Graphical abstract
Keywords: Biomarkers, Cadmium, Landfill, Lead, Population, Nephrotoxicity
Highlights
-
•
Health risk of the neighboring population of the Mbeubeuss landfill (Senegal).
-
•
Assessment of the impact of Cd/Pb exposure through dysfunction renal biomarkers.
-
•
Specific increases of a set of early dysfunction renal biomarkers in exposed subjects.
-
•
Glomerular and tubular dysfunction in exposed subjects.
Abstract
The aim of this study was to assess the integrity and kidney overall functional capacity of subjects exposed to landfill emissions. Urine and blood levels of Pb and Cd, and several of the newly biomarkers of nephrotoxicity (Kim Injury Molecule 1 (KIM-1), alpha-1 Microglobulin (α1 M), beta-2 Microglobulin (β2 M), Cystatin-C (Cyst C), Clusterin, alpha-glutathione S-transferase (GSTα), pi-glutathione S-transferase (GSTπ), Tissue Inhibitor of Metalloproteinase-1 (TIMP1), Calbindin, Neutrophil Gelatinase-Associated Lipocalin (NGAL), Osteopontin (OPN), (Retinol Binding Protein(RBP), Liver-type Fatty Acid-Binding Protein (FABP-1), Trefoil Factor 3 (TFF3), Collagen VI) were measured in order to assess glomerular and tubule damage in adults living near a landfill.
Our results indicate glomerular dysfunction in exposed subjects, and supported evidence of necrosis of proximal and distal tubule epithelial cells as specific biomarkers began to appear in the urine.
Positive correlation by Pearson test were obtained between : blood Pb and B-OPN, B-Cyst C, Calbindin, U-KIM-1, TIMP1, U-OPN, and U-Clusterin; and also, between urinary Cd and TIMP1, B-Clusterin, U-OPN, FABP-1, Albumin, and U-Clusterin. The relation between biomarkers of Cd/Pb exposure and early effect biomarkers in this study clearly predicts the future risk of severe kidney injury in subjects living close to the landfill.
1. Introduction
Heavy metals are ubiquitous in the environment and are widely employed in a variety of human activities to meet the needs of accelerated global growth. They are then cause of a very worrying multisectoral contamination with serious consequences. All natural environments, namely waters, soils and the atmosphere are affected by pollution with heavy metals [1].
Open dumps are sites known for their high levels of heavy metal contamination. Unfortunately, in developing countries, landfilling remains the most economical means of waste disposal, and therefore the most widespread. However, this inappropriate method of waste elimination poses potential risks of environmental degradation and mainly represents high-risk areas for local populations.
The possible risks related to housing near these sites on health of populations have been the subject of numerous studies published in recent years [[2], [3], [4], [5]]. Studies have even reported high mortality caused by several types of cancer (liver, pancreas, kidney) and non-Hodgkin's lymphoma [2]. Unfortunately, the data from these studies have not been confirmed [6] but respiratory illnesses have been reported in some residents living near close to biodegradable waste facilities [7]. Despite all this evidence, opinions differ on the link between living near a landfill and harmful health effects. However, in previous works, we assessed the adverse effects of the Mbeubeuss landfill, a wild open dump located near downtown of Dakar, on the population living in proximity [8,9].
Due to their cumulative properties, long-term exposure to Cd and Pb may cause chronic adverse health effects. Indeed, several studies have already shown that the kidney is a main site of organ damage caused by Cd and/or Pb toxicity, and that renal damage are often associated with ROS overproduction [20,50,51]
Kidneys is an organ with a very high level of complexity. Kidneys consisting of very special functional units called nephrons. Human kidneys each contain about 1 million nephrons which collectively provide daily filtration of 150–180 liters of plasma. Nephrons also processing this filtrate to regulate water, electrolyte and acid-base balance and eliminate waste products at the same time. Kidneys are particularly sensitive to pollutants toxicity. This character can be attributed to its anatomy but more precisely to its function. Filtrate components can be concentrated in excess fo three-fold in proximal tubule during filtrate move along the complex tubular structure of nephrons. High levels of concentration of this components can be reach in the distal tubule and collecting duct, in some cases [10].
Thus, to the early diagnostic of kidney damage one approach consist defining different biomarkers that involved on the mechanisms of toxicity of each pollutant. Indeed, to emphasize the link between environmental pollution and public health, Cabral et al. [9] have already conducted a study aiming to assess to what extent the residents living in proximity of Mbeubeuss landfill (Dakar, Senegal) were exposed to pollutants (Cd, Pb) known to affect human health. In order to detect an eventual impact of the waste dump towards the neighboring population, they have also investigated (i) blood and urine levels of Cd and Pb, and the subsequent changes in biomarkers of hemoglobin synthesis, (ii) oxidative stress biomarkers and (iii) the renal function [9]. The results of their study showed that exposed subjects had significantly higher levels of Cd and Pb in blood and urine. Furthermore, changes in several sensitive markers of nephrotoxicity (Total protein, Albumin, RBP, CC16, GSTα and LDH) clearly indicated early signs of impaired renal function for the landfill neighboring population [9].
Exploration of anomalies of renal function by measuring serum creatinine or blood urea nitrogen has long been the main tool for assessing nephrotoxicity. However, these markers used in routine analyzes do not appear until late, due to the functional reserve of the kidney. They are therefore not reliable indicators of acute kidney injury. Thus, the development of new biomarkers with ability of measuring minute changes on renal tubule integrity rather than reduced kidney function has become a major challenge for earlier and more precise detection of nephrotoxicity [11].
In a large-scale initiative by the Predictive Safety Testing Consortium, 23 exploratory kidney markers were assessed to determine their value for improved detection of kidney injury [12,13]. These biomarkers of kidney damage can be utilized to delineate the nature, and site of injury based on their specificity. Several studies have described the predictive utility of biomarkers in earlier diagnosis of kidney damage.
