Abstract
BACKGROUND
Moderate-to-severe pain exists in the early postoperative period after laparoscopic renal surgery.
OBJECTIVE
We investigated the analgesic effect of quadratus lumborum block (QLB) via two approaches in patients undergoing laparoscopic renal nephrectomy.
DESIGN
A randomised controlled trial.
SETTING
An academic tertiary care hospital in Beijing, China.
PARTICIPANTS
Ninety-six patients aged 18 to 70 years who were scheduled for elective laparoscopic radical or partial nephrectomy.
INTERVENTIONS
Eligible patients were allocated randomly to a control group (no block), lateral QLB group or posterior QLB group. Ultrasound-guided QLB was performed via either the lateral or posterior approach with 30 ml of 0.4% ropivacaine before surgery.
MAIN OUTCOME MEASURES
The primary outcome was sufentanil equivalent consumption within 24 h. Among secondary outcomes, somatic and visceral pain intensity at rest and on coughing were assessed with a numerical rating scale (where 0 = no pain and 10 = the worst pain) until 24 h postoperatively.
RESULTS
Sufentanil equivalent consumption did not differ among the three groups (118 ± 36 μg in the control group, 115 ± 47 μg in the lateral QLB group and 119 ± 40 μg in the posterior QLB group; P = 0.955). However, both somatic (lateral QLB vs. control, median difference −1, P < 0.001 at rest and −2 to −1, P < 0.001 on coughing; posterior QLB vs. control, −1, P < 0.001 at rest and −2 to −1, P < 0.001 on coughing) and visceral pain scores (lateral QLB vs. control, −1 to 0, P < 0.001 at rest and −1, P < 0.001 on coughing; posterior QLB vs. control, −1 to 0, P < 0.001 at rest and −2 to −1, P < 0.001 on coughing) were significantly lower in the two QLB groups than in the control group.
CONCLUSION
For patients undergoing laparoscopic renal surgery, a pre-operative single-shot QLB via the lateral or posterior approach did not decrease opioid consumption, but improved analgesia for up to 24 h after surgery.
TRIAL REGISTRATION
www.chictr.org.cn identifier: ChiCTR1800019883.
Introduction
Laparoscopic renal surgery (LRS), either via the retroperitoneal or transperitoneal approach, is a popular method for renal cancer therapy.1,2 However, despite less surgical trauma with LRS compared with open surgery, moderate-to-severe pain remains in the early postoperative period.3,4 Adequate pain control is important for rapid recovery after surgery. Although opioids are the most commonly used analgesics, they bring side effects such as postoperative nausea and vomiting, gastro-intestinal ileus and respiratory depression. Therefore, multimodal analgesia including regional nerve block is advocated to decrease opioid consumption and improve analgesia after LRS.5
Quadratus lumborum block (QLB) is a novel method of trunk block that aims to control both somatic and visceral pain in the lateral and anterior abdomen.6 There are two classic methods of QLB based on the site of the local anaesthetic injection: lateral QLB (local anaesthetic injected lateral to quadratus lumborum, also named QLB1) and posterior QLB (local anaesthetic injected posterior to quadratus lumborum, also named QLB2).7 Both approaches have been shown to reduce opioid consumption and relieve pain intensity after lower abdominal surgery,6,8 but evidence is limited in urological surgery.9 This study was designed to compare the analgesic effect of single-shot lateral or posterior QLBs versus no block in patients undergoing LRS. The primary endpoint was cumulative opioid consumption within 24 h after surgery.
Methods
The current randomised, observer-blinded trial was approved by the Biomedical Research Ethics Committee of Peking University First Hospital (2018-69) and registered with the Chinese Clinical Trial Registry (http://www.chictr.org.cn, ChiCTR1800019883; 5 December 2018) before the enrolment process started on 10 December 2018. The study adhered to the CONSORT guidelines.
We enrolled patients aged 18 to 70 years who were scheduled for elective laparoscopic radical or partial nephrectomy. Exclusion criteria were: chronic opioid addiction or use of other analgesic drugs for more than 3 months; persistent pain in the renal region; presence of tumour thrombus in the renal vein or inferior vena cava, or receiving renal artery embolisation before surgery; inability to communicate due to severe dementia, language barrier or end-stage disease; nerve block contraindications, such as local infection, coagulopathy and anatomical abnormalities; planned ICU admission after surgery; American Society of Anesthesiologists physical status at least 4; or allergy to local anaesthetics. Written informed consent was obtained from each participant.
Patients were assigned randomly to a control group, a lateral QLB group or a posterior QLB group according to randomisation information sealed in sequentially numbered envelopes. Random numbers were generated using the SAS statistical package version 9.3 (SAS Institute, Cary, North Carolina, USA) with a block size of 6 in a 1 : 1 : 1 ratio. Randomisation was stratified according to the planned type of surgery (radical or partial nephrectomy).
In the pre-operative preparation room on the day of surgery, a study co-ordinator (Z-YL) opened the envelopes consecutively according to the recruitment sequence, but did not participate in the rest of the trial. While anaesthesiologists were aware of group allocation, patients would not know which QLB they had received. However, other healthcare team members and investigators in charge of postoperative following-ups (Z-ZX and Z-ML) were blinded to group assignments.
Patients allocated to the QLB groups were given premedication with midazolam 0.02 mg kg−1 or sufentanil 0.08 μg kg−1 administered intravenously. All patients were placed in a lateral decubitus position with the operative side up. Ultrasound-guided QLB was performed by one of two experienced anaesthetists (DH or HK) who did not participate in the rest of the study. A curvilinear ultrasound probe (3 to 5 MHz, GE Healthcare, Boston, Massachusetts, USA) was first placed at the midaxillary line between the lower costal margin and the iliac crest, at approximately the L3/L4 vertebral level. After recognising the three layers of abdominal wall muscles, the transversus abdominis was traced more posteriorly until the transversus aponeurosis appeared. By slightly tilting the probe caudally, the view was improved to distinguish between the retroperitoneal fat and quadratus lumborum. The probe was then moved more posteriorly to identify the lumbar interfascial triangle.
