Abstract
Background: There is scant clinical data of electrolyte analyses in the pleural fluid under heart failure (HF) pathophysiology.
Methods and Results: This study retrospectively analyzed data from 17 consecutive patients who presented with pleural effusion and underwent thoracentesis. A diagnosis of worsening HF was established by clinical criteria (presentation, echocardiography, serum B-type natriuretic peptide, and response to therapy). Samples of non-heparinized pleural fluid and peripheral venous blood, obtained within 2 h of each other, were subjected to biochemical analysis. The source of pleural effusion was determined as transudate or exudate according to Light’s criteria. Fifteen patients (53% men; mean [±SD] age 85±11 years) had HF-associated pleural effusion, 10 of whom had transudative effusion and 5 who had exudative effusion (fulfilling only 1 [n=4] or both [n=1] lactate dehydrogenase criteria). The effusion-serum gradient (calculated by subtracting the serum electrolyte concentration from the effusion electrolyte concentration) was significantly higher for chloride (mean [±SD] 7.4±2.6 mEq/L; range 4–14 mEq/L) than sodium (0.9±1.4 mEq/L; ranging from −1 to 4 mEq/L) and potassium (−0.1±0.3 mEq/L; ranging from −0.8 to 0.2 mEq/L; P<0.001 for each).
Conclusions: In HF-associated pleural effusion, the chloride concentration is higher in the pleural effusion than the serum, indicating that chloride may have an important role in the formation and retention of body fluid in the pleural space.
Key Words: Body fluid, Chloride, Electrolyte, Heart failure, Pleural effusion
In heart failure (HF) pathophysiology, regulation of the body fluid volume is a complex process involving the interaction of a variety of afferent (sensory) and neurohumoral efferent (effector) mechanisms.1–3 Until recently, most studies focused on body fluid dynamics in HF as controlled by sodium (Na), potassium, and water balance in the body.4–7 However, a unifying hypothesis for HF pathophysiology based on serum biochemical solute(s) has not yet been fully developed.
Recent studies indicate that changes in vascular8–10 and red blood cell11 volumes are independently associated with serum chloride (Cl), but not serum Na, concentrations during worsening HF and its recovery. Consistent with the established central role of Cl in the renin-angiotensin-aldosterone system,1–3 a unifying hypothesis for HF pathophysiology, namely the “chloride theory”, has been proposed, whereby changes in serum Cl concentrations are the primary determinants of changes in plasma volume, and presumably the distribution of fluid in each body compartment12 (i.e., intracellular, intravascular, and interstitial compartments13,14).
Transudative pleural and pericardial effusions are not uncommon in patients with worsening HF.15,16 Cl– may be involved in the accumulation of body fluid in the pleural space, but clinical data regarding pleural fluid electrolytes under HF pathophysiology are scarce. Vascular and pleural spaces are dynamic interfaces for body fluid distribution. Thus, the present study tested the hypothesis that there are differential Cl– concentrations between the pleural fluid and blood serum in patients with worsening HF.
Methods
Study Patients
The present study was a retrospective single-center observational study evaluating the nature of HF-related pleural effusion. Seventeen patients who presented with pleural effusion at the Cardiology Section of Nishida Hospital and underwent thoracentesis from May 2017 to December 2017 were studied retrospectively.
Evaluation of HF Status and Thoracentesis
Diagnosis of worsening HF was established by standard clinical criteria of presentation, echocardiography, serum B-type natriuretic peptide (BNP), and response to HF therapy.17 Additional routine tests included thoracic ultrasound to evaluate the presence of pleural effusion18 and monitoring changes in body weight during follow-up (HBF-352-W; Omron Healthcare, Kyoto, Japan).17,19 Worsening HF was treated by conventional therapy with a combination of loop diuretics, aldosterone blockade, thiazide diuretics, an oral vasopressin antagonist, acetazolamide, and/or inotropic drugs administered via oral and/or intravenous routes in the hospital or outpatient clinic. The response of worsening HF to treatment and the return of the clinical presentation to stable HF status were determined on the basis of follow-up examinations.
Etiologies of pleural effusion other than worsening HF were determined according to well-established clinical criteria using appropriate combinations of laboratory tests on blood and pleural fluid samples,20–26 as well as chest X-ray computed tomography (CT) to search for inflammatory and tumorous lesions.
Under thoracic sonographic guidance,18 diagnostic thoracentesis using a standard intramuscular 21-gauge needle was performed in seated patients. After injection of a local anesthetic, a sample of approximately 20 mL pleural fluid was obtained.
Laboratory Tests of Peripheral Blood and Pleural Fluid
Biochemical measurements were performed on samples of non-heparinized pleural fluid and peripheral venous blood obtained within 2 h of each other. Both samples were immediately centrifuged at 3,500 r.p.m. for 5 min at 20℃. Total protein, albumin, lactate dehydrogenase (LDH), and electrolyte concentrations in the supernatant were tested within 48 h using an automatic analyzer (Hitachi 7180 type; Hitachi, Tokyo, Japan); total protein and albumin were measured using the Biuret method, LDH was measured using an enzyme method, and electrolytes were measured using ion-selective electrodes. Other main laboratory tests included measurements of adenosine deaminase activity (normal range 10–30 U/L) using an enzyme method21 (Serotec, Sapporo, Japan) and real-time polymerase chain reaction (PCR) detection of Mycobacterium tuberculosis (Roche Diagnostics, Basel, Switzerland) in the pleural fluid, as well as determination of serum BNP concentrations (normal range <6 pg/mL) using a chemiluminescent immunoassay (Abbott JAPAN, Tokyo, Japan). In this study, the upper normal limit of serum LDH was 245 IU.