The purpose of our study is therefore to assess the integrity and kidney overall functional capacity of subjects exposed to landfill emissions.
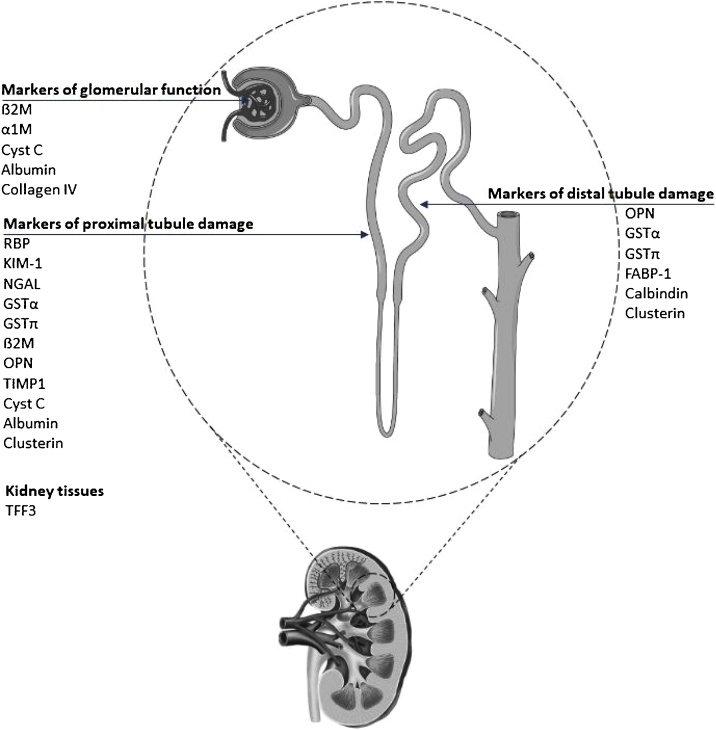
For that, several of the newly accepted makers (KIM-1, α1 M, β2 M, Cyst C, Clusterin) along with additional blood and urinary key proteins of kidney injury (GSTα, GSTpi, TIMP1, Calbindin, NGAL, OPN, RBP, FABP-1, TFF3, Uromodulin, Collagen VI) to detect tubule damage were used (Fig. 1).
Fig. 1.
Specific biomarkers to detect injury to specific nephron segments affected by various nephrotoxicants such as heavy metals.
KIM-1 (Kim Injury Molecule 1), α1 M (alpha-1 Microglobulin), β2 M (beta-2 Microglobulin), Cyst C (Cystatin-C), Clusterin, GSTα (alpha-glutathione S-transferase), GSTπ (pi-glutathione S-transferase), TIMP1 (Tissue Inhibitor of Metalloproteinase-1), Calbindin, NGAL (Neutrophil Gelatinase-Associated Lipocalin), OPN (Osteopontin), RBP (Retinol Binding Protein), FABP-1 (Liver-type Fatty Acid-Binding Protein), TFF3 (Trefoil Factor 3), Collagen VI, Albumin).
2. Materials and methods
2.1. The Mbeubeuss waste dumping site
Mbeubeuss landfill covers an area of about 175 ha, on the outskirts of Dakar city at a distance of about 30 km from the city center. Since 1970, this landfill has received more than 400,000 tons per year of household solid waste. The waste is not covered with inert material making the health risks more marked, and the waste dump is not closed, thereby facilitating access to the site for people. « Darou Salam 6 Nder », the control site (about 3.5 km from the Mbeubeuss landfill) was selected as being far enough from the landfill to not be influenced by its pollution [8,9].
2.2. Population under study
After The national Biomedical Ethics Committee of the Department of Health and Preventive Medicine of Dakar (Senegal) approved the epidemiological study, the participants were informed about the aim of this research.
The subjects were asked to fill in a personal data questionnaire, including detailed information on their place of residence, duration of residence, occupational history, medical history (e.g., kidney diseases), present status, etc. Persons with kidney diseases, diabetes or known occupational exposure to Cd and/or Pb as well as those living on the respective sites for less than 5 years were excluded from the study based on the questionnaire data. In total, 87 persons (i.e., 52 exposed subjects and 35 control subjects) were enrolled in the study (Table 1).
Table 1.
Descriptive information and biomarkers of control and exposed subjects included in the study.
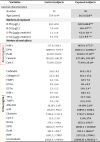 |
General characteristics are expressed either as number, or as mean value ± SD. Blood and urine levels of lead (i.e. B-Pb and U-Pb, respectively) and cadmium (B-Cd and U-Cd, respectively), and markers of renal effects, Retinol Binding Protein(RBP), alpha-Glutathione S-Transferase (GSTα), pi-Glutathione S-Transferase (GSTπ), Cystatin-C, Neutrophil Gelatinase-Associated Lipocalin (NGAL), Kim Injury Molecule 1 (KIM1), Liver-type Fatty Acid-Binding Protein (FABP-1), Trefoil Factor 3 (TFF3), Tissue Inhibitor of Metalloproteinase-1 (TIMP1),Clusterin, Osteopontin (OPN), Calbindin, Uromodulin, Collagen VI, α1 Microglobulin (α1 M), beta-2 microglobulin (β2 M) are expressed as mean value ± SD.
(* = p < 0.05, ** = p < 0.01, *** = p < 0.001; Mann-Whitney U test).
2.3. Analytical methods
2.3.1. Biological sample collection
Blood and spot urine were collected with disposable collection material certified for trace metal determination. After taking, blood and urine samples were quickly placed in a cooling box at 4 °C and transported to the laboratory where they were divided into aliquots and stored at −80 °C until required for analyses.
2.3.2. Markers of cadmium and lead exposure
Cadmium and lead in blood (B-Cd, B-Pb) and urine (U-Cd, U-Pb) were determined by inductively coupled plasma-mass spectrometry as described by Heitland and Koster [52,53]. The detection limits were for B-Cd, B-Pb, U-Cd and U-Pb 0.02, 0.07, 0.03 and 0.09 μg/L, respectively.
