Abstract
The present paper reports the fabrication of inverse opal photonic crystals (IOPCs) by using SiO2 spherical particles with a diameter of 300 nm as an opal photonic crystal template and poly(ethylene glycol) diacrylate (PEGDA) as an inverse opal material. Characteristics and fluorescence properties of the fabricated IOPCs were investigated by using the Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), X-ray diffraction (XRD), reflection spectroscopy, and fluorescence microscopy. The results clearly showed that the IOPCs were formed comprising of air spheres with a diameter of ∼270 nm. The decrease in size led to a decrease in the average refractive indexes from 1.40 to 1.12, and a remarkable stopband blue shift for the IOPCs was thus achieved. In addition, the obtained results also showed a fluorescence enhancement over 7.7-fold for the Fluor® 488 dye infiltrated onto the IOPCs sample in comparison with onto the control sample.
1. Introduction
Photonic crystals (PhC) with a periodic structure have significant effects on the propagation of electromagnetic (EM) waves due to the diffraction of photons in a limited wavelength range from the lattice planes [1, 2], leading to the allowance or restriction of the propagation of EM waves through the material structure. When the EM radiation is forbidden in the wavelength region, it cannot be transmitted, resulting in the reflection from the crystal lattice, known as Bragg diffraction [3]. As the result, the photonic bandgap (PBG) is formed. The changes in the refractive index of PhC lead to the adjustment of the PBG wavelengths and reflection intensity. Consequently, the photonic especially fluorescent properties of the materials are changed [4, 5].
SiO2 material with a porous structure, known as a stably, nontoxically, harmlessly and low-costly photonic crystalline material, has been widely applied to various areas, such as catalyst supports [6–8], adsorbents [9–11], chromatographic materials [12, 13], and biosensors [14, 15]. In particular, the SiO2 inverse opal photonic crystal (IOPC) biosensors have been fabricated based on changes in their photoluminescence properties or reflective spectra. It has been shown in the literature [16] that the IOPCs can change not only the wavelengths of PBGs but also enhance the fluorescent intensity. Materials used as the inverse opal materials for fabrication of IOPCs are varieties of hydrogels, such as polystyrene [5, 17], poly(4-vinylbenzyl chloride-co-methyl methacrylate) [18], and poly(styrene-co-methyl methacrylate-co-acrylic acid) [19]. Although poly(ethylene glycol) diacrylate (PEGDA) is a derivative of polyethylene glycol and has been widely used for various biomedical applications due to its biocompatibility [20] and easy excretion from the body [21], the use of this hydrogel as an inverse opal material in IOPCs has still been limited. For instance, studies of Park et al. focused on the fabrication of humidity sensors and biosensors for the detection of immunoglobulin G [22, 23]. Though the obtained results clearly show the change in colour of the fabricated IOPCs with the presence of detection targets, the improvement of fluorescence intensity of IOPCs is still required to enhance the sensitivity of target detections.
In order to fabricate the IOPCs, two techniques have been widely used, namely, thermal degradation [5, 17–19, 24] and chemical etching [25, 26]. The thermal degradation has been commonly used for the treatment of organic templates, while the chemical etching has been regularly applied to remove the inorganic templates, such as TiO2, ZnO, and SiO2. Among the chemical etchants, the buffered oxide etch (BOE), a mixture of hydrofluoric acid (HF) and ammonium fluoride (NH4F), is often used for etching silicon dioxide on the silicon wafers. In this work, SiO2 spherical particles are used as an opal photonic crystal material, whereas PEGDA is utilized as an inverse opal material to fabricate the IOPCs having two-dimensional periodical and microporous structure with the lattice spacing on the order of the wavelength of light. These IOPCs thus not only induce a stopband shift but also exhibit a fluorescence enhancement for Alexa Fluor® 488 dye, which is a green fluorophore and has been commonly used in applications such as immunolabeling, fluorescence microscopy, and flow cytometry.
2. Materials and Methods
2.1. Chemicals
Nonfunctionalized silica microspheres with a diameter of 300 nm were purchased from the Polyscience Asia Pacific Inc. Poly(ethylene glycol) diacrylate (Mn = 250), ethanol 95%, 2-hydroxy-2-methylpropiophenone 97% (Irgacure 1173), donkey anti-rabbit IgG (H + L), Alexa Fluor® 488 conjugate (A-21206, Invitrogen), and buffered oxide etchant (BOE) were supplied by Sigma-Aldrich. Irgacure 1173, a photoinitiator, was used in radiation curing in the polymerization of PEGDA.