The source of the pleural effusions (i.e., transudate or exudate) was determined using either the traditional Light’s criteria20 or other proposed criteria for the serum-effusion albumin gradient.22,25 Pleural effusion was classified as transudative by Light’s criteria20 when none of the following criteria was met: pleural to serum protein ratio >0.5, pleural fluid LDH >200 IU, and pleural fluid-to-serum LDH ratio >0.6. Pleural effusion was classified as transudative by the serum-effusion albumin gradient22 if this value was >1.2 g/dL.
The effusion-serum electrolyte gradient was calculated by subtracting the serum electrolyte concentration from the pleural fluid electrolyte concentration.
Statistical Analysis
All statistical analyses were performed using GraphPad Prism 4 (GraphPad, San Diego, CA, USA). Continuous data are expressed as the mean±SD, whereas categorical data are expressed as percentages. The significance of differences in intragroup continuous data was analyzed using paired Student’s t-tests. The significance of differences between groups was analyzed using 2-way analysis of variance (ANOVA) with Tukey’s post hoc test. In all cases, 2-tailed P<0.05 was considered significant.
Ethical Considerations
The Research Ethics Committee of Nishida Hospital (Chairman: Dr K. Okamura) approved the study protocol (Reference no. 201803-03). Given that the study was a retrospective study, the requirement for written informed consent was waived, but an opt-out method was always taken into consideration during the study period. The present study was performed in accordance with the Declaration of Helsinki.
Results
Of the 17 study patients, 2 were excluded from the present analysis because the etiology of pleural effusion was not due to HF, but to M. tuberculosis infection in 1 patient (left ventricular ejection fraction [LVEF] 72%, serum BNP 32 pg/mL) and severe nutritional hypoalbuminemia in the other patient (LVEF 65%, serum BNP 67 pg/mL). The remaining 15 patients (53% men; mean age 85±11 years) were determined to have HF-related pleural effusion and were included in the present analysis (Table 1). The primary causes of worsening HF varied, and atrial fibrillation was observed in 10 patients. Serum BNP concentrations were definitely elevated (≥500 pg/mL) in 10 patients and moderately to mildly elevated (100–500 pg/mL) in 5.
Table 1.
Clinical Characteristics of the Study Patients (n=15)
| Age (years) | |
| Mean±SD | 85±11 |
| Range | 62–99 |
| Male sex | 8 (53) |
| Primary cause of HF | |
| Ischemic or dilated cardiomyopathy | 5 (33) |
| Valvular disease | 4 (26) |
| Hypertension | 3 (20) |
| Hypertrophic cardiomyopathy | 1 (7) |
| Arrhythmia | 1 (7) |
| Congenital heart disease | 1 (7) |
| LVEF (%) | 51.4±17.8 |
| LVEF >50% | 8 (53) |
| Atrial fibrillation | 10 (67) |
| Serum creatinine (mg/dL) | |
| Mean±SD | 1.27±0.55 |
| Range | 0.41–2.32 |
| Serum albumin (g/dL) | |
| Mean±SD | 3.41±0.54 |
| Range | 2.4–4.7 |
| Cardiovascular medication at baseline | |
| Data not available | 5 (33) |
| Using cardiovascular medication | 10 (67) |
| Loop diuretics | 8 (53) |
| Thiazide diuretics | 1 (7) |
| MRA | 7 (47) |
| ACEI/ARB | 3 (20) |
| β-blockers | 3 (20) |
| Calcium antagonists | 3 (20) |
| Vasopressin antagonist | 4 (26) |
| HF-related physical findings | |
| Bilateral leg edema around or above the ankle | 15 (100) |
| Bilateral pulmonary rales beyond the basal lung | 9 (60) |
| B-type natriuretic peptide (pg/mL) | |
| ≥500 | 10 (67) |
| 300–500 | 2 (13) |
| 200–300 | 2 (13) |
| 100–200 | 1 (7) |
| Moderate elevation (≥5 mg/dL) of CRP | 3 (20) |
Unless specified otherwise, data presented as n (%). ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CRP, C-reactive protein; HF, heart failure; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist.
As indicated in Table 2, worsening HF was determined on the basis of leg edema at initial presentation in all 15 patients and ≥1 of the following: definitely higher (≥500 pg/mL) serum BNP concentrations at initial presentation of worsening HF (n=10), and resolution of lower leg edema (n=4), ultrasound pleural effusion (n=4), and/or weight reduction ≥1.4 kg (n=4) after diuretic therapy in patients with moderately to mildly elevated (100–500 pg/mL) serum BNP concentrations (n=5). Thoracic X-ray CT (n=10) revealed no inflammation or malignancy. Worsening HF after decongestion therapy was resolved in 11 HF patients, but 2 HF patients died from advanced HF (Patients 8 and 9), and another 2 patients were transferred to other hospitals after completion of the initial evaluation (Patients 1 and 12).
Table 2.