A special needle designed for nerve block (80 mm, Stimuplex D, B.Braun, Melsungen, Germany) was inserted using an in-plane technique in the posteroanterior direction. In the lateral QLB group, the needle tip was in the thoracolumbar fascia just anterolateral to quadratus lumborum, whereas in the posterior QLB group it was in the lumbar interfascial triangle. After aspiration, 1 to 2 ml of 0.9% saline was injected first to ensure correct positioning of the needle tip; 30 ml of 0.4% ropivacaine was then injected. Successful injection was confirmed by the appearance of a hypoechoic ellipsoid anterolateral to quadratus lumborum and ventral displacement of the transversalis fascia in the lateral QLB group (Fig. 1a) or a hypoechoic ellipsoid between quadratus lumborum and erector spinae without any intramuscular injection imaging in the posterior QLB group (Fig. 1b).
Fig. 1.
Sonography of lateral quadratus lumborum block (a) and posterior quadratus lumborum block (b) after local anaesthetic injection
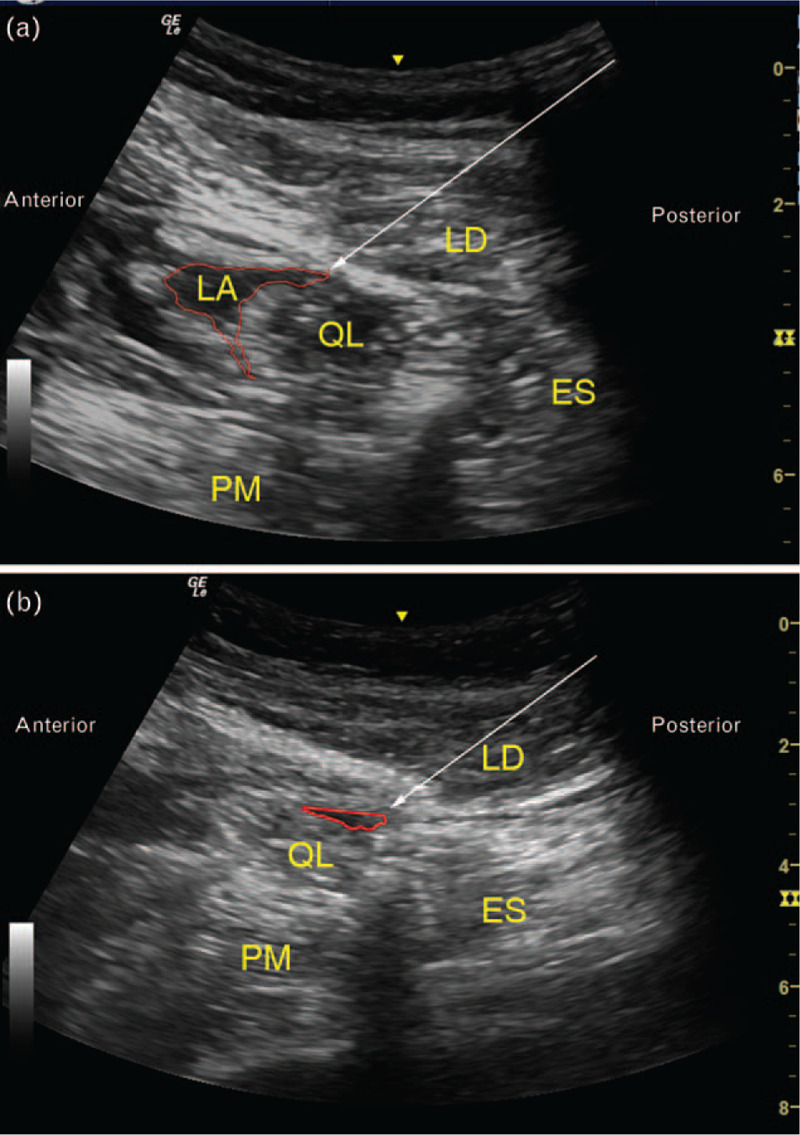
The white arrow indicates the needle trajectory. ES, erector spinae; LA, local anaesthetic; LD, latissimus dorsi; PM, psoas major; QLB, quadratus lumborum block.
From 5 min after injection, sensory block was evaluated via a pinprick test every 10 min at the anterior midline, midclavicular and anterior axillary line in the anterior abdomen, at the midaxillary and posterior axillary lines in the lateral abdomen, at the mid-scapula and posterior midline in the back area, and at the L1 area in the lateral thigh. Blocked dermatomes were recorded in a craniocaudal direction. Successful block was defined as decreased or loss of pinprick sensation in the area covering the main surgical incision in the lateral abdomen just below the tip of the 12th rib. Both onset time and fixed time of the block were recorded. Sensory block was evaluated again at 24 h after surgery in the general ward.
Anaesthesia and peri-operative management
No pre-emptive oral analgesic drug was given before anaesthesia. A single dose of methylprednisolone 40 mg was administered intravenously before anaesthesia induction. General anaesthesia was induced with sufentanil, propofol/etomidate and rocuronium, and maintained with a propofol infusion, remifentanil infusion/sufentanil injection, with or without nitrous oxide or sevoflurane inhalation. An intravenous injection of midazolam (1 to 2 mg before induction) or an infusion of dexmedetomidine (0.3 to 0.7 μg kg−1 h−1, initiated after induction and stopped at least 1 h before the end of surgery) was administered at the discretion of the anaesthesiologists. The target was to keep the bispectral index between 40 and 60. Fluid therapy was provided according to routine practice. Vasoactive drugs were administered when necessary; the target was to maintain mean arterial pressure and heart rate within 20% of baseline (average value in the ward). At 30 min before the end of surgery, flurbiprofen axetil 50 mg and tropisetron 5 mg were administered intravenously. After anaesthesia emergence, all patients were monitored in the postanaesthesia care unit for at least 1 h before being sent back to the general ward.
Laparoscopic procedures were performed by the same surgical team members. For procedures with a retroperitoneal approach, the patient was placed in the flank decubitus position and three ports were needed. The first port was just below the 12th rib on the posterior axillary line; the second was 2 cm above the iliac crest in the midaxillary line; the third was under the costal margin on the anterior axillary line. In case of radical nephrectomy, the first incision was extended ventrally for kidney removal. For procedures with a transperitoneal approach, the patient was placed in the semi-oblique decubitus position with three or four trocars distributed between the umbilicus and the xiphoid process from the midline to the anterior axillary line in the anterior abdomen. The pneumoperitoneum pressure was maintained at 12 to 16 mmHg throughout the procedure.
