Abstract
Introduction
Preoperative anemia is associated with increased morbidity, mortality, and healthcare costs. As a result of the increased incidence of chronic blood loss and iron deficiency anemia in abdominal surgery patients and its impact on patient outcomes, we systematically evaluated the quality of evidence for preoperative intravenous (IV) administration of iron to patients with anemia undergoing major abdominal surgery with the focus on clinical outcomes.
Methods
In this systematic review, PubMed, Cochrane, The Cumulative Index to Nursing and Allied Health Literature, Web Of Science, and Excerpta Medica Database databases were searched up to 2019 using specific keywords. Inclusion criteria were patients that were over 18 years of age, underwent abdominal surgery, and received an IV iron treatment in the preoperative setting.
Results
The nine studies included in the final systematic review do not provide consistent evidence of a reduced incidence of allogeneic blood transfusions with preoperative IV iron administration. However, IV iron administration did consistently cause a significant increase in hemoglobin levels relative to oral iron therapy or no iron.
Conclusion
Overall, these findings are consistent in that IV iron administration is highly effective at rapidly increasing hemoglobin levels in patients with iron deficiency anemia undergoing major abdominal surgery. Unfortunately, there is currently no evidence of reduced incidence of allogeneic blood transfusions or other enhanced outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-021-01628-7.
Keywords: Abdominal surgery, Anemia, Hemoglobin, Iron, Preoperative
Key Summary Points
| The studies identified and evaluated in this review demonstrate a consistent, significant increase in hemoglobin levels in the intravenous iron group, but do not consistently report a significant reduction in the number of red blood cell transfusions intraoperatively. |
| Thus, we recommend that abdominal surgery patients be evaluated and treated for iron deficiency anemia prior to receiving surgery. |
| More studies are necessary to definitively determine the efficacy of IV iron administration in decreasing morbidity and mortality for abdominal surgery patients. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.13580687.
Introduction
Preoperative anemia remains a common problem in patients scheduled to undergo major abdominal surgery [1]. Many patients with colorectal cancer or uterine disease have lesions that predispose them to acute or chronic blood loss. One review found anemia present in 40–50% of patients scheduled for gynecological or colorectal surgery [1]. Much of this anemia is likely iron deficiency anemia (IDA) due to the high incidence of chronic blood loss in these patients. This is supported by Third National Health and Nutrition Examination Survey (NHANES III) data showing that iron deficiency is the cause of at least 20% of anemia in adults over 65 [2].
Preoperative anemia is associated with increased healthcare costs, morbidity, and mortality [3–10]. Unoptimized red blood cell mass in the preoperative setting is also associated with increased blood transfusions and greater morbidity and financial burden [8, 11, 12]. In patients with colorectal cancer, blood transfusions have been associated with increased infection rates, longer hospital stays, higher mortality, and a greater risk of cancer recurrence [13, 14]. One meta-analysis showed a dose-dependent correlation between the amount of blood transfused intraoperatively and the incidence of colorectal cancer recurrence [15].
Given these findings, the 2018 Frankfurt Consensus Conference statement strongly recommends early detection and management of anemia before major elective surgeries with the use of iron supplementation for patients with iron deficiency anemia [16]. Similarly, a prior consensus statement released by the British Society for Haemotology recommends intravenous (IV) administration of iron if IDA is diagnosed near the day of surgery; otherwise, oral iron therapy could be used [17]. However, there remains a lack of robust evidence for this intervention because of a limited number of randomized controlled trials (RCTs) and inadequately powered studies. Several systematic reviews evaluating preoperative IV iron therapy use have reported similar limitations, but these were not focused on patients receiving abdominal surgery [18–20].
Therefore, we performed a systematic review to evaluate the current evidence for clinical effectiveness of preoperative IV iron administration as a treatment for anemia in patients undergoing major abdominal surgery.
Methods
Protocol Registration
The protocol for this systematic review was designed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We enlisted the assistance of a research librarian and statistician in developing the protocol at Countway Library of Medicine, Boston, Massachusetts. The protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO Application Number 160868). This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Eligibility Criteria
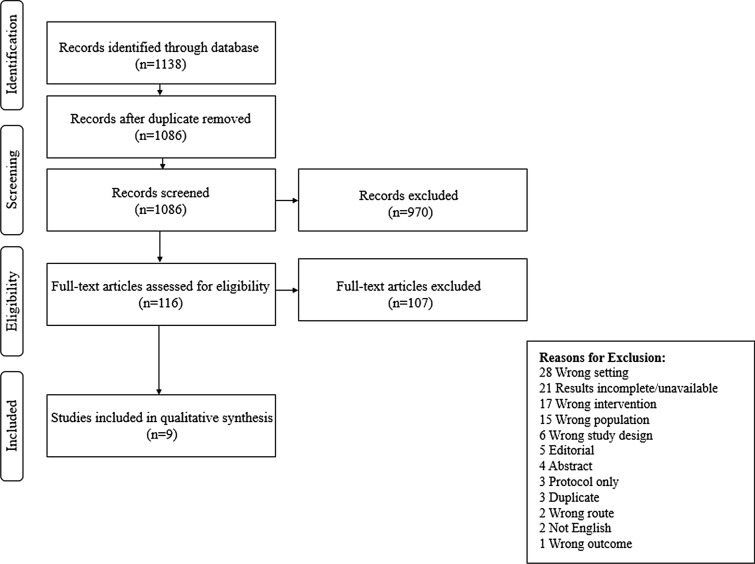
The original studies considered for this systematic review included patients that were over 18 years of age, underwent major abdominal surgery, and received an intravenous iron treatment in the preoperative setting. Duplicate and non-English studies were removed before analysis. Review articles, incomplete trials, published abstracts, letters to the editor, study protocols, and case reports were excluded from this systematic review. Studies evaluating the wrong setting intervention, population, study design, and outcome were also excluded. The study flow diagram and all exclusion criteria can be found in Fig. 1.
Fig. 1.
Study flow diagram with exclusion criteria. Of the 1138 studies initially identified through the database search, only nine were eventually included in the qualitative analysis
Information Sources and Data Analysis
We searched several research databases, including PubMed, Cochrane, CLINAHL, WOS, and EMBASE, on September 20, 2019, for all publications through November 1, 2020. The search term list (see Appendix A in the supplementary material) included the following terms: “anemia”, “hemoglobin”, “hematocrit”, “preoperative period”, “preoperative care”, “perioperative period”, “perioperative care”, “postoperative period”, “postoperative care”, “iron”, “iron compounds”, “hematinics”, “treatment outcome”, “outcome assessment”, “outcome and process assessment”, and “patient outcome assessment”.