Clinical Picture at Admission and After Decongestive Therapy in Patients With HF-Associated Pleural Effusion
| Patient no. |
Age (years)/ sex |
Primary diagnosis of HF |
ECG | EF (%) | Before decongestion therapy | After decongestion therapy | X-ray CT | Clinical course | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rales | Leg edema | BW (kg) | BNP (pg/mL) | Rales | Leg edema | ΔBW (kg) | BNP (pg/mL) | US-PLE | |||||||
| 1 | 91/F | Systolic HF | Af | 24 | Yes | Yes | NA | 598 | NA | NA | NA | NA | NA | NA | Transfer |
| 2 | 91/F | Systolic HF | Af | 47 | Yes | Yes | 33.6 | 897 | No | No | −1.4 | 294 | Absent | No | Improved |
| 3 | 85/F | Systolic HF | Af | 49 | No | Yes | 69.3 | 771 | No | No | −3.9 | 146 | Absent | No | Improved |
| 4 | 86/F | AR | SR | 53 | No | Yes | 36 | 589 | No | No | −1.4 | 387 | Reduced | NA | Improved |
| 5 | 91/M | HHD | SR | 80 | Yes | Yes | 58.4 | 471 | No | No | −9.3 | 224 | Absent | No | Improved |
| 6 | 91/M | HHD | SR | 64 | Yes | Yes | 57.9 | 806 | Yes | No | −5.6 | 383 | Absent | No | Improved |
| 7 | 83/M | Arrhythmia | Af | 60 | Yes | Yes | NA | 576 | No | No | NA | 56 | Reduced | No | Improved |
| 8 | 92/M | HCM | Af | 48 | No | Yes | NA | 958 | NA | NA | NA | NA | NA | No | Died |
| 9 | 99/F | AS | Af | 40 | Yes | Yes | 38.2 | 2,864 | NA | NA | NA | 1,553 | NA | No | Died |
| 10 | 62/M | CHD | SR | 57 | No | Yes | 46.2 | 631 | No | No | −4.8 | 123 | Absent | No | Improved |
| 11 | 69/F | MS | Af | 54 | No | Yes | 33.3 | 132 | No | No | −2.3 | 78 | Reduced | No | Improved |
| 12 | 93/M | HHD | SR | 81 | Yes | Yes | 76.9 | 211 | NA | NA | NA | NA | NA | NA | Transfer |
| 13 | 85/F | AS | Af | 60 | No | Yes | 39.5 | 237 | No | No | −4.2 | 97 | Absent | NA | Improved |
| 14 | 86/M | Systolic HF | Af | 39 | Yes | Yes | 43.6 | 460 | No | No | −6 | 179 | Absent | NA | Improved |
| 15 | 65/M | Systolic HF | Af | 15 | Yes | Yes | 50.2 | 596 | Yes | No | −5.7 | 92 | Absent | No | Improved |
Systolic heart failure (HF) included ischemic and dilated cardiomyopathy. Af, atrial fibrillation; AR, aortic regurgitation; AS, aortic stenosis; BNP, B-type natriuretic peptide; BW, body weight; CHD, congenital heart disease; CT, computed tomography; ECG, electrocardiography; EF, ejection fraction; F, female; HCM, hypertrophic cardiomyopathy; HHD, hypertensive heart disease; M, male; MS, mitral stenosis; NA, not available; SR, sinus rhythm; US-PLE, ultrasound pleural effusion.
The results of biochemical measurements of blood serum and pleural fluid samples to characterize the pleural effusion are given in Table 3. According to the Light criteria, 10 of 15 patients with HF-related pleural effusion were classified as having transudative effusion, and the remaining 5 were classified as having exudative effusion, fulfilling only 1 (n=4) or both (n=1) LDH criteria. Based on the ‘albumin criteria’, 14 of 15 patients (93%) were classified as having transudative pleural effusion. Of the 3 patients with moderately elevated C-reactive protein, only 1 was classified as having exudative effusion according to both Light’s and the albumin criteria. The results of M. tuberculosis PCR tests were negative in all study patients.
Table 3.
Biochemical Measurements of Blood Serum and Pleural Fluid Samples in Patients With HF-Associated Pleural Effusion
| Patient no. |
Total protein (g/dL) |
Lactate dehydrogenase (U/L) |
Albumin (g/dL) |
Other peripheral blood measurements |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum | PlF | PlF/ serum ratio |
Serum | PlF | PlF/ serum ratio |
Serum | PlF | Serum-PlF gradient |
WBC (/μL) |
CRP (mg/dL) |
ADA (U/L) |
TB DNA-PCR |
|
