Abstract
Temperature is a universal cue and regulates many essential processes ranging from enzymatic reactions to species migration. Due to the profound impact of temperature on physiology and behavior, animals and humans have evolved sophisticated mechanisms to detect temperature changes. Studies from animal models, such as mouse, Drosophila, and C. elegans, have revealed many exciting principles of thermosensation. For example, conserved molecular thermosensors, including thermosensitive channels and receptors, act as the initial detectors of temperature changes across taxa. Additionally, thermosensory neurons and circuits in different species appear to adopt similar logic to transduce and process temperature information. Here, we present the current understanding of thermosensation at the molecular and cellular levels. We also discuss the fundamental coding strategies of thermosensation at the circuit level. A thorough understanding of thermosensation not only provides key insights into sensory biology but also builds a foundation for developing better treatments for various sensory disorders.
Keywords: heat, cold, somatosensation, pain, TRP, thermoregulation
INTRODUCTION
Many ancient cultures considered heat (fire) a classical element of nature (1). To quantify the heat present in an object, modern sciences use temperature (defined by the third law of thermodynamics) as a measure of the motions and vibrations of the particle constituents of the object. All biochemical reactions have a temperature coefficient that refers to the rate of change of a biochemical reaction as a consequence of temperature increase. As a result, temperature essentially regulates all physiological processes of living organisms. For example, many essential enzymes have an optimal temperature range for their enzymatic activities. In addition, temperature regulates development, reproduction, aging, and the sex ratio of many species (2). Furthermore, temperature has a profound impact on many animal behaviors ranging from acute thermal avoidance to hibernation and animal migration (3).
Temperature sensation has been studied for over a century (4). In the late nineteenth century, the prominent neurologist Charles-Édouard Brown-Séquard found that hemisection of the spinal cord could lead to the loss of temperature sensation on the contralateral side (5), suggesting an essential role of human spinal cord in temperature sensation. Since the 1920s, with the development of amplifiers suitable for electrophysiological recording of nerve fibers (6), many researchers started to record the electric signals induced by temperature changes in humans and other mammals (7-10). These pioneering studies confirmed the involvement of the spinal cord in sensing both cooling and warming. More importantly, they demonstrated the existence of cold nerve fibers and warm nerve fibers and revealed that the unmyelinated C-fibers and thinly myelinated Aδ-fibers convey the temperature information in ascending afferent pathways. Interestingly, distinct types of nerve fibers can display different temperature sensitivities, ranging from painful heat to noxious cold. More recently, genetic and physiological studies have identified multiple types of temperature-sensitive ion channels and receptors on the cell membrane, which can convert temperature cues into electrical signals and function as molecular thermometers (11, 12).
To detect temperature changes, animals have evolved sophisticated mechanisms that are coupled with sensory physiology and energy metabolism (13). Simple organisms such as Caenorhabditis elegans and Drosophila melanogaster rely on a few temperature-sensitive neurons to sense temperature and initiate complex thermal behaviors, including thermotaxis and thermal avoidance (14). For homeotherms like mammals, in general, thermosensitive primary sensory neurons located in the dorsal root ganglia (DRG) and trigeminal ganglia (TG) convey the temperature input from the skin and viscera to the brain and then trigger thermosensory behaviors.
Thermosensation and thermoregulation are intuitively interconnected (13). In the meantime, studies in model organisms have shown that temperature can have a major impact on the aging process of both poikilotherms and homeotherms (15-18). As these topics were recently discussed in several other review articles (13, 17), we focus on the molecular, cellular, and circuit mechanisms of temperature sensation.
MOLECULAR THERMOSENSORS INVOLVED IN TEMPERATURE SENSATION
A common principle of sensory biology is that various membrane ion channels and receptors act as the initial detectors of environmental cues (3). The same logic applies to temperature sensation. As temperature regulates nearly all biochemical reactions through thermodynamics, the Q10 coefficient is typically used to measure the reaction rate change as a function of temperature increase by 10°C (19). Although most temperature-insensitive enzymatic reactions and ion channel openings have a Q10 value of 1–3 (20), many temperature-sensitive ion channels display a remarkably high Q10 value. Therefore, to define molecular temperature sensors, these molecules should be activated or potentiated by temperature changes (either warming or cooling) and exhibit a Q10 value >3. A heat sensor would be activated or potentiated by warming, while a cold sensor should exhibit an elevated activity in response to cooling. In this section, we discuss the molecular thermosensors involved in thermosensation based on their temperature sensitivity and activation threshold.
Noxious Heat Sensors
TRPV1, the founding member of the vanilloid subtype of TRP channels (a class of evolutionarily conserved cation channels that were first identified in Drosophila; 21), is probably the best characterized ion channel involved in sensing noxious heat (22). Initially cloned as a capsaicin receptor, TRPV1 turns out to be activated by both chili pepper and heat (Figure 1a) (22). Through candidate gene approaches, a large collection of heat sensors has been identified in mammals, nearly all of which belong to the TRP channel family, including TRPV1, TRPV2, TRPV3, TRPV4, TRPM2, TRPM3, TRPM4, TRPM5, and TRPA1 (23). Summaries of all of these heat sensors are shown in Tables 1 and 2.
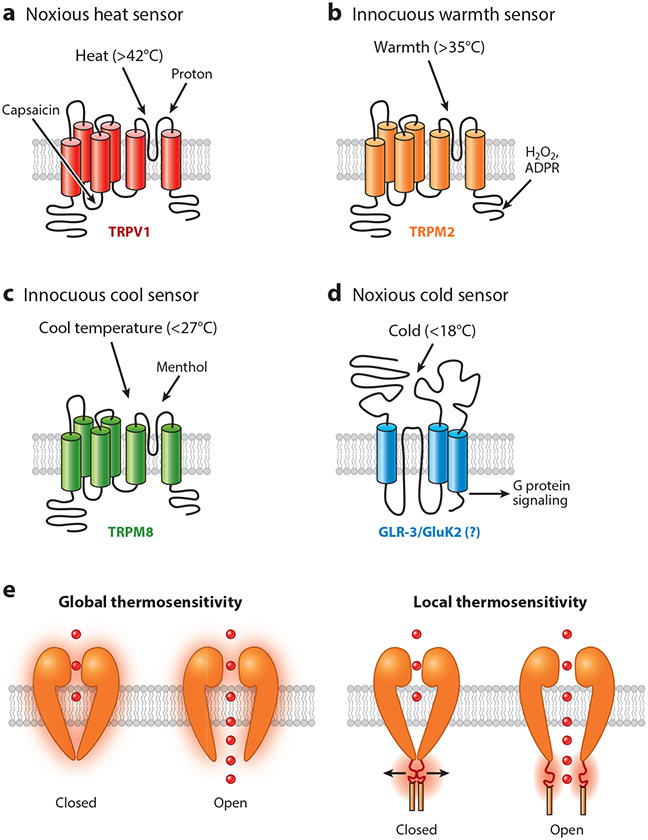
Figure 1.
Molecular thermosensors involved in temperature sensation. (a) Shown is the representative noxious heat sensor TRPV1,which can be regulated by multiple environmental and endogenous stimuli, including heat (>42°C), proton, and capsaicin. (b) TRPM2 plays an important role in sensing innocuous warmth (>35°C). (c) Mammalian TRPM8 is the best-studied innocuous cool sensor (<27°C). (d) The ionotropic glutamate receptor GLR-3/GluK2 might act as a noxious cold receptor in both C. elegans and mammals (< 18°C). Notably, GLR-3/GluK2 is a metabotropic cold receptor (G protein signaling mediates its role in cold sensation), and the cold sensitivity of GLR-3/GluK2 is independent of its channel activity. (e) Molecular thermosensors may use both global conformation and local motif mechanisms to respond to temperature changes. Abbreviation: ADPR, adenosine diphosphate ribose.
Table 1.
Summary of molecular heat sensors in different model systems
| Heat sensors | Species | Tact | Q10 | Remarks | References |
|---|---|---|---|---|---|
| TRPV1 | Mouse | ~42°C | >15 | Mediate noxious heat sensation and pain and warmth sensation | 22, 47, 160 |
| TRPM3 | Mouse | ~40°C | ~7 | Mediate noxious heat sensation and pain | 32, 35 |
| TRPA1 | Mouse | variable | ~10 | Mediate noxious heat sensation and pain | 35, 161 |
| ANO1 | Mouse | ~44°C | 20 | May play a redundant role in noxious heat sensation | 34 |
| TRPV2 | Mouse | ~52°C | ~100 | Heat sensitive in vitro No notable thermal phenotype in KO mice |
95, 162 |
| TRPM2 | Mouse | ~35°C | ~15.6 | Mediate warmth sensation, also a redox sensor | 33, 48, 49 |
| STIM1 | Mouse | ~35°C | 6.8 | Act in skin keratinocytes to mediate warmth sensation | 52 |
| TRPV3 | Mouse | ~32°C | 33 | Heat sensitive in vitro No notable thermal phenotype in KO mice |
42, 46 |
| TRPV4 | Mouse | ~27°C | ~10 | Heat sensitive in vitro No notable thermal phenotype in KO mice |
44, 46 |
| TRPM4 | Mouse | ~15°C | ~8 | Heat sensitive in vitro | 163 |
| TRPM5 | Mouse | ~15°C | ~10 | Contribute to the temperature effect on perceived sweet taste | 163 |
| Painless | Drosophila | 42–44°C | ~29 | Mediate heat-induced nocifensive behavior in larvae | 36, 37 |
| TRPA1 | Drosophila | ~25°C | ~9 [TRPA1(A)], ~116[TRPA1(B)] | A warmth sensor required for slow thermotaxis in flies Also used as a thermogenetic tool in flies |
54, 105 |
| Gr28b(D) | Drosophila | ~26°C | ~25 | A warmth sensor required for quick thermotaxis in flies | 56 |
| Rhodopsins (Rh1, Rh5, Rh6) | Drosophila | ND | ND | Important for thermal preference in fly larva | 58, 60 |
| GCY-8, -18, -23 | C. elegans | Varies with cultivation temperature | ~53 | Mediate the warmth sensitivity of the AFD neuron | 62 |
Abbreviations: KO, knockout; ND, not determined; Tact, temperature activation threshold.
Table 2.
Summary of molecular cold sensors in different model systems
| Cold sensors | Species | Tact | Q10 | Remarks | References |
|---|---|---|---|---|---|
| TRPM8 | Mouse | ~27°C | 24 | Cool sensor mediating cool sensation | 65, 68 |
| TRPC5 | Mouse | ~25°C | ~10 | Cool sensitive in vitro No notable thermal phenotype in KO mice |
72 |
| GC-G | Mouse | Over 30°C | ND | Cool sensing in Grueneberg ganglion | 73 |
| TRPA1 | Mouse, human | Variable | ~10 | Heat sensitive under physiological conditions, but cold sensitive under oxidative stress conditions | 84, 161 |
| GluK2 | Mouse | ~18°C | ND | Cold sensitive in vitro Knockdown of GluK2 suppresses noxious cold, but not cool, sensation in DRG neurons |
86 |
| IR21a, −25a, −93a | Drosophila | ND | ND | May form a heteromeric cool sensor | 76, 77 |
| TRPA1 | C. elegans | ~20°C | ND | Promotes longevity at low temperatures | 15, 80, 148 |
| GLR-3 | C. elegans | ~18°C | ND | A metabotropic cold sensor driving cold avoidance behavior | 86 |
Abbreviations: DRG, dorsal root ganglia; GC-G, guanylyl cyclase G; KO, knockout; ND, not determined; Tact, temperature activation threshold.