A patient-controlled analgesia (PCA) pump (sufentanil 1.25 μg ml−1) was provided for all patients after surgery. It was programmed to deliver 4-ml boluses with a background infusion rate at 0.5 ml h−1 and a 10-min lockout interval. Patients were instructed to control their pain with self-press boluses when the numerical rating scale (NRS; an 11-point scale where 0 = no pain and 10 = the worst pain) for pain was at least 4. If the pain was not relieved, NSAIDs or other analgesics were prescribed as rescue medication.
Two investigators (XL and Y-TL) were designated to assess the range of blocked dermatomes in the QLB groups; another two investigators (Z-ZX and Z-ML), who were blinded to the study group assignment, were responsible for pain assessment and data collection in all patients at 0, 0.5, 1, 2, 6, 12 and 24 h after surgery. Somatic pain indicated incisional pain with definite locations in the abdominal wall that the patient could touch; visceral pain indicated deep, dull pain inside the abdomen that was difficult to localise.10 During the postoperative follow-up, patients were asked to discriminate between incisional (trocar sites) and deep abdominal pain. Each was assessed with the NRS.
The primary outcome was total opioid consumption (sufentanil equivalent dose11,12) during surgery and within the first 24 h after surgery. Secondary outcomes included: somatic and visceral pain intensity assessed with NRS both at rest and on coughing at the above timepoints postoperatively; time to first required PCA bolus; use of rescue analgesics; subjective sleep quality on the night of surgery evaluated with the NRS (where 0 = the best sleep quality and 10 = the worst sleep quality); time to first ambulation after surgery; self-reported comfort at 24 h after surgery assessed with the NRS; quality of recovery score at 24 h after surgery (assessed with the QoR-40 scale, scores from 0 to 200, with higher scores indicating better postsurgical recovery13); and length of hospital stay after surgery. Other predefined outcomes included the following: rate of successful block before surgery and at 24 h after surgery; onset time of QLB (defined as the time to the first detection of a blocked dermatome); time to fixed sensory block (defined as the time when there was no further extension of the blocked dermatomes); and range of blocked dermatomes.
Safety outcomes were monitored from the beginning of the block until 24 h after surgery. Potential adverse events associated with QLB included but were not limited to the following: numbness in the lower extremities, haematoma and bleeding in the needle trajectory, visceral organ injury, anaphylaxis and local anaesthetic toxicity. Other peri-operative adverse events were also documented.
Sample size estimation
Based on a preliminary survey in our hospital, sufentanil consumption during surgery and within the first 24 h after LRS was 120 ± 31 μg in 10 patients without QLB and was 98 ± 30 μg in another 10 patients who received a posterior QLB. With a significance level of α = 0.05 and a power of 1 − β = 80% using the one-way analysis of variance test, the sample size required to detect this differences was 90 patients (30 in each group). Taking into account a drop-out rate of approximately 5%, we planned to enrol 96 patients. Sample size calculation was performed with the PASS 15.0 software (Stata Corp. LP, College Station, Texas, USA).
Statistical analysis
Normally distributed continuous variables were compared using analysis of variance if all data satisfied the homogeneity of variance assumptions, otherwise the nonparametric Kruskal–Wallis test was used. Nonnormally distributed continuous variables and ordinal data were compared using the Kruskal–Wallis test. Categorical variables were compared with χ2 analysis or Fischer's exact test. Repeatedly measured variables (somatic and visceral NRS pain scores) were compared with the generalised estimating equation method. Missing data were not replaced. Time-to-event data were analysed by the Kaplan–Meier estimator, with the difference among groups compared by the log-rank test. We also performed post hoc pairwise comparison of the randomised groups, and the significance criterion for each pairwise comparison was P less than 0.0167 after Bonferroni correction. Outcome analyses were performed in the intention-to-treat population. A two-sided P value less than 0.05 was considered statistically significant. Statistical analysis was performed on SPSS 25.0 software package (IBM SPSS, Chicago, Illinois, USA).
Results
From 10 December 2018 to 17 September 2019, 96 patients were enrolled and allocated randomly to the control, lateral QLB or posterior QLB groups, with 32 patients in each group. The block failed in three patients in each of the lateral QLB and posterior QLB groups. Patients with a failed block were included in the intention-to-treat analysis but excluded for the per-protocol analysis (Fig. 2).
Fig. 2.
Flow diagram of the study
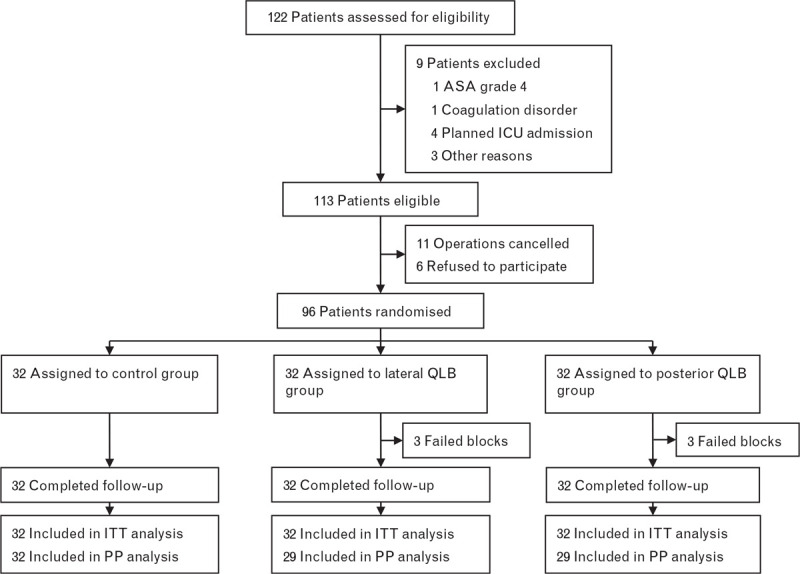
ITT, intention to treat; PP, per protocol.
