Abstract
Background:
With increasing intention to treat HIV as early as possible, evidence to confirm the safety and therapeutic drug concentrations of a nevirapine-based antiretroviral regimen in the early neonatal period is needed. This study aims to establish dosing of nevirapine for very early treatment of HIV-exposed neonates at high risk of HIV acquisition.
Methods:
IMPAACT P1115 is a multinational trial in which presumptive treatment for in utero HIV infection is initiated within 48 hours of birth in HIV-exposed neonates at high risk of HIV acquisition. The primary aim is achievement of remission, defined as no confirmed HIV RNA greater than the limit of detection for 48 weeks following antiretroviral therapy cessation. We report here results of the secondary aim of establishing a safe and therapeutic dosing regimen for nevirapine in very early treatment. The regimen used consisted of two nucleoside reverse transcriptase inhibitors plus nevirapine dosed at 6 mg/kg twice daily for infants ≥37 weeks gestational age (GA); infants 34 to ≥37 weeks GA received 4 mg/kg twice-daily for one week and 6 mg/kg twice-daily thereafter. A population pharmacokinetic model was developed from nevirapine concentrations at weeks 1 and 2. Safety was assessed through week 4.
Findings:
Of 438 infants analysed (36 with in utero HIV infection), 90% (389/434) were ≥37 weeks GA. Infants without confirmed in utero HIV infection received nevirapine for a median (Q1,Q3) of 13 (7,14) days. Measured dried-blood-spot nevirapine levels were above the minimum HIV treatment target (3 mcg/mL) in 90% [314/349; 95%CI (88%, 94%)] of infants at Week 1 and 87% (174/201, 95%CI (81 %, 91%)] at Week 2. In Monte Carlo simulations, Week 1 nevirapine levels exceeded 3 mcg/mL in 80% and 82% infants of ≥37 and 34-37 weeks GA, respectively. DAIDS Grade 3/4 adverse events possibly related to antiretrovirals occurred in 30/438 (7% [95%CI (5%, 10%)]) infants but never led to nevirapine cessation; neutropenia and anaemia were most common.
Interpretation:
Nevirapine at the dose studied was confirmed to be safe and provides therapeutic exposures. These data support nevirapine as a component of presumptive HIV treatment or short-term prophylaxis against HIV acquisition in high-risk neonates.
Keywords: nevirapine dosing, neonatal, HIV
Introduction
Despite great gains in the antiretroviral (ARV) treatment coverage of pregnant women with HIV, estimated at 82% globally in 20181 many women are still diagnosed late in pregnancy, leaving neonates at high risk of vertical acquisition in both resource-rich2 and resource-limited settings.3 For the past decade, guidelines from the World Health Organization4 have recommended combination two-agent regimens of zidovudine and nevirapine (NVP) as prophylaxis against HIV transmission for infants at high risk of acquisition.5 However, there is increasing interest in ARV regimens that can also be used for early treatment for those with in utero HIV.6 The case of a very early-treated infant who experienced HIV remission for 27 months without detectable plasma HIV despite not receiving ARVs raised the possibility that very early treatment has the potential to induce control of HIV without constant ARV treatment.7 Subsequent studies have shown that early treatment can lead to smaller latent HIV reservoirs in infants.8 as well as post-treatment control in adults.
One of the key barriers to early treatment of infants is the lack of antiretroviral agents with a formulation suitable for use in neonates and adequate neonatal safety and pharmacokinetic data to comprise a three-agent combination regimen that safely and reliably achieves treatment plasma concentration targets in the first days of life. Ritonavir-boosted lopinavir is recommended as first line for treatment of infants, but not until 2 weeks of life and 42 weeks post-menstrual age due concerns for toxicity.9 Treatment dosing of zidovudine and lamivudine is approved for neonates, but nevirapine pharmacokinetic and safety data in neonates are only available for prophylaxis in which the exposure goal is a plasma nevirapine concentration through the second week of life of 0·1 mcg/mL.10 In contrast, for older children and adults with HIV receiving nevirapine for treatment, the trough concentration target is 3·0 mcg/mL.11 In this study, we aimed to confirm a neonatal nevirapine dosing regimen that achieves treatment levels of ≥3·0 mcg/mL and is safe, within a three-drug combination begun soon after birth in neonates at high risk of HIV acquisition.
Methods
Study design and participants
The International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network has implemented a study titled, a “Very Early Intensive Treatment of HIV-Infected Infants to Achieve HIV Remission: A Phase I/II Proof of Concept Study (P1115),” (NCT02140255). The primary objective of this ongoing study is to assess HIV remission among neonates with in utero HIV who initiate antiretroviral therapy (ART) within 48 hours of birth. Here we present the results of the secondary objectives to determine the nevirapine dose needed to maintain concentrations adequate for treatment, and to assess the safety of very early ART in neonates.
Participants were infants ≥34 weeks gestational age (GA) at birth enrolled at ≤48 hours of age, with undetermined HIV-infection status born to women with presumed (at least one positive antibody test) or confirmed (two positive results collected at different time points, including HIV antibody or RNA) HIV infection who had not received ARVs during this pregnancy. We recruited participants at IMPAACT Network sites in North America, Africa, Asia, and South America from January 23, 2015 to September 4, 2017. The National Institutes of Health and each site’s local Institutional Review Board reviewed the protocol. Written informed consent was obtained from each participant’s mother or legal guardian for participation, in English or local language.