2.3.3. Determination of blood and urinary kidney markers
Second-generation biomarkers for renal injury, selected for their sensitivity to early renal changes and their specificity of nephron different segments were used for this study. These are mainly indicators of tubular segment function. Blood and urinary levels of RBP, GSTα, GSTπ, Cyst C, KIM-1, NGAL, FABP-1, TFF3, TIMP1, Clusterin, OPN, Calbindin, Uromodulin, Collagen VI, α1 M, and β2 M were determined by microsphere based Luminex xMAP® technology using the Human kidney Toxicity Panel 1, 2, and 3, following the manufacturer’s instructions. This technique is an adaptation of the developed and validated sandwich enzyme-linked immunosorbant assay, that was described at the 2005 American Society of Nephrology Meeting [14]. The pre-validated assays are based on the Luminex® xMAP® technology [11]. Urine and blood samples were thawed approximately 1 h before assays performance. For quantitative analysis of all markers per panel in an optimal range, it was necessary to adjust the dilution factors according to the manufacturer’s recommendations.
2.4. Statistical analysis
Group general characteristics, Pb and Cd exposure levels, and blood and urine biomarker levels were expressed as mean value ± standard deviation. Data analyses were performed using SPSS 25.0 for Windows (SPSS, Paris, France). Comparisons were carried out between the control and the exposed subjects (Mann-Whitney U test). Thereafter, we looked for the blood or urinary renal injury biomarkers significantly associated with Pb and Cd exposure levels with single linear regression models (Pearson test).
3. Results and discussion
This study dealt with the health impact of Mbeubeuss landfill on adults living close to this waste dump. Our previous results [8] showed that the two metals of interest, Cadmium (Cd) and lead (Pb), were detected at highest levels in the soil and air samples collected in the exposed site comparing to usual environmental values. Exposure to these elements, particularly at chronic low doses, is still a major public health concern.
3.1. Cd and Pb exposure
The present study involved 87 subjects aged from 17 to 67 years (35 controls and 52 exposed). Table 1 presents the mean and the deviation values for biological parameters in the control and exposed site. Among the adults under study, 43 (49.5 %) were males and 44 (50.5 %) were females.
B-Pb levels were 65.27 μg/L for control subjects, and 120.95 μg/L for the exposed ones, showing a significant difference (p < 0.001) between exposed and control subjects (Table 1). This result highlighted the greater absorption of adults living near the landfill, who exhibited a high B-Pb level. In agreement with our previous statement about B-Pb levels, U-Pb levels were significantly higher (p < 0.01) for exposed subjects (4.21 μg/g creatinine) than controls (1.1 μg/g creatinine) (Table 1).
The results of B-Cd (Table 1) showed a significant difference (p < 0.001) between controls (1.17 μg/L) and exposed (1.78 μg/L) subjects, independently of the gender. B-Cd is a indicator of recent exposure, and it is less well correlated with the intensity of exposure compared to U-Cd. In our study, there was significant difference (p <0.001) in U-Cd between individuals in the polluted area (1.25 μg/g creatinine) and controls (0.34 μg/g creatinine) (Table 1).
These levels are below the 2.5 μg / g creatinine threshold advised by the Joint FAO/WHO Expert Committee on Food Additives (JECFA). They are also below the rate of 2 μg/g recommended by European Scientific Committee on Toxicity and the Environnement (ESCTEE) to prevent kidney damage. However, they are much higher than the rates noted in France during investigations by InVS in 2009 national study on incinerators (0.27 μg/g creatinine) [15] and in 2011 the National health and Nutrition Survey (ENNS) (0.3 μg/g creatinine) [16].
Cadmium and lead are preoccupying industrial and environmental pollutants. Their pleomorphic effects on several organ systems are known. Cd and Pb can cause severe damage to various organs depending on the dose, route and duration of exposure. Lungs, liver, testes, bones and kidneys are susceptible to irreversible damage from these heavy metals. According to several recent studies, renal damage due to negative effects of Cd and Pb can result from even very low exposure levels [17,18].
3.2. Biomarkers of renal damage
Regarding to the results of previous studies [8,9], changes in several sensitive and specific markers of nephrotoxicity clearly suggested the occurrence of early signs of impaired renal function for the landfill neighboring population. Kidneys show a great capacity to accumulate Cd and Pb. They are also characterized by a great sensitivity to injuries. For these reasons, kidneys are a sentinel of exposure to Cd and Pb. Much attention was therefore paid to identification of urinary biomarkers of the early stages of the nephrotoxicity of these metals [54].
Oxidative stress has been termed as cause and effect in heavy metal-induced kidney toxicity and is suggested to play a key role in the early process of glomerular and tubular damage in the kidney induced by heavy metal [19,48]. The detection of renal lesions at an earlier stage has known a major advance in recent years by the implementation of new reliable biomarkers rather than conventional tests. Several authors have also described several early biomarkers measurable in biological samples and their usefulness in differentiation of the nature and severity of injury, or providing prognostic information on the course and outcomes of renal damage [[21], [22], [23]].
The purpose of our study is therefore to evaluate the integrity and kidney overall functional capacity of subjects exposed to Mbeubeuss landfill emissions. For that, several of the newly accepted biomakers to detect tubule damage were used.
3.3. Markers of glomerular dysfunction
As defined in Fig. 1, the glomerular function was evaluated by different parameters such as α1 M, β2 M, Cyst C and albumin. The increase of urinary/blood levels of α1 M, β2 M and Cyst C (Table 1) in exposed subjects suggests a better glomerular filtration of protein in control. Trzcinka-Ochocka et al. (2004) have shown that a proteinuria induced by Cd or Pb can lead to glomerular damage with a significant increase in the urinary level of high molecular weight proteins, such as albumin. However, no change was found in albumin excretion between exposed and control unexposed populations (Table 1), but it is important to note that urinary albumin concentrations were below the microalbuminuria threshold value defined as 2 mg/mmol creatinine.