Baseline data were balanced among the three groups except that the duration of education was shorter in the lateral QLB group (vs. the control group) but longer in the posterior QLB group (vs. the lateral QLB group). However, since this variable was not correlated with analgesic outcomes, no further adjustment was performed (Table 1). Intra-operative variables did not differ significantly among the three groups (Table 2).
Table 1.
Baseline data
| Control, n=32 | Lateral QLB, n=32 | Posterior QLB, n=32 | P | |
| Age (years) | 56 [51 to 62] | 56 [51 to 62] | 53 [45 to 59] | 0.206 |
| BMI (kg m−2) | 25.3 ± 3.5 | 25.9 ± 3.8 | 24.6 ± 2.7 | 0.283 |
| Male | 21 (65.6%) | 22 (68.8%) | 23 (71.9%) | 0.865 |
| Duration of education (years) | 14 [12 to 16] | 12 [9 to 12]∗ | 16 [12 to 16]∗∗ | 0.005 |
| Comorbidities | ||||
| Stroke | 2 (6.3%) | 2 (6.3%) | 0 (0.0%) | 0.541 |
| Hypertension | 9 (28.1%) | 14 (43.8%) | 11 (34.4%) | 0.421 |
| Chronic heart diseasea | 1 (3.1%) | 4 (12.5%) | 2 (6.3%) | 0.495 |
| Diabetes mellitus | 6 (18.8%) | 3 (9.4%) | 2 (6.3%) | 0.369 |
| Abnormal kidney functionb | 1 (3.1%) | 0 (0.0%) | 2 (6.3%) | 0.771 |
| Laboratory tests | ||||
| Haemoglobin (g l−1) | 146 [132 to 154] | 151 [134 to 159] | 145 [132 to 155] | 0.320 |
| Albumin (g l−1) | 46 [43 to 47] [1] | 46 [44 to 47] | 44 [43 to 47] | 0.432 |
| Creatinine (μmol l−1) | 73 [64 to 80] [1] | 75 [63 to 84] | 75 [69 to 93] | 0.134 |
| ASA physical status | 0.802 | |||
| 1 | 10 (31.3%) | 8 (25.0%) | 10 (31.3%) | |
| 2 | 21 (65.6%) | 22 (68.8%) | 19 (59.4%) | |
| 3 | 1 (3.1%) | 2 (6.3%) | 3 (9.4%) | |
| Maximum tumour diameter (cm) | 3.4 [2.3 to 5.1] | 3.8 [3.0 to 5.9] [3] | 3.0 [2.5 to 4.8] [1] | 0.147 |
| Hospital anxiety and depression, scorec | 3 [1 to 5] | 3 [1 to 5] | 3 [1 to 6] | 0.752 |
Data are mean ± SD, median [IQR], or number (%). Single numbers in square brackets indicate patients with missing data. ASA, American Society of Anesthesiologists; QLB, quadratus lumborum block.
Chronic heart disease included coronary heart disease, arrhythmia, valvular heart disease.
Serum creatinine more than 133 μmol l−1.
Assessed with the hospital anxiety and depression scale (scores range from 0 to 21 for either anxiety or depression, with higher scores indicating more severe anxiety and depression status) on the day before surgery.
P < 0.0167 vs. control group (P < 0.0167 was considered statistically significant after Bonferroni correction).
P < 0.0167 vs. lateral QLB group.
Table 2.
Intra-operative data
| Control, n=32 | Lateral QLB, n=32 | Posterior QLB, n=32 | P | |
| Duration of anaesthesia (min) | 153 [136 to 183] | 145 [126 to 170] | 153 [123 to 174] | 0.516 |
| Dose of methylprednisolone (mg) | 40 [40 to 40] | 40 [40 to 40] | 40 [40 to 40] | >0.999 |
| Use of midazolam | 8 (25.0%) | 9 (28.1%) | 17 (53.1%) | 0.036 |
| Dose of midazolam (mg) | 0 [0 to 0] | 0 [0 to 1] | 1 [0 to 2] | 0.034 |
| Use of etomidate | 23 (71.9%) | 22 (68.8%) | 26 (81.3%) | 0.495 |
| Dose of etomidate (mg) | 10 [0 to 12] | 8 [0 to 10] | 10 [6 to 18] | 0.086 |
| Dose of propofol (mg) | 500 [375 to 641] | 493 [378 to 575] | 528 [403 to 699] | 0.556 |
| Dose of sufentanil (μg) | 25 [20 to 30] | 25 [16 to 32] | 20 [16 to 30] | 0.614 |
| Dose of remifentanil (μg) | 590 [395 to 759] | 557 [353 to 749] | 562 [398 to 754] | 0.913 |
| Use of dexmedetomidine | 6 (18.8%) | 6 (18.8%) | 8 (25.0%) | 0.777 |
| Dose of dexmedetomidine (μg kg−1 h−1) | 0 [0 to 0] | 0 [0 to 0] | 0 [0 to 15] | 0.843 |
| Use of N2O | 29 (90.6%) | 27 (84.4%) | 29 (90.6%) | 0.782 |
| Use of sevoflurane | 7 (21.9%) | 5 (15.6%) | 11 (34.4%) | 0.202 |
| Duration of surgery (min) | 84 [65 to 115] | 79 [67 to 107] | 92 [67 to 110] | 0.685 |
| Surgical side | 0.272 | |||
| Left | 17 (53.1%) | 11 (34.4%) | 16 (50.0%) | |
| Right | 15 (46.9%) | 21 (65.6%) | 16 (50.0%) | |
| Type of surgery | 0.607 | |||
| Radical nephrectomy | 14 (43.8%) | 16 (50.0%) | 18 (56.3%) | |
| Partial nephrectomy | 18 (56.3%) | 16 (50.0%) | 14 (43.8%) | |
| Laparoscopic approach | 0.868 | |||
| Retroperitoneal | 30 (96.9%) | 30 (96.9%) | 30 (96.9%) | |
| Transperitoneal | 2 (3.1%) | 2 (3.1%) | 2 (3.1%) | |
| Pneumoperitoneum pressure (cmH2O) | 15 [14 to 16] | 14 [14 to 16] | 14 [13 to 15] | 0.117 |
Data are median [IQR] or number (%). QLB, quadratus lumborum block.