Procedures
Upon enrolment, participants received nevirapine at study doses (6 mg/kg/dose orally twice daily (BID) for term (≥37 weeks GA) neonates and 4 mg/kg/dose BID for the first week and thereafter increased to 6 mg/kg/dose BID for pre-term (34 to <37 weeks GA) neonates plus two nucleoside reverse transcriptase inhibitors (NRTIs) chosen by the primary provider and dosed according to World Health Organization or local guidelines. If in utero HIV-infection was confirmed from two positive nucleic acid tests (NATs, either DNA or RNA) from separate blood draws at least one hour apart and within 48 hours of birth, a fourth ARV agent, ritonavir-boosted lopinavir, was added at ≥14 days of life and ≥42 weeks postmenstrual age. If two such NATs were negative, study dosing of nevirapine was stopped and ARVs were changed to local standard-of-care prophylaxis between study weeks one and two.
We evaluated participants at enrolment and at weeks 1 and 2 (each +/− 2 days), and 4 (+/− 7 days) for clinical history and physical examination; laboratory evaluations at enrolment and week 2 included complete blood counts, alanine transaminase and aspartate transaminase, with additional testing at week 4 if any week 2 laboratory values were ≥ Grade 1 per the Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events (Version 1·0).12 Additional laboratory evaluations occurred when in utero HIV was confirmed, and at the 2 week visit. Participants for whom in utero HIV was not confirmed discontinued the study at week 4 except for continued follow-up of Grade ≥2 events until resolution or stabilization.
We selected the investigational dose of nevirapine using existing population models based on pharmacokinetic (PK) data from prophylaxis dosing among pre-term13 and term neonates, and treatment dosing in older children.14 The dosing regimen was predicted to maintain nevirapine concentrations above 3 mcg/mL, a level shown efficacious in adults, and limit the number with troughs greater than 10 mcg/mL, approximately the upper limit of adult ranges.11 We obtained plasma and dried blood spot (DBS) specimens for PK simultaneously at weeks 1 and 2 (if still taking nevirapine and not taking ritonavir-boosted lopinavir) from the first 30 participants; specimens were not drawn according to a schedule but the times of two preceding doses were recorded. In pre-specified interim assessment of the first 30 participants, nevirapine concentrations were observed to be highly variable, above 3 mcg/mL in 89% but also exceeding 10 mcg/mL in 43%; the dose was well tolerated by all.15 Given the wide range in trough concentrations, there was no single dose that would be predicted to achieve 80% success within the originally targeted window. Because the clinical evidence that troughs above 3 mcg/mL result in virologic suppression is much greater than evidence for a specific threshold concentration for toxicity, and because we did not see toxicity related to higher concentrations in the interim analysis, the investigational dose was approved for utilization in the remainder of the study, with continued monitoring for toxicity. After that point, we collected no additional plasma specimens; DBS specimens were collected for all participants at week 1 and for those still taking nevirapine, but not taking ritonavir-boosted lopinavir, at week 2.
We assayed plasma and DBS samples for nevirapine concentrations using high performance liquid chromatography with UV detection and lower limits of quantitation of 0·043 and 0·687 mcg/mL, respectively, at the IMPAACT pharmacology laboratory at the University of California, San Diego.16 The NIAID clinical pharmacology quality assurance (CPQA) program reviewed and approved plasma and DBS assays; within run variability of all controls were less than 15%. CYP2B6 pharmacogenomics were determined using real time PCR.17
Outcomes:
The primary outcome of this study is achievement of HIV remission, measured as no confirmed plasma HIV RNA above the limit of detection for 48 weeks following antiretroviral therapy cessation. Here we evaluate the following secondary outcome measures: 1) significant (grade 3 or 4 severity) signs/symptoms, laboratory values or diagnoses at least possibly, probably or definitely related to antiretroviral therapy; 2) nevirapine concentrations among treated neonates and young infants using study dosing; 3) lopinavir concentrations in combination with NVP. Additional secondary outcomes of the study are: 4) meeting study-defined eligibility criteria for antiretroviral therapy cessation. 5) meeting the selected study defined eligibility criteria for ART cessation among infants who also met the viral suppression criterion for ART cessation; 6) Among participants who discontinue ART and do not rebound within 12 weeks of discontinuation, a) HIV persistence as measured by plasma viremia (single copy), digital droplet DNA, replication competent HIV reservoirs and b) immune activation markers (%CD8+/DR+ T cells) and HIV-specific immune responses: HIV-specific antibodies and HIV-specific T cell responses.
Statistical Analysis
We determined the P1115 study overall sample size for the primary objective of the study, based on having a high probability of observing events of interest, such as remission or adverse events. We aimed to enrol at least 22 infants with in utero HIV to allow a probability >80% for observing events of interest occurring with frequencies of ≥1 in 100. Assuming an in utero transmission rate between 5 to 7%5 between 314 and 440 neonates would be needed to identify 22 participants with HIV; target enrolment was thus 440 infants. We made no formal sample size calculations for the PK objectives; association analyses were considered exploratory.
We evaluated the nevirapine dose using descriptive statistics by study week; in addition, a population PK analysis was performed using the computer program NONMEM version 7·4 (ICON, Dublin Ireland) to evaluate the potential impact of GA at birth, breast feeding status, treatment duration, and CYP2B6 genotype at position 516 on nevirapine PK with the 516 TT genotype deemed as poor metabolizer. We used a two-stage approach to assess these covariates. The first stage was a univariate assessment; covariates that improved the model’s objective function (MOF) by at least 3·84 (~p<0·05) were included in the second multivariate (backwards elimination) stage assessment. Covariates that improved the MOF by at least 7·88 (~p<0·005) in the multivariate evaluation were retained in the final population PK model. Bootstrap evaluation with 1000 replications generated model parameter confidence intervals using Wings for NONMEM version 7·4. We used Monte Carlo simulations to evaluate the nevirapine dosing regimen used in the current study. Nevirapine concentration time profiles for 4000 infants, 2000 term and 2000 pre-term, were simulated based on our derived model that included prematurity and treatment duration.
