 9 |
| 4 | 30 | 1 | 1 | 2 | 0 | 0 | 0 |
| 5 | 30 | 1 | 1 | 2 | 0 | 2 | 7 |
| 6 | 48 | 2 | 2 | 2 | 0 | 3 | 18 |
| 7 | 120 | 3 | 1 | 2 | 13 | 7 | 11 |
| 8 | 36 | 2 | 2 | 2 | 0 | 5 | 4 |
| 9 | 40 | 2 | 2 | 2 | 0 | 1 | 0 |
| 10 | 84 | 3 | 1 | 2 | 0 | 0 | 4 |
| 11 | 38 | 2 | 2 | 2 | 12 | 11 | 4 |
| 12 | 26 | 1 | 2 | 2 | 7 | 0 | 0 |
| 13 | 30 | 1 | 1 | 2 | 0 | 0 | 0 |
| 14 | 84 | 3 | 2 | 2 | 1 | 0 | 1 |
| 15 | 78 | 3 | 2 | 2 | 0 | 0 | 0 |
| 16 | 30 | 1 | 1 | 2 | 0 | 0 | 0 |
| 17 | 24 | 1 | 2 | 2 | 0 | 0 | 0 |
| 18 | 52 | 2 | 1 | 1 | 1 | 1 | 0 |
| 19 | 90 | 3 | 2 | 2 | 0 | 0 | 0 |
| 20 | 42 | 2 | 2 | 2 | 0 | 1 | 0 |
| 21 | 30 | 1 | 1 | 2 | 0 | 0 | 0 |
| 22 | 30 | 1 | 1 | 1 | 0 | 0 | 0 |
| 23 | 100 | 3 | 1 | 1 | 0 | 0 | 5 |
| 24 | 42 | 2 | 2 | 2 | 0 | 0 | 0 |
| 25 | 90 | 3 | 1 | 2 | 0 | 0 | 0 |
| 26 | 114 | 3 | 2 | 1 | 0 | 0 | 0 |
| 27 | 30 | 1 | 2 | 2 | 15 | 5 | 0 |
| 28 | 29 | 1 | 2 | 2 | 0 | 0 | 0 |
| 29 | 32 | 1 | 2 | 2 | 0 | 0 | 12 |
| 30 | 20 | 1 | 2 | 2 | 7 | 24 | 12 |
| 31 | 45 | 2 | 2 | 2 | 0 | 0 | 3 |
| 32 | 132 | 3 | 2 | 2 | 0 | 0 | 12 |
| 33 | 52 | 2 | 2 | 2 | 3 | 0 | 4 |
| 34 | 39 | 2 | 2 | 1 | 12 | 20 | 22 |
| 35 | 33 | 1 | 1 | 2 | 21 | 6 | 0 |
| 36 | 32 | 1 | 1 | 2 | 10 | 11 | 20 |
| 37 | 34 | 1 | 2 | 2 | 0 | 17 | 0 |
| 38 | 25 | 1 | 2 | 2 | 12 | 0 | 21 |
FNEs: free nerve endings per 50 mm2; HJC: hip joint capsule; LT: ligamentum teres
Anteroposterior and frog-leg radiographs were obtained, and the initial radiographs were evaluated using the Tonnis classification system (20). All patients were considered as exhibiting Tonnis grade 4 position. Patients with a neuromuscular or teratological dislocation had been previously excluded.
Pre-operative traction was not attempted. Combined procedures including open reduction and pelvic and/or femoral osteotomy were performed on 38 hips (21, 22). As in a previous study (10), the HJC was opened using a T-shaped incision, leaving in place a redundant proximal part of the superolateral flap of the capsule. This triangular part of the flap was excised with the scalpel for plication and used as a biopsy specimen. The LT was excised, and a mark suture was placed on the acetabular side. The hypertrophic fibrous fatty tissue of the pulvinar was removed with pituitary rongeurs, as in our previous study (18).
Histopathological assessment
All studied specimens were assessed macroscopically, and none showed signs of damage or structural abnormality. The pulvinar materials were fixed in 10% formaldehyde solution and then embedded in paraffin. Subsequently, they were prepared using routine tissue processing and cut into 4-μm sections.
Histological analysis of the stained tissue sections was performed using an Olympus light microscope (Olympus BX 51 TF, Tokyo, Japan). H&E staining was used to determine tissue morphology, and all specimens were evaluated to exclude signs of specimen lesions before starting the SNE analysis. SNEs were analyzed immunohistochemically using a mouse monoclonal antibody against S-100 Beta Protein (Genetex, USA). This was an acidic, dimeric calcium-binding protein with a molecular weight of 21,000 kD composed of various combinations of a and p sub-units, which was first isolated in the central nervous system (23).