The studies were independently identified and subsequently reviewed by six authors in two different phases. The systematic review results were collected and processed using Covidence software (Melbourne, Australia) [20]. During the first phase, one author (BP) screened all titles and abstracts produced by the databases’ search criteria to determine eligibility. A second author (TP) read the full text of each article that made it past the screening phase and independently decided if the publication should be included. After this, a third author (TM) evaluated the validity of reasons for the excluded articles. Disagreements were harmonized by consensus, and a final set of studies was chosen using the aforementioned methods. A qualitative analysis was performed in the second phase instead of a meta-analysis because of the high heterogeneity across the nine studies [21]. One reviewer extracted data (AS), and another (RU) verified the data. Extracted data included publication title, author(s), year of publication, location of publication design of the study, population characteristics, intervention given to the patient population, comparator(s), and outcome results (Table 1). We then performed another review of the publishded data, and another author (TB) re-queried the databases and, in consultation with the senior author (RU), added three more studies to the systematic review, following the aforementioned process.
Table 1.
Study characteristics
| First author (location, year) | Study design | Patient characteristics | Intervention | Comparator | Outcomes | |||
|---|---|---|---|---|---|---|---|---|
| Anemia definition | Surgery type | N | IV iron therapy (dose); duration | Primary | Secondary | |||
| Richards (UK, 2020) | Multicenter randomized double-blinded controlled | Hb < 13 g/dL in men or < 12 g/dL in women | Elective major open abdominal surgery | 487 | FMC (1000 mg × 1 dose); 10–42 days preop | Placebo | Composite risk of blood transfusion or death; number of transfusion episodes from randomization until 30 days postop | Total units RBCs or other blood component received at 30 days and 6 months postop, Hb change from randomization to surgery and 8 weeks and 6 months postop, ICU and total hospital LOS |
| Edwards (UK, 2009) | Single-center randomized blinded prospective cohort | Hb < 13.5 g/dl in men or < 12.5 g/dL in women | Elective resection for colorectal carcinoma | 60 |
Iron sucrose (300 mg × 2 doses); 2 weeks before surgery |
Placebo | Hb levels between recruitment and day of randomization | Transfusion rate, changes in serum iron markers, LOS, AEs |
| Keeler (UK, 2017) | Multicenter randomized open-label prospective cohort | Hb < 12 g/dL in men or < 11 g/dL in women | Elective surgery for non-metastatic colorectal carcinoma | 116 | FCM (1000 mg × 3 doses); 2 weeks preop | Ferrous sulfate (20 mg BID) | Differences in the mean volume of transfusions | Changes in Hb and hematinic profiles |
| Froessler (UK, 2018) | Single-center randomized non-blinded prospective cohort study | Hb < 13 g/dL in men or < 12 g/dL in women | Elective and urgent major abdominal surgery | 72 | FCM (1000 mg); preop and (0.5 mg/L blood lost); 2 days postop | No IV iron therapy | Incidence of transfusion | Hb levels, change in Hb between time points, LOS, morbidity, 30-day mortality, QoL score |
| Kim (South Korea, 2009) | Single-center randomized open-label controlled trial | Hb < 9 g/dL | Surgery for menorrhagia | 76 | Iron sucrose (dose according to Ganzoni’s formula 3 times per week); 3 weeks preop |
Oral iron succinylate therapy (80 mg) daily for 3 weeks |
Recruitment and admission Hb | None |
| Laso-Morales (Spain, 2017) | Multicenter retrospective cohort | Hb < 13 g/dL | Elective resection for colorectal carcinoma | 322 | Iron sucrose or FCM (dose according to Ganzoni equation); 4–6 weeks preop | Oral iron therapy or no iron | RBC transfusion requirement | Postop infection rate, hospital LOS |
| Wilson (the Netherlands, 2018) | Single-center retrospective cohort | Hb < 12.9 g/dL in men or < 12 g/dL in women | Elective resection for colorectal carcinoma | 318 | FCM (1000–2000 mg); time from diagnosis to surgery | No IV iron therapy | Change in Hb | Percentage of patients who received a blood transfusion, AEs |
| Calleja (Spain, 2015) | Multicenter retrospective and prospective cohort | Hb < 13 g/dL in men or < 12 g/dL in women | Elective resection for colorectal carcinoma | 266 | FCM (1000 mg); 2–4 weeks before surgery | No IV iron therapy | Periop and 30-day postop transfusion requirements | LOS, postop complication, Hb and iron parameters, percentage of patients with normalized Hb |
| Kam (China, 2020) | Single-center prospective propensity-score matched cohort | Hb < 10 g/dL before transfusion or Hb < 12 g/dL after recent transfusion | Elective resection for colorectal carcinoma | 100 | Iron sucrose (500 mg) or iron isomaltoside (1000 mg); at least 2 weeks preop | No IV iron therapy | Change in Hb | Percentage of patients who received an RBC transfusion |
AEs adverse events, BID twice a day, FCM ferric carboxymaltose, Hb hemoglobin, ICU intensive care unit, IV intravenous, LOS length of stay, periop perioperative, preop preoperative, postop postoperative, QoL quality of life, RBC red blood cell, UK United Kingdom
Outcomes
The primary outcome examined in the included studies was change in serum Hb. The secondary areas of interest were transfusion rates, morbidity and mortality, and hospital length of stay (LOS).
Quality Assessment
The quality and risk of bias of the four randomized and two non-randomized studies were assessed with the Cochrane Risk Assessment tool and Newcastle–Ottawa Scale (NOS), respectively. The Cochrane Risk Assessment tool assesses bias in randomized studies by assigning a rank of “high,” “low,” or “clear” in five subcategories [22]. Subcategories include selection bias, which is evaluated on random sequence generation and allocation concealment; reporting bias, which is evaluated on selective reporting; performance bias, which is evaluated on blinding of participants and personnel; detection bias, which is evaluated on blinding of outcome assessment; attrition bias which is evaluated on incomplete outcome data; and other sources of bias. Study quality was defined as “good” if the study had no “high” ratings for risk of bias. Study quality was defined as “fair” if the study had 1–2 “high” ratings for bias. Study quality was defined as “poor” if the study had three or more “high” ratings.
The NOS is used as a tool to assess the quality of non-randomized studies using a scoring system that rates studies on the basis of selection, comparability, and outcome [23]. Study quality is defined as “good” if the study scored in the ranges of 3–4 for selection, 1–2 for comparability, and 2–3 for outcome. Study quality was defined as “fair” if the study scored 2 for selection, 1–2 for comparability, and 2–3 for outcome. Study quality was defined as “poor” if the score did not meet criteria for either “good” or “fair.”
Results
Literature Search
A total of 1138 articles were identified and screened. After removal of duplicates, case reports, animal studies, and conference abstracts, the remaining 116 full-text studies were assessed for eligibility. Of those, 107 were excluded and nine studies remained that met the criteria for this systematic review (Fig. 1).