The safety analysis included data from enrolment through infant age <37 days, the upper limit of study week 4 visit window, with censoring at the time ritonavir-boosted lopinavir was initiated among participants with confirmed in utero HIV, death or subsequently determined to not have been exposed to HIV. Pre-specified safety outcome measures included DAIDS12 grade 3 or 4 signs/symptoms, laboratory values or diagnoses that were assessed as possibly, probably or definitely related to a drug in the ARV regimen per site clinicians with team adjudication, death, and permanent treatment discontinuation due to ARV toxicity. Time to event survival distributions were estimated by the Kaplan-Meier method and incidence rates assumed a Poisson distribution. CYP2B6 genotype and week 1 nevirapine concentration associations with the safety outcome measure were assessed with logistic regression. Summaries are presented overall and by HIV status. Safety analyses were performed with SAS version 9·4 (SAS Institute Inc., Cary, North Carolina, USA).
The P1115 study team reviewed safety data at least monthly. A Study Monitoring Committee (SMC) that did not include study team members reviewed safety data least annually; an SMC review could also be triggered based on guidelines outlined in the protocol. This trial was registered at clinicaltrials.gov (NCT02140255).
Role of the funding source
This trial was funded by that National Institutes of Health (NIH). The funder of the study contributed to study design but had no role in data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders (NIFH).
Results
Of 440 infants enrolled, 438 were included in this analysis (Figure 1), 389 of whom were born at term (Table 1). Nevirapine was initiated by 48 hours of age in 422 infants (97% of the 435 infants with ARV data). Zidovudine and lamivudine were given with nevirapine for most participants (422/435, 97%). For six infants, mothers had single positive HIV test results that were not subsequently confirmed. Among the 432 infants of mothers confirmed to have HIV, 36 infants [8·3%, (95% confidence interval: 5·9%, 11·4%)] were documented to have had in utero HIV infection of whom 66% (24/36) had ritonavir-boosted lopinavir added as a fourth agent before 37 days of age. Protocol specified safety follow-up was completed by 93% (409/438) of participants. The median (Q1, Q3) follow-up time was 28 (27, 33) days total (28 (27, 30) among infants without confirmed HIV; all but two infants with HIV were followed ≥35 days).
Figure 1. Participant Flow Chart.

^ Dried blood spots for pharmacokinetic testing were obtained from 349 infants at Week 1 and 201 infants at Week 2; *Initial HIV testing of mother was positive, but subsequent confirmatory testing negative. ~Safety follow-up data were included from all participants up to 37 days of age or at the time of study exit prior; data censored at time of initiating LPV/r prior for those with in utero HIV.
Table 1.
Participant Characteristics at Enrolment
| Participants with in utero HIV infection | Participants withoutin utero HIV infection | Total | ||
|---|---|---|---|---|
| (N=36) | (N=402) | (N=438) | ||
| Infants | ||||
| Sex | ||||
| Female | 24 (67%) | 194 (48%) | 218 (50%) | |
| Male | 12 (33%) | 208 (52%) | 220 (50%) | |
| Weight (kg)* | 2·8 (2·5,3·1) | 2·9 (2·6,3·2) | 2·9 (2·6,3·2) | |
| Twin Gestations | 1 (2.8%) | 5 (1.3%) | 6 (1.4%) | |
| Gestational Age* | 34 to < 37 weeks | 4 (11%) | 41 (10%) | 45 (10%) |
| ≥ 37 weeks | 32 (89%) | 357 (90%) | 389 (90%) | |
| Breastfeeding | 31 (86%) | 336 (84%) | 367 (84%) | |
| Region | Africa | 34 (94%) | 345 (86%) | 379 (87%) |
| Asia | 1 (3%) | 3 (1%) | 4 (1%) | |
| North America | 0 | 27 (7%) | 27 (6%) | |
| South America | 1 (3%) | 27 (7%) | 28 (6%) | |
| CYP2B6*(position 516) | GG | 5 (17%) | 124 (37%) | 129 (35%) |
| GT | 19 (66%) | 162 (48%) | 181 (49%) | |
| TT | 5 (17%) | 52 (15%) | 57 (16%) | |
| Background ARV* | ZDV+3TC | 36 (100%) | 386 (97%) | 422 (96%) |
| ABC+3TC | 0 (0%) | 12 (3%) | 12 (3%) | |
| ZDV+FTC | 0 (0%) | 1 (0·3%) | 1 (0·2%) | |
| Mothers | ||||
| Age (years) | 23·5 (20·5,28·5) | 27 (22,31) | 27 (22,31) | |
| Plasma HIV RNA [Logio(copies per mL)] | 4·7 (4·1,5·2) | 4.1 (3·5, 4·7) | 4.2 (3·5,4·8) | |
| <10,000 | 7 (19%) | 176 (45%) | 183 (43%) | |
| 10,000 to < 100,000 | 17 (47%) | 148 (38%) | 165 (39%) | |
| ≥ 100,000 | 12 (33%) | 65 (17%) | 77 (18%) | |
Values are n(%) or median (Q1, Q3). ARV=Antiretroviral; ZDV=zidovudine; 3TC=lamivudine; FTC=emtricitabine; ABC=abacavir.
Data missing on weight (n=4), gestational age (n=4), CYP2B6 genotype (n=71), background ARV (n=3), maternal HIV RNA (n=8).