SNEs were analyzed according to the classification of Freeman and Wyke after staining with H&E and S-100 protein (24). We examined the specimens for the presence of the four types of SNEs: type I, Ruffini corpuscles; type II, Pacini corpuscles; type III Golgi organs; and type IVa, unmyelinated FNEs. An area of 50 mm2 was investigated in each anatomical structure under a light microscope in a high-power field, as described by Leunig et al. (23). The SNE values were evaluated based on the mean of four sections of each pulvinar specimen. All specimens were blinded for cell counts.
Statistical analysis
All analyses were conducted using the Statistical Package for Social Sciences version 22.0 (IBM SPSS Corp.; Armonk, NY, USA). Continuous variables are expressed as the mean±SD. The normality of distributions was confirmed using the one-sample Kolmogorov–Smirnov test. Comparisons of the categorical and continuous variables were performed using the χ2 test and Mann–Whitney U-test, respectively. The correlations of age group, gender, and operated side with SNEs were evaluated using the Spearman test. Two-tailed p values of <0.05 were considered significant.
Results
Types I–III mechanoreceptors were not found in the pulvinar, LT, or HJC specimens. FNEs (type IVa) were observed in 13 (34%) pulvinar, 19 (50%) LT, and 16 (42%) HJC specimens. Patient data including the presence of FNEs are provided in Table I. The total density of FNEs was 3.31±5.70 (range 0–21)/50 mm2 for the pulvinar specimens (Figure 1), 3.18±5.92 (range 0–24)/50 mm2 for the HJC specimens (Figure 2), and 4.51 ± 6.61 (range 0–22)/50 mm2 for the LT specimens (Figure 3).
Figure 1.
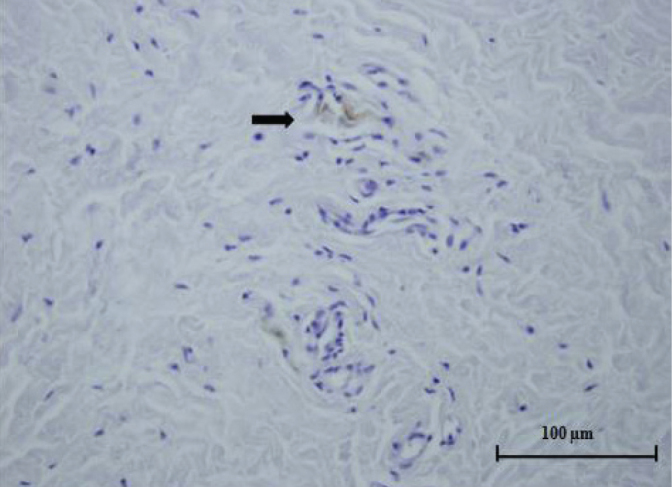
Histological specimen from the pulvinar. Free nerve ending (arrow) can be seen in the fibrovascular connective tissue (light microscope x400, S-100 protein)
Figure 2.

Histological specimen of the hip joint capsule. Several free nerve endings (arrows) can be seen in the fibrous connective tissue (light microscope x400, S-100 protein)
Figure 3.

Histological specimen of the ligamentum teres. Several free nerve endings (arrows) can be seen in the fibrovascular connective tissue (light microscope x400, S-100 protein)
The following results were obtained for the presence of FNEs in the pulvinar, HJC, and LT specimens of various age groups. The detection ratios of FNEs in group 1 (<3 years, 19 hips) were 7/19 for pulvinar specimens, 8/19 for LT specimens, and 8/19 for HJC specimens. In group 2 (3–5 years, 10 hips), the ratios were 4/10 for pulvinar specimens, 8/10 for LT specimens, and 7/10 for HJC specimens. In group 3 (>5 years, 9 hips), the ratios were 2/9 for pulvinar specimens, 4/9 for LT specimens, and 1/9 for HJC specimens. No significant differences were observed among the groups (p>0.05).
Furthermore, the operated side, gender, and the number of FNEs in the specimens did not differ significantly among the age groups (p>0.05 for all). However, we observed positive correlations (r=0.396, p<0.05) between FNEs in the pulvinar and in the HJC and between those in the LT and in the HJC (r=0.382, p<0.05).
Discussion
We identified no types I–III mechanoreceptors in the pulvinar; however, we identified FNEs (type Va) in all specimens. In addition, we found that the HJC and LT were similar to previous studies in that only FNEs were identified in these structures. Finally, we found no correlations between age group and the operated side, gender, or number of FNEs in any of the specimens.
