Abstract
Background: Oral anticoagulants (OACs) are effective in preventing stroke in patients with atrial fibrillation (AF), but are challenging for elderly patients because of the higher risk of bleeding complications.
Methods and Results: The ANAFIE Registry is a prospective multicenter observational study of elderly (≥75 years) Japanese AF patients. This subanalysis evaluated the current use of OACs. Of 32,713 patients (mean age 81.5 years), 30,068 (91.9%) were receiving OACs, including 8,354 (25.5%) on warfarin and 21,714 (66.4%) on direct OACs (DOACs); 2,645 (8.1%) were not receiving OACs. The most common prescribed dose was a reduced dose for all DOACs. A substantial proportion of patients receiving the reduced dose did not fulfill dose reduction criteria (underdosing): apixaban, 25.1%; rivaroxaban, 26.3%; and edoxaban, 13.7%. Some patients received a lower off-label dose rather than the reduced dose: apixaban, 5.9%; rivaroxaban, 0.3%; edoxaban, 5.3%; and dabigatran, 13.6%. In multivariate analyses, advanced age, history of hemorrhage, paroxysmal AF, and antiplatelet drug use were significantly associated with no OAC. Advanced age, persistent or permanent AF, chronic kidney disease, and concomitant antiplatelet drugs were associated with warfarin rather than DOAC use.
Conclusions: In the ANAFIE Registry, >90% of elderly Japanese AF patients received OAC therapy, mostly DOACs. Inappropriate low doses of DOACs that did not fulfill dose reduction criteria were prescribed in 20–30% of patients.
Key Words: Anticoagulants, Atrial fibrillation, Direct oral anticoagulants, Elderly, Warfarin
Atrial fibrillation (AF) is a common arrhythmic disorder in the elderly, with the incidence of AF increasing with age. The prevalence of AF in Japan is estimated to be 0.56% of the general population.1 In addition, to the prevalence of AF increasing with age, it is more common in men than in women.1,2 Based on current demographic trends, the number of people with AF in Japan is projected to increase from 716,000 to 1,034,000 in 2050.1
According to a report from the Fushimi AF registry, patients with AF in Japan are mostly elderly with a mean age of 74.2 years, although one-third are ≥80 years of age.3
AF is associated with an increased risk of stroke.4–6 Although anticoagulation is important for stroke prevention, only 60.0% of Japanese patients with AF aged 74–84 years and 41.3% of patients aged ≥85 years are treated with oral anticoagulants (OACs).5 The associated risk of bleeding complications is one of the most common reasons cited by physicians for withholding OAC therapy, especially in elderly patients.7 The vitamin K antagonist warfarin has been used commonly over the past 5 decades, but since 2011 direct OACs (DOACs) have been rapidly adopted into daily practice because they overcome some of the limitations of warfarin.4,8
The All Nippon Atrial Fibrillation In the Elderly (ANAFIE) Registry is a large-scale multicenter prospective observational study being conducted in Japanese patients aged ≥75 years with non-valvular AF (NVAF) in order to collect real-world clinical information in this patient population. This subanalysis of the ANAFIE Registry describes the current status of OAC therapy, with a particular focus on compliance with on-label dosing of DOACs, among elderly Japanese patients with NVAF included in the registry.
Methods
Study Population
Detailed methodology for the ANAFIE registry is provided elsewhere.9,10 Briefly, Japanese patients aged ≥75 years who were diagnosed with NVAF and were able to attend hospital visits, regardless of whether they received OACs and when they initiated OAC therapy, and who provided written informed consent were included in the study. The mean age of the study population was 81.5 years, and 57.2% were male. The most common type of AF was paroxysmal (42.0%), followed by persistent (30.1%) and permanent (27.9%).
This study was conducted in accordance with the Declaration of Helsinki, local regulatory requirements and ethical guidelines for clinical studies applicable in Japan. All patients provided written informed consent prior to enrollment. The study has been registered with the UMIN Clinical Trials Registry (ID: UMIN000024006).
Data Collection
Information regarding patient demographics and medical history, including AF, type of AF, date and method of diagnosis, symptoms, treatment decisions, type of OACs used, CHADS2 and CHA2DS2-VASc scores for stroke risk and HAS-BLED score for bleeding risk were collected at baseline. Each patient was followed up for at least 2 years from the time of enrollment.
Definitions of OAC Use
The status of anticoagulant use at baseline has also been reported previously10 and is briefly summarized in Figure 1. Of 32,713 patients, 30,068 (91.9%) were receiving OACs (8,354 [25.5%] on warfarin and 21,714 [66.4%] on DOACs); 2,645 (8.1%) were not receiving OACs. In the present analysis, antiplatelet drugs included aspirin, clopidogrel, ticlopidine, and prasugrel. OACs included warfarin and 4 DOACs (dabigatran, rivaroxaban, apixaban, and edoxaban). The prothrombin time (PT) and International Normalized Ratio (INR) were determined up to 6 times during the 6 months prior to the time of enrollment for patients taking warfarin.
Figure 1.
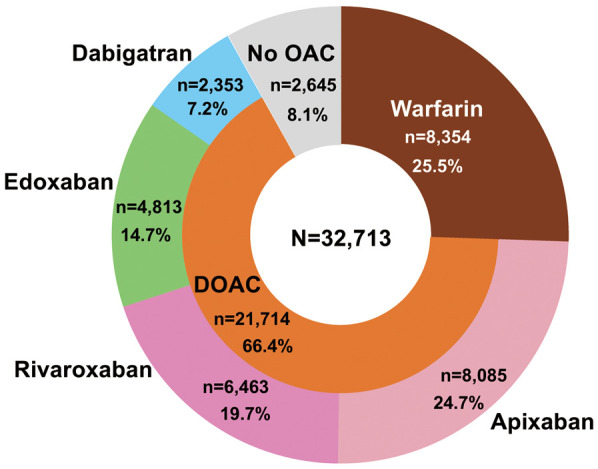
Oral anticoagulant (OAC) use by patients in the All Nippon Atrial Fibrillation In the Elderly (ANAFIE) Registry. Thirteen patients receiving non-oral anticoagulants were excluded from the entire cohort (n=32,726). DOAC, direct oral anticoagulant.