TRPV1 is highly expressed in TG and DRG sensory neurons, both of which play a pivotal role in somatosensation (22). When expressed in heterologous expression systems, TRPV1 displays a steep temperature-dependent activation curve with the temperature activation threshold of ~42°C (22). Coincidently, 42–45°C is commonly considered as the threshold of heat pain for humans (24, 25). Thus, TRPV1 may simultaneously mediate two somatosensory modalities, temperature sensation and pain. In line with this view, TRPV1 is a polymodal sensor that can be activated or modulated by multiple exogenous and endogenous factors involved in temperature sensation and pain, including heat, capsaicin, proton, and various inflammation-related G protein–coupled receptors (Figure 1a) (26, 27).
The important role of TRPV1 in mediating heat sensation is confirmed in vivo using Trpv1 knockout (KO) mice (28). Intriguingly, although Trpv1 KO mice only display modest behavioral deficits in temperature discrimination >50°C, genetic ablation of TRPV1-positive cells essentially abolished the temperature sensitivity above 37°C (28-30). This indicates that other noxious heat sensors are present in TRPV1-positive cells. Indeed, many other temperature-sensitive ion channels are coexpressed with TRPV1 in a subset of small-diameter primary afferent neurons, including TRPA1, TRPM2, TRPM3, and anoctamin/TMEM16 (31-34). Among them, triple KO of Trpv1, Trpm3, and Trpa1, but not individual KO, selectively abolishes acute noxious heat (>50°C) sensing without affecting warmth sensing, cold sensing, or mechanosensation, supporting that TRPV1, TRPM3, and TRPA1 act redundantly in sensing noxious heat (35). Besides TRP channels, the calcium-activated chloride channel anoctamin 1 (ANO1/TMEM16A) seems to be also involved in sensing noxious heat (34). When expressed in HEK293T cells, ANO1 can be activated by temperatures >44°C, and it features a Q10 value of ~20 (34). In DRG neurons, 78% of ANO1+ neurons also express TRPV1, indicating that ANO1 and TRPV1 might play a redundant role in these neurons (34). Collectively, multiple ion channels expressed in small-diameter DRG neurons participate in sensing noxious heat, which is understandable because noxious heat is an immediate threat to life and redundant noxious heat sensors will ensure proper detection of this great danger.
Invertebrate animals such as Drosophila and C. elegans are great model systems to identify and characterize molecular thermal sensors. Evolutionarily conserved TRP channels are also important for temperature sensation in invertebrates. Painless, a TRPA channel in Drosophila, is a noxious heat sensor both in vitro and in vivo (36, 37). painless mutant flies are defective in nociceptive behaviors and Painless-expressing sensory neurons in painless mutant flies fail to fire action potentials upon heat stimulation (>42°C) (36). When expressed in HEK293 cells, Painless displays a temperature activation threshold of 42–44°C and Q10 value of 28.5, and excised membrane exhibits heat sensitivity with a similar activation threshold (37). Thus, Painless may act as an intrinsic noxious heat sensor in flies.
Although a large number (at least 17) of TRP channels are present in C. elegans, none of them have been verified as a direct heat sensor (38). The TRPV channels OSM-9 and OCR-2 have been shown to play a role in sensing noxious heat (−35–38°C) (39, 40). However, TRP channels are known to be regulated by G protein signaling and many other intracellular signaling pathways. Therefore, OSM-9 and OCR-2 might act as transduction channels downstream of the heat sensor. To demonstrate that OSM-9 and/or OCR-2 are bona fide heat sensors, they need to be tested in heterologous expression systems. If OSM-9 and OCR-2 are direct heat-activated ion channels, their expression should confer heat sensitivity.
Innocuous Warmth Sensors
Although several thermosensitive TRP channels have the temperature activation threshold in the warm range in vitro, genetic evidence argues against their participation in sensing warmth under physiological conditions. For example, TRPV3 is activated by temperatures >32–39°C, and TRPV4 also responds to warmth (26–34°C) (41-45). However, Trpv3 and Trpv4 double KO mice in a pure genetic background do not exhibit a notable defect in temperature sensation (46). Thus, TRPV3 and TRPV4 may not be major players in warmth sensation. On the other hand, although TRPV1 is well established to be activated by noxious heat, surprisingly, it is also involved in sensing innocuous warmth in TG neurons (47). It is possible that under physiological conditions, modulators of TRPV1, as discussed above, shift the temperature sensitivity of TRPV1 to lower temperatures, making it function as a warmth sensor. Nevertheless, it remains to be determined whether TRPV1 is also required for sensing warmth in DRG sensory neurons.
TRPM2 features a temperature activation threshold of ~35°C and Q10 value of ~15.6 (Figure 1b) (48). Through its expression in both peripheral and central nervous systems, TRPM2 contributes to sensing ambient and central warm temperatures, respectively (33, 49). A subset of DRG sensory neurons expresses TRPM2 but not other known heat-activated TRPV1–4 or TRPM3 channels (33). Compared to wild-type mice that typically avoid the innocuous warm temperature of 38°C in the thermal preference test, Trpm2 KO mice exhibit no such avoidance, showing that TRPM2 is involved in peripheral warmth sensation. Notably, a small portion (3%) of DRG neurons in Trpm2 KO mice are still activated by warming, although they are insensitive to agonists of heat-activated TRPV1–4 or TRPM3, indicating the presence of other warmth sensors in the peripheral nervous system (33). On the other hand, while TRPM2 is considered a warmth sensor in vivo, it surprisingly functions as a noxious heat sensor in a subpopulation of dissociated DRG and TG neurons, as its activation threshold is elevated to the noxious heat range (>45°C) in such neurons for unknown reasons (50, 51). Whether TRPM2 contributes to noxious heat sensation in vivo remains an open question. Besides TRPM2, the endoplasmic reticulum calcium sensor STIM1 acts in skin keratinocytes, but not sensory neurons, to mediate the peripheral warmth sensation (52). In the brain, TRPM2 can be detected in the preoptic area (POA) of the hypothalamus, an area known to play a central role in monitoring central temperatures and thermoregulation (49). These TRPM2-expressing POA neurons can be activated by a warm temperature of 38°C, and H2O2 (a known agonist of TRPM2) greatly potentiates the warming-induced calcium responses (49). During high-grade fever, TRPM2 may act as a brake in the central thermoregulatory circuit to reduce core temperatures (49). Notably, while ~30% of POA neurons are temperature sensitive, the expression of TRPM2 is not limited to these thermosensitive POA neurons (49, 53).
In Drosophila, TRPA1 is a warmth sensor and can be directly activated by temperatures over 25°C, which is the preferred temperature for flies (54, 55). Additionally, an ionotropic gustatory receptor Gr28b(D) may also act as a warmth-activated ion channel in flies. Gr28b(D) has a temperature activation threshold of ~26°C and Q10 value of ~25 (56). Importantly, ectopic expression of Gr28b(D) in fly muscle or its heterologous expression in Xenopus oocytes confers innocuous warmth sensitivity to these otherwise temperature-insensitive cells (56, 57), suggesting that Gr28b(D) may not require other auxiliary components for its temperature sensitivity. Besides TRPA1 and Gr28b(D), the Drosophila rhodopsin (Rh1, Rh5, and Rh6), phospholipase Cβ (PLCβ), and its downstream phosphoinositide metabolism enzymes are all involved in sensing innocuous warmth in the range of 18–24°C (58-60). These rhodopsins might act as a warmth sensor in Drosophila larvae, which then regulates TRPA1 through G protein signaling.
In C. elegans, the AFD neuron is arguably the best-studied heat-sensitive neuron (61). Interestingly, several transmembrane guanylyl cyclases (GCY-8, GCY-18, and GCY-23) are required for warmth sensing in the temperature range of 15–25°C, as their mutants greatly affect the temperature sensitivity of AFD (62). Importantly, ectopic expression of GCY-18 and GCY-23 confers temperature responses to chemosensory neurons and muscle cells, suggesting that these guanylyl cyclases are warmth sensors (62). After activation by warming, guanylyl cyclases can catalyze the conversion of GTP to the second messenger cGMP, which then acts as an agonist for the cGMP-gated CNG channel TAX-2/TAX-4. Thus, TAX-2/TAX-4 acts as transduction channels in the thermosensitive AFD neuron (61).
Innocuous Cool Sensors
Similar to heat sensors, the best-studied cold sensor TRPM8 also belongs to the TRP channel family (63, 64). Coincidently, TRPM8 is the receptor for menthol, the cool sensation-inducing substance found in peppermint (Figure 1c) (63, 64). When expressed in heterologous expression systems, TRPM8 exhibits a temperature activation threshold of 25–27°C and Q10 value of 24 (65). TRPM8 is endogenously expressed in a subpopulation of small-diameter DRG neurons and TG neurons (66, 67). Trpm8 KO mice are defective in the temperature preference test within the cool range (15–25°C) (68, 69). However, they still avoid contact with surfaces within the noxious cold range (<15°C), and a subpopulation of DRG neurons in Trpm8 KO mice still exhibits cold sensitivity (68, 69). Furthermore, TRPM8+ neuron-ablated mice display a more severe defect in cold sensation compared to Trpm8 KO mice (70, 71). Therefore, the TRPM8-independent cold sensor(s), particularly those sensing noxious cold, must exist in mammals. In addition to TRPM8, TRPC5 exhibits cold sensitivity in vitro and can be activated by cooling in the temperature range of 37–25°C (72). However, no severe temperature phenotype is detected in Trpc5 KO mice (72), suggesting that TRPC5 may not be a major player in sensing cool temperatures in vivo. Besides TRP channels, the transmembrane guanylyl cyclase G (GC-G) may directly respond to cooling and mediate the cool sensation in the mouse Grueneberg ganglion that is located at the rostral tip of the nose (73). Therefore, transmembrane guanylyl cyclases can serve as molecular thermosensors in both C. elegans and mammals.
Drosophila display robust avoidance behavior against cold temperatures (74). TRPP channels Brivido1–3 were initially reported to be expressed in the aristal Cool Cells and important for sensing low temperatures (25–11°C) in Drosophila (75). However, a more recent study showed that these Brivido channels may not be required for the temperature sensitivity of aristal Cool Cells (76). Instead, a group of ionotropic receptors (IRs), IR21a, IR25a, and IR93a, are expressed in the aristal Cool Cells of adult flies to sense innocuous cool temperatures (76). In larval flies, IR21a and IR25a are expressed in the dorsal organ Cool Cells, where they mediate cellular cooling sensitivity and cool avoidance behavior (77). Importantly, misexpression of IR21a in Warm Cells can confer cool sensitivity if IR25a is coexpressed (77). Thus, IR21a, IR25a, and IR93a might act as heteromers in cool transduction. One interesting finding is that these IRs are required for the morphogenesis of Cool Cells, and ectopic expression of IR21a in Warm Cells is sufficient to transform their morphology to Cool Cell-like (76). Thus, these IRs appear to have an instructive role in specifying the fate of thermosensory neurons. Since functional expression of these IRs in heterologous systems was unsuccessful, alternative approaches are needed to verify whether they can directly sense cooling. Notably, together with IR40a, IR25a and IR93a have also been shown to be required for Drosophila hygrosensation (78, 79). Thus, different combinations of IRs may mediate both cool sensation and hygrosensation.
In C. elegans, TRPA-1 is a cool receptor that can be activated by cooling at 20°C (80), the standard laboratory cultivation temperature of C. elegans. TRPA-1 is endogenously expressed in several sensory neurons and the intestine, where it mediates cold sensation and regulates longevity (15, 81). Since ectopic expression of TRPA-1 in cold-insensitive C. elegans neurons and HEK293T cells confers cold sensitivity (80), the C. elegans TRPA-1 seems to act as a cooling-activated ion channel. Although the C. elegans intestine is typically not considered as an excitable organ, it is highly cold sensitive even without neuronal input (15). The intestinal TRPA-1 plays an important role in cool sensing, as the cooling-induced calcium response in the trpa-1 mutant background is reduced but not diminished (15). Therefore, additional cold sensors must be present in C. elegans, offering an opportunity to identify novel cold sensors using C. elegans as a model.
