Abstract
Introduction
Although clinical guidelines are broadly available, the relationship between adherence and outcomes is not well studied. This study aimed to assess the association between adherence to National Comprehensive Cancer Network (NCCN) guidelines and clinical outcomes for adult patients with advanced non-small-cell lung cancer (aNSCLC).
Methods
This was a retrospective cohort study of adult patients with aNSCLC (stages IIIB, IIIC, and IV) from a de-identified real-world database. The objective was accomplished in a two-step analysis process. We first assessed adherence to NCCN recommendations for biomarker testing and overall survival (OS). Next, we assessed adherence to NCCN-recommended first-line therapy and time to treatment discontinuation (TTD). Multivariable Cox regression analyses were conducted to evaluate the association between guideline adherence and patient outcomes. Kaplan–Meier analyses were used to assess median OS and TTD.
Results
A total of 28,784 patients with a diagnosis for aNSCLC between January 1, 2011 and July 31, 2019 met the inclusion criteria for the analysis of NCCN-recommended biomarker testing adherence. Two-thirds of these patients (n = 19,787) had evidence of biomarker testing (adherent). Multivariable Cox models found that testing-adherent patients had a significantly lower risk of mortality [hazard ratio (HR) = 0.89, 95% confidence interval (CI) 0.86, 0.92; p < 0.01]. Median OS was modestly longer in the testing-adherent group compared to the testing-non-adherent group (15.4 vs. 14.2 months; p < 0.01). For the first-line therapy analysis, 15,898 patients met the inclusion criteria, of which 69.9% had evidence of appropriate first-line therapy (first-line-adherent). The multivariable Cox model found that adherent patients had significantly lower risk of treatment discontinuation versus non-adherent patients (HR = 0.60, 95% CI 0.57, 0.62; p < 0.01). First-line-adherent patients had a modest, yet significantly longer median TTD compared to first-line-non-adherent patients (3.45 vs. 2.40 months; p < 0.01).
Conclusions
Improved clinical outcomes were observed in patients who were adherent to NCCN-recommended biomarker testing and first-line therapy. This study demonstrated the value of following NCCN guideline recommendations and the need to prioritize timely access to biomarker testing and individualized treatment.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-020-01617-2.
Keywords: Adherence, Biomarker, First-line treatment, Guidelines, Non-small cell lung cancer, Precision medicine
Key Summary Points
| Two-thirds of advanced non-small cell lung cancer patients from a de-identified real-world database between 2011 and 2019 had evidence of adherence to National Comprehensive Cancer Network (NCCN)-recommended biomarker testing and first-line therapy based on biomarker testing. |
| Adherence to NCCN-recommended biomarker testing was associated with significantly lower risk of mortality among these patients. |
| Patients adherent to NCCN-recommended first-line therapy based on biomarker results had significantly lower risk of treatment discontinuation versus non-adherent patients. |
| The findings of this real-world data study demonstrate that, even among a large, heterogeneous population across the US, where testing and drug availability may differ, testing guidelines drive treatment decisions, resulting in a reduced risk of mortality and a moderate, yet significant, increased duration of treatment, collectively supporting the practice of precision medicine. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.13476120.
Introduction
Precision oncology focuses on identifying appropriate therapies based on the genetic and molecular characterization of the cancer [1, 2]. As the interest for this type of medicine grows, the number of clinical trials and therapies developed with companion diagnostics to support personalized medicine have also increased [1]. Keeping abreast of available and emerging biomarkers and the ensuing treatments driven by the heterogeneity of the disease is challenging. Guidelines provide clinicians a practical guide to individualize these complex treatment pathways for patients, helping them navigate biomarkers and the ensuing treatment specifically based on their stage of disease. There is a growing body of evidence suggesting that adherence to guideline-recommended biomarker-driven therapies may improve patient outcomes. These improvements in patient outcomes may be even more critical in indications such as advanced non-small cell lung cancer (aNSCLC), where prognosis is generally poor [3, 4].
Guidelines from the National Comprehensive Cancer Network (NCCN), as well as from the American Society of Clinical Oncology (ASCO) and the European Society of Medicinal Oncology (ESMO), recommend the use of biomarker-driven therapies in stage IV (metastatic) lung cancer patients, while recommendations for biomarker-driven therapies are less clear in stages I–III, early stage, or locally-advanced patients [4–8]. A major strength of the NCCN guidelines is the frequency of updates, sometimes multiple times a year, as seen in NSCLC [5]. However, keeping up with these guideline updates and then incorporating them to changes in clinical practice may be challenging and time-consuming.
Multiple studies have indicated variability in adherence to the guidelines [9–13]. Most studies demonstrated improved outcomes in patients adherent to guideline-recommended first-line therapy, with many studies focusing on a specific biomarker and first-line therapy pairing [9, 12, 13]. However, there is a gap in assessing clinically meaningful impact of adherence holistically across multiple biomarkers.
This study aimed to comprehensively assess the association between NCCN guideline adherence to biomarker testing and first-line therapies in patients with aNSCLC and patient outcomes, overall survival (OS) and time to treatment discontinuation (TTD), using real-world data from the United States.
Methods
Study Objective
This study aimed to assess the association between adherence to NCCN guidelines and clinical outcomes among adult patients with NSCLC in the advanced setting (stage IIIB or higher), based on the most recent 2019 NCCN guidelines (V.7) available at the time of analysis [4]. We conducted two analyses within this study. For the first analysis, we assessed the association between adherence to guideline-recommended biomarker testing and OS. For the second analysis, we assessed the association between adherence to guideline-recommended first-line therapy and TTD.