The dose selection for each DOAC was evaluated based on the manufacturer label recommendations in Japan, with standard doses defined as dabigatran, 300 mg/day; rivaroxaban, 15 mg/day; apixaban, 10 mg/day; and edoxaban, 60 mg/day. Reduced doses were defined as dabigatran, 220 mg/day; rivaroxaban, 10 mg/day; apixaban, 5 mg/day; and edoxaban, 30 mg/day. Compliance with on-label dosing was evaluated according to whether the dose was adjusted in accordance with current package insert labeling in Japan. Dabigatran does not have defined dose reduction criteria, but dose reductions are suggested if a patient meets any 1 of the following criteria: age ≥70 years; creatinine clearance 30–50 mL/min; history of major bleeding; or the use of P-glycoprotein inhibitors (verapamil). There are dose reduction criteria for rivaroxaban, apixaban, and edoxaban. Rivaroxaban dose reductions are indicated in patients with creatinine clearance 15–49 mL/min. Apixaban dose reductions are indicated if the patient meets any 2 of the following criteria: body weight ≤60 kg, age ≥80 years, or serum creatinine ≥1.5 mg/dL. Edoxaban dose reductions are indicated if a patient has creatinine clearance 15–49 mL/min, body weight ≤60 kg, or is receiving concomitant P-glycoprotein inhibitor therapy.
Administration of DOACs was regarded as “appropriate” if the dosing complied with the on-label standard or reduced-dose regimen. “Underdosing” was defined as the administration of a reduced dose of DOAC despite the standard dose criteria being fulfilled. “Overdosing” was defined as the administration of a standard dose of DOAC despite a patient fulfilling the reduced dose regimen criteria. “Off-label dosing” was defined as the administration of a dose lower than the reduced dose.
Statistical Analyses
Baseline variables are described using summary statistics, including the mean±SD, and were compared using t-tests or the Chi-squared test. Univariate and multivariate logistic regression analyses were used to estimate odds ratios (ORs) and 95% confidence intervals between patients receiving OACs and those not receiving OACs, as well as between patients receiving warfarin and those receiving DOACs. All clinically relevant covariates were included in the multivariate analysis. All statistical tests were 2-sided, with the level of significance set at 0.05. Statistical analyses were performed using SAS release 9.4 (SAS Institute, Cary, NC, USA).
Results
Patient Characteristics and OAC Therapy
Most patients who received OACs at baseline had started this therapy >1 month before enrollment in the study (98.5%, 96.9%, 95.7%, 96.3%, and 86.4% of patients taking warfarin, dabigatran, rivaroxaban, apixaban, and edoxaban, respectively).
Baseline demographics of the population have been reported in detail elsewhere.10
Table 1 compares baseline characteristics between patients receiving and those not receiving OAC therapy, as well as between those receiving warfarin and those receiving DOACs. Patients who did not receive OAC therapy were more likely to be females and to have low creatinine clearance, a history of hemorrhage, and paroxysmal AF than those on OAC therapy. Small but statistically significant differences in CHADS2, CHA2DS2-VASc, and HAS-BLED scores were found between patients receiving and those not receiving OAC therapy.
Table 1.
Baseline Patient Characteristics
| OAC use (n=30,068) |
No OAC use (n=2,645) |
P value | Warfarin (n=8,354) |
DOAC (n=21,714) |
P value | |
|---|---|---|---|---|---|---|
| Male sex | 17,348 (57.7) | 1,376 (52.0) | <0.0001 | 5,178 (62.0) | 12,170 (56.0) | <0.0001 |
| Age (years) | 81.4±4.8 | 82.3±5.5 | <0.0001 | 81.9±4.9 | 81.2±4.7 | <0.0001 |
| Age groups (years) | ||||||
| ≥75 to <80 | 12,075 (40.2) | 978 (37.0) | <0.0001 | 3,030 (36.3) | 9,045 (41.7) | <0.0001 |
| ≥80 to <85 | 10,317 (34.3) | 783 (29.6) | 2,887 (34.6) | 7,430 (34.2) | ||
| ≥85 to <90 | 5,806 (19.3) | 591 (22.3) | 1,781 (21.3) | 4,025 (18.5) | ||
| ≥90 to <95 | 1,647 (5.5) | 230 (8.7) | 567 (6.8) | 1,080 (5.0) | ||
| ≥95 to <100 | 218 (0.7) | 57 (2.2) | 86 (1.0) | 132 (0.6) | ||
| ≥100 | 5 (<0.1) | 6 (0.2) | 3 (<0.1) | 2 (<0.1) | ||