Noxious Cold Sensors
Mammalian TRPA1 was initially cloned as a noxious cold sensor and exhibits a cold temperature activation threshold of ~17°C in vitro (31). However, there is a debate on the physiological role of TRPA1 in noxious cold sensation, as Trpa1 KO mice are largely normal in avoiding noxious cold temperatures (82, 83). Paradoxically, recent work shows that TRPA1 contributes to noxious heat rather than cold sensation in mice, suggesting mammalian TRPA1 as a heat sensor (35). Indeed, purified human TRPA1, when reconstituted in lipid bilayer, is redox sensitive, acting as a heat sensor under reducing conditions but functioning as a cold sensor under oxidative stresses (84). It is possible that under physiological conditions, mammalian TRPA1 mediates heat sensation in vivo, whereas upon injury or under other inflammatory conditions, TRPA1 contributes to cold allodynia (35, 85). Collectively, TRPA1 probably does not mediate acute noxious cold sensation in mammals.
Previous efforts to identify temperature sensors mostly focused on the use of candidate gene approaches, leading to the identification of a large number of thermosensitive TRP channels. This approach, though highly successful in identifying heat sensors, has not been very fruitful in identifying cold sensors. Those cold sensors not falling into the category of known thermosensors (e.g., TRP channels) would thus have eluded detection. A recent study performed an unbiased forward genetic screen to identify cold sensors in C. elegans. GLR-3, a kainate-type glutamate receptor, was identified as a cold-sensing receptor in this screen (Figure 1d) (86). In addition to the intestine, GLR-3 is expressed in the ASER sensory neuron and RIA interneurons (86). In ASER, GLR-3 is required for its cold sensitivity (≤18°C) and cold-induced avoidance response. Moreover, ectopic expression of either GLR-3 or its mouse homolog GluK2 confers cold sensitivity to the otherwise cold-insensitive C. elegans muscle cells. Furthermore, GLR-3 and its vertebrate homolog GluK2 from zebrafish, mouse, and human can all function as a cold-sensing receptor when expressed in heterologous expression systems (86), strongly suggesting that GLR-3/GluK2 are evolutionarily conserved cold sensors. An interesting feature of mammalian kainate-type glutamate receptors is that they have both metabotropic and ionotropic functions (87). The channel activity of GLR-3/GluK2 is not required for its cold sensitivity because channel-dead GLR-3/GluK2 mutants are still cold sensitive. Instead, GLR-3/GluK2 relies on G protein signaling to transmit cold signals, and ion channels acting downstream of G protein signaling may function as transduction channels (86). Overall, these results identify GLR-3/GluK2 as a new type of metabotropic cold sensor.
In mouse DRG, GluK2 is expressed in a subset of small- to medium-diameter sensory neurons. Intriguingly, siRNA knockdown of GluK2 in cultured DRG neurons specifically suppresses noxious cold- but not cool temperature-induced calcium responses (86). Therefore, GluK2 may be a noxious cold receptor in mammalian peripheral nervous systems. Nevertheless, further studies are required to determine whether GluK2 functions as a noxious cold sensor in vivo.
Several two-pore domain potassium (K2P) channels such as TREK1,TREK2, andTRAAK are expressed in sensory neurons and suppressed by low temperatures (88, 89). As the opening of these K2Ps hyperpolarizes cells, cold-induced suppression of K2Ps will excite sensory neurons. Indeed, Trek1 and Traak double KO mice are sensitized to noxious cold (20–10°C) (89). On the other hand, unlike most voltage-gated sodium channels, Nav1.8 in DRG neurons shows little inactivation at low temperatures (90). As a result, Nav1.8 is particularly important for transducing noxious cold signals, and Nav1.8 KO mice are largely insensitive to noxious cold (90). Nevertheless, K2P and Nav1.8 channels are general regulators of membrane excitability. Although they participate in temperature sensation, they are typically not considered as bona fide thermosensors.
Activation Mechanisms of Molecular Thermosensors
While all temperature-sensitive ion channels can be activated or potentiated by temperature changes, it is not clear whether temperature itself is the gating force. Indeed, both heat-activated TRPV1 and cold-activated TRPM8 were previously proposed to be gated by membrane voltage, but not temperature (91). In this theory, temperature changes only potentiate the voltage-dependent gating process (91). However, voltage was later shown to be a partial activator of TRPV1 and TRPM8 (92). For full activation, voltage, temperature changes, and agonists are all independently coupled to gate these thermosensitive TRP channels (92). In this regard, temperature can indeed act as a gating force of temperature-sensitive ion channels. A key parameter to understand the exceptional temperature sensitivity of molecular thermosensors is the change of heat capacity (ΔCp) between the activation and inactivation conformations (19). The simplest definition of heat capacity can be expressed as the increase in enthalpy with temperature Cp = dH/dT. In theory, any part of a protein can contribute to ΔCp through solvation of hydrophobic or hydrophilic residues. Namely, solvation of a hydrophobic residue increases ΔCp, whereas that of a polar residue decreases it (93). An overall positive ΔCp will render the channel cold sensitive, whereas a negative one makes it heat sensitive. Thus, molecular thermosensors might use a global sensing mechanism to detect temperature changes, as many residues may contribute to ΔCp (Figure 1e). In support of this view, one can rationally design a temperature-sensitive ion channel guided by structural biology and ΔCp calculation. The well-studied voltage-gated Shaker potassium channel exhibits little temperature-dependent gating under normal conditions. However, since the S1–S4 voltage-sensing domain (VSD) of the Shaker channel contains water-accessible crevices, mutating different residues within the VSD to the ones with distinct polarity can greatly change ΔCp and sensitize the channel to temperature changes (94). Remarkably, manipulating the hydrophobicity of several residues can make the Shaker channel either heat sensitive or cold sensitive, supporting the heat capacity theory of temperature-sensitive ion channels (19, 94).
Nonetheless, it should be noted that the global temperature-sensing mechanism does not rule out the possibility that individual amino acid residues can play a predominant role in the temperature sensitivity of molecular thermosensors, because it is plausible that certain residues or intramolecular motifs can make a major contribution to ΔCp. Indeed, the pore turret and/or the N-terminal region that links ankyrin repeats to the S1 helix of TPRV1 have been proposed to contain intrinsic heat sensitivity (95-97). Additionally, heat activation, but not chemical activation or voltage modulation, of TRPV3 specifically requires its pore loop (98). Furthermore, the C-terminal region and several nonconserved residues within the pore loop of TRPM8 appear to be particularly important for its cold sensitivity (99-101). Lastly, the N-terminal ATD domain of GluK2 is required for its cold sensitivity, as mutating this domain abolishes the cold-evoked, but not glutamate-evoked, response (86).
Is there an evolutionarily conserved temperature-sensing module in thermosensitive ion channels and receptors like the voltage-sensing S4 helix in voltage-gated potassium channels (19)? Interestingly, the bacterial voltage-gated sodium channel BacNav exhibits a strong temperature-dependent gating through a temperature-dependent reversible structural transition in a C-terminal “neck” domain proximal to the pore (102). Intriguingly, a similar architecture composed of the channel pore domain and the proximal C-terminal coiled-coiled domain is also found in TRPA1 and TRPM8 (103, 104), raising the possibility that the neck domain proximal to the pore might act as a general temperature-sensing module (Figure 1e). Nevertheless, not all temperature-sensitive ion channels contain such a neck domain. Thus, if intramolecular temperature-sensing modules are present in all temperature-sensitive ion channels, they may have evolved independently.
Traditionally, the absolute temperature activation threshold (often obtained from heterologous expression studies) is commonly used to evaluate the temperature sensitivity of molecular thermosensors, which is in line with the heat capacity (ΔCp)-dependent activation theory of molecular thermosensors. However, an important concept to clarify for these thermosensors is that their temperature sensitivity can be variable, depending on the experimental conditions, protein modifications, species, and splicing isoforms. For example, different isoforms of Drosophila TRPA1 can range from highly heat sensitive to completely temperature insensitive (105). Another good example is that the rodent TRPV1 with a typical heat activation threshold of 42°C becomes active at 37°C at a moderately acidic cellular condition (26). Moreover, many inflammatory factors can potentiate TRPV1 and shift its activation threshold to a lower temperature (~32°C) by activating protein kinase C (106). Furthermore, phosphatidylinositol 4,5-bisphosphate (PIP2) and other phospholipids are well established to be able to modulate the temperature activation threshold of many thermosensitive TRP channels (100, 107). On the other hand, besides the absolute temperature, the rate of temperature change may also modulate the activity of thermosensors. Drosophila TRPA1 can detect the rate of temperature increase, as a faster heat ramp induces a much larger cellular response in a TRPA1-dependent manner (108). Moreover, the rate of temperature changes can also alter the activation threshold of thermosensitive channels, as the faster heat rate lowers the temperature activation threshold of Drosophila TRPA1 (108). Therefore, physical factors can also affect the activation threshold. Nevertheless, the molecular nature of this thermosensory feature is unclear because the rate of temperature change is typically not considered in the heat capacity theory of temperature sensing. Meanwhile, it remains to be determined whether other temperature-sensitive ion channels and receptors can also detect the rate of temperature change. Together, these thermosensory features may allow a limited number of molecular sensors to encode distinct temperature information, and a single temperature sensor may encode different temperature cues when expressed in distinct tissues and cells under different physiological and pathophysiological conditions. Thus, we should consider the activation threshold of a molecular thermosensor as a parameter that is actively regulated by many chemical, physical, and genetic factors. A heat capacity-based model incorporating other parameters such as the rate of temperature changes, variable activation threshold, and other regulatory mechanisms may be needed to better explain the temperature sensitivity of molecular thermosensors.
CELLULAR AND CIRCUIT MECHANISMS OF TEMPERATURE SENSATION
After activation, thermosensitive ion channels and receptors transduce the temperature input into electrical signals, which are then transmitted through thermosensory circuits to trigger various thermal behaviors. While a great amount of information on temperature sensation has been revealed by studying mammalian thermosensory circuits, the powerful genetic tools in combination with the simplicity of the nervous systems of model organisms can often discover in-depth circuit mechanisms of sensory processing. Importantly, the fundamental logic of temperature sensation tends to be similar across taxa. In this section, we discuss the cellular and circuit mechanisms of temperature sensation in both mammals and invertebrate model organisms (i.e., Drosophila and C. elegans).
Mammalian Thermosensation
In general, three layers of neuronal hubs are involved in mammalian temperature information relay: peripheral thermosensory neurons, the superficial dorsal horn of the spinal cord, and supraspinal centers of temperature information processing (Figure 2) (3). Peripheral thermosensory neurons are pseudounipolar cells, and their cell bodies are clustered in either the DRG (innervating the majority of the body) or TG (mainly innervating the head area). Early electrophysiological recordings of peripheral nerve fibers demonstrated that distinct types of afferent sensory neurons can respond to either heat, cold, or heat/cold (to a much lesser extent) (109-112). These elegant studies also showed that the unmyelinated C-fibers and thinly myelinated Aδ-fibers conduct noxious cold, cool, and noxious hot temperatures, whereas innocuous warmth is mainly transduced by C-fibers (Figure 2) (113-116).