The total opioid consumption during surgery and within the first 24 h after surgery did not differ among the three groups (sufentanil equivalent dose 118 ± 36 μg in the control group, 115 ± 47 μg in the lateral QLB group and 119 ± 40 μg in the posterior QLB group, P = 0.955). Per-protocol analysis also showed no difference in the total opioid consumption among the three groups (Table 3 and Supplemental Table S1).
Table 3.
Efficacy outcomes
| Control, n=32 | Lateral QLB, n=32 | Posterior QLB, n=32 | P | |
| Primary outcome | ||||
| Total sufentanil equivalent dose (μg)a | 118 ± 36 | 115 ± 47 | 119 ± 40 | 0.955 |
| Total sufentanil equivalent dose (μg)a (PP analysis) | 118 ± 36 | 116 ± 48 | 120 ± 42 | 0.953 |
| Secondary outcomes | ||||
| Time to first required PCA bolus (h)b | 3.0 (0.2 to 5.8) | 8.0 (0.0 to 17.6) | 13.5 (1.7 to 25.3) | 0.034 |
| Number of required PCA bolus | 3 [1 to 5] | 2 [0 to 9] | 2 [0 to 10] | 0.763 |
| Number of administered PCA bolus | 2 [1 to 5] | 2 [0 to 9] | 2 [0 to 8] | 0.741 |
| Use of rescue analgesics besides PCA | 4 (12.5%) | 0 (0.0%) | 3 (9.4%) | 0.156 |
| Flurbiprofen axetil | 4 (12.5%) | 0 (0.0%) | 0 (0.0%) | 0.032 |
| Oral analgesicsc | 0 (0.0%) | 0 (0.0%) | 3 (9.4%) | 0.104 |
| Subjective sleep quality (night of surgery), scored | 6 [4 to 7] | 5 [4 to 8] | 5 [3 to 8] | 0.766 |
| Time to first ambulation (h)b | 21 (19 to 23) | 21 (19 to 23) | 20 (17 to 22) | 0.669 |
| Self-reported comfort at 24 h after surgery, scoree | 6 ± 2 | 6 ± 2 | 6 ± 3 | 0.291 |
| Quality of recovery at 24 h after surgery, scoref | 180 [173 to 184] | 178 [175 to 190] | 180 [174 to 189] | 0.748 |
| Length of hospital stay after surgery (day)b | 4.0 (3.4 to 4.6) | 5.0 (4.7 to 5.3) | 4.0 (3.5 to 4.5) | 0.423 |
Data are mean ± SD, median [IQR], median (95% CI) or number (%). PCA, patient-controlled analgesia; PP, per protocol; QLB, quadratus lumborum block.
The total sufentanil equivalent dose consumed during surgery and within 24 h after surgery (including PCA pump). 1 μg sufentanil = 10 μg remifentanil12; 1 Tylox tablet (containing 4.5-mg oxycodone) = 6.7-μg sufentanil.
Analysed by Kaplan–Meier analysis and tested by log-rank method.
Tylox tablet; one tablet contains 4.5-mg oxycodone and paracetamol 325 mg.
Assessed with the numerical rating scale where 0 indicates the best sleep and 10 indicates the worst sleep.
Assessed with the numerical rating scale where 0 indicates the most discomfort and 10 indicates the best comfort.
Assessed with QoR-40 scale, which contains 40 items and its scores ranges from 0 to 200, with higher scores indicating better postoperative recovery.
Compared with the control group, postsurgical somatic pain scores both at rest and on coughing were significantly lower in the lateral QLB group (at rest, median difference −1, P < 0.001; on coughing, median difference from −2 to −1, P < 0.001) and posterior QLB group (at rest, median difference −1, P < 0.001; on coughing, median difference from −2 to −1, P < 0.001) (Fig. 3 a and b; Supplemental Tables S2 and S3). Compared with the control group, postsurgical visceral pain scores both at rest and on coughing were also significantly lower in the lateral (at rest, median difference from −1 to 0, P < 0.001; on coughing, median difference −1, P < 0.001) and posterior QLB groups (at rest, median difference from −1 to 0, P < 0.001; on coughing, median difference from −2 to −1, P < 0.001) (Fig. 3 c and d; Supplemental Table S4 and S5).
Fig. 3.
Numerical rating scale somatic pain and visceral pain scores within the first 24 h after surgery
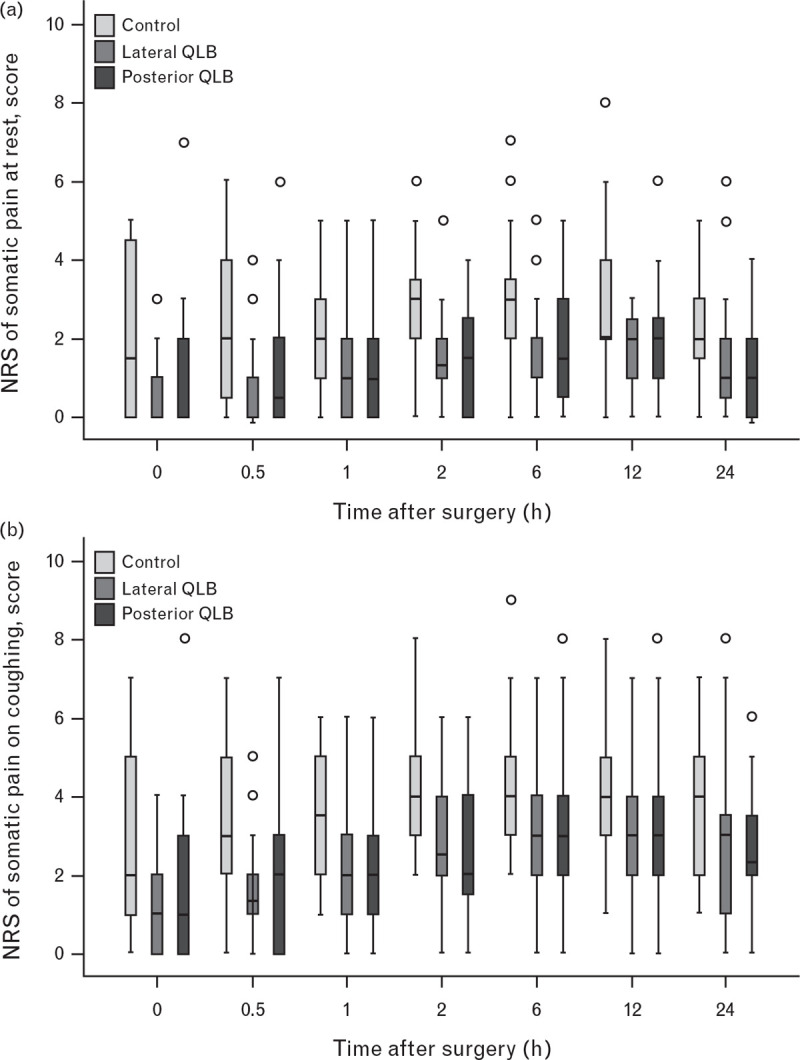
Somatic pain scores at rest (a) and on coughing (b), and visceral pain scores at rest (c) and on coughing (d) were all significantly lower in the lateral and posterior quadratus lumborum block groups than in the control group (all P < 0.001; compared using the generalised estimating equation method). NRS, numerical rating scale; QLB, quadratus lumborum block.