| BMI (kg/m2) | 23.4±3.6 | 22.8±3.5 | <0.0001 | 23.3±3.6 | 23.4±3.6 | 0.3070 |
| SBP (mmHg) | 127.2±17.0 | 129.7±17.4 | <0.0001 | 125.9±17.1 | 127.7±16.9 | <0.0001 |
| DBP (mmHg) | 70.7±11.6 | 70.3±11.5 | 0.1489 | 69.9±11.8 | 71.0±11.6 | <0.0001 |
| CCr (mL/min) | 48.6±22.0 | 46.0±18.3 | <0.0001 | 44.9±28.9 | 50.1±18.5 | <0.0001 |
| CHADS2 score | 2.87±1.18 | 2.63±1.17 | <0.0001 | 2.95±1.18 | 2.84±1.18 | <0.0001 |
| CHA2DS2-VASc score | 4.47±1.38 | 4.28±1.40 | <0.0001 | 4.54±1.40 | 4.44±1.37 | <0.0001 |
| HAS-BLED score | 1.85±0.85 | 1.94±0.89 | <0.0001 | 1.95±0.88 | 1.82±0.84 | <0.0001 |
| History of hemorrhage | 1,106 (3.7) | 158 (6.0) | <0.0001 | 342 (4.1) | 764 (3.5) | 0.0176 |
| AF type | ||||||
| Paroxysmal | 11,930 (39.7) | 1,814 (68.6) | <0.0001 | 2,461 (29.5) | 9,469 (43.6) | <0.0001 |
| Persistent | 9,335 (31.0) | 510 (19.3) | 2,719 (32.5) | 6,616 (30.5) | ||
| Permanent | 8,803 (29.3) | 321 (12.1) | 3,174 (38.0) | 5,629 (25.9) | ||
| History of non-pharmaceutical therapy for AF |
5,111 (17.0) | 618 (23.4) | <0.0001 | 1,356 (16.2) | 3,755 (17.3) | 0.0282 |
| Comorbidities | ||||||
| Hypertension | 22,683 (75.4) | 1,922 (72.7) | 0.0015 | 6,249 (74.8) | 16,434 (75.7) | 0.1117 |
| Diabetes | 8,196 (27.3) | 633 (23.9) | 0.0002 | 2,449 (29.3) | 5,747 (26.5) | <0.0001 |
| CKD | 6,325 (21.0) | 460 (17.4) | <0.0001 | 2,182 (26.1) | 4,143 (19.1) | <0.0001 |
| Respiratory disease | 3,850 (12.8) | 343 (13.0) | 0.8094 | 1,116 (13.4) | 2,734 (12.6) | 0.0743 |
| MI | 1,720 (5.7) | 153 (5.8) | 0.8917 | 621 (7.4) | 1,099 (5.1) | <0.0001 |
| Heart failure | 11,471 (38.2) | 788 (29.8) | <0.0001 | 3,656 (43.8) | 7,815 (36.0) | <0.0001 |
| Cerebrovascular disease | 6,897 (22.9) | 512 (19.4) | <0.0001 | 1,901 (22.8) | 4,996 (23.0) | 0.6407 |
| Peripheral arterial disease | 1,786 (5.9) | 158 (6.0) | 0.9440 | 567 (6.8) | 1,219 (5.6) | 0.0001 |
| Gastrointestinal disorder | 8,731 (29.0) | 852 (32.2) | 0.0006 | 2,363 (28.3) | 6,368 (29.3) | 0.0749 |
| Malignant tumor (primary cancer only) |
3,295 (11.0) | 293 (11.1) | 0.8510 | 857 (10.3) | 2,438 (11.2) | 0.0160 |
| Dementia | 2,293 (7.6) | 266 (10.1) | <0.0001 | 588 (7.0) | 1,705 (7.9) | 0.0173 |
| Fall within 1 year | 2,198 (7.3) | 179 (6.8) | 0.3795 | 672 (8.0) | 1,526 (7.0) | 0.0025 |
| No. concomitant drugs | 6.7±3.1 | 5.8±3.3 | <0.0001 | 7.2±3.3 | 6.5±3.1 | <0.0001 |
Unless indicated otherwise, data are presented as n (%) or mean±SD. AF, atrial fibrillation; BMI, body mass index; CCr, creatinine clearance; CKD, chronic kidney disease; DBP, diastolic blood pressure; DOAC, direct oral anticoagulant; MI, myocardial infarction; OAC, oral anticoagulant; SBP, systolic blood pressure.
Differences were noted in characteristics between patients on warfarin and those on DOACs. The proportion of male patients treated with warfarin was significantly higher than the proportion of male patients treated with DOACs. Patients in the warfarin group were older, had lower systolic and diastolic blood pressures, a lower prevalence of dementia, lower creatinine clearance, and received more concomitant drugs. Similarly, small but statistically significant differences were found in the CHADS2, CHA2DS2-VASc, and HAS-BLED scores between patients being treated with warfarin and those being treated with DOACs.
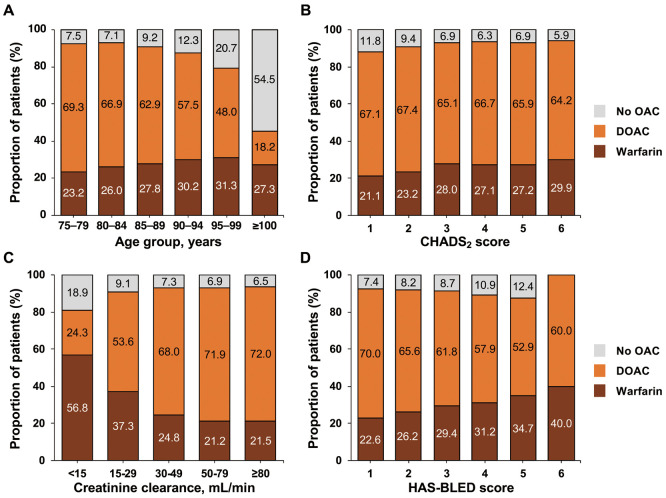
The pattern of OAC use differed by age, CHADS2 score, creatinine clearance, and HAS-BLED score (Figure 2). The use of DOACs tended to decrease with age, whereas warfarin use increased with age. Up to 90 years of age, more than 90% of patients were receiving an OAC, but this proportion was lower in older age groups (Figure 2A). DOAC was relatively constant across all categories of CHADS2 score, whereas warfarin use was higher and no OAC was lower in patients with higher CHADS2 scores (Figure 2B). DOAC use decreased and warfarin or no OAC increased with worsening creatinine clearance (Figure 2C), and 24% of patients with creatinine clearance <15 mL/min were receiving a DOAC. A similar pattern was seen with increasing HAS-BLED score (Figure 2D); namely, less DOAC use and more warfarin or no OAC with higher HAS-BLED scores. For HAS-BLED scores ≥3, more than 87% of patients were receiving an OAC.
