Abstract
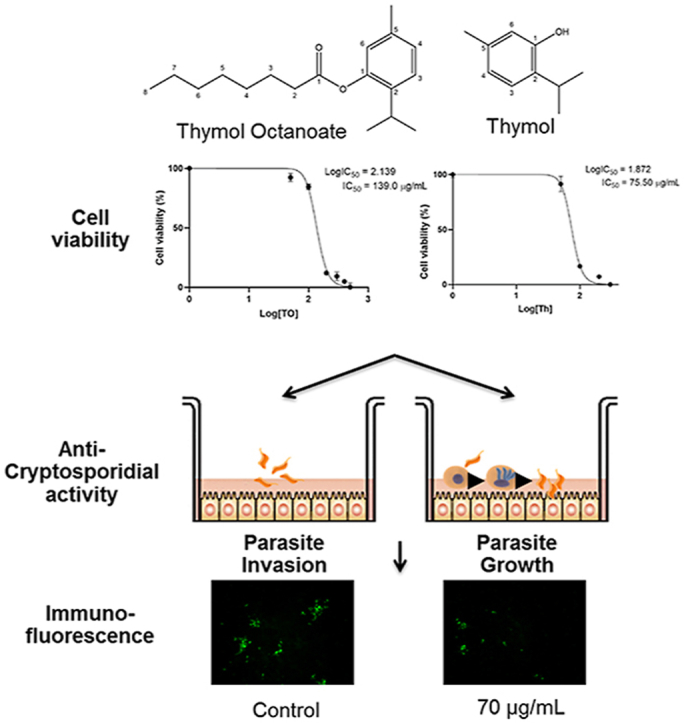
Cryptosporidium parvum is a protozoan parasite that infects intestinal epithelial cells causing malabsorption and severe diarrhea. The monoterpene thymol has been reported to have antifungal and antibacterial properties but less is known about the antiparasitic effect of this compound. Terpenes are sometimes unsuitable for therapeutic and food applications because of their instability. Esterification of terpenes eliminates this disadvantage. The present study evaluates the effects of thymol (Th) and a thymol ester, thymol octanoate (TO), against C. parvum infectivity in vitro. The cytotoxicity IC50 value for TO after 24 h of treatment was 309.6 μg/mL, significantly higher than that of Th (122.5 μg/mL) in a human adenocarcinoma cell line (HCT-8). In the same way, following 48 h of treatment, the cytotoxicity IC50 value for TO was significantly higher (139 μg/mL) than that of Th (75.5 μg/mL). These results indicate that esterification significantly reduces Th cytotoxicity. Dose-dependent effects were observed for TO and Th when both parasite invasion and parasite growth assays were evaluated. When evaluated for their activity against C. parvum growth cultured in vitro in HCT-8 cells, the anti-cryptosporidial IC50 values were 35.5 and 7.5 μg/mL, for TO and Th, respectively. Together, these findings indicate that esterified thymol has anti-cryptosporidial effect comparable with its parental compound thymol, but with improved safety margins in mammalian cells and better physicochemical properties that could make it more suitable for diverse applications as an antiparasitic agent.
Keywords: Cryptosporidium parvum, Thymol, Esterification, Thymol octanoate, Antiparasitic
Graphical abstract
Highlights
•Thymol and thymol octanoate were active against C. parvum in vitro, with low cytotoxicity in HCT-8 cells.
•Esterified thymol has anti-cryptosporidial effect comparable with its parental compound but with improved physicochemical properties.
•Thymol had a stronger effect than thymol octanoate against C. parvum invasion but similar effects when parasite growth was evaluated.
1. Introduction
Cryptosporidium parvum is a zoonotic protozoan parasite belonging to the phylum Apicomplexa that has world-wide prevalence, and the second leading cause of water and foodborne diarrheal diseases worldwide (Ryan et al., 2016). The impact is especially devastating among immunocompromised individuals (Z. D. Wang et al., 2018) as well as infants living in resource-constrained regions, and is associated with an estimated annual death rate of >200,000 children under 2 years of age (Checkley et al., 2015; Kotloff, 2017; Sow et al., 2016). This parasite replicates within intestinal epithelial cells, compromising the function of the intestinal barrier (Kumar et al., 2019), which leads to progressive atrophy of the villi, nutrient malabsorption, and severe diarrhea. The parasite is mostly transmitted through water contaminated with parasite oocysts shed by infected livestock. C. parvum is difficult to eliminate because oocysts are resistant to most chemical disinfectants as well as to commonly used water treatments such as chlorination (Cabada and White, 2010; Daniels et al., 2016). Nitazoxanide is the only moderately effective drug approved by the United States Food and Drug Administration (FDA), but whose effect is questionable, especially among immunocompromised patients (Abubakar et al., 2007; Sears and Kirkpatrick, 2007). Paromomycin, an antibiotic commonly used to treat C. parvum infections in mice, has also been determined to be ineffective in both immunocompetent and immunocompromised human populations (Hewitt et al., 2000). Therefore, further efforts into the discovery of novel therapeutics from either synthetic or natural sources are a high priority to address C. parvum infection (Anthony et al., 2005; Jin et al., 2019).
Scientific interest in natural products, primarily plant essential oils, with anticryptosporidial properties has been increasing in recent years (Jin et al., 2019). The antimicrobial properties of essential oils and their individual compounds have been widely studied against numerous bacteria, viruses, fungi, parasites, and insects (Mérillon, 2018; Monzote et al., 2012; Rao et al., 2019). Plant essential oils and their compounds can be used as alternatives or adjuvants to current antiparasitic therapies.
Thymol (2-isopropyl-5-methylphenol) is a monoterpene and is the main component (30–40%) of thyme (Thymus vulgaris) and oregano (Origanum vulgaris) (1–30%) essentials oils (Youssefi et al., 2019). Thymol has been reported to have antibacterial (Kachur and Suntres, 2019), antifungal (K. Wang et al., 2018), larvicidal (López et al., 2018), and acaricidal (Araújo et al., 2015) properties, but less is known about its anti-protozoal properties. Thymol, which is generally recognized as safe (GRAS), has the advantage of being readily available from many natural sources and has relatively low toxicity to mammalian cells. Therefore, thymol and similar compounds could be considered as potential starting points for the development of new treatments against parasites (Chauhan and Kang, 2014).
Despite their strong bioactivity, monoterpenes are quite unstable in formulations, showing high volatility and strong flavor, which limits their suitability for food applications. Recently, studies have focused on developing more stable formulations to overcome these disadvantages. In order to improve their stability, promising strategies have included encapsulation, emulsification, and chemical modifications like esterification (Bilia et al., 2014; Rao et al., 2019; Tharamak et al., 2019). In this study, we tested thymol (Th) and a thymol ester, 2-Isopropyl-5-methylphenyl octanoate, or thymol octanoate (TO) against C. parvum infection in vitro.