Study Characteristics
Study characteristics are summarized in Table 1. All of the studies in this systematic review included cohorts of patients undergoing abdominal surgery who had a preoperative diagnosis of anemia. A total of nine studies were included. The studies involved a total of 1817 patients. The studies did not all examine the same postoperative outcomes. Three of the studies were conducted in the UK, two in Spain, one in Australia, one in South Korea, one in the Netherlands, and one in China. The design of the studies included two randomized and blinded studies from Edwards et al. and Richards et al., three randomized non-blinded studies from Froessler et al., Keeler et al., and Kim et al., one non-randomized non-blinded study from Calleja et al., two retrospective cohort studies from Wilson et al. and Laso-Morales et al., and a prospective propensity-matched study from Kam et al. Six of the studies were limited to patients with planned colorectal cancer resection. In contrast, Froessler et al. and Richards et al. used patients from a broader category of major abdominal surgery. The study from Kim et al. was limited to patients scheduled for surgical treatment of menorrhagia.
Definitions and Measures
The definition of anemia varied between studies. The 1968 World Health Organization (WHO) criteria of baseline Hb levels less than 13 g/dL for men, and less than 12 g/dL for women were used to define anemia in two of the studies [24, 25]. Four studies defined anemia below the WHO Hb levels [26–29]. The Hb inclusion criterion in Keeler et al. was 12 g/dL in men and 11 g/dL in women; Wilson et al. restricted Hb levels to less than 12.9 g/dL in men, Kim et al. only enrolled patients with Hb levels below 9.0 g/dL and established IDA, and Kam et al. recruited patients with Hb less than 10 g/dL before transfusion or less than 12 g/dL after recent transfusion. Two studies used definitions for anemia slightly higher than the WHO criteria: Edwards et al. defined anemia below 13.5 g/dL for men and 12.5 g/dL for women, and Laso-Morales et al. defined anemia as less than 13 g/dL for both sexes.
Association of Iron Therapy and Hemoglobin Levels
Changes in Hb for each cohort in each study are summarized in Table 2. All nine of the reviewed studies assessed the effect of preoperative intravenous iron therapy on Hb levels. Edwards et al., Kim et al., Wilson et al., and Kam et al. used changes in Hb levels as their primary outcome, while Richards et al., Laso-Morales et al., Froessler et al., Keeler et al., and Calleja et al. made this a secondary outcome. Of the nine studies answering this question, four of the five RCTs and three of four non-randomized studies found a significant increase in average Hb levels in the IV iron arm of the study compared to the control group.
Table 2.
Results of included studies
| Study | Transfusion rate (%) | Blood transfused per patient | Change in Hb (g/dL) from IDA diagnosis to admission | Length of stay (days) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | IV iron therapy | p | Control | IV iron therapy | p | Control | IV iron therapy | p | Control | IV iron therapy | p | |
| Richards et al., 2020 | 28 | 29 | NS | 0.65 units | 0.61 units | NS | – | – | < 0.05a | 9 | 9 | NS |
| Edwards et al., 2009 | 19.2 | 14.7 | NS | 2 units | 0 units | NS | 0.1 | 0.5 | NS | – | – | – |
| Keeler et al., 2016 | 23 | 18 | NS | 0.632 mL | 0.693 mL | NS | 0.5 | 1.55 | 0.001 | 6 | 6 | NS |
| Froessler et al., 2018 | 31.3 | 12.5 | NS | 3 units | 2 units | 0.016 | 0.1 | 0.8 | 0.01 | 9.7 | 7 | 0.026 |
| Kim et al., 2009 | – | – | – | – | – | – | 0.8 | 3.9 | < 0.0001 | – | – | – |
| Laso-Morales et al., 2017 | 16 | 17 | NS | 0.3 units | 0.4 units | NS | – | – | – | 9 | 9 | NS |
| Wilson et al., 2018 | – | – | NS | – | – | – | 0.16 | 1.05 | < 0.0001 | – | – | – |
| Calleja et al., 2015 | 38.7 | 9.9 | < 0.001 | 0.8 units | 0.2 units | < 0.0001 | 0.5 | 1.5 | < 0.0001 | 10.9 | 8.4 | < 0.001 |
| Kam et al., 2020 | 21.1 | 48.4 | 0.006 | 0.55 units | 1.42 units | 0.076 | 0.6 | 1.9 | < 0.001 | 8.5 | 9 | NS |
All studies reported mean values with the exception of Richards et al. and Kam et al., who reported median values for length of stay
Hb hemoglobin, IDA iron deficiency anemia, NS non-significant, – not reported, CI confidence interval
aMean Hb values for control and IV iron groups not reported; however, mean difference on day of surgery was noted to be 0.47 g/dL between groups with CI 0.27–0.68
In contrast to the other studies, Edwards et al. found no significant change in mean Hb levels between groups for either the whole study population or the subgroup of patients with anemia [30]. The iron sucrose group’s median Hb increased by 0.5 g/dL from enrollment to hospital admission, while the usual care cohort increased by 0.1 g/dL.
Froessler et al. found a significant increase in mean Hb from enrollment to hospital admission in the IV ferric carboxymaltose (FCM) group versus usual care. The FCM group increased by 0.8 g/dL, while the usual care group increased by 0.1 g/dL [25]. Keeler et al. and Kim et al. compared oral iron therapy to IV iron therapy and found a significant increase in the mean Hb from enrollment to hospital admission in their IV iron cohorts compared to the oral iron cohorts. In Keeler et al., the FCM group’s Hb increased by 1.55 g/dL while the oral iron group’s only increased by 0.50 g/dL [26]. In Kim et al., the iron sucrose-treated group’s mean Hb increased by 3.0 g/dL, while the oral iron group only increased by 0.8 g/dL [28]. Laso-Morales et al. noted that, compared to patients on either oral iron therapy or no supplemental iron, patients with anemia on IV iron therapy presented with significantly lower baseline Hb (10.8 g/dL vs 12.0 g/dL) but similar Hb on the day of surgery, immediately postoperatively, at discharge, and 30 days postoperatively, implying that IV iron therapy was more effective in treating preoperative anemia [31]. Calleja et al. found a significant increase in mean Hb levels from enrollment to hospital admission in the FCM group versus usual care. This significant advantage is still present 30 days after surgery [24]. The FCM cohort mean Hb increased by 1.5 g/dL from enrollment to hospital admission versus only 0.5 g/dL in the usual care group. At 30 days postoperatively, these differences were 3.1 g/dL and 1.9 g/dL, respectively. Richards et al. similarly found a statistically significant increase in Hb from randomization to the time of surgery (mean difference 4.7 g/L) and at 8 weeks postoperatively (mean difference 10.7 g/L) [32]. Wilson et al. also found a significant increase in mean Hb levels from enrollment to hospital admission in the FCM group (1.05 g/dL) compared to usual care (0.16 g/dL) [27].
Association of Iron Therapy and Transfusion Rates
Transfusion rates and volumes for each cohort in each study are summarized in Table 2. Eight of the nine studies evaluated transfusion rates as an outcome. Of the five RCTs, three studies evaluated transfusion rates as a primary outcome, one as a secondary outcome, and one not at all. All non-randomized studies measured transfusion rates, two as a primary outcome, and two as a secondary outcome. Of the eight studies answering this question, only one found a significant improvement in transfusion rates.
