Abstract
Background:
Somatosensory deficits are prevalent after stroke, but effective interventions are limited. Brain stimulation of the contralesional primary somatosensory cortex (S1) is a promising adjunct to peripherally administered rehabilitation therapies.
Objective:
To assess short term effects of repetitive Transcranial Magnetic Stimulation (rTMS) targeting contralesional (S1) of the upper extremity.
Methods:
Using a single session randomized cross-over design, stroke survivors with upper extremity somatosensory loss participated in 3 rTMS treatments targeting contralesional S1: Sham, 5 Hz and 1 Hz. rTMS was delivered concurrently with peripheral of sensory electrical stimulation and vibration of the affected hand. Outcomes included 2-point discrimination (2PD), proprioception, vibration perception threshold, monofilament threshold(size) and Somatosensory Evoked Potential (SEP). Measures were collected before, immediately after- and 1 hour after-treatment. Mixed models were fit to analyze the effects of the three interventions.
Results:
Subjects were 59.8±8.1 years old and 45±39 months post-stroke. There was improvement in 2PD after 5Hz rTMS for the stroke-affected (F(2, 76.163)=3.5, p = .035) and unaffected arm (F(2, 192.786)=10.6, p<0.0001). Peak-to-peak SEP amplitudes were greater after 5Hz rTMS for N33-P45 (F(2, 133.027)=3.518, p=0.032) and N45-P60 (F(2, 67.353)=3.212, p=0.047). Latencies shortened after 5Hz rTMS for N20 (F(2, 69.64)=3.37, p=0.04), N60 (F(2, 47.343)=4.375, p=0.018) and P100(F(2, 37.608)=3.537, p=0.039) peaks. There were no differences between changes immediately after the intervention and an hour later.
Conclusions:
Short-term application of facilitatory high frequency rTMS (5Hz) to contralesional S1 combined with peripheral somatosensory stimulation may promote somatosensory function. This intervention may serve as a useful adjunct in somatosensory rehabilitation after stroke.
Keywords: Stroke, Sensory deficits, Somatosensory deficits, repetitive Transcranial Magnetic Stimulation (rTMS), two-point discrimination, Sensory Evoked Potentials
INTRODUCTION
Loss of somatosensation is present in upwards of 90% of stroke survivors1–3. Impairment of proprioception, perception of touch, or pain limits an individual’s ability to avoid injury and impacts how they experience the world around them. Furthermore, somatosensory deficits significantly impair motor control, impede functional performance2,4–7 and negatively impact quality of life8. Though somatosensation has an important role in the functioning of the upper limb7 and is routinely assessed clinically7, interventions that address somatosensory deficits are lacking.
Peripherally directed therapies9,10 (such as electrical stimulation, vibration and others) can produce some improvement in upper limb somatosensory function, but restoration is typically not realized. One limitation of these methods is that they target the periphery (e.g. upper limbs)11,12 rather than the brain which is the source of the deficit and the key area for targeting therapies to promote upper limb functional recovery and re-organization13.
Recovery after stroke is driven by functional and structural brain re-organization14–16. Several possible neuroplastic patterns have been observed during rehabilitation, e.g. functional re-mapping of surviving pathways or shift of function to homologous and functionally related regions13,17. It may be possible to promote changes in motor performance by stimulating the brain with electrical currents18,19. Non-invasive methods using repetitive TMS (rTMS) have been shown to induce brain changes and enhance functional recovery by directly modulating brain activity20. Most non-invasive brain stimulation studies have focused on movement-related rehabilitation interventions targeting the primary motor area (M1). After stroke, there is functional interhemispheric imbalance between the right and left motor regions. A diminished activity of the ipsilesional M1 by stroke is further suppressed by overactivity of the contralesional M121. rTMS has been used to correct this imbalance. A common approach involves facilitating activity of ipsilesional M1 and/or inhibiting activity of contralesional M1 (believed to have strong inter-hemispheric inhibitory effects)20. Although facilitatory (5Hz) rTMS of the ipsilesional S1 paired with motor therapy showed improvement of both motor abilities and somatosensory discrimination abilities22 , the role of rTMS in post-stroke somatosensory recovery has not been fully tested. Promisingly, it has been shown that rTMS can affect sensory perception in healthy subjects23.
Targeting primary somatosensory cortex (S1) to restore somatosensory function may be a logical corollary of the approaches adopted in the area of motor recovery. Contralesional S1 may be a more reasonable target than its ipsilesional counterpart for many reasons. First, modulating structurally intact brain may produce a more reliable and consistent effect across patients given the lack of confounding influence of differing lesion sizes, locations and geometry24. Second, although the majority of sensory processing is in the contralateral hemisphere, it is believed that there is a bihemispheric involvement in sensory processing as demonstrated by functional Magnetic Resonance imaging (fMRI)25,26 and electroencephalography (EEG) studies27 and because unilateral infarct may result in bilateral somatosensory deficits1,28.
The objective of this proof-of-principle study was to explore the effect of rTMS targeting contralesional S1. Using a single-session randomized crossover design, participants received high frequency rTMS (5-Hz, H-rTMS), low frequency rTMS (1Hz, L-rTMS) and sham rTMS (S-rTMS) targeting contralesional S1. High frequency rTMS increases cortical excitability and thus is facilitatory while low frequency rTMS decreases cortical excitability and is inhibitory29. Both high and low rTMS frequencies were tested because it is unclear whether facilitating or inhibiting contralesional S1 is beneficial for somatosensory function30, and because effects of inhibiting contralesional M1 have been equivocal in studies of post-stroke motor recovery20,31. Somatosensory processing of the various somatosensory modalities take different paths in the brain32 and the response of different somatosensory modalities to rehabilitation intervention can vary2,33. Therefore, we included a range of somatosensory outcomes probing both primary somatosensation and discriminatory abilities as well as testing both posterior column and the spinothalamic pathways. Immediate and delayed effects of rTMS on clinical somatosensory measures and neurophysiologic indices characterizing somatosensory cortical processing, i.e. Somatosensory Evoked Potentials (SEPs) were studied.
METHODS
Participants.
Subjects were recruited by word of mouth and through use of flyers from the local population. Sixteen patients with upper extremity somatosensory deficits following first-ever stroke (>6 months post) were enrolled. Subjects were screened for study entry and deemed eligible to enroll if they had a detectable difference between the affected and unaffected upper limb in ≥1 study measure of somatosensation. The inclusion criteria were intentionally broad because of the nature of the study and therefore some individuals with the most severe deficits did not perceive all the sensory modalities. Exclusion criteria were contraindications for TMS such as metal implants, history of seizures, or use of substances that lower seizure threshold34. A description of limbs in the text is as follows: affected side – contralateral to the stroke lesions and unaffected side – ipsilateral to the stroke lesions.
Overview of Study Design.
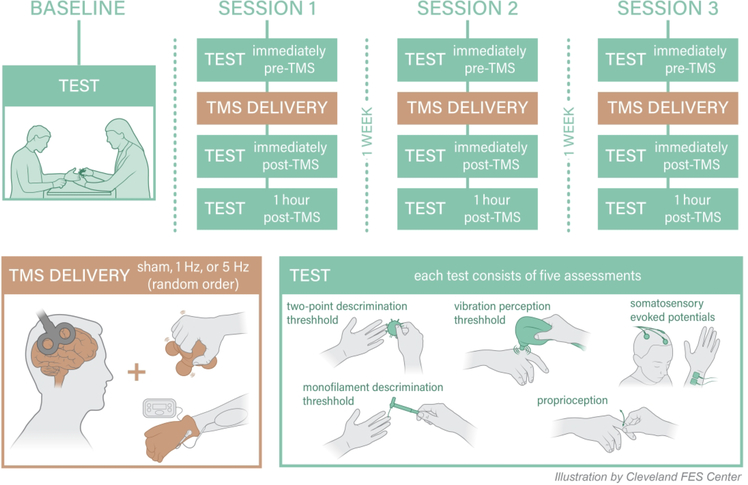
In a randomized, crossover experiment, participants underwent single-sessions of three different types of rTMS at intervals ≥1wk: (1) facilitatory H-rTMS, (2) inhibitory L-rTMS and (3) sham. H-rTMS involved application of 5Hz rTMS while L-rTMS involved 1Hz rTMS to contralesional S1. Sham rTMS was delivered using a placebo coil. In every session, rTMS was delivered concurrently while participants received peripheral sensory stimulation of the affected hand. Measurements were performed before (Pre), immediately after- (Post 1) and one hour after- (Post 2) each session. Figure 1 depicts an overview of the study design. The rationale for the timing of data collection was based on prior reports of rTMS effects lasting up to one hour35. Participants underwent a baseline session to familiarize them with the testing protocol. A randomized-block design using the 6 order permutations within the block was used to randomize the order of the different rTMS sessions. The rTMS protocols were delivered ≥1 week apart to allow for a washout period36. The study enrolled patients from two hospitals in Cleveland, Ohio: the Louis Stokes Cleveland Veterans Affairs Medical Center (LSCVAMC) and the Cleveland Clinic (CC). The protocol at both sites was identical unless otherwise noted. Local IRBs of each hospital approved the study. Participants provided written informed consent before participating.
Figure 1.