Study Design and Data Source
This was a retrospective cohort study of adult patients with aNSCLC (stages IIIB, IIIC, IVA, and IVB) from the Flatiron Health database. Flatiron Health is a nationwide longitudinal, de-identified database derived from electronic health record (EHR) data. During the study period, the de-identified data originated from approximately 280 US cancer clinics (~ 800 sites of care). Structured data include data on diagnosis, laboratory values, and medication administrations, and unstructured data include clinic notes, both curated via technology-enabled abstraction [14, 15]. This database provides longitudinal data that allow individual patients to be followed over several years after their date of advanced cancer diagnosis.
No ethics committee or IRB approval is required for this study using de-identified retrospective data.
Study Population
Adult patients, aged 18 years or older, diagnosed with non-squamous or not otherwise specified (NOS) aNSCLC from January 1, 2011 through July 31, 2019 were included in this analysis. To minimize bias, patients must have had recorded medical activity within 90 days following aNSCLC diagnosis, and at least a 90-day follow-up period after diagnosis. Patients were excluded if age and sex were unknown. Further, patients with evidence of a biomarker test in their EHR record, but without a biomarker result date recorded, were excluded from this study.
An additional set of exclusion criteria were applied for the first-line therapy analysis. Firstly, patients must have had a recorded first-line therapy within 90 days of aNSCLC diagnosis, and at least 90 days of follow-up following initiation of the first-line therapy. Patients who received a clinical study drug as part of the first-line therapy were excluded. Further, patients who received a biomarker-driven targeted or immunotherapy as first-line without evidence of a positive biomarker test result were excluded, as testing information may be incomplete or missing in these patients’ records or in the database.
Definitions of Key Exposure Variables
Adherence to Biomarker Testing
For the analysis on adherence to biomarker testing, patients were grouped into either a testing-adherent cohort or testing-non-adherent cohort. The testing-adherent group consisted of patients with evidence of testing for any biomarkers including EGFR, ALK, BRAF, KRAS, ROS1, or PD-L1 between 14-days prior to and 90-days after aNSCLC diagnosis. Patients who had no recorded evidence of testing were classified as testing-non-adherent.
Adherence to Biomarker-Driven First-Line Therapy
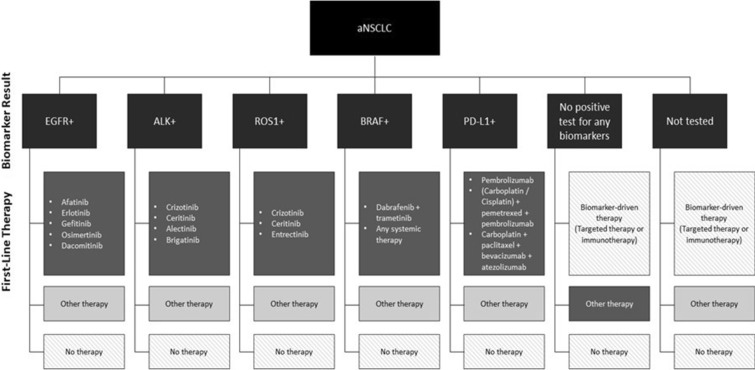
For the analysis on adherence to biomarker-driven first-line therapy selection, patients who received NCCN guideline-recommended first-line therapy consistent with their testing results were classified as the first-line-adherent group (including those with a negative or indeterminate biomarker result and receiving other therapies, e.g., chemotherapy, as their first-line treatment). In instances where a patient tested positive for two or more of the biomarkers, they were considered adherent if their first-line therapy was as per guideline recommendation for any of the positive biomarkers. First-line-non-adherent patients were those with no testing, or those who tested positive but did not receive recommended first-line treatment. Figure 1 shows the a priori adherence grouping assignments across analyses.
Fig. 1.
NCCN guideline-recommended first-line treatments based on biomarker diagnostic tests; aNSCLC advanced non-small-cell lung cancer. Patients were considered adherent to NCCN first-line treatment if they received an appropriate treatment based on one of their biomarker test results (dark gray). Patients were considered non-adherent if they received any therapy in first-line other than the NCCN-recommended therapy (light gray). Patients who received no therapy or who received a biomarker-driven therapy without evidence of a positive biomarker result were excluded from analysis, as adherence could not be adequately assessed
(Source: Non-Small Cell Lung Cancer (version 7.2019). National Comprehensive Cancer Network)
Definitions of Key Outcome Variables
Overall Survival
In the analysis on adherence to biomarker testing, the main outcome of interest was OS, agnostic to subsequent lines of treatment, to assess the relationship between initial biomarker testing and overall survival holistically. OS was defined as the duration between the initial advanced-stage diagnosis date and the end of follow-up, which was either the date of death or date of last observation in the database.
Time to Treatment Discontinuation
In the analysis on adherence to biomarker-driven first-line therapy, the main outcome of interest was TTD of first-line therapy after aNSCLC diagnosis, defined as the duration between the first and the last observed drug episode in the first-line setting. Patients who met any of the following three criteria were considered as having their first-line therapy discontinued: (1) advancement to a new line of therapy, (2) no evidence of advancement to a new line of therapy, and a recorded date of death was present, (3) no evidence of advancement to a new line of therapy, no recorded date of death, but evidence of structured activity more than 120 days after the last drug episode within first line.
Statistical Analysis
Overall Analyses
Descriptive statistics were used to summarize the demographic and clinical characteristics of both cohorts. Continuous variables were summarized using mean and standard deviation (SD), as well as median and interquartile range (IQR). Categorical variables were summarized using frequency and percentage. Chi-squared tests, Student’s t tests, and Mood’s tests were used to compare the characteristics between adherent and non-adherent groups. Cumulative distribution plots to determine the proportion of patients of achieving the outcome variables (e.g., TTD) by adherence groups were also generated.