2.2. Protocol for IOPC Fabrication
The fabrication of IOPCs was performed following Scheme 1 [22, 23]. Herein, 0.5 mL silica sol suspension was dropped onto a microscope slide after covering with a hydrophobic thin layer and dried in air for 1 day at room temperature. The mixture of 99 wt% PEGDA and 1 wt% 2-hydroxy-2-methylpropiophenone was then added into the dried SiO2 for 5 minutes and exposed under the UV light (312 nm) for 5 minutes for the polymerization process. Finally, the chip was etched by BOE for few hours to obtain the IOPCs before being washed by pure water several times.
Scheme 1.
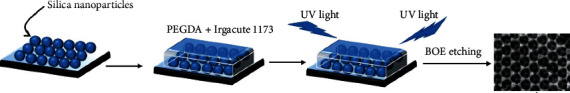
Protocol for the fabrication of IOPCs.
2.3. Instruments
The spectrometer processor (Ocean Optics QE Pro-FL), which has a wavelength range of 350 nm–1100 nm, in conjunction with a halogen light source (Ocean Optics HL-2000) was used to determine the reflected spectra. The fluorescent images of the samples were measured using a fluorescent microscope (BX51, Olympus), whereas the fluorescent intensity was analysed by the ImageJ software. Scanning electron microscopy (SEM, Hitachi S-4800; acceleration of 15–20 kV and working electrode distance of 4–5 mm) was used to characterize the surface morphology. Fourier transform infrared spectroscopy (FTIR, FTIR-PerkinElmer Spectrum 10.5.2) measurements were recorded at the atmospheric pressure with a resolution of 4 cm−1 using p-polarized radiation. X-ray diffraction data were collected using Bruker D8 (20 kV, 5 mA) equipped with a LynxEye detector and a conventional Cu anode. Diffractograms were collected at a step of 0.25 s.
3. Results and Discussion
3.1. Characterizations of PEGDA/SiO2 and IOPCs
3.1.1. FT-IR Spectrum
The interactions between PEGDA and SiO2 are determined via the FT-IR analysis. Figure 1 shows the FT-IR spectra of SiO2, PEGDA, and PEGDA/SiO2. The characteristic peaks belonging to SiO2 are clearly observed in curve (a). The bending vibration of the Si–O group is recorded at the wavenumber of 478 cm−1, while the peak at 949 cm−1 is assigned to the vibration of Si–OH. The peaks at 801 cm−1 and 1099 cm−1 are related to the vibration of the Si–O–Si group. The vibrations of –OH groups are found at 1634 cm−1 and 3423 cm−1 [27, 28]. The FT-IR spectrum of PEGDA (curve (b)) shows a typical peak of the carbonyl group (C=O) at the wavelength of 1724 cm−1, whereas the peak at the wavelength of 2921 cm−1 is attributed to the CH2 group of PEGDA [29]. The spectrum of PEGDA/SiO2 (curve (c)) shows all dominant peaks corresponding to both PEGDA and SiO2. Noticeably, those are related to the methylene group (CH2), C=O, and Si-O functional surface groups. These results clearly indicate that PEGDA particles have been successfully grafted to SiO2 spheres via physical interactions [27, 30].
Figure 1.
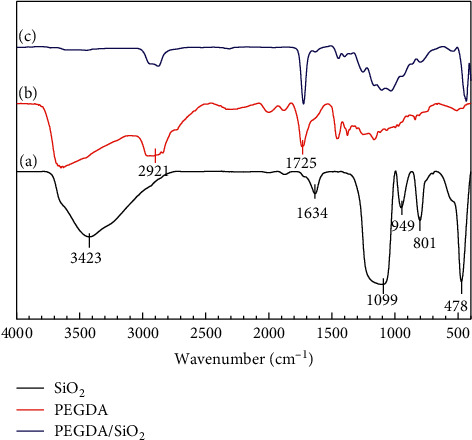
FT-IR spectra of SiO2 (curve (a)), PEGDA (curve (b)), and PEGDA/SiO2 (curve (c)).