2. Materials and methods
2.1. Materials
Unless otherwise indicated, most reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA) and were of reagent grade or highest purity. Thymol octanoate (2-Isopropyl-5-methylphenyl octanoate) was kindly donated by Tyratech (Morrisville, NC, USA). RPMI 1640 culture media was obtained from Gibco (Grand Island, NY, USA) and supplemented with 10% Horse Serum, 2 g/L of sodium bicarbonate, 2.5 g/L of glucose, 1 × antibiotic–antimycotic (Gibco), and 1 × sodium pyruvate (Gibco). Human adenocarcinoma cells (HCT-8) were obtained from the ATCC (CCL 244). C. parvum oocysts extracted from fresh feces of an infected male Holstein calf were kindly provided by Dr. William Witola (Department of Pathobiology, University of Illinois at Urbana-Champaign, USA).
2.2. Compound cytotoxicity by flow cytometry
Cell cytotoxicity of Th and TO was measured by flow cytometry. Briefly, HCT-8 cells were cultured in T-25 flasks (Thermo Scientific™, MA, USA) in 5 mL of RPMI 1640 complete medium in an atmosphere containing 5% CO2. Th and TO were tested at various concentrations (50, 100, 200, 300, 400, and 500 μg/mL). Compounds were dissolved in DMSO and further diluted with RPMI 1640 complete medium and tested in triplicate. The DMSO concentration did not exceed 0.1% in any of the dilutions. Cells were grown to 80% confluence and then treated for 24 or 48 h at 37 °C. Culture medium with 0.1% DMSO was used as negative control. After treatment, cells were detached by trypsinization with TrypLE™ Express, quenched with media, centrifuged at 800 rpm for 5 min, and resuspended in fresh RPMI medium at a density of 106 cells/mL. Samples were analyzed using a BD LSR II Flow Cytometry Analyzer (BD Biosciences, San Jose, CA, USA). Briefly, cells were stained with 2 μg/mL of propidium iodide (488 nm) for 5 min in the dark and snap-vortexed immediately before placement in a flow cytometer. Forward scatter area (FSC-A) versus side scatter area (SSC-A) dot plots for each cell sample were generated using the flow cytometry software (FACSDiVa™ v6.1). Voltages for FSC-A and SSC-A were adjusted to achieve gating on single-cell populations. Forward and Side Scatter gates were established to exclude debris. The population on the left side (expressing no fluorescence) was gated as live cells, while the population on the lower right was gated as dead cells.
2.3. Parasites
Sporozoites were excysted from C. parvum oocysts following the method described by (Kuhlenschmidt et al., 2016). Briefly, 3 × 108 purified C. parvum oocysts were suspended in 500 μl of phosphate-buffered saline (PBS), and an equal volume of 40% commercial laundry bleach was added and incubated for 10 min at 4 °C. After incubation, the pellet was washed four times in PBS containing 1% (w/v) bovine serum albumin and resuspended in Hanks balanced salt solution (HBSS), incubated for 60 min at 37 °C, and mixed with an equal volume of warm 1.5% sodium taurocholate in HBSS for another 60 min at 37 °C. The excysted sporozoites were collected by centrifugation, suspended in RPMI 1640 medium, and purified by passing the suspension through a sterile 5.0 μM syringe filter (Millex-SV, USA), followed by enumeration by hemocytometer. Sporozoites were maintained on ice until use.
2.4. In vitro anti-cryptosporidial activity
To evaluate the efficacy of TO and Th against C. parvum, concentrations lower than the compounds’ cytotoxicity IC50 values in HCT-8 cells were used. Compounds were dissolved in 0.1% DMSO and further diluted in RPMI 1640 medium. For evaluating the effect on the parasite invasion, host cell HCT-8 monolayers were cultured in 96-well plates and incubated with 200 μL of different concentrations of both Th and TO (10, 20, 40, 70, and 100 μg/mL) shortly before infecting them with C. parvum sporozoites (4 × 104 sporozoites/well) for 48 h, to assess if the compounds would block host cell invasion by sporozoites. For evaluating the effect on parasite growth, host cell HCT-8 monolayers were incubated with C. parvum sporozoites (4 × 104 sporozoites/well) suspended in 200 μL of RPMI 1640 medium for 2 h, the medium was replaced with 200 μL of RPMI 1640 medium with different concentrations of each compound (from 0 to 100 μg/mL) for additional 48 h. In all experiments, negative controls were treated with 0.1% DMSO for the same duration of corresponding treatment groups. Paromomycin was used as a reference control drug. Host cell HCT-8 monolayers were cultured in 96-well plates and incubated for 48 h with various concentrations of paromomycin (50, 100, 200, 300, 400 and 500 μg/mL) either shortly after infection (0 h p.i.) with C. parvum sporozoites (4 × 104 sporozoites/well), or 2 h post-infection (2 h p.i) to replicate the conditions of parasite invasion and parasite growth assays, respectively.
2.5. Immunofluorescence assays
Cells were processed for immunofluorescence analysis as described previously (Kuhlenschmidt et al., 2016). The medium was removed from culture wells, and cells were fixed with methanol-acetic acid (9:1v/v) for 5 min at room temperature. Cells were permeabilized by successive washes with buffer (0.1% Triton X-100, 0.35 M NaCl, 0.13 M Tris-base, pH 7.6) and blocked with 5% normal goat serum, followed by overnight staining with fluorescein (FL)-labeled antibody against C. parvum (SporoGlo; Waterborn, Inc., New Orleans, LA, USA) at 4 °C. The stained cells were washed twice in PBS, and then 200 μL of water was added to each well. Plates were imaged by using a 20X objective of an inverted fluorescence microscope. Parasite fluorescence quantification in the captured images was done using ImageJ version 1.37v software (NIH, USA).
2.6. Statistical analyses
Statistical analyses were done using JMP version 7.0 (SAS, Inc. Cary, NC). Statistical analyses were performed using Student's t-test. P < 0.05 was considered significant. Non-linear regression using GraphPad Prism® version 5.0 (GraphPad Software Inc., La Jolla, CA, USA) was used to calculate the 50% inhibitory concentration (IC50) values.