Edwards et al. did not find a significant reduction in transfusion rates with iron sucrose administration, even when limiting their analysis to a small anemic subgroup [30]. Overall, 14.7% of patients from the iron sucrose group required transfusion, while 19.2% of the control group patients required transfusion. Similarly, Froessler et al. did not find a significant reduction in transfusion rates. Only 12.5% of FCM patients received transfusion, while 31.3% of usual care patients were transfused. They did find a significant reduction in the median number of red blood cell (RBC) units transfused in the FCM group with a median of 2 units transfused in the FCM group and 3 units in the usual care group [25]. Keeler et al. also found no significant difference in the number of patients transfused or volume transfused over the course of the study [26]. In absolute terms, the FCM group received more units of blood on average (0.698 vs 0.632), while fewer patients were transfused (18% vs 23%). Wilson et al. did not find any statistically significant differences in postoperative transfusion rates between their two cohorts after multivariate analysis with a 46% reduction in postoperative transfusion rates in the IV iron cohort [27]. Laso-Morales et al. also found no significant difference in the number of patients who required RBC transfusion between patients with anemia on IV iron therapy and those on standard care (16% vs 17%) [31]. Richards et al. also similarly found that between the placebo and IV iron groups, mean units of transfused blood were not statistically different from randomization to 30 days postoperatively (0.65 vs 0.61 units) and 6 months postoperatively (0.94 vs 0.75 units) [32]. Similarly, Kam et al. found that significantly fewer patients in the IV iron group required transfusions than in the non-IV iron group (8 vs 30) [29].
The outlier was in the study by Calleja et al., which found that the perioperative and 30-day postoperative percentages of patients transfused and RBC units transfused were significantly reduced in their FCM cohort [24]. Only 9.9% of patients in the FCM group required transfusion, while 38.7% of patients in the usual care cohort required transfusion. Mean units transfused decreased from 0.8 to 0.2. These reductions were found to be independent of the surgical approach (laparoscopic versus open).
Association of Iron Therapy and LOS
Patient LOS for each cohort in each study are summarized in Table 2. Four studies measured patient LOS as an outcome. Froessler et al. and Calleja et al. showed a significant reduction in the LOS in FCM-treated patients versus the usual care cohort, which was 2.5 days and 4 days, respectively. In contrast, Keeler et al., Richards et al., and Laso-Morales et al. reported that the LOS was equivalent between the IV iron groups and the controls.
Quality of Included Studies and Risk for Bias
The grading schemes for the randomized and non-randomized studies are specified in Tables 3 and 4, respectively. All nine studies were classified as good owing to a low risk of bias in blinding, randomization, and other sources of bias for the RCTs and the good comparability of cohorts and appropriate statistical analysis in the non-randomized studies.
Table 3.
Cochrane risk of bias tool for randomized studies
| Bias | Support for judgment | Author’s judgment |
|---|---|---|
| Richards et al. (UK, 2020) | ||
| Random sequence generation | Quote: “Randomisation was done by trained staff members using a secure web-based service through the Clinical Trials Unit at the London School of Hygiene & Tropical Medicine. The web-based service was provided by an independent research support organization” | Low risk |
| Allocation concealment | Quote: “Randomisation was 1:1 with allocation concealment that used minimisation, considering baseline haemoglobin (< 100 vs ≥ 100 g/L), age (< 70 vs ≥ 70 years), centre, and operation type (major, major plus, complex major)” | Low risk |
| Blinding of participants and personnel | Quote: “Because the intravenous iron was a dark-brown solution that is easily distinguishable from the saline placebo, dedicated unblinded study personnel were responsible for the preparation and administration of the study drug but had no other involvement in the trial. To ensure blinding of participants, their skin was swabbed with iodine, and the study treatment was shielded from vision (light protection bags) and infused through black tubing. Other clinical and research staff were blinded to the treatment allocated” | Low risk |
| Blinding of outcome assessment | Comment: No blinding of coprimary outcome assessment (composite endpoint of blood transfusion or death and the number of blood transfusion episodes). These objective outcomes are unlikely to change under unblinded conditions | Low risk |
| Incomplete outcome data | Comment: 3 patients withdrew from the study between randomization and surgery. Between surgery and follow-up visits (8 week and 6 month), 5 patients withdrew and 5 were lost to follow-up. Also, between surgery and follow-up visits, 22 deaths occurred, 3 of which had missing information needed for intention to treat analysis for coprimary endpoints | Unclear risk |
| Selective reporting | Comment: None identified | Unclear risk |
| Other bias | Comment: None identified | Unclear risk |
| Quality | Good | |
| Edwards et al. (UK, 2009) | ||
| Random sequence generation | Quote: “Computer-generated randomization sequence” | Low risk |
| Allocation concealment | Quote: “Sealed in sequentially numbered opaque envelopes” | Low risk |
| Blinding of participants and personnel | Quote: “Concealed from the patient by using an opaque sheath to cover the drug giving set” | Low risk |
| Blinding of outcome assessment | Quote: “Chief investigator and clinicians involved remained blinded to the treatment group for the duration of the trial” | Low risk |
| Incomplete outcome data | Quote: “One patient from each group failed to attend for the second infusion” | Low risk |
| Selective reporting | Comment: None identified | Unclear risk |
| Other bias | Comment: None identified | Unclear risk |
| Quality | Good | |
| Keeler et al. (UK, 2017) | ||
| Random sequence generation | Quote: “Recruited patients were randomized in a 1:1 fashion via a web-based system using variable block allocation” | Low risk |
| Allocation concealment | Quote: “The system was designed, set up and run by a unit independent of the study” | Low risk |
| Blinding of participants and personnel | Comment: Open label study but lack of blinding unlikely to influence objective outcomes | Low risk |
| Blinding of outcome assessment | Comment: No blinding unlikely to influence perioperative changes in hemoglobin, ferritin, transferrin saturation, and blood transfusion | Low risk |
| Incomplete outcome data |
Quote: “Four patients had their operation cancelled on the day of surgery… one patient died during induction of anesthesia, and the operation was abandoned at initial laparotomy in one patient as inoperable disease was found” Comment: No incomplete outcome data was reported |
Low risk |
| Selective reporting | Comment: None identified | Unclear risk |
| Other bias | Comment None identified | Unclear risk |
| Quality | Good | |
| Froessler et al. (UK, 2009) | ||
| Random sequence generation | Quote: “Computer-generated number sequence” | Low risk |
| Allocation concealment | Quote: “Allocation was conducted by telephone” | Low risk |
| Blinding of participants and personnel | Quote: “Surgeon performing the operation was informed of patient participation in the study but group allocation was not revealed” | Low risk |
| Blinding of outcome assessment | Comment: No blinding but objective measurement of transfusion incidence and hemoglobin unlikely to be influenced | Low risk |
| Incomplete outcome data | Quote: “The study was terminated early due to higher than expected rates of poor outcome in the usual care group” | Low risk |
| Selective reporting | Comment: None identified | Unclear risk |
| Other bias | Comment: None identified | Unclear risk |
| Quality | Good | |
| Kim et al. (South Korea, 2009) | ||
| Random sequence generation | Quote: “Computer-generated randomization tables” | Low risk |
| Allocation concealment | Quote: “Group allocation was determined by one of the authors who was not involved in patient care” | Low risk |
| Blinding of participants and personnel | Comment: Open label study but lack of blinding unlikely to influence objective outcomes | Low risk |
| Blinding of outcome assessment | Comment: No blinding but objective outcome of change in hemoglobin level unlikely to be influenced | Low risk |
| Incomplete outcome data | Comment: None identified | Unclear risk |
| Selective reporting | Quote: “Participants who had ≥ 80% compliance were included in the analysis for efficacy” | Low risk |
| Other bias | Comment: None identified | Unclear risk |
| Quality | Good | |
Table 4.