Univariate analyses were conducted to determine the association of baseline factors with the outcome variable for each analysis to inform the multivariable analyses. Adjusted Cox Proportional Hazards models were performed to determine factors associated with OS or TTD. For both analyses, age, sex, smoking status, and stage at initial diagnosis were included as a priori variables. Additional variables, such as the year of advanced diagnosis, insurance status, and tumor histology, were evaluated as potential confounders; only those which materially changed the effect estimates were included in the final model. The likelihood ratio test was used to assess the proportional hazards (PH) assumption by comparing models with and without an interaction term between exposure and time. Kaplan–Meier (KM) survival curves were drawn for each cohort in both analyses with the number of patients at risk over time. The median OS and TTD were presented with their 95% confidence intervals (CI) by group.
We additionally conducted a sensitivity analysis and ran the Cox model among patients who were initially diagnosed with stage IV NSCLC, adjusting for age, sex, and smoking status.
All analyses were performed using SAS v.9.4 (SAS Institute, Cary, NC, USA) and R v.1.2.5019-7.
Additional Analyses for Adherence to First-Line Therapy
In the first-line therapy analysis, we assessed the association of guideline adherence and risk of treatment discontinuation stratified by the type of first-line treatment, and additionally plotted KM curves by type of first-line treatment in the adherent group. Given the known heterogeneity of treatments, we were concerned that there may be differences in the type of treatment within the two groups. Using the Herfindahl–Hirschman Index (HHI), we assessed the level of variability by the type of treatment within the two groups. While the cutoff value of treatment heterogeneity for aNSCLC is undefined, previous studies have considered HHI values < 0.1000 to be very heterogeneous and values > 0.2000 to be more homogenous [16].
Results
Adherence to NCCN Biomarker-Testing Analysis
Cohort Demographics and Baseline Characteristics
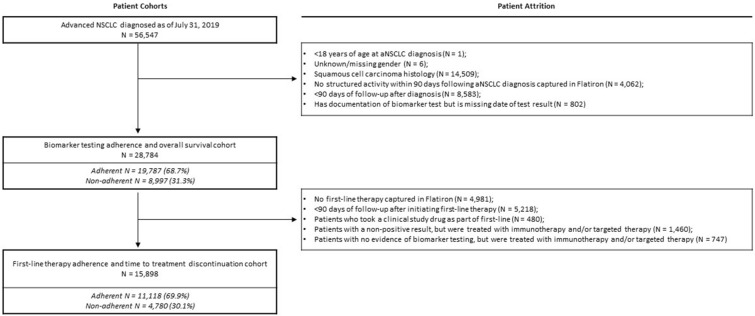
A total of 56,747 patients were identified with a diagnosis of aNSCLC between January 1, 2011 and July 31, 2019. Of these, 28,784 patients met the inclusion criteria for the analysis of NCCN biomarker adherence (for full cohort attrition, see Fig. 2). Approximately two-thirds of these patients (n = 19,787; 68.7%) had evidence of biomarker testing (testing-adherent) (Fig. 2; Supplementary Table 1). The testing-adherent group was younger than the testing-non-adherent group (mean age at diagnosis 67.7 vs. 68.2 years; p < 0.01), and more likely to be recently diagnosed from 2016 to 2019 (50.8% vs. 28.9%; p < 0.01) (Supplementary Table 1). The median time from aNSCLC diagnosis to the first biomarker test result was 19 days [interquartile range (IQR): 11–31].
Fig. 2.
Attrition diagram for NCCN biomarker-testing adherence analysis and biomarker-driven first-line therapy analysis among patients with advanced non-small-cell lung cancer (aNSCLC) in Flatiron Health, 2011–2019
Biomarker-Testing Adherence and Overall Survival
The median follow-up time across all patients assessed for NCCN biomarker-testing adherence was 11.7 months (IQR 6.3–22.8), and the results were similar across both testing-adherent and testing-non-adherent groups.
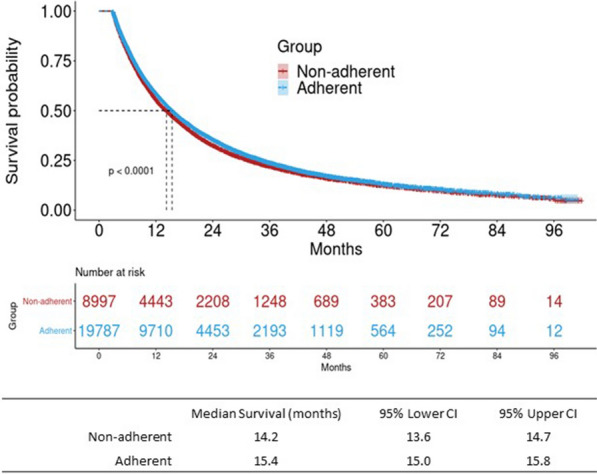
The multivariable model, adjusting for age at aNSCLC diagnosis, sex, smoking status and stage at initial NSCLC diagnosis, showed that testing-adherent patients had a significantly lower risk of mortality (HR = 0.89, 95% CI 0.86, 0.92); this was also seen for the subset of the cohort with stage IV diagnosis only (HR = 0.80, 95% CI 0.77, 0.84; Table 1). However, the proportional hazards assumption was violated likely due to small sample size sometime after 5 or 6 years. A sensitivity sub-analysis was conducted where follow-up was truncated at 1-year post-diagnosis; the results remained significant and the PH assumption was met. The KM survival curve demonstrated improved survival in the testing-adherent group (15.4 vs. 14.2 months, p < 0.01), with median survival reached shortly after 1-year (Fig. 3).
Table 1.