Overview of the study design.
Intervention
rTMS.
Patients were seated in a chair with a headrest and arms resting on a table. rTMS was applied using a biphasic stimulator [Magstim Super Rapid 2002 magnetic stimulator (Magstim Company Ltd., Wales, UK) at LSCDVAMC site and MagPro R30 (Dantec, Denmark) at CC site]. Coil targeting was guided by frameless stereotactic navigation (Brainsight2, Rogue Research, Inc., Montreal, QC). At the beginning of each session, evoked response to single pulses of TMS, called motor evoked potentials (MEPs), were collected from contralateral first dorsal interosseus muscle (FDI). Surface electromyography was acquired via silver-silver chloride 8 mm bipolar electrodes [Powerlab 4/25T (AD Instruments Inc. Colorado Springs, CO)] with a reference electrode over the lateral epicondyle (CC) or with Brainsight2 EMG pod via 10mm surface gel adhesive electrodes with reference electrode over the lateral forearm (LSCDVAMC). Hotspot for FDI was identified as the site that evoked ≥50μV MEPs at the lowest TMS intensity (motor threshold, MT) reliably in 6 out of 10 trials37. Motor hotspot for FDI served as a guide to identify the anatomic location of S1 and MT intensity served as a means to establish rTMS intensities.
The site for S1 stimulation was identified 2 cm posterior to the FDI hotspot in M123,30. The intensity of rTMS application was subthreshold, applied at 90% of the resting MT required to elicit FDI responses in accordance with safety guidelines34. The H-rTMS protocol consisted of 5Hz rTMS at 90% resting MT for a total of 1250 pulses as follows: 5 blocks of 250 pulses with an inter-block interval of 60 s; each block consisted of five 50-pulse trains with an inter-train interval of 2 s.23,38 This protocol showed effect on sensory function in healthy controls.23,38 The L-rTMS protocol involved continuous 1Hz rTMS of 1200 pulses which is the most commonly used inhibitory rTMS paradigm. S-rTMS was provided using a sham coil and 5Hz protocol. At LSCDVAMC, the active coil was a flat 70 mm figure-of-eight magnetic air-cooled coil with a maximum magnetic field strength of 1.5 T (Tesla) and an average inductance of 15.5 μH; the sham coil was identical in appearance to the active coil but had a maximum magnetic field of 0.2T and an average inductance of 2.8 μH. At CC, both sham and active rTMS were provided with the dual-sided active/sham 70mm figure-eight MagVenture A/P coil that has an inductance of 11.3–12.0 mH (for both active and sham) and a maximum magnetic field of 1.4 T on the active side and 0.07 T on the sham side. The outputs of both sham coils are well below stimulus threshold while producing similar tactile and auditory effects.
Peripheral sensory stimulation.
During rTMS, peripheral sensory stimulation consisting of 5 minutes of sensory level electrical stimulation and 5 minutes of vibratory stimulation to the affected hand was administered. Sensory-level electrical stimulation was delivered by an EMPI 300 PV Neuromuscular Stimulator at a frequency of 50 Hz at sub-motor intensity with an Electro-Mesh glove (Prizm Medical, Modesto, CA); 1 sec ramp on/off; 20secs on and 5 seconds off. For vibratory stimulation, the subject’s hand was positioned with the palmar surface in contact with the handle of a mini massager (Homedics, Commerce Township, MI) and fingers gently curled over the handle (Figure 1). Subjects were asked to not actively grasp the massager, rather to rest their hand in this position.
Outcome measures
Clinical measures
Somatosensory modalities included two-point, proprioception, monofilament size and vibratory discrimination (Figure 1). Subjects were blindfolded during testing and skin temperature was maintained at a constant level. Both the clinical assessor and the study participant were blinded to the order of rTMS protocol delivery and the same assessor tested a given subject for each of the three rTMS sessions.
Two-point discrimination was measured with Disk-Criminator disks (Baltimore, MD) by determining the subjects’ ability to perceive two points on the disk as two separate points rather than as a single point39. The distances between the two points ranged between 2 and 15 mm. One and two sensory points were presented in a pseudo-random order to subjects’ 4th digit volar fingertip surface. A threshold is determined when seventy percent accuracy is exhibited for identifying the difference between single versus double point stimulation39. Accuracy of threshold was confirmed by retesting the level above and below the determined threshold level. A score of 16mm was assigned when the subject could not accurately differentiate one versus two points at the maximum distance (15mm). Mean average for healthy controls has been reported to be 3.2 (±.9) for the 4th digit of the dominant hand40. The volar surface of the 4th digit was assessed due to the high incidence of median nerve entrapment common in this population which could confound the findings41.
For proprioception, an examiner held the subjects’ 2nd digit at the medial and lateral surfaces of the proximal interphalangeal joint (forearm pronated) and flexed or extended the subject’s 2nd metacarpophalangeal joint in a random sequence of approximately 20° flexion and extension. Subjects were asked whether the joint was moved upwards (extension) or downwards (flexion). Proprioception accuracy was expressed as a percent of correct responses42 and a chance score for the testing as was conducted was 50%.
The monofilament test assessed the threshold for perceiving tactile stimulation at the 4th digit volar fingertip with Tactile Semmes-Weinstein Monofilaments43. Monofilaments of varying sizes (2.83, 3.61, 4.31, 4.56, 6.65) were presented in random time intervals achieving filament bent into “C” or blanched skin underneath for 6.65. We report the smallest filament reliably detected using standard clinical testing procedures. For individuals who could not sense the thickest filament, a score of 7.6 was recorded. Filaments were presented in descending order thickest to finest. Normal monofilament score is ≤2.8343.
Vibration perception threshold was determined using the Biothesiometer (Bio-Medical Instrument Co, Newbury, OH). The Biothesiometer pestle, which vibrates at a frequency of 120 Hz, was placed at the second metacarpophalangeal joint44. Vibration amplitude was gradually increased. Test score corresponded to device units (vibration amplitude) at which subjects detected vibration averaged across three trials44. A normal score is <7V45.
Somatosensory Evoked Potentials (SEP).
SEPs were recorded with a Cadwell Sierra Wave (Cadwell, Kennewick, WA) (LSCDVAMC) or with Powerlab 4/25T (AD Instruments Inc. Colorado Springs, CO) and a Grass Stimulator (Natus Neurology, Middleton, WI) (CC)46. The recording electrodes (1 cm diameter, gold cup electrodes filled with conductive paste) were placed 2 cm posterior to C3 & C4 (10–20 international system of EEG electrode placement) and the reference electrode at Fz (Figure 1). Electrode impedance was kept below 5 kΩ. Stimulus was applied to the median nerve at the wrist (anode at the wrist crease and cathode 2cm proximal). Ground electrodes were placed at the lateral epicondyle of the stimulated arm. Square wave stimulus of 200 μsec pulse width and frequency of 5.1 Hz was applied with sufficient amplitude to cause 1–2 cm of thumb movement. The evoked response from 500 stimuli were recorded and averaged for a single trial. Three SEP trials were recorded then analyzed. Latencies (in milliseconds) were determined for N20, P25, N33, P45, N60, P100, and N120. Peak-to-peak amplitudes (in μV) were extracted for N20-P25, P25-N33, N33-P45, P45-N60, N60-P100, P100-N12046.
Statistical analysis.
Descriptive statistics were applied to determine the shape of data distributions. Baseline scores (Pre) were compared using non-parametric Friedman test for repeated measurements and Wilcoxon signed rank test for paired comparisons. Linear mixed models were fit to analyze the response of both clinical and SEP outcome measures to the three intervention protocols. We included subject-level random intercept, as well as compound symmetry repeated covariance type structure, to reflect within-subject and serial correlation. The outcome (dependent) variables in the mixed models were the difference in respective outcome measure score from Pre to Post 1 and Pre to Post 2 (for example, change in 2-point discrimination at Post1 and at Post2). The two explanatory variables were: 1) a categorical variable denoted as rTMS session type: either S-rTMS, L-rTMS or H-rTMS, and 2) a categorical time variable - indicating whether the outcome value is a difference from Pre to Post 1 or Pre to Post 2. Note that statistically significant treatment variable association indicates that the change over time differs by rTMS type. We also conducted pairwise analysis of the treatment effects, to ascertain directionality. Pairwise comparison based on estimated marginal means included Sidak’s method for correction for multiple comparisons. The reported p-values were adjusted for multiple comparisons.
RESULTS
Subject characteristics are in Table 1. Mean differences between the stroke-affected and unaffected hand were statistically significant for 2-point discrimination, monofilament threshold, proprioception and vibration detection (p<0.05) (Table 2). The un-affected hand had diminished somatosensation with frequency of 94% compared with the normative values (Table 2).
Table 1.
