Abstract
Background
Little is known about MS patients’ acceptability of a COVID-19 vaccine.
Objective and methods
An online survey was conducted among MS patients to study COVID-19 vaccine acceptability and its associated factors.
Results and conclusion
Among 256 participants, 80.9% of the patients were either definitely or probably willing to receive a COVID-19 vaccine. Most hesitant patients would consider being vaccinated under physician recommendation. Older patients and those with comorbidities seem to be more willing to get vaccinated. Moreover, vaccine acceptability was associated with participants’ convictions and concerns about COVID-19, as well as previous vaccination practices.
Keywords: Multiple sclerosis, COVID-19, Vaccine
Introduction
The availability of COVID-19 vaccines has been heralded as key to control the COVID-19 pandemic; yet little is known about MS patients’ acceptability of a COVID-19 vaccine. Common concerns may include vaccine safety and effectiveness (Reiter et al., 2020; Neumann et al., 2020; Bell et al., 2020).
The main objective of our study was to evaluate the willingness to get a COVID-19 vaccine among MS patients, and also to investigate possible association with demographic, clinical and psychosocial factors.
Methods
We performed an observational, cross-sectional, hospital-based, single-center study, using an online survey. A voluntary, anonymized, self-administered web-based survey was conducted from 21st December 2020 (the day when EMA recommended first COVID-19 vaccine for authorization in the European Union) through 3rd January 2021. Patients diagnosed with MS according to the 2017 McDonald criteria, followed at Egas Moniz Hospital (a tertiary hospital in Lisbon), were invited to answer the survey by an email with a link to the survey website, in Google Forms. Patients who are not fluent in Portuguese and those with dementia were excluded.
The questionnaire was designed based on scientific literature (Reiter et al., 2020; Neumann et al., 2020). We assessed participants’ vaccine acceptability by asking how willing they would be to get a COVID-19 vaccine; response options included “definitely willing”, “probably willing”, “probably not willing” and “definitely not willing”.
Statistical analysis
Statistical analysis was performed using SPSS (V.25.0; IBM Corporation®). Baseline analysis of our population was performed using descriptive statistics. In order to study the factors associated with the willingness to get a COVID-19 vaccine, patients’ answers were dichotomized regarding vaccine acceptability into “willing” (definitely or probably willing) or “not willing” (definitely not willing or probably not willing). Continuous data were evaluated using T or Mann-Whitney U tests and Chi-square test was performed to compare categorical data between groups. P values < 0.05 were considered statistically significant.
Results
Among 337 MS patients followed-up in our department, 21 were eliminated based on the exclusion criteria and 316 patients were invited to participate. We obtained 256 valid responses (response rate of 81%). The majority of our survey participants were females (187, 73.0%), with a median age of 45 years [18–77], in line with the demographic features of the patients followed-up in our department (p = 0.584 for gender and p = 0.371 for age).
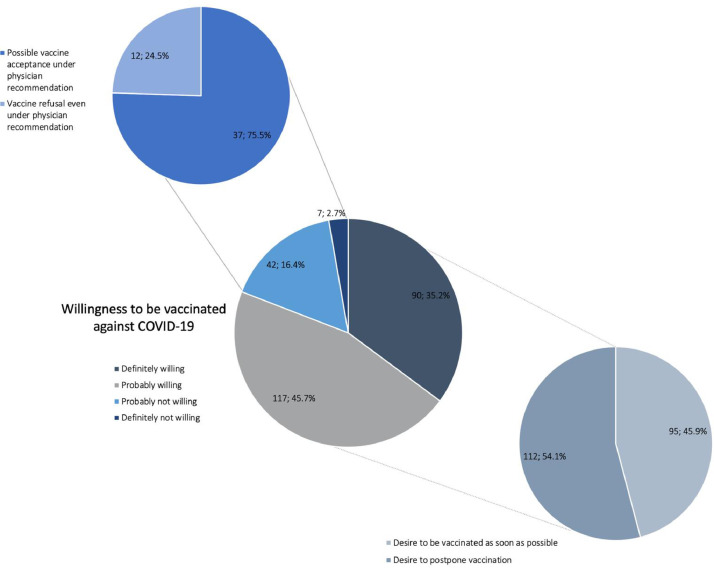
Overall, 207 (80.9%) of MS participants were either definitely (35.2%) or probably willing (45.7%) to receive a COVID-19 vaccine, however 112 (54.1%) of them would prefer to postpone vaccination. Conversely, 49 (19.1%) patients said they definitely (2.7%) or probably (16.4%) would not get a COVID-19 vaccine although most of them (37, 75.5%) would consider being vaccinated under recommendation of their physician. ( Fig. 1 )
Fig. 1.