Association between adherence to NCCN-recommended biomarker testing guidelines and overall survival (OS)a among advanced non-small-cell lung cancer (aNSCLC) patients
| Testing-non-adherent | Testing-adherent | |
|---|---|---|
| All aNSCLC patients (n = 28,784) | ||
| Number of events | 6760 | 13,190 |
| Person-months | 165,258 | 337,887 |
| Model | HR (95% CI) | HR (95% CI) |
| Unadjusted | 1.00 (reference) | 0.94 (0.91, 0.96) |
| Adjusted for age at aNSCLC diagnosis, sex, smoking status, and stage at initial NSCLC diagnosis | 1.00 (reference) | 0.89 (0.86, 0.92) |
| aNSCLC patients initially diagnosed at stage IVb (n = 18,761) | ||
| Number of events | 3300 | 10,195 |
| Person-months | 59,958 | 238,480 |
| Model | HR (95% CI) | HR (95% CI) |
| Unadjusted | 1.00 (reference) | 0.76 (0.73, 0.79) |
| Adjusted for age at aNSCLC diagnosis, sex, and smoking status | 1.00 (reference) | 0.80 (0.77, 0.84) |
Flatiron Health, 2011–2019
CI confidence interval, HR hazard ratio
aOverall survival is defined as the time from aNSCLC diagnosis to end of follow-up
bStage IV includes stages IV, IVA, and IVB
Fig. 3.
Kaplan–Meier (KM) results for overall survival (OS)a by adherence to NCCN-recommended biomarker testing guidelines among advanced non-small-cell lung cancer (aNSCLC) patients (n = 28,784). Flatiron Health, 2011–2019; CI confidence interval. aOverall survival is defined as the time from advanced non-small cell lung cancer diagnosis to end of follow-up
Adherence to NCCN First-Line Therapy Analysis
Cohort Demographics and Baseline Characteristics
A subset of 15,898 patients from the biomarker-testing analysis met the inclusion criteria for the NCCN first-line therapy adherence analysis (for full attrition, see Fig. 2). Over two-thirds of these patients (n = 11,118; 69.9%) had evidence of appropriate first-line therapy (first-line-adherent) (Fig. 2; Supplementary Table 1).
In these patients, the median time from first positive-biomarker test result to first-line therapy initiation was 18 days (IQR 9–29). The median time from aNSCLC diagnosis to first-line therapy initiation was 31 days (IQR 20–45). Median follow-up time post first-line therapy initiation was 3.2 months (IQR 1.9–5.0).
First-Line Therapy Adherence and Time to Treatment Discontinuation
The type of first-line therapy differed significantly across the two groups (Supplementary Table 1). More patients in the first-line of therapy non-adherent cohort initiated chemotherapy monotherapy (72.4%) compared to the adherent group (45.0%). A considerably higher percentage of first-line therapy adherent patients initiated on targeted monotherapy (20.1%) than non-adherent patients (0.8%). However, combination treatment with chemotherapy and targeted therapy (~ 24%) was similar across both groups.
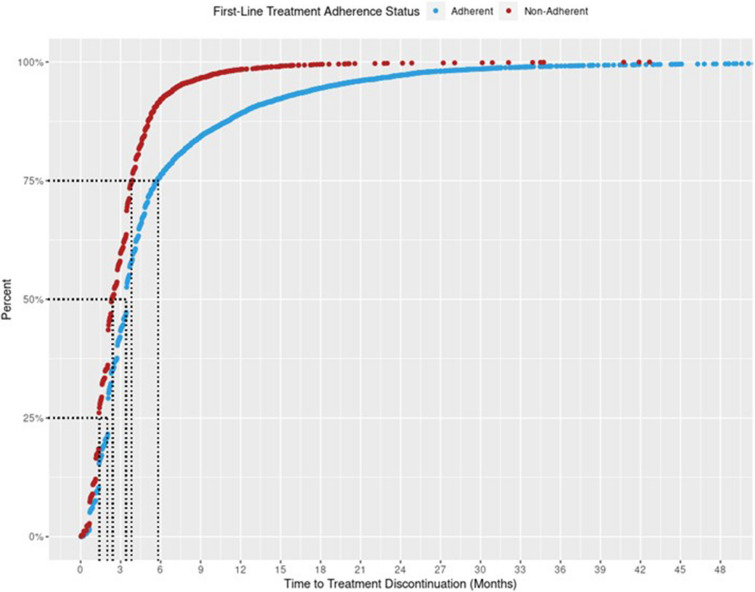
The cumulative frequency distribution of TTD for first-line treatment by the two groups is shown in Fig. 4. Approximately 75% of patients in the first-line therapy adherent group discontinued their treatment at 6 months, compared to just over 3 months in the non-adherent cohort.
Fig. 4.
Cumulative frequency distribution plot for time to treatment discontinuationa by adherence to NCCN-recommended first-line treatment among advanced non-small-cell lung cancer (aNSCLC) patients (n = 15,898). Flatiron Health, 2011–2019. aPatients who met any of the following are considered as having their first-line treatment discontinued (event): 1. Advanced to a new line of therapy; 2. Not advanced to a new line of therapy, but has a recorded date of death; 3. Not advanced to a new line of therapy and has no recorded date of death, but has evidence of structured activity more than 120 days after the last drug episode within the first line
A multivariable Cox Proportional Hazards model for the overall cohort, adjusting for age, sex, stage at initial diagnosis, and smoking status, found that first-line therapy adherent patients had significantly lower risk of treatment discontinuation versus non-adherent patients (HR = 0.60, 95% CI 0.57, 0.62). Similar results were observed when only including patients initially diagnosed in stage IV (Table 2). When stratified by type of first-line therapy, the reduced risk of discontinuation was more pronounced in patients who received immunotherapy or targeted therapy, rather than chemotherapy alone (Fig. 5). The risk of treatment discontinuation were similar to the overall cohort in patients initiated on immunotherapy and targeted therapy (HR = 0.54, 95% CI 0.44, 0.68 and HR = 0.52, 95% CI 0.49, 0.56, respectively), but was closer to the null for chemotherapy (HR = 0.95, 95% CI 0.91, 1.00).