3.1.2. SEM Images
As seen in Figure 2(a), PEGDA/SiO2 template comprises many SiO2 spherical particles with a diameter of 300 nm, which are covered by a PEGDA thin film. After etching, the inverse opal photonic structure is created, which contains a large number of micropores with a diameter of ∼270 nm, as seen in Figure 2(b). It is obvious that the diameter of air spherical is shrunk, leading to the variation of the wavelength of the reflected light due to effects on the propagation of electromagnetic waves in a limited frequency range, as discussed below.
Figure 2.
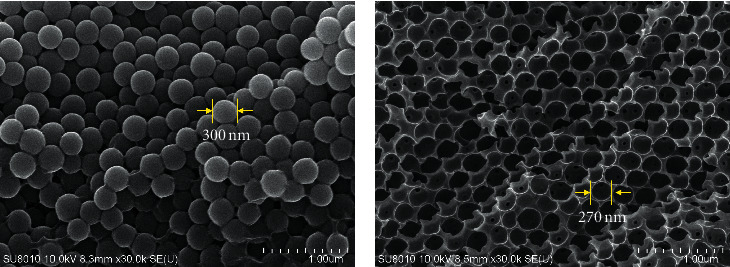
SEM images of PEGDA/SiO2 before (a) and after etching with BOE (b).
3.1.3. XRD Analysis
XRD measurement was performed to study the characteristics of PEGDA/SiO2 before and after etching with BOE. The obtained XRD results for SiO2, PEGDA/SiO2 template, and the IOPCs showed only one peak at 2θ = 22° (see supplemental information (available here)). The appearance of this peak is a feature of the amorphous state of silica materials [31] and indicates that the fabrication of IOPCs is not influenced by the etching process.
3.2. Reflectance Spectra
The wavelength of reflection peaks (λ) can be estimated based on Bragg's equation as follows [32]:
| (1) |
where d (nm) is the center-to-center distance between two neighbouring mesopores and naverage is the average refractive index of the studied materials. According to the product specification sheet, the refractive indexes of SiO2 particles and PEGDA materials are about 1.378 and 1.463, respectively. The average refractive index of the PEGDA/SiO2 and IOPCs in the air can be calculated using the following equation [32, 33]:
| (2) |
where n1 is the refractive index of colloidal crystal, n2 is the refractive index of the surrounding environment, and Φ is the void ratio of colloidal crystal.
The calculated average refractive indexes of the PEGDA/SiO2 and the IOPCs were 1.40 and 1.12, respectively. Obviously, the refractive index of the PEGDA/SiO2 is decreased after etching because of the replacement of SiO2 spheres in the template by voids. In addition, the wavelengths of reflection peaks of SiO2, PEGDA/SiO2, and the IOPCs calculated from equation (1) are 632, 686, and 548, respectively, which are slightly different from the position of reflection peaks at 619, 688, and 539 nm, respectively, as shown in Figure 3.
Figure 3.
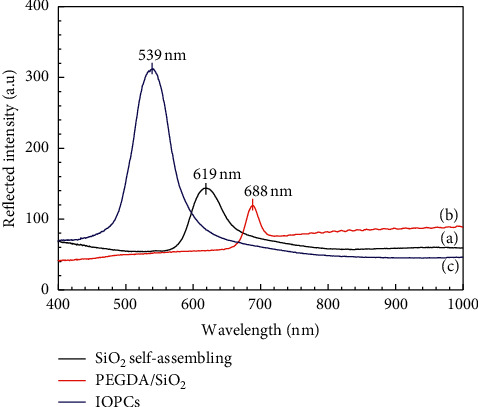
Reflection spectra for SiO2 self-assembling (curve (a)), PEGDA/SiO2 (curve (b)), and the IOPCs (curve (c)).
This difference is due to the decrease in the diameter of micropores as seen in the SEM images. Thus, there is a good agreement between the wavelengths of reflection peaks shown in the reflection spectra and those calculated by Bragg's equation. The reflection spectra shown in Figure 3 exhibit a remarkable stopband blue shift for the IOPCs when the average refractive index decreases and there is a decrease in the diameter of micropores. This can be clearly observed in images shown in Figure 4, in which the observed colour of SiO2 self-assembling, PEGDA/SiO2, and the IOPCs is changed from the red colour to orange and green colours correspond to the wavelengths of their photonic stopbands at 688 nm, 619 nm, and 539 nm, respectively. These colour changes exhibit how PhCs made of different materials modify the propagation of light through the photonic stopband. These obtained results are similar to those reported by Subramania and coworkers [34], in which the authors have shown a systematic shift of the reflection peak to longer wavelengths with increasing the diameter of spheres.