Willingness to be vaccinated against COVID-19 among MS patients. 80.9% of the patients were either definitely or probably willing to receive a COVID-19 vaccine; most of them would like to postpone vaccination (54.1%). Most hesitant patients (75.5%) would consider being vaccinated under physician recommendation.
Older patients (p = 0.016) and those with comorbidities (p = 0.047) seem to be more willing to get vaccinated. Also, men (p = 0.062) and patients who are unable to walk independently (p = 0.083) were more likely to be interested in getting a COVID-19 vaccine but both results did not reach statistical significance. Patients who wanted to be vaccinated had a higher desire of acquiring self-protection from the vaccine (p<0.001), as well as protection of family (p<0.001) and community (p<0.001). Also, vaccine acceptability was associated with COVID-19 information-seeking behavior (p = 0.001) and the belief that population COVID-19 vaccination will bring life to normal (p = 0.001). On the other hand, patients who were reluctant to receive a COVID-19 vaccine were less concerned about COVID-19 disease (p = 0.004) and they reported that COVID-19 pandemic has had less impact in their lives (p = 0.032) when compared with the group of patients who wanted to be vaccinated. Hesitant patients also manifested more concerns about potential side effects (p<0.001) and the vaccine safety-profile in patients with MS (p=<0.001) or under their DMT (p = 0.020). Patients up to date on their vaccination schedule (according to the Portuguese National Vaccination Programme) reported more willingness to get a COVID-19 vaccine (p<0.001) while patients with previous history of vaccine refusal were less inclined to get a COVID-19 vaccine (though it did not reach statistical significance; p = 0.06). A previous diagnosis of COVID-19 disease (found in 6 participants; 2.3%) did not seem to influence the intent to receive a COVID-19 vaccine (p = 0.876). (Table 1 ; Supplementary results)
Table 1.
Demographic, clinical and psychosocial features of participants regarding the willingness to get COVID-19 a vaccine
| Total (256), n (%) | Willing to get vaccinated (207, 80.9%), n (%) | Not willing to get vaccinated (49, 19.1%), n (%) | p value | |
|---|---|---|---|---|
| Demographic factors | ||||
| Female gender | 187 (73) | 146 (70.5) | 41 (83.7) | 0.062⁎ |
| Age, years (median, IQR [min-max]) | 45 (17) [18-77] | 46 (17) [18-76] | 41 (16) [18-77] | 0.016# |
| Clinical factors | ||||
| DMT+ | 0.148⁎ | |||
| - Modest efficacy | 158 (61.7) | 122 (59.2) | 36 (73.5) | |
| - High efficacy | 75 (29.3) | 66 (32.0) | 9 (18.4) | |
| - Without therapy | 22 (8.6) | 18 (8.7) | 4 (8.2) | |
| Disease duration, years (median, IQR [min-max]) | 11.5 (12) [0-57] | 11 (12) [0-57] | 12 (10) [1-28] | 0.946# |
| Disability related to MS | 0.083⁎ | |||
| Unassisted walking | 214 (83.6) | 169 (81.6) | 45 (91.8) | |
| Unable to walk independently | 42 (16.4) | 38 (18.4) | 4 (8.2) | |
| Presence of co-morbidities∇ | 65 (25.4) | 58 (28) | 7 (14.3) | 0.047⁎ |
| COVID-19 disease and vaccination related-factors | ||||
| Concerns about COVID-19 infection: | 0.004⁎ | |||
| Quite worried | 86 (33.6) | 73 (35.3) | 13 (26.5) | |
| Worried | 140 (54.7) | 113 (54.6) | 27 (55.1) | |