The population PK analysis included 389 (89%) infants with a total of 602 DBS and 80 plasma concentrations (Appendix, page 1). Concentrations from paired DBS and plasma samples were similar, with plasma concentrations 9.3% greater than those from DBS (Appendix, page 2). Fifty-two (8·6%) of the DBS samples had nevirapine concentrations below the lower quantification limit (BQL) of the assay. All DBS samples that were not BQL had nevirapine concentrations more than double the quantitative limit. Thus, BQL concentrations were assumed to indicate substantial non-adherence and were excluded from the analyses. Among the concentrations from term and preterm infants above the lower quantification limit, the median [Q1, Q3] DBS nevirapine concentration was 8·36 [4·82,12·86] mcg/mL at week 1 (n=349), decreasing to 5·89 [3·92,8·73] mcg/mL at week 2 (n=201)(Figure 2). Overall, nevirapine DBS concentrations were above the treatment target lower limit (3 mcg/mL) in 90% (314/349, 95%CI (86%, 93%)) of samples at week 1 and 87% (174/201, 95%CI (81%, 91%)) at week 2. Week 1 DBS were greater than 10 mcg/mL in 38% (132/349, 95% CI (33%, 43%)) of samples and in 19% (39/201, 95% CI (14%, 26%)) at week 2. When stratified by GA at birth, DBS nevirapine levels in pre-term infants (n=36), who received a lower nevirapine dosage of ~4mg/kg for the first 7 days, overlap with concentrations seen in term infants (Figure 2).
Figure 2. Nevirapine Concentrations at Weeks 1 and 2 Visits.

Dried Blood Spot (DBS) nevirapine concentrations, excluding those deemed non-adherent with concentrations below the assay quantitative limit. Dashed lines represent target exposure range. The centred line is the line is the median, the box is the interquartile range, and the whiskers are the 5th and 95th percentiles.
A nevirapine one-compartment population PK model with first order absorption was developed from the combined DBS and plasma PK data to assess factors associated with nevirapine exposure (Appendix, pages 3-6). After 52 BQL samples were excluded from the modelling analysis, there were 630 nevirapine DBS or plasma concentrations from 389 participants available for the population PK modelling. Nevirapine treatment duration (log transformed) was an independently significant covariate, with apparent clearance more than doubling across the first 3 weeks of study. Among the 365/389 (94%) with available genotyping, CYP2B6 poor metabolizer (PM) status (516 TT genotype) appeared to be associated with a 26% [95% CI (10%, 40%)] lower apparent clearance (CL/F), although this association did not reach the a priori model improvement criterion (7·88 or p-value<0·005) for retention in the final population pharmacokinetic model. The sample matrix effect estimated from the population PK model was found to be modest and similar to that seen in the paired samples, with plasma on average 12% [95% CI (5%,18%)] higher than DBS concentrations. Monte Carlo simulations of study nevirapine dosing demonstrate achievement of median trough concentrations within the target range but with large variability (Appendix, pages 7,8). Simulation predicted median trough [10th, 90th percentiles] values in term infants were 7·98 [1·54, 23·35] mcg/mL and 5·35 [0·66, 24·6] mcg/mL at weeks 1 and 2, respectively. With the lower initial dose, pre-term infants had trough concentrations similar to term infants at week 1 and about 50% greater at week 2 once the preterm infants received the dose increase from 4 to 6 mg/kg. Based on the simulations approximately 80% of term and 82% of pre-term are expected to have trough concentration >3 mcg/mL at 1 week, declining to 66% and 76% at week 2. At week 2, 39% of term and 70% of pre-term infants troughs predicted to be <10 mcg/mL. Overall, 30 infants (7% of 438 [95% CI (5%, 10%)]) experienced at least one laboratory DAIDS Grade 3 or 4 adverse event considered at least possibly related to the ARV regimen for an overall incidence rate per person-year of 0·86 [95% CI (0·67, 1·09)] (Figure 3, Table 2). Nineteen percent [95% CI (8%, 36%)] of the 36 participants with in utero HIV experienced such events over a median (Q1, Q3) exposure time to the three-drug regimen of 20 (15·5, 36) days. Infants without in utero HIV stopped study dosing of nevirapine at a median (Q1, Q3) of 13 (7,14) days of life; 6% [95% CI (4%, 8%)] of the 402 infants without in utero HIV experienced Grade 3 or 4 events. Neutrophil counts decreased in 25 infants reaching Grade 3 in 16 infants and Grade 4 in nine infants (Figure 4). There was an overall decline in haemoglobin consistent with neonatal physiologic anaemia with 3 infants reaching Grade 3 and 3 infants Grade 4 (Figure 4). There was 1 episode of Grade 4 thrombocytopenia. No Grade 3 or 4 clinical (sign, symptom or diagnosis) adverse events were observed, but one Grade 2 rash considered possibly treatment-related spontaneously resolved. In a post-hoc analysis, safety was not significantly associated with CYP2B6 (odds ratio (OR) of grade 3 or 4 adverse event at least possibly related to study treatment (95% CI) 1·32 (0·48-3·67) for CYP2B6 TT vs. non-TT); infants with Week 1 nevirapine DBS concentration (>10mcg/mL vs. ≤10mcg/mL) had increased odds of experiencing a Grade 3 or 4 event on or after the concentration draw date at least possibly related to study drug [11% (95% CI 6%-17%) vs. 5% (95% CI 3%-9%), respectively, OR 2.20 (95%CI 0.97-4.98)]. Toxicity led to permanent zidovudine discontinuation in 2% (9/438) of participants (4 with in utero HIV, 5 without); in 3 cases for neutropenia (grades 2, 3, and 4), and in 6 cases for anaemia (grades 1, 2, 3, 3, 4, and 4). No other ARVs were permanently discontinued for toxicity. ZDV alone was temporarily held and restarted in 4 infants; twice for anaemia (grades 3 and 4) and twice for neutropenia (both grade 3). A grade 4 elevated lipase led to the full regimen of zidovudine/lamivudine/nevirapine being temporarily held, but was ultimately judged not related. One infant without in utero HIV died 11 days after enrolment from aspiration which was judged to be not related to study treatment. All adverse events, without regard to association with study drug, are summarized in the appendix (page 9).