Newcastle–Ottawa Scale for non-randomized studies
| Author’s judgment | Support for judgment | Score | |
|---|---|---|---|
| Laso-Morales et al. (Spain, 2017) | |||
| Selection | |||
| Representativeness of the exposed cohort | Truly representative of patients with iron deficiency anemia undergoing resection for colorectal cancer | Non-elective procedures, non-anemic cases, and patients who had already received RBC transfusions were excluded | 1 |
| Selection of the non-exposed cohort | Drawn from the same community as the exposed cohort | Both exposed and non-exposed cohorts drawn from two Spanish centers from January 2012 to December 2013 | 1 |
| Ascertainment of exposure | Secure record | Clinical characteristics and laboratory data collected from medical record | 1 |
| Demonstration that outcome of interest was not present at start of study | Yes | Primary outcome was relative RBC transfusion rate (%) and RBC transfusion index (units/patient) | 1 |
| 4 | |||
| Comparability | |||
| Comparability of cohorts on the basis of the design or analysis | Study controls for differences in blood transfusion criteria | The following transfusion protocol was uniformly applied across participating centers: a restrictive transfusion trigger of Hb < 8 g/dL unless “active cardiac disease or symptoms of acute anemia” in which a less restrictive transfusion trigger, Hb < 9 g/dL, was used | 1 |
| 1 | |||
| Outcome | |||
| Assessment of outcome | Record linkage | Clinical characteristics and laboratory data were collected from medical records | 1 |
| Was follow-up long enough for outcomes to occur? | Yes | Follow-up extended to 30 days post-surgery | 1 |
| Adequacy of follow-up of cohorts | Yes | Complete follow-up with all subjects accounted for | 1 |
| 3 | |||
| Overall quality | Good | ||
| Wilson et al. (the Netherlands, 2006) | |||
| Selection | |||
| Representativeness of the exposed cohort | Yes, truly representative of patients with anemia undergoing resection for colorectal cancer | Only patients who had surgery in the emergency setting and those with missing data with respect to baseline Hb levels and blood transfusions were excluded | 1 |
| Selection of the non-exposed cohort | Drawn from the same community as the exposed cohort | Both exposed and non-exposed cohorts drawn from the Reiner De Graaf Hospital from January 1, 2010 to July 1, 2016 | 1 |
| Ascertainment of exposure | Secure record | Hb values manually obtained from medical records | 1 |
| Demonstration that outcome of interest was not present at start of study | Yes | Primary outcome was relative change in allogenic blood transfusion requirements between exposed and non-exposed cohorts | 1 |
| 4 | |||
| Comparability | |||
| Comparability of cohorts on the basis of the design or analysis | Study controls for differences in blood transfusion criteria | Performed multivariate analysis to control for differences in the severity of the anemia and condition of the patient | 1 |
| 1 | |||
| Outcome | |||
| Assessment of outcome | Independent blind assessment | Clinical and pathologic data collected by the Dutch Surgical Colorectal Audit, a disease-specific national audit. The data set is based on evidenced-based guidelines and is crosschecked on a yearly basis with data from the Netherlands Cancer Registry | 1 |
| Was follow-up long enough for outcomes to occur? | Yes | Follow-up extended to 30 days post-surgery | 1 |
| Adequacy of follow-up of cohorts | Yes | Data set collected by the Dutch Surgical Colorectal Audit includes information on patient, tumor, treatment, and 30-day and in-hospital outcome characteristics of all patients undergoing a resection for primary colorectal carcinoma in the Netherlands | 1 |
| 3 | |||
| Overall quality | Good | ||
| Calleja et al. (Spain, 2015) | |||
| Selection | |||
| Representativeness of the exposed cohort | Yes, somewhat representative of colon cancer patients with anemia | Inclusion criteria limited to patients “diagnosed with colon adenocarcinoma located at least 15 cm above anal margin” | 1 |
| Selection of the non-exposed cohort | Drawn from the same community as the exposed cohort | Although exposed cohort was drawn prospectively and the non-exposed cohort retrospectively, both cohorts were drawn from the same participant centers. To avoid selection bias, the retrospective cohort was obtained in a sequential manner independently of outcomes | 1 |
| Ascertainment of exposure | Secure record | Collected from surgical intervention 2011 registries | 1 |
| Demonstration that outcome of interest was not present at start of study | Yes | Primary outcome was relative reduction in allogenic blood transfusion requirements between exposed and non-exposed cohorts | 1 |
| 4 | |||
| Comparability | |||
| Comparability of cohorts on the basis of the design or analysis | Study controls for differences in blood transfusion criteria | Standardized blood transfusion protocol of being “always performed in patients with hemoglobin levels under 7 g/dL, under physician criteria between 7 and 9 g/dL, and not recommended over 9 g/dL.” Defined iron deficiency anemia using WHO criteria of Hb level < 13 g/dL in men and < 12 g/dL in women | 1 |
| 1 | |||
| Outcome | |||
| Assessment of outcome | Record linkage | Outcomes collected from surgical intervention 2011 registries | 1 |
| Was follow-up long enough for outcomes to occur? | Yes | Follow-up extended to 30 days post-surgery in the exposed cohort. 30-day time frame supported by a previous study which “found evidence for improved hemoglobin concentrations and reduced transfusions” in the 30-day post-surgery period in patients with gastrointestinal cancer and with anemia treated with FCM | 1 |
| Adequacy of follow-up of cohorts | No statement | 0 | |
| 2 | |||
| Overall quality | Good | ||
| Kam et al. (China, 2020) | |||
| Selection | |||
| Representativeness of the exposed cohort | Yes, somewhat representative of patients undergoing resection for colorectal adenocarcinoma with iron deficiency anemia | Patients were excluded if tumor histology was not consistent with adenocarcinoma or if anemia was not due to iron deficiency | 1 |
| Selection of the non-exposed cohort | Drawn from the same community as the exposed cohort | Data for non-exposed cohort was collected retrospectively from a database of all patients treated in Hospital Authority hospitals in Hong Kong, which includes Queen Elizabeth Hospital, where the exposed cohort’s data was obtained | 1 |
| Ascertainment of exposure | Secure record | Data collected prospectively from surgical records | 1 |
| Demonstration that outcome of interest was not present at start of study | Yes | Primary outcome was mean Hb change after use of preoperative IV iron therapy. At baseline preoperatively, there was no statistical difference between mean Hb in the exposed and non-exposed cohorts | 1 |
| 4 | |||
| Comparability | |||
| Comparability of cohorts on the basis of the design or analysis | Study used 2:1 propensity score matching to control for first Hb level, age, sex, transfusion of RBC prior to IV iron therapy, use of oral iron supplement, and tumor location | After matching there were no statistical differences between median age, sex, premorbid status, medical comorbidities, use of antiplatelet or anticoagulant medication, RBC transfusions, or percentage taking oral iron therapy prior to surgery | 1 |
| 1 | |||
| Outcome | |||
| Assessment of outcome | Record linkage | Outcomes prospectively collected from medical records as patients underwent elective operations | 1 |
| Was follow-up long enough for outcomes to occur? | Yes | For the outcome of interest, mean Hb change from the time of initial IV iron administration (at least 2 weeks prior to surgery) and admission for surgery, there was adequate follow-up | 1 |