Subjects’ characteristics
| Subject | Affected arm | Age | Gender | Months Post Stroke | Stroke Type | Lesion location |
|---|---|---|---|---|---|---|
| 1 | L | 67 | M | 25 | I | C |
| 2 | R | 55 | M | 33 | I | S |
| 3 | L | 65 | M | 19 | H | C |
| 4 | R | 64 | F | 16 | I | S |
| 5 | L | 57 | M | 92 | I | B |
| 6 | R | 57 | M | 79 | H | S |
| 7 | R | 58 | M | 95 | I | S |
| 8 | R | 55 | M | 47 | I | S |
| 9 | R | 51 | M | 75 | I | B |
| 10 | R | 55 | M | 35 | H | S |
| 11 | R | 48 | M | 42 | H | S |
| 12 | R | 54 | M | 15 | H | S |
| 13 | R | 59 | F | 9 | I | B |
| 14 | R | 83 | M | 8 | H | S |
| 15 | R | 55 | M | 141 | I | B |
| 16 | L | 61 | M | 7 | H | S |
| Summary | R = 68.7% | 59 (8.1) | M = 87.5% | 45 (39) | I = 56% | |
Key: L = left arm affect, R = right arm affected, M = male, F = female, I = ischemic, H = hemorrhagic, C = cortical, S = subcortical, B = both cortical and subcortical
Table 2.
Baseline testing values averaged across the three rTMS sessions for both stroke-affected and unaffected arms (mean (SD))
| 2-point discrimination threshold (mm) | Monofilament threshold (size) | Proprioception (% correct) | Vibration perception threshold(V) | |||||
|---|---|---|---|---|---|---|---|---|
| Subject | Affected | Unaffected | Affected | Unaffected | Affected | Unaffected | Affected | Unaffected |
| 1 | 16.0 (0.0) | 4.7 (0.6) | 3.6 (0.7) | 3.1 (0.5) | 46.7 (5.8) | 96.7 (5.8) | 6.3 (1.1) | 8.5 (1.5) |
| 2 | 4.7 (0.6) | 5.0 (1.0) | 3.4 (0.5) | 3.1 (0.5) | 96.7 (5.8) | 93.3 (5.8) | 3.6 (0.2) | 3.8 (0.5) |
| 3 | 10.7 (2.3) | 4.3 (2.1) | 3.3 (0.9) | 3.4 (0.5) | 96.7 (5.8) | 100.0 (0.0) | 4.1 (0.9) | 4.6 (1.0) |
| 4 | 2.3 (0.6) | 2.0 (0.0) | 4.1 (0.4) | 3.6 (0.0) | 100.0 (0.0) | 100.0 (0.0) | 6.8 (1.1) | 8.7 (1.0) |
| 5 | 16.0 (0.0) | 3.7 (0.6) | 7.3 (0.5) | 3.6 (0.0) | 20.0 (20.0) | 100.0 (0.0) | 34.0 (15.4) | 2.9 (0.9) |
| 6 | 15.7 (0.6) | 3.0 (1.0) | 6.0 (2.1) | 5.4 (1.9) | 46.7 (15.3) | 100.0 (0.0) | 9.1 (0.9) | 3.3 (0.9) |
| 7 | 14.0 (2.0) | 2.0 (0.0) | 3.6 (0.0) | 3.6 (0.0) | 100.0 (0.0) | 100.0 (0.0) | 4.7 (0.2) | 3.8 (0.5) |
| 8 | 16.0 (0.0) | 2.3 (0.6) | 6.5 (1.9) | 3.8 (0.4) | 53.3 (15.3) | 100.0 (0.0) | 6.5 (4.1) | 4.1 (0.4) |
| 9 | 15.7 (0.6) | 2.0 (0.0) | 6.6 (1.8) | 3.6 (0.0) | 63.3 (23.1) | 100.0 (0.0) | 3.0 (0.3) | 2.7 (0.6) |
| 10 | 7.0 (0.0) | 2.0 (0.0) | 3.4 (0.5) | 3.6 (0.0) | 100.0 (0.0) | 100.0 (0.0) | 4.1 (0.5) | 4.5 (1.7) |
| 11 | 6.7 (8.1) | 2.3 (0.6) | 2.8 (0.0) | 3.4 (0.5) | 76.7 (25.2) | 100.0 (0.0) | 7.9 (0.9) | 4.2 (0.3) |
| 12 | 15.7 (0.6) | 2.0 (0.0) | 4.3 (0.0) | 3.4 (0.5) | 55.0 (17.3) | 100.0 (0.0) | 23.7 (9.8) | 5.8 (0.9) |
| 13 | 11.7 (1.2) | 2.7 (0.6) | 4.4 (0.1) | 3.4 (0.5) | 66.7 (11.5) | 100.0 (0.0) | 3.8 (0.3) | 3.1 (0.7) |
| 14 | 16.0 (0.0) | 3.0 (1.0) | 5.3 (1.2) | 3.6 (0.0) | 95.0 (5.0) | 100.0 (0.0) | 15.8 (2.1) | 6.3 (2.0) |
| 15 | 15.7 (0.6) | 2.3 (0.6) | 7.6 (0.0) | 3.6 (0.0) | 73.3 (10.4) | 100.0 (0.0) | 6.0 (1.4) | 4.7 (0.5) |
| 16 | 16.0 (0.0) | 2.3 (0.6) | 7.6 (0.0) | 2.8 (0.0) | 56.7 (10.4) | 100.0 (0.0) | 15.9 (2.7) | 4.9 (0.3) |
| Group Mean (SD) | 12.5 (5.0) | 2.9 (1.2)* | 5.0 (1.8) | 3.6 (0.7)* | 71.7 (26.4) | 99.4 (2.4)* | 9.7 (9.3) | 4.7 (1.9)* |
affected vs. unaffected arm testing difference, Wilcoxon test p < 0.05
Baseline Stability of Measures.
For the stroke affected arm, pre-intervention somatosensory measurements were similar at all three rTMS sessions for 2-point discrimination, monofilament threshold and vibration threshold (Table 3). However, for proprioception, there was a slight decrease in proprioception accuracy over the three sessions (Friedman test p=0.03). For the unaffected arm, there were no differences among pre-intervention somatosensory test scores. There were no differences in baseline values for any SEP components (Table 3). The average duration between session 1 and 2 was 16.7(10.4) (mean(SD)) days and between sessions 2 and 3, 16.4(18.5) days.
Table 3.
Pre-intervention (baseline) measurements across the three consecutive intervention sessions
| Measures | Affected arm | Unaffected arm | ||||||
|---|---|---|---|---|---|---|---|---|
| rTMS Sessions | rTMS Sessions | |||||||
| 1 | 2 | 3 | p | 1 | 2 | 3 | p | |
| Clinical | ||||||||
| 2pt-discr (mm) | 13.0 (4.5) | 12.6 (5.4) | 11.9 (5.3) | ns | 3.0 (1.5) | 2.8 (1.0) | 2.8 (1.1) | ns |
| Monofilament | 4.8 (1.9) | 5.0 (1.9) | 5.1 (1.8) | ns | 3.4 (0.5) | 3.8 (1.0) | 3.5 (0.4) | ns |
| Proprioception (% correct) | 78.4 (22.3) | 70.6 (29.1) | 65.9 (27.6) | 0.03 | 98.8 (3.4) | 99.4 (2.5) | 100.0 (0.0) | ns |
| Vibration (V) | 8.5 (5.6) | 11.0 (11.9) | 9.6 (9.7) | ns | 4.4 (1.7) | 5.1 (2.2) | 4.8 (2.0) | ns |
| SEP | ||||||||
| N20 latency (msec) | 16.0 (10.4) | 16.7 (10.5) | 16.3 (10.8) | ns | 21.8 (1.1) | 21.7 (0.9) | 21.8 (1.2) | ns |
| P25 latency | 21.1 (14.0) | 22.2 (13.7) | 20.7 (13.9) | ns | 27.5 (2.9) | 27.9 (3.4) | 27.9 (3.7) | ns |
| N33 latency | 27.3 (17.9) | 29.0 (19.1) | 27.0 (18.7) | ns | 37.4 (8.3) | 38.9 (10.1) | 37.6 (5.3) | ns |
| P45 latency | 33.2 (25.0) | 37.3 (23.5) | 34.5 (23.6) | ns | 42.0 (12.4) | 44.6 (12.8) | 47.6 (5.1) | ns |
| N60 latency | 45.4 (35.6) | 51.5 (37.6) | 51.6 (35.7) | ns | 59.6 (26.9) | 59.5 (25.5) | 64.3 (19.9) | ns |
| P100 latency | 78.3 (46.3) | 79.2 (52.5) | 84.3 (49.0) | ns | 104.0 (25.8) | 101.6 (24.9) | 104.8 (21.7) | ns |
| N120 latency | 104.4 (63.7) | 109.6 (70.9) | 107.7 (60.2) | ns | 139.8 (25.1) | 116.6 (58.8) | 126.5 (46.9) | ns |
| N20-P25 amplitude (μV) | 0.9 (1.7) | 1.0 (1.9) | 0.6 (1.1) | ns | 3.2 (1.8) | 3.4 (1.6) | 3.2 (2.1) | ns |
| P25-N33 amplitude | 0.9 (1.4) | 1.1 (1.9) | 0.7 (1.1) | ns | 2.9 (2.0) | 3.0 (1.9) | 2.9 (1.9) | ns |
| N33-P45 amplitude | 0.9 (1.5) | 0.9 (1.2) | 0.8 (1.3) | ns | 2.3 (1.7) | 2.6 (1.9) | 2.5 (1.9) | ns |
| P45-N60 amplitude | 0.9 (1.2) | 1.1 (1.3) | 1.4 (1.7) | ns | 3.7 (2.8) | 3.7 (2.8) | 3.7 (2.9) | ns |
| N60-P100 amplitude | 0.7 (0.7) | 0.7 (0.6) | 0.9 (0.8) | ns | 3.6 (2.2) | 3.5 (1.6) | 3.0 (1.8) | ns |
| P100-N120 amplitude | 0.4 (0.4) | 0.5 (0.6) | 0.7 (0.8) | ns | 1.6 (1.0) | 1.3 (0.8) | 1.7 (1.0) | ns |
All values are expressed as mean (standard deviation)
p = Friedman test p-value
ns = p > 0.05
Effects of rTMS on Clinical Somatosensory Function.