Figure 3. Infant Time to Grade 3/4 Safety Event (at least Possibly Related).
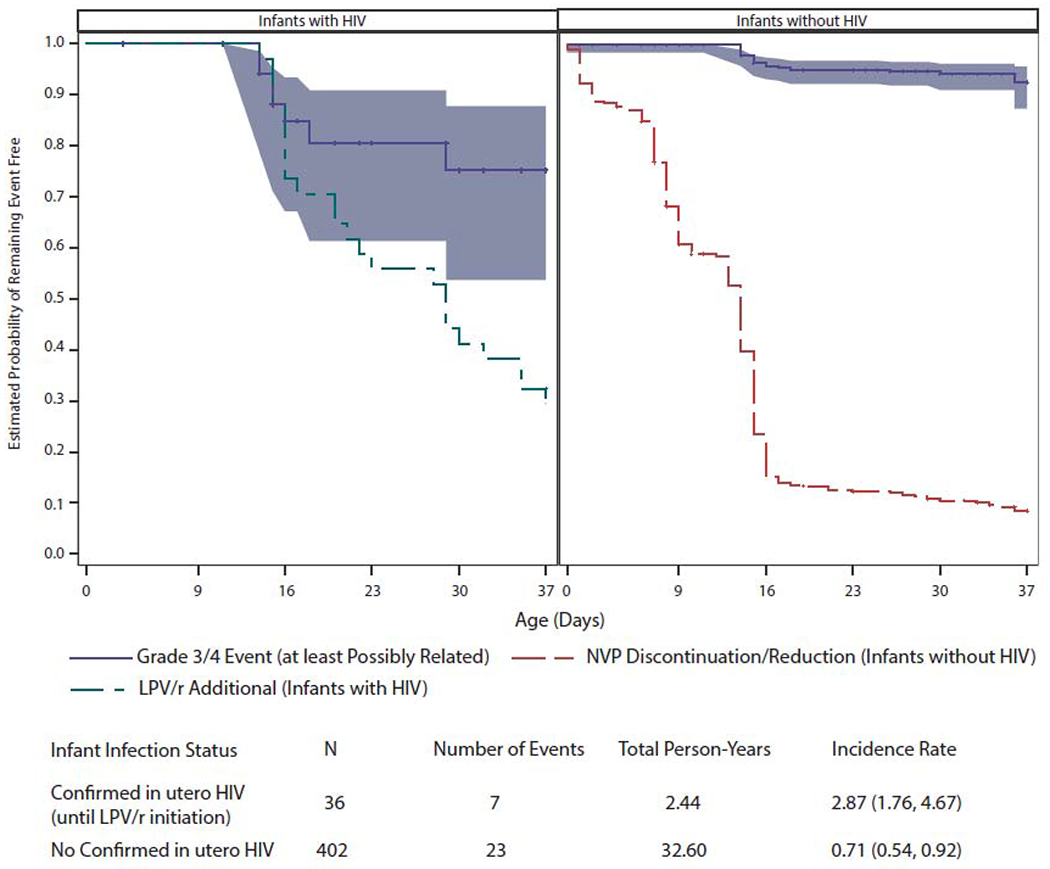
Age at first Grade 3 or 4 safety event (at least possibly related to one or more study ARV drugs) for infants with and without in utero HIV infection. Each blue line presents the estimated probability of remaining Grade 3/4 event free, where event time is censored (at tick marks) at the earlier of study follow-up discontinuation and 37 days of age. For infants with in utero HIV infection the Grade 3/4 event time is additionally censored at LPV/r initiation. The blue shaded band presents the 95% CI for the Grade 3/4 event free probabilities. The black dashed line presents the estimated probability of not yet initiating LPV/r among infants with in utero HIV infection. The dotted black line presents the estimated probability of not yet reducing/discontinuing NVP among infants without in utero HIV infection.
Table 2.
Grade 3 or higher adverse events assessed as possibly, probably or definitely related to study drug
| Infant in utero Infection Status | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Infected (N=36) Grade | Not Infected (N=402) Grade | Total (N=438) Grade | |||||||
| Event | 3 | 4 | Total (%) (95% CI) | 3 | 4 | Total N (%) (95% CI) | 3 | 4 | Total N (%) (95% CI) |
| Neutrophil count decreased | 4 | 1 | 5 | 13 | 7 | 20 | 17 | 8 | 25 |
| Haemoglobin decreased | 1 | 2 | 3 | 2 | 1 | 3 | 3 | 3 | 6 |
| Platelet count decreased | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 |
| Overall | 4 | 3 | 7 (19%) (8%, 36%) | 15 | 8 | 23 (6%) (4%, 8%) | 19 | 11 | 30 (7%) (5%, 10%) |
Each participant is counted once for the event class and overall total. For any given participant, the highest grade and relatedness for each safety event is counted. Safety data was censored at onset prior to initiation of ritonavir-boosted lopinavir or 37 days of age. Clopper-Pearson (exact) method used for confidence intervals.
Figure 4. Infant Absolute Neutrophil Count and Haemoglobin at Weeks 0 and 2 Visits.

Infants’ absolute neutrophil count (ANC) and Hemoglobin values at scheduled study visits entry and week 2 (triangles). The red line is a loess smooth.
Discussion
Our study demonstrates that a three-drug treatment regimen including nevirapine at the dose of 6 mg/kg twice daily for term infants appears safe and resulted in median nevirapine levels above the treatment trough target of 3 mcg/mL over the first 2 weeks of life in most neonates. Similar exposures were achieved among pre-term infants given 4 mg/kg twice daily for the first week, followed by 6 mg/kg thereafter. This nevirapine dosing paradigm also limited the frequency of high nevirapine levels (>10 mcg/mL) at Weeks 1 and 2 to 40% or less.