| 1 | 6.8 | 2.1 | 0.31 | 207 | 72 | 0.35 | 3.3 | 1.2 | 2.1 | 6,930 | 2.36 | NA | Negative |
| 2 | 5.2 | 2.2 | 0.42 | 161 | 182 | 1.13A | 2.4 | 1.2 | 1.2B | 9,310 | 18.3 | NA | Negative |
| 3 | 6.2 | 2.7 | 0.44 | 160 | 81 | 0.51 | 2.7 | 1.4 | 1.3 | 5,540 | 1.63 | NA | Negative |
| 4 | 6.8 | 2.6 | 0.38 | 245 | 159 | 0.65A | 3.7 | 1.6 | 2.1 | 4,170 | 0.1 | NA | Negative |
| 5 | 5.6 | 1.7 | 0.3 | 295 | 96 | 0.33 | 3.4 | 1.1 | 2.3 | 7,490 | 0.5 | 3.2 | Negative |
| 6 | 5.7 | 2.5 | 0.44 | 245 | 166 | 0.68A | 3.6 | 1.8 | 1.8 | 8,820 | 0.03 | 8.6 | Negative |
| 7 | 5.8 | 2.5 | 0.43 | 173 | 67 | 0.39 | 3.4 | 1.5 | 1.9 | 6,830 | 0.69 | 16 | Negative |
| 8 | 6.7 | 2.9 | 0.43 | 322 | 352A | 1.09A | 3.6 | 1.7 | 1.9 | 3,420 | 2.93 | 10.5 | Negative |
| 9 | 6.5 | 1.8 | 0.28 | 182 | 119 | 0.65A | 3.2 | 1.1 | 2.1 | 7,010 | 4.45 | 10.4 | Negative |
| 10 | 5.9 | 2.1 | 0.36 | 253 | 93 | 0.37 | 3.3 | 1.4 | 1.9 | 7,230 | 6.56 | 10.2 | Negative |
| 11 | 8 | 1.7 | 0.21 | 265 | 62 | 0.23 | 4.7 | 1.1 | 3.6 | 7,430 | 6.86 | 6.8 | Negative |
| 12 | 6.7 | 2.6 | 0.39 | 234 | 108 | 0.46 | 2.9 | 1.4 | 1.5 | 4,210 | 0.36 | 13 | Negative |
| 13 | 6.7 | 1 | 0.15 | 261 | 112 | 0.43 | 4 | 0.1 | 3.9 | 3,280 | 0.02 | 8.5 | Negative |
| 14 | 6.6 | 1 | 0.15 | 189 | 64 | 0.34 | 3.4 | 1.1 | 2.3 | 4,910 | 0.14 | 6 | Negative |
| 15 | 5.8 | 1.1 | 0.19 | 295 | 66 | 0.22 | 3.5 | 0.8 | 2.7 | 7,340 | 0.21 | 4.4 | Negative |
AFulfills Light’s criteria. BFulfills the serum-pleural fluid (PlF) albumin gradient. ADA, adenosine deaminase activity; PCR, polymerase chain reaction; TB, tuberculosis; WBC, white cell count. Other abbreviations as in Tables 1,2.
Comparing pleural with serum concentrations for each electrolyte (Table 4) indicated significantly higher pleural than serum Cl concentrations (111±5 vs. 104±6 mEq/L; P<0.0001) and slightly higher pleural than serum Na concentrations (140±4 vs. 139±5 mEq/L; P<0.027). There was no significant difference between pleural and serum potassium concentrations (4.2±0.6 vs. 4.3±0.6 mEq/L; P=0.09).
Table 4.
Pleural Effusion-Serum Electrolyte Gradient in Patients With HF-Associated Pleural Effusion
| Chloride (mEq/L) | Sodium (mEq/L) | Potassium (mEq/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Serum | PlF | PlF-serum gradient |
Serum | PlF | PlF-serum gradient |
Serum | PlF | PlF-serum gradient |
|
| Patient no. | |||||||||
| 1 | 97 | 106 | 9 | 131 | 133 | 2 | 5.4 | 5.2 | −0.2 |
| 2 | 102 | 116 | 14 | 143 | 145 | 2 | 3.4 | 3.3 | −0.1 |
| 3 | 105 | 111 | 6 | 138 | 138 | 0 | 5 | 4.7 | −0.3 |
| 4 | 106 | 113 | 7 | 144 | 145 | 1 | 4.1 | 4 | −0.1 |
| 5 | 103 | 110 | 7 | 137 | 138 | 1 | 3.9 | 3.8 | −0.1 |
| 6 | 114 | 118 | 4 | 139 | 141 | 2 | 4.2 | 4.3 | 0.1 |
| 7 | 95 | 101 | 6 | 128 | 132 | 4 | 5.7 | 5.7 | 0 |
| 8 | 96 | 101 | 5 | 135 | 135 | 0 | 4 | 4.1 | 0.1 |
| 9 | 106 | 114 | 8 | 139 | 141 | 2 | 3.9 | 3.8 | −0.1 |
| 10 | 100 | 107 | 7 | 139 | 138 | −1 | 4.5 | 3.7 | −0.8 |
| 11 | 97 | 108 | 11 | 137 | 137 | 0 | 4.3 | 3.9 | −0.4 |
| 12 | 111 | 115 | 4 | 144 | 144 | 0 | 4.2 | 4.1 | −0.1 |
| 13 | 106 | 113 | 7 | 143 | 143 | 0 | 3.6 | 3.8 | 0.2 |
| 14 | 104 | 113 | 9 | 140 | 141 | 1 | 4.2 | 4.4 | 0.2 |
| 15 | 110 | 117 | 7 | 146 | 145 | −1 | 4.2 | 4 | −0.2 |
| Mean±SD | 104±5.7 | 111±5.3 | 7.4±2.6 | 139±4.9 | 140±4.3 | 0.9±1.4 | 4.3±0.6 | 4.2±0.6 | −0.1±0.3 |
HF, heart failure; PlF, pleural fluid.
As shown in Figure, the effusion-serum gradient of electrolytes, calculated by subtracting the concentration of serum electrolytes from that of pleural fluid electrolytes, was significantly higher for Cl (7.4±2.6 mEq/L; range 4–14 mEq/L) than for Na (0.9±1.4 mEq/L; ranging from −1 to 4 mEq/L) and potassium (−0.1±0.3 mEq/L; ranging from −0.8 to 0.2 mEq/L; P<0.001 for each).
Figure.