Fig. 3 (Continued).
Numerical rating scale somatic pain and visceral pain scores within the first 24 h after surgery
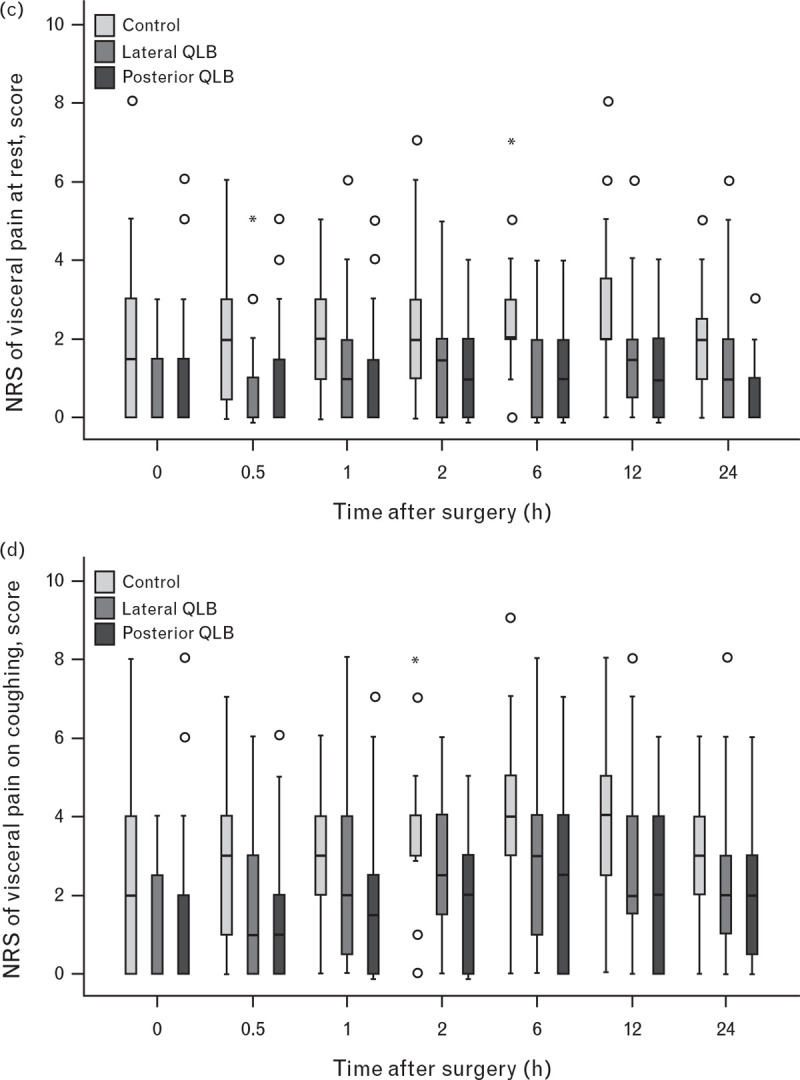
Somatic pain scores at rest (a) and on coughing (b), and visceral pain scores at rest (c) and on coughing (d) were all significantly lower in the lateral and posterior quadratus lumborum block groups than in the control group (all P < 0.001; compared using the generalised estimating equation method). NRS, numerical rating scale; QLB, quadratus lumborum block.
Time to first required PCA bolus was earlier (P = 0.034) whereas the percentage of patients who required rescue flurbiprofen axetil was higher (P = 0.032) in the control group than in the QLB groups; however, the difference was not statistically different when compared with each of the QLB groups after Bonferroni correction. Other secondary outcomes were not significantly different among groups (Table 3 and Supplemental Table S1). The overall successful block rate before surgery was 90.6% (58/64) in the QLB groups. Block-related data did not differ between two QLB groups (Table 4). The incidences of adverse events were similar among the three groups (Table 5).
Table 4.
Block-related data
| Lateral QLB, n=32 | Posterior QLB, n=32 | P | |
| Successful block before surgerya | 29 (90.6%) | 29 (90.6%) | >0.999 |
| Onset time (min) | 10 [6 to 15] [2] | 10 [5 to 13] [4] | 0.758 |
| Time to fixed sensory block (min) | 19 [15 to 25] [6] | 21 [15 to 30] [8] | 0.290 |
| Range of blocked dermatomes, n | 5 [3 to 6] | 4 [3 to 6] | 0.189 |
| Successful block at 24 h after surgerya | 4 (12.5%) | 9 (28.1%) | 0.120 |
Data are number (%) or median [IQR]. Single numbers in square brackets indicate patients with missing data. QLB, quadratus lumborum block.
Defined as decreased or loss of pinprick sensation in the area, which covered the main incision of surgery in the lateral abdomen just below the tip of the 12th rib.
Table 5.