Safety and PK studies of nevirapine in infants ≥ 8 weeks of age established nevirapine treatment dosing in the late 1990s,18 but similar studies of nevirapine in the first weeks of life focused on regimens for perinatal prophylaxis, targeting troughs of 0·1 mcg/mL.10 Data from a case series of three infants from one institution suggested 6 mg/kg twice daily could achieve treatment levels.19 Average nevirapine trough concentrations from the BHP074 study were above 3 mcg/mL using 6mg/kg twice daily but were highly variable and frequently below at 3 mcg/mL at 2 weeks of age.20 Other neonatal nevirapine PK studies have been performed but not using treatment level dosing. In studies utilizing lower nevirapine infant doses, the initial neonatal apparent clearance is lower than in the current study. This may be due to the effect of dose on auto-induction of nevirapine metabolism. The apparent clearance from these prior nevirapine neonatal studies also show high variability which increases substantially over the first 2-4 weeks of life.21 USA guidelines currently recommend the dosing used in our trial, however acknowledge that it is investigational and based on modelling.22 Our results represent the first robust PK evaluation of this nevirapine dosing approach for treatment of HIV infection in neonates through 2 weeks of life, after which ritonavir-boosted lopinavir can be safely used.
While remaining above the target trough threshold in most infants through week 1, nevirapine declined significantly with a meaningful proportion falling below 3 mcg/mL by two weeks. This suggests additional nevirapine dosing considerations may be needed for infants extending nevirapine therapy beyond 2 weeks. At both weeks 1 and 2 there was high variability in nevirapine concentrations. This likely reflects a combination of maturation and nevirapine-based induction of cytochrome P450 liver isoenzymes CYP2B6 and CYP3A4.23 Genetic polymorphisms in liver isoenzymes are associated with differences in nevirapine metabolism; specifically the 516 TT variant of CYP2B6 leads to slower nevirapine apparent clearance in adults and older children17. In our study CYP2B6 516 TT was associated with lower apparent clearance in but did not reach the threshold (p<0·005) for PK model improvement in the multivariate analysis. The frequency of 516 TT in our study PK samples was modest (16%) and the rapid changes in nevirapine apparent clearance due to maturation and auto-induction along with the impact of other enzymes involved in nevirapine metabolism may have limited our ability to define CYP2B6 pharmacogenomic associations in this population. More importantly, the estimated magnitude of the PM status on CL/F is too small to warrant separate dosing recommendations based on CYP2B6 genotype (Supplemental Tables 2 and 3). We did not examine other CYP2B6 mutations or polymorphism in fetal CYP3A7.
The most common toxicities of nevirapine based regimens among older children with HIV are rash followed by granulocytopenia24 In HIV Prevention Trials Network study 040, high-risk HIV-exposed neonates randomized to receive 2 weeks of nelfinavir plus lamivudine and zidovudine experienced higher rates of ≥ grade 2 neutropenia (28%) than those receiving 3 doses of nevirapine plus daily ZDV over two weeks (15%), raising concern about increased bone marrow toxicity from 3-drug ARV combinations in neonates.5 The nevirapine-based treatment regimen of our study was well-tolerated by the infants. While only 6% of participants without in utero HIV infection experienced Grade 3 or 4 toxicitycompared to 19% among participants with in utero HIV. Higher event frequencies could be expected in this group in part due to the longer ARV exposure and higher intensity of safety evaluations (median of 3 versus 2 laboratory evaluations) which provide more opportunity to detect adverse events. It is challenging to determine the clinical significance of neutropenia in our study population, of whom 87% were African. Neutropenia, based on US reference values, is common among healthy African infants;25 one study of Zimbabwean infants found that 13% of healthy 10-day old infants met criteria for neutropenia per DAIDS 2004 guidelines.26 Safety was not significantly associated with CYP2B6 in this study, but the wide confidence interval on the odds ratio limits our ability to draw conclusions about the genotype comparison. We observed a higher risk of adverse events among infants who had Week 1 nevirapine concentrations in excess of 10mcg/mL but the confidence interval was wide and did not demonstrate a significant association; in addition, there were no nevirapine discontinuations for toxicity (i.e., nevirapine was managed as per protocol, either stopping when in utero infection was ruled out, or continued for those with in utero HIV). The prospective study design – enrolling infants at high risk of HIV acquisition and treating them with three ARVs dosed to achieve treatment levels until in utero infection is ruled out – generalizes to a scenario faced by clinicians globally. Until more rapid HIV nucleic acid testing is available, clinicians in both resource-rich and -poor settings must decide upon an ARV regimen for infants at high risk of in utero transmission while awaiting results of NAT birth testing. In our cohort, 8·3% of infants acquired HIV in utero; a high rate that likely reflects the elevated risk of transmission from mothers who received no ARVs during pregnancy. But one must also consider scenarios in which pregnant women may be taking ARVs but experience breakthrough viremia due to suboptimal adherence or ARV resistance. Observational cohort studies in South Africa found that 15% of women taking ART at conception experienced viremia (> 1,000 copies per mL) during pregnancy27. Even when provided same-day initiation of ART during antenatal care, up to 26% of women remain viremic at the time of delivery.28 While the aims for a regimen to treat infants born to viremic mothers receiving ARVs are the same, it is possible that the rate of adverse events and neonatal nevirapine metabolism could differ among infants who experienced transplacental exposure to maternal ARVs. A study of daily prophylaxis in Thai infants found that exposure to maternal efavirenz, a substrate and inducer of cytochrome P450 2B6, did not affect infant nevirapine levels.29
Our study had limitations. The limited follow-up of infants without in utero HIV infection does not allow for assessment of intra- or postpartum transmission. When in utero HIV was excluded by two negative NATs, infants stopped the study regimen by two weeks of age and left the study after the week 4 visit, making ascertainment of ultimate infection status of infants impossible. The study was not designed to assess prophylaxis or treatment efficacy and the non-randomized design for this proof-of-concept study limits inference on the regimen’s efficacy; furthermore, the PK targets were based on extrapolation from efficacy data in adults.