Beta-2-microglobulin is a marker of glomerular filtration and proximal tubular injury. Measurement of values in both blood and urine exhibited concentrations significantly higher in exposed subjects (blood: 39.105 vs 32.105 pg/mL, p < 0.01; urine: 421.12 vs 304.29 ng/mL, p < 0.05; Table 1). Fig. 2B also highlight a significant increase in β2M both for exposed women (p < 0.05) and exposed men (p < 0.01) in comparison with their respective controls.
Fig. 2.
Renal biomarkers in blood and urine (between control and exposed, women and men) illustrated by the median, the upper and lower quartiles, the range and the outliers (°). Significant differences between control and exposed women or men are shown as follow: * = p < 0.05; ** = p < 0.01; Mann-Whitney U test.
A:OPN (Osteopontin), B: Blood β2 M level (beta-2 microglobulin), C:Clusterin, D: (Cyst C) Cystatin C, E: pi-glutathione S-transferase (GSTπ), F:NGAL (neutrophil gelatinase-associated lipocalin).
 Control women
Control women  Exposed women
Exposed women  Control men
Control men  Exposed men
Exposed men
To predict the glomerular filtration rate and diagnose disorders of tubular function, measurement of serum and urinary β2 M was carried out with variable results.
Furthermore, urinary β2 M can also be an effective potential biomarker for early detection of acute renal injury. However, data obtained from animal and human models suggest that it may be a more predictive biomarker of glomerular rather than tubular lesions [24]. Uncertainties are notable on the increase in the urinary excretion of β2 M. It is not certain that it is only related to the dysfunction of the proximal tubule or if it is the result of the plasma levels of the protein, which can be influenced by actions of Cd at the glomerulus or on organs other than the kidney.
Cystatin C is a 13 kDa protein. Cysteine proteinase inhibitor, Cystatin C (Cyst C) is freely filtered through the glomerulus and fully reabsorbed at proximal tubule cells. Several recent studies have shown much interest in the potential of Cyst C as biomarkers of Cd nephrotoxicity [25,26]. Authors of these studies just measured serum Cystatin C levels and correlated them to glomerular function. Data on the possible effects of Cd on the urinary excretion of Cystatin C were almost absent.
Concerning urinary/serum Cyst C levels, no statistical difference was observed between exposed and control subjects in the whole population (Table 1). However, Fig. 2D showed that serum Cyst C level was significantly higher in exposed males compared with controls.
As showed in a recent study (Valcke et al. (2019), both serum Cyst C and β2 M were better biomarkers compared to creatinine in the detection of glomerular damage. Therefore, our results seemed to indicate a glomerular dysfunction in exposed subjects, but it should be noted that, regardless of residence, Fig. 2B and D showed a difference in sex. This could be justified by different B-Pb and U-Cd concentrations between men and women.
As shown by the PCA (Fig. 3), these two glomerular damage markers (Cyst C, B2 M) are correlated with monitor heavy metals exposure. Indeed, there is a significant correlation between B-Pb and Cyst C (p = 0.025) and between U-Cd and albumin (p = 0.005). In disagreement with Wanigasuriyal et al. (2017), our results showed a significant correlation between U-Cd and β2M (p = 0.03), itself significantly linked to Cyst C (p = 0.008), KIM (p = 0.016), and OPN (p = 0.007) in exposed subjects (Table 2). This result is in accordance with the findings of Valcke et al. (2019) who showed positive correlation between these biomarkers and U-Cd.
Fig. 3.
Significant correlations between renal biomarkers and blood Pb (A) and urine Cd (B).
Blood level of lead, B-Pb ; urine level of cadmium, CdU ; retinol binding protein, RBP ; alpha-glutathione S-transferase, GSTα ; pi-glutathione S-transferase, GSTπ ; Cystatin-C ; Neutrophil gelatinase-associated lipocalin, NGAL ; Kim injury molecule 1, KIM-1 ; liver-type fatty acid-binding protein, FABP-1 ; Tissue inhibitor of metalloproteinase-1, TIMP1 ; Clusterin ; Osteopontin, OPN ; Calbindin ; Uromodulin ; Collagen VI ; alpha1microglobulin, α 1 M ; beta-2 microglobulin, β2 M ; Albumin.
Table 2.
Pearson’s correlation coefficients between biomarkers of Pb/Cd exposure and renal markers measured in biological sample of participants.
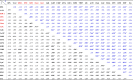 |
Relationships between the biomarkers of Pb/Cd exposure and renal markers of the subjects under study (i.e. blood level of lead. B-Pb ; urine level of cadmium.U-Cd ;retinol binding protein. RBP ; alpha-glutathione S-transferase. GST α ; pi-glutathione S-transferase. GSTπ ; Cystatin-C ; Neutrophil gelatinase-associated lipocalin. NGAL ; Kim injury molecule 1. KIM1 ; liver-type fatty acid-binding protein. FABP-1 ;Tissue inhibitor of metalloproteinase-1. TIMP1 ; Clusterin ; Osteopontin. OPN; Calbindin ; Uromodulin ; Collagen VI ; α1μglobulin. α 1 M ; Beta-2 microglobulin. β2 M. Albumin.
* = p < 0.05 ; ** = p < 0.01.
3.4. Markers of tubular lesion
In addition to the glomerular function, we have also highlighted any tubular lesion through a panel of specific biomarkers to proximal and distal tubules (Fig. 1). The levels of RBP, GSTα, GSTπ, OPN, β2 M, NGAL, KIM-1, TIMP1, Clusterin and Osteopontin were quantified to detect lesions in proximal tubules. Although there is no significant difference between exposed and control populations for some markers such as RBP, GSTα, GSTπ, and TIMP1, Table 1 and Fig. 2A, C, E, and F showed significant differences between the two groups of individuals but also between gender.