| Adequacy of follow-up of cohorts | Complete follow-up—all subjects accounted for | After propensity score matching, 38 patients where included in the IV iron group and 62 patients in the historic (non-IV iron) cohort, all of whom were followed through duration of the study | 1 |
| 3 | |||
| Overall quality | Good | ||
Hb hemoglobin, IV intravenous, RBC red blood cell
Discussion
Our goal in this systematic review was to determine if preoperative IV iron therapy for patients with anemia undergoing abdominal surgery could improve clinical outcomes. Most studies reviewed demonstrate a rapid improvement in serum Hb with the administration of IV iron therapy, as evidenced by mean pre- and post-treatment mean Hb change. However, these studies did not provide consistent evidence of a reduced transfusion rate with preoperative IV iron administration. Thus, while we recommend the preoperative treatment of anemia with oral iron supplementation when possible, there is currently insufficient clinical evidence to justify IV iron administration as a standard treatment for all patients with anemia scheduled for major abdominal surgery.
The best evidence for reduction in transfusion frequency comes from Calleja et al., which showed significant reductions in transfusion rates, and Froessler et al., which showed a significant decrease in units transfused [24, 25]. This is in contrast to the other studies examining this outcome that showed no significant changes. In contrast to the mixed transfusion results, eight out of nine studies showed that IV iron therapy caused a significant increase in preoperative Hb relative to oral iron therapy or usual care. As Hb level is a key predictor for blood transfusion, it stands to reason that an intervention that reliably increases Hb should also reduce the need for transfusions if large enough cohorts are examined.
There are several potential reasons for the heterogeneity between the studies. Edwards et al. had a good experimental design but was very much an outlier in study goals compared to the other five studies. This study included very few subjects with anemia and had a less stringent definition of anemia than WHO guidelines. This is reflected in the relatively high average ferritin levels (100.5 ng/mL) in the patients with anemia receiving IV iron therapy [30]. The amount of iron administered was also the lowest in this study at 600 mg total. Together, these factors limit our ability to make conclusions about the preoperative treatment of anemia as so few patients with anemia were treated in this study. Froessler et al. had a similar design to Edwards et al. but required included patients to have IDA and administered a much higher dose of IV iron therapy. This may explain why they found a significant improvement in Hb, units transfused, and LOS in their IV iron arm [25].
Keeler et al. was similar to Froessler et al. in study design and compared oral iron therapy to IV iron therapy but administered the lowest dose of IV iron therapy [26]. This reduced dose and the administration of oral iron therapy to the control group may have contributed to the lack of a significant reduction in transfusion rate or LOS in this study.
Kim et al. also compared oral iron therapy to IV iron therapy but included only patients with known IDA and administered one of the largest overall doses of IV iron therapy. This is likely why their study showed the largest increase in Hb concentrations with IV iron administration. However, they did not report transfusion rates [28].
Calleja et al. had a large experimental group, and the control group in this study was also receiving oral iron supplementation [24]. Unfortunately, interpretation of the results from Calleja et al. was limited by the fact that the control cohort is a retrospective cohort and the FCM cohort is a prospective cohort, separated from one another in time. Appropriate efforts were made to match the two cohorts, but the chronological differences between the cohorts and lack of multivariate analysis limited the comparability between groups. This chronological effect was likely responsible for the increased rate of laparoscopic surgery in the more recent FCM cohort. These issues may explain why Calleja et al. observed such a large effect from FCM treatment on the transfusion rate. This may also explain why different lengths of stay were observed between the study arms. However, intraoperative blood loss was equal between groups, if not marginally higher in the FCM cohort. When the two surgical approaches were analyzed separately, the reduced need for transfusions is maintained. Thus, changes in surgical approach were less likely to be responsible for the findings of Calleja et al.
Wilson et al. utilized a fully retrospective study design with cohort assignment dependent upon provider preference [27]. As such, patients receiving IV iron therapy in this study were more likely to have more severe anemia, comorbidities, laparoscopic surgery, male gender, and to have been treated after the institution of a new patient blood management protocol that recommended increased usage of IV iron therapy. These factors make it challenging to make any definitive conclusions about their results, although they use an appropriate statistical approach to analyze it.
A high frequency of transfusion in the usual care group is a commonality in studies demonstrating a significant reduction in transfusion rate. Both Calleja et al. and Froessler et al. reported blood transfusion rates higher than 30% in control cohorts, while Edwards et al. and Keeler et al. report rates lower than 25%. This may indicate that preoperative IDA screening and IV iron treatment may be most effective for centers and surgeries that have especially high rates of transfusion.
Overall, these studies are consistent with IV iron therapy being highly efficacious for increasing levels of Hb in patients with IDA. Two of the studies also compared oral iron therapy to IV iron therapy. Both showed that IV iron therapy was significantly more efficacious at raising Hb than oral iron therapy, even in experimental circumstances where oral iron therapy compliance would be expected to be higher than normal. However, this effect on serum Hb does not consistently lead to a significantly reduced frequency of transfusions in these studies. Thus, more research is necessary to definitively determine whether IV iron therapy reduces the need for transfusions in major abdominal surgery.
Recent reviews and meta-analyses of the efficacy of IV iron administration in patients with anemia have been performed looking at orthopedic surgery patients, cardiac surgery patients, and surgical patients generally. In their meta-analysis, Shin et al. found that there was insufficient evidence for benefit from perioperative IV iron administration on transfusion rates or recovery in major orthopedic surgery, similar to our findings [33]. Hogan et al. performed a systematic review looking at this question for cardiac surgery and also found a lack of evidence for improved outcomes with IV iron administration as a result of a lack of studies examining that question [34]. In a Cochrane meta-analysis, Ng et al. examined the efficacy of IV iron therapy in surgical patients with anemia and found that while IV iron therapy did cause a significant increase in Hb and ferritin, there was no significant decrease in transfusion rates [35]. They also reported that the evidence for a significant improvement in Hb was weak. Another recent meta-analysis from Peters et al. also evaluated IV iron administration in all surgical patients, with similar findings to Ng et al. [36]. Thus, the results we report here are similar to prior meta-analyses and systematic reviews looking at similar questions.