The selection of the optimal very early ARV regimen for infants born at high risk of HIV transmission must address a challenging risk-benefit balance. Many infants of women with sustained viremia during pregnancy will be born with HIV already acquired from in utero transmission and require ARV drug exposures appropriate for treatment. While some data suggest that early treatment with a three-drug, nevirapine-based regimen alone may be insufficient to achieve remission,30 other data underscore the potential benefits of early treatment on the immune response of infants.8 However, the majority of these infants will ultimately be uninfected and their ARV regimen must be safe while awaiting testing results and also provide effective postnatal prophylaxis against HIV. To this point, only 6% of infants without in utero HIV in our study experienced toxicity, in contrast to 19% of infants with in utero HIV who needed to continue treatment. A single regimen must thus satisfy two distinct objectives across a heterogeneous population of high-risk infants awaiting the results of HIV nucleic acid testing.
In conclusion, our data demonstrate that a three-drug treatment regimen with nevirapine dosed at 6 mg/kg twice daily for infants ≥37 weeks GA (and 4 mg/kg twice daily for one week and 6 mg/kg twice daily thereafter for infants 34 to <37 weeks GA) achieved concentrations appropriate for treatment and is well-tolerated. Although progress in newborn ARV therapy lags behind that of older children and adults, these data lay the groundwork for the ongoing study of antiretroviral drugs for early treatment of infants with HIV infection, and provide a starting point for clinical guidelines to manage high risk infants globally.
Supplementary Material
Putting research into context.
Evidence before this study
Pubmed was with searched using the terms “safety,” “pharmacokinetics,” “nevirapine” and “neonates” in preparation for the study (2012) and for this manuscript (September 5, 2020). Nevirapine has been used to treat children since the 1990’s but pharmacokinetic and safety data in neonates have been limited to regimens designed to achieve plasma concentration targets for prophylaxis rather than treatment. While there has been increasing desire to provide antiretroviral treatment to neonates with HIV infection as early as possible after birth, the small number of antiretrovirals with suitable formulations and sufficient neonatal safety and pharmacokinetic data is a barrier.
Added value of this study:
This study contributes novel comprehensive pharmacokinetic and safety data about nevirapine dosing to meet treatment concentration targets as part of a three-drug antiretroviral regimen in the first weeks of life for newborn infants with a gestational age of 34 weeks or higher at birth and at high risk of acquiring HIV. The study utilizes a simple dosing approach in a large international cohort of 438 neonates initiating presumptive 3-drug antiretroviral treatment within the first 2 days of life.
Implications of all the available evidence:
The pharmacokinetic and safety data about nevirapine presented here should form the basis for dosing guidelines for nevirapine-based antiretroviral regimens in the management of neonates with or at high-risk of perinatal HIV transmission globally and inform policy by the World Health Organization.
Acknowledgments
Funding: Support was provided by the National Institute of Allergy and Infectious Diseases (NIAID) with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (NIMH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration: This trial is registered at ClinicalTrials.gov (NCT02140255)
Data sharing statement
The data cannot be made publicly available due the ethical restrictions in the study’s informed consent documents and in the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network’s approved human subjects protection plan; public availability may compromise participant confidentiality. However, data are available to all interested researchers upon request to the IMPAACT Statistical and Data Management Center’s data access committee (email address: sdac.data@fstrf.org) with the agreement of the IMPAACT Network.
References
- 1.UNAIDS. Global HIV & AIDS statistics — 2019 fact sheet. 2019. https://www.unaids.org/en/resources/fact-sheet (accessed May 1 2020).
- 2.Camacho-Gonzalez AF, Kingbo MH, Boylan A, Eckard AR, Chahroudi A, Chakraborty R. Missed opportunities for prevention of mother-to-child transmission in the United States. AIDS 2015; 29(12): 1511–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chetty T, Vandormael A, Thorne C, Coutsoudis A. Incident HIV during pregnancy and early postpartum period: a population-based cohort study in a rural area in KwaZulu-Natal, South Africa. BMC Pregnancy Childbirth 2017; 17(1): 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach – 2nd edition. http://www.who.int/hiv/pub/arv/arv-2016/en/, 2016. [PubMed]
- 5.Nielsen-Saines K, Watts DH, Veloso VG, et al. Three postpartum antiretroviral regimens to prevent intrapartum HIV infection. N Engl J Med 2012; 366(25): 2368–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rainwater-Lovett K, Luzuriaga K, Persaud D. Very early combination antiretroviral therapy in infants: prospects for cure. Curr Opin HIV AIDS 2015; 10(1): 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luzuriaga K, Gay H, Ziemniak C, et al. Viremic relapse after HIV-1 remission in a perinatally infected child. N Engl J Med 2015; 372(8): 786–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Broncano P, Maddali S, Einkauf KB, et al. Early antiretroviral therapy in neonates with HIV-1 infection restricts viral reservoir size and induces a distinct innate immune profile. Scl Transl Med 2019; 11(520). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. 2020. https://aidsinfo.nih.gov/guidelines/html/2/pediatric-arv/0.