| Slightly worried | 23 (9.0) | 19 (9.2) | 4 (8.2) | |
| Not worried | 7 (2.7) | 2 (1.0) | 5 (10.2) | |
| Perceived risk to be infected | 0.634⁎ | |||
| High chance | 43 (16.8) | 36 (17.4) | 7 (14.3) | |
| Moderate chance | 147 (57.4) | 118 (57.0) | 29 (59.2) | |
| Low chance | 61 (23.8) | 50 (24.2) | 11 (22.4) | |
| No chance | 5 (2.0) | 3 (1.4) | 2 (4.1) | |
| Expectations about the severity of an eventual future COVID-19 infection: | 0.561⁎ | |||
| Severe | 197 (77) | 162 (78.3) | 35 (71.4) | |
| Not Severe | 59 (23) | 45 (21.7) | 14 (28.6) | |
| COVID-19 information-seeking behaviorθ | 81 (31.6) | 75 (36.2) | 6 (12.2) | 0.001⁎ |
| Impact of COVID-19 pandemic on patients' lifestyle | 0.032⁎ | |||
| Extreme | 40 (15.6) | 32 (15.5) | 8 (16.3) | |
| High | 165 (64.5) | 136 (65.7) | 29 (59.2) | |
| Low | 49 (19.1) | 39 (18.8) | 10 (20.4) | |
| None | 2 (0.8) | 0 (0) | 2 (4.1) | |
| Importance of acquiring self-protection from the vaccine | < 0.001⁎ | |||
| Extremely or highly important | 239 (93.4) | 204 (98.6) | 35 (71.4) | |
| Mildly or not important | 17 (6.6) | 3 (1.4) | 14 (28.6) | |
| Importance of acquiring protection of family from the vaccine | < 0.001⁎ | |||
| Extremely or highly important | 251 (98) | 207 (100) | 44 (89.8) | |
| Mildly or not important | 5 (2.9) | 0 (0) | 5 (10.2) | |
| Importance of acquiring protection of community from the vaccine | < 0.001⁎ | |||
| Extremely or highly important | 252 (98.4) | 207 (100) | 45 (91.8) | |
| Mildly or not important | 4 (1.6) | 0 (0) | 4 (8.2) | |
| Concerns regarding side effects of COVID-19 vaccines | < 0.001⁎ | |||
| Worried | 226 (88.3) | 178 (86) | 48 (98) | |
| Not worried | 30 (11.7) | 29 (14) | 1 (2.0) | |
| Concerns regarding safety of COVID-19 vaccines in MS patients | < 0.001⁎ | |||
| Worried | 234 (91.4) | 185 (89.4) | 49 (100) | |
| Not worried | 22 (8.6) | 22 (10.6) | 0 (0) | |
| Concerns regarding safety of COVID-19 vaccines in MS patients under DMT | 0.020⁎ | |||
| Worried | 227 (88.7) | 180 (87.0) | 47 (95.9) | |
| Not worried | 29 (11.3) | 27 (13.0) | 2 (4.1) | |
| Belief that population COVID-19 vaccination will bring life to normal | 205 (80.1) | 174 (84.1) | 31 (63.3) | 0.001⁎ |
| Up-to-date vaccination schedule∑ | 226 (88.3) | 186 (89.9) | 40 (81.6) | < 0.001⁎ |
Chi-Square test
Mann-Whitney test
DMT – Disease Modifying Therapy. Modest efficacy: Glatiramer Acetate, Interferon beta-1a, Interferon beta-1b, Peginterferon beta-1a, Teriflunomide, Dimethyl fumarate. High efficacy: Alemtuzumab, Cladribine, Fingolimod, Natalizumab, Ocrelizumab
Co-morbidities included heart failure, cardiac disease, chronic kidney disease, chronic pulmonary disease, diabetes, active neoplasm, obesity and hypertension.
Patients who searched more data about COVID-19 disease, beyond the information made available by the media.
Patients who complied with all mandatory vaccines appropriate to their age group (according to the Portuguese National Vaccination Programme)
Discussion
To our knowledge, this is the first European study reporting the acceptability of COVID-19 vaccines in a MS population. The high response rate obtained in this survey may reflect the significant interest in this subject among MS patients.