In our study, we found no type I–III mechanoreceptors in the pulvinar, LT, and HJC. However, FNEs were detected in some specimens. This may be because the joint surfaces are not in contact with the dislocated hip. All of our patients had high-grade DDH (Tonnis type IV), and it is possible that mechanoreceptor types I–III develop during hip joint growth following reduction; hence, concentric reduction is essential for normal hip development (1, 15, 17). To increase our understanding of this condition, patients with Crowne type IV dysplastic hip and adult DDH should be compared with patients who have undergone hip surgery for other reasons.
Leunig et al., who examined the LT in a wide age range of patients (9–94 years) who underwent hip surgery for a variety of reasons, identified FNEs in all patients; however, no types I–III mechanoreceptors were detected (23). In Leunig’s study, only 2 of the 18 patients were 9 years old, and they did not suffer from DDH patients. FNEs were reported to occur in larger numbers in these patients. One patient with DDH was 18 years old, and the FNE ratio was very low. This may be due to disruption of nerve ending development due to dislocation of the hip. Similar to Leunig’s study, we observed low-density FNEs in three of four DDH patients aged 9–10 years, and one had no FNEs. We also observed FNEs in 50% of the LT specimens. Although the same staining technique was used, this discrepancy may reflect differences in patient ages and the populations sampled. Moraes et al. used gold chloride staining to assess the HJC, LT, and acetabular labrum of patients (mean age: 56.5) who were operated for coxarthrosis and younger cadavers (mean age: 35.5) with no arthrosis (7). They identified all receptor types (types I–IVa) (7). Thereafter, Gerhardt et al. used gold chloride staining to assess the HJC, LT, transverse acetabular ligament (TAL), and acetabular labrum (14). They found all mechanoreceptors in the labrum and capsule, but only FNEs in the TAL. In addition, they found no significant SNEs in the LT, and reported that mechanoreceptors were strongly present in the superolateral area of the capsule. On the other hand, they reported no receptors in the posterior capsule and decreased SNEs in the anterior and inferior regions. This may explain our inability to find types I–III mechanoreceptors in HJC specimens. In our study, we evaluated excised capsule material, from the antero-inferior zone. The difference of our results may be explained in part by the use of different staining techniques. Kılıçarslan et al. examined labrum and TAL specimens with S-100 staining in patients with total hip arthroplasty (13). All types of SNEs were identified in the labrum, but only FNEs were identified in the TAL. These studies show the presence of FNEs in hip joint tissues. Therefore, these FNEs may role in pain sensation in the hip joint.
In DDH patients (mean age: 10.3 months), Muratlı et al. found no receptors in the HJC and LT (10). They suggested that specimens excised from the anterior capsule (0.5 × 0.5 cm) may be inadequate for examination and suggested that the receptors may increase with age. We found no significant differences between age groups in our study. Moraes et al. identified all receptors in their study of adult patients and cadavers (7). Sarban et al. studied the LT in patients with luxated or sub-luxated DDH and found only FNEs, which agrees with our results (11). Later, Desteli et al. conducted a study comparing HJC and LT specimens in DDH patients with in utero ex fetus samples (12). Similar to our study, no types I–III receptors were found, although FNEs were present. In addition, both studies suggested that the ligamentum and pulvinar may contribute to joint stability through the FNEs; they proposed preserving these tissues instead of excising them (11, 12). Considering the importance of concentric hip joint reduction in the treatment of DDH, we do not agree with this recommendation for two reasons: first, we found no types I–III mechanoreceptors; second, FNEs were not present in all specimens.
Our study has various assumptions and limitations. First, our study is only an investigation of the SNEs in a pediatric population with DDH. Therefore, our study does not explore the presence of SNEs in the hip joints of healthy children or adults. However, no control group was included due to ethical constraints. In keeping with the tenets of the Declaration of Helsinki, we did not use a control group of healthy children (25). Second, our results may be related to different staining techniques. We investigated the specimens with immunohistochemical analysis using mouse monoclonal antibody against S-100 protein. Although the cost of immunohistology for the S-100protein is high, it revealed greater detail (26). Gold chloride staining was found to be less efficacious and non-specific for staining thin neuronal structures, as it stains vascular and other structures containing collagen and elastin (27). On the other hand, using only S-100 as a specific neural marker is likely not sufficient to precisely classify SNEs. Numerous studies have recommended the use of a combination of immunohistochemical antibodies to classify SNEs because the morphology can be highly variable, and recent studies have revealed a large number of unclassifiable corpuscles according to the classification of Hagert et al. (28).