3.4.1. Proximal tubule damage
Concerning urinary RBP levels, according to our previous results [9], no statistical difference was observed between exposed and control subjects (Table 1), nor after gender segregation. Similar results were reported by Hambach et al. [19], and it was suggested that GSTα and GSTπ activity could be more suitable for early detection of even small lesions in the proximal tubule [27]. Fig. 2E showed that GSTπ activity was significantly lower in exposed females, compared to the control ones.
While urinary β2 M is a common marker of kidney damage, concentrations of kidney injury molecule (KIM-1) in blood and urine are extensively used as biomarkers of early kidney dysfunction [28]. KIM-1 has been widely studied in recent years, as a tubular injury biomarker. Its rate is high in the primary stage of acute renal injury, which would be an effective alert for chronic renal damage [29]. Several studies concluded that urinary KIM-1 levels were significantly elevated within few hours after kidney damage [30].
Interestingly, KIM-1 blood concentrations were significantly higher in exposed subjects in whole population (145.44 vs 117.40 pg/mL; Table 1) but also when the gender is considered.
Kim-1 is a biomarker specifically produced by damaged epithelial cells of proximal tubule and then transported to the urine. This fact suggests that in the event of a proximal tubular lesion, Kim-1 could be the most specific biomarker [55]. So, it is not surprising that urinary KIM-1 concentration (Table 1) in exposed subjects (0.82 ng/mL) was higher than in control (0.68 ng/mL). Furthermore, Fig. 3 shows a positive relation between KIM-1 and B-Pb.
Therefore, combining with other sensitive markers such as NGAL, Clusterin, and ONP, KIM-1 levels can suggest impaired proximal tubule for the population living close to the landfill.
One interesting aspect of the data in Table 1 is that these 3 markers showed a significant difference between exposed subjects and control group. Indeed, there was significant difference (p < 0.05) in U-NGAL, B-Clusterin and B-OPN between individuals in the polluted area (135.18 ng /mL; 237.107 pg /mL; 18231.1 pg /mL) and in the control ones (176.10 ng /mL; 150.107 pg /mL; 12887.9 pg /mL) (Table 1).
In addition to the significant difference between exposed and control subjects according to these biomarkers, Fig. 2A, 2C and 2 F highlights the difference between women and men (p < 0.05).
The expression of NGAL, is known, at fairly low scales in different tissues and organs such as kidney and various types of human cells [49]. But, after toxic renal injury, significant upregulation of NGAL is noted in the kidney. It is the most regulated transcript in this sense and this observation has been made in animal models as well as in adult humans, which validates NGAL as an early biomarker of structural renal tubular lesion (in particular proximal renal tubular [31,32]. According to very recently published data, NGAL is mainly produced by the intercalated cells of the collecting duct and thick ascending branch of the loop of Henle [33]. In normal and healthy subjects' serum and urine, NGAL concentrations are very low, on the order of nanograms (∼ 20 ng / mL at steady state) [33]. Additionally, a recent study showed that after 6 h of contrast media exposure, urinary NGAL < 20 ng/mL and blood NGAL < 179 ng/mL is a reliable sign that ruled out contrast agent-induced AKI [34]. However, the concentrations of NGAL in our study show concentrations 6 times higher than this value, for both control and exposed subjects (Table 1). This result does not reconsider the choice of the control population, but highlights the panel of toxic agents that may induce kidney damage. The increase of NGAL concentration is detectable as 3 h after a tubular injury and remains high approximately 6–12, depending of the acuity of the damage [35]. According to the data of Parikh et al. [36] this elevation can peak up to 5 days after the initial injury, particularly when the injury is severe [36]. Morever, Haase et al. [37] and Wagene et al. (2008) have shown that urine and plasma NGAL levels are correlated to the severity and duration of the injury. NGAL high levels claim that population of our study is in contact with nephrotoxic agents. Fig. 2F showed that women have higher concentrations of NGAL in urine, and this result is consistent with other studies [38,39] showing that NGAL production increases with age and is higher in women than men.
Several studies have confirmed that serum Cyst C can diagnose kidney injury earlier than serum creatinin [40,41], but later than NGAL [42,43]. This finding suggests that NGAL is one of the most valuable biomarkers for renal failure, with high sensitivity and specificity, and this is in agreement with our study. Indeed, no significant difference was observed for Cyst C while there was a significant difference between exposed and control subjects for NGAL.
3.4.2. Distal tubule damage
To assess tubular kidney damage, we also determined OPN and Clusterin levels in blood and urine. Mann-Whitney U test analysis revealed that there is a significant difference (p < 0.05) between exposed and control groups in the B-ONP concentration (18231.1 versus 12887.9 pg/mL) and B-Clusterin concentration (237.107 versus 150.107pg/mL) (Table 1). This difference is also highlighted by Figs. 2A and 2C between exposed and control men (p < 0.05) and among exposed and control women (p < 0.05).
According to a study which took place in Sri Lanka, in subjects suffering from chronic kidney disease, the urinary markers clusterine, cyst C and β2 M were significantly higher compared to the controls [44]. Excepted β2 M, these results are not in agreement with ours, although Pb and Cd exposure levels of the different study populations are similar.
Referring to Wanigasuriya et al. [44]; OPN and Clusterin in urine are useful in screening for early tubular (proximal and distal) dysfunction induced by heavy metals such as Cd/Pb. A significant increase of their excretion in exposed subjects may occur even with lesions of the renal tubule.
Fig. 2C highlights a significant increase in Clusterin both for exposed women (p < 0.05) and exposed men (p < 0.05) in comparison with their respective controls. However, for OPN, the difference only occurred between exposed and control men (p < 0.05) (Fig. 2A). These results indicated an alteration in proximal and distal function.
In agreement with lIU Liu et al. (2016) [56], the predictive effect of urinary and blood NGAL, KIM-1, Clusterin, Cyst C, OPN, β2 M was stronger than other biomarkers (FABP-1, Collagen IV). From data in Table 1, it can be seen that FABP-1, although slightly increased in the exposed population, was not significantly different compared to controls.