Figure 2.
Distribution of oral anticoagulant (OAC) use according to (A) age, (B) CHADS2 score, (C) creatinine clearance, and (D) HAS-BLED score. DOAC, direct oral anticoagulant.
Table 2 shows baseline characteristics of patients receiving DOACs. Apixaban was the most frequently used DOAC in this Registry; the mean age those in the apixaban group was the highest among all DOACs and the mean creatinine clearance was the lowest. Dabigatran was more likely to be used in relatively younger patients and in those with higher creatinine clearance.
Table 2.
Baseline Characteristics of Patients on DOACs
| Apixaban (n=8,085) |
Rivaroxaban (n=6,463) |
Edoxaban (n=4,813) |
Dabigatran (n=2,353) |
|
|---|---|---|---|---|
| Male sex | 4,369 (54.0) | 3,721 (57.6) | 2,522 (52.4) | 1,558 (66.2) |
| Age (years) | 81.8±4.7 | 81.0±4.6 | 81.2±4.7 | 79.9±4.1 |
| Age ≥85 years | 2,264 (28.0) | 1,465 (22.7) | 1,181 (24.5) | 327 (13.9) |
| Body weight (kg) | 57.2±11.0 | 58.7±11.1 | 56.9±11.0 | 60.8±10.8 |
| BMI (kg/m2) | 23.2±3.5 | 23.6±3.6 | 23.1±3.5 | 23.9±3.4 |
| SBP (mmHg) | 127±17 | 128±16 | 128±17 | 128±16 |
| DBP (mmHg) | 70±12 | 71±11 | 72±12 | 71±12 |
| Dementia | 640 (7.9) | 541 (8.4) | 416 (8.6) | 108 (4.6) |
| History of cerebrovascular disease | 1,989 (24.6) | 1,448 (22.4) | 1,015 (21.1) | 544 (23.1) |
| Concomitant drugs used | 6.9±3.2 | 6.4±3.0 | 6.2±3.1 | 6.4±2.9 |
| CCr (mL/min) | 47.2±17.4 | 51.7±20.6 | 49.7±16.7 | 56.5±17.8 |
| CHADS2 score | 2.9±1.2 | 2.8±1.2 | 2.8±1.2 | 2.8±1.2 |
| CHA2DS2-VASc score | 4.5±1.4 | 4.4±1.4 | 4.4±1.4 | 4.3±1.3 |
| HAS-BLED score | 1.9±0.9 | 1.8±0.8 | 1.8±0.8 | 1.8±0.8 |
Unless indicated otherwise, data are presented as n (%) or mean±SD. Abbreviations as in Table 1.
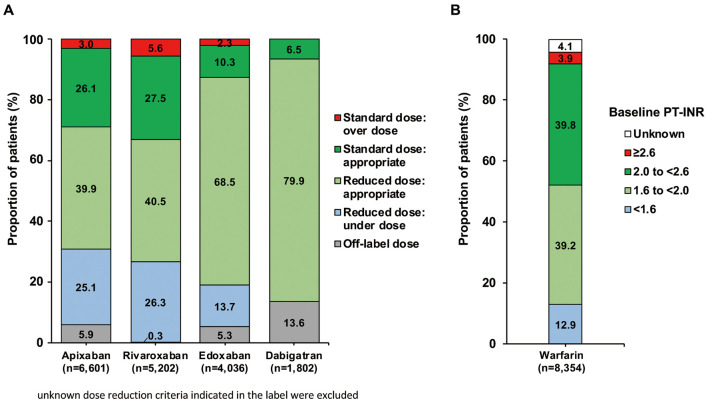
The most common daily dose was a reduced dose for all DOACs; 5 mg apixaban (64.1%), 10 mg rivaroxaban (66.7%), 30 mg edoxaban (82.2%), and 220 mg dabigatran (78.9%; data are given in Koretsune et al10). A substantial proportion of patients who were receiving the reduced dose did not fulfill the dose reduction criteria indicated in their packaging insert (underdosing): apixaban, 25.1%; rivaroxaban, 26.3%; and edoxaban, 13.7%. Some patients received an off-label dose: apixaban, 5.9%; rivaroxaban, 0.3%; edoxaban, 5.3%; and dabigatran, 13.6% (Figure 3A).
Figure 3.
(A) Compliance with on-label dosing for direct oral anticoagulants. (B) Distribution of baseline prothrombin time (PT) and International Normalized Ratio (INR) in patients receiving warfarin.
In the warfarin group, most patients had a PT-INR value ranging from 1.6 to <2.0 (39.2%) or from 2.0 to <2.6 (39.8%), with 12.9% of patients having a PT-INR <1.6 and 3.9% having a PT-INR ≥2.6 (Figure 3B).