- 10.Mirochnick M, Nielsen-Saines K, Pilotto JH, et al. Nevirapine concentrations in newborns receiving an extended prophylactic regimen. J Acqulr Immune Deflc Syndr 2008; 47(3): 334–7. [PubMed] [Google Scholar]
- 11.de Vries-Sluijs TE, Dieleman JP, Arts D, et al. Low nevirapine plasma concentrations predict virological failure in an unselected HIV-1-infected population. Clin Pharmacokinet 2003; 42(6): 599–605. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Department of Health and Human Services NIoH, National Institute of Allergy and Infectious, Diseases Division of AIDS,. Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 1.0. [Updated August 2009], 2004. http://rsc.techres.com/docs/default-source/safety/table_for_grading_severity_of_adult_pediatric_adverse_events.pdf (accessed September 10 2018).
- 13.de Waal R, Kroon SM, Holgate SL, et al. Nevirapine concentrations in preterm and low birth weight HIV-exposed infants: implications for dosing recommendations. Pediatr Infect Dis J 2014; 33(12): 1231–3. [DOI] [PubMed] [Google Scholar]
- 14.Mirochnick M, Nielsen-Saines K, Pilotto J, Musoke P, Shetty A, Luzuriaga K, Capparelli E Nevirapine Dosing for Treatment in the First Month of Life. Conference on Retrovirus and Opportunistic Infection. Boston, MA; 2016. [Google Scholar]
- 15.Chadwick EG Qin MBY, Mirochnick M, Ruel T, Zadzilka A, Coletti A, Zimmer B, Tierney C, Persaud D, Cotton MF, Jean-Philippe P, Hazra R, Jennings C and Capparelli EV for the IMPAACT P1115 Team. Establishing a treatment dose of nevirapine for full-term neonates with perinatal HIV infection: IMPAACT P1115. International AIDS Society. Durbanm South Africa; 2016. [Google Scholar]
- 16.Dross SE, Rossi SS, Beck IA, et al. Variable and suboptimal nevirapine levels in infants given single-dose nevirapine at birth without maternal prophylaxis. AIDS 2014; 28(16): 2491–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saitoh A, Sarles E, Capparelli E, et al. CYP2B6 genetic variants are associated with nevirapine pharmacokinetics and clinical response in HIV-1-infected children. AIDS 2007; 21(16): 2191–9. [DOI] [PubMed] [Google Scholar]
- 18.Luzuriaga K, Bryson Y, Krogstad P, et al. Combination treatment with zidovudine, didanosine, and nevirapine in infants with human immunodeficiency virus type 1 infection. N Engl J Med 1997; 336(19): 1343–9. [DOI] [PubMed] [Google Scholar]
- 19.Bolaris MA, Keller MA, Robbins BL, Podany AT, Fletcher CV. Nevirapine Plasma Concentrations in Human Immunodeficiency Virus-Exposed Neonates Receiving High-Dose Nevirapine Prophylaxis as Part of 3-Drug Regimen. J Pediatric Infect Dis Soc 2017; 6(1): 102–4. [DOI] [PubMed] [Google Scholar]
- 20.Maswabi K, Ajibola G, Bennett K, et al. Safety and Efficacy of Starting Antiretroviral Therapy in the First Week of Life. Clin Infect Dis 2020; (Online ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cressey TR, Punyawudho B, Le Coeur S, et al. Assessment of Nevirapine Prophylactic and Therapeutic Dosing Regimens for Neonates. J Acquir Immune Defic Syndr 2017; 75(5): 554–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panel on Treatment of Pregnant Women With HIV Infection and Prevention of Perinatal Transmission. Recommendations for the Use of Antiretroviral Drugs in Pregnant Women with HIV Infection and Interventions to Reduce Perinatal HIV Transmission in the United States. 2020. https://aidsinfo.nih.gov/guidelines/html/3/perinatal/187/antiretroviral-management-of-newborns-with-perinatal-hiv-exposure-or-hiv-infection (accessed July 28 2020).
- 23.Riska P, Lamson M, MacGregor T, et al. Disposition and biotransformation of the antiretroviral drug nevirapine in humans. Drug Metab Dispos 1999; 27(8): 895–901. [PubMed] [Google Scholar]
- 24.Pollard RB, Robinson P, Dransfield K. Safety profile of nevirapine, a nonnucleoside reverse transcriptase inhibitor for the treatment of human immunodeficiency virus infection. Clin Ther 1998; 20(6): 1071–92. [DOI] [PubMed] [Google Scholar]
- 25.Scott-Emuakpor AB, Okolo AA, Omene JA, Ukpe SI. Pattern of leukocytes in the blood of healthy African neonates. Acta Haematol 1985; 74(2): 104–7. [DOI] [PubMed] [Google Scholar]
- 26.Wells J, Shetty AK, Stranix L, et al. Range of normal neutrophil counts in healthy Zimbabwean infants: implications for monitoring antiretroviral drug toxicity. J Acquir Immune Defic Syndr 2006; 42(4): 460–3. [DOI] [PubMed] [Google Scholar]
- 27.Chetty T, Newell ML, Thorne C, Coutsoudis A. Viraemia before, during and after pregnancy in HIV-infected women on antiretroviral therapy in rural KwaZulu-Natal, South Africa, 2010-2015. Trop Med Int Health 2018; 23(1): 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langwenya N, Phillips TK, Brittain K, Zerbe A, Abrams EJ, Myer L. Same-day antiretroviral therapy (ART) initiation in pregnancy is not associated with viral suppression or engagement in care: A cohort study. J Int AIDS Soc 2018; 21(6): e25133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anugulruengkitt S, Cressey TR, Suntarattiwong P, et al. Nevirapine Concentrations During the First Month of Life and Maternal Efavirenz Washout in High-Risk HIV-Exposed Infants Receiving Triple Antiretroviral Prophylaxis. Pediatr Infect Dis J 2019; 38(2): 152–6. [DOI] [PubMed] [Google Scholar]
- 30.Kuhn L, Strehlau R, Shiau S, et al. Early antiretroviral treatment of infants to attain HIV remission. EClinicalMedicine 2020; 18: 100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