Morever, our data showed that B-Pb was correlated with B-OPN (p = 0.042) and strongly correlated with U-OPN (Fig. 3) (p = 0.001). Likewise, U-Cd was correlated with Clusterin (p = 0.02) and U-OPN (p = 0.044), itself significantly correlated to KIM-1, TIMP1, albumin, α1 M and Calbindin (Table 2). The relations observed herein between nephrotoxic metal exposures and OPN is stronger than with other biomarkers of renal effect. This is coherent with a greater sensitivity of OPN to early kidney effects as compared to other effect biomarkers. Indeed, it appears logical that a correlation between an exposure biomarker and an effect biomarker appears sooner for the most sensitive effect biomarker.
4. Conclusion
This study investigated a set of biomarkers on interactions between toxic metal exposure and renal function in a group of Senegalese subjects living near a waste dump.
Specific increases of KIM-1, NGAL, Clusterin, Cystatin C, OPN, β2 M, Uromodulin and Collagen IV following the exposure to nephrotoxicants agents of Mbeubeus landfill have been observed. Taken together these results suggest a glomerular and tubule dysfunction in exposed subjects.
The results supported the possible occurrence of necrosis of proximal and distal tubule epithelial cells since biomarkers such as KIM-1, NGAL, Clusterin, Cyst C, OPN, β 2 M, Uromodulin, began to be significantly excreted in urine. Pearson’s correlation matrix between the various parameters measured in the biological samples showed significant correlations between a panel of effect biomarkers of exposure to metals. More specifically, significant correlations were noted for the same metal with different renal biomarkers.
The relations between biomarkers of Cd and /or Pb exposure and early effect biomarkers predict the future risk of severe kidney injury if the exposure continues. Its findings warrant further investigations of longitudinal data in a greater number of participants, taking into account genetic variations. Indeed, some researchers believe that these genetic variations may be one of the main causes of the sensitivity of different inter-individuals to heavy metals. In general, it is assumed that polymorphisms in genes responsible for heavy metal metabolisms, may play an important role in body levels, sensitivity and severity of the health effects caused by these materials.
However, overall, these results closely supported the usefulness of this panel of early effect biomarkers of kidney injury to better monitor exposure environmental to relatively low to moderate levels of Cd and/or Pb.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
The research described in this article benefited from grants from the “Centre de Recherche pour le Dévelopement International” (CRDI) of Dakar (Senegal). The “Ministère de l’Enseignement Supérieur et de la Recherche” of Dakar (Senegal) “ and the Agence Universitaire de la Francophonie” (AUF) provided Mathilde Cabral’s mobility financial support. We are particularly grateful to Dr Mbaye Diaw Dioum for his useful suggestions and careful rereading of the article.
Edited by Dr. A.M Tsatsaka
References
- 1.Muedi V.Ma.K.L. Heavy Metals. IntechOpen; 2018. Environmental contamination by heavy metals. Editor. [Google Scholar]
- 2.Ancona C. Mortality and morbidity in a population exposed to multiple sources of air pollution: a retrospective cohort study using air dispersion models. Environ. Res. 2015;137:467–474. doi: 10.1016/j.envres.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 3.Forastiere F. Health impact assessment of waste management facilities in three European countries. Environ. Health. 2011;10:53. doi: 10.1186/1476-069X-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mataloni F. Morbidity and mortality of people who live close to municipal waste landfills: a multisite cohort study. Int. J. Epidemiol. 2016;45(3):806–815. doi: 10.1093/ije/dyw052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porta D. Systematic review of epidemiological studies on health effects associated with management of solid waste. Environ. Health. 2009;8:60. doi: 10.1186/1476-069X-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarup L. Cancer risks in populations living near landfill sites in Great Britain. Br. J. Cancer. 2002;86(11):1732–1736. doi: 10.1038/sj.bjc.6600311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanes-Vidal V. Respiratory and sensory irritation symptoms among residents exposed to low-to-moderate air pollution from biodegradable wastes. J. Expo. Sci. Environ. Epidemiol. 2014;24(4):388–397. doi: 10.1038/jes.2014.20. [DOI] [PubMed] [Google Scholar]
- 8.Cabral M. Low-level environmental exposure to lead and renal adverse effects: a cross-sectional study in the population of children bordering the Mbeubeuss landfill near Dakar, Senegal. Hum Exp Toxicol. 2012;31(12):1280–1291. doi: 10.1177/0960327112446815. [DOI] [PubMed] [Google Scholar]
- 9.Cabral M. Effects of environmental cadmium and lead exposure on adults neighboring a discharge: evidences of adverse health effects. Environ. Pollut. 2015;206:247–255. doi: 10.1016/j.envpol.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 10.Bonventre J.V., Vaidya V.S., Schmouder R. Next-generation biomarkers for detecting kidney toxicity. Nat. Biotechnol. 2010;28:436–440. doi: 10.1038/nbt0510-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann D., FuchsT Henzler T., Matheis K., Herget T., Dekant W., Hewitt P., Mally A. Evaluation of a urinary kidney biomarker panel in rat models of acute and subchronic nephrotoxicity. Toxicology. 2010;277:49–58. doi: 10.1016/j.tox.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Dieterle F., Perentes E., Cordier A., Roth D.R., Verdes P., Grenet O. Urinary Clusterin, Cystatin C, β2-microglobulin and total protein as markers to detect drug-induced kidney injury. Nat. Biotechnol. 2010;28:463–469. doi: 10.1038/nbt.1622. doi:10.1038/ nbt.1622. [DOI] [PubMed] [Google Scholar]