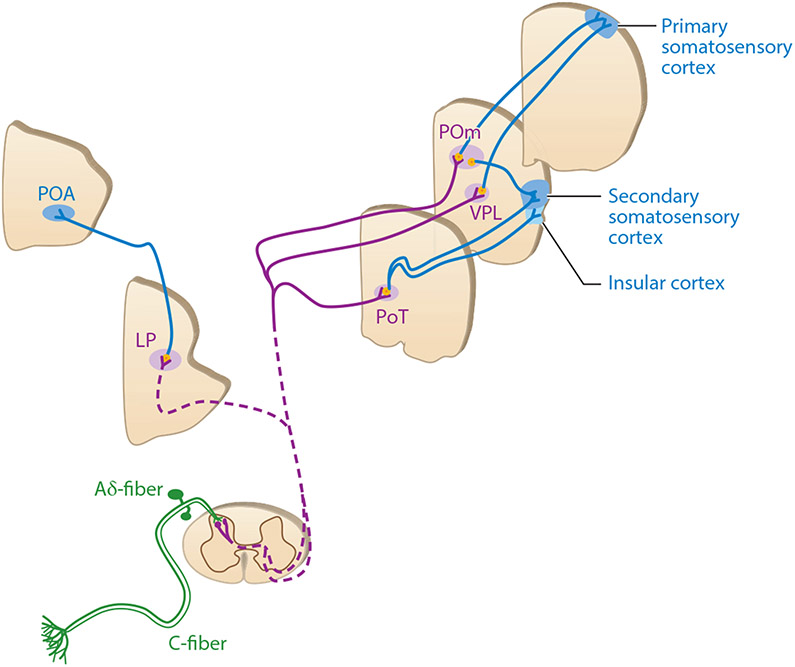
Figure 2.
Thermosensory circuits in mammals. Overall, three layers of neurons are involved in transmitting the temperature information (color coded in green, purple, and blue, respectively) (127). Abbreviations: LP, lateral parabrachial; POA, preoptic area; PoT, posterior thalamic; POm, posterior medial; VPL, ventral posterolateral.
As discussed earlier, TRPV1 and TRPM8 are the best characterized heat sensor and cold sensor, respectively. Although exhibiting some overlapping expression at different developmental stages, TRPV1 and TRPM8 are mostly expressed in separate populations of small-diameter afferent neurons, which contribute to our sensory discrimination of heat versus cold (66, 67). In mouse TG, multiple types of cold-sensitive neurons were found to express TRPM8, supporting a key role of TRPM8 in cool sensation in the TG (47). On the other hand, the vast majority (~90%) of heat-sensitive TG neurons only respond to noxious heat (>43°C), while a small portion (~10%) display graded responses to warming, ranging from innocuous warmth to painful heat (47). These two distinct types of heat-sensitive neurons are generally referred to as noxious heat sensors and innocuous warmth sensors, respectively. Similar to TG neurons, multiple types of heat-sensitive and cold-sensitive DRG neurons exist. For example, some DRG neurons only respond to noxious heat (50°C) but not innocuous warmth (38°C), while other DRG neurons can respond to both (117). Another important feature of temperature-sensitive DRG neurons is that most of them are polymodal sensors, as they can respond to both temperature and mechanical stimuli (117). Collectively, temperature-sensitive afferent neurons are rather heterogeneous in their thermoresponsive patterns. Identifying additional cellular markers and temperature sensors in thermosensory neurons is definitely needed to refine the cellular basis of temperature sensation.
In the spinal cord, temperature-sensitive afferent neurons send their central-branch axon mainly to the superficial laminae (lamina I and lamina II) of the dorsal horn. Notably, many neuronal types are present in these laminae to transmit temperature information. For example, primary thermosensory neurons form synapses with at least three different types of neurons in lamina I: fusiform cells (responding to noxious heat and pinching), pyramidal cells (responding to innocuous cold), and multipolar cells (responding to heat, cold, and pinching) (118, 119). Although most primary sensory neurons exhibit some specificity toward temperature stimulation, there is considerable cross talk of thermal inputs in the spinal cord. For instance, the capsaicin-sensitive TRPV1+ primary neurons can suppress inhibitory interneurons (e.g., GABAergic substantia gelatinosa neurons) in the spinal cord to enhance the cold sensitivity through a disinhibition mechanism (120-122).
Similar to DRG and TG neurons, spinal neurons have been studied for their temperature sensitivity using functional calcium imaging. Notably, ablating TRPV1-expressing DRG neurons greatly reduces (80% inhibition) the number of spinal neurons that respond to both 50°C and 37°C, illustrating the essential role of TRPV1-expressing sensory neurons in detecting both noxious heat and innocuous warmth (123). However, ablating TRPM8-expressing DRG neurons only selectively reduced the number of spinal neurons responding to cooling but not to noxious cold (123). It would be interesting to determine whether GluK2-expressing DRG neurons mediate the noxious cold sensation and, if so, the identity of their postsynaptic neurons in the spinal cord. Another interesting observation from this study is that a significant number of temperature-sensitive spinal neurons can respond to both heat and cold (123). These broadly tuned spinal neurons might receive thermal inputs from multiple types of DRG neurons. Overall, although the temperature information is clearly integrated and computed at the spinal cord level, the cellular identities and local circuits of thermosensitive dorsal horn neurons are little known.
After synaptic activation by primary sensory neurons, the second-order spinal neurons then compute and relay temperature information through the contralateral spinothalamic tract to supraspinal centers for further information integration. In humans, the dorsal margins of the left insular cortex and orbitofrontal cortex are activated by innocuous temperatures, while the anterior cingulate cortex and posterior insula are activated by noxious temperatures (124). In rodents, temperature-responding spinal neurons first project to several somatosensory nuclei in the thalamus [i.e., ventral posterolateral (VPL), posterior medial (POm), and posterior thalamic (PoT) nuclei] as well as the lateral parabrachial (LP) nucleus in the brainstem (Figure 2) (125-127). Next, these thalamus and brainstem nuclei send projections to the cortex via different pathways (Figure 2) (127). Taken together, distinct brain areas are employed to process the temperature information of different ranges in mammals.
Drosophila Thermosensation
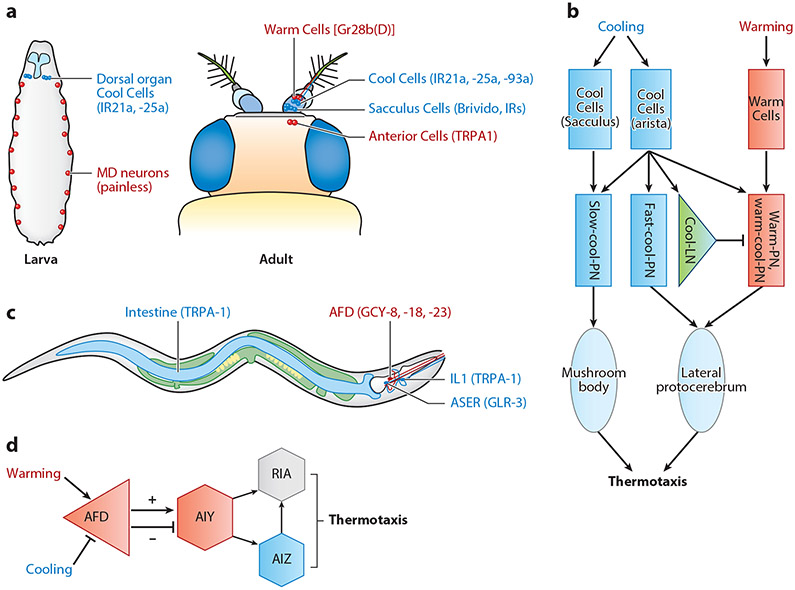
Drosophila larvae have a preferred growth temperature of 18–24°C. Under unfavorable temperatures, larvae explore the temperature gradient and avoid both cool and warm temperatures during thermotaxis (74). At the cellular level, the dorsal organ ganglion on each side of the body contains three highly cool-sensitive neurons that are largely responsible for avoiding cool temperatures (Figure 3a) (128). Remarkably, these neurons can be activated by cooling as little as 0.005°C/s (128). Two IRs, IR21a and IR25a, are expressed in the dorsal organ Cool Cells to act as the molecular cool sensors in these neurons (77). For warmth sensation during thermotaxis, larvae rely on TRPA1 expressed in a small number of central brain neurons (129). In addition to the thermotactic behavior under innocuous temperatures, Drosophila larvae display a characteristic rolling escape behavior upon noxious heat stimulation (~39°C) (36). The TRPA channel Painless is responsible for detecting noxious heat in the multiple dendritic neurons that tile the larval body wall (36) (Figure 3a). However, the neural circuit downstream of sensory neurons in larval temperature sensation is less understood.
Figure 3.
Cellular and circuit mechanisms of temperature sensation in Drosophila and C. elegans. (a) Larval and adult flies use different temperature-sensitive neurons for temperature sensation (130). (b) The neural circuit of temperature sensation in adult flies (131, 132). (c) In C. elegans, the AFD neuron is the primary warmth-sensing neuron. Meanwhile, the head neuron ASER and the intestine are cold sensitive through the cold sensors GLR-3 and TRPA-1, respectively. The other head neuron IL1 senses cooling through TRPA-1. (d) The neural circuit of thermotaxis in C. elegans. Abbreviations: IR, ionotropic receptor; LN, local neuron; md, multiple dendritic; PN, projection neuron.
In adult flies, the Anterior Cells in the head and three Warm Cells in the arista detect innocuous warmth (>25°C), while Sacculus Cells in the antenna and three Cool Cells in the arista sense innocuous cool temperatures (Figure 3a) (130). At the molecular level, the Anterior Cells use TRPA1, and Warm Cells employ Gr28b(D) as warmth sensors (56, 129). By contrast, the Cool Cells use IR21a, IR25a, and IR93a as cooling sensors, while Sacculus Cells might use Brivido channels and/or IRs to detect cool temperatures (75, 76). Notably, adult flies can exhibit two types of avoidance behaviors upon warmth stimulation: the rapid (<1 min) avoidance on steep thermal gradients and the slow (>20 min) avoidance on shallow thermal gradients. Mechanistically, the peripheral Warm Cells mediate rapid thermotaxis while the internal Anterior Cells mediate slow thermotaxis (56, 129). In addition to innocuous warmth sensation, adult flies exhibit the jumping escape behavior upon noxious heat stimulation; however, little is known about the cellular basis of this noxious heat avoidance behavior.
Compared to mammals, the circuit mechanisms by which the fly brain processes thermal cues are better understood. At the primary sensory neuron level, the Sacculus Cells, aristal Warm Cells, and aristal Cool Cells all project to the posterior antennal lobe (PAL), where two distinct, but adjacent, glomeruli represent warming and cooling temperature inputs, respectively (75, 131, 132). In the PAL area, the second-order thermosensory projection neurons (PNs) form synapses with primary sensory neurons and then relay temperature information to three higher-level brain centers: the mushroom body, the lateral horn, and the posterior lateral protocerebrum (Figure 3b) (131, 132). Interestingly, the cool temperature-responsive PNs may only receive synaptic inputs from cooling-sensitive primary neurons but not warming-sensitive neurons (131). However, the warm temperature-responsive PNs seem to form synapses with multiple types of neurons, including warming-sensitive primary neurons, cooling-sensitive primary neurons, and cooling-sensitive interneurons (131). Taken together, like mammals, adult flies use multiple brain regions to integrate and compute thermosensory inputs from the antenna.