Strengths and Limitations
Our systematic review has several strengths. We performed a comprehensive search with broad search terms, enlisted methodology expertise, and did not limit the search to elective surgeries or by time frame. We included all experimental designs to avoid selection bias. We assessed quality using the Cochrane risk of bias tool and the Newcastle–Ottawa Scale assessment of study quality.
Our systematic review is the most recent and comprehensive on this topic but was limited to only nine articles, including five good-quality RCTs and four good-quality non-randomized studies. One of our systematic review’s main limitations is the lack of publications investigating iron therapy as a preoperative intervention for major abdominal surgery. We acknowledge the surgical heterogeneity inherent in the broad category of major abdominal surgery. However, most studies presented in this systematic review involve patients undergoing bowel resection for colorectal cancer, and the additional studies are of good quality. We felt that excluding studies that did not specifically include patients with colorectal cancer would detract from an already sparse body of literature. In all, we were only able to identify nine good-quality studies that met our inclusion and exclusion criteria; nevertheless, strong conclusions could not be drawn from these studies given the heterogeneity both in surgical populations and in specific outcomes data. Individual studies may not have given high enough doses of the treatment or have been inadequately powered to identify significant differences between groups.
There are several studies that discuss the safety of IV iron treatment that were excluded from this systematic review since they did not meet our inclusion criteria [37–40].
Conclusions
There is a paucity of RCTs evaluating the merits of IV iron supplementation in patients with anemia undergoing major abdominal surgery. The studies identified and evaluated in this systematic review demonstrate a consistent, significant increase in hemoglobin levels in the intravenous iron group but do not consistently report a significant reduction in the number of transfusions intraoperatively. More studies are necessary to definitively determine the efficacy of IV iron therapy in decreasing morbidity and mortality for patients. Thus, we recommend that patients be evaluated and treated for IDA before receiving abdominal surgery. There is currently insufficient evidence of improved outcomes to support the administration of IV iron therapy in all cases of IDA. However, when patients are diagnosed with significant IDA close to their day of surgery, IV iron therapy appears to be far more efficacious at resolving anemia than oral iron therapy and would be a reasonable intervention.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Tiffany Moon, Aaron Smith, Taylor Pak, Brian H. Park, Sascha S. Beutler, Travis Brown, Alan D. Kaye have nothing to disclose. Richard D. Urman discloses fees/funding from Merck, Medtronic/Covidien, AcelRx, Pfizer and Acacia.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Muñoz M, Gómez-Ramírez S, Campos A, Ruiz J, Liumbruno GM. Pre-operative anaemia: prevalence, consequences and approaches to management. Blood Transfus. 2015;13(3):370–379. doi: 10.2450/2015.0014-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104(8):2263–2268. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 3.Karkouti K, Wijeysundera DN, Beattie WS, Reducing Bleeding in Cardiac Surgery (RBC) Investigators. Risk associated with preoperative anemia in cardiac surgery: a multicenter cohort study. Circulation. 2008;117(4):478–84. [DOI] [PubMed]
- 4.Theusinger OM, Kind SL, Seifert B, Borgeat L, Gerber C, Spahn DR. Patient blood management in orthopaedic surgery: a four-year follow-up of transfusion requirements and blood loss from 2008 to 2011 at the Balgrist University Hospital in Zurich, Switzerland. Blood Transfus. 2014;12(2):195–203. doi: 10.2450/2014.0306-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliver JC, Griffin RL, Hannon T, Marques MB. The success of our patient blood management program depended on an institution-wide change in transfusion practices. Transfusion. 2014;54(10 Pt 2):2617–2624. doi: 10.1111/trf.12536. [DOI] [PubMed] [Google Scholar]
- 6.Roubinian NH, Escobar GJ, Liu V, et al. Trends in red blood cell transfusion and 30-day mortality among hospitalized patients. Transfusion. 2014;54(10 Pt 2):2678–2686. doi: 10.1111/trf.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leahy MF, Roberts H, Mukhtar SA, et al. A pragmatic approach to embedding patient blood management in a tertiary hospital. Transfusion. 2014;54(4):1133–1145. doi: 10.1111/trf.12362. [DOI] [PubMed] [Google Scholar]
- 8.Goodnough LT, Maggio P, Hadhazy E, et al. Restrictive blood transfusion practices are associated with improved patient outcomes. Transfusion. 2014;54(10 Pt 2):2753–2759. doi: 10.1111/trf.12723. [DOI] [PubMed] [Google Scholar]
- 9.Gross I, Seifert B, Hofmann A, Spahn DR. Patient blood management in cardiac surgery results in fewer transfusions and better outcome. Transfusion. 2015;55(5):1075–1081. doi: 10.1111/trf.12946. [DOI] [PubMed] [Google Scholar]
- 10.Goodnough LT, Shieh L, Hadhazy E, Cheng N, Khari P, Maggio P. Improved blood utilization using real-time clinical decision support. Transfusion. 2014;54(5):1358–1365. doi: 10.1111/trf.12445. [DOI] [PubMed] [Google Scholar]
- 11.Moskowitz DM, McCullough JN, Shander A, et al. The impact of blood conservation on outcomes in cardiac surgery: is it safe and effective? Ann Thorac Surg. 2010;90(2):451–458. doi: 10.1016/j.athoracsur.2010.04.089. [DOI] [PubMed] [Google Scholar]
- 12.Leahy MF, Hofmann A, Towler S, et al. Improved outcomes and reduced costs associated with a health-system-wide patient blood management program: a retrospective observational study in four major adult tertiary-care hospitals. Transfusion. 2017;57(6):1347–1358. doi: 10.1111/trf.14006. [DOI] [PubMed] [Google Scholar]
- 13.Cuenca J, García-Erce JA, Martínez F, Pérez-Serrano L, Herrera A, Muñoz M. Perioperative intravenous iron, with or without erythropoietin, plus restrictive transfusion protocol reduce the need for allogeneic blood after knee replacement surgery. Transfusion. 2006;46(7):1112–1119. doi: 10.1111/j.1537-2995.2006.00859.x. [DOI] [PubMed] [Google Scholar]
- 14.Acheson AG, Brookes MJ, Spahn DR. Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery: a systematic review and meta-analysis. Ann Surg. 2012;256(2):235–244. doi: 10.1097/SLA.0b013e31825b35d5. [DOI] [PubMed] [Google Scholar]
- 15.Amato A, Pescatori M. Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane Database Syst Rev. 2006;1:CD005033. [DOI] [PMC free article] [PubMed]
- 16.Mueller MM, Van Remoortel H, Meybohm P, et al. Patient blood management: recommendations from the 2018 Frankfurt Consensus Conference. JAMA. 2019;321(10):983–997. doi: 10.1001/jama.2019.0554. [DOI] [PubMed] [Google Scholar]
- 17.Kotzé A, Harris A, Baker C, et al. British Committee for Standards in Haematology guidelines on the identification and management of pre-operative anaemia. Br J Haematol. 2015;171(3):322–331. doi: 10.1111/bjh.13623. [DOI] [PubMed] [Google Scholar]
- 18.Shah A, Palmer AJR, Fisher SA, et al. What is the effect of perioperative intravenous iron therapy in patients undergoing non-elective surgery? A systematic review with meta-analysis and trial sequential analysis. Perioper Med. 2018;7(1):30. doi: 10.1186/s13741-018-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schack A, Berkfors AA, Ekeloef S, Gögenur I, Burcharth J. The effect of perioperative iron therapy in acute major non-cardiac surgery on allogenic blood transfusion and postoperative haemoglobin levels: a systematic review and meta-analysis. World J Surg. 2019;43(7):1677–1691. doi: 10.1007/s00268-019-04971-7. [DOI] [PubMed] [Google Scholar]
- 20.Perelman I, Winter R, Sikora L, Martel G, Saidenberg E, Fergusson D. The efficacy of postoperative iron therapy in improving clinical and patient-centered outcomes following surgery: a systematic review and meta-analysis. Transfus Med Rev. 2018;32(2):89–101. doi: 10.1016/j.tmrv.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Ioannidis JPA, Patsopoulos NA, Rothstein HR. Reasons or excuses for avoiding meta-analysis in forest plots. BMJ. 2008;336(7658):1413–1415. doi: 10.1136/bmj.a117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;18(343):d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wells G, Shea B, O’Connell B, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 10 Dec 2020.