Two-point discrimination.
Affected arm.
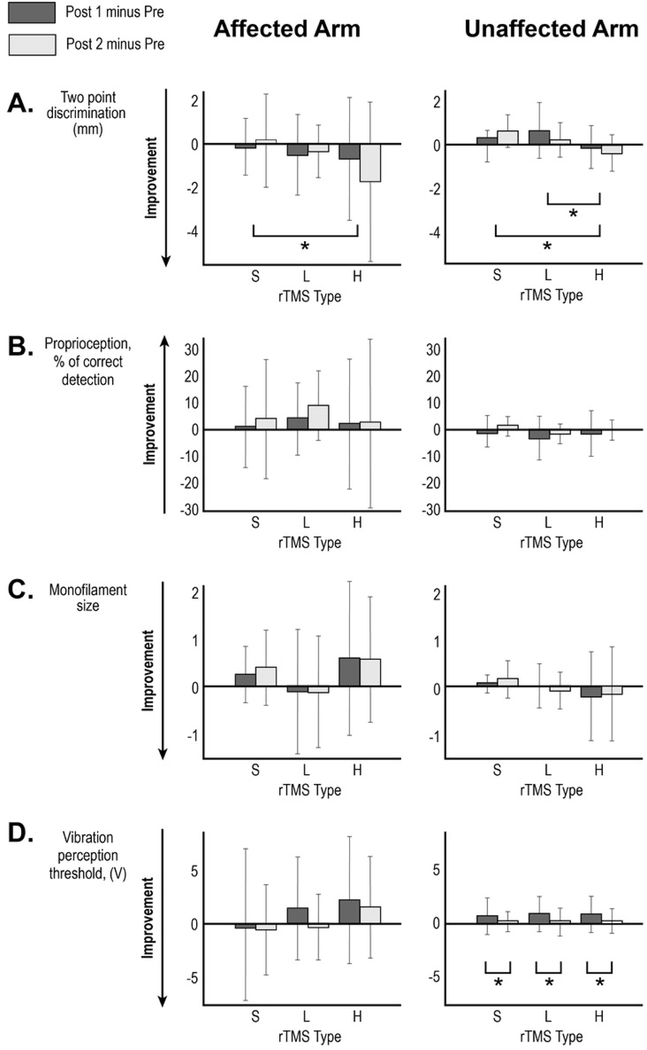
Based on the mixed model analysis, we found for the primary outcome measure, 2-point discrimination, a statistically significant treatment effect for session type (F(2, 76.163)=3.5, p = .035). The pairwise analysis demonstrated an improvement of 2-point discrimination of the affected arm in response to H-rTMS compared to S-rTMS (estimated mean difference of −1.187mm, SE=0.459; p = 0.011). There was a trend toward greater improvement after H-rTMS compared with L-rTMS responses (estimated mean difference = −0.773mm, SE=0.459, p = .096) but no difference between L-rTMS and S-rTMS. Figure 2A and Table 4 depict a change in 2-point discrimination ability in response to different rTMS interventions.
Figure 2.
Changes in clinical test scores following the three interventions performed ≥1 week apart. Mean changes for (A) two-point discrimination, (B) proprioception, (C) monofilament size and (D) vibration perception threshold at Post 1 (immediately after rTMS; dark bar) and Post 2 (1 hour after rTMS;light bar) are shown with error bar representing standard deviation. Stroke-affected arm data is in the left-sided graphs and stroke-unaffected arm is the right-sided graphs. * Brackets indicate statistically significant post-hoc paired comparisons in the mixed model analysis based on estimated marginal means. S- sham rTMS, L-low frequency rTMS, H- high frequency rTMS.
Table 4.
Baseline values and changes of clinical and SEP measures in response to different rTMS interventions (affected arm)
| Measure | Baseline (avg. across the 3 sessions) | High frequency rTMS session | Low frequency rTMS session | Sham rTMS session | |||
|---|---|---|---|---|---|---|---|
| Change at Post 1 | Change at Post 2 | Change at Post 1 | Change at Post 2 | Change at Post 1 | Change at Post 2 | ||
| Clinical | |||||||
| 2pt. discrimination* (mm) | 12.5 (5.0) | −0.7 (2.7) | −1.7 (3.5) | −0.5 (1.8) | −0.3 (1.2) | −0.1 (1.3) | 0.1 (2.1) |
| Monofilament | 5.0 (1.8) | 0.6 (1.6) | 0.6 (1.3) | −0.1 (1.3) | −0.1 (1.2) | 0.3 (0.6) | 0.4 (0.8) |
| Proprioception (% correct) | 71.7 (26.4) | 2.3 (24.8) | 2.5 (32.0) | 4.4 (13.8) | 9.3 (13.3) | 1.2 (15.4) | 4.1 (22.7) |
| Vibration (V) | 9.7 (9.3) | 2.4 (6.1) | 1.7 (4.9) | 1.5 (5.0) | −0.2 (3.2) | −0.4 (7.6) | −0.5 (4.4) |
| SEP | |||||||
| N20 latency* (msec) | 16.3 (10.3) | −1.1 (2.3) | 0.1 (2.0) | 0.1 (2.2) | −1.8 (5.8) | 0.7 (3.0) | 1.2 (2.5) |
| P25 latency | 21.3 (13.6) | −1.7 (5.5) | −1.6 (3.1) | −1.3 (1.9) | −3.1 (8.3) | −0.1 (5.1) | −0.7 (4.1) |
| N33 latency | 27.8 (18.2) | −4.2 (6.5) | −2.0 (5.5) | −0.7 (3.0) | 0.8 (5.6) | −0.1 (7.0) | −0.8 (4.8) |
| P45 latency | 35.1 (23.6) | 0.9 (15.2) | 0.9 (18.1) | −2.1 (5.9) | 1.9 (4.3) | 2.8 (9.8) | 0.2 (7.4) |
| N60 latency* | 49.5 (35.7) | 3.0 (20.7) | 3.2 (19.6) | −7.1 (22.6) | −4.9 (22.5) | 6.6 (15.1) | −0.1 (20.8) |
| P100 latency* | 80.6 (48.0) | 2.1 (25.4) | −0.6 (9.0) | 4.1 (15.1) | 1.0 (9.6) | 14.7 (19.3) | 5.6 (15.9) |
| N120 latency | 107.4 (63.3) | −21.2 (59.5) | −6.4 (22.5) | 4.8 (11.9) | −0.2 (20.4) | −7.5 (51.2) | −10.4 (51.7) |
| N20-P25 amplitude (μV) | 0.8 (1.6) | −0.1 (0.6) | 0.1 (0.5) | 0.1 (0.3) | −0.0 (0.3) | 0.1 (0.3) | 0.1 (0.3) |
| P25-N33 amplitude | 0.9 (1.5) | −0.0 (0.5) | 0.4 (1.0) | −0.1 (0.3) | −0.1 (0.3) | 0.1 (0.5) | 0.1 (0.3) |
| N33-P45 amplitude* | 0.9 (1.3) | 0.2 (0.5) | 0.5 (1.0) | −0.0 (0.3) | 0.1 (0.3) | 0.2 (0.5) | 0.1 (0.5) |
| P45-N60 amplitude* | 1.1 (1.4) | 0.2 (1.0) | 0.4 (0.9) | −0.3 (0.6) | −0.3 (1.4) | 0.3 (0.9) | 0.0 (0.5) |
| N60-P100 amplitude | 0.8 (0.7) | −0.2 (0.8) | −0.2 (0.9) | −0.1 (0.4) | 0.0 (0.4) | −0.1 (0.5) | 0.0 (0.2) |
| P100-N120 amplitude | 0.5 (0.6) | −0.4 (1.0) | −0.3 (1.0) | −0.1 (0.4) | −0.0 (0.3) | −0.0 (0.4) | −0.0 (0.1) |
All values are expressed as mean (standard deviation)
Indicates the variable that demonstrated statistical significance in the mixed model analysis at p ≤ 0.05
Change at Post 1 = testing value at Baseline at each session minus testing value at Post 1 data collection
Change at Post 2 = testing value at Baseline at each session minus testing value at Post 2 data collection
Unaffected arm.
Mixed model analysis showed a statistically significant improvement of 2-point discrimination of the unaffected arm following H-rTMS intervention (F(2, 192.786)=10.6, p<0.0001). Based on the pairwise analysis, H-rTMS produced greater improvement compared with S-rTMS (estimated mean difference =−.710mm, SE=0.173; p<0.0001) and greater improvement compared with L-rTMS (estimated mean difference = −.663mm, SE=0.173; p <0.0001). Table 5 and Figure 2A depict a change in 2-point discrimination ability in response to different rTMS.