Table 2.
Association between adherence to NCCN-recommended first-line therapy and risk of treatment discontinuationa among advanced non-small-cell lung cancer (aNSCLC) patients
| First-line-non-adherent | First-line-adherent | |
|---|---|---|
| All aNSCLC patients (n = 15,898) | ||
| Number of events | 4641 | 9889 |
| Person-months | 15,001 | 62,001 |
| Models | HR (95% CI) | HR (95% CI) |
| Unadjusted | 1.00 (reference) | 0.57 (0.55, 0.60) |
| Adjusted for age at first-line therapy start, sex, smoking status, stage at initial NSCLC diagnosis | 1.00 (reference) | 0.60 (0.57, 0.62) |
| aNSCLC patients initially diagnosed at stage IVb (n = 11,121) | ||
| Number of events | 2548 | 7469 |
| Person-months | 8365 | 49,048 |
| Models | HR (95% CI) | HR (95% CI) |
| Unadjusted | 1.00 (reference) | 0.56 (0.53, 0.59) |
| Adjusted for age at first-line therapy start, sex, smoking status | 1.00 (reference) | 0.59 (0.56, 0.62) |
Flatiron Health, 2011–2019
CI confidence interval, HR hazard ratio
aTime from start to discontinuation of first-line treatment; patients who met any of the following are considered as having their first-line treatment discontinued (event): 1. Advanced to a new line of therapy; 2. Not advanced to a new line of therapy, but has a recorded date of death; 3. Not advanced to a new line of therapy and has no recorded date of death, but has evidence of structured activity more than 120 days after the last drug episode within the first line
bStage IV includes stages IV, IVA, and IVB
Fig. 5.
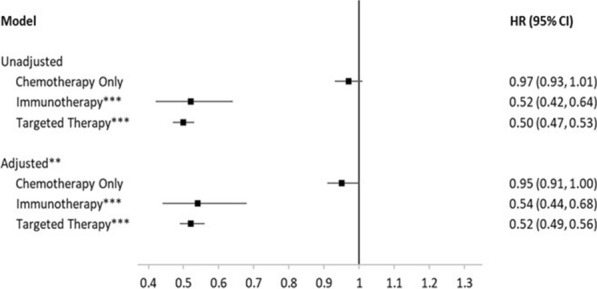
Association between adherence to NCCN-recommended by type of first-line therapy and risk of treatment discontinuationa among advanced non-small-cell lung cancer (aNSCLC) patients (n = 15,898). Flatiron Health, 2011–2019. CI confidence interval, HR hazard ratio. Reference group was non-adherent patients. aTime from start to discontinuation of first-line treatment; patients who met any of the following are considered as having their first-line treatment discontinued (event): 1. Advanced to a new line of therapy; 2. Not advanced to a new line of therapy, but has a recorded date of death; 3. Not advanced to a new line of therapy and has no recorded date of death, but has evidence of structured activity more than 120 days after the last drug episode within the first line. **Adjusted for age at first-line therapy start, sex, stage at initial NSCLC diagnosis, smoking status. ***Immunotherapy and targeted therapy may have been administered alone or in combination with chemotherapy
The KM curve for first-line therapy adherent patients demonstrated a modest, significantly longer median duration of treatment compared to non-adherent patients (3.45 months, 95% CI 3.45, 3.45 vs. 2.40 months, 95% CI 2.30, 2.53; p < 0.01) (Fig. 6). Among the first-line therapy adherent patients, when stratified by type of first-line therapy, both targeted therapy and immunotherapy had more pronounced, significantly longer TTD than chemotherapy alone; the median treatment duration was 9.40 months (95% CI 8.28, 10.58) for patients receiving immunotherapy, 4.57 months (95% CI 4.40, 4.67) for patients receiving targeted therapy, and 2.30 months (95% CI 2.27, 2.30) for patients receiving chemotherapy (Fig. 7).
Fig. 6.
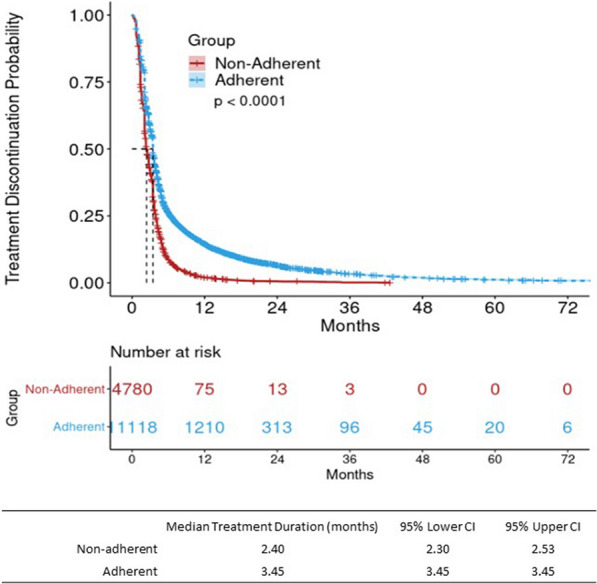
Kaplan–Meier (KM) results for treatment discontinuationa by adherence to NCCN-recommended first-line therapy among advanced non-small-cell lung cancer (aNSCLC) patients (n = 15,898). Flatiron Health, 2011–2019; CI confidence interval. aTime from start to discontinuation of first-line treatment; patients who met any of the following are considered as having their first-line treatment discontinued (event): 1. Advanced to a new line of therapy; 2. Not advanced to a new line of therapy, but has a recorded date of death; 3. Not advanced to a new line of therapy and has no recorded date of death, but has evidence of structured activity more than 120 days after the last drug episode within the first line
Fig. 7.