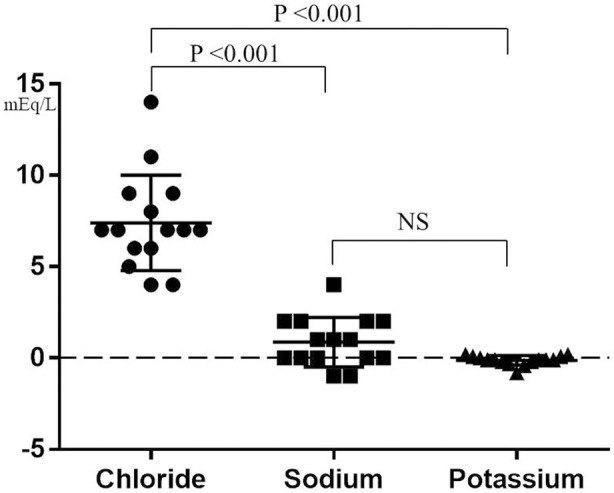
Effusion-serum electrolyte gradient in heart failure-associated pleural effusion. The effusion-serum electrolyte gradient was selectively and significantly higher for chloride than for sodium and potassium concentrations. Symbols show values for individual patients, with the horizontal lines indicating mean values and whiskers indicating the standard deviation.
Discussion
Interpretation of Results
When considering the “chloride theory” for HF pathophysiology,12 it would be of considerable interest to know how Cl dynamics affect the formation of pleural effusion under HF pathophysiology. There have been few experimental and clinical analyses of pleural fluidal electrolytes under both physiologic and pathophysiologic states, including worsening HF. Experimental studies have revealed low pleural compared with serum Cl concentrations under normal physiological conditions (Table 5).27,28 The finding of higher Cl concentrations in HF-associated pleural fluid in the present study raises a new idea regarding pleural fluid dynamics under HF pathophysiology.
Table 5.
Electrolyte Concentrations in Pleural/Interstitial Fluids Compared With Blood Serum Under Normal Physiological Conditions and in Heart Failure
| Pleural space | Reference | Interstitial space | Reference | |
|---|---|---|---|---|
| Normal physiology | ||||
| Sodium | Lower | Sahn et al,27 | Lower | Edelman et al13 |
| Chloride | Lower | Zocchi et al28 | Higher | |
| Potassium | Equivalent | Lower | ||
| Heart failure | ||||
| Sodium | Slightly higher | Present study | Unclear | Not available |
| Chloride | Selectively and greatly higher | |||
| Potassium | Equivalent | |||
Mechanisms of Pleural Fluid Formation by the Classical Starling Equation
The physiology of pleural fluid formation and absorption remains controversial. The most accepted model of pleural exchange in the normal state involves formation primarily via filtration through the capillaries in the parietal pleura lining the chest wall and drainage of the pleural liquid via lymphatic stomata in the parietal pleura.29–32 Transitionally, the formation of pleural fluid has been explained physiologically by the classical Starling equation and the solute flux equation, which calculate the hydrostatic and colloidal osmotic pressures as the main determinants of filtration and absorption across the endothelium.31,33 Under pathophysiologic conditions, the accumulation of transudative or exudative pleural fluid results from an imbalance between the fluid leaking into the pleural space and its removal. In the case of worsening HF, the production of transudative pleural effusion would result from increased leakage of fluid into the pulmonary interstitium and its accumulation in the pleural space, as well as increased venous pressure, which decreases lymphatic flow and therefore decreases pleural fluid absorption.22,34 However, the classical Starling and solute flux equations do not consider the electrolyte balance in the formation of the pleural fluid.
Contribution of Electrolytes to Pleural Fluid Formation
The present study revealed higher Cl concentrations in the HF-associated pleural effusion than in the serum. Although the severity of the hemodynamic imbalance would be the primary determinant of HF-associated pleural effusion,15,29–32 special attention should be paid to changes in Cl concentrations in both the serum and pleural space to gain a better understanding of the production of HF-related pleural effusion under a given hemodynamic state. What is the contribution of the higher Cl concentration in HF-associated pleural effusion than serum to HF-related pathophysiology in the present study?
The anatomic architecture differs between the pleural and interstitial spaces; the pleural space is nearly empty and is surrounded by the pleural mesothelium, whereas the interstitial space comprises a rich network of proteoglycans and collagen and/or elastic fibers.14,35–37 Regardless, the pathway of body fluid is similar between the pleural29–32 and interstitial14 spaces. Specifically, filtered plasma in both spaces reportedly drains primarily through the lymphatic pathway, ultimately into the blood stream. However, as indicated in Table 5, the Cl– concentrations are quite different between the interstitial and pleural spaces in the normal physiological state, and change in pleural spaces during the transition from normal physiology to worsening HF.
Reports in the literature13,27,28 (Table 5) indicate that, under normal physiological conditions, Cl concentrations are high in the interstitial space compared with serum in the human due to the Donnan effect,14 or possibly because of a negatively charged network of glycosaminoglycans.14,35–37 Conversely, several experimental studies reported that the Cl concentration in the pleural space is low due to active transport of Cl– out of the pleural space.27 Therefore, under normal physiological conditions, it may be that different Cl concentrations between the interstitial and pleural spaces produce differential amounts of body fluid (i.e., wetter conditions in the interstitial space and lubricant conditions with less fluid in the pleural space). Under worsening HF, as shown in the present study, there is a high pleural Cl concentration compared with the low Cl concentration in the normal physiological state,27,28 suggesting that Cl has an active role in the formation of pleural fluid, in accordance with the “chloride theory”, which predicts that Cl is the key electrolyte for regulating the distribution of body fluid or water in each body compartment.12 In fact, experimental studies have demonstrated the contribution of Cl to the formation of cardiogenic alveolar edema38,39 or vascular endothelial glycocalyx swelling,40 which supports the regulation of body water distribution by Cl−.