Table 5.
Baseline values and changes of clinical and SEP measures in response to different rTMS interventions (unaffected arm)
| Measure | Baseline (avg. across the 3 sessions) | High frequency rTMS session | Low frequency rTMS session | Sham rTMS session | |||
|---|---|---|---|---|---|---|---|
| Change at Post 1 | Change at Post 2 | Change at Post 1 | Change at Post 2 | Change at Post1 | Change at Post 2 | ||
| Clinical | |||||||
| 2pt. discrimination* (mm) | 2.9 (1.2) | −0.1 (1.0) | −0.4 (0.8) | 0.6 (1.3) | 0.2 (0.8) | 0.3 (0.6) | 0.6 (0.7) |
| Monofilament | 3.6 (0.7) | −0.2 (0.9) | −0.2 (1.0) | 0.0 (0.5) | −0.1 (0.4) | 0.0 (0.2) | 0.1 (0.4) |
| Proprioception (%correct) | 99.4 (2.4) | −1.3 (8.3) | 0.0 (3.7) | −3.1 (7.9) | −1.3 (3.5) | −0.6 (5.7) | 1.2 (3.4) |
| Vibration* (V) | 4.7 (1.9) | 0.8 (1.7) | 0.2 (1.1) | 0.9 (1.6) | 0.1 (1.3) | 0.7 (1.7) | 0.1 (0.9) |
| SEP | |||||||
| N20 latency (msec) | 21.7 (1.0) | 0.3 (1.1) | 0.6 (1.3) | −0.1 (0.4) | 0.5 (1.1) | 0.4 (1.0) | 0.2 (0.4) |
| P25 latency | 27.8 (3.3) | −0.1 (2.5) | 0.6 (1.8) | −0.8 (2.6) | −0.5 (2.7) | 0.5 (1.1) | 0.2 (0.6) |
| N33 latency | 38.0 (8.1) | 0.5 (1.7) | 0.7 (1.4) | 1.6 (5.5) | 0.5 (0.9) | −1.0 (6.9) | −0.2 (7.2) |
| P45 latency | 44.7 (10.7) | −0.3 (1.7) | −3.9 (13.6) | −3.5 (15.3) | 2.3 (3.5) | 1.3 (2.0) | 4.5 (15.0) |
| N60 latency* | 61.1 (23.9) | −0.8 (3.7) | −2.9 (5.6) | −5.3 (18.6) | 0.0 (3.7) | 1.2 (6.2) | 11.6 (25.3) |
| P100 latency | 103.4 (23.5) | −6.2 (16.1) | −7.3 (17.4) | −2.4 (11.8) | −1.9 (10.7) | −2.6 (11.9) | 2.0 (9.5) |
| N120 latency | 127.3 (45.9) | −0.2 (18.9) | −0.6 (17.6) | 10.9 (39.7) | 12.6 (40.1) | −4.6 (11.6) | −0.8 (9.6) |
| N20-P25 amplitude (μV) | 3.3 (1.8) | 0.1 (0.8) | 0.0 (0.7) | 0.3 (0.8) | 0.1 (1.3) | 0.0 (0.9) | 0.0 (0.6) |
| P25-N33 amplitude | 2.9 (1.9) | 0.1 (0.7) | 0.1 (0.7) | 0.4 (0.6) | 0.5 (0.7) | −0.1(0.9) | 0.3 (1.0) |
| N33-P45 amplitude | 2.5 (1.8) | 0.1 (0.4) | −0.1 (0.7) | 0.2 (0.7) | 0.4 (0.7) | 0.0 (0.5) | 0.5 (0.7) |
| P45-N60 amplitude | 3.7 (2.7) | 0.1 (0.8) | −0.3 (0.7) | 0.0 (1.0) | 0.1 (1.0) | 0.0 (0.8) | 0.3 (1.3) |
| N60-P100 amplitude | 3.4 (1.8) | 0.3 (1.4) | −0.4 (1.5) | 0.1 (0.9) | 0.3 (1.0) | 0.1 (0.9) | 0.1 (0.8) |
| P100-N120 amplitude | 1.5 (0.9) | 0.5 (1.1) | 0.5 (1.0) | 0.6 (1.5) | 0.7 (1.3) | 0.3 (1.3) | 0.6 (1.1) |
All values are expressed as mean (standard deviation)
Indicates statistical significance in the mixed model analysis at p ≤ 0.05
Change at Post 1 = testing value at Baseline at each session minus testing value at Post 1 data collection
Change at Post 2 = testing value at Baseline at each session minus testing value at Post 2 data collection
Proprioception and monofilament threshold.
For both affected and unaffected arms, mixed model analysis did not show a statistically significant difference between responses to the three types of rTMS for proprioception ( Figure 2B;Tables 4 and 5) or monofilament threshold (Figure 2C; Tables 4 and 5). However results for monofilament threshold were trending toward significance affected: F(2, 75.303)=2.769, p=0.069; unaffected: (F(2, 74.34)=2.737, p=0.071).
Vibration.
Affected arm.
There were no statistically significant mixed model results for vibration for the affected arm (F(2, 75.545)=2.026, p=0.139). (Table 4 and Figure 2D).
Unaffected arm.
Mixed model analysis demonstrated a statistically significant reduction in vibration perception at Post 1 compared with Post 2 (F(2, 153.350)=7.843, p=0.006). There was no effect of session type (p=0.928). These transient changes in vibration perception were similar following all treatment types (Table 5, Figure 2D).
Effect of rTMS on Somatosensory Evoked Potential (SEP).
Affected arm SEP.
We used mixed model analysis to evaluate changes in SEP component measures in response to the three types of rTMS. Table 4 shows data for SEP at baseline and changes observed at Post 1 and Post 2. There were statistically significant findings for session type in models where independent variables were peak to peak amplitude changes for N33-P45 (F(2, 133.027)=3.518, p=0.032) and P45-N60 (F(2, 67.353)=3.212, p=0.047) as well as changes in N20 latency (F(2, 69.64)=3.37, p=0.04), N60 latency (F(2, 47.343)=4.375, p=0.018) and P100 latency (F(2, 37.608)=3.537, p=0.039). For N33-P45 amplitude change, margin of mean test demonstrated that an amplitude change following H-rTMS was greater compared to L-rTMS (estimated marginal mean difference = 0.291μV, SE= 0.113 p=0.032). For P45-N60, there was a trend toward H-rTMS producing greater response compared to L-rTMS (estimated marginal mean difference =0.611μV, SE=0.253, p=0.054). For N20 latency change, H-rTMS resulted in a shorter latency for N20 compared to change following S-rTMS (estimated marginal means difference = −2.049msec, SE=0.8, p=0.037). For N60 latency change, H-rTMS also resulted in a shorter latency for N60 compared to change following S-rTMS (estimated marginal means difference = −10.288msec, SE=3.7, p=0.025). Similarly, for P100 latency change, H-rTMS resulted in a shorter latency compared to change following S-rTMS (estimated marginal means difference = −16.215msec, SE=6.1, p=0.035).
Unaffected arm SEP.
Table 5 demonstrates both baseline SEP components values and changes in these measures in response to each of the rTMS interventions. Based on mixed model analysis, N60 latency showed a statistically significant result for session type (F(2, 64.76)=3.875, p=0.026). Marginal mean estimate tests showed that following H-rTMS, there was a shortening of latency for N60 peak compared with S-rTMS (estimated marginal means = −3.56μV, SE=1.29, p=0.022).
DISCUSSION
This proof-of-principle study evaluates for the first time whether facilitation or inhibition of contralesional S1 using rTMS paired with peripheral sensory stimulation can elicit improvement in somatosensory function in chronic stroke. Our results suggest that facilitatory H-rTMS (5 Hz) plus simultaneous peripheral sensory stimulation to the affected arm improves 2-point discrimination. In addition, our exploratory analysis of SEP indicates enhancement of somatosensory evoked responses following H-rTMS. Both clinical and physiological effects lasted at least an hour as suggested by the lack of differences between the changes found immediately after and one hour following the interventions. There were no differences among the pre-intervention testing for all sessions.
Somatosensory modality specific response.