3. Results
3.1. Compounds cytotoxicity
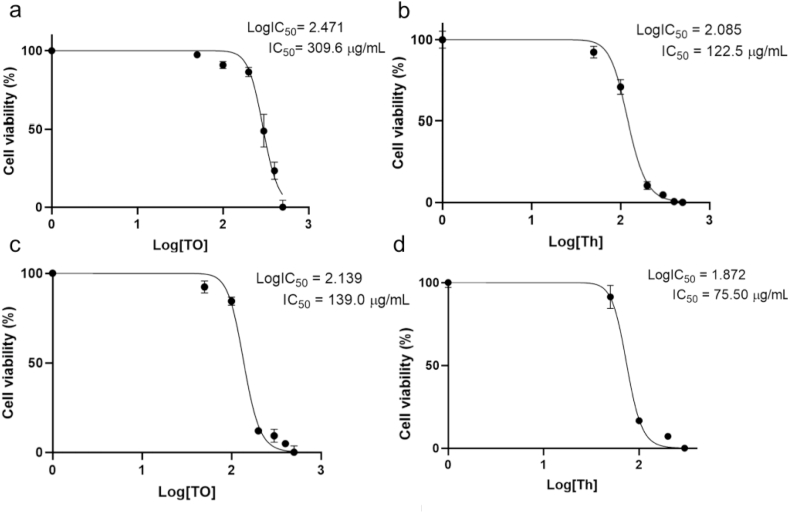
The cytotoxic effect of Th and TO in HCT-8 cells was evaluated by flow cytometry using propidium iodide (PI) staining. Dose-response curves for the compounds after 24 h and 48 h of treatment of the cell cultures were derived (Fig. 1a and Fig. 1b, respectively). Both Th and TO showed a dose-dependent decrease in the viability of the cells with IC50 values 2.5- and 1.8 fold higher for 24 and 48 h exposure, respectively. The cytotoxicity of TO was less severe than that of Th; however, doses of 500 μg/mL for both compounds showed almost 100% decrease in cell viability, with no significant difference between the treatments. Interestingly, cells showed an increase in their viability when exposed to lower concentrations of the compounds. When cells were exposed to compounds for 48 h (Fig. 1b), the cell survival rate was lower for both compounds, which indicates that the cytotoxic effect of both compounds increases with time.
Fig. 1.
Analysis of HCT-8 cell viability after treatment with varying amounts of Thymol (Th) and Thymol Octanoate (TO). Cells were incubated with increasing concentrations (50, 100, 200, 300, 400, and 500 μg/mL) of compounds for 24 h (a) or 48 h (b). Data points represent means of three independent experiments ±SD (bars). Letter superscripts represent statistical differences among treatments at each concentration after ANOVA and Tukey post hoc test (P < 0.05).
The cell viability half-maximal inhibitory concentration (IC50) value for TO after 24 h of treatment was 309.6 μg/mL (Fig. 2a), significantly higher than the cytotoxicity IC50 for thymol (122.5 μg/mL) (Fig. 2b). Following 48 h of treatment, the IC50 for TO was significantly higher (139 μg/mL) (Fig. 2c) than that of Th (75.5 μg/mL) (Fig. 2d). These findings indicate that esterification significantly reduces thymol cytotoxicity.
Fig. 2.
Analysis of the Log-dose cell viability response to Thymol octanoate (TO) or Thymol (Th) after 24 h (a and b, respectively) or after 48 h (c and d, respectively) of culture. Values were estimated with nonlinear curve-fitting analysis with Prism Software (GraphPad, La Jolla, CA, USA). IC50 = Concentration (μg/mL) that inhibit 50% of cell viability. All experiments were performed in triplicate. Data points represent mean ± SD (bars).
3.2. Anti-cryptosporidial activity
To evaluate the effect of varying concentrations (from 0 to 100 μg/mL) of each compound against C. parvum in vitro, an immunofluorescence assay was performed. The inhibitory effects of Th and TO on parasite invasion in cultures when the compounds were added at the time of infection (Fig. 3a) were significantly higher (P < 0.05) than when the compounds were added 2 h post-infection of the HCT-8 cells with parasites (Fig. 3b). This difference was particularly evident at concentrations of 10, 20, and 40 μg/mL, but as the concentration increased further, the effect was similar (Fig. 3a and b). This indicated that exposure of extracellular parasites to compounds before host cell invasion enhanced the efficacy of the compounds in retarding the growth of the parasites after they invaded host cells. Both Th and TO depicted dose-dependent inhibitory effects on the growth of the parasites in culture.
Fig. 3.
Analysis of the effect of varying concentrations of Thymol octanoate (TO) and Thymol (Th) on the growth of Cryptosporidium parvum in HCT-8 cells. Equal amounts of freshly excysted sporozoites of C. parvum were inoculated into HCT-8 cells and different concentrations of compounds (0, 10, 20, 40, 70, and 100 μg/mL) were added at the time of infection (a) or 2 h post-infection (b). Cultures were analyzed for parasite infectivity and proliferation by an immunofluorescence assay after 48 h. Fluorescence generated is shown on the Y-axis representing the parasite load. Data points represent the mean of three independent experiments ±SD (bars). Letter superscripts represent statistical differences among treatments at each concentration after ANOVA and Tukey post hoc test (P < 0.05).
Results showed a slightly less dramatic effect when different concentrations of Th and TO were evaluated on parasite growth compared to the results obtained for parasite invasion assay. Infected HCT-8 cultures treated with 70 μg/mL displayed a significant decrease in parasite growth of 66% and 80% when exposed to and Th, respectively. The IC50 values of Th and TO's inhibitory effect on the growth of C. parvum in vitro were derived from the dose-response curves using GraphPad PRISM software. The TO and Th IC50 concentrations against parasite growth when parasites were exposed to compounds before host cell invasion were 35.5 and 7.5 μg/mL, respectively (Table 1). On the other hand, when parasites were allowed to invade host cells before compound treatment of the cultures, the TO and Th IC50 concentrations were 19 and 20.3 μg/mL, respectively (Table 1). As a positive control drug, paromomycin treatment depicted a concentration-dependent effect against C. parvum growth (Fig. 4) with IC50 concentrations of 402.1 and 365.5 μg/mL with no significant differences (P > 0.05) when the drug was added to the culture at the time of infection or 2 h post-infection, respectively.
Table 1.
IC50 values (μg/mL) for TO and Th against HCT-8 cell viability (24 and 48 h) and C. parvum invasion or growth in vitro.
| Cell viability |
Anti-cryptosporidial activity |
|||
|---|---|---|---|---|
| IC50 24 h | IC50 48 h | IC50 Invasion | IC50 Growth | |
| TO | 309.6 | 139.0 | 35.56 | 19.03 |
| Th | 122.5 | 75.5 | 7.51 | 20.31 |
Fig. 4.
Dose-response curves of various concentrations of paromomycin on the infectivity of C. parvum in HCT-8 cells. Equal amounts of freshly excysted C. parvum sporozoites were inoculated into HCT-8 cells in culture and different concentrations of paromomycin (0–500 μg/mL) dissolved in sterile distilled water were added at the time of infection (0 h p.i) or 2 h post-infection (2 h p.i.). Cultures were analyzed for parasite infectivity and proliferation by an immunofluorescence assay after 48 h. Fluorescence generated is shown on the Y-axis representing the parasite load. Data points represent means of three independent experiments ±SD (bars). Superscript above data points (*) indicate statistical differences between treatments after t-test at each concentration (P < 0.05).
4. Discussion
Despite several developments in therapeutic approaches against cryptosporidiosis, current drugs are still unreliable (Miyamoto and Eckmann, 2015). Some efforts have been focused on evaluating natural compounds, particularly essential oils against C. parvum (Jin et al., 2019). Essential oil monoterpenes such as carvacrol, linalool, thymol, and eugenol are known to exhibit antimicrobial activity, but their antiprotozoal activity has not been extensively studied (Tasdemir et al., 2019). Convincing evidence shows that Th possesses potent antimicrobial, antifungal, antibacterial, and antiparasitic properties (Marchese et al., 2016; Nagoor et al., 2017; Pinheiro et al., 2017; K. Wang et al., 2018), however, information about its anti-cryptosporidial properties is limited.