Multivariate Analysis
OAC vs. No OAC Therapy Results of the univariate and multivariate analyses of factors associated with OAC vs. no OAC therapy are summarized in Table 3. In the multivariate analysis, OAC therapy was significantly associated with patients having persistent or permanent AF (OR 3.21), hypertension (OR 1.27), diabetes (OR 1.18), heart failure (OR 1.26), and cerebrovascular disease (OR 1.37). In contrast, no OAC therapy was significantly associated with patients being female (OR 0.82), aged ≥85 years (OR 0.63), having a history of major hemorrhage (OR 0.48), and antiplatelet drug use (OR 0.34).
Table 3.
Univariate and Multivariate Analyses of Factors Associated With OAC Use vs. No OAC Use and Warfarin Use vs. DOAC Use
| OAC vs. no OAC | Warfarin vs. DOAC | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysisA | Univariate analysis | Multivariate analysisA | |||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Female sex | 0.80 (0.73–0.86) |
<0.0001 | 0.82 (0.75–0.89) |
<0.0001 | 0.78 (0.74–0.82) |
<0.0001 | 0.75 (0.70–0.79) |
<0.0001 |
| Age ≥85 years | 0.68 (0.63–0.74) |
<0.0001 | 0.63 (0.57–0.68) |
<0.0001 | 1.30 (1.22–1.37) |
<0.0001 | 1.24 (1.17–1.31) |
<0.0001 |
| History of major hemorrhage | 0.60 (0.51–0.71) |
<0.0001 | 0.48 (0.40–0.58) |
<0.0001 | 1.17 (1.03–1.33) |
0.0177 | 1.07 (0.94–1.23) |
0.3103 |
| Persistent or permanent AF | 3.32 (3.05–3.61) |
<0.0001 | 3.21 (2.94–3.50) |
<0.0001 | 1.85 (1.75–1.96) |
<0.0001 | 1.74 (1.65–1.84) |
<0.0001 |
| Hypertension | 1.16 (1.06–1.26) |
0.0015 | 1.27 (1.16–1.39) |
<0.0001 | 0.95 (0.90–1.01) |
0.1099 | 0.92 (0.87–0.98) |
0.0097 |
| Diabetes | 1.19 (1.09–1.31) |
0.0002 | 1.18 (1.07–1.30) |
<0.0001 | 1.15 (1.09–1.22) |
<0.0001 | 1.06 (1.00–1.12) |
0.0664 |
| CKD | 1.11 (1.00–1.22) |
0.0421 | 1.09 (0.98–1.20) |
0.1144 | 1.67 (1.58–1.77) |
<0.0001 | 1.52 (1.43–1.61) |
<0.0001 |
| Heart failure | 1.45 (1.33–1.59) |
<0.0001 | 1.26 (1.15–1.38) |
<0.0001 | 1.38 (1.32–1.46) |
<0.0001 | 1.17 (1.11–1.24) |
<0.0001 |
| Cerebrovascular disease | 1.24 (1.12–1.37) |
<0.0001 | 1.37 (1.23–1.52) |
<0.0001 | 0.99 (0.93–1.05) |
0.6413 | 0.89 (0.84–0.95) |
0.0002 |
| Antiplatelet use | 0.38 (0.35–0.42) |
<0.0001 | 0.34 (0.31–0.38) |
<0.0001 | 1.52 (1.43–1.63) |
<0.0001 | 1.48 (1.39–1.59) |
<0.0001 |
AFactors included in the analysis were sex, age, body mass index, smoking, alcohol consumption, major surgery, history of major bleeding, drug allergy, AF type, non-pharmacotherapy for AF, hypertension, diabetes, dyslipidemia, hyperuricemia, kidney disease, severe liver disorder, respiratory disorder, cardiac disease, cerebral disease, other vascular disease, thyroid disease, digestive disease, active cancer, dementia, other disease, fall within past year, antiarrhythmic agent, antiplatelet agent, anticancer agent, P-glycoprotein inhibitor, polypharmacy, and creatinine clearance. CI, confidence interval; OR, odds ratio. Other abbreviations as in Table 1.
Warfarin vs. DOAC Therapy Results of the univariate and multivariate analysis of factors associated with warfarin vs. DOAC therapy are summarized in Table 3. The multivariate analysis indicated that warfarin therapy was significantly associated with patient age ≥85 years (OR 1.24), having persistent or permanent AF (OR 1.74), chronic kidney disease (CKD; OR 1.52), heart failure (OR 1.17), and antiplatelet drug use (OR 1.48). Conversely, DOAC therapy was significantly associated with female sex (OR 0.75), having hypertension (OR 0.92), and cerebrovascular disease (OR 0.89).
Discussion
The ANAFIE Registry is a prospective multicenter observational registry study that has enrolled 32,726 patients with NVAF. In this study population, which had a mean age of 81.5 years, most patients (91.7%) were receiving OAC therapy at baseline, including warfarin in 25.5% of patients and DOACs in 66.4%. The present subanalysis of the ANAFIE Registry illustrated the current status of OAC therapy and revealed factors associated with OAC or DOAC use, and demonstrated that substantial numbers of patients received off-label underdosing with DOACs.
OAC Use or Non-Use in Japanese Elderly AF Patients
The prevalence of OAC prescriptions in the ANAFIE Registry was remarkably high, and indeed higher than that reported in the older age groups of the Fushimi AF registry,5 in which only 41.3% of patients aged ≥85 years were receiving OACs,5 compared with 89.7% of patients aged ≥85 years in the ANAFIE Registry. There may be a number of reasons for this difference in OAC use between the ANAFIE and Fushimi AF registries. First, it is possible that the use of OACs in very elderly patients in Japan has increased between when the Fushimi AF Registry (2011–2014) and ANAFIE Registry (2016–2018) data were collected. However, the time between data collections would seem too short to see such a marked difference. Second, the difference in OAC use may reflect differences in the prescribing practices of different physicians, because 84% of the centers participating in the Fushimi AF Registry were primary care practices,5 whereas in the ANAFIE Registry many patients were prescribed OACs by specialist physicians.