A statistically significant response to facilitatory H-rTMS (5 Hz) plus simultaneous peripheral sensory stimulation of the affected arm was observed for 2-point discrimination ability on the stroke-affected side. Other somatosensory modalities (proprioception, vibration and monofilament) did not demonstrate a statistically significant change. This modality-specific response maybe due to differences in somatosensory signal processing, i.e. proprioceptive vs. tactile, primary somatosensory perception (monofilament) vs. associative (2-point discrimination). It is possible that discriminative somatosensory function is more likely to be impacted by an ipsilateral intervention due to a bilateral processing of somatosensory discrimination compared to the processing of primary somatosensory input. In other words, tactile sensing is involved in monofilament test vs. 2-point discrimination. Others have also reported a modality-specific response to brain stimulation following 10Hz rTMS of the primary motor region where facilitatory stimulation reduced painful perception of thermal stimuli but did not alter ability to sense non-painful tactile stimulation47. Another factor that may be influencing the modality specific response is the choice of the peripheral stimulus paired with brain stimulation. For example, a modality specific response in healthy controls receiving rTMS over M1 paired with passive joint movement resulted in a significant change of corticospinal excitability while stimulation with rTMS alone did not48. In our study, we used sensory level electrical stimulation and vibration. Perhaps, other combinations of peripheral somatosensory therapy and brain treatment protocols need to be evaluated to determine if they have a specific effect on other somatosensory modalities. Somatosensation is complex, and thus an array of measures was employed to assess various aspects of somatosensation. At baseline, 2-point discrimination had the largest difference between affected and unaffected hand and thus the tool may have been sensitive enough to detect this change. It may be that the other measurement tools were not sensitive enough to detect change in the other somatosensory modalities assessed. Indeed, available somatosensory testing has many limitations that include a lack of sensitivity, poor responsiveness, decreased validity and a lack of objectiveness49 and this may have contributed to having only 1 out of 4 sensory measures demonstrating a statistically significant response to the intervention. Additionally, the study employed impairment level measures and no measurement of function was directly assessed. Therefore, the impact of this observed change in 2-point discrimination on overall function was not studied. However, the fact that we found an effect on 2-pt discrimination in a small sample using standard clinical tests is intriguing and suggests future study is warranted with measures that more finely assess somatosensory function.
Unilateral stimulation, bilateral effect.
Importantly, our findings suggest that contralesional S1 is playing a role in processing of tactile discrimination in individuals in the chronic stage after stroke. This finding might contradict the classic functional sensory anatomy teaching stating that peripheral signals evoke contralateral activity and, therefore, are processed in the contralateral hemisphere50. Indeed, recent functional imaging studies describe bihemispheric activation following unilateral somatosensory stimulation as seen with both fMRI and somatosensory evoked potential tests51,52. The pathways of sensory signal transfer to bilateral hemispheres may involve transcallosal connections and possibly direct ipsilateral uncrossed afferent pathways27,53–55. Therefore, it is reasonable to expect that after stroke, these secondary contralesional somatosensory processing regions play a role in restoring the lost function.
The concept of focal rTMS influencing function of bilateral somatosensory networks and bilateral somatosensory abilities has been previously evaluated in healthy adults and in pain-related research. In healthy adults, Premji et. al56 demonstrated that unilateral facilitatory stimulation using an intermittent theta-burst stimulation (iTBS) protocol increased somatosensory evoked potential amplitude at the site of stimulation and also on the non-stimulated hemisphere. Another study of healthy adults showed that five-pulse 10Hz TMS burst targeting right parietal region when paired with same side peripheral median nerve stimulation increased fMRI activation57. In pain therapy, unilateral 10 Hz rTMS targeting primary motor area (M1) reduced thermal pain threshold in both hands47. Overall, our findings and those of others support contralesional somatosensory cortex modulation as a reasonable brain stimulation target to enhance post-stroke somatosensory restoration.
Facilitatory, not Inhibitory, rTMS targeting contralesional S1.
Brain reorganization may take several paths to support recovery of somatosensory function after stroke. If we consider a paradigm of interhemispheric balance that is disturbed after stroke, one pattern of plasticity may be toward rebalance of interhemispheric interactions21. Indeed, in motor control studies using rTMS, inhibition of the contralesional hemisphere has been shown to enhance motor function of the stroke affected upper limb presumably by correcting imbalance21. Interhemispheric interactions have been demonstrated for healthy controls where inhibition of somatosensory pathways on one side resulted in enhanced excitability of SEP on the opposite side30,58 and improved somatosensory function58–60. After stroke, anesthesia of the unaffected upper limb improved somatosensory perception on the affected side61. However, these were peripherally administered inhibitory interventions of the un-affected limb and we cannot expect the same response for modulation of the brain somatosensory networks. In fact, contralesional S1 may be supporting the lost function of the opposite hemisphere since somatosensory signal processing is bilateral. It is possible the mechanism of somatosensory improvement following contralesional S1 facilitation is tapping into pathways not related to transcallosal inhibition. These mechanisms may involve facilitation of alternative somatosensory processing centers. Therefore, it is quite reasonable to find that the facilitation of contralesional S1 and not its inhibition benefits recovery of somatosensory function after stroke.
Unaffected upper limb response to stimulation supports protocol feasibility.
We observed a statistically significant improvement of 2-point discrimination of the unaffected-by-stroke arm. This finding is an important confirmation that our protocol achieves modulation of somatosensory function. H-rTMS, which is known to enhance cortical activity, improved 2-point discrimination of the unaffected hand, contralaterally to stimulated cortex. The positive changes in response to H-rTMS were contrasted with both S-rTMS and L-rTMS. Others demonstrated in healthy adults that two types of facilitatory TMS (5Hz rTMS and iTBS) can transiently increase 2-point discrimination59,60. Notably, in our cohort of stroke survivors we observed changes in 2-point discrimination even though we did not use a more sensitive 2-point discrimination set up with inter-point distances less than 2mm as was applied in studies with healthy controls. Perhaps, the response is more robust in individuals with stroke deficits compared with healthy controls.
A transient decrease in vibration sense was observed immediately after each intervention. This was not a lasting effect confirmed by the lack of differences among the testing at each session’s baseline. It may be that adaptation of the cutaneous mechanoreceptors during the vibratory stimulation of the hand was the cause for this temporary change 62. Although, vibration was applied to the stroke-affected hand, the unaffected hand may have inadvertently received vibratory stimulation while both arms were resting on a table where the affected hand was placed on a vibrating device.
SEP changes in response to rTMS.
Our exploratory analysis suggests that H-rTMS may enhance the SEP signal induced by peripheral stimulation of the median nerve. Specifically, for the stroke affected side there was a reduction of N20, N60, P100 peak latencies and greater amplitudes of N25-P33 and N33-P45 peaks following H-rTMS. The stroke-unaffected SEP pathway demonstrated changes for the N60 peak latency following H-rTMS. Changes in both early and late SEP components for both stroke-affected and -unaffected somatosensory tracts suggest unilateral modulation of contralesional S1 may have an impact on the bilateral somatosensory processing network. The earlier peaks (N20, P25, N33, P45) may reflect changes at the level of thalamocortical projections to S1 cortex areas 3b and area163. Changes in the late SEP components (N60 and P100) suggest involvement of SII and other higher-order sensory27,64 and sensory-motor65 integration regions. SEP changes provide additional support of potential therapeutic feasibility of our stimulation paradigm to evoke response in the function of the somatosensory network.
After-effect duration.
There were no differences between Post 1 and Post 2 test results. Thus, when the changes were achieved, they lasted at least 60 minutes. This finding is expected and suggests the interventions induced an after-effect of up to 60 minutes (reviewed in24). Physiological mechanisms of the after-effect are unclear but are thought to involve long-term potentiation and long-term depression(reviewed in 24,66). In fact, analysis of SEP changes also demonstrated lasting after-effects in both increased amplitude and decreased latencies, and thus provide indirect evidence for functional brain changes remaining after stimulation for at least an hour (reviewed in 24,66). It is expected that a single rehabilitation session would not provide a permanent functional recovery and future multisession studies are needed to evaluate a long-lasting of this intervention.
Limitations.
Three main limitations may have led to the limited response of different somatosensory modalities. First, that we only demonstrated a significant change on 2-point discrimination may be because for this measure there was the largest separation between the affected and unaffected sides. Therefore, it had the highest opportunity for improvement compared to the other measures. Second, the outcome measures may have not been sensitive enough to detect change. For instance, the proprioception measure may have been too subjective in nature to detect change. Third, different forms of peripheral stimulation paired with NIBS may yield better results. It should also be noted the study cohort was younger than average for the stroke population and this may limit the generalizing study results to the greater stroke population. Future research using more sensitive somatosensory measuring tools67, testing a population with a broader range of sensory deficits and multisession design of complimentary peripherally directed stimulation paired with NIBS should overcome these limitations.
Conclusion.
Using a single session randomized cross-over design, we report preliminary evidence in support of the effect of facilitatory contralesional rTMS on 2-point discrimination in chronic stroke. This may be due to the higher incidence of 2-point discrimination deficit compared with other sensory modalities in our patient cohort. In motor rehabilitation literature, a single session crossover intervention paradigm has been widely used as a first step. In these studies, different brain stimulation interventions were compared on the same patients with the goal of identifying a therapy for application in multisession clinical trials. Further studies are needed to explore the role of rTMS in rehabilitation of somatosensory function after stroke.
Acknowledgments
Funding: This work was supported by the Award Number I01BX007080 from the Rehabilitation Research & Development Service of the VA Office of Research and Development; by the Case Western Reserve University/Cleveland Clinic CTSA Grant Number UL1 RR024989 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health and NIH roadmap for Medical Research.
Footnotes
Conflict of Interest. None of the authors declare conflict of interest.