C. elegans Thermosensation
Similar to flies, C. elegans exhibits both thermotaxis at innocuous temperatures and quick avoidance at noxious temperatures. After worms are cultivated at certain temperatures (15–25°C) for a long period (>4 h), they adopt this cultivation temperature (Tc) as the thermostatic set point (133). Once placed on a thermal gradient, worms exhibit either positive thermotactic or negative thermotactic behaviors to seek the Tc spot. After reaching the Tc location, worms then display isothermal tracking. At the cellular level, the warmth-sensing AFD thermosensory neuron plays a central role in temperature sensation (Figure 3c), as the ablation of AFD greatly disrupts both positive and negative thermotaxis (134, 135). Multiple signaling molecules mediate the temperature sensitivity of AFD, including the warmth-activated guanylyl cyclases GCY-8, GCY-18, and GCY-23, the CNG transduction channel TAX-2/TAX-4, and the Ca2+/calmodulin-dependent protein kinase 1 (61). Acting downstream of AFD, the first-layer interneuron AIY is a synaptic partner of multiple sensory neurons, including AWA, AWC, ASI, and ASE (136, 137). Thus, AIY may integrate temperature, olfactory, and gustatory cues. Ablation of AIY makes the worm cryophilic in thermotaxis (134, 135). Interestingly, AFD can either excite or inhibit AIY through different synaptic mechanisms. AIY expresses a glutamate-gated chloride channel GLC-3, and glutamate released from AFD can inhibit AIY through GLC-3 (138). Although the excitatory mechanism of AFD on AIY is still unknown, AFD might use a neuropeptide to activate AIY, as multiple neuropeptides (e.g., FLP-6, NLP-7, NLP-21) are expressed in AFD, and the excitatory synaptic transmission between AFD and AIY is disrupted in the unc-31 mutant with defective neuropeptide release (139-141). Besides AIY, two other interneurons, RIA and AIZ, have also been suggested to act downstream of AFD in the thermotaxis circuit (Figure 3d), as ablation of RIA makes the worm cryophilic, while AIZ-ablated worms are thermophilic (134). However, the essential role of RIA and AIZ in thermotaxis was recently questioned because the removal of these two interneurons seems not to have a major impact on thermotactic behaviors (135). This discrepancy may be due to differences in experimental conditions. Alternatively, AFD and AIY are more important for thermotaxis, whereas RIA and AIZ may play additional roles in mediating the isothermal tracking behavior. Further analysis is clearly needed to clarify this issue. On the other hand, a recent study suggested that depending on whether the current temperature is above or below the Tc and how big a difference there is between the current temperature and the Tc, different sets of neurons and behavioral components may be employed to steer the worms toward the Tc, manifesting a context-dependent action of thermosensory neural circuits (141). Notably, different thermal contexts can induce bidirectional cellular responses in the first-layer interneuron AIB, suggesting a critical role of AIB in context-dependent thermosensory behaviors (141).
Interestingly, the memory of Tc can last for hours (133). Again, AFD plays a key role in this temperature memory (142-145). AFD only exhibits the warming-induced calcium transient when the temperature is around the cultivation temperature, and the temperature threshold of this calcium response varies, depending on the cultivation temperature (142, 143). Remarkably, isolated AFD without any neuronal connections can still memorize the cultivation temperature, suggesting the presence of single-cell temperature memory in AFD (144). Notably, although the thermosensory behavioral memory lasts for several hours, AFD can adapt to a new holding temperature within minutes (133, 146, 147). The synaptic plasticity between the AFD sensory neuron and the AIY interneuron seems to determine the final outcome of cultivation temperature memory (145).
Cool temperatures are well established to extend life span in poikilotherms. Additionally, recent studies have shown that low core body temperatures are correlated with longevity in homeotherms such as rodents and humans (17, 18). In C. elegans, the cool sensor TRPA-1 acts in both the IL1 sensory neuron and the intestine to promote longevity at cool temperatures (Figure 3c) (15, 148). Specifically, cooling activates TRPA-1 that is expressed in IL1 and the intestine, and both tissues contribute to the cold-promoted longevity in a DAF-16/FOXO-dependent manner (15, 148). The activation of TRPA-1 in IL1 triggers glutamate release from IL1, which then activates a downstream neuron, NSM. Next, NSM secretes the neurotransmitter serotonin to activate the intestine through a serotonin receptor SER-7 (148). Thus, a thermosensory circuit from IL1 to the intestine promotes C. elegans longevity at cool temperatures.
C. elegans exhibits avoidance behaviors in response to noxious temperature shock. At least three sensory neurons are involved in sensing noxious heat, including AFD, FLP, and PHC (40). Interestingly, these neurons use different ion channels to transduce noxious heat. Namely, AFD relies on the CNG channel TAX-2/TAX-4, while FLP and PHC use the TRPV channels OSM-9 and OCR-2 (40). However, none of these channels have been demonstrated to be directly activated by heat. Therefore, the molecular sensor of noxious heat remains elusive in C. elegans. At the circuit level, AFD might act through the AIB interneuron, whereas PHC may act through PVC and DVA to trigger noxious heat avoidance (40). For acute cold sensation, the peripheral chemosensory neuron ASER mediates the cold shock–triggered reversals and turns (86). The other sensory neuron PVD has also been suggested to promote turns in response to cooling (80). Interestingly, optogenetic activation of PVD in fact suppresses rather than promotes turns by stimulating forward movement (149, 150). Notably, GLR-3 acts as the cold sensor in the ASER neuron to trigger cold avoidance behavior (86).
CODING PRINCIPLES IN TEMPERATURE SENSATION
While much progress has been made on the cellular and circuit mechanisms of temperature sensation, several fundamental questions remain. For example, do thermosensory systems detect the absolute temperature or temperature changes? How do primary sensory neurons encode the temperature signal? How are temperature inputs processed at the circuit level and eventually represented in the brain as a thermal perception? After many decades of research, several coding principles in temperature sensation have been recently revealed. In this section, we briefly discuss how temperature information is encoded and processed in sensory neurons and thermosensory circuits.
Absolute Temperature Versus Temperature Changes
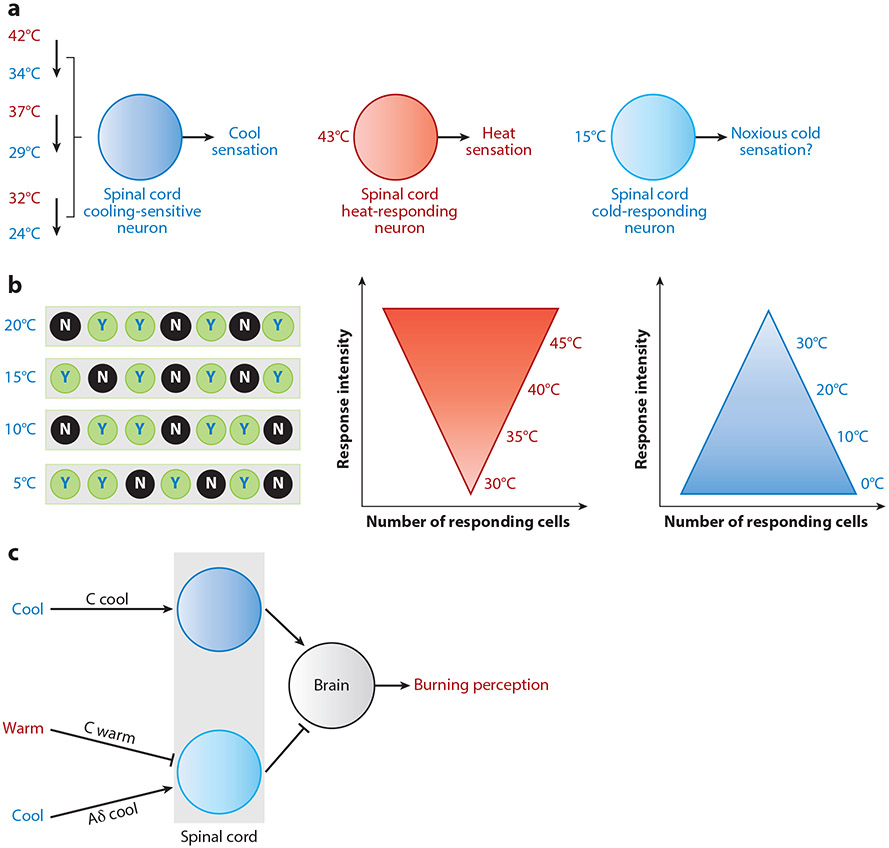
An important question of temperature coding is whether the thermosensory system detects absolute temperatures or temperature changes. Interestingly, cold sensation and heat sensation might utilize different mechanisms. In mice, both temperature-sensitive DRG neurons and spinal cord neurons encode absolute skin temperatures during heat sensation (Figure 4a) (117, 123). By contrast, cooling-sensitive neurons are often activated by the cooling process rather than fixed temperatures (117, 123). Thus, one model is that cold sensation may detect the decrease of temperature in these cases (Figure 4a). Notably, a similar coding strategy may also apply to Drosophila cool sensation. The aristal Cool Cells are activated by cooling and inhibited by warming (76, 131). Moreover, the firing frequency of Cool Cells is coupled to the change of temperature but not the absolute temperature (76). In contrast to the aristal Cool Cells in adult flies, the cooling-sensitive dorsal organ ganglion cells in larval flies do exhibit a defined low temperature activation threshold (128). Moreover, unlike in the aristal Cool Cells, cold-induced cellular responses in dorsal organ ganglion cells do not adapt (128). Thus, different cooling-sensitive neurons might be able to encode both absolute temperatures and the decrease of temperature. Intriguingly, while cool sensation is generally more indefinite than heat sensation, noxious cold sensation in humans is commonly associated with a threshold of 15°C (151). Therefore, an interesting model on low temperature sensation is that cool sensation detects temperature decreases, while noxious cold sensation detects the absolute temperature on top of temperature decreases.
Figure 4.
The coding strategies of temperature sensation. (a) The cooling process but not the absolute low temperature seems to trigger cool sensation, while the absolute high temperature activates spinal cord neurons and leads to heat sensation (123). For noxious cold sensation, it is possible that both the cooling process and the absolute temperature are involved in thermal information processing. (b) At the primary sensory neuron level, cold sensation might adopt a combinatorial coding strategy (N denotes nonresponding neurons, and Y indicates responding neurons). By contrast, heat sensation employs a graded response strategy (117). Meanwhile, some sensory neurons also exhibit graded responses upon cold stimulation (47). (c) In the thermal grill scenario, multiple temperature-sensitive afferent fibers transmit innocuous cool and warm temperature information simultaneously, the cross talk of which can eventually induce a burning hot perception in the brain.
Graded Coding Versus Combinatorial Coding
At the primary sensory neuron level, cooling- and warming-sensitive neurons might adopt different strategies to encode temperature stimuli. For example, in mouse DRG neurons, a digitallike all-or-none signal seems to encode low temperatures, whereas an analog-like graded signal encodes high temperatures (117) (Figure 4b). Specifically, a growing number of DRG neurons are recruited in heat sensation, and the peak amplitude of warming-induced calcium transients increases with temperature (117). By contrast, different cooling temperatures activate distinct groups of cold-sensitive neurons, and these neurons exhibit calcium transients of similar amplitude even when temperature drops to different degrees (117). These observations suggest that cooling-sensitive neurons in the DRG employ a combinatorial code, while warming-sensitive neurons adopt a graded coding strategy. However, the same coding principles might not apply to TG neurons, particularly for cooling-sensitive neurons. Specifically, the peak amplitude of cooling-evoked calcium transients increases with temperature drops, and different cooling temperatures can activate all major types of cooling-sensitive neurons, though the activation kinetics appear different, arguing that cooling-sensitive neurons adopt a graded coding strategy in the TG (47). This seems to be consistent with the observation that TRPM8-expressing neurons can sense both innocuous cool and noxious cold temperatures (152), through the cool sensor TRPM8 and the elusive noxious cold sensor in these neurons, respectively. Thus, one possibility is that while a graded coding strategy is utilized by warming-sensitive neurons, both graded and combinatorial strategies may be adopted by cooling-sensitive neurons, depending on the type of sensory organs.