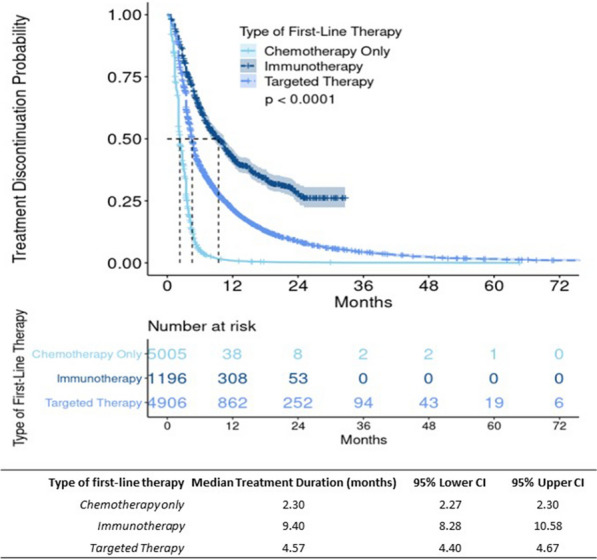
Kaplan–Meier (KM) results by type of first-line therapy and treatment discontinuationa among adherent advanced non-small-cell lung cancer (aNSCLC) patients (n = 11,107). Flatiron Health, 2011–2019; CI confidence interval. aTime from start to discontinuation of first-line treatment; patients who met any of the following are considered as having their first-line treatment discontinued (event): 1. Advanced to a new line of therapy; 2. Not advanced to a new line of therapy, but has a recorded date of death; 3. Not advanced to a new line of therapy and has no recorded date of death, but has evidence of structured activity more than 120 days after the last drug episode within the first line. **Only patients who were adherent to NCCN-recommended first-line therapy were included in this sub-analysis (n = 11,107). Patients concurrently on immunotherapy and targeted therapy were not included (n = 11). Immunotherapy and targeted therapy may have been administered alone or in combination with chemotherapy
The HHI, an index of heterogeneity, revealed that there was significant variability by the type of first-line treatment within each of the two cohorts (HHI = 0.17 for the adherent group vs. 0.22 for the non-adherent group).
Discussion
This study assessed adherence to NCCN-recommended biomarker testing and first-line therapy and its association with clinical outcomes in a national sample of adult patients with aNSCLC over a period of 8 years. To our knowledge, this is the largest study using real-world data to evaluate the impact of adherence to NCCN-recommended testing and treatment on clinical outcomes. Non-homogeneous sites contributed data from across the US, both small and large, and in regions where testing and drug availability may differ. Our study was biomarker- and treatment-agnostic, meaning it did not focus on one specific biomarker and therapy combination, but rather on all the biomarker and therapy combinations available to patients with aNSCLC, and the results demonstrated favorable clinical benefits of adherence to NCCN guideline recommendations from a holistic perspective.
Adherence to Biomarker-Testing Analysis
These analyses found that there was strong evidence supporting adherence to NCCN biomarker testing guidelines: approximately two-thirds (68.7%) of patients received a recommended biomarker test, highlighting the need for improvement in the utilization of biomarker testing as an integral component of routine practice. There may be several reasons for lack of adherence to guideline-recommended biomarker testing, including slow clinician uptake of tests, difficulties assessing samples, and payer coverage [17]. Only recently has the US Centers for Medicare and Medicaid Services approved a national coverage decision for Next-generation Sequencing (NGS) in patients with aNSCLC [18]. Additionally, the US Food and Drug Administration has recently approved several NGS-based platforms for biomarker testing [17]. As such, in the coming years, it is expected that adherence to testing guidelines will be increased. This is already observable in this study, as patients in the biomarker testing-adherent group were more likely to be diagnosed in recent years. Mason et al. published a similar study in 2018 using a smaller patient sample size (n = 379) across seven academic and community centers, and found higher rates of biomarker testing ranging from 85 to 100%, including ALK, EGFR, and ROS1, but lower rates of PD-L1 testing (~ 57%) [19]. This study demonstrates that adherence to biomarker testing may vary drastically based on the type of biomarker. While Mason et al. used data from primarily large, academic and community institutions, our study collected data across ~ 800 national sites, varying in size, affiliations, and regions. Regardless, our study finds relatively high overall adherence to biomarker testing given a non-homogenous sample.
Adherence to NCCN-recommended biomarker testing was associated with 10% reduced risk of mortality for patients with advanced diagnosis in this analysis. Furthermore, in a subset of patients with a more advanced initial diagnosis (initial stage IV diagnosis), where biomarker testing is well-specified in the guidelines, the risk of mortality was reduced by 20% compared to non-adherent patients. These results reaffirmed findings from various studies reporting the benefits of individual biomarkers and associated treatment [9, 12, 13, 20–22].
Adherence to Biomarker-Driven First-Line Therapy
This analysis assessed the association between adherence to NCCN-recommended first-line therapy and TTD in adult patients with aNSCLC. The majority of patients in this analysis were adherent to NCCN-recommended first-line therapy. Of the patients included in this analysis, 69.9% were treated with NCCN-recommended first-line therapy based on their biomarker test results, agnostic of mutation. Mason et al. reported varying adherence to first-line treatment dependent on mutation: 96.8% adherence to recommended first-line treatment among patients with ALK, EGFR, or ROS1 mutations and 9.6% among patients with PD-L1 mutations [19].
There may be several reasons for treatment non-adherence that are not captured in this study, including physician preference, patient safety profile, and therapy availability. We believe that non-adherence may not necessarily be intentional and may be related to many unmeasured factors such as patient’s underlying health, safety reasons, patient and/or physician preference, compliance, access to testing or treatment, reimbursement barriers, and other healthcare system-related factors (clinical pathways), etc. Access to biomarker testing and the appropriate therapy may also serve as a barrier to adherence across non-homogenous US sites included in the database; certain biomarker tests and therapies may be more widely available in some regions or institutions versus others. Because data on the availability of each biomarker test and therapy at the contributing institutions were not captured, the authors cannot conclude that all patients in the present study had equal access to NCCN-recommended biomarker tests and therapies. These may be a few of the reasons for a lower observed adherence in this study, given the general understanding of guideline importance. Further studies are needed to better understand why patients may not receive NCCN-recommended biomarker tests or first-line therapy.