Of note, the results of the present study do not support the Donnan effect14 in the production of higher Cl concentrations in HF-related pleural effusions because Pearson’s correlation analysis indicated there was no significant correlation between the effusion-serum gradient of Cl and the serum-effusion albumin gradient22 (r=0.019, n=15, P=0.63).
Differential Role of Na or Cl in Pleural Fluid Formation Under HF Pathophysiology
What is the contribution of Cl− to the regulation of water distribution12 in the human body? Solutes in the human body are classified as effective or ineffective osmoles on the basis of their ability to generate osmotic water movement, and osmotic water flux requires a solute concentration gradient.14 “Tonicity” is the effective osmolality across a barrier, and thus regulates body water distribution to each body space compartment.14 At the capillary interface, small Na+, Cl−, and K+ solutes are considered to be fundamentally ineffective osmoles that freely move across the interendothelial spaces.14 However, in the human body the electrolytes have a distinctly different distribution (i.e., a relatively homogeneous distribution of Na and K, but an inhomogeneous distribution of Cl across the vascular space and interstitial or pleural spaces; Table 5). The fact that there are considerable differences in Cl− concentrations in each compartment of the extracellular body space under both normal and pathophysiologic conditions strongly suggests that Cl− has “tonicity” potential in each compartment of the body space, thus regulating the flow or distribution of water across each body space compartment. The exact mechanism(s) leading to the different Cl concentrations across human body compartments, including the pleural space, remains unclear, but may involve fluid dynamics through capillary vessels to the pleural space and the mesothelium covering the inner surface of the pleura.27–31
Recent Developments in Microvascular Fluid Exchange
According to the recently developed revised Starling equation and the glycocalyx model of transvascular fluid exchange, the endothelial glycocalyx layer is semipermeable with regard to anionic macromolecules such as albumin and other plasma proteins and generates an effective oncotic gradient within a very small space.41 However, this theory does not take into account the small Cl molecule in the mechanism of pleural fluid formation under worsening HF. The pleura is comprised of a layer of mesothelial cells and underlying connective tissue.42 These mesothelial cells are recognized as active cells, involved in many structural and metabolic functions.30,43 In addition to these mesothelial cell functions,30,43 it is important to determine whether anionic Cl− truly and selectively penetrate the pleural-associated capillary endothelial glycocalyx layer,44 diffuse into the pleural space, and hold pleural fluid as a result of their tonic effect under conditions of insufficient drainage via venous and/or lymphatic channels in association with the effects of HF status on the function of the endothelial glycocalyx layer.45,46 Other related mechanisms of transcapillary exchange of solutes47,48 in and around the pleural space should also be examined with regard to the formation of HF-related pleural effusion.
Study Limitations
Because this study was performed on a relatively small number of patients and was a single-center observational study, it should be considered a hypothesis-generating study. In addition, this study lacked control pleural effusion samples obtained from patients in a steady HF state (without acute decompensation) or recovering from worsening HF after diuretic therapy because performing thoracentesis under such HF status with little or near absent pleural effusion is extremely dangerous. It would also be helpful if information was available regarding electrolyte concentrations in pleural fluid in healthy people, but, to the best of the author’s knowledge, there have been no such a human studies. Furthermore, additional studies are required to clarify whether the HF medication affected serum and pleural electrolyte concentrations, as well as the effusion-serum Cl gradient (e.g., a diuretic effect on the proteins and other components of pleural fluid49,50). Finally, the data used in this study were derived from a selected patient population with acutely worsening HF. Therefore, other etiologies for changes in pleural electrolytes, particularly inflammation and malignancy, should be examined to determine the integrity of pleural function on the effusion-serum Cl gradient. In the present study, the effusion-serum Cl gradient was low (2 mEq/L) in 1 patient with inflammatory tuberculous pleuritis, suggesting impaired mesothelial integrity. In another patient with severe hypoalbuminemia (serum albumin 1.4 g/dL) due to malnutrition, the effusion-serum Cl gradient was high (6 mEq/L), suggesting preservation of mesothelial integrity. If there are differences in pleural Cl concentrations among different etiologies of pleural effusion, determination of the effusion-serum gradient of Cl may contribute to the diagnosis, classification, and management of the corresponding etiology of pleural effusion.
Conclusions
In acutely worsening HF patients, there is a higher effusion-serum Cl gradient, indicating that Cl may have an important and active role in the formation and retention of body fluid in the pleural space, and possibly in the interstitial space. Future studies of the movement of Cl across each compartment of the body fluid space13,14 are needed to explore the pathophysiologic mechanisms of body fluid redistribution into each body fluid space.
Sources of Funding
This work did not receive any external funding.
Disclosures
The author declares to have no conflict of interest.
IRB Information
This study was approved by the Institutional Review Board of Nishida Hospital (Reference no. 201803-03).