In the present study, we show that both Th and TO have a strong effect against C. parvum infectivity and growth in the HCT-8 cell culture model. To the best of our knowledge, this is the first study that evaluates Th and TO's anti-cryptosporidial activity and cytotoxicity in the HCT-8 cell line. The results indicate that both Th and TO have high activity against C. parvum.
We first established the compounds' cytotoxicity against HCT-8 cells in order to determine the safety margins of the compounds in mammalian cells (Armson et al., 1999). HCT-8 cell line is an excellent model for in vitro culture of C. parvum (Karanis and Aldeyarbi, 2011). Our results revealed a dose-dependent, cytotoxic effect after 24 and 48 h exposure, with TO being less cytotoxic than Th. Esterification of the phenolic hydroxyl group on terpenes has been used as a strategy to reduce their volatility. The impact of the structural configuration and functional groups of terpenes on their cytotoxicity and bioactivity remains uncertain (Rao et al., 2019). The molecular weight of TO is 276.41 g/mol, almost 2-fold greater than thymol's (150.22 g/mol). During the formation of TO, the hydroxyl group of Th is esterified with ethyl octanoate. It is possible that the lower cytotoxicity of TO is due to the limited action of non-specific esterases, its size, and differential lipophilicity, which might affect solubility and uptake. Monoterpene esters are hydrolyzed by the action of several lipase-type enzymes as earlier described (Chatterjee et al., 2001). However, the specific lipase in humans that can hydrolyze monoterpene esters is not known.
Flow cytometry-based assay allows for sensitive, rapid, and accurate counting and categorizing cell populations based on physical and biochemical cellular characteristics with fewer treatment steps and free radioactivity. It has been reported that there is an excellent agreement between the widely used MTT assay and the flow cytometry-based assay (Wang and Zheng, 2002). Khadir et al. (2016) used the MTT assay to evaluate the cytotoxicity of the essential oil from Thymus lanceolatus, containing ~70% thymol in Caco-2 cells. After 24 h exposure, the IC50 value was 2.4-fold higher (293.53 μg/mL) than the values presented herein. This discrepancy can partially be attributed to the difference in the concentration of thymol present in that oil compared to the pure compound we used in this study. It might also be due to the different metabolic abilities of each cell line (Llana-ruiz-cabello et al., 2014). Caco-2 cells overexpress the ABC transporter P-glycoprotein, which exports xenobiotics from the cytosol to the lumen of the intestines. This might explain the low activity of Th against Caco-2 cells (Wink et al., 2012). Similarly, thymol has shown cytotoxic effects on the growth of different human cancer cell lines. Th IC50 values of 134.29 μg/mL and 304.8 μg/mL for human cervical cancer HeLa cells and MCF-7 breast cancer cells, respectively, have been previously reported (Khadir et al., 2016). Amirghofran et al. (2011) also reported IC50 values of 200 μg/mL against peripheral blood lymphocytes proliferation. Other studies showed that concentrations of 150 μg/mL for Th did not affect the viability of primary cultures of mouse cortical neurons measured by MTT assay. Interestingly, at lower concentrations, thymol appeared to stimulate cell growth, promoting cell viability greater than 100%. Similar results have been previously reported for thymol and other phenolic compounds (Costa et al., 2019). This can be due to the protective effect of these types of compounds against oxidative stress. It has been shown that phenolic compounds can reduce the production of H2O2-induced ROS exerting antioxidant effects (Liu et al., 2019). Although the findings in this study showed higher Th cytotoxicity in HCT-8 cells, the mechanisms of action are unknown.
Plants bioactives like the polyphenols rutin, quercetin, and curcumin have shown anti-parasitic activities in vitro and in vivo (Asadpour et al., 2018a; Da Silva et al., 2019). In addition to polyphenols, some monoterpenes have also been studied as anti-parasitic agents (Gaur et al., 2018; Marchese et al., 2017). Thymol has shown better antioxidant, anti-inflammatory, and anti-apoptotic effects than its isomer carvacrol (El-Sayed et al., 2016). Though it is known that thymol has several antibiotic activities (Kachur and Suntres, 2019), less is known about its anti-cryptosporidial properties. Results from the present study show that both Th and TO are effective against C. parvum in vitro. It is worth noting that for parasite growth, IC50 values for TO and Th were not different (Table 1, P > 0.05).
Studies on the effect of monoterpenes against several other protozoan parasites have been reported, but information about their effects on Cryptosporidium are scarce. Recently, Gaur et al. (2018) evaluated the effect of oregano essential oil and carvacrol on inhibition of C. parvum infectivity in vitro and found reduced relative C. parvum infectivity after treatment in a dose-dependent manner (55.6l ± 10.4% and 45.8 ± 4.1% at 60 and 30 μg/mL of oregano essential oil and carvacrol, respectively). Previous studies have reported the antiplasmodial activity of oregano essential oil containing 25% thymol. The whole oil, as well as its constituents, thymol and carvacrol, showed in vitro activity against Plasmodium falciparum FCR-3 strain, with an IC50 value of 10 μg/mL (Fujisaki et al., 2012). The activity of thymol against P. falciparum K1 strain with an IC50 value of 4.5 μg/mL has also been reported (Mota et al., 2012). Several Origanum spp. essential oils, as well as thymol and carvacrol alone, have been evaluated against different Leishmania species. Thymol and carvacrol showed activity against promastigote forms of L. chagasi with IC50 values of 9.8 μg/mL and 2.3 μg/mL, respectively (Oliveira De Melo et al., 2013). The moderate and comparable leishmanicidal effects of both carvacrol and thymol against L. infantum promastigotes (IC50 values of 9.8 and 7.2 μg/mL, respectively) was recently reported (Youssefi et al., 2019). Other studies evaluated the effect of thymol on different Trypanosome species. Thymol was found to be active against intracellular amastigotes of T. cruzi-infected Vero cells, with IC50 values of 3.2 μg/mL (Escobar et al., 2010). Carvacrol and thymol demonstrated activity against T. brucei (life stage was not reported) with IC50 values of 11.3 and 22.9 μg/mL, respectively (Nibret and Wink, 2010). Thymol showed in vitro activity against three different protozoan parasites, Trypanosoma brucei, Leishmania donovani, and P. falciparum, with IC50 values of 0.11, 17.3, and 5.7 μg/mL, respectively (Tasdemir et al., 2019). These values correlate favorably with the results we obtained for Th against C. parvum. Interestingly, in the same study, in a T. brucei mouse model, thymol, but not carvacrol, extended the mean survival of animals. Overall, essential oils have bioactivities against several parasite types. Thus, they could be used as broad-spectrum antiparasitic agents.