Figure 4.
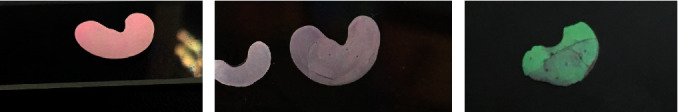
Photographs of SiO2 self-assembling (a), PEGDA/SiO2 (b), and the IOPCs (c).
3.3. Fluorescent Properties
In order to investigate the effects of the IOPCs on the fluorescence enhancement for Fluor® 488 dye, an accurate amount of 1.0 mg/mL this dye was dropped onto the control PEGDA/SiO2 template and the IOPCs sample. The Fluor® 488 dye, which is a bright and green-fluorescent dye, has an absorption peak at a wavelength of 496 nm and a maximum emission around 519 nm. Figures 5 and 6 compare the fluorescence intensity of Fluor® 488 dye infiltrated onto the PEGDA/SiO2 template (non-IOPCs) and the IOPCs sample. The experimental data clearly indicate that over 7.7-fold fluorescence enhancement was achieved for Fluor® 488 dye infiltrated onto the IOPCs sample in comparison with onto the control template. The fluorescence enhancement occurs owing to the fact that the stopband of the IOPCs (539 nm) overlaps the emission of fluorescent Fluor® 488 dye (519 nm), leading to a local resonance mode for the propagation of emission.
Figure 5.
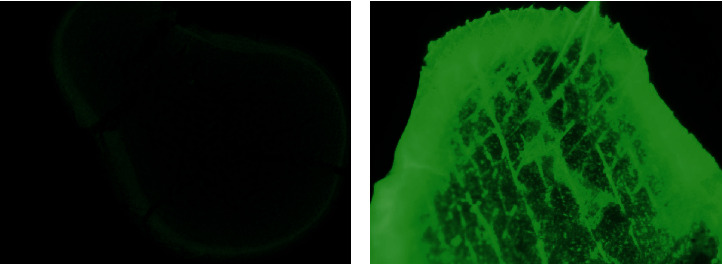
Fluorescence images of PEGDA/SiO2 (a) and the IOPCs, for Fluor® 488 dye (b).
Figure 6.
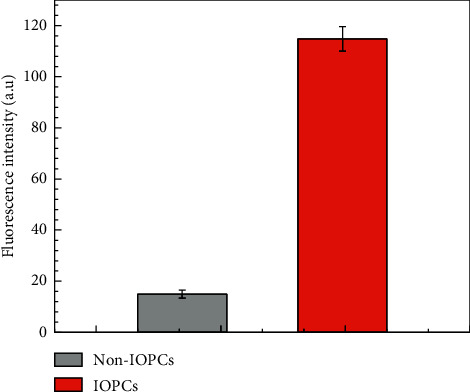
Fluorescence intensities of PEGDA/SiO2 (non-IOPCs) and the IOPCs for Fluor® 488 dye.
In the context that many studies have been attempting to improve the fluorescence enhancement of PhCs, such as studies of the introduction of dyes into the PhCs by different methods [35], effects of lattice period, resonant mode polarization, and symmetry on the enhancement effect of PhCs comprised of colloidal quantum dots [36], the resonant interactions between the localized surface plasmon of gold nanoparticles and the emission of the dye infiltrated into the PhCs [37], or the formation of host-guest complexes between Rhodamine B and cucurbituril [38], our obtained results demonstrate for the first time the fluorescence enhancement of Fluor® 488 dye infiltrated onto the IOPCs which is made of PEGDA/SiO2. In addition, Fluor® 488 dye is one of the Alexa Fluor dyes, which can be conjugated directly to primary antibodies or to secondary antibodies to amplify signal and sensitivity, since we believe the results of this work can be extended to studies in the field of biological system that might benefit from the added sensitivity afforded by this approach.