References
- 1. Schrier RW.. Body fluid volume regulation in health and disease: A unifying hypothesis. Ann Intern Med 1990; 113: 155–159. [DOI] [PubMed] [Google Scholar]
- 2. Schrier RW, Abraham WT.. Hormones and hemodynamics in heart failure. N Engl J Med 1999; 341: 577–585. [DOI] [PubMed] [Google Scholar]
- 3. Kalra PR, Anagnostopoulos C, Bolger AP, Coats AJS, Anker SD.. The regulation and measurement of plasma volume in heart failure. J Am Coll Cardiol 2002; 39: 1901–1908. [DOI] [PubMed] [Google Scholar]
- 4. Cody RJ, Covit AB, Schaer GL, Laragh JH, Sealey JE, Feldschuh J.. Sodium and water balance in chronic congestive heart failure. J Clin Invest 1986; 77: 1441–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Volpe M, Tritto C, DeLuca N, Rao MAE, Lamenza F, Mirante A, et al.. Abnormalities of sodium handling and of cardiovascular adaptations during high salt diet in patients with mild heart failure. Circulation 1993; 88: 1620–1627. [DOI] [PubMed] [Google Scholar]
- 6. Cadnapaphornchai MA, Gurevich AK, Weinberger HD, Schrier RW.. Pathophysiology of sodium and water retention in heart failure. Cardiology 2001; 96: 122–131. [DOI] [PubMed] [Google Scholar]
- 7. Sica DA.. Sodium and water retention in heart failure and diuretic therapy: Basic mechanisms. Cleve Clin J Med 2006; 73(Suppl 2): S2–S7. [DOI] [PubMed] [Google Scholar]
- 8. Kataoka H.. Vascular expansion during worsening of heart failure: Effects on clinical features and its determinants. Int J Cardiol 2017; 230: 556–561. [DOI] [PubMed] [Google Scholar]
- 9. Kataoka H.. Proposal for heart failure progression based on the “chloride theory”: Worsening heart failure with increased vs. non-increased serum chloride concentration. ESC Heart Fail 2017; 4: 623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kataoka H.. Biochemical determinants of changes in plasma volume after decongestion therapy for worsening heart failure. J Card Fail 2019; 25: 213–217. [DOI] [PubMed] [Google Scholar]
- 11. Kataoka H.. Changes in red blood cell volume during transition of heart failure status: A reflection of cellular hydration status? Scand J Clin Lab Invest 2018; 78: 305–311. [DOI] [PubMed] [Google Scholar]
- 12. Kataoka H.. The “chloride theory”, a unifying hypothesis for renal handling and body fluid distribution in heart failure pathophysiology. Med Hypotheses 2017; 104: 170–173. [DOI] [PubMed] [Google Scholar]
- 13. Edelman IS, Leibman J.. Anatomy of body water and electrolytes. Am J Med 1959; 27: 256–277. [DOI] [PubMed] [Google Scholar]
- 14. Bhave G, Neilson EG.. Body fluid dynamics: Back to the future. J Am Soc Nephrol 2011; 22: 2166–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wiener-Kronish JP, Matthay MA, Callen PW, Filly RA, Gamsu G, Staub NC.. Relationship of pleural effusions to pulmonary hemodynamics in patients with congestive heart failure. Am Rev Respir Dis 1985; 132: 1253–1256. [DOI] [PubMed] [Google Scholar]
- 16. Kataoka H.. Pericardial and pleural effusions in decompensated chronic heart failure. Am Heart J 2000; 139: 918–923. [DOI] [PubMed] [Google Scholar]
- 17. Kataoka H.. Clinical significance of bilateral leg edema and added value of monitoring weight gain during follow-up of patients with established heart failure. ESC Heart Fail 2015; 2: 106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kataoka H, Takada S.. The role of thoracic ultrasonography for evaluation of patients with decompensated chronic heart failure. J Am Coll Cardiol 2000; 35: 1638–1646. [DOI] [PubMed] [Google Scholar]
- 19. Kataoka H.. A new monitoring method for the estimation of body fluid status by digital weight scale incorporating bioelectrical impedance analyzer in definite heart failure patients. J Card Fail 2009; 15: 410–418. [DOI] [PubMed] [Google Scholar]
- 20. Light RW, MacGregor I, Luchsinger PC, Ball WC.. Pleural effusions: The diagnostic separation of transudates and exudates. Ann Intern Med 1972; 77: 507–513. [DOI] [PubMed] [Google Scholar]
- 21. Baganha MF, Pêgo A, Lima MA, Gaspar EV, Cordeiro AR.. Serum and pleural adenosine deaminase: Correlation with lymphocytic populations. Chest 1990; 97: 605–610. [DOI] [PubMed] [Google Scholar]
- 22. Roth BJ, O’Meara TF, Cragun WH.. The serum-effusion albumin gradient in the evaluation of pleural effusions. Chest 1990; 98: 546–549. [DOI] [PubMed] [Google Scholar]
- 23. Vives M, Porcel JM, Vicente de Vera M, Ribelles E, Rubio M.. A study of Light’s criteria and possible modifications for distinguishing exudative from transudative pleural effusions. Chest 1996; 109: 1503–1507. [DOI] [PubMed] [Google Scholar]
- 24. Mattison LE, Coppage L, Alderman DF, Herlong JO, Sahn SA.. Pleural effusions in the medical ICU: Prevalence, causes, and clinical implications. Chest 1997; 111: 1018–1023. [DOI] [PubMed] [Google Scholar]