Safety outcomes
| Control, n=32 | Lateral QLB, n=32 | Posterior QLB, n=32 | P | |
| Intra-operative bradycardiaa | 1 (3.1%) | 1 (3.1%) | 1 (3.1%) | >0.999 |
| Intra-operative hypotensionb | 9 (28.1%) | 8 (25.0%) | 9 (28.1%) | 0.949 |
| Intra-operative arrhythmiac | 1 (3.1%) | 0 (0.0%) | 0 (0.0%) | >0.999 |
| Emergence deliriumd | 0 (0.0%) | 1 (3.1%) | 0 (0.0%) | >0.999 |
| PONV within 24 h | 12 (37.5%) | 11 (34.4%) | 9 (28.1%) | 0.720 |
| Antiemetic therapy within 24 h | 2 (6.3%) | 2 (6.3%) | 5 (15.6%) | 0.496 |
Data are number (%). PONV, postoperative nausea and vomiting; QLB, quadratus lumborum block.
Defined as HR less than 45 bpm and required intervention.
Defined as a decrease of SBP more than 30% from baseline level (average value in the ward) or SBP less than 90 mmHg and required intervention.
New onset atrial premature beats.
Assessed with the Confusion Assessment Method for the ICU in the postanaesthesia care unit.
Discussion
Our results showed that pre-operative QLB using either the lateral or posterior approach did relieve somatic and visceral pain intensity both at rest and on coughing for up to 24 h after surgery, although they did not decrease total opioid consumption. Thus, both lateral and posterior QLB might be promising trunk blocks for LRS.
Previous studies have reported that pain scores were significantly decreased by lateral or posterior QLB compared with various controls after caesarean section14,15 and other low abdominal procedures.16,17 Our results were consistent with these findings. We found that lateral and posterior QLBs provide effective analgesia for both somatic and visceral pain. Compared with no block, the median difference of pain scores in QLB groups, especially on coughing, ranged from −2 to −1, which was greater than the minimal clinically important difference and thus had important clinical significance.18 The analgesic efficacy of QLB was probably due to the diffusion of local anaesthetics into the thoracic paravertebral space along the thoracolumbar fascia surrounding the quadratus lumborum and endothoracic fascia,6,19 as well as peripheral sympathetic field block on the basis of abundant A/C-fibre nociceptors and mechanoreceptors in the thoracolumbar fascia.20,21 However, the exact mechanisms of QLB remained ambiguous because there is some doubt as to whether local anaesthetics diffuse into the thoracic paravertebral space.22,23
In the current study, the median time interval to first required PCA bolus was 8.0 h in the lateral QLB group and 13.5 h in the posterior QLB group; these were similar to the results reported by Ahmed et al.24 (12.0 h with the posterior QLB approach). To some extent, this time interval could be deemed as the clinical duration of QLB; thus, the analgesic effect of QLB with 0.4% ropivacaine did not exceed 24 h. This was verified by the fact that the successful block rate had decreased dramatically at postsurgical 24 h in our patients.
Significantly, we did not find significant differences in opioid consumption among the three groups; this conflicts with the results of Zhu et al.25 This may be attributable in part to the background infusion setting of the PCA pump, which might have concealed the individual differences in our patients.26 Furthermore, subcostal anterior QLB was performed as the intervention in Zhu's study,25 which was different from ours. Compared with posterior QLB in a cadaveric study, subcostal anterior QLB had a more cranial intrathoracic distribution, consistently rising to the T7/8 level.23 A recent preliminary case series reported that subcostal anterior QLB could provide an appropriate thoracic dermatomal block for analgesia in open urological surgical procedures.27 It should be noted that approaches to QLB block affect local anaesthetic diffusion and thus determine analgesic effect.7 Therefore, when comparing results among different trials, it is crucial to take the block approach into consideration.
As for safety outcomes, we did not observe significant adverse events related to QLB. Other authors have reported hypotension and lower limb weakness, perhaps due to bilateral QLB performed in their patients.28,29 The incidences of postoperative nausea and vomiting (PONV) in our patients in the two QLB groups were 28.1 and 34.4%, which were similar to those in a previous study.25 As expected, we did not find any difference in the incidence of PONV among the three groups, because opioid consumption was comparable.
Our study had two prominent strengths. First, we checked sensory block dermatomes after local anaesthetic injection to confirm the effectiveness of QLB before the start of surgery. The relatively high success rate (90.6%) guaranteed the reliability of subsequent efficacy analysis of QLB. Second, we assessed somatic and visceral pain separately. Incisional wound pain and deep intra-abdominal pain co-exist after laparoscopic nephrectomy.30 Assessing pain of two different origins separately helped to verify whether QLB could provide visceral analgesia.
Our study has some limitations. First, patients were not blinded as they did not receive a sham block; consequently, reported bias of pain score might exist. However, investigators responsible for pain assessment were blinded. Second, intra-operative opioids were not standardised.
Conclusion
Results of this randomised trial showed that a pre-operative single-shot QLB using either the lateral or posterior approach did not decrease opioid consumption, but it effectively relieved somatic and visceral pain intensity both at rest and on coughing for up to 24 h in patients undergoing LRS. Our study added new evidence to the current knowledge of QLB analgesic efficacy.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements relating to this article
Assistance with the study: the authors sincerely appreciate the efforts of Dr Da Huang and Hao Kong (Department of Anesthesiology, Peking University First Hospital) for performing the QLBs, the surgical team (Department of Urology, Peking University First Hospital) for supporting the research and Ms Xue-Ying Li (Department of Biostatistics, Peking University First Hospital) for statistical consultation.
Financial support and sponsorship: this trial was supported by Seed Funding of Peking University First Hospital (2018SF062).
Conflicts of interest: none.
Presentation: none.
Xue Li and Zhen-Zhen Xu contributed equally to the work.
Published online 4 January 2021
Supplemental digital content is available for this article.