4. Conclusions
The inverse opal photonic crystals (IOPCs) fabricated by etching the SiO2 spheres from the template composed of poly(ethylene glycol) diacrylate (PEGDA) and SiO2 within this thin layer (PEGDA/SiO2) have been fabricated. This process induced a reduction of air sphere diameter lowering to ∼270 nm. This led to a decrease of the average refractive index of the fabricated IOPCs from 1.40 to 1.12. Consequently, a blue shift of the stopband was achieved. In addition, the IOPCs exhibited a significant enhancement for Fluor® 488 dye up to 7.7-fold in comparison with the control PEGDA/SiO2 template. Such obtained results can be helpful for studies in the field of biological system to improve the sensitivity of sensing.
Acknowledgments
This study was funded by a Grant-in-Aid for the Project no. NCVCC 06.13/20-20 approved by the decision of 2589/QĐ-VHL of Vietnam Academy of Science and Technology.
Data Availability
All the data and supporting materials are included within the article.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Supplementary Materials
Supp. 1: XRD patterns of SiO2 (a), PEGDA/SiO2 (b), and the IOPCs (c).
References
- 1.Erola M. O. A., Philip A., Ahmed T., Suvanto S., Pakkanen T. T. Fabrication of Au- and Ag-SiO2 inverse opals having both localized surface plasmon resonance and Bragg diffraction. Journal of Solid State Chemistry. 2015;230:209–217. doi: 10.1016/j.jssc.2015.06.036. [DOI] [Google Scholar]
- 2.Josephson D. P., Miller M., Stein A. Inverse opal SiO2 photonic crystals as structurally-colored pigments with additive primary colors. Zeitschrift für anorganische und allgemeine Chemie. 2014;640(3-4):655–662. doi: 10.1002/zaac.201300578. [DOI] [Google Scholar]
- 3.Waterhouse G. I. N., Waterland M. R. Opal and inverse opal photonic crystals: fabrication and characterization. Polyhedron. 2007;26(2):356–368. doi: 10.1016/j.poly.2006.06.024. [DOI] [Google Scholar]
- 4.Schroden R. C., Al-Daous M., Blanford C. F., Stein A. Optical properties of inverse opal photonic crystals. Chemistry of Materials. 2002;14(8):3305–3315. doi: 10.1021/cm020100z. [DOI] [Google Scholar]
- 5.Zhang Y., Mu L., Zhou R., et al. Fluoral-p infiltrated SiO2 inverse opal photonic crystals as fluorescent film sensors for detecting formaldehyde vapor. Journal of Materials Chemistry C. 2016;4(41):9841–9847. doi: 10.1039/c6tc03862j. [DOI] [Google Scholar]
- 6.Tomiyama S., Takahashi R., Sato S., Sodesawa T., Yoshida S. Preparation of Ni/SiO2 catalyst with high thermal stability for CO2-reforming of CH4. Applied Catalysis A: General. 2003;241(1-2):349–361. doi: 10.1016/s0926-860x(02)00493-3. [DOI] [Google Scholar]
- 7.Radivojević D., Seshan K., Lefferts L. Pt/SiO2 catalyst preparation: high platinum dispersions by using low-temperature treatments. In: Gaigneaux E. M., Devillers M., De Vos D. E., et al., editors. Studies in Surface Science and Catalysis. Amsterdam, Netherlands: Elsevier; 2006. pp. 529–536. [Google Scholar]
- 8.Xue F., Chen W., Song X., Cheng X., Ding Y. Promotional effects of Cr and Fe on Rh/SiO2 catalyst for the preparation of ethanol from CO hydrogenation. RSC Advances. 2016;6(42):35348–35353. doi: 10.1039/c5ra28075c. [DOI] [Google Scholar]
- 9.Sahu O., Singh N. 13—significance of bioadsorption process on textile industry wastewater. In: Shahid ul I., Butola B. S., editors. The Impact and Prospects of Green Chemistry for Textile Technology. Sawston,UK: Woodhead Publishing; 2019. pp. 367–416. [Google Scholar]
- 10.Zhao M., Yang X., Church T. L., Harris A. T. Novel CaO-SiO2 sorbent and bifunctional Ni/Co-CaO/SiO2 complex for selective H2 synthesis from cellulose. Environmental Science & Technology. 2012;46(5):2976–2983. doi: 10.1021/es300135d. [DOI] [PubMed] [Google Scholar]