Specificity Theory Versus Pattern Theory
After activating primary sensory neurons, temperature cues are further processed at different levels of the thermosensory circuit. For mammals, sensory-to-spinal transmission, spinal cord processing, spinal-to-brain transmission, and brain processing all contribute to the final representation of temperature cues in the brain. There are two popular theories on thermosensory coding at the circuit level: specificity theory and pattern theory, both receiving strong experimental evidence (153). Notably, these two coding theories may apply to both the peripheral and central nervous systems. In the specificity theory, labeled lines of thermosensory transmission are thought to specifically encode heat and cold in the central nervous system. In support of this view, ablation of TRPM8-positive sensory neurons in mice largely diminishes their cold sensitivity, but heat sensation remains normal (152). By contrast, killing TRPV1-positive primary neurons greatly affects heat sensation, but cool sensation stays normal (29). Similarly, Drosophila was also proposed to use labeled lines for thermosensation. For example, the aristal Warm Cells and Cool Cells are activated by warming and cooling, respectively, and they send projections to the adjacent but distinct thermosensory glomeruli in the PAL region (75). Notably, some thermosensitive afferent fibers are polymodal nociceptors (153). As a result, a given stimulus might activate multiple labeled lines, and a single labeled line might receive inputs from different stimuli. To some extent, this resembles the strategy of olfactory information encoding where a given odorant can activate multiple odorant receptors and a single odorant receptor may be sensitive to several odorants (154).
In contrast to the specificity theory, the pattern theory of temperature sensation considers that the final perception of temperature is generated by a combination of inputs (population coding) from multiple primary sensory afferents and cross talk occurs at different levels of thermosensory circuits (153). Thus, according to the pattern theory, the uncoupling of temperature inputs and temperature perception could occur and a temperature stimulus may be misinterpreted in the brain, leading to temperature illusion (153). A famous example for the pattern theory of temperature sensation is the synthetic heat or thermal grill phenomenon that was independently discovered by Thunberg and Alrutz over a century ago (155). Specifically, the application of alternating cold and warm tubing (thermal grill) or concurrent stimulation of cold and warm spots on the human skin leads to noxious heat sensation. Mechanistically, the cooling-sensitive Aδ-fibers (a cold-labeled line) may mask other cooling-sensitive C-fibers, which evoke hot burning pain (a heat pain-labeled line), by cross inhibition in the central nervous system (Figure 4c). With the concurrent activation of warming-sensitive C-fibers (a warmth-labeled line) that can block the cooling-sensitive Aδ-fibers, the combination of cold and warm stimulation will give rise to the sense of burning heat (Figure 4c) (155). In addition to humans, the pattern theory of temperature sensation may also apply to other species. A recent study argued that the labeled line for innocuous warmth may not exist in mice. Instead, the innocuous warmth is encoded by the combination of warm-activated C-fibers (expressing TRPM2 and likely other warm-sensitive ion channels) and warm-inhibited, cold-sensitive C-fibers (expressing TRPM8) (156). How does one reconcile the specificity theory with the pattern theory in temperature sensation? One possibility is that since the population coding strategy requires a more complex nervous system compared to the dedicated labeled line strategy, labeled lines of thermosensory transmission might emerge first during evolution. In higher species like humans, both mechanisms may coexist, and the population coding strategy could be utilized to expand sensory modalities and promote sensory integration. Another possibility is that as sensory information is mainly processed and integrated in the central nervous system, the labeled line strategy might be more frequently used in the peripheral nervous system, while the population coding is mainly utilized in the central nervous system.
CONCLUSION AND FUTURE DIRECTIONS
For both humans and animals, temperature sensation provides essential information on their environment. A key advance in our understanding of temperature sensation is the cloning and characterization of diverse molecular thermosensors in different species. Among them, a group of evolutionarily conserved thermosensitive TRP channels are arguably the most well-established molecular thermosensors across species. Nevertheless, the molecular identities of some key temperature sensors remain elusive. For example, the mammalian noxious cold sensor has not been definitively identified. Moreover, a TRPM2-independent warmth sensor seems to exist in mammals. Identifying these thermosensors will help bridge the knowledge gap in temperature sensation. On the other hand, model organisms provide an excellent platform to perform genetic screens for novel players involved in temperature sensation. For instance, several IRs function as temperature sensors in the peripheral nervous system of Drosophila. Since these IRs are insect specific and thermosensation plays an important role in the host-seeking behavior of many disease-vector insects (157-159), targeting these temperature-sensitive IRs may provide a new avenue for pest control.
In both mammals and invertebrates, temperature information is relayed through several layers of neurons. While considerable progress has been made in understanding the temperature sensitivity of primary sensory neurons, how temperature information is computed and integrated in the central nervous system is still an open question. For example, what are the synaptic partners of DRG neurons in the spinal cord and how is the temperature information processed in the spinal cord? How are spinal neurons projected to distinct brain regions, and how does the brain process and encode temperature information? Finally, how is the thermosensory circuit connected to thermoregulation and other physiological processes such as aging? Due to the complexity of the mammalian nervous system, genetic and functional characterization of thermosensory circuits in Drosophila and C. elegans may reveal some fundamental principles of temperature sensation.
Notably, some broadly tuned neurons in thermosensory circuits are sensitive to both warming and cooling. Meanwhile, many temperature-sensitive neurons can be activated by other types of stimuli such as mechanical forces and chemicals. Therefore, different somatosensory modalities appear to be able to cross talk at the circuit level. Additional studies are required to reveal how temperature sensation can interact with pain, touch, and potentially itch. Understanding these interactions may not only reveal novel mechanisms of sensory transduction and processing but also provide important guidance for developing better treatment for sensory processing disorders and pain.
ACKNOWLEDGMENTS
We thank Bo Duan and Mingmin Zhang for critically reading the manuscript. The authors have been supported by the US National Institutes of Health (AG063766 and AG028740 to R.X.; GM126917, NS118769, DC018167, and AG067753 to X.Z.S.X.), the American Cancer Society (RSG-17-171-01-DMC to R.X.), the American Federation for Aging Research (to R.X.), the University of Florida Center for Smell and Taste (to R.X.), and the MCubed Program from the University of Michigan (to X.Z.S.X.). The authors would like to acknowledge and apologize that not all of the relevant literature has been cited due to space constraints.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, funding, or financial interests that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Frazer JG. 1930. Myths of the Origin of Fire—An Essay. London: Macmillan & Co. [Google Scholar]
- 2.Hall JE. 2016. Guyton and Hall Textbook of Medical Physiology. Philadelphia: Elsevier [Google Scholar]
- 3.Kandel ER. 2013. Principles of Neural Science. New York/London: McGraw-Hill Med. [Google Scholar]
- 4.Gowers WR. 1888. A Manual of Diseases of the Nervous System. Philadelphia: Blakiston [Google Scholar]
- 5.Brown-Séquard E. 1858. Dr. E. Brown-Séquard on the physiology and pathology of the nervous system. J. Psychol. Med. Ment. Pathol 11:i–xvi [PMC free article] [PubMed] [Google Scholar]
- 6.Adrian EDA. 1928. The Basis of Sensation, the Action of the Sense Organs. New York: W.W Norton [Google Scholar]
- 7.Iggo A. 1959. Cutaneous heat and cold receptors with slowly conducting (C) afferent fibres. Q. J. Exp.Physiol Cogn. Med. Sci 44:362–70 [DOI] [PubMed] [Google Scholar]
- 8.Darian-Smith I, Johnson KO, Dykes R. 1973. “Cold” fiber population innervating palmar and digital skin of the monkey: responses to cooling pulses. J. Neurophysiol. 36:325–46 [DOI] [PubMed] [Google Scholar]
- 9.Hensel H, Boman KK. 1960. Afferent impulses in cutaneous sensory nerves in human subjects. J. Neurophysiol. 23:564–78 [DOI] [PubMed] [Google Scholar]
- 10.Brown AG, Iggo A. 1967. A quantitative study of cutaneous receptors and afferent fibres in the cat and rabbit. J. Physiol. 193:707–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhaka A, Viswanath V, Patapoutian A. 2006. Trp ion channels and temperature sensation. Annu. Rev. Neurosci 29:135–61 [DOI] [PubMed] [Google Scholar]
- 12.Vriens J, Nilius B, Voets T. 2014. Peripheral thermosensation in mammals. Nat. Rev. Neurosci 15:573–89 [DOI] [PubMed] [Google Scholar]
- 13.Morrison SF, Nakamura K. 2019. Central mechanisms for thermoregulation. Annu. Rev. Physiol 81:285–308 [DOI] [PubMed] [Google Scholar]
- 14.Garrity PA, Goodman MB, Samuel AD, Sengupta P. 2010. Running hot and cold: behavioral strategies, neural circuits, and the molecular machinery for thermotaxis in C. elegans and Drosophila. Genes Dev. 24:2365–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao R, Zhang B, Dong Y, Gong J, Xu T, et al. 2013. A genetic program promotes C. elegans longevity at cold temperatures via a thermosensitive TRP channel. Cell 152:806–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang B, Xiao R, Ronan EA, He Y, Hsu A-L, et al. 2015. Environmental temperature differentially modulates C. elegans longevity through a thermosensitive TRP channel. Cell Rep. 11:1414–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao R, Liu J, Xu XZS. 2015. Thermosensation and longevity. J. Comp. Physiol A 201:857–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conti B 2008. Considerations on temperature, longevity and aging. Cell. Mol. Life Sci 65:1626–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clapham DE, Miller C. 2011. A thermodynamic framework for understanding temperature sensing by transient receptor potential (TRP) channels. PNAS 108:19492–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hille B. 2001. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer [Google Scholar]
- 21.Montell C, Rubin GM. 1989. Molecular characterization of the Drosophila TRP locus: a putative integral membrane protein required for phototransduction. Neuron 2:1313–23 [DOI] [PubMed] [Google Scholar]
- 22.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. 1997. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–24 [DOI] [PubMed] [Google Scholar]
- 23.Venkatachalam K, Montell C. 2007. TRP channels. Annu. Rev. Biochem 76:387–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yarnitsky D, Sprecher E, Zaslansky R, Hemli JA. 1995. Heat pain thresholds: normative data and repeatability. Pain 60:329–32 [DOI] [PubMed] [Google Scholar]
- 25.Defrin R, Ohry A, Blumen N, Urca G. 2002. Sensory determinants of thermal pain. Brain 125:501–10 [DOI] [PubMed] [Google Scholar]
- 26.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, et al. 1998. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21:531–43 [DOI] [PubMed] [Google Scholar]
- 27.Julius D 2013. TRP channels and pain. Annu. Rev. Cell Dev. Biol 29:355–84 [DOI] [PubMed] [Google Scholar]
- 28.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, et al. 2000. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288:306–13 [DOI] [PubMed] [Google Scholar]