Overall, our study found that patients who were adherent to first-line recommendations had a 40% lower probability of discontinuing treatment, and were more likely to remain on their treatment for about a month longer than non-adherent patients. Wang et al. [9] similarly found that patients who received guideline-recommended first-line therapy had significantly longer follow-up periods on first-line therapy than patients who were not administered guideline-recommended therapy [9]. In a study conducted in Spain, patients with guideline-recommended diagnosis and treatment had improved survival, with 1- and 2-year relative survival of 51% and 28.2% versus 34.1% and 17.5%, respectively, in patients who did not receive guideline-recommended diagnosis and treatment, and non-adherence may have been due to bad performance status, advanced age, exitus, and patient preference [12]. These may also be contributing factors in our study. In addition to OS, adherence to guideline-recommended precision medicine has been shown to improve progression-free survival in patients with advanced-stage cancer [20].
In this study, there was significant heterogeneity by the types of treatment for both groups, and this effect was seen for risk of and time to discontinuation. More than half of the patients received chemotherapy alone as their first-line treatment; however, the difference in risk for patients receiving chemotherapy alone was null. Among patients who received immunotherapy or targeted therapy (as monotherapy or in combination with chemotherapy) as first-line treatment, adherence was associated with more than 50% reduced risk of discontinuation. Chemotherapy had the shortest treatment duration of just over 2 months, followed by targeted therapy with 4 months and immunotherapy with about 9 months.
Despite the heterogeneity of treatment, these findings suggest that patients who are adherent to guidelines had more favorable overall outcomes, especially if initiating treatment on immunotherapy or targeted therapy, adding to the growing body of literature outlining improved outcomes based on individual biomarker-driven treatment [9, 12, 13, 20–22].
Strengths and Limitations
This real-world study assessed adherence to guidelines using a holistic approach, agnostic of biomarkers and treatments. This study comprised a large cohort of patients with aNSCLC who were treated primarily in a community-based setting, reflecting clinical practice in the real world. Moreover, the use of EHR data captured as part of routine care enabled extended follow-up periods and the ability to include additional variables as potential confounders.
Despite the large sample size and availability of longitudinal data, there are several limitations associated with the use of real-world data; therefore, our study findings should be interpreted within the context of these noted limitations (e.g., missingness, unmeasured confounders, misclassification). For example, patients may have undergone biomarker testing or received guideline-recommended treatment outside of the Flatiron Health network; however, as this study was conducted only with data from this EHR system, these patients may have been inaccurately placed in the non-adherent cohorts and hence may potentially bias the estimation. One limitation of selecting OS as the outcome of interest for this analysis is the number of potential factors intervening between the exposure of interest (in this case, biomarker testing) and the outcome (OS) over time. Several clinical factors, including choice of first-line therapy, tolerance to therapy, switching, discontinuation, etc. that occur after initial biomarker testing, may have a significant impact on OS; however, it is likely that both groups would be subject to this limitation. Another limitation of this study was our inability to account for the changes to NCCN guidelines that likely occurred over the 8.5-year timeframe. For instance, many more new biomarkers emerged over time, or immunotherapies as a class were not introduced to the market until 2015. Therefore, adherence in patients recruited from earlier in the study time frame (e.g., 2011–2015) may be underestimated as the options within the guidelines were not as fully developed as they are today. This limitation may explain why patients diagnosed in earlier years were more likely to be classified as non-adherent. Finally, rationale for non-adherence was not available; these may be related to underlying patient health, safety, or cancer progression characteristics, patient and physician preference, compliance, physician, reimbursement, and other healthcare system-related factors, etc.
Conclusions
Guidelines provide clinicians a practical tool to individualize treatments and enable them to manage complex treatment pathways for oncology patients. Keeping abreast of guideline updates for emerging biomarkers and treatments in precision oncology care and management can be challenging. The findings of this study demonstrate that, in a large and hetrogenous population, biomarker testing drives treatment decisions and is associated with a modest but significant OS and TTD gain, even when considering the number of relevant local and regional differences in testing and drug availability; all collectively supporting the practice of precision medicine. These findings further support the importance of initiation of care through diagnostic testing and biomarker-driven treatment strategies by prioritizing timely access to biomarker testing and individualized treatment for patients. Therefore, having access to up-to-date and easy-to-digest guideline information incorporated within the workflow is much needed. This raises the potential need for tools integrated into practice to enable use of guidelines seamlessly into routine care, as such efforts can also help build a body of evidence to further inform evidence-based practice and improve quality of care for patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study was sponsored/funded by Roche Diagnostics, Santa Clara, CA, USA. Roche Diagnostics is also funding the publishing of this manuscript, including the Rapid Service and Open Access fees for Advances in Therapy.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical Writing and Editorial Assistance
Writing and editorial assistance was provided by Darrin Benjumea and Patrick Callahan, of Genesis Research, LLC (Hoboken, NJ, USA) and paid for by Roche Diagnostics.
Disclosures
Ani John, Baiyu Yang and Roma Shah are employees of Roche Diagnostics.
Compliance with Ethics Guidelines
Data used in this study is from Flatiron Health, a large, national, de-identified database. Roche Diagnostics purchased access to Flatiron Health data. No ethics committee or IRB approval is required for this study using de-identified retrospective data.
Data Availability
The datasets generated and/or analyzed during the current study are not publicly available. They are the property of Flatiron Health and have been licensed by Roche Diagnostics for this study.