- 11.Ko T.-H., Wang S., Chang F.-H., Chu C.-Y. Performance of ZnMn2O4/SiO2 sorbent for high temperature H2S removal from hot coal gas. RSC Advances. 2017;7(57):35795–35804. doi: 10.1039/c7ra06785b. [DOI] [Google Scholar]
- 12.Loy D. A. Sol–Gel processing. In: Meyers R. A., editor. Encyclopedia of Physical Science and Technology. Third. New York, NY, USA: Academic Press; 2003. pp. 257–276. [Google Scholar]
- 13.Ehrling S., Kutzscher C., Freund P., Müller P., Senkovska I., Kaskel S. MOF@SiO2 core-shell composites as stationary phase in high performance liquid chromatography. Microporous and Mesoporous Materials. 2018;263:268–274. doi: 10.1016/j.micromeso.2018.01.003. [DOI] [Google Scholar]
- 14.Li C., Li J., Tang H., Yang X., Fei Q., Sun C. A non-enzymatic electrochemical biosensor based on SiO2-Au nanoparticles for hemoglobin detection. Analytical Methods. 2017;9(8):1265–1272. doi: 10.1039/c6ay03032g. [DOI] [Google Scholar]
- 15.Massad-Ivanir N., Shtenberg G., Zeidman T., Segal E. Construction and characterization of porous SiO2/hydrogel hybrids as optical biosensors for rapid detection of bacteria. Advanced Functional Materials. 2010;20(14):2269–2277. doi: 10.1002/adfm.201000406. [DOI] [Google Scholar]
- 16.Lee W., Kang T., Kim S.-H., Jeong J. An antibody-immobilized silica inverse opal nanostructure for label-free optical biosensors. Sensors. 2018;18(1):p. 307. doi: 10.3390/s18010307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y., Li Q., Guo P., et al. Fluorescence-enhancing film sensor for highly effective detection of Bi3+ ions based on SiO2 inverse opal photonic crystals. Journal of Materials Chemistry C. 2018;6(27):7326–7332. doi: 10.1039/c8tc01461b. [DOI] [Google Scholar]
- 18.Zhang Y., Sun Y., Liu J., Guo P., Cai Z., Wang J.-J. Polymer-infiltrated SiO2 inverse opal photonic crystals for colorimetrically selective detection of xylene vapors. Sensors and Actuators B: Chemical. 2019;291:67–73. doi: 10.1016/j.snb.2019.04.036. [DOI] [Google Scholar]
- 19.Li H., Wang J., Pan Z., et al. Amplifying fluorescence sensing based on inverse opal photonic crystal toward trace TNT detection. Journal of Materials Chemistry. 2011;21(6):1730–1735. doi: 10.1039/c0jm02554b. [DOI] [Google Scholar]
- 20.Stillman Z., Jarai B. M., Raman N., Patel P., Fromen C. A. Degradation profiles of poly (ethylene glycol) diacrylate (PEGDA)-based hydrogel nanoparticles. Polymer Chemistry. 2020;11(2):568–580. doi: 10.1039/c9py01206k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavallo A., Madaghiele M., Masullo U., Lionetto M. G., Sannino A. Photo-crosslinked poly (ethylene glycol) diacrylate (PEGDA) hydrogels from low molecular weight prepolymer: swelling and permeation studies. Journal of Applied Polymer Science. 2017;134:443801–443809. doi: 10.1002/app.44380. [DOI] [Google Scholar]
- 22.Kim J. H., Moon J. H., Lee S.-Y., Park J. Biologically inspired humidity sensor based on three-dimensional photonic crystals. Applied Physics Letters. 2010;97(10):p. 103701. doi: 10.1063/1.3486115. [DOI] [Google Scholar]
- 23.Choi E., Choi Y., Nejad Y. H. P., Shin K., Park J. Label-free specific detection of immunoglobulin G antibody using nanoporous hydrogel photonic crystals. Sensors and Actuators B: Chemical. 2013;180:107–113. doi: 10.1016/j.snb.2012.03.053. [DOI] [Google Scholar]
- 24.Huang K.-M., Ho C.-L., Chang H.-J., Wu M.-C. Fabrication of inverted zinc oxide photonic crystal using sol-gel solution by spin coating method. Nanoscale Research Letters. 2013;8(1):p. 306. doi: 10.1186/1556-276x-8-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varghese L. T., Xuan Y., Niu B., Fan L., Bermel P., Qi M. Enhanced photon management of thin-film silicon solar cells using inverse opal photonic crystals with 3D photonic bandgaps. Advanced Optical Materials. 2013;1(10):692–698. doi: 10.1002/adom.201300254. [DOI] [Google Scholar]