- 25. Romero-Candeira S, Hernández L, Romero-Brufao S, Orts D, Fernández C, Martín C.. Is it meaningful to use biochemical parameters to discriminate between transudative and exudative pleural effusions? Chest 2002; 122: 1524–1529. [DOI] [PubMed] [Google Scholar]
- 26. Porcel JM, Vives M.. Etiology and pleural fluid characteristics of large and massive effusions. Chest 2003; 124: 978–983. [DOI] [PubMed] [Google Scholar]
- 27. Sahn SA, Willcox ML, Good JT Jr, Potts DE, Filley GF.. Characteristics of normal rabbit pleural fluid: Physiologic and biochemical implications. Lung 1979; 156: 63–69. [DOI] [PubMed] [Google Scholar]
- 28. Zocchi L, Agostoni E, Cremaschi D.. Electrolyte transport across the pleura of rabbits. Respir Physiol 1991; 86: 125–138. [DOI] [PubMed] [Google Scholar]
- 29. Miserocchi G.. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J 1997; 10: 219–225. [DOI] [PubMed] [Google Scholar]
- 30. Zocchi L.. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J 2002; 20: 1545–1558. [DOI] [PubMed] [Google Scholar]
- 31. Lai-Fook SJ.. Pleural mechanics and fluid exchange. Physiol Rev 2004; 84: 385–410. [DOI] [PubMed] [Google Scholar]
- 32. Natanzon A, Kronzon I.. Pericardial and pleural effusions in congestive heart failure: Anatomical, pathophysiologic, and clinical considerations. Am J Med Sci 2009; 338: 211–216. [DOI] [PubMed] [Google Scholar]
- 33. Curry FE.. Mechanics and thermodynamics of capillary exchange. In: Renkin EM, Michel CC, editors. Handbook of physiology, section 2: Cardiovascular, Vol. IV: Microcirculation. Bethesda: American Physiological Society, 1984; 309–374. [Google Scholar]
- 34. Pistolesi M, Miniati M, Giuntini C.. Pleural liquid and solute exchange. Am Rev Respir Dis 1989; 140: 825–847. [DOI] [PubMed] [Google Scholar]
- 35. Nijst P, Verbrugge FH, Grieten L, Dupont M, Steels P, Tang WHW, et al.. The pathophysiological role of interstitial sodium in heart failure. J Am Coll Cardiol 2015; 65: 378–388. [DOI] [PubMed] [Google Scholar]
- 36. Nijst P, Olinevich M, Hilkens P, Martens P, Dupont M, Tang WHW, et al.. Dermal interstitial alterations in patients with heart failure and reduced ejection fraction. Circ Heart Fail 2018; 11: e004763. [DOI] [PubMed] [Google Scholar]
- 37. Wiig H.. Regulation of fluid volume from the outside: A role of glycosaminoglycans in the skin interstitium? Circ Heart Fail 2018; 11: e005135. [DOI] [PubMed] [Google Scholar]
- 38. Solymosi EA, Kaestle-Gembardt SM, Vadász I, Wang L, Neye N, Chupin CJA, et al.. Chloride transport-driven alveolar fluid secretion is a major contributor to cardiogenic lung edema. Proc Natl Acad Sci USA 2013; 110: E2308–E2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Londino JD, Matalon S.. Chloride secretion across adult alveolar epithelial cells contributes to cardiogenic edema. Proc Natl Acad Sci USA 2013; 110: 10055–10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Peters W, Kusche-Vihrog K, Oberleithner H, Schillers H.. Cystic fibrosis transmembrane conductance regulator is involved in polyphenol-induced swelling of the endothelial glycocalyx. Nanomedicine 2015; 11: 1521–1530. [DOI] [PubMed] [Google Scholar]
- 41. Michel CC.. Starling: The formulation of his hypothesis of microvascular fluid exchange and its significance after 100 years. Exp Physiol 1997; 82: 1–30. [DOI] [PubMed] [Google Scholar]
- 42. Wang NS.. Anatomy of the pleura. Clin Chest Med 1998; 19: 229–240. [DOI] [PubMed] [Google Scholar]
- 43. Ji HL, Nie HG.. Electrolyte and fluid transport in mesothelial cells. J Epithel Biol Pharmacol 2008; 1: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vink H, Duling BR.. Capillary endothelial surface layer selectively reduces plasma solute distribution volume. Am J Physiol Heart Circ Physiol 2000; 278: H285–H289. [DOI] [PubMed] [Google Scholar]
- 45. Bruegger D, Jacob M, Rehm M, Loetsch M, Welsch U, Conzen P, et al.. Atrial natriuretic peptide induces shedding of endothelial glycocalyx in coronary vascular bed of guinea pig hearts. Am J Physiol Heart Circ Physiol 2005; 289: H1993–H1999. [DOI] [PubMed] [Google Scholar]
- 46. Curry FR, Noll T.. Spotlight on microvascular permeability. Cardiovasc Res 2010; 87: 195–197. [DOI] [PubMed] [Google Scholar]
- 47. Levick JR, Michel CC.. Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res 2010; 87: 198–210. [DOI] [PubMed] [Google Scholar]
- 48. Reed RK, Rubin K.. Transcapillary exchange: Role and importance of the interstitial fluid pressure and the extracellular matrix. Cardiovasc Res 2010; 87: 211–217. [DOI] [PubMed] [Google Scholar]
- 49. Chakko SC, Caldwell SH, Sforza PP.. Treatment of congestive heart failure: Its effect on pleural fluid chemistry. Chest 1989; 95: 798–802. [DOI] [PubMed] [Google Scholar]
- 50. Romero-Candeira S, Fernández C, Martín C, Sánchez-Paya J, Hernández L.. Influence of diuretics on the concentration of proteins and other components of pleural transudates in patients with heart failure. Am J Med 2001; 110: 681–686. [DOI] [PubMed] [Google Scholar]