The mechanisms underlying the antiprotozoal effect of essential oils and their monoterpenes are not fully understood (Oliveira De Melo et al., 2013). Due to their high lipophilic nature, which permits easy absorption by the cell membrane, it has been generally accepted that they are involved in the alteration and disruption of lipophilic membranes and inhibition of the lipid metabolism of parasites (de Medeiros et al., 2011). Another mode of action includes membrane permeation first, followed by modulation of cytoplasmic metabolic pathways or the function of organelles, rather than compromising the parasite's membrane integrity (Santoro et al., 2007). Essential oils can interfere with membrane-catalyzed enzymes and with enzymes responsible for energy and protein production, leading to cell death (Elshafie et al., 2017). The most recent proposed mechanism of action argues that essential oils and their monoterpenes form free radicals that may interfere with essential enzymes of the parasite such as lactate dehydrogenase, glucose phosphate isomerase, and glyceraldehyde-3-phosphate dehydrogenase that are related to energy metabolism, or protein phosphatase, threonine peptidase, peptide deformylase, N-myristoyltransferase, cysteine proteases and protein farnesyltransferase related to protein metabolism (Liu et al., 2019; Mérillon, 2018; Witola et al., 2017). Some new studies investigated potential targets using in silico methods and included enzyme and protein targets (Lazarević et al., 2017; Pandey et al., 2019). In contrast, it is well known that paromomycin inhibits protein synthesis in bacteria, but its anti-cryptosporidial mode of action also remains to be determined (Pund and Joshi, 2017). Preliminary studies support that paromomycin's anti-cryptosporidial activity is variable (Asadpour et al., 2018b). Paromomycin has been reported to have IC50 values between 400 and 700 μg/mL in in vitro assays (Downey et al., 2008; Li et al., 2019). The values found in this study do agree with this evidence, however, lower concentrations of paromomycin have been used as positive controls in other in vitro studies. In previous studies, investigators used a positive control of paromomycin at 200 μg/mL, which consistently inhibited C. parvum growth by 60–70% (Kayser et al., 2002). In a more recent study, a concentration of 150 μM (92 μg/mL) paromomycin was used as a positive control for the evaluation of diverse natural products against the growth of C. parvum in vitro (Jin et al., 2019). Paromomycin has been shown to be effective in reducing oocyst shedding and some clinical signs in animal models of natural and experimental cryptosporidiosis (Asadpour et al., 2018a; Mammeri et al., 2018). It is important to note that the IC50 values reported by us and others for paromomycin are considerably higher than the ones found for Th and TO, ranging from 11 to 53-fold higher for parasite invasion and, 18 to 19-fold higher for parasite growth.
Although results of this study are promising, further studies are necessary to clarify the mechanism by which Th and TO exert their effect against C. parvum in infected cells. Moreover, their effect in vivo should also be determined. Discrepancies between in vitro and in vivo efficacy is often a hurdle for oral delivery of supplements and drugs targeting the gut, especially for essential oils and monoterpenes due to their low solubility, in vivo instability, hydrolysis and oxidation, very rapid absorption and elimination rate (Rao et al., 2019). According to previous reports, esterification of monoterpenes can improve these disadvantages (Youssefi et al., 2019). Thus, the testing and evaluation of esterified monoterpenes in vivo could provide data about their potential as effective anticryptosporidial drugs.
Essential oils and their main components are characterized by their high volatility. Recently, studies have been focusing on developing more stable formulations to overcome this disadvantage and improve their efficacy (Rao et al., 2019). In order to improve their stability, promising strategies include the development of better delivery systems (i.e. encapsulation, emulsification) (De Matos et al., 2019) or chemical modifications in the carbon structure (e.g. esterification), and the formulation of semisynthetic derivatives (Jansen and Shenvi, 2014; Talavera-Alemán et al., 2016). Talavera-Alemán et al. (2016) reported that only 10% of known thymol derivatives have been employed in biological testing and shown a vast array of activities including antimicrobial, antioxidant, antinociceptive, antileishmanial, antiprotozoal, insecticidal, and piscicidal activity. The usefulness of thymol derivatives as transdermal drug delivery enhancers has also been reported (Zhao et al., 2010). Thymol ester derivatives were found to be more effective against streptococcus species (Mathela et al., 2010). An in silico study also predicted antimicrobial activity of some thymol esters (Lazarević et al., 2017). In the case of monoterpene esters, they have already proven to be promising due to their amenability to hydrolysis in vivo and their enhanced lipophilicity and passive membrane transport (Lazarević et al., 2017).
5. Conclusions
Taken together, our results showed that both thymol and thymol octanoate were biologically active against C. parvum in vitro, with low cytotoxicity levels in HCT-8 cells. Thymol had a stronger effect than thymol octanoate against C. parvum invasion but effects were similar when parasite growth was evaluated. Esterified thymol has anti-cryptosporidial effect comparable with its parental compound, thymol, but with improved physicochemical properties that could make it more suitable for diverse applications as antiparasitic agent.
Authors contributions
A.D.U., W.H.W. and J.E.A.L. conceived and planned the experiments. A.D.U., D.F.A., and K.L. carried out the experiments. K.L. and W.H.W trained personnel in specific protozoan assays. A.D.U., D.F.A., K.L., W.H.W. and J.E.A.L. contributed to the interpretation of the results. A.D.U. took the lead in writing the manuscript and creation of tables and figures. All authors provided critical feedback and helped shape the final manuscript.
Declaration of competing interest
Authors declare no conflicts of interest.