- 26.Shin J., Braun P. V., Lee W. Fast response photonic crystal pH sensor based on templated photo-polymerized hydrogel inverse opal. Sensors and Actuators B: Chemical. 2010;150(1):183–190. doi: 10.1016/j.snb.2010.07.018. [DOI] [Google Scholar]
- 27.Qian T., Li J., Ma H., Yang J. The preparation of a green shape-stabilized composite phase change material of polyethylene glycol/SiO2 with enhanced thermal performance based on oil shale ash via temperature-assisted sol-gel method. Solar Energy Materials and Solar Cells. 2015;132:29–39. doi: 10.1016/j.solmat.2014.08.017. [DOI] [Google Scholar]
- 28.Liang Y., Ouyang J., Wang H., Wang W., Chui P., Sun K. Synthesis and characterization of core-shell structured SiO2@YVO4:Yb3+, Er3+ microspheres. Applied Surface Science. 2012;258(8):3689–3694. doi: 10.1016/j.apsusc.2011.12.006. [DOI] [Google Scholar]
- 29.Askari F., Zandi M., Shokrolahi P., Tabatabaei M. H., Hajirasoliha E. Reduction in protein absorption on ophthalmic lenses by PEGDA bulk modification of silicone acrylate-based formulation. Progress in Biomaterials. 2019;8(3):169–183. doi: 10.1007/s40204-019-00119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin B., Zhou S. Poly (ethylene glycol)-grafted silica nanoparticles for highly hydrophilic acrylic-based polyurethane coatings. Progress in Organic Coatings. 2017;106:145–154. doi: 10.1016/j.porgcoat.2017.02.008. [DOI] [Google Scholar]
- 31.Zhang G., Xu Y., Xu D., Wang D., Xue Y., Su W. Pressure-induced crystallization of amorphous SiO2 with silicon-hydroxy group and the quick synthesis of coesite under lower temperature. High Pressure Research. 2008;28(4):641–650. doi: 10.1080/08957950802510091. [DOI] [Google Scholar]
- 32.Zhang B., Cheng Y., Wang H., et al. Multifunctional inverse opal particles for drug delivery and monitoring. Nanoscale. 2015;7(24):10590–10594. doi: 10.1039/c5nr02324f. [DOI] [PubMed] [Google Scholar]
- 33.Yu B., Cong H., Yang Z., et al. Preparation of humidity-sensitive poly(ethylene glycol) inverse opal micropatterns using colloidal lithography. Materials. 2017;10(9):p. 1035. doi: 10.3390/ma10091035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subramania G., Constant K., Biswas R., Sigalas M. M., Ho K.-M. Inverse face-centered cubic thin film photonic crystals. Advanced Materials. 2001;13(6):443–446. doi: 10.1002/1521-4095(200103)13:6<443::aid-adma443>3.0.co;2-k. [DOI] [Google Scholar]
- 35.Eftekhari E., Cole I. S., Li Q. The effect of fluorophore incorporation on fluorescence enhancement in colloidal photonic crystal. Physical Chemistry Chemical Physics. 2015;18:1743–1749. doi: 10.1039/c5cp06489a. [DOI] [PubMed] [Google Scholar]
- 36.Ganesh N., Block I. D., Mathias P. C., et al. Leaky-mode assisted fluorescence extraction: application to fluorescence enhancement biosensors. Optics Express. 2008;16(26):21626–21640. doi: 10.1364/oe.16.021626. [DOI] [PubMed] [Google Scholar]
- 37.Rout D., Vijaya R. Localized surface plasmon-influenced fluorescence decay in dye-doped metallo-dielectric opals. Journal of Applied Physics. 2016;119:023108–023116. doi: 10.1063/1.4939775. [DOI] [Google Scholar]
- 38.Mohamed A. P., Pillai S. Photonic bandgap effect and dye-encapsulated cucurbituril-triggered enhanced fluorescence using monolic colloidal photonic crystals. New Journal of Chemistry. 2019;43:16264–16272. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp. 1: XRD patterns of SiO2 (a), PEGDA/SiO2 (b), and the IOPCs (c).
Data Availability Statement
All the data and supporting materials are included within the article.