- 13.Vaidya V.S., Waikar S.S., Ferguson M.A. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin. Transl. Sci. 2008;1:200–208. doi: 10.1111/j.1752-8062.2008.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaidya V.S., Bonventre J.V. Mechanistic biomarkers for cytotoxic acute kidney injury. Expert Opin Drug MetabToxicol. 2006;2:697–713. doi: 10.1517/17425255.2.5.697. [DOI] [PubMed] [Google Scholar]
- 15.Fréry N., Volatier J.L., Zeghnoun A., Sarter H., Falq G., Thébault A., Pascal M., Bérat B., De Crouy-Chanel P. Rapport d’étude. Institut de veille sanitaire; Saint-Maurice: 2009. Etude d’imprégnation par les dioxines des populations vivant à proximité d’usines d’incinération d’ordures ménagères; p. 228.http://www.invs.sante.fr [Google Scholar]
- 16.Fréry N., Saoudi A., Garnier R., Zeghnoun A., Falq G. 2011. Exposition de la population française aux substances chimiques de l’environnement. Saint-Maurice: Institut de veille sanitaire. p.151.http://www.invs.sante.fr consulté en 08/2019. [Google Scholar]
- 17.Huang M., Seong-Jin C., Dong-Won K., Na-Young K., Bae Hye-Sun, Byung-Sun C., Il-Je Y., Jung-Duck P. Evaluation of factors associated with cadmium exposure and kidney function in the general population. Environ. Toxicol. 2011 doi: 10.1002/tox.20750. DOI 10.1002/tox. [DOI] [PubMed] [Google Scholar]
- 18.Kim S., Kwon H.J., Cheong H.K., Choi K., Jang J.Y., Jeong W.C., Kim D.S., Yu S., Kim Y.W., Lee K.Y., Yang S.O., Jhung I.J., Yang W.H., Hong Y.C. Investigation on health effects of an abandoned metal mine. J. Korean Med. Sci. 2008;23:452–458. doi: 10.3346/jkms.2008.23.3.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hambach R., Lison D., D’Haese P.C., Weyler J., De Graef E., De Schryver A., Lamberts L.V., Van Sprundel M. Co-exposure to lead increases the renal response to low levels of cadmium in metallurgy workers. Toxicol. Lett. 2013;222(2):233–238. doi: 10.1016/j.toxlet.2013.06.218. [DOI] [PubMed] [Google Scholar]
- 20.Wieloch M., Kamin’ski P., Ossowska A. Do toxic heavy metals affect antioxidant defense mechanisms in humans? Ecotoxicology and Environment Safety. 2012;78:195–205. doi: 10.1016/j.ecoenv.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 21.Nickolas T.L., Schmidt-Ott K.M., Canetta P. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J. Am. Coll. Cardiol. 2012;59:246–255. doi: 10.1016/j.jacc.2011.10.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singer E., Elger A., Elitok S. Urinary neutrophil gelatinase-associated lipocalin distinguishes pre-renal from intrinsic renal failure and predicts outcomes. Kidney Int. 2011;80:405–414. doi: 10.1038/ki.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Endre Z.H., Walker R.J., Pickering J.W. Early intervention with erythropoietin does not affect the outcome of acute kidney injury (the EARLYARF trial) Kidney Int. 2010;77:1020–1030. doi: 10.1038/ki.2010.25. [DOI] [PubMed] [Google Scholar]
- 24.Argyropoulos C.P., Chen S.S., Ng Y.-H., Roumelioti M.-E., Shaffi K., Singh P.P., Tzamaloukas A.H. Rediscovering Beta-2 microglobulin As a biomarker across the Spectrum of kidney diseases. Front. Med. (Lausanne) 2017;4:73. doi: 10.3389/fmed.2017.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harisa G.I., Attia S.M., Ashour A.E., Abdallah G.M., Omran G.A., Touliabah H.E. Cigarette smoking and hyperglycemia increase renal response to low levels of cadmium in welders: cystatin C as a sensitive marker. Biol. Trace Elem. Res. 2014;158:289–296. doi: 10.1007/s12011-014-9939-1. [DOI] [PubMed] [Google Scholar]
- 26.Wallin M., Sallsten G., Lundh T., Barregard L. Low-level cadmium exposure and effects on kidney function. Occup. Environ. Med. 2014;71:848–854. doi: 10.1136/oemed-2014-102279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garçon G., Leleu B., Marez T., Zerimech F., Haguenoer J.M., Furon D., Shirali P. Biomonitoring of the adverse effects induced by the chronic exposure to lead and cadmium on kidney function: usefulness of alpha-glutathione S-transferase. Sc. Tot. Environ. 2007;377:165–172. doi: 10.1016/j.scitotenv.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Ruangyuttikarn W., Panyamoon A., Nambunmee K., Honda R., Swaddiwudhipong W., Nishijo M. Use of the kidney injury molecule-1 as a biomarker for early detectionof renal tubular dysfunction in a population chronically exposed to cadmiumin the environment. Springerplus. 2013;17(Oct. (2)):533. doi: 10.1186/2193-1801-2-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kadioglu T., Uzunlulu M., Yigit Kaya S., Oguz A., Gonenli G., Isbilen B., Isman F.K. Urinary kidney injury molecule-1 levels as a marker of early kidney injury in hypertensive patients. Minerva UrolNefrol. 2015 [PubMed] [Google Scholar]
- 30.Lim A.I., Tang S.C., Lai K.N., Leung J.C. Kidney injury molecule -1, more than just an injury marker of tubularepithelial cells? J. Cell. Physiol. 2013;228:917–924. doi: 10.1002/jcp.24267. [DOI] [PubMed] [Google Scholar]