In conclusion, on the basis of our results, the pulvinar, LT, and HJC have no types I–III mechanoreceptors, but do contain FNEs, which are known to play a role in pain sensation. Accordingly, surgical excision of these tissues to achieve concentric reduction may not cause a significant loss of sensory functions in the hip joint in DDH patients. To increase our understanding of the mechanoreceptor characteristics of the hip joint, it is important to investigate healthy hips as a control group and to evaluate extra-articular tissues. The most appropriate treatment algorithms for DDH or other diseases of the hip could be defined by considering the dynamic functions of the hip joint.
HIGHLIGHTS
Pulvinar, ligamentum teres (LT) and hip joint capsule (HJC) excised from open surgery of DDH patients were examined for sensory nerve endings (SNEs).
This study is the first, to investigate SNEs in the pulvinar.
No spesific SNEs (type I, II, III mechanoreceptors) were identified in any specimens.
Type IVa mechanoreceptors were characterized in 34% of pulvinar, 50% of LT, and 42% of HJC specimens, and not differ significantly among the age groups.
Excision of these tissues to achieve concentric reduction may not lead significant loss of sensory function in the hip joint of DDH patients.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of Harran University (approval number: B.30.2. HRÜ.0.20.05.00.101.5/031).
Informed Consent: Written informed consent was obtained from the parents of the patients who participated in this study.
Author Contributions: Concept - C.E.; Design - C.E., M.A.A.; Supervision - S.K.; Materials - C.E. S.K.; Data Collection and/or Processing - S.K. H.B.; Analysis and/or Interpretation - C.E. S.K.; Literature Review - S.K. H.B. M.A.A.; Writing - C.E., H.B.; Critical Review - M.A.A.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Connolly P, Weinstein SL. The natural history of acetabular development in developmental dysplasia of the hip. Acta Orthop Traumatol Turc. 2007;41(Suppl1):1–5. [PubMed] [Google Scholar]
- 2.Frank CB. Ligament structure, physiology and function. J Musculoskelet Neuronal Interact. 2004;4:199–201. [PubMed] [Google Scholar]
- 3.Zimny ML. Mechanoreceptors in articular tissues. Am J Anat. 1988;182:16–32. doi: 10.1002/aja.1001820103. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy JC, Alexander IJ, Hayes KC. Nerve supply of the human knee and its functional importance. Am J Sports Med. 1982;10:329–35. doi: 10.1177/036354658201000601. [DOI] [PubMed] [Google Scholar]
- 5.Johansson H, Sjolander P, Sojka P. Receptors in the knee joint ligaments and their role in the biomechanics of the joint. Crit Rev Biomed Eng. 1991;18:341–68. [PubMed] [Google Scholar]
- 6.Rein S, Hagert E, Hanisch U, Lwowski S, Fieguth A, Zwipp H. Immunohistochemical analysis of sensory nerve endings in ankle ligaments: A cadaver study. Cells Tissues Organs. 2013;197:64–76. doi: 10.1159/000339877. [DOI] [PubMed] [Google Scholar]
- 7.Moraes MR, Cavalcante ML, Leite JA, et al. The characteristics of the mechanoreceptors of the hip with arthrosis. J Orthop Surg Res. 2011;16:58. doi: 10.1186/1749-799X-6-58. doi: 10.1186/1749-799X-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He XH, Tay SS, Ling EA. Sensory nerve endings in monkey hip joint capsule: A morphological investigation. Clin Anat. 1998;11:81–5. doi: 10.1002/(SICI)1098-2353(1998)11:2<81::AID-CA2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 9.Carli G, Farabollini F, Fontani G, Meucci M. Slowly adapting receptors in cat hip joint. J Neurophysiol. 1979;42:767–78. doi: 10.1152/jn.1979.42.3.767. [DOI] [PubMed] [Google Scholar]
- 10.Muratli HH, Bicimoglu A, Tabak YA, Celebi L, Paker I. Mechanoreceptor evaluation of hip joint capsule and ligamentum capitis femoris in developmental hip dysplasia: A preliminary study. J Pediatr Orthop B. 2004;13:299–302. doi: 10.1097/01202412-200409000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Sarban S, Baba F, Kocabey Y, Cengiz M, Isikan UE. Free nerve endings and morphological features of the ligamentum capitis femoris in developmental dysplasia of the hip. J Pediatr Orthop B. 2007;16:351–6. doi: 10.1097/01.bpb.0000243830.99681.3e. [DOI] [PubMed] [Google Scholar]