References
- 1.Millner LM, Strotman LN. The future of precision medicine in oncology. Clin Lab Med. 2016;36(3):557–573. doi: 10.1016/j.cll.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Bailey AM, Mao Y, Zeng J, et al. Implementation of biomarker-driven cancer therapy: existing tools and remaining gaps. Discov Med. 2014;17(92):101–114. https://pubmed.ncbi.nlm.nih.gov/24534473. [PMC free article] [PubMed]
- 3.Noone A, Howlader N, Krapcho M. SEER cancer statistics review, 1975–2015: lung and bronchus cancer. https://seer.cancer.gov/archive/csr/1975_2015/results_merged/sect_15_lung_bronchus.pdf. Published 2017. Accessed 29 June 2020.
- 4.Non-small cell lung cancer (version 7.2019). National Comprehensive Cancer Network. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed 1 Nov 2019.
- 5.Bironzo P, Di Maio M. A review of guidelines for lung cancer. J Thorac Dis. 2018;10(Suppl 13):S1556–S1563. doi: 10.21037/jtd.2018.03.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanna NH, Schneider BJ, Temin S, et al. Therapy for stage IV non–small-cell lung cancer without driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol. 2020;38(14):1608–1632. doi: 10.1200/JCO.19.03022. [DOI] [PubMed] [Google Scholar]
- 7.Narod A. Guideline on stage IV non-small-cell lung cancer therapy updated. 2017. https://www.asco.org/about-asco/press-center/news-releases/guideline-stage-iv-non-small-cell-lung-cancer-therapy-updated. Accessed 1 June 2020.
- 8.Planchard D, Popat S, Kerr K, et al. Metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. European Society for Medical Oncology. https://www.esmo.org/guidelines/lung-and-chest-tumours/metastatic-non-small-cell-lung-cancer. Published 2019. Accessed 26 June 2020. [DOI] [PubMed]
- 9.Wang Z, Askamit I, Tuscher L, Bergstrom K. Rates of guideline adherence among US community oncologists treating NSCLC. Am J Manag Care. 2013;19(3):185–192. [PubMed] [Google Scholar]
- 10.Malin JL, Schneider EC, Epstein AM, Adams J, Emanuel EJ, Kahn KL. Results of the National Initiative for Cancer Care Quality: how can we improve the quality of cancer care in the United States? J Clin Oncol. 2006;24(4):626–634. doi: 10.1200/JCO.2005.03.3365. [DOI] [PubMed] [Google Scholar]
- 11.Salloum RG, Smith TJ, Jensen GA, Lafata JE. Factors associated with adherence to chemotherapy guidelines in patients with non-small cell lung cancer. Lung Cancer. 2012;75(2):255–260. doi: 10.1016/j.lungcan.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linares I, Sanchez M-J, Pérez-Alija J, et al. EP-1221: adherence to lung cancer guidelines and its impact on survival. Radiother Oncol. 2017;123:S659. doi: 10.1016/S0167-8140(17)31656-0. [DOI] [Google Scholar]
- 13.Elegbede AA, Gibson AJ, Fu H, et al. Real-world adherence to guideline-recommended treatment for small cell lung cancer. Am J Clin Oncol. 2020;43(4):236–242. doi: 10.1097/COC.0000000000000657. [DOI] [PubMed] [Google Scholar]
- 14.Birnbaum B, Nussbaum N, Seidl-Rathkopf K, Agrawal M, Estevez M, et al. Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research. arXiv.org. Submitted 13 Jan 2020. https://arxiv.org/abs/2001.09765. Accessed 28 Oct 2020.
- 15.Ma X, Long L, Moon S, Adamson BJS, Baxi SS. Comparison of population characteristics in real-world clinical oncology databases in the US: Flatiron Health, SEER, and NPCR. medRxiv. 2020 doi: 10.1101/2020.03.16.20037143. [DOI] [Google Scholar]
- 16.Paulson S, Waycaster C, Baidoo B, Chatterjee A, Liepa A, Hess A. [Poster] The impact of gastric cancer clinical pathways based on national comprehensive cancer network guidelines on treatment heterogeneity and clinical outcomes in the US Oncology Network. In: NCCN 2020: 25th annual conference, Orlando, FL; 2020. https://www.nccn.org/professionals/meetings/2020/pdf/014_GPS_NCCNac20.pdf.
- 17.Pennell NA, Arcila ME, Gandara DR, West H. Biomarker testing for patients with advanced non-small cell lung cancer: real-world issues and tough choices. Am Soc Clin Oncol Educ Book. 2019;39:531–542. doi: 10.1200/EDBK_237863. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Medicare & Medicare Services. Decision memo for next generation sequencing (NGS) for medicare beneficiaries with advanced cancer (CAG-00450N). https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=290. Accessed 20 Feb 2019.
- 19.Mason C, Ellis PG, Lokay K, et al. Patterns of biomarker testing rates and appropriate use of targeted therapy in the first-line, metastatic non-small cell lung cancer treatment setting. J Clin Pathw. 2018;4(1):49–54. doi: 10.25270/jcp.2018.02.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haslem DS, Van Norman SB, Fulde G, et al. A retrospective analysis of precision medicine outcomes in patients with advanced cancer reveals improved progression-free survival without increased health care costs. J Oncol Pract. 2017;13(2):e108–e119. doi: 10.1200/JOP.2016.011486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsimberidou A-M, Iskander NG, Hong DS, et al. Personalized medicine in a phase I clinical trials program: the MD Anderson Cancer Center initiative. Clin Cancer Res. 2012;18(22):6373–6383. doi: 10.1158/1078-0432.CCR-12-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311(19):1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available. They are the property of Flatiron Health and have been licensed by Roche Diagnostics for this study.