In the multivariate analysis, factors significantly associated with the use of OACs vs. no OAC therapy were persistent or permanent AF, hypertension, diabetes, heart failure, or cerebrovascular disease, whereas factors significantly associated with no OAC therapy were female sex, advanced age (≥85 years), a history of major hemorrhage, and antiplatelet drug use. These findings are consistent with those reported in previous studies. In the Euro Heart Survey, valvular heart disease, persistent or permanent AF, and diabetes were significantly associated with OAC prescription.11 Similarly, a study from the US reported that a diagnosis of persistent or permanent AF and a history of stroke or transient ischemic attack or systemic embolism were positive predictive factors for warfarin prescription, whereas age >80 years and perceived bleeding risk were negative predictive factors.12 The J-RHYTHM Registry of 7,937 Japanese AF patients demonstrated that patients on warfarin were significantly older and more commonly had hypertension, non-paroxysmal AF, cardiomyopathy, valvular disease, diabetes, and a history of stroke or transient ischemic attack compared with patients not receiving warfarin.13
Warfarin or DOAC Use in Japanese Elderly AF Patients
In the ANAFIE Registry cohort, clinical characteristics differed between DOAC and warfarin users. Similar findings were reported for the international GARFIELD-AF Registry, a prospective non-interventional registry of patients with newly diagnosed AF that is currently being conducted in 35 countries.14 In the GARFIELD-AF Registry, DOAC prescribing seemed to be favored in lower-risk groups (i.e., patients with paroxysmal AF) and warfarin was preferentially used in patients with permanent AF, moderate to severe kidney disease, heart failure, vascular disease, or diabetes, and with concomitant antiplatelet drug use.14 The Danish nationwide cohort of 18,611 AF patients initiating OAC showed that older age, female sex, and prior stroke were associated with DOAC use, whereas CKD, myocardial infarction, and heart failure were associated with warfarin use.15 The higher proportion of warfarin users among patients with CKD may be due to the fact that dose adjustment and contraindications for DOACs are determined by a patient’s creatinine clearance. The lower prevalence of DOAC use in patients with concomitant antiplatelet therapy may be associated with a lack of evidence for the efficacy and safety of DOACs in these patients, in whom the use of warfarin with lower PT-INR intensity is preferred.
Underdosing of DOACs in Japanese Elderly AF Patients
In the ANAFIE Registry, many patients were prescribed underdose DOAC therapy (i.e., they received a reduced DOAC dose without fulfilling the dose reduction criteria). The frequency of underdosing, including off-label underdosing of DOACs (30.0% for apixaban, 29.1% for rivaroxaban, 19.6% for edoxaban, and 13.6% for dabigatran), was similar to that reported in other Japanese AF registries.16,17 In the SAKURA AF Registry, approximately 20–28% of patients were inappropriately underdosed with DOACs, and in the Fushimi AF Registry, underdosing was seen in 21% of patients on rivaroxaban and 26% of patients on apixaban.16,17 The ANAFIE Registry was limited to patients aged ≥75 years, many of whom would be candidates for appropriate (on-label) reduced doses of DOACs, but underdosing of DOACs remained prevalent. The high proportion of Japanese AF patients receiving underdoses of DOAC therapy probably reflects a cautious approach to dosing among physicians in this country, particularly in the elderly. An analysis of SAKURA AF Registry data found that age ≥75 years and impaired renal function were significant predictors of underdosing DOAC therapy.16
The main problem of prescribing underdosing DOAC therapy is that it may be associated with poor outcomes. In the US, underdosing DOAC therapy in the ORBIT-AF II Registry was associated with increased hospitalization for cardiovascular events.18 Another study from a large US administrative database also showed a higher risk of stroke in patients receiving underdosing apixaban, but not in those receiving underdosing dabigatran or rivaroxaban.19 In contrast, the SAKURA AF Registry showed an apparently conflicting result, in which underdosing DOAC may provide potential benefits in Japanese AF patients; stroke or systemic embolism were equivalent between the on-label dosing and underdosing groups, but major bleeding events tended to be lower in the underdosing group.20 Recent research using the Korean National Health Insurance Service database also demonstrated that 31% of DOAC-treated patients were underdosed, and that underdosing was not associated with worse clinical outcomes compared with on-label DOAC dosing, after adjusting for confounding factors.21 Although these results may appeal to many physicians because underdosing of DOACs is an easy and convenient way to avoid bleeding, it should be interpreted with caution, because this was an observational study and the baseline clinical characteristics of the 2 groups (on-label dosing vs. underdosing) were different.20 Outcome data from the present ANAFIE Registry subanalysis provide additional information regarding this clinically important issue.
Study Limitations
The present study has several limitations. First, this is an observational study and provides only associative not causative evidence. Second, there may have been selection bias because the enrolled patients did not constitute a consecutive patient series. Physicians may have chosen patients who were otherwise relatively healthy and could visit hospitals or clinics regularly in order to complete the 2-year observation period. Physicians may not have enrolled patients with a very high bleeding risk who were not suitable for long-term OAC therapy. Third, the study consisted of patients with relatively well-managed AF, because they were enrolled from medical institutions with physicians who were familiar with OAC therapy. The data that more than 90% of elderly patients received OAC therapy could be explained by these biases. Finally, this study only included Japanese elderly AF patients, and so the findings may not be generalizable to younger AF patients or patients in other countries or with other ethnicities.