- 12.Desteli EE, Gülman AB, Imren Y, Kaymaz F. Comparison of mechanoreceptor quantities in hip joints of developmental dysplasia of the hip patients with normal hips. Hip Int. 2014;24:44–8. doi: 10.5301/hipint.5000091. [DOI] [PubMed] [Google Scholar]
- 13.Kilicarslan K, Kilicarslan A, Demirkale İ, Aytekin MN, Aksekili MA, Uğurlu M. Immunohistochemical analysis of mechanoreceptors in transverse acetabular ligament and labrum: A prospective analysis of 35 cases. Acta Orthop Traumatol Turc. 2015;49:394–8. doi: 10.3944/AOTT.2015.14.0366. [DOI] [PubMed] [Google Scholar]
- 14.Gerhardt M, Johnson K, Atkinson R, et al. Characterisation and classification of the neural anatomy in the human hip joint. Hip Int. 2012;22:75–81. doi: 10.5301/HIP.2012.9042. [DOI] [PubMed] [Google Scholar]
- 15.Weinstein SL, Ponseti IV. Congenital dislocation of the hip. J Bone Joint Surg Am. 1979;61:119–24. doi: 10.2106/00004623-197961010-00021. [DOI] [PubMed] [Google Scholar]
- 16.Erturk C, Altay MA, İsikan UE. A radiological comparison of Salter and Pemberton osteotomies to improve acetabular deformations in developmental dysplasia of the hip. J Pediatr Orthop B. 2013;22:527–32. doi: 10.1097/BPB.0b013e32836337cd. [DOI] [PubMed] [Google Scholar]
- 17.Herring JA. Developmental dysplasia of the hip. In: Herring JA, editor. Tachdjian’s Pediatric Orthopaedics. 4th ed. Philadelphia: WB Saunders; 2008. pp. 637–770. [DOI] [Google Scholar]
- 18.Erturk C, Altay MA, Yarimpapuc R, Isikan UE. Medial open reduction of developmental dysplasia of the hip using the Weinstein-Ponseti approach. Saudi Med J. 2011;32:901–6. [PubMed] [Google Scholar]
- 19.Tezeren G, Tukenmez M, Bulut O, Percin S, Cekin T. The surgical treatment of developmental dislocation of the hip in older children: A comparative study. Acta Orthop Belg. 2005;71:678–85. [PubMed] [Google Scholar]
- 20.Tonnis D. Congenital dysplasia and dislocation of the hip in children and adults. Berlin: Springer-Verlag; 1987. pp. 100–42. [DOI] [Google Scholar]
- 21.Erturk C, Altay MA, Yarimpapuc R, Koruk I, Isikan UE. One-stage treatment of developmental dysplasia of the hip in untreated children from two to five years old. A comparative study. Acta Orthop Belg. 2011;77:464–71. [PubMed] [Google Scholar]
- 22.Erturk C, Altay MA, Isikan UE. Femoral segment graft is a suitable alternative to stabilize pelvic osteotomies in developmental dysplasia of the hip: A comparative study. J Pediatr Orthop B. 2012;21:200–5. doi: 10.1097/BPB.0b013e32834f7f9d. [DOI] [PubMed] [Google Scholar]
- 23.Leunig M, Beck M, Stauffer E, Hertel R, Ganz R. Free nerve endings in the ligamentum capitis femoris. Acta Orthop Scand. 2000;71:452–4. doi: 10.1080/000164700317381117. [DOI] [PubMed] [Google Scholar]
- 24.Freeman MA, Wyke B. The innervation of the knee joint. An anatomical and histological study in the cat. Acta Anat (Basel) 1967;68:321–33. doi: 10.1159/000143037. [DOI] [PubMed] [Google Scholar]
- 25.World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–4. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 26.Syed SN, Phillips A, Van Pitius DG. Assessing regeneration of mechanoreceptors in human hip pseudocapsule after primary total hip arthroplasty. J Orthop Trauma Rehab. 2014;18:12–4. doi: 10.1016/j.jotr.2013.12.007. [DOI] [Google Scholar]
- 27.Hogervorst T, Brand RA. Mechanoreceptors in joint function. J Bone Joint Surg Am. 1998;80:1365–78. doi: 10.2106/00004623-199809000-00018. [DOI] [PubMed] [Google Scholar]
- 28.Hagert E. Department of clinical science and education. Stockholm, Sweden: Karolinska Institutet; 2008. Wrist ligaments-innervation patterns and ligamento-muscular reflexes; pp. 1–93. [Google Scholar]