We found that approximately 81% of MS patients were willing to receive a COVID-19 vaccine. This is in line with the findings of a European study conducted in the general population in April 2020 reporting 73.9% vaccine acceptability (Neumann et al., 2020). Moreover, a US study conducted in the same period among MS patients found that 66% of the participants were willing to get a future COVID-19 vaccine whereas 15.4% of the sample was unwilling (Ehde et al., 2021). Interestingly, many of our patients would prefer to postpone vaccination, probably due to uncertainty about efficacy and risks of COVID-19 vaccines. In fact, most participants reported concerns regarding the safety of COVID-19 vaccines suggesting that MS patients might be particularly apprehensive about adverse effects. Despite that, most hesitant patients would consider being vaccinated under recommendation of their physician. Interestingly, the US study found that the two COVID-19 information sources most highly trusted, and also highly associated with COVID-19 vaccine willingness among MS patients, were healthcare providers and the National MS Society (Ehde et al., 2021). These findings emphasize the primary role of physicians in promoting the uptake of COVID-19 vaccines.
Our survey revealed that older patients and participants with comorbidities seem to be more willing to get vaccinated. The acceptability of COVID-19 vaccines among older, disabled MS patients with comorbidities is of utmost relevance given their high risk for severe COVID-19 disease (Bensa et al., 2020). There was also a trend towards a higher vaccine acceptability among males and more disabled patients. Furthermore, our data suggest that participants’ convictions and concerns about COVID-19, as well as previous vaccination practices, are linked to willingness to get vaccinated. Some of these findings are in agreement with previous studies conducted in the general population (Reiter et al., 2020), (Neumann et al., 2020), (Guidry et al., 2020), (Detoc et al., 2020). On the other hand, a US study found that greater willingness to receive the vaccine among MS patients was associated with having a higher level of education and a higher perception of risk of catching COVID-19 (Ehde et al., 2021).
As far as we know this is the first study evaluating the acceptability of COVID-19 vaccines among MS patients that was conducted after EU's first COVID-19 vaccine approval. Nevertheless, the design of the current study is subject to limitations. It was conducted with a convenience sample from a single-center with a relatively small population size. In addition, all tests should be interpreted as part of exploratory data analysis.
Conclusion
We found that a large majority of MS patients are likely to accept COVID-19 vaccination. Still, 19,1% of the participants revealed vaccine hesitancy and, in those cases, strong healthcare provider recommendations will be critical to promoting vaccine uptake.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Ethics approval
This study protocol was approved by the Institutional Ethics Committee. All participants consented to the use of recorded surveys for scientific purposes.
Funding
This study received no funding.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.msard.2021.102880.
Appendix. Supplementary materials
References
- Bell S., Clarke R., Mounier-jack S., Walker J.L., Paterson P., Health P., et al. Parents’ and guardians’ views on the acceptability of a future COVID- 19 vaccine: a multi-methods study in England. Vaccine. 2020;38:7789–7798. doi: 10.1016/j.vaccine.2020.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensa C., Deschamps R., Créange A., Wahab A., Pelletier J., Bigaut K., et al. Clinical characteristics and outcomes in patients with Coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020;77(9):1079–1088. doi: 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detoc M., Bruel S., Frappe P., Tardy B., Botelho-Nevers E., Gagneux-Brunon A. Intention to participate in a COVID-19 vaccine clinical trial and to get vaccinated against COVID-19 in france during the pandemic. Vaccine. 2020;38(45):7002–7006. doi: 10.1016/j.vaccine.2020.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehde D.M., Roberts M.K., Herring T.E., Alschuler K.N. Willingness to obtain COVID-19 vaccination in adults with multiple sclerosis in the United States. Mult Scler Relat Disord. 2021;49(January) doi: 10.1016/j.msard.2021.102788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidry J.P.D., Laestadius L.I., Vraga E.K., Miller C.A., Perrin P.B., Burton C.W., et al. Willingness to get the COVID-19 vaccine with and without emergency use authorization. Am J Infect Control. 2020;S0196-6553(20):31002–31006. doi: 10.1016/j.ajic.2020.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S., Nirosha B., Varghese E., Sabat I., Pita P., Werner B., et al. Once we have it, will we use it? A European survey on willingness to be vaccinated against COVID-19. Eur J Health Econ. 2020;21(7):977–982. doi: 10.1007/s10198-020-01208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter P.L., Pennell M.L., Katz M.L. Acceptability of a COVID-19 vaccine among adults in the United States: how many people would get vaccinated? Vaccine. 2020;38(42):6500–6507. doi: 10.1016/j.vaccine.2020.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.