Financial disclosures: None
References
- 1.Kim JS, Choi-Kwon S. Discriminative sensory dysfunction after unilateral stroke. Stroke. 1996;27(0039–2499 (Print)):677–682. [DOI] [PubMed] [Google Scholar]
- 2.Carey LM, Matyas TA, Oke LE. Sensory loss in stroke patients: effective training of tactile and proprioceptive discrimination. ArchPhysMedRehabil. 1993;74(0003–9993 (Print)):602–611. [DOI] [PubMed] [Google Scholar]
- 3.Doyle S, Bennett S, Fasoli SE, McKenna KT. Interventions for sensory impairment in the upper limb after stroke. CochraneDatabaseSystRev. 2010;(1469–493X (Electronic)):CD006331. doi: 10.1002/14651858.CD006331.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dukelow SP, Herter TM, Bagg SD, Scott SH. The independence of deficits in position sense and visually guided reaching following stroke. JNeuroengRehabil. 2012;9(1743–0003 (Electronic)):72. doi: 10.1186/1743-0003-9-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desrosiers J, Noreau L, Rochette A, Bourbonnais D, Bravo G, Bourget A. Predictors of long-term participation after stroke. DisabilRehabil. 2006;28(0963–8288 (Print)):221–230. doi: 10.1080/09638280500158372 [DOI] [PubMed] [Google Scholar]
- 6.Molle Da Costa RD, Luvizutto GJ, Martins LG, et al. Clinical factors associated with the development of nonuse learned after stroke: a prospective study. Top Stroke Rehabil. Published online June 22, 2019:1–7. doi: 10.1080/10749357.2019.1631605 [DOI] [PubMed] [Google Scholar]
- 7.Meyer S, Karttunen AH, Thijs V, Feys H, Verheyden G. How Do Somatosensory Deficits in the Arm and Hand Relate to Upper Limb Impairment, Activity, and Participation Problems After Stroke? A Systematic Review. PhysTher. 2014;(1538–6724 (Electronic)). doi: 10.2522/ptj.20130271 [DOI] [PubMed] [Google Scholar]
- 8.Serrada I, Hordacre B, Hillier SL. Does Sensory Retraining Improve Sensation and Sensorimotor Function Following Stroke: A Systematic Review and Meta-Analysis. Front Neurosci. 2019;13. doi: 10.3389/fnins.2019.00402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yozbatiran N, Donmez B, Kayak N, Bozan O. Electrical stimulation of wrist and fingers for sensory and functional recovery in acute hemiplegia. ClinRehabil. 2006;20(0269–2155 (Print)):4–11. [DOI] [PubMed] [Google Scholar]
- 10.Peurala SH, Pitkanen K, Sivenius J, Tarkka IM. Cutaneous electrical stimulation may enhance sensorimotor recovery in chronic stroke. ClinRehabil. 2002;16(0269–2155 (Print)):709–716. [DOI] [PubMed] [Google Scholar]
- 11.Bhatt E, Nagpal A, Greer KH, et al. Effect of finger tracking combined with electrical stimulation on brain reorganization and hand function in subjects with stroke. ExpBrain Res. 2007;182(1432–1106 (Electronic)):435–447. doi: 10.1007/s00221-007-1001-5 [DOI] [PubMed] [Google Scholar]
- 12.Cauraugh JH, Kim S. Two coupled motor recovery protocols are better than one: electromyogram-triggered neuromuscular stimulation and bilateral movements. Stroke. 2002;33(1524–4628 (Electronic)):1589–1594. [DOI] [PubMed] [Google Scholar]
- 13.Cassidy JM, Cramer SC. Spontaneous and Therapeutic-Induced Mechanisms of Functional Recovery After Stroke. Transl Stroke Res. 2017;8(1):33–46. doi: 10.1007/s12975-016-0467-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taskin B, Jungehulsing GJ, Ruben J, et al. Preserved responsiveness of secondary somatosensory cortex in patients with thalamic stroke. CerebCortex. 2006;16(1047–3211 (Print)):1431–1439. doi: 10.1093/cercor/bhj080 [DOI] [PubMed] [Google Scholar]
- 15.Carey LM, Abbott DF, Puce A, Jackson GD, Syngeniotis A, Donnan GA. Reemergence of activation with poststroke somatosensory recovery: a serial fMRI case study. Neurology. 2002;59(0028–3878 (Print)):749–752. [DOI] [PubMed] [Google Scholar]
- 16.Carey L, Walsh A, Adikari A, et al. Finding the Intersection of Neuroplasticity, Stroke Recovery, and Learning: Scope and Contributions to Stroke Rehabilitation. Neural Plast. 2019;2019:1–15. doi: 10.1155/2019/5232374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cramer SC. Treatments to Promote Neural Repair after Stroke. J Stroke. 2018;20(1):57–70. doi: 10.5853/jos.2017.02796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bao S, Khan A, Song R, Kai-yu Tong R. Rewiring the Lesioned Brain: Electrical Stimulation for Post-Stroke Motor Restoration. J Stroke. 2020;22(1):47–63. doi: 10.5853/jos.2019.03027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lefaucheur J-P, Aleman A, Baeken C, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin Neurophysiol. 2020;131(2):474–528. doi: 10.1016/j.clinph.2019.11.002 [DOI] [PubMed] [Google Scholar]
- 20.Hsu WY, Cheng CH, Liao KK, Lee IH, Lin YY. Effects of repetitive transcranial magnetic stimulation on motor functions in patients with stroke: a meta-analysis. Stroke. 2012;43(1524–4628 (Electronic)):1849–1857. doi: 10.1161/STROKEAHA.111.649756 [DOI] [PubMed] [Google Scholar]
- 21.Liew SL, Santarnecchi E, Buch ER, Cohen LG. Non-invasive brain stimulation in neurorehabilitation: local and distant effects for motor recovery. Front HumNeurosci. 2014;8(1662–5161 (Electronic)):378. doi: 10.3389/fnhum.2014.00378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brodie SM, Meehan S, Borich MR, Boyd LA. 5 Hz repetitive transcranial magnetic stimulation over the ipsilesional sensory cortex enhances motor learning after stroke. Front HumNeurosci. 2014;8(1662–5161 (Electronic)):143. doi: 10.3389/fnhum.2014.00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pleger B, Blankenburg F, Bestmann S, et al. Repetitive transcranial magnetic stimulation-induced changes in sensorimotor coupling parallel improvements of somatosensation in humans. JNeurosci. 2006;26(1529–2401 (Electronic)):1945–1952. doi: 10.1523/JNEUROSCI.4097-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernandez-Pavon JC, Harvey RL. Noninvasive Transcranial Magnetic Brain Stimulation in Stroke. Phys Med Rehabil Clin N Am. 2019;30(2):319–335. doi: 10.1016/j.pmr.2018.12.010 [DOI] [PubMed] [Google Scholar]
- 25.Bjornsdotter M, Gordon I, Pelphrey KA, Olausson H, Kaiser MD. Development of brain mechanisms for processing affective touch. Front BehavNeurosci. 2014;8(1662–5153 (Electronic)):24. doi: 10.3389/fnbeh.2014.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van EF, de Lange FP, Maris E. Anticipation Increases Tactile Stimulus Processing in the Ipsilateral Primary Somatosensory Cortex. CerebCortex. 2013;(1460–2199 (Electronic)). doi: 10.1093/cercor/bht111 [DOI] [PubMed] [Google Scholar]
- 27.Allison T, McCarthy G, Wood CC, Williamson PD, Spencer DD. Human cortical potentials evoked by stimulation of the median nerve. II. Cytoarchitectonic areas generating long-latency activity. J Neurophysiol. 1989;62(3):711–722. doi: 10.1152/jn.1989.62.3.711 [DOI] [PubMed] [Google Scholar]
- 28.Jones RD, Donaldson IMacg, Parkin PJ. IMPAIRMENT AND RECOVERY OF IPSILATERAL SENSORY-MOTOR FUNCTION FOLLOWING UNILATERAL CEREBRAL INFARCTION. Brain. 1989;112(1):113–132. doi: 10.1093/brain/112.1.113 [DOI] [PubMed] [Google Scholar]
- 29.Lefaucheur J-P, André-Obadia N, Antal A, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol. 2014;125(11):2150–2206. doi: 10.1016/j.clinph.2014.05.021 [DOI] [PubMed] [Google Scholar]
- 30.Meehan SK, Dao E, Linsdell MA, Boyd LA. Continuous theta burst stimulation over the contralesional sensory and motor cortex enhances motor learning post-stroke. NeurosciLett. 2011;500(1872–7972 (Electronic)):26–30. doi: 10.1016/j.neulet.2011.05.237 [DOI] [PubMed] [Google Scholar]
- 31.Harris-Love ML, Perez MA, Chen R, Cohen LG. Interhemispheric inhibition in distal and proximal arm representations in the primary motor cortex. JNeurophysiol. 2007;97(0022–3077 (Print)):2511–2515. doi: 10.1152/jn.01331.2006 [DOI] [PubMed] [Google Scholar]
- 32.Vanderah TW, Gould DJ, eds. Nolte’s the Human Brain: An Introduction to Its Functional Anatomy. 8th ed. Elsevier; 2020. [Google Scholar]
- 33.Carey LM, Matyas TA. Training of somatosensory discrimination after stroke: facilitation of stimulus generalization. AmJ PhysMedRehabil. 2005;84(0894–9115 (Print)):428–442. [DOI] [PubMed] [Google Scholar]