Conclusions
In the ANAFIE Registry, more than 90% of elderly Japanese patients with NVAF were receiving OAC therapy, mostly DOACs. Advanced age, a history of hemorrhage, paroxysmal AF, and antiplatelet drug use were significantly associated with the non-use of OACs. Advanced age, persistent or permanent AF, CKD, and concomitant antiplatelet drugs were associated with warfarin use rather than DOAC use. Inappropriate low doses of DOACs that did not fulfill dose reduction criteria were prescribed in 20–30% of patients receiving DOAC therapy.
Sources of Funding
This research was sponsored by Daiichi Sankyo.
Disclosures
M.A. has received research funding from Bayer and Daiichi Sankyo; and remuneration from Bristol-Myers Squibb, Nippon Boehringer Ingelheim, Bayer, and Daiichi Sankyo. W.S. has received research funding from Bristol-Myers Squibb, Daiichi Sankyo, and Nippon Boehringer Ingelheim; remuneration from Daiichi Sankyo; and patent royalties/licensing fees from Daiichi Sankyo, Pfizer Japan, Bristol-Myers Squibb, Bayer, and Nippon Boehringer Ingelheim. H.A. has received remuneration from Daiichi Sankyo. T.I. has received research funding from Daiichi Sankyo, Medtronic, and Japan Lifeline; and remuneration from Daiichi Sankyo, Bayer, Nippon Boehringer Ingelheim, and Bristol-Myers Squibb. H.I. has received remuneration from Daiichi Sankyo, Bayer, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim. K.O. has received remuneration from Nippon Boehringer Ingelheim, Daiichi Sankyo, Johnson & Johnson, and Medtronic. Y.K. has received remuneration from Daiichi Sankyo. H.T. has received research funding from Nippon Boehringer Ingelheim, Daiichi Sankyo, and IQVIA Services Japan; remuneration from Daiichi Sankyo, Nippon Boehringer Ingelheim, Bayer, Pfizer, and Bristol-Myers Squibb; donations from Daiichi Sankyo; and consultancy fees from Nippon Boehringer Ingelheim and Bayer. K.T. has received remuneration from Daiichi Sankyo, Bayer, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim. A.H. has participated in courses sponsored by Boston Scientific Japan, Fukuda Denshi, St. Jude Medical, Medtronic, and Japan Lifeline; has received remuneration from Bayer, Daiichi Sankyo, Bristol-Myers Squibb and Nippon Boehringer Ingelheim; and is a member of Circulation Reports’ Editorial Team. M.Y. has received research funding from Nippon Boehringer Ingelheim and remuneration from Nippon Boehringer Ingelheim, Daiichi Sankyo, Bayer, and Bristol-Myers Squibb. T. Yamashita has received research funding from Bristol-Myers Squibb, Bayer, and Daiichi Sankyo; manuscript fees from Daiichi Sankyo and Bristol-Myers Squibb; donations from Daiichi Sankyo; and remuneration from Daiichi Sankyo, Bayer, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim. T. Yamaguchi has received manuscript fees from Bristol-Myers Squibb and remuneration from Daiichi Sankyo. S.T. has received research funding from Nippon Boehringer Ingelheim and remuneration from Daiichi Sankyo. T.K., J.K., and A.T. are employees of Daiichi Sankyo.
IRB Information
This study was approved by the Ethics Committee of The Cardiovascular Institute (Reference no. 299) and has been registered with the UMIN Clinical Trials Registry (ID: UMIN000024006).
Author Contributions
M.A., W.S., H.A., T.I., H.I., K.O., Y.K., H.T., K.T., A.H., M.S., T. Yamashita, T. Yamaguchi, and S.T. designed and conducted the study and interpreted the data analysis; S.T. carried out statistical analyses; T.K., J.K., and A.T. supported the study and reviewed the manuscript; all authors approved the final version.
Acknowledgments
The authors thank all the centers that participated in the Registry and all the patients who gave their consent to participate. The authors also thank Georgii Filatov and Sarah Greig, PhD, of inScience Communications, Springer Healthcare, who wrote the outline and first draft of this manuscript, respectively. This medical writing assistance was funded by Daiichi Sankyo.
Data Availability
The study protocol will be made available. The deidentified participant data used in this study will be shared with researchers who participated in the study and provide a methodologically sound proposal for 36 months after article publication. The proposal may be reviewed by a committee led by Daiichi Sankyo. For any purpose, requests must be in writing and should be sent to yamt-tky@umin.ac.jp. To gain access, those requesting the data will need to sign a data access agreement.