- 24.Calleja JL, Delgado S, del Val A, et al. Ferric carboxymaltose reduces transfusions and hospital stay in patients with colon cancer and anemia. Int J Colorect Dis. 2016;31(3):543–551. doi: 10.1007/s00384-015-2461-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Froessler B, Palm P, Weber I, Hodyl NA, Singh R, Murphy EM. The important role for intravenous iron in perioperative patient blood management in major abdominal surgery: a randomized controlled trial. Ann Surg. 2016;264(1):41–46. doi: 10.1097/SLA.0000000000001646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keeler BD, Simpson JA, Ng O, et al. Randomized clinical trial of preoperative oral versus intravenous iron in anaemic patients with colorectal cancer. Br J Surg. 2017;104(3):214–221. doi: 10.1002/bjs.10328. [DOI] [PubMed] [Google Scholar]
- 27.Wilson MJ, Dekker JW, Bruns E, et al. Short-term effect of preoperative intravenous iron therapy in colorectal cancer patients with anemia: results of a cohort study. Transfusion. 2018;58(3):795–803. doi: 10.1111/trf.14456. [DOI] [PubMed] [Google Scholar]
- 28.Kim YH, Chung HH, Kang S-B, Kim SC, Kim YT. Safety and usefulness of intravenous iron sucrose in the management of preoperative anemia in patients with menorrhagia: a phase IV, open-label, prospective, randomized study. Acta Haematol. 2009;121(1):37–41. doi: 10.1159/000210062. [DOI] [PubMed] [Google Scholar]
- 29.Kam PM-H, Chu CW-H, Chan EM-Y, Liu O-L, Kwok K-H. Use of intravenous iron therapy in colorectal cancer patient with iron deficiency anemia: a propensity-score matched study. Int J Colorect Dis. 2020;35(3):521–7. [DOI] [PubMed]
- 30.Edwards TJ, Noble EJ, Durran A, Mellor N, Hosie KB. Randomized clinical trial of preoperative intravenous iron sucrose to reduce blood transfusion in anaemic patients after colorectal cancer surgery. Br J Surg. 2009;96(10):1122–1128. doi: 10.1002/bjs.6688. [DOI] [PubMed] [Google Scholar]
- 31.Laso-Morales M, Jericó C, Gómez-Ramírez S, et al. Preoperative management of colorectal cancer-induced iron deficiency anemia in clinical practice: data from a large observational cohort. Transfusion. 2017;57(12):3040–3048. doi: 10.1111/trf.14278. [DOI] [PubMed] [Google Scholar]
- 32.Richards T, Baikady RR, Clevenger B, et al. Preoperative intravenous iron to treat anaemia before major abdominal surgery (PREVENTT): a randomised, double-blind, controlled trial. Lancet. 2020;396(10259):1353–1361. doi: 10.1016/S0140-6736(20)31539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin HW, Park JJ, Kim HJ, You HS, Choi SU, Lee MJ. Efficacy of perioperative intravenous iron therapy for transfusion in orthopedic surgery: a systematic review and meta-analysis. PLoS One. 2019;14(5). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6502310/. Accessed 10 Dec 2020. [DOI] [PMC free article] [PubMed]
- 34.Hogan M, Klein AA, Richards T. The impact of anaemia and intravenous iron replacement therapy on outcomes in cardiac surgery. Eur J Cardiothorac Surg. 2015;47(2):218–226. doi: 10.1093/ejcts/ezu200. [DOI] [PubMed] [Google Scholar]
- 35.Ng O, Keeler BD, Mishra A, et al. Iron therapy for preoperative anaemia. Cochrane Datab Syst Rev. 2019;2019(12). [DOI] [PMC free article] [PubMed]
- 36.Peters F, Ellermann I, Steinbicker AU. Intravenous iron for treatment of anemia in the 3 perisurgical phases: a review and analysis of the current literature. Anesth Analg. 2018;126(4):1268–1282. doi: 10.1213/ANE.0000000000002591. [DOI] [PubMed] [Google Scholar]
- 37.Muñoz M, Gómez-Ramírez S, Bhandari S. The safety of available treatment options for iron-deficiency anemia. Expert Opin Drug Saf. 2018;17(2):149–159. doi: 10.1080/14740338.2018.1400009. [DOI] [PubMed] [Google Scholar]
- 38.Kalra PA, Bhandari S. Safety of intravenous iron use in chronic kidney disease. Curr Opin Nephrol Hypertens. 2016;25(6):529–535. doi: 10.1097/MNH.0000000000000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhandari S, Allgar V, Lamplugh A, Macdougall IC, Kalra PA. Protocol and baseline data of a multicentre prospective double-blinded randomized study of intravenous iron on functional status in patients with chronic kidney disease. Am J Nephrol. 2020;51(6):493–500. doi: 10.1159/000507872. [DOI] [PubMed] [Google Scholar]
- 40.Macdougall IC, Bhandari S, White C, et al. Intravenous iron dosing and infection risk in patients on hemodialysis: a prespecified secondary analysis of the PIVOTAL trial. J Am Soc Nephrol. 2020;31(5):1118–1127. doi: 10.1681/ASN.2019090972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.