- 34.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siebner H, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res. 2003;148(1):1–16. doi: 10.1007/s00221-002-1234-2 [DOI] [PubMed] [Google Scholar]
- 36.Goh H-T, Chan H-Y, Abdul-Latif L. Aftereffects of 2 Noninvasive Brain Stimulation Techniques on Corticospinal Excitability in Persons With Chronic Stroke: A Pilot Study. J Neurol Phys Ther. 2015;39(1):15–22. doi: 10.1097/NPT.0000000000000064 [DOI] [PubMed] [Google Scholar]
- 37.Rossini PM, Burke D, Chen R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126(6):1071–1107. doi: 10.1016/j.clinph.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ragert P, Dinse HR, Pleger B, et al. Combination of 5 Hz repetitive transcranial magnetic stimulation (rTMS) and tactile coactivation boosts tactile discrimination in humans. NeurosciLett. 2003;348(0304–3940 (Print)):105–108. [DOI] [PubMed] [Google Scholar]
- 39.Dellon AL, Mackinnon SE, Crosby PM. Reliability of two-point discrimination measurements. JHand SurgAm. 1987;12(0363–5023 (Print)):693–696. [DOI] [PubMed] [Google Scholar]
- 40.Sims SEG, Engel L, Hammert WC, Elfar JC. Hand Sensibility, Strength, and Laxity of High-Level Musicians Compared to Nonmusicians. J Hand Surg. 2015;40(10):1996–2002.e5. doi: 10.1016/j.jhsa.2015.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Odabas FO, Sayin R, Milanlioglu A, Tombul T, Cögen EE, Yildirim G. Electrophysciological analysis of entrapment neuropathies developed in acute and subacute period in paretic and non-paretic extremities in patients with stroke. JPMA J Pak Med Assoc. 2012;62(7):649–652. [PubMed] [Google Scholar]
- 42.Julkunen L, Tenovuo O, Jaaskelainen SK, Hamalainen H. Recovery of somatosensory deficits in acute stroke. Acta NeurolScand. 2005;111(0001–6314 (Print)):366–372. doi: 10.1111/j.1600-0404.2005.00393.x [DOI] [PubMed] [Google Scholar]
- 43.Bell-Krotoski JA, Fess EE, Figarola JH, Hiltz D. Threshold detection and Semmes-Weinstein monofilaments. JHand Ther. 1995;8(0894–1130 (Print)):155–162. [DOI] [PubMed] [Google Scholar]
- 44.Goldberg JM, Lindblom U. Standardised method of determining vibratory perception thresholds for diagnosis and screening in neurological investigation. J Neurol Neurosurg Psychiatry. 1979;42(9):793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gregg EC. ABSOLUTE MEASUREMENT OF THE VIBRATORY THRESHOLD. Arch Neurol Psychiatry. 1951;66(4):403. doi: 10.1001/archneurpsyc.1951.02320100003001 [DOI] [PubMed] [Google Scholar]
- 46.Nuwer MR, Aminoff M, Desmedt J, et al. IFCN recommended standards for short latency somatosensory evoked potentials. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91(1):6–11. [DOI] [PubMed] [Google Scholar]
- 47.Nahmias F, Debes C, de Andrade DC, Mhalla A, Bouhassira D. Diffuse analgesic effects of unilateral repetitive transcranial magnetic stimulation (rTMS) in healthy volunteers. Pain. 2009;147(1–3):224–232. doi: 10.1016/j.pain.2009.09.016 [DOI] [PubMed] [Google Scholar]
- 48.Edwards DJ, Dipietro L, Demirtas-Tatlidede A, et al. Movement-generated afference paired with transcranial magnetic stimulation: an associative stimulation paradigm. J NeuroEngineering Rehabil. 2014;11(1):31. doi: 10.1186/1743-0003-11-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sullivan JE, Hedman LD. Sensory dysfunction following stroke: incidence, significance, examination, and intervention. TopStroke Rehabil. 2008;15(1074–9357 (Print)):200–217. doi: 10.1310/tsr1503-200 [DOI] [PubMed] [Google Scholar]
- 50.Kandel ER, Schwartz JH, Jessell T, Siegelbaum SA, Hudspeth AJ, Mack S, eds. Principles of Neural Science. Fifth edition. McGraw-Hill Medical; 2013. [Google Scholar]
- 51.Hlushchuk Y, Hari R. Transient suppression of ipsilateral primary somatosensory cortex during tactile finger stimulation. JNeurosci. 2006;26(1529–2401 (Electronic)):5819–5824. doi: 10.1523/JNEUROSCI.5536-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lamp G, Goodin P, Palmer S, Low E, Barutchu A, Carey LM. Activation of Bilateral Secondary Somatosensory Cortex With Right Hand Touch Stimulation: A Meta-Analysis of Functional Neuroimaging Studies. Front Neurol. 2019;9:1129. doi: 10.3389/fneur.2018.01129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sutherland MT. The hand and the ipsilateral primary somatosensory cortex. JNeurosci. 2006;26(1529–2401 (Electronic)):8217–8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tommerdahl M, Simons SB, Chiu JS, Favorov O, Whitsel BL. Ipsilateral input modifies the primary somatosensory cortex response to contralateral skin flutter. JNeurosci. 2006;26(1529–2401 (Electronic)):5970–5977. doi: 10.1523/JNEUROSCI.5270-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kanno A, Nakasato N, Nagamine Y, Tominaga T. Non-transcallosal ipsilateral area 3b responses to median nerve stimulus. J ClinNeurosci. 2004;11(0967–5868 (Print)):868–871. doi: 10.1016/j.jocn.2004.01.007 [DOI] [PubMed] [Google Scholar]
- 56.Premji A, Ziluk A, Nelson AJ. Bilateral somatosensory evoked potentials following intermittent theta-burst repetitive transcranial magnetic stimulation. BMCNeurosci. 2010;11(1471–2202 (Electronic)):91. doi: 10.1186/1471-2202-11-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blankenburg F, Ruff CC, Bestmann S, et al. Interhemispheric effect of parietal TMS on somatosensory response confirmed directly with concurrent TMS-fMRI. J Neurosci. 2008;28(1529–2401 (Electronic)):13202–13208. doi: 10.1523/JNEUROSCI.3043-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Werhahn KJ, Mortensen J, Kaelin-Lang A, Boroojerdi B, Cohen LG. Cortical excitability changes induced by deafferentation of the contralateral hemisphere. Brain. 2002;125(0006–8950 (Print)):1402–1413. [DOI] [PubMed] [Google Scholar]
- 59.Tegenthoff M, Ragert P, Pleger B, et al. Improvement of tactile discrimination performance and enlargement of cortical somatosensory maps after 5 Hz rTMS. PLoSBiol. 2005;3(1545–7885 (Electronic)):e362. doi: 10.1371/journal.pbio.0030362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ragert P, Franzkowiak S, Schwenkreis P, Tegenthoff M, Dinse HR. Improvement of tactile perception and enhancement of cortical excitability through intermittent theta burst rTMS over human primary somatosensory cortex. ExpBrain Res. 2008;184(1432–1106 (Electronic)):1–11. doi: 10.1007/s00221-007-1073-2 [DOI] [PubMed] [Google Scholar]
- 61.Voller B, Floel A, Werhahn KJ, Ravindran S, Wu CW, Cohen LG. Contralateral hand anesthesia transiently improves poststroke sensory deficits. AnnNeurol. 2006;59(0364–5134 (Print)):385–388. doi: 10.1002/ana.20689 [DOI] [PubMed] [Google Scholar]
- 62.Janz Vernoski JL, Bjorkland JR, Kramer TJ, Oczak ST, Borstad AL. A Simple Non-invasive Method for Temporary Knockdown of Upper Limb Proprioception. J Vis Exp JoVE. 2018;(133). doi: 10.3791/57218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Allison T, McCarthy G, Wood CC, Darcey TM, Spencer DD, Williamson PD. Human cortical potentials evoked by stimulation of the median nerve. I. Cytoarchitectonic areas generating short-latency activity. JNeurophysiol. 1989;62(0022–3077 (Print)):694–710. [DOI] [PubMed] [Google Scholar]
- 64.Desmedt JE, Huy NT, Bourguet M. The cognitive P40, N60 and P100 components of somatosensory evoked potentials and the earliest electrical signs of sensory processing in man. Electroencephalogr Clin Neurophysiol. 1983;56(4):272–282. doi: 10.1016/0013-4694(83)90252-3 [DOI] [PubMed] [Google Scholar]
- 65.Cebolla AM, Palmero-Soler E, Dan B, Cheron G. Frontal phasic and oscillatory generators of the N30 somatosensory evoked potential. NeuroImage. 2011;54(2):1297–1306. doi: 10.1016/j.neuroimage.2010.08.060 [DOI] [PubMed] [Google Scholar]
- 66.Edwardson MA, Lucas TH, Carey JR, Fetz EE. New modalities of brain stimulation for stroke rehabilitation. Exp Brain Res. 2013;224(3):335–358. doi: 10.1007/s00221-012-3315-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carey LM, Matyas TA, Oke LE. Evaluation of impaired fingertip texture discrimination and wrist position sense in patients affected by stroke: comparison of clinical and new quantitative measures. J Hand Ther. 2002;15(0894–1130 (Print)):71–82. [DOI] [PubMed] [Google Scholar]