- 29.Mishra SK, Hoon MA. 2010. Ablation of TrpV1 neurons reveals their selective role in thermal pain sensation. Mol. Cell. Neurosci 43:157–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mishra SK, Tisel SM, Orestes P, Bhangoo SK, Hoon MA. 2011. TRPV1-lineage neurons are required for thermal sensation. EMBO J. 30:582–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, et al. 2003. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112:819–29 [DOI] [PubMed] [Google Scholar]
- 32.Vriens J, Owsianik G, Hofmann T, Philipp SE, Stab J, et al. 2011. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 70:482–94 [DOI] [PubMed] [Google Scholar]
- 33.Tan C-H, McNaughton PA. 2016. The TRPM2 ion channel is required for sensitivity to warmth. Nature 536:460–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho H, Yang YD, Lee J, Lee B, Kim T, et al. 2012. The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nat. Neurosci 15:1015–21 [DOI] [PubMed] [Google Scholar]
- 35.Vandewauw I, De Clercq K, Mulier M, Held K, Pinto S, et al. 2018. A TRP channel trio mediates acute noxious heat sensing. Nature 555:662–66 [DOI] [PubMed] [Google Scholar]
- 36.Tracey WD Jr., Wilson RI, Laurent G, Benzer S. 2003. painless, a Drosophila gene essential for nociception. Cell 113:261–73 [DOI] [PubMed] [Google Scholar]
- 37.Sokabe T, Tsujiuchi S, Kadowaki T, Tominaga M. 2008. Drosophila Painless is a Ca2+-requiring channel activated by noxious heat. J. Neurosci. 28:9929–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao R, Xu XZ. 2009. Function and regulation of TRP family channels in C. elegans. Pflügers Arch. 458:851–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chatzigeorgiou M, Schafer WR. 2011. Lateral facilitation between primary mechanosensory neurons controls nose touch perception in C. elegans. Neuron 70:299–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu S, Schulze E, Baumeister R. 2012. Temperature- and touch-sensitive neurons couple CNG and TRPV channel activities to control heat avoidance in Caenorhabditis elegans. PLOS ONE 7:e32360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, et al. 2002. A heat-sensitive TRP channel expressed in keratinocytes. Science 296:2046–49 [DOI] [PubMed] [Google Scholar]
- 42.Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, et al. 2002. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 418:181–86 [DOI] [PubMed] [Google Scholar]
- 43.Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, et al. 2002. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 418:186–90 [DOI] [PubMed] [Google Scholar]
- 44.Guler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. 2002. Heat-evoked activation of the ion channel, TRPV4. J. Neurosci 22:6408–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. 2002. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J. Biol. Chem 277:47044–51 [DOI] [PubMed] [Google Scholar]
- 46.Huang SM, Li X, Yu Y, Wang J, Caterina MJ. 2011. TRPV3 and TRPV4 ion channels are not major contributors to mouse heat sensation. Mol. Pain 7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yarmolinsky DA, Peng Y, Pogorzala LA, Rutlin M, Hoon MA, Zuker CS. 2016. Coding and plasticity in the mammalian thermosensory system. Neuron 92:1079–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Togashi K, Hara Y, Tominaga T, Higashi T, Konishi Y, et al. 2006. TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. EMBO J. 25:1804–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song K, Wang H, Kamm GB, Pohle J, de Castro Reis F, et al. 2016. The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science 353:1393–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vilar B, Tan CH, McNaughton PA. 2020. Heat detection by the TRPM2 ion channel. Nature 584 (7820):E5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mulier M, Vandewauw I, Vriens J, Voets T. 2020. Reply to: Heat detection by the TRPM2 ion channel. Nature 584(7820):E13–15 [DOI] [PubMed] [Google Scholar]
- 52.Liu X, Wang H, Jiang Y, Zheng Q, Petrus M, et al. 2019. STIM1 thermosensitivity defines the optimal preference temperature for warm sensation in mice. Cell Res. 29:95–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang TA, Teo CF, Åkerblom M, Chen C, Tynan-La Fontaine M, et al. 2019. Thermoregulation via temperature-dependent PGD2 production in mouse preoptic area. Neuron 103:309–22.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosenzweig M, Brennan KM, Tayler TD, Phelps PO, Patapoutian A, Garrity PA. 2005. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev. 19:419–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Viswanath V, Story GM, Peier AM, Petrus MJ, Lee VM, et al. 2003. Opposite thermosensor in fruitfly and mouse. Nature 423:822–23 [DOI] [PubMed] [Google Scholar]
- 56.Ni L, Bronk P, Chang EC, Lowell AM, Flam JO, et al. 2013. A gustatory receptor paralogue controls rapid warmth avoidance in Drosophila. Nature 500:580–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mishra A, Salari A, Berigan BR, Miguel KC, Amirshenava M, et al. 2018. The Drosophila Gr28bD product is a non-specific cation channel that can be used as a novel thermogenetic tool. Sci. Rep 8:901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shen WL, Kwon Y, Adegbola AA, Luo J, Chess A, Montell C. 2011. Function of rhodopsin in temperature discrimination in Drosophila. Science 331:1333–36 [DOI] [PubMed] [Google Scholar]
- 59.Kwon Y, Shim HS, Wang X, Montell C. 2008. Control of thermotactic behavior via coupling of a TRP channel to a phospholipase C signaling cascade. Nat. Neurosci 11:871–73 [DOI] [PubMed] [Google Scholar]
- 60.Sokabe T, Chen HC, Luo J, Montell C. 2016. A switch in thermal preference in Drosophila larvae depends on multiple rhodopsins. Cell Rep. 17:336–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goodman MB, Sengupta P 2018. The extraordinary AFD thermosensor of C. elegans. Pflügers Arch. 470:839–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takeishi A, Yu YV, Hapiak VM, Bell HW, O’Leary T, Sengupta P. 2016. Receptor-type guanylyl cyclases confer thermosensory responses in C. elegans. Neuron 90:235–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, et al. 2002. A TRP channel that senses cold stimuli and menthol. Cell 108:705–15 [DOI] [PubMed] [Google Scholar]
- 64.McKemy DD, Neuhausser WM, Julius D. 2002. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416:52–58 [DOI] [PubMed] [Google Scholar]
- 65.Brauchi S, Orio P, Latorre R. 2004. Clues to understanding cold sensation: thermodynamics and electrophysiological analysis of the cold receptor TRPM8. PNAS 101:15494–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dhaka A, Earley TJ, Watson J, Patapoutian A. 2008. Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J. Neurosci. 28:566–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takashima Y, Ma L, McKemy DD. 2010. The development of peripheral cold neural circuits based on TRPM8 expression. Neuroscience 169:828–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, et al. 2007. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448:204–8 [DOI] [PubMed] [Google Scholar]
- 69.Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. 2007. TRPM8 is required for cold sensation in mice. Neuron 54:371–78 [DOI] [PubMed] [Google Scholar]
- 70.Knowlton WM, Palkar R, Lippoldt EK, McCoy DD, Baluch F, et al. 2013. A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. J. Neurosci. 33:2837–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pogorzala LA, Mishra SK, Hoon MA. 2013. The cellular code for mammalian thermosensation. J. Neurosci. 33:5533–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zimmermann K, Lennerz JK, Hein A, Link AS, Kaczmarek JS, et al. 2011. Transient receptor potential cation channel, subfamily C, member 5 (TRPC5) is a cold-transducer in the peripheral nervous system. PNAS 108:18114–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chao Y-C, Chen C-C, Lin Y-C, Breer H, Fleischer J, Yang R-B. 2015. Receptor guanylyl cyclase-G is a novel thermosensory protein activated by cool temperatures. EMBO J. 34:294–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sayeed O, Benzer S. 1996. Behavioral genetics of thermosensation and hygrosensation in Drosophila. PNAS 93:6079–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gallio M, Ofstad TA, Macpherson LJ, Wang JW, Zuker CS. 2011. The coding of temperature in the Drosophila brain. Cell 144:614–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Budelli G, Ni L, Berciu C, van Giesen L, Knecht ZA, et al. 2019. Ionotropic receptors specify the morphogenesis of phasic sensors controlling rapid thermal preference in Drosophila. Neuron 101:738–47.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ni L, Klein M, Svec KV, Budelli G, Chang EC, et al. 2016. The ionotropic receptors IR21a and IR25a mediate cool sensing in Drosophila. eLife 5:e13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Enjin A, Zaharieva EE, Frank DD, Mansourian S, Suh GS, et al. 2016. Humidity sensing in Drosophila. Curr. Biol. 26:1352–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Knecht ZA, Silbering AF, Ni L, Klein M, Budelli G, et al. 2016. Distinct combinations of variant ionotropic glutamate receptors mediate thermosensation and hygrosensation in Drosophila. eLife 5:e17879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chatzigeorgiou M, Yoo S, Watson JD, Lee WH, Spencer WC, et al. 2010. Specific roles for DEG/ENaC and TRP channels in touch and thermosensation in C. elegans nociceptors. Nat. Neurosci 13:861–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kindt KS, Viswanath V, Macpherson L, Quast K, Hu H, et al. 2007. Caenorhabditis elegans TRPA-1 functions in mechanosensation. Nat. Neurosci 10:568–77 [DOI] [PubMed] [Google Scholar]
- 82.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang D-S, et al. 2006. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 50:277–89 [DOI] [PubMed] [Google Scholar]
- 83.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, et al. 2006. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124:1269–82 [DOI] [PubMed] [Google Scholar]
- 84.Moparthi L, Kichko TI, Eberhardt M, Hogestatt ED, Kjellbom P, et al. 2016. Human TRPA1 is a heat sensor displaying intrinsic U-shaped thermosensitivity. Sci. Rep 6:28763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.del Camino D, Murphy S, Heiry M, Barrett LB, Earley TJ, et al. 2010. TRPA1 contributes to cold hypersensitivity. J. Neurosci 30:15165–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gong J, Liu J, Ronan EA, He F, Cai W, et al. 2019. A cold-sensing receptor encoded by a glutamate receptor gene. Cell 178:1375–86.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rodriguez-Moreno A, Lerma J. 1998. Kainate receptor modulation of GABA release involves a metabotropic function. Neuron 20:1211–18 [DOI] [PubMed] [Google Scholar]
- 88.Kang D, Choe C, Kim D. 2005. Thermosensitivity of the two-pore domain K+ channels TREK-2 and TRAAK. J. Physiol. 564:103–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Noel J, Zimmermann K, Busserolles J, Deval E, Alloui A, et al. 2009. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J. 28:1308–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zimmermann K, Leffler A, Babes A, Cendan CM, Carr RW, et al. 2007. Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature 447:855–58 [DOI] [PubMed] [Google Scholar]
- 91.Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. 2004. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 430:748–54 [DOI] [PubMed] [Google Scholar]
- 92.Matta JA, Ahern GP 2007. Voltage is a partial activator of rat thermosensitive TRP channels. J. Physiol 585:469–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Makhatadze GI, Privalov PL. 1990. Heat capacity of proteins. I. Partial molar heat capacity of individual amino acid residues in aqueous solution: hydration effect. J. Mol. Biol 213:375–84 [DOI] [PubMed] [Google Scholar]