Acknowledgments
This work was supported by United States Department of Agriculture (USDA) - Hatch ILLU-698-904. Author A.D.-U. was supported by CONACYT – Mexico.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2021.02.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abubakar I., Aliyu S.H., Arumugam C., Usman N.K., Hunter P.R. Treatment of cryptosporidiosis in immunocompromised individuals: systematic review and meta-analysis. 2007. [DOI] [PMC free article] [PubMed]
- Amirghofran Z., Hashemzadeh R., Javidnia K., Golmoghaddam H., Esmaeilbeig A. In vitro immunomodulatory effects of extracts from three plants of the Labiatae family and isolation of the active compound(s) J. Immunot. 2011;8:265–273. doi: 10.3109/1547691X.2011.590828. [DOI] [PubMed] [Google Scholar]
- Anthony J.P., Fyfe L., Smith H. Plant active components - a resource for antiparasitic agents? Trends Parasitol. 2005;21:462–468. doi: 10.1016/j.pt.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Araújo L.X., Novato T.P.L., Zeringota V., Matos R.S., Senra T.O.S., Maturano R., Prata M.C.A., Daemon E., Monteiro C.M.O. Acaricidal activity of thymol against larvae of Rhipicephalus microplus (Acari: ixodidae) under semi-natural conditions. Parasitol. Res. 2015;114:3271–3276. doi: 10.1007/s00436-015-4547-3. [DOI] [PubMed] [Google Scholar]
- Armson A., Meloni B.P., Reynoldson J.A., Thompson R.C.A. Vol. 178. 1999. pp. 227–233. (Assessment of Drugs against Cryptosporidium Parvum Using a Simple in Vitro Screening Method). [DOI] [PubMed] [Google Scholar]
- Asadpour M., Namazi F., Razavi S.M., Nazifi S. Curcumin: a promising treatment for Cryptosporidium parvum infection in immunosuppressed BALB/c mice. Exp. Parasitol. 2018;195:59–65. doi: 10.1016/j.exppara.2018.10.008. [DOI] [PubMed] [Google Scholar]
- Asadpour M., Namazi F., Razavi S.M., Nazifi S. Comparative efficacy of curcumin and paromomycin against Cryptosporidium parvum infection in a BALB/c model. Vet. Parasitol. 2018;250:7–14. doi: 10.1016/j.vetpar.2017.12.008. [DOI] [PubMed] [Google Scholar]
- Bilia A.R., Guccione C., Isacchi B., Righeschi C., Firenzuoli F., Bergonzi M.C. Essential oils loaded in nanosystems: a developing strategy for a successful therapeutic approach. Event Based. Compl. Alter. Med. 2014 doi: 10.1155/2014/651593. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Cabada M.M., White A.C. Treatment of cryptosporidiosis: do we know what we think we know? Curr. Opin. Infect. Dis. 2010;23:494–499. doi: 10.1097/QCO.0b013e32833de052. [DOI] [PubMed] [Google Scholar]
- Chatterjee T., Chatterjee B.K., Bhattacharyya D.K. Study of lipase-catalyzed hydrolysis of some monoterpene esters. Can. J. Microbiol. 2001;47:397–403. doi: 10.1139/cjm-47-5-397. [DOI] [PubMed] [Google Scholar]
- Chauhan A.K., Kang S.C. Thymol disrupts the membrane integrity of Salmonella ser. typhimurium invitro and recovers infected macrophages from oxidative stress in an ex vivo model. Res. Microbiol. 2014;165:559–565. doi: 10.1016/j.resmic.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Checkley W., White A.C., Jaganath D., Arrowood M.J., Chalmers R.M., Chen X.M., Fayer R., Griffiths J.K., Guerrant R.L., Hedstrom L., Huston C.D., Kotloff K.L., Kang G., Mead J.R., Miller M., Petri W.A., Priest J.W., Roos D.S., Striepen B., Thompson R.C.A., Ward H.D., Van Voorhis W.A., Xiao L., Zhu G., Houpt E.R. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for cryptosporidium. Lancet Infect. Dis. 2015;15:85–94. doi: 10.1016/S1473-3099(14)70772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M.F., Durço A.O., Rabelo T.K., Barreto R. de S.S., Guimarães A.G. Effects of Carvacrol, Thymol and essential oils containing such monoterpenes on wound healing: a systematic review. J. Pharm. Pharmacol. 2019;71:141–155. doi: 10.1111/jphp.13054. [DOI] [PubMed] [Google Scholar]
- Da Silva E.R., Brogi S., Lucon-Júnior J.F., Campiani G., Gemma S., Maquiaveli C.D.C. Dietary polyphenols rutin, taxifolin and quercetin related compounds target: Leishmania amazonensis arginase. Food Funct. 2019;10:3172–3180. doi: 10.1039/c9fo00265k. [DOI] [PubMed] [Google Scholar]
- Daniels M.E., Smith W.A., Schmidt W.P., Clasen T., Jenkins M.W. Modeling cryptosporidium and giardia in ground and surface water sources in rural India: associations with latrines, livestock, damaged wells, and rainfall patterns. Environ. Sci. Technol. 2016;50:7498–7507. doi: 10.1021/acs.est.5b05797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matos S.P., Teixeira H.F., De Lima Á.A.N., Veiga-Junior V.F., Koester L.S. Essential oils and isolated terpenes in nanosystems designed for topical administration: a review. Biomolecules. 2019;9 doi: 10.3390/biom9040138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Medeiros M., das G.F., da Silva A.C., Citó A.M., das G.L., Borges A.R., de Lima S.G., Lopes J.A.D., Figueiredo R.C.B.Q. In vitro antileishmanial activity and cytotoxicity of essential oil from Lippia sidoides Cham. Parasitol. Int. 2011;60:237–241. doi: 10.1016/j.parint.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Downey A.S., Chong C.R., Graczyk T.K., Sullivan D.J. Efficacy of pyrvinium pamoate against Cryptosporidium parvum infection in vitro and in a neonatal mouse model. Antimicrob. Agents Chemother. 2008;52:3106–3112. doi: 10.1128/AAC.00207-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed E.-S.M., Mansour A., Abdul-Hameed M.S. Thymol and carvacrol prevent doxorubicin-induced cardiotoxicity by abrogation of oxidative stress, inflammation, and apoptosis in rats. J. Biochem. Mol. Toxicol. 2016;30:37–44. doi: 10.1002/jbt. [DOI] [PubMed] [Google Scholar]
- Elshafie H.S., Armentano M.F., Carmosino M., Bufo S.A., De Feo V., Camele I. Cytotoxic activity of origanum vulgare L. on Hepatocellular carcinoma cell line HepG2 and evaluation of its biological activity. Molecules. 2017;22 doi: 10.3390/molecules22091435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar P., Leal S.M., Herrera L.V., Martinez J.R., Stashenko E. Chemical composition and antiprotozoal activities of Colombian Lippia spp essential oils and their major components. Mem. Inst. Oswaldo Cruz. 2010;105:184–190. doi: 10.1590/s0074-02762010000200013. [DOI] [PubMed] [Google Scholar]
- Fujisaki R., Kamei K., Yamamura M., Nishiya H., Inouye S., Takahashi M., Abe S. In vitro and in vivo anti-plasmodial activity of essential oils, including hinokitiol. Southeast Asian J. Trop. Med. Publ. Health. 2012;43:270–279. [PubMed] [Google Scholar]