- 31.Zappitelli M., Washburn K.K., Arikan A.A., Loftis L., Ma Q., Devarajan P. Urine neutrophil gelatinase-associated lipocalin is anearly marker of acute kidney injury in critically ill children: a prospectivecohort study. Crit Care (London, England) 2007;11:R84. doi: 10.1186/cc6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mishra J., Mori K., Ma Q., Kelly C., Barasch J., Devarajan P. Neutrophil gelatinase-associated lipocalin: a novel earlyurinary biomarker for cisplatin nephrotoxicity. Am. J. Nephrol. 2004;24:307–315. doi: 10.1159/000078452. [DOI] [PubMed] [Google Scholar]
- 33.Charlton J.R., Portilla D., Okusa M.D. A basic science view of acutekidney injury biomarkers. Nephrol. Dial. Transplant. 2014;29:1301–1311. doi: 10.1093/ndt/gft510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quintavalle C., Anselmi C.V., De Micco F., Roscigno G., Visconti G., Golia B., Focaccio A., Ricciardelli B., Perna E., Papa L., Donnarumma E., Condorelli G., Briguori C. Neutrophil gelatinase-associated lipocalin and contrastinducedacute kidney injury. CircCardiovascInterv. 2015;8 doi: 10.1161/CIRCINTERVENTIONS.115.002673. [DOI] [PubMed] [Google Scholar]
- 35.Devarajan P. Review: neutrophil gelatinase-associated lipocalin:a troponin-like biomarker for human acute kidney injury. Nephrology(Carlton, Vic) 2010;15:419–428. doi: 10.1111/j.1440-1797.2010.01317.x. [DOI] [PubMed] [Google Scholar]
- 36.Parikh C.R., Coca S.G., Thiessen-Philbrook H., Shlipak M.G., Koyner J.L., Wang Z. Postoperative biomarkers predict acutekidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol. 2011;22:1748–1757. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haase M., Devarajan P., Haase-Fielitz A., Bellomo R., Cruz D.N., Wagener G. The outcome of neutrophil gelatinaseassociatedlipocalin-positive subclinical acute kidney injury: amulticenter pooled analysis of prospective studies. J. Am. Coll.Cardiol. 2011;57:1752–1761. doi: 10.1016/j.jacc.2010.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cullen M.R., Murray P.T., Fitzgibbon M.C. Establishment of areference interval for urinary neutrophil gelatinase-associatedlipocalin. Ann. Clin. Biochem. 2012;49(Pt 2):190–193. doi: 10.1258/acb.2011.011105. [DOI] [PubMed] [Google Scholar]
- 39.Pennemans V., Rigo J.M., Faes C., Reynders C., Penders J., Swennen Q. Establishment of reference values for novel urinary biomarkersfor renal damage in the healthy population: are age andgender an issue? Clin. Chem. Lab. Med. 2013;51:1795–1802. doi: 10.1515/cclm-2013-0157. [DOI] [PubMed] [Google Scholar]
- 40.Thongprayoon C., Cheungpasitporn W., Kashani K. Serum creatininelevel, a surrogate of muscle mass, predicts mortalityin critically ill patients. J. Thorac. Dis. 2016;8:E305–11. doi: 10.21037/jtd.2016.03.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wagener G., Minhaz M., Mattis F.A., Kim M., Emond J.C., Lee H.T. Urinary neutrophil gelatinase-associated lipocalin as a markerof acute kidney injury after orthotopic liver transplantation. Nephrol. Dial. Transplant. 2011;26:1717–1723. doi: 10.1093/ndt/gfq770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medic B., Rovcanin B., Vujovic K.S., Obradovic D., Duric D., Prostran M. Evaluation of novel biomarkers of acute kidney injury:the possibilities and limitations. Curr. Med. Chem. 2016;23:1981–1997. doi: 10.2174/0929867323666160210130256. [DOI] [PubMed] [Google Scholar]
- 43.Szerlip H.M., Chawla L.S. Predicting acute kidney injury prognosis. Curr. Opin. Nephrol. Hypertens. 2016;25:226–231. doi: 10.1097/MNH.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 44.Wanigasuriya l K., Jayawardene I., Amaraslrlwardanav C., Wickremasinghe R. Novel urinary biomarkers and their association with urinary heavy metals in chronic kidney disease of unknown aetiology in Sri Lank: a pilot study. Ceylon Med. J. 2017;62:210–217. doi: 10.4038/cmj.v62i4.8568. DOI: http://doLorgllO.4038/cmj.v62i4.8568. [DOI] [PubMed] [Google Scholar]
- 50.Whittaker M.H., Wang G., Chen X.Q., Lipsky M., Smith D., Gwiazda R., Fowler B.A. Exposure to Pb, Cd, and As mixtures potentiates the production of oxidative stress precursors: 30-day, 90-day, and 180-day drinking water studies in rats. Toxicol. Appl. Pharmacol. 2011;254:154–166. doi: 10.1016/j.taap.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 51.Lee J.C., Son Y.O., Pratheeshkumar P., Shi X. Oxidative stress and metal carcinogenesis. Free Radical Biology and Medicine. 2012;52:742–757. doi: 10.1016/j.freeradbiomed.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 52.Heitland P., Köster H.D. Biomonitoring of 37 trace elements in blood samples from inhabitants of northern Germany by ICP–MS. J. Trace Elements Med. Biol. 2006;20:253–262. doi: 10.1016/j.jtemb.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 53.Heitland P., Köster H.D. Biomonitoring of 30 trace elements in urine of children and adults by ICP-MS. Clin. Chim. Acta. 2006;365:310–318. doi: 10.1016/j.cca.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 54.Bernard A., Lauwerys R. Cadmium in human population. Experientia. 1984;40:143–152. doi: 10.1007/BF01963577. [DOI] [PubMed] [Google Scholar]
- 55.Prozialeck W.C., Edwards J.R., Lamar P.C., Liu J., Vaidya V.S., Bonventre J.V. Expression of kidney injury molecule-1 (Kim-1) in relation to necrosis and apoptosis during the early stages of Cd-induced proximal tubule injury. Toxicology and Applied Pharmacology. 2009;238:306–314. doi: 10.1016/j.taap.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu X., Foster M.C., Tighiouart H., Anderson A.H., Beck G.J. Non-GFR determinants of low-molecular-weight serum protein filtration markers in CKD. Am J Kidney Dis. 2016;68:892–900. doi: 10.1053/j.ajkd.2016.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]