- 94.Chowdhury S, Jarecki BW, Chanda B. 2014. A molecular framework for temperature-dependent gating of ion channels. Cell 158:1148–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yao J, Liu B, Qin F. 2011. Modular thermal sensors in temperature-gated transient receptor potential (TRP) channels. PNAS 108:11109–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cui Y, Yang F, Cao X, Yarov-Yarovoy V, Wang K, Zheng J. 2012. Selective disruption of high sensitivity heat activation but not capsaicin activation of TRPV1 channels by pore turret mutations. J. Gen. Physiol 139:273–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grandl J, Kim SE, Uzzell V, Bursulaya B, Petrus M, et al. 2010. Temperature-induced opening of TRPVl ion channel is stabilized by the pore domain. Nat. Neurosci 13:708–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grandl J, Hu H, Bandell M, Bursulaya B, Schmidt M, et al. 2008. Pore region of TRPV3 ion channel is specifically required for heat activation. Nat. Neurosci 11:1007–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pertusa M, Rivera B, Gonzalez A, Ugarte G, Madrid R. 2018. Critical role of the pore domain in the cold response of TRPM8 channels identified by ortholog functional comparison. J. Biol. Chem 293:12454–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brauchi S, Orta G, Mascayano C, Salazar M, Raddatz N, et al. 2007. Dissection of the components for PIP2 activation and thermosensation in TRP channels. PNAS 104:10246–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brauchi S, Orta G, Salazar M, Rosenmann E, Latorre R. 2006. A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels. J. Neurosci 26:4835–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Arrigoni C, Rohaim A, Shaya D, Findeisen F, Stein RA, et al. 2016. Unfolding of a temperature-sensitive domain controls voltage-gated channel activation. Cell 164:922–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Paulsen CE, Armache JP, Gao Y, Cheng Y, Julius D. 2015. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 520:511–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tsuruda PR, Julius D, Minor DL Jr. 2006. Coiled coils direct assembly of a cold-activated TRP channel. Neuron 51:201–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kang K, Panzano VC, Chang EC, Ni L, Dainis AM, et al. 2011. Modulation of TRPA1 thermal sensitivity enables sensory discrimination in Drosophila. Nature 481:76–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Numazaki M, Tominaga T, Toyooka H, Tominaga M. 2002. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cε and identification of two target serine residues. J. Biol. Chem 277:13375–78 [DOI] [PubMed] [Google Scholar]
- 107.Cao E, Cordero-Morales JF, Liu B, Qin F, Julius D. 2013. TRPV1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids. Neuron 77:667–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Luo J, Shen WL, Montell C. 2017. TRPA1 mediates sensation of the rate of temperature change in Drosophila larvae. Nat. Neurosci 20:34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bessou P, Perl ER. 1969. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J. Neurophysiol 32:1025–43 [DOI] [PubMed] [Google Scholar]
- 110.Kenton B, Coger R, Crue B, Pinsky J, Friedman Y, Carmon A. 1980. Peripheral fiber correlates to noxious thermal stimulation in humans. Neurosci. Lett 17:301–6 [DOI] [PubMed] [Google Scholar]
- 111.Konietzny F. 1984. Peripheral neural correlates of temperature sensations in man. Hum. Neurobiol 3:21–32 [PubMed] [Google Scholar]
- 112.Kenshalo DR, Duclaux R. 1977. Response characteristics of cutaneous cold receptors in the monkey. J. Neurophysiol 40:319–32 [DOI] [PubMed] [Google Scholar]
- 113.Darian-Smith I, Johnson KO, LaMotte C, Shigenaga Y, Kenins P, Champness P. 1979. Warm fibers innervating palmar and digital skin of the monkey: responses to thermal stimuli. J. Neurophysiol 42:1297–315 [DOI] [PubMed] [Google Scholar]
- 114.Simone DA, Kajander KC. 1997. Responses of cutaneous A-fiber nociceptors to noxious cold. J. Neurophysiol 77:2049–60 [DOI] [PubMed] [Google Scholar]
- 115.Campero M, Serra J, Ochoa JL. 1996. C-polymodal nociceptors activated by noxious low temperature in human skin. J. Physiol 497(Part 2):565–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Price DD, Hu JW, Dubner R, Gracely RH. 1977. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain 3:57–68 [DOI] [PubMed] [Google Scholar]
- 117.Wang F, Belanger E, Cote SL, Desrosiers P, Prescott SA, et al. 2018. Sensory afferents use different coding strategies for heat and cold. Cell Rep. 23:2001–13 [DOI] [PubMed] [Google Scholar]
- 118.Zhang ET, Han ZS, Craig AD. 1996. Morphological classes of spinothalamic lamina I neurons in the cat. J. Comp. Neurol 367:537–49 [DOI] [PubMed] [Google Scholar]
- 119.Han ZS, Zhang ET, Craig AD. 1998. Nociceptive and thermoreceptive lamina I neurons are anatomically distinct. Nat. Neurosci 1:218–25 [DOI] [PubMed] [Google Scholar]
- 120.Todd AJ. 2010. Neuronal circuitry for pain processing in the dorsal horn. Nat. Rev. Neurosci 11:823–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zheng J, Lu Y, Perl ER. 2010. Inhibitory neurones of the spinal substantia gelatinosa mediate interaction of signals from primary afferents. J. Physiol. 588:2065–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McCoy ES, Taylor-Blake B, Street SE, Pribisko AL, Zheng J, Zylka MJ. 2013. Peptidergic CGRPα primary sensory neurons encode heat and itch and tonically suppress sensitivity to cold. Neuron 78(1):138–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ran C, Hoon MA, Chen X. 2016. The coding of cutaneous temperature in the spinal cord. Nat. Neurosci 19:1201–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Craig AD, Chen K, Bandy D, Reiman EM. 2000. Thermosensory activation of insular cortex. Nat. Neurosci 3:184–90 [DOI] [PubMed] [Google Scholar]
- 125.Gauriau C, Bernard J-F. 2004. A comparative reappraisal of projections from the superficial laminae of the dorsal horn in the rat: the forebrain. J. Comp. Neurol 468:24–56 [DOI] [PubMed] [Google Scholar]
- 126.Craig AD, Bushnell MC, Zhang ET, Blomqvist A. 1994. A thalamic nucleus specific for pain and temperature sensation. Nature 372:770–73 [DOI] [PubMed] [Google Scholar]
- 127.Bokiniec P, Zampieri N, Lewin GR, Poulet JF. 2018. The neural circuits of thermal perception. Curr. Opin. Neurobiol 52:98–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Klein M, Afonso B, Vonner AJ, Hernandez-Nunez L, Berck M, et al. 2015. Sensory determinants of behavioral dynamics in Drosophila thermotaxis. PNAS 112:E220–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, et al. 2008. An internal thermal sensor controlling temperature preference in Drosophila. Nature 454:217–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Barbagallo B, Garrity PA. 2015. Temperature sensation in Drosophila. Curr Opin. Neurobiol 34:8–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu WW, Mazor O, Wilson RI. 2015. Thermosensory processing in the Drosophila brain. Nature 519:353–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Frank DD, Jouandet GC, Kearney PJ, Macpherson LJ, Gallio M. 2015. Temperature representation in the Drosophila brain. Nature 519:358–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hedgecock EM, Russell RL. 1975. Normal and mutant thermotaxis in the nematode Caenorhabditis elegans. PNAS 72:4061–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mori I, Ohshima Y. 1995. Neural regulation of thermotaxis in Caenorhabditis elegans. Nature 376:344–48 [DOI] [PubMed] [Google Scholar]
- 135.Luo L, Cook N, Venkatachalam V, Martinez-Velazquez LA, Zhang X, et al. 2014. Bidirectional thermotaxis in Caenorhabditis elegans is mediated by distinct sensorimotor strategies driven by the AFD thermosensory neurons. PNAS 111:2776–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cook SJ, Jarrell TA, Brittin CA, Wang Y, Bloniarz AE, et al. 2019. Whole-animal connectomes of both Caenorhabditis elegans sexes. Nature 571:63–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.White JG, Southgate E, Thomson JN, Brenner S. 1986. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. B 314:1–340 [DOI] [PubMed] [Google Scholar]
- 138.Ohnishi N, Kuhara A, Nakamura F, Okochi Y, Mori I. 2011. Bidirectional regulation of thermotaxis by glutamate transmissions in Caenorhabditis elegans. EMBO. 30:1376–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li C, Kim K. 2008. Neuropeptides. WormBook 2008:1–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Narayan A, Laurent G, Sternberg PW. 2011. Transfer characteristics of a thermosensory synapse in Caenorhabditis elegans. PNAS 108:9667–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ikeda M, Nakano S, Giles AC, Xu L, Costa WS, et al. 2020. Context-dependent operation of neural circuits underlies a navigation behavior in Caenorhabditis elegans. PNAS 117:6178–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kimura KD, Miyawaki A, Matsumoto K, Mori I. 2004. The C. elegans thermosensory neuron AFD responds to warming. Curr. Biol. 14:1291–95 [DOI] [PubMed] [Google Scholar]
- 143.Clark DA, Biron D, Sengupta P, Samuel AD. 2006. The AFD sensory neurons encode multiple functions underlying thermotactic behavior in Caenorhabditis elegans. J. Neurosci 26:7444–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kobayashi K, Nakano S, Amano M, Tsuboi D, Nishioka T, et al. 2016. Single-cell memory regulates a neural circuit for sensory behavior. Cell Rep. 14:11–21 [DOI] [PubMed] [Google Scholar]
- 145.Hawk JD, Calvo AC, Liu P, Almoril-Porras A, Aljobeh A, et al. 2018. Integration of plasticity mechanisms within a single sensory neuron of C. elegans actuates a memory. Neuron 97:356–67.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chi CA, Clark DA, Lee S, Biron D, Luo L, et al. 2007. Temperature and food mediate long-term thermotactic behavioral plasticity by association-independent mechanisms in C. elegans. J. Exp. Biol 210:4043–52 [DOI] [PubMed] [Google Scholar]
- 147.Ramot D, MacInnis BL, Goodman MB. 2008. Bidirectional temperature-sensing by a single thermosensory neuron in C. elegans. Nat. Neurosci 11:908–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang B, Gong J, Zhang W, Xiao R, Liu J, Xu XZS. 2018. Brain-gut communications via distinct neuroendocrine signals bidirectionally regulate longevity in C. elegans. Genes Dev. 32:258–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li W, Kang L, Piggott BJ, Feng Z, Xu XZ. 2011. The neural circuits and sensory channels mediating harsh touch sensation in Caenorhabditis elegans. Nat. Commun 2:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Husson SJ, Costa WS, Wabnig S, Stirman JN, Watson JD, et al. 2012. Optogenetic analysis of a nociceptor neuron and network reveals ion channels acting downstream of primary sensors. Curr. Biol 22:743–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Davis KD, Pope GE. 2002. Noxious cold evokes multiple sensations with distinct time courses. Pain 98:179–85 [DOI] [PubMed] [Google Scholar]
- 152.Knowlton WM, Palkar R, Lippoldt EK, McCoy DD, Baluch F, et al. 2013. A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. J. Neurosci 33:2837–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ma Q. 2010. Labeled lines meet and talk: population coding of somatic sensations. J. Clin. Investig 120:3773–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Uchida N, Poo C, Haddad R. 2014. Coding and transformations in the olfactory system. Annu. Rev. Neurosci 37:363–85 [DOI] [PubMed] [Google Scholar]
- 155.Craig AD, Bushnell MC. 1994. The thermal grill illusion: unmasking the burn of cold pain. Science 265:252–55 [DOI] [PubMed] [Google Scholar]
- 156.Paricio-Montesinos R, Schwaller F, Udhayachandran A, Rau F, Walcher J, et al. 2020. The sensory coding of warm perception. Neuron 106:830–41.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Elliot SL, Blanford S, Thomas MB. 2002. Host-pathogen interactions in a varying environment: temperature, behavioural fever and fitness. Proc. Biol. Sci 269:1599–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Corfas RA, Vosshall LB. 2015. The cation channel TRPA1 tunes mosquito thermotaxis to host temperatures. eLife 4:e11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Greppi C, Laursen WJ, Budelli G, Chang EC, Daniels AM, et al. 2020. Mosquito heat seeking is driven by an ancestral cooling receptor. Science 367:681–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liu B, Hui K, Qin F. 2003. Thermodynamics of heat activation of single capsaicin ion channels VR1. Biophys. J 85:2988–3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Karashima Y, Talavera K, Everaerts W, Janssens A, Kwan KY, et al. 2009. TRPA1 acts as a cold sensor in vitro and in vivo. PNAS 106:1273–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Park U, Vastani N, Guan Y, Raja SN, Koltzenburg M, Caterina MJ. 2011. TRP vanilloid 2 knockout mice are susceptible to perinatal lethality but display normal thermal and mechanical nociception. J. Neurosci. 31:11425–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, et al. 2005. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature 438:1022–25 [DOI] [PubMed] [Google Scholar]