- Gaur S., Kuhlenschmidt T.B., Kuhlenschmidt M.S., Andrade J.E. Effect of oregano essential oil and carvacrol on Cryptosporidium parvum infectivity in HCT-8 cells. Parasitol. Int. 2018;67:170–175. doi: 10.1016/j.parint.2017.11.001. [DOI] [PubMed] [Google Scholar]
- Hewitt R.G., Yiannoutsos C.T., Higgs E.S., Carey J.T., Geiseler P.J., Soave R., Rosenberg R., Vazquez G.J., Wheat L.J., Fass R.J., Antoninievic Z., Walawander A.L., Flanigan T.P. 2000. Paromomycin : No more effective than placebo for treatment of cryptosporidiosis in patients with advanced human immunodeficiency Virus Infection; pp. 1084–1092. [DOI] [PubMed] [Google Scholar]
- Jansen D.J., Shenvi R.A. Synthesis of medicinally relevant terpenes: reducing the cost and time of drug discovery. Future Med. Chem. 2014;6:1127–1148. doi: 10.4155/fmc.14.71.Synthesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z., Ma J., Zhu G., Zhang H. Discovery of novel anti-cryptosporidial activities from natural products by in vitro high-throughput phenotypic screening. Front. Microbiol. 2019;10:1–11. doi: 10.3389/fmicb.2019.01999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachur K., Suntres Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2019:1–12. doi: 10.1080/10408398.2019.1675585. 0. [DOI] [PubMed] [Google Scholar]
- Karanis P., Aldeyarbi H.M. Evolution of Cryptosporidium in vitro culture. Int. J. Parasitol. 2011;41:1231–1242. doi: 10.1016/j.ijpara.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Kayser O., Kiderlen A.F., Croft S.L. Natural products as potential antiparasitic drugs. Stud. Nat. Prod. Chem. 2002;26:779–848. doi: 10.1016/S1572-5995(02)80019-9. [DOI] [Google Scholar]
- Khadir A., Sobeh M., Gad H.A., Benbelaid F., Bendahou M., Peixoto H., Sporer F., Ashour M.L., Wink M. Chemical composition and biological activity of the essential oil from Thymus lanceolatus. Zeitschrift fur Naturforsch. - Sect. C J. Biosci. 2016;71:155–163. doi: 10.1515/znc-2016-0005. [DOI] [PubMed] [Google Scholar]
- Kotloff K.L. The burden and etiology of diarrheal illness in developing countries. Pediatr. Clin. 2017;64:799–814. doi: 10.1016/j.pcl.2017.03.006. [DOI] [PubMed] [Google Scholar]
- Kuhlenschmidt T.B., Rutaganira F.U., Long S., Tang K., Shokat K.M., Kuhlenschmidt M.S., Sibley L.D. Inhibition of Calcium-Dependent Protein Kinase 1 (CDPK1) in Vitro by pyrazolopyrimidine derivatives does not correlate with sensitivity of Cryptosporidium parvum growth in cell culture. Antimicrob. Agents Chemother. 2016;60:570–579. doi: 10.1128/AAC.01915-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Chatterjee I., Anbazhagan A.N., Jayawardena D., Alrefai W.A., Sun J., Borthakur A., Dudeja P.K. Cryptosporidium parvum disrupts intestinal epithelial barrier function via altering expression of key tight junction and adherens junction proteins. Cell Microbiol. 2019;20:1–29. doi: 10.1111/cmi.12830.Cryptosporidium. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarević J., Kolarević A., Dordević A., Stojanović G., Šmelcerović A., Ciuffreda P., Santaniello E. Synthesis, antimicrobial activity and in silico studies on thymol esters. Acta Chim. Slov. 2017;64:603–612. doi: 10.17344/acsi.2017.3356. [DOI] [PubMed] [Google Scholar]
- Li K., Nader S.M., Zhang X., Ray B.C., Kim C.Y., Das A., Witola W.H. Novel lactate dehydrogenase inhibitors with in vivo efficacy against Cryptosporidium parvum. PLoS Pathog. 2019;15:1–25. doi: 10.1371/journal.ppat.1007953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Guo H., DaSilva N.A., Li D., Zhang K., Wan Y., Gao X.H., Chen H.D., Seeram N.P., Ma H. Pomegranate (Punica granatum) phenolics ameliorate hydrogen peroxide-induced oxidative stress and cytotoxicity in human keratinocytes. J. Funct. Foods. 2019;54:559–567. doi: 10.1016/j.jff.2019.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llana-ruiz-cabello M., Gutiérrez-praena D., Pichardo S., Moreno F.J., María J., Aucejo S., María A. 2014. Cytotoxicity and Morphological Effects Induced by Carvacrol and Thymol on the Human Cell Line Caco-2 64; pp. 281–290. [DOI] [PubMed] [Google Scholar]
- López V., Cascella M., Benelli G., Maggi F., Gómez-Rincón C. Green drugs in the fight against Anisakis simplex—larvicidal activity and acetylcholinesterase inhibition of Origanum compactum essential oil. Parasitol. Res. 2018;117:861–867. doi: 10.1007/s00436-018-5764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammeri M., Chevillot A., Thomas M., Polack B., Julien C., Marden J.P., Auclair E., Vallée I., Adjou K.T. Efficacy of chitosan, a natural polysaccharide, against Cryptosporidium parvum in vitro and in vivo in neonatal mice. Exp. Parasitol. 2018;194:1–8. doi: 10.1016/j.exppara.2018.09.003. [DOI] [PubMed] [Google Scholar]
- Marchese A., Arciola C.R., Barbieri R., Silva A.S., Nabavi S.F., Sokeng A.J.T., Izadi M., Jafari N.J., Suntar I., Daglia M., Nabavi S.M. Update on monoterpenes as antimicrobial agents: a particular focus on p-cymene. Materials. 2017;10:1–15. doi: 10.3390/ma10080947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchese A., Orhan I.E., Daglia M., Barbieri R., Di Lorenzo A., Nabavi S.F., Gortzi O., Izadi M., Nabavi S.M. Antibacterial and antifungal activities of thymol: a brief review of the literature. Food Chem. 2016;210:402–414. doi: 10.1016/j.foodchem.2016.04.111. [DOI] [PubMed] [Google Scholar]
- Mathela C.S., Singh K.K., Gupta V.K. Synthesis and in vitro antibacterial activity of new. Acta Pol. Pharm. 2010;67:375–380. doi: 10.1016/j.jscs.2011.05.019. [DOI] [PubMed] [Google Scholar]
- Mérillon J. Natural antimicrobial agents. 2018. [DOI]
- Miyamoto Y., Eckmann L. Drug development against the major diarrhea-causing parasites of the small intestine, cryptosporidium and giardia. Front. Microbiol. 2015;19:1208. doi: 10.3389/fmicb.2015.01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzote L., Alarcón O., Setzer W.N. Antiprotozoal activity of essential oils. Agric. Conspectus Sci. 2012;77:167–175. [Google Scholar]
- Mota M.L., Coeltho Lobo L.T., da Costa J.G., Costa L.S., Rocha H.A.O., Rocha e Silva L.F., Pohlit A.M., Adrade Neto V.F. In vitro and in vivo antimalarial activity of essential oils and chemical components from three medicinal plants found in northeastern Brazil. Planta Med. 2012:658–664. doi: 10.1055/s-0031-1298333. [DOI] [PubMed] [Google Scholar]