References
- 1. Inoue H, Fujiki A, Origasa H, Ogawa S, Okumura K, Kubota I, et al.. Prevalence of atrial fibrillation in the general population of Japan: An analysis based on periodic health examination. Int J Cardiol 2009; 137: 102–107. [DOI] [PubMed] [Google Scholar]
- 2. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al.. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285: 2370–2375. [DOI] [PubMed] [Google Scholar]
- 3. Akao M, Chun YH, Wada H, Esato M, Hashimoto T, Abe M, et al.. Current status of clinical background of patients with atrial fibrillation in a community-based survey: The Fushimi AF Registry. J Cardiol 2013; 61: 260–266. [DOI] [PubMed] [Google Scholar]
- 4. Wolf PA, Abbott RD, Kannel WB.. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991; 22: 983–988. [DOI] [PubMed] [Google Scholar]
- 5. Yamashita Y, Hamatani Y, Esato M, Chun YH, Tsuji H, Wada H, et al.. Clinical characteristics and outcomes in extreme elderly (age ≥85 years) Japanese patients with atrial fibrillation: The Fushimi AF Registry. Chest 2016; 149: 401–412. [DOI] [PubMed] [Google Scholar]
- 6. Hart RG, Pearce LA, Rothbart RM, McAnulty JH, Asinger RW, Halperin JL, et al.. Stroke with intermittent atrial fibrillation: Incidence and predictors during aspirin therapy. Am J Coll Cardiol 2000; 35: 183–187. [DOI] [PubMed] [Google Scholar]
- 7. Hylek EM, D’Antonio J, Evans-Molina C, Shea C, Henault LE, Regan S.. Translating the results of randomized trials into clinical practice: The challenge of warfarin candidacy among hospitalized elderly patients with atrial fibrillation. Stroke 2006; 37: 1075–1080. [DOI] [PubMed] [Google Scholar]
- 8. Park J, Lee SR, Choi EK, Kwon S, Jung JH, Han KD, et al.. Effectiveness and safety of direct oral anticoagulant for secondary prevention in Asians with atrial fibrillation. J Clin Med 2019; 8: 2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Inoue H, Yamashita T, Akao M, Atarashi H, Ikeda T, Okumura K, et al.. Prospective observational study in elderly patients with non-valvular atrial fibrillation: Rationale and design of the All Nippon AF In the Elderly (ANAFIE) Registry. J Cardiol 2018; 72: 300–306. [DOI] [PubMed] [Google Scholar]
- 10. Koretsune Y, Yamashita T, Akao M, Atarashi H, Ikeda T, Okumura K, et al.. Baseline demographics and clinical characteristics in the All Nippon AF in the Elderly (ANAFIE) Registry. Circ J 2019; 83: 1538–1545. [DOI] [PubMed] [Google Scholar]
- 11. Nieuwlaat R, Capucci A, Lip GY, Olsson SB, Prins MH, Nieman FH, et al.. Antithrombotic treatment in real-life atrial fibrillation patients: A report from the Euro Heart Survey on Atrial Fibrillation. Eur Heart J 2006; 27: 3018–3026. [DOI] [PubMed] [Google Scholar]
- 12. Waldo AL, Becker RC, Tapson VF, Colgan KJ.. Hospitalized patients with atrial fibrillation and a high risk of stroke are not being provided with adequate anticoagulation. Am J Coll Cardiol 2005; 46: 1729–1736. [DOI] [PubMed] [Google Scholar]
- 13. Atarashi H, Inoue H, Okumura K, Yamashita T, Kumagai N, Origasa H.. Present status of anticoagulation treatment in Japanese patients with atrial fibrillation: A report from the J-RHYTHM Registry. Circ J 2011; 75: 1328–1333. [DOI] [PubMed] [Google Scholar]
- 14. Haas S, Camm AJ, Bassand JP, Angchaisuksiri P, Cools F, Corbalan R, et al.. Predictors of NOAC versus VKA use for stroke prevention in patients with newly diagnosed atrial fibrillation: Results from GARFIELD-AF. Am Heart J 2019; 213: 35–46. [DOI] [PubMed] [Google Scholar]
- 15. Olesen JB, Sorensen R, Hansen ML, Lamberts M, Weeke P, Mikkelsen AP, et al.. Non-vitamin K antagonist oral anticoagulation agents in anticoagulant naive atrial fibrillation patients: Danish nationwide descriptive data 2011–2013. Europace 2015; 17: 187–193. [DOI] [PubMed] [Google Scholar]
- 16. Okumura Y, Yokoyama K, Matsumoto N, Tachibana E, Kuronuma K, Oiwa K, et al.. Current use of direct oral anticoagulants for atrial fibrillation in Japan: Findings from the SAKURA AF Registry. J Arrhythm 2017; 33: 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamashita Y, Uozumi R, Hamatani Y, Esato M, Chun YH, Tsuji H, et al.. Current status and outcomes of direct oral anticoagulant use in real-world atrial fibrillation patients: Fushimi AF Registry. Circ J 2017; 81: 1278–1285. [DOI] [PubMed] [Google Scholar]
- 18. Steinberg BA, Shrader P, Thomas L, Ansell J, Fonarow GC, Gersh BJ, et al.. Off-label dosing of non-vitamin K antagonist oral anticoagulants and adverse outcomes: The ORBIT-AF II registry. Am J Coll Cardiol 2016; 68: 2597–2604. [DOI] [PubMed] [Google Scholar]
- 19. Yao X, Shah ND, Sangaralingham LR, Gersh BJ, Noseworthy PA.. Non-vitamin K antagonist oral anticoagulant dosing in patients with atrial fibrillation and renal dysfunction. Am J Coll Cardiol 2017; 69: 2779–2790. [DOI] [PubMed] [Google Scholar]
- 20. Murata N, Okumura Y, Yokoyama K, Matsumoto N, Tachibana E, Kuronuma K, et al.. Clinical outcomes of off-label dosing of direct oral anticoagulant therapy among Japanese patients with atrial fibrillation identified from the SAKURA AF Registry. Circ J 2019; 83: 727–735. [DOI] [PubMed] [Google Scholar]
- 21. Yu HT, Yang PS, Jang E, Kim TH, Uhm JS, Kim JY, et al.. Label adherence of direct oral anticoagulants dosing and clinical outcomes in patients with atrial fibrillation. J Am Heart Assoc 2020; 9: e014177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study protocol will be made available. The deidentified participant data used in this study will be shared with researchers who participated in the study and provide a methodologically sound proposal for 36 months after article publication. The proposal may be reviewed by a committee led by Daiichi Sankyo. For any purpose, requests must be in writing and should be sent to yamt-tky@umin.ac.jp. To gain access, those requesting the data will need to sign a data access agreement.