References
- 1.Ren T, Liu Y, Zhao X, et al. Transperitoneal approach versus retroperitoneal approach: a meta-analysis of laparoscopic partial nephrectomy for renal cell carcinoma. PLoS One 2014; 9:e91978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan X, Xu K, Lin T, et al. Comparison of transperitoneal and retroperitoneal laparoscopic nephrectomy for renal cell carcinoma: a systematic review and meta-analysis. BJU Int 2013; 111:611–621. [DOI] [PubMed] [Google Scholar]
- 3.Alper I, Yüksel E. Comparison of acute and chronic pain after open nephrectomy versus laparoscopic nephrectomy: a prospective clinical trial. Medicine 2016; 95:e3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin SJ, Park JY, Kim DH, et al. Comparison of postoperative pain between laparoscopic and robot-assisted partial nephrectomies for renal tumors: a propensity score matching analysis. Medicine 2017; 96:e7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nasrallah G, Souki FG. Perianesthetic management of laparoscopic kidney surgery. Curr Urol Rep 2018; 19:1. [DOI] [PubMed] [Google Scholar]
- 6.Elsharkawy H, El-Boghdadly K, Barrington M. Quadratus lumborum block: anatomical concepts, mechanisms, and techniques. Anesthesiology 2019; 130:322–335. [DOI] [PubMed] [Google Scholar]
- 7.Ueshima H, Otake H, Lin JA. Ultrasound-guided quadratus lumborum block: an updated review of anatomy and techniques. Biomed Res Int 2017; 2017:2752876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin Z, Liu J, Li R, et al. Single injection quadratus lumborum block for postoperative analgesia in adult surgical population: a systematic review and meta-analysis. J Clin Anesth 2020; 62:109715. [DOI] [PubMed] [Google Scholar]
- 9.Sindwani G, Suri A, Shrivastava D, et al. Laparoscopic guided continuous type 1 quadratus lumborum block – ‘Sindwani technique with case series’. J Clin Anesth 2017; 42:93–94. [DOI] [PubMed] [Google Scholar]
- 10.Joris J, Thiry E, Paris P, et al. Pain after laparoscopic cholecystectomy: characteristics and effect of intraperitoneal bupivacaine. Anesth Analg 1995; 81:379–384. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen S, Degenhardt L, Hoban B, et al. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf 2016; 25:733–737. [DOI] [PubMed] [Google Scholar]
- 12.Lang E, Kapila A, Shlugman D, et al. Reduction of isoflurane minimal alveolar concentration by remifentanil. Anesthesiology 1996; 85:721–728. [DOI] [PubMed] [Google Scholar]
- 13.Myles PS, Myles DB, Galagher W, et al. Minimal clinically important difference for three quality of recovery scales. Anesthesiology 2016; 125:39–45. [DOI] [PubMed] [Google Scholar]
- 14.Mieszkowski MM, Mayzner-Zawadzka E, Tuyakov B, et al. Evaluation of the effectiveness of the quadratus lumborum Block type 1 using ropivacaine in postoperative analgesia after a cesarean section – a controlled clinical study. Ginekol Pol 2018; 89:89–96. [DOI] [PubMed] [Google Scholar]
- 15.Blanco R, Ansari T, Girgis E. Quadratus lumborum block for postoperative pain after caesarean section: a randomised controlled trial. Eur J Anaesthesiol 2015; 32:812–818. [DOI] [PubMed] [Google Scholar]
- 16.Öksüz G, Arslan M, Urfalioğlu A, et al. Comparison of quadratus lumborum block and caudal block for postoperative analgesia in pediatric patients undergoing inguinal hernia repair and orchiopexy surgeries: a randomized controlled trial. Reg Anesth Pain Med 2020; 45:187–191. [DOI] [PubMed] [Google Scholar]
- 17.Huang D, Song L, Li Y, et al. Posteromedial quadratus lumborum block versus transversus abdominal plane block for postoperative analgesia following laparoscopic colorectal surgery: a randomized controlled trial. J Clin Anesth 2020; 62:109716. [DOI] [PubMed] [Google Scholar]
- 18.Myles PS, Myles DB, Galagher W, et al. Measuring acute postoperative pain using the visual analog scale: the minimal clinically important difference and patient acceptable symptom state. Br J Anaes 2017; 118:424–429. [DOI] [PubMed] [Google Scholar]
- 19.Tamura T, Yokota S, Ito S, et al. Local anesthetic spread into the paravertebral space with two types of quadratus lumborum blocks: a crossover volunteer study. J Anesth 2019; 33:26–32. [DOI] [PubMed] [Google Scholar]
- 20.Blanco R. The mechanism of the quadratus lumborum block: a peripheral sympathetic field block? Br J Anaes 2016; 117: Suppl: EL_13593. [Google Scholar]
- 21.Yahia L, Rhalmi S, Newman N, et al. Sensory innervation of human thoracolumbar fascia. An immunohistochemical study. Acta Orthop Scand 1992; 63:195–197. [DOI] [PubMed] [Google Scholar]
- 22.Kumar A, Sadeghi N, Wahal C, et al. Quadratus lumborum spares paravertebral space in fresh cadaver injection. Anesth Analg 2017; 125:708–709. [DOI] [PubMed] [Google Scholar]
- 23.Elsharkawy H, El-Boghdadly K, Kolli S, et al. Injectate spread following anterior sub-costal and posterior approaches to the quadratus lumborum block: a comparative cadaveric study. Eur J Anaesthesiol 2017; 34:587–595. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed A, Fawzy M, Nasr MAR, et al. Ultrasound-guided quadratus lumborum block for postoperative pain control in patients undergoing unilateral inguinal hernia repair, a comparative study between two approaches. BMC Anesthesiol 2019; 19:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu M, Qi Y, He H, et al. Analgesic effect of the ultrasound-guided subcostal approach to transmuscular quadratus lumborum block in patients undergoing laparoscopic nephrectomy: a randomized controlled trial. BMC Anesthesiol 2019; 19:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain 2016; 17:131–157. [DOI] [PubMed] [Google Scholar]
- 27.Elsharkawy H, Ahuja S, DeGrande S, et al. Subcostal approach to anterior quadratus lumborum block for pain control following open urological procedures. J Anesth 2019; 33:148–154. [DOI] [PubMed] [Google Scholar]
- 28.Ueshima H, Hiroshi O. Incidence of lower-extremity muscle weakness after quadratus lumborum block. J Clin Anesth 2018; 44:104. [DOI] [PubMed] [Google Scholar]
- 29.Sá M, Cardoso JM, Reis H, et al. Quadratus lumborum block: are we aware of its side effects? A report of 2 cases. Rev Bras Anestesiol 2018; 68:396–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ergün M, Berkers AW, van der Jagt MF, et al. Components of pain assessment after laparoscopic donor nephrectomy. Acta Anaesthesiol Scand 2014; 58:219–222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