- Nagoor M.F., Javed H., Taee H. Al, Azimullah S., Ojha S.K. Pharmacological properties and molecular mechanisms of thymol: prospects for its therapeutic potential and pharmaceutical development. Front. Pharmacol. 2017;8:1–34. doi: 10.3389/fphar.2017.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibret E., Wink M. Phytomedicine Trypanocidal and antileukaemic effects of the essential oils of Hagenia abyssinica , Leonotis ocymifolia , Moringa stenopetala , and their main individual constituents. Eur. J. Integr. Med. 2010;17:911–920. doi: 10.1016/j.phymed.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Oliveira De Melo J., Aparecida Bitencourt T., Fachin A.L., Oliveira Cruz E.M., Ramos De Jesus H.C., Barreto Alves P., Arrigoni-blank M.D.F., De Castro Franca S., Oliveira Beleboni R., Miranda Fernandes R.P., Fitzgerald Blank A., Scher R. Antidermatophytic and antileishmanial activities of essential oils from Lippia gracilis Schauer genotypes. Acta Trop. 2013;128:110–115. doi: 10.1016/j.actatropica.2013.06.024. [DOI] [PubMed] [Google Scholar]
- Pandey S.C., Jha A., Kumar A., Samant M. Evaluation of antileishmanial potential of computationally screened compounds targeting DEAD-box RNA helicase of Leishmania donovani. Int. J. Biol. Macromol. 2019;121:480–487. doi: 10.1016/j.ijbiomac.2018.10.053. [DOI] [PubMed] [Google Scholar]
- Pinheiro W.P., Cavalcante G.S., Correia Ribeiro W.L., Leite dos Santos J.M., Freitas Macedo I.T., Beserra de Paula H.C., de Morais S.M. de, de Melo J.V., Leal Bevilaqua C.M. Anthelmintic effect of thymol and thymol acetate on sheep gastrointestinal nematodes and their toxicity in mice. Brazilian J. Parasitol. 2017;26:323–330. doi: 10.1590/S1984-29612017056. [DOI] [PubMed] [Google Scholar]
- Pund S., Joshi A. Elsevier Inc; 2017. Nanoarchitectures for Neglected Tropical Protozoal Diseases: Challenges and State of the Art, Nano- and Microscale Drug Delivery Systems: Design and Fabrication. [DOI] [Google Scholar]
- Rao J., Chen B., Mcclements D.J. 2019. Improving the Efficacy of Essential Oils as Antimicrobials in Food: Mechanisms of Action 1–23. [DOI] [PubMed] [Google Scholar]
- Ryan U., Zahedi A., Paparini A. Cryptosporidium in humans and animals—a one health approach to prophylaxis. Parasite Immunol. 2016;38:535–547. doi: 10.1111/pim.12350. [DOI] [PubMed] [Google Scholar]
- Santoro G.F., Das Graças Cardoso M., Guimarães L.G.L., Salgado A.P.S.P., Menna-Barreto R.F.S., Soares M.J. Effect of oregano (Origanum vulgare L.) and thyme (Thymus vulgaris L.) essential oils on Trypanosoma cruzi (Protozoa: kinetoplastida) growth and ultrastructure. Parasitol. Res. 2007;100:783–790. doi: 10.1007/s00436-006-0326-5. [DOI] [PubMed] [Google Scholar]
- Sears C.L., Kirkpatrick B.D. Is nitazoxanide an effective treatment for patients with acquired immune deficiency syndrome-related cryptosporidiosis? Nat. Clin. Pract. Gastroenterol. Hepatol. 2007;4:136–137. doi: 10.1038/ncpgasthep0737. [DOI] [PubMed] [Google Scholar]
- Sow S.O., Muhsen K., Nasrin D., Blackwelder W.C., Wu Y., Farag T.H., Panchalingam S., Sur D., Zaidi A.K.M., Faruque A.S.G., Saha D., Adegbola R., Alonso P.L., Breiman R.F., Bassat Q., Tamboura B., Sanogo D., Onwuchekwa U., Manna B., Ramamurthy T., Kanungo S., Ahmed S., Qureshi S., Quadri F., Hossain A., Das S.K., Antonio M., Hossain M.J., Mandomando I., Nhampossa T., Acácio S., Omore R., Oundo J.O., Ochieng J.B., Mintz E.D., O'Reilly C.E., Berkeley L.Y., Livio S., Tennant S.M., Sommerfelt H., Nataro J.P., Ziv-Baran T., Robins-Browne R.M., Mishcherkin V., Zhang J., Liu J., Houpt E.R., Kotloff K.L., Levine M.M. The burden of cryptosporidium diarrheal disease among children < 24 Months of age in moderate/high mortality regions of sub-saharan africa and south asia, utilizing data from the global enteric multicenter study (GEMS) PLoS Neglected Trop. Dis. 2016;10:1–20. doi: 10.1371/journal.pntd.0004729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera-Alemán A., Rodríguez-García G., López Y., García-Gutiérrez H.A., Torres-Valencia J.M., del Río R.E., Cerda-García-Rojas C.M., Joseph-Nathan P., Gómez-Hurtado M.A. Systematic evaluation of thymol derivatives possessing stereogenic or prostereogenic centers. Phytochemistry Rev. 2016;15:251–277. doi: 10.1007/s11101-015-9412-6. [DOI] [Google Scholar]
- Tasdemir D., Kaiser M., Demirci B., Demirci F., Baser H.C. Antiprotozoal activity of Turkish origanum onites essential oil components. Molecules. 2019;24:1–16. doi: 10.3390/molecules24234421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharamak S., Yooboon T., Pengsook A., Ratwatthananon A., Kumrungsee N., Bullangpoti V., Pluempanupat W. Synthesis of thymyl esters and their insecticidal activity against Spodoptera litura (Lepidoptera: noctuidae) Pest Manag. Sci. 2019 doi: 10.1002/ps.5598. [DOI] [PubMed] [Google Scholar]
- Wang K., Jiang S., Yang Y., Fan L., Su F., Ye M. Synthesis and antifungal activity of carvacrol and thymol esters with heteroaromatic carboxylic acids. Nat. Prod. Res. 2018;6419:1–7. doi: 10.1080/14786419.2018.1480618. [DOI] [PubMed] [Google Scholar]
- Wang Y.Y., Zheng X.X. A flow cytometry-based assay for quantitative analysis of cellular proliferation and cytotoxicity in vitro. J. Immunol. Methods. 2002;268:179–188. doi: 10.1016/S0022-1759(02)00190-4. [DOI] [PubMed] [Google Scholar]
- Wang Z.D., Liu Q., Liu H.H., Li S., Zhang L., Zhao Y.K., Zhu X.Q. Prevalence of Cryptosporidium, microsporidia and Isospora infection in HIV-infected people: a global systematic review and meta-analysis. Parasites Vectors. 2018;11:1–19. doi: 10.1186/s13071-017-2558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink M., Ashour M.L., El-Readi M.Z. Secondary metabolites from plants inhibiting ABC transporters and reversing resistance of cancer cells and microbes to cytotoxic and antimicrobial agents. Front. Microbiol. 2012;3:1–15. doi: 10.3389/fmicb.2012.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witola W.H., Zhang X., Kim C.Y. Targeted gene knockdown validates the essential role of lactate dehydrogenase in Cryptosporidium parvum. Int. J. Parasitol. 2017;47:867–874. doi: 10.1016/j.ijpara.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssefi M.R., Moghaddas E., Tabari M.A., Moghadamnia A.A., Hosseini S.M., Hosseini Farash B.R., Ebrahimi M.A., Mousavi N.N., Fata A., Maggi F., Petrelli R., Dall'Acqua S., Benelli G., Sut S. In vitro and in vivo effectiveness of carvacrol, thymol and linalool against Leishmania infantum. Molecules. 2019;24:1–11. doi: 10.3390/molecules24112072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Li Y., Liu Q., Gao K. Antimicrobial activities of some thymol derivatives from the roots of Inula hupehensis. Food Chem. 2010;120:512–516. doi: 10.1016/j.foodchem.2009